Pharmacologic Adjuncts to Intubation
In 1979, Taryle and coworkers1 reported that complications occurred in more than half the patients intubated in a university hospital ED. They called for improved house officer training in ET intubation, including “more liberal use of the procedures and agents used in the operating room (OR), including sedatives and muscle relaxers.”1 Since this report, the use of neuromuscular blockade and rapid-sequence intubation (RSI) has become the standard for emergency medicine practice.2,3 In addition to RSI, emergency physicians now use airway devices such as videoscopes and flexible fiberoptic bronchoscopes to manage difficult and complex airways.
Overview of Rapid-Sequence Intubation
The sequential process for quickly intubating a patient in an emergency situation is referred to as rapid-sequence intubation. The steps in performing RSI are often described by the six “P’s”: preparation, preoxygenation, pretreatment and induction, paralysis, placement of the tube, and postintubation management (Fig. 5-1). This sequential technique of rapidly inducing unconsciousness (induction) combined with muscular paralysis and optimal conditions for intubation has gained broad acceptance among ED clinicians. Obviously, many patients do not afford the clinician the time or opportunity to comply with the ideal scenario of trachael intubation described in this chapter. RSI, as described in this chapter, is the ideal method of emergency airway management for intubations not anticipated to be difficult.
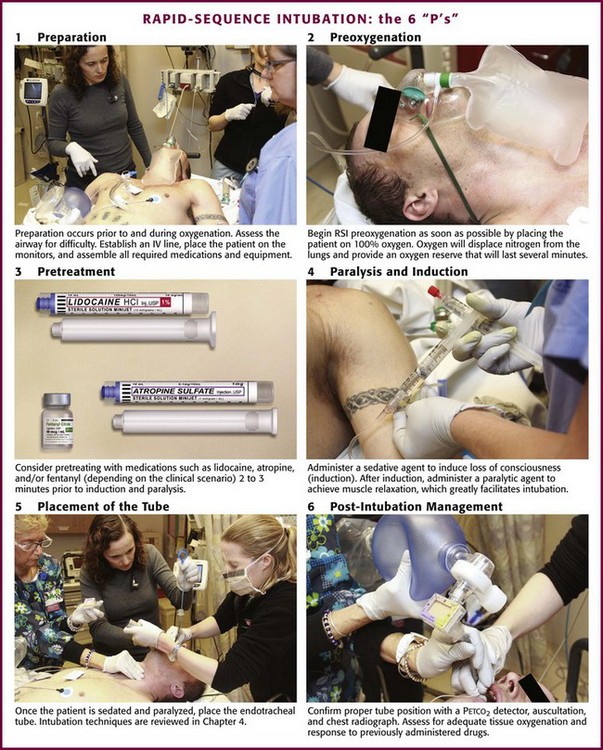
Figure 5-1 The 6 “P’s” of rapid sequence intubation. IV, intravenous; RSI, rapid-sequence intubation.
Begin RSI preoxygenation as soon as possible by administering 100% oxygen. The intent is to displace nitrogen from the lungs and replace it with an oxygen reserve that will last several minutes. Under optimal conditions, breathing 100% oxygen for 3 minutes has been demonstrated to maintain acceptable oxygen saturation for up to 8 minutes in previously healthy apneic individuals.4 Another method is to give four maximal breaths of 100% oxygen from a face mask, which can also maintain acceptable saturation for 6 minutes.4 Comparable results may not be expected in the ED setting, though, because of differences in the underlying health and cooperation of the patient population.
Paralysis and induction involve the induction of a state of unconsciousness with a sedative agent, followed immediately by muscle paralysis. A protocol for ED-based RSI is summarized in Box 5-1. ET intubation and RSI have also expanded beyond the ED into the prehospital setting. Prehospital RSI protocols use a sedative plus a paralytic for patients not in cardiac arrest, with success rates as high as 92% to 98% being demonstrated.5–9 As in the ED setting, without a full complement of medications, prehospital intubation becomes significantly more difficult, and success rates drop to approximately 60%.10 Rates of misplaced ET tubes and complications by paramedics may be much higher than previously reported.11,12 Recent studies indicate that outcomes may be worse for patients with traumatic brain injury intubated in the prehospital setting than in the ED.13 For these reasons many prehospital systems have moved away from the use of RSI.
The technique for proper ET tube placement is discussed in Chapter 2.1–3 Postintubation monitoring should assess for proper tube placement, adequate tissue oxygenation, and response to previously administered drugs. After laryngoscopy, the clinician should ensure ongoing analgesic and anxiolytic therapy.
Pretreatment: preventing the Complications of Intubation
The Pressor Response
In addition to the ubiquitous sinus tachycardia, a number of dysrhythmias have been reported after intubation. They are primarily ventricular in origin and include ectopic beats, bigeminy, and short runs of ventricular tachycardia. Bradyarrhythmias have been reported uncommonly. Electrocardiographic changes suggestive of ischemia have been documented, particularly in patients with dramatic increases in blood pressure.14,15 In 1977, Fox and colleagues16 reported two patients, both of whom deteriorated after induction of anesthesia and orotracheal intubation. This report has been widely quoted as evidence that the pressor response should be prevented. No studies have reported comparative data, and none have established a direct relationship between the response and subsequent clinical deterioration in a large patient population. It is also unclear whether attenuation of the pressor response will prevent dysrhythmias or electrocardiographic evidence of ischemia, although it is prudent to avoid sudden increases in blood pressure in unstable patients with acute cardiac or vascular disease.
Multiple studies have evaluated procedures to pharmacologically block the pressor response. Lidocaine has been the most extensively evaluated, but the results of these studies are inconclusive.17–23 No standards are universally accepted. Although it appears that administration of lidocaine, 1.5 to 2 mg/kg intravenously before intubation, may blunt the response, it is not clear that the reductions reported (10 to 15 mm Hg and 20 beats/min) are of any clinical significance. Other drugs, including thiopental, sodium nitroprusside, labetalol, nitroglycerin, verapamil, nifedipine, clonidine, fentanyl, sufentanil, etomidate, and magnesium, have shown variable responses.24–33
Of these drugs, fentanyl may be the most effective. It completely suppresses the pressor response at large anesthetic doses of 50 µg/kg,34–36 but considerably smaller doses may be effective. Two studies have shown marked suppression of the pressor response at doses of 5 to 6 µg/kg, although in both studies patients also received 5 mg/kg of thiopental.18,37 Fentanyl has blunted the pressor response when administered in conjunction with etomidate.32 The 1998 SHRED (Sedatives and Hemodynamics during Rapid-Sequence Intubation in the Emergency Department) study evaluated the pressor response to RSI and compared thiopental, fentanyl, and midazolam as induction agents.38
Midazolam, which is associated with the poorest intubating conditions and the most attempts required for intubation, showed a mean increase in heart rate of 17 beats/min. Thiopental, probably because of its direct myocardial depressant and venodilatory effects, decreased mean arterial pressure by about 40 mm Hg. Fentanyl recipients maintained a relatively neutral hemodynamic profile during intubation. Use of paralytics during intubation did not appear to alter the hemodynamic response associated with each sedative agent. One study isolated the effect of laryngoscopy from the effect of tube passage into the trachea. Adachi and colleagues39 found that pretreatment with 2 µg/kg of fentanyl could blunt the hemodynamic effects of tracheal tube passage, but not the hemodynamic effects of laryngoscopy.
It is important to note that even studies demonstrating blunting of the pressor response failed to show that this provided any real benefit to patients. It is likely that the pressor response is innocuous in the vast majority of patients, but it may be exaggerated and potentially harmful in those with preexisting hypertension, cardiovascular disease,40 or other vascular comorbid conditions, in whom sudden changes in hemodynamics may be detrimental.41 The pressor response may contribute to the rise in ICP that follows laryngoscopy, and it is potentially harmful in patients with intracranial pathology. Administration of lidocaine or fentanyl to blunt the pressor response may be appropriate in these subsets of patients, but no universal standards exist.
Intracranial Hypertension
Physical stimulation of the respiratory tract by maneuvers such as laryngoscopy, tracheal intubation, and ET suctioning is commonly associated with a brief rise in ICP. The exact mechanism responsible for this rise is unknown. One potential mechanism is the coughing and gagging that frequently follow manipulation of the upper airway and subsequent transmission of intrathoracic pressure to the cerebral circulation. An alternative explanation is the release of catecholamine that accompanies laryngoscopy, which causes a rise in mean arterial pressure and cerebral perfusion pressure. A small rise in ICP has been reported after the administration of succinylcholine. The value of pretreatment with defasciculating doses of neuromuscular blockers (NMBs) to prevent rises in ICP is unknown.42
Good evidence suggests that deep general anesthesia prevents the rise in ICP associated with intubation. Depending on the drug used, anesthesia may compromise cardiovascular performance and critically reduce cerebral blood flow.43–45 Consequently, the ideal anesthetic agents to facilitate intubation of patients with acute intracranial pathology may be those that have minimal effects on cardiovascular performance, such as etomidate or fentanyl. Etomidate has been demonstrated to prevent changes in both cerebral perfusion pressure and ICP after tracheal intubation of patients with space-occupying intracranial lesions.46
At the present time the clinical consequences of intubation-induced physiologic changes are not thoroughly understood. The role of drugs in preventing these changes is equally unclear. Despite this lack of data, it may be intuitively reasonable to attempt to protect patients at theoretical risk. The approach outlined in Box 5-2 is recommended.
Induction Agents
After premedication, a sedative agent is used to induce loss of consciousness. A number of diverse drugs are available in the ED to induce unconsciousness before intubation, including barbiturates, benzodiazepines, etomidate, ketamine, opiates, and propofol. The choice of a particular induction agent depends on the experience and training of the clinician, the patient’s clinical status, drug characteristics, and institutional protocols (Box 5-3).
Considerable evidence indicates that the sedative agent selected influences the quality of intubation conditions and the rapidity of their attainment. These effects persist even when paralytic agents are used. Commonly used drugs and their doses are summarized in Table 5-1.
TABLE 5-1
Recommended Anesthetic Dosing for Rapid-Sequence Intubation and Clinical Considerations
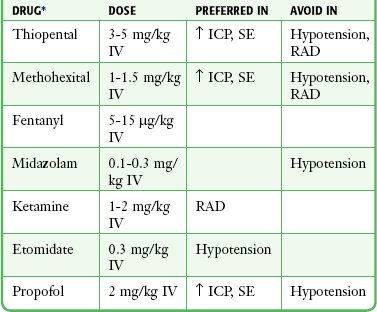
ICP, intracranial pressure; IV, intravenously; RAD, reactive airway disease; SE, status epilepticus.
*Any of these can drugs be used before the administration of a neuromuscular blocking agent to induce anesthesia (see text).
Barbiturates: Thiopental and Methohexital
The barbiturates rapidly cross the blood-brain barrier and induce unconsciousness in less than a minute. They are rapidly redistributed and then ultimately degraded in the liver. After a single IV dose of thiopental, anesthesia lasts 5 to 10 minutes, as compared with 4 to 6 minutes for methohexital.47,48 The recommended dose of thiopental is 3 to 5 mg/kg intravenously administered over a 60-second period. Methohexital is dosed at 1 to 1.5 mg/kg intravenously over a 30- to 60-second period.
Advantages of barbiturates as induction agents include their high potency, rapid onset, and short duration of action. They reduce cerebral metabolism, oxygen consumption, cerebral blood flow, and ICP.49,50 For this reason, thiopental is considered the agent of choice for induction of anesthesia and maintenance of long-term anesthesia in patients with elevated ICP. Short-term use of barbiturates during RSI has not been shown to have a protective effect on the CNS. Barbiturate use in hypovolemic patients may lead to systemic hypotension and impaired cerebral perfusion pressure, which may offset any cerebral-protective characteristics.38 Some evidence indicates that for ED RSI, thiopental may produce the best intubating conditions when used in conjunction with succinylcholine.51
The most significant complication of barbiturate therapy is depression of the vasomotor center and myocardial contractility, which can lead to significant hypotension. One study showed the average decrease in mean arterial pressure during RSI with thiopental to be 40 mm Hg.38 This is particularly pronounced in the presence of hypovolemia or cardiovascular disease.
Etomidate
Etomidate is an ultrashort-acting nonbarbiturate hypnotic agent that has been used successfully as an anesthesia induction agent in Europe since the mid-1970s and in the United States since 1983. A significant benefit of etomidate in the emergency setting is its lack of cardiodepressant effects.52,53 Several case series have now demonstrated its safety and effective use in ED RSI.54–58 Extensive experience with this agent now exists in both pediatric59 and adult patients, and it is an agent of choice for most ED intubations.
Etomidate is a carboxylated imidazole that is both water and lipid soluble. The drug reaches peak brain concentrations within 1 minute of IV infusion60 and induces unconsciousness within 30 seconds of administration. Its effects last less than 10 minutes after a single bolus dose.10 Redistribution of the drug is quite rapid, which accounts for its short duration of action. Etomidate is rapidly hydrolyzed in the liver and plasma and forms an inactive metabolite excreted primarily in urine.60 The recommended dose is 0.3 mg/kg via rapid IV bolus. There is virtually no accumulation of the drug, and anesthesia may be maintained through repeated doses; however, etomidate should not be used as an infusion or in repeated bolus doses for maintenance of sedation after intubation in the ED.61
Etomidate acts on the CNS by stimulating γ-aminobutyric acid (GABA) receptors and depressing the reticular activating system. It produces electroencephalographic changes similar to those produced by barbiturates as patients pass rapidly through light to deep levels of surgical anesthesia. Because etomidate has no analgesic activity,60 it should be used in conjunction with a parenteral analgesic when painful conditions are being treated, although it is most commonly used as a sole induction agent for intubation. Etomidate decreases cerebral oxygen consumption, cerebral blood flow, and ICP but appears to have minimal effects on cerebral perfusion pressure.46 Most importantly in the ED setting, etomidate is characterized by hemodynamic stability without significant changes in mean arterial pressure,53,54,57 although a slightly increased heart rate may be observed.61 Etomidate is suggested for induction of patients with significant cardiovascular disease requiring RSI. The hemodynamic stability that it provides and the absence of induced hypertension make it preferable to other sedatives. This hemodynamic stability persists even in patients with preexisting hypotension.62 The most common immediate side effects of etomidate are pain on injection, nausea, vomiting, and myoclonic jerks.63 Pain on injection is reported in up to two thirds of patients. Use of a large vein, simultaneous saline infusion, and opioid premedication can reduce the discomfort in appropriate situations.64 Myoclonic activity has been reported in about one third of cases and is believed to be caused by disinhibition of subcortical activity rather than CNS stimulation and does not represent seizure activity.60 This sometimes dramatic effect can be avoided through the use of NMBs and is rarely seen in the ED, where paralytic agents are regularly used with RSI. No treatment of myoclonus is usually necessary, and it is generally of no clinical significance. If persistent, an intravenous benzodiazepine may be administered. Etomidate need not be avoided in patients with seizure disorders, status epilepticus, head injury, or stroke.
Some degree of altered adrenal function has been demonstrated even after a single dose of etomidate.65,66 The true clinical effect is unknown, but because the alteration in adrenal function appears to persist for 12 to 24 hours, there is theoretical concern about potential clinical consequences.10,67,68 Etomidate is a reversible inhibitor of 11β-hydroxylase, the enzyme that converts 11-deoxycortisol to cortisol. Although cortisol levels do not fall below the normal physiologic range, even a single induction dose of etomidate causes a measurable decrease in the level of circulating cortisol that occurs in response to the administration of exogenous adrenocorticotropic hormone (ACTH). Despite concerns regarding the safety of etomidate in the specific setting of adrenal insufficiency related to sepsis, no well-designed, prospective trial has shown adverse effects from a single dose of etomidate used for intubation in patients with sepsis or septic shock. Overall, however, the literature on this subject provides conflicting results.69–86
When comparing etomidate with ketamine, a multicenter randomized trial of critically ill patients requiring emergency intubation found no significant difference in organ failure score, 28-day mortality, or intubating conditions between patients given etomidate for induction and those given ketamine.83 No serious, drug-related adverse events were reported with either medication. Even though adrenal insufficiency occurred at a higher rate in the etomidate group (86%), it also developed in approximately 48% of patients receiving ketamine.
Although historically no individual study is sufficiently powered to detect differences in mortality from the adrenal effects of etomidate, a systematic review of 20 studies in which etomidate was given in a bolus dose as part of induction for tracheal intubation found that etomidate does not have a significant proven effect on mortality.84 Declines in serum cortisol concentration were more prevalent in etomidate recipients than in those who did not receive etomidate in the large majority of studies, but the effect did not persist beyond 5 hours. When intubating a critically ill patient with possible adrenal insufficiency, the clinician must weigh the theoretical risk of cortisol suppression against the hemodynamic instability that may be caused by alternative induction agents. Overall, the mixed results in the current literature do not justify recommendations to avoid using etomidate for induction in patients with sepsis. Pending more definitive studies and subject to change as additional evidence is forthcoming, etomidate is an acceptable induction agent for patients with severe sepsis.87
Ketamine
Ketamine is a water- and lipid-soluble drug that rapidly penetrates into the CNS. Like the barbiturates, ketamine accumulates rapidly and then undergoes redistribution with subsequent degradation in the liver.88 The recommended dose of ketamine before intubation is 1 to 2 mg/kg administered intravenously over a 1-minute period. Anesthesia occurs within 1 minute of completing the infusion and lasts 5 to 10 minutes. A smaller additional dose (0.5 to 1 mg/kg) may be given 5 minutes after the initial dose if needed to maintain anesthesia. Simultaneous administration of succinylcholine and midazolam is recommended to provide adequate muscle relaxation and to decrease the incidence of postanesthesia emergence reactions, respectively. The intramuscular dose for intubation has not been well studied, but a suggested dose is 4 to 5 mg/kg. Onset of action occurs within 2 to 3 minutes. Because of its good vascularity, the anterior thigh muscle is theoretically the preferred site for administration.
Unlike other anesthetic agents that depress the reticular activating system, ketamine acts by interrupting association pathways between the thalamocortical and limbic systems. Characteristically, the eyes remain open, and patients exhibit spontaneous, though not purposeful movements. Increases in blood pressure, heart rate, cardiac output, and myocardial oxygen consumption are seen, most likely mediated through the CNS. In vitro studies indicate that ketamine is a myocardial depressant, but the CNS-mediated pressor effects generally mask the direct cardiac effects,89,90 thus making it potentially useful in patients with hemorrhagic shock or hypotension. Respirations are initially rapid and shallow after ketamine administration, but they soon return to normal.
In one study, eight patients with traumatic brain injury and elevated ICP who were sedated with propofol were given different doses of ketamine (1.5, 3, or 5 mg/kg).91 Ketamine did not alter cerebral hemodynamics at any time. In another study, researchers randomly assigned 25 patients with traumatic brain injury to sedation with either ketamine and midazolam or sufentanil and midazolam.85
Other studies suggest that ketamine does not interfere with cerebral metabolism or increase cerebral oxygen consumption and does not reduce regional glucose metabolism.86 Ketamine can also offset any decrease in mean arterial pressure caused by fentanyl, a drug commonly used as part of RSI in patients with a head injury.92
The most promising use of ketamine as an intubation adjunct has been in the setting of acute bronchospastic disease. Ketamine relaxes bronchial smooth muscle either directly through enhancement of sympathomimetic effects or indirectly through inhibition of vagal effects. Ketamine also increases bronchial secretions and may decrease the incidence of mucous plugging, which is commonly seen in decompensating asthmatic patients.93 Clinical reports have demonstrated a reduction in airway resistance and an increase in pulmonary compliance within minutes of ketamine administration.88,94 Bronchospastic patients struggling to breathe and unable to tolerate oxygen masks or bronchodilators because of hypoxic encephalopathy will continue to breath deeply and rapidly with ketamine anesthesia, thereby allowing the maximum delivery of oxygen before a more elective intubation (Fig. 5-2).
A potential side effect that has raised some concern about the use of ketamine for RSI is its tendency to produce postanesthesia emergence reactions, a characteristic that it shares with the structurally similar drug phencyclidine. The reemergence phenomenon, such as disturbing dreams as patients emerge from ketamine-induced anesthesia, is much less of a concern when the drug is used for RSI. In fact, there are no convincing data indicating that when used for RSI, ketamine produces unpleasant reemergence reactions that are significant or common enough to limit its use for this purpose. One study found that although dreams occurred frequently following sedative doses of ketamine, they were generally pleasant and the frequency of reemergence phenomena and delirium was markedly reduced by the concomitant use of a benzodiazepine.95 Rarely, reactions may be marked and distressing, with symptoms including floating sensations, dizziness, blurred vision, out-of-body experiences, and vivid dreams or nightmares. The true incidence of emergence reactions following RSI is unknown, and clinical experience suggests that it is not an issue for RSI in the ED. Such reactions are less common in children than in adults and may be suppressed with benzodiazepines. Both diazepam and lorazepam appear to be useful in adults, but the latter is more effective, most likely because of its enhanced amnestic effect. Midazolam is effective in adult patients at doses of 0.07 mg/kg96 and may be the preferred agent because it has potent amnestic effects and a short duration of action. Studies in children have failed to show a reduction in the rate of emergence reactions in those treated with both ketamine and midazolam.97,98
Despite preservation of pharyngeal and laryngeal reflexes in patients sedated with ketamine, aspiration can still occur.99,100 Ketamine does not relax skeletal muscles, and production of the desired intubating conditions requires the simultaneous administration of a paralytic agent, thereby removing all upper airway reflexes.
Propofol
Propofol is a popular drug among anesthesiologists for OR-based induction and is ideal for ED use. Multiple reports have demonstrated the safety and efficacy of propofol for ED procedural sedation.101,102 Although some ED clinicians now intubate only with propofol, eschewing longer-acting agents and paralysis, its role as an adjunct to intubation in the ED is undergoing evolution.
Propofol is an alkylphenol sedative-hypnotic used for induction and maintenance of general anesthesia. The drug has no analgesic activity, but it does exert a powerful amnestic effect. Propofol also tends to decrease vomiting through an unknown antiemetic effect. It produces dose-dependent depression of consciousness ranging from light sedation to coma. Propofol is a highly lipophilic, water-insoluble compound that undergoes rapid uptake by vascular tissues, including the brain, followed soon afterward by redistribution to muscle and fat. The drug is metabolized by the liver and excreted in urine.103,104 After an induction dose of 2 mg/kg intravenously, unconsciousness occurs within 1 minute and lasts for 5 to 10 minutes. A smaller dose (1 to 1.5 mg/kg) is recommended in the elderly and when simultaneously administering other CNS depressants. Because propofol has a short duration of action and patients rapidly regain consciousness, repeat boluses are not a practical way to maintain a desired level of sedation.103,104 Therefore, a slow drip infusion of 3 to 5 mg/kg/hr titrated to effect is preferred.
Propofol suppresses sympathetic activity, thereby causing myocardial depression and peripheral vasodilation, particularly in the elderly or hypovolemic patients and when administered simultaneously with opioids. Hypotension can be minimized with fluid loading or reversal with 5 to 10 mg of IV ephedrine. Propofol reduces cerebral blood flow and may cause mild CNS excitatory activity (e.g., myoclonus, tremors, hiccups) during induction. Pain on injection occurs commonly, even when the drug is infused slowly.103,104 Pretreating the infusing vein with 3 mL of 1% lidocaine (30 mg) injected over a 30-second period or choosing a large antecubital vein will ameliorate this pain.
Benzodiazepines (Midazolam)
Midazolam has replaced diazepam as a preoperative sedative agent, even in elderly patients.105–109 When compared with diazepam, the primary advantages of midazolam include a twofold increase in potency, shorter half-life, and decreased potential for cardiorespiratory depression. Midazolam is water soluble in an acid medium and does not require suspension in propylene glycol like other benzodiazepines. It is rarely associated with phlebitis and can be given intramuscularly when a very rapid onset of action is not required. At physiologic pH, midazolam is lipophilic and rapidly accumulates in the CNS, with onset of sedation occurring in as little as 1 to 2 minutes. Outside the CNS it accumulates in fatty tissue and extensively binds to plasma, which accounts for the paucity of non-CNS side effects. Its half-life of elimination is 1 to 4 hours and is dependent on release of the drug from adipose tissue and protein-binding sites. The period of sedation after a single IV dose is considerably shorter. Emergence from a 0.15-mg/kg dose occurs in 15 to 20 minutes.110
Clinical experience using midazolam with or without fentanyl for procedural sedation is considerable, and it is considered both safe and effective in the ED setting. The recommended dose for moderate sedation with midazolam is 0.05 to 0.1 mg/kg given in 1-mg boluses and not exceeding 2.5 mg over a period of 2 minutes. Doses upward of 0.1 mg/kg are often needed to produce good conditions for intubation.38,06
The potential for adverse effects with midazolam is similar to that with other benzodiazepines. A small increase in heart rate is seen frequently, as is a small decrease in systolic blood pressure.111 Changes in blood pressure may be exaggerated in the presence of hypovolemia.112 An ED-based study reported a mean 10% decrease in systolic blood pressure, with 19% of intubated patients having systolic blood pressure lower than 90 mm Hg.111 The cardiac index and coronary artery blood flow are not generally affected. In the prehospital setting, hypotension with midazolam was found to be dose related,113 and it should be used cautiously in patients with hypovolemia or traumatic brain injury. Respiratory depression may occur even at standard doses, but it most often follows rapid administration of an excessive dose. Respiratory depression is also more likely to occur in debilitated or elderly patients and in those simultaneously receiving opioids. The effects of midazolam are rapidly reversed by administration of the benzodiazepine antagonist flumazenil. A study of various induction agents for ET intubation suggested that the use of midazolam alone for RSI may be associated with suboptimal intubating conditions and increased difficulty.51 Other induction agents may be preferred over midazolam alone during RSI.
Opioids (Fentanyl)
Although any of several opioids administered intravenously could be used to induce unconsciousness, fentanyl has significant advantages over other opioid agents. A synthetic opioid, it has been widely used since its introduction in 1968. Its favorable pharmacologic properties include rapid serum clearance, high potency, and minimal release of histamine.114–117 Fentanyl quickly crosses the blood-brain barrier and produces analgesia in as little as 1 to 2 minutes. Serum levels decline rapidly from peak concentrations because of extensive tissue uptake.118,119 Unlike morphine, the brain concentration of fentanyl falls in conjunction with the serum level. The duration of analgesic action is 30 to 40 minutes, although at high doses a second peak of activity may be seen several hours later because of release of the bound drug from tissue stores. Fentanyl is about 50 to 100 times as potent as morphine.120 This unique combination of potency and short half-life permits the administration of numerous small doses that can be titrated to the desired clinical effect. Similar to other opioids, fentanyl is competitively reversed with naloxone.
The relative safety of fentanyl permits considerable latitude in dosing. When used as a primary anesthetic agent for major surgical procedures, doses ranging from 50 to 100 µg/kg produce minimal side effects.121 Comparatively tiny doses produce sedation, and 3 to 5 µg/kg, given at a rate of 1 to 2 µg/kg/min, is generally an effective analgesic dose. More rapid administration will cause greater depression of the level of consciousness. Mostert and coworkers114 reported successful awake intubation in 99 of 103 patients who were administered an average cumulative dose of 3.7 µg/kg. Most of these patients were able to follow commands, and many recalled events surrounding the intubation. A small percentage could not be intubated even after receiving 500 µg of fentanyl.
Unlike other opioids, fentanyl causes little or no release of histamine, and its use is seldom associated with emesis or hypotension. It is probably the safest opioid to use in hypovolemic patients. Fentanyl also has significantly fewer emetic effects than other opioids do. Adverse effects that have been reported with fentanyl are few and primarily follow the rapid IV infusion of very large doses. Like other opioids, fentanyl may cause rigidity of the skeletal musculature, including the chest wall and diaphragm. Rigidity occurs with doses in excess of 15 µg/kg, but it has also been reported with doses as low as 10 µg/kg and may also be related to rapid administration.114,122 The muscular rigidity may be prevented or treated with standard doses of succinylcholine or naloxone.123 The most common significant complication with fentanyl is respiratory depression, and it generally occurs when fentanyl is given in combination with other CNS depressants or in excessive amounts.124
Neuromuscular Blocking Agents
NMBs are used to achieve muscle relaxation for intubation. They permit complete airway control and greatly simplify visualization of the vocal cords. This is particularly important when intubation must be performed quickly under less than ideal circumstances. Sedatives may provide some muscle relaxation, but this requires rapid administration of large doses, which poses a risk for depression of the cardiovascular system. The combination of a paralytic agent and a sedative or analgesic agent is generally superior to the use of either agent alone. A 1999 report showed an 18% failure-to-intubate rate with a sedative alone versus a 0% failure rate for sedatives plus paralytics.125 Procedural complication rates such as significant airway trauma and aspiration were also markedly higher in the group receiving sedation only.
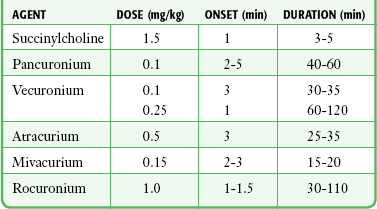
NMBs are classified as either depolarizing or nondepolarizing. Depolarizing agents mimic the action of acetylcholine (ACh) and produce sustained depolarization of the neuromuscular junction, during which time muscle contractions cannot occur. Nondepolarizing agents competitively block the action of ACh at the neuromuscular junction and prevent depolarization and therefore muscle contractions. Commonly used NMBs and their dosages and characteristics are listed in Table 5-2.
Succinylcholine
The standard depolarizing agent currently in use is succinylcholine.126 It has a chemical structure similar to that of ACh and depolarizes the postjunctional neuromuscular membrane. Administration is followed by a brief period of muscle fasciculations that correspond to the initial membrane depolarization and muscle fiber activation. Unlike ACh, which is released in minute amounts and hydrolyzed in milliseconds, succinylcholine requires several minutes for breakdown to occur. During this time the neuromuscular junction remains depolarized, but the muscles relax and will not contract again until the neuromuscular end plate and adjacent sarcoplasmic reticulum return to the resting state and are again depolarized. Relaxation proceeds from the small, distal, rapidly moving muscles to the proximal, slowly moving muscles. The diaphragm is one of the last muscles to relax.127
Succinylcholine is rapidly degraded in serum by the enzyme pseudocholinesterase, and the duration of action of a single dose is 3 to 5 minutes. Relaxation may be maintained by repeated IV injections. Prolonged or repeated use of the drug enhances its vagal stimulatory effects, thereby resulting in bradycardia, hypotension, and other muscarinic effects. These effects may be seen even at normal doses, particularly in children.128 For this reason, atropine pretreatment at a dose of 0.02 mg/kg has been recommended in all small children and for adults receiving multiple doses, although the need and optimal dose are still in question.128 Repeated dosing of succinylcholine may produce a desensitization blockade and create a scenario in which the neuromuscular membrane returns to the resting state and becomes resistant to further depolarization by succinylcholine.129,130 In general, there is little need for repeated doses of succinylcholine if appropriately dosed the first time. If paralysis in excess of 3 to 5 minutes is desired, longer-acting, nondepolarizing agents such as vecuronium or pancuronium should be used.
The recommended dose of succinylcholine is 1 to 1.5 mg/kg given intravenously. Dosages at the upper end of this range are suggested to guarantee complete relaxation and avoid the need for repeated dosing. Dosage calculations should also be based on actual, not lean body mass because of alterations in both volume of distribution and pseudocholinesterase activity.131 Neonates and infants require a slightly higher dose of succinylcholine (2 mg/kg intravenously) as a result of their higher volume of distribution.61,132 It is crucial that succinylcholine be administered as a rapid bolus because slow administration may lead to incomplete relaxation. Use of a rapid 20- to 30-mL saline flush after IV administration may enhance its desired effect.
There are a number of potential adverse effects of the use of succinylcholine ranging from minor to life-threatening. Muscle fasciculations accompany the initial depolarization of the neuromuscular membrane. Fasciculations are most prominent in muscular patients and create deep, aching muscle pain that may last for days.133 It is unclear whether any regimen will totally prevent the succinylcholine-induced fasciculations (seen in 73% to 100% of patients) and myalgias (seen in 10% to 83% of patients), with varying effects reported after numerous interventions.134 Interestingly, higher doses of succinylcholine may be associated with less myalgia. Traditionally, fasciculations have been prevented by the preadministration of a defasciculating dose (0.01 mg/kg) of pancuronium or vecuronium. The evidence available does not suggest that succinylcholine worsens outcomes in at-risk patients, nor does any evidence suggest that defasciculation improves outcomes in at-risk patients.135
A major consideration in the use of succinylcholine is its propensity to cause hyperkalemia. This electrolyte disturbance is believed to occur secondary to asynchronous depolarization of muscle cells and resulting cellular injury. Elevation in serum potassium occurs in normal patients after standard doses but is typically clinically inconsequential, with increases of less than 0.5 mmol/L (mEq/L).136 Increases in potassium are not prevented with defasciculating doses of nondepolarizing agents. Marked hyperkalemia is associated with increased extrajunctional muscle ACh receptors, which develop in patients with prolonged diseases of the neuromuscular system. Susceptibility may occur within as little as 5 to 7 days and persist indefinitely. In these cases the hyperkalemic response may be as much as 5 mmol/L (mEq/L). Such conditions include late severe burns,137 major muscle trauma,138 spinal cord injury, muscular dystrophy, multiple sclerosis, and other upper motor neuron diseases139,140 such as amyotrophic lateral sclerosis. These large elevations occur only in patients who have had significant tissue injury or muscle denervation for several days or weeks before the use of succinylcholine. Importantly, succinylcholine is not contraindicated in the initial management of patients with acute injuries, including burns, major crush injuries, and spinal cord injuries. Succinylcholine is also not contraindicated in normokalemic patients with renal failure because the magnitude of the rise in serum potassium is the same as in patients with normally functioning kidneys.141 A retrospective review of the use of succinylcholine in 38 operative cases with moderate pre-RSI hyperkalemia (5.6 to 7.6 mmol/L) suggested that the risk for hyperkalemia-related complications may be lower than feared.142 In a review of more than 41,000 intubations (38 patients had hyperkalemia with a mean serum potassium level of 5.9 mmol/L), Schow and colleagues142 concluded that it is safe to administer succinylcholine to patients with a potassium level of 5.5 to 6.0 mEq/L. Regardless, succinylcholine is best avoided (if other equally effective pharmacologic options such as rocuronium exist) in the setting of known or suspected preexisting hyperkalemia (e.g., renal failure patients not receiving regular dialysis or demonstrating a wide QRS complex). Concern has also been raised over the use of succinylcholine in the pediatric population because of rare case reports of hyperkalemic cardiac arrest after administration to children with undiagnosed myopathies. Succinylcholine is currently recommended for use in pediatric patients only under emergency circumstances when the airway must be secured rapidly.132
Malignant hyperthermia is a rare complication with an autosomal dominant inheritance pattern that is triggered by multiple anesthetic agents, including succinylcholine. Most provocative agents, such as halothane, are not used in the ED setting, and it is extremely rare for the ED physician to encounter this complication. It occurs in approximately 1 in 15,000 children and 1 in 50,000 adults.143 The clinical syndrome consists of high fever, tachypnea, tachycardia, cardiac arrhythmias, hypoxia, acidosis, myoglobinuria, and impaired coagulation. Unabated muscle contractions mediated by abnormal calcium channels are the physiologic basis for this condition.144 Treatment includes aggressive cooling measures, volume replacement, and correction of hypoxia and acid-base and electrolyte abnormalities. Dantrolene sodium, a direct-acting skeletal muscle relaxant, is thought to be effective in reducing the muscle hypermetabolism that causes the dramatic hyperpyrexia.145 An associated abnormal response to succinylcholine is isolated masseter spasm,146 but it can occur in isolation or portend the subsequent development of malignant hyperthermia. Though rare, it has been reported in the emergency medicine literature.147 In this condition, forcible sustained contraction of the masseter muscles occurs and prevents mouth opening and oral intubation. Management is controversial, with recommendations ranging from use of a bag valve mask, securing a surgical airway until the contraction abates, to attempting to suppress the contractions through administration of a nondepolarizing NMB.
Prolonged paralysis after the administration of succinylcholine may occur in clinical conditions that result in decreased pseudocholinesterase levels and subsequent decreased metabolism of succinylcholine. Physiologic states associated with this condition include hepatic disease, anemia, renal failure, organophosphate poisoning, pregnancy, chronic cocaine use, advanced age, bronchogenic carcinoma, and connective tissue disorders. Patients with these conditions experience a twofold to threefold increase in the duration of apnea.148 Patients with cocaine intoxication may also experience prolonged muscle relaxation because cocaine is competitively metabolized by the cholinesterases. An inherited deficiency of pseudocholinesterase is also present in about 0.03% of the population and can lead to prolonged paralysis from the administration of succinylcholine.145
Succinylcholine can also result in an increase in ICP, but the magnitude and significance of the increase in ICP remains controversial.149–153 Increases in the range of 5 to 10 mm Hg have been reported by several investigators, but other researchers have shown no increase. There is no evidence of neurologic deterioration associated with these transient elevations in ICP. Mechanisms that have been proposed to explain the elevated ICP include (1) a direct effect of fasciculations, (2) an increase in cortical electrical activity with a resultant increase in cerebral blood flow and blood volume, and (3) sympathetic postganglionic stimulation. Limited studies have been performed to evaluate the significance of this rise in ICP in a brain-injured human patient population. These studies have shown no significant change in electroencephalographic activity or ICP with succinylcholine, but the small size of the studies limits the ability to draw conclusions about clinical outcomes.132,154,155
Nondepolarizing Agents
Because nondepolarizing agents act competitively at neuromuscular junction receptors, increasing the concentration of ACh may reverse their effects. Cholinesterase inhibitors such as neostigmine or edrophonium may be used but will not be effective until some spontaneous signs of reversal are seen. The concept of reversal is of limited clinical importance in the ED with rare exceptions, such as performance of a neurologic examination on a previously paralyzed patient. When reversal is required, neostigmine, 0.02 to 0.04 mg/kg, is given by slow IV push. An additional dose of 0.01 to 0.02 mg/kg may be given in 5 minutes if reversal is incomplete, but the total dose should not exceed 5 mg in adults. Atropine, 0.01 mg/kg (with a minimum dose of 0.1 mg in children and a maximum dose of 1 mg in adults), should be given concurrently with neostigmine to block its systemic cholinergic effects.156–158 Another potential reversing agent, sugammadex, binds directly to the aminosteroid NMB rather than to cholinesterases.159 For reversal of shallow or profound neuromuscular blockade, 2 or 4 mg/kg intravenously, respectively, is recommended. Unfortunately, no studies currently address its use in emergency situations. It is approved for use in Europe but not the United States.
Long-Acting Agents: Pancuronium
Pancuronium is an aminosteroid that is primarily excreted in urine within 1 hour of IV administration.159 Classified as a long-acting agent, its onset and duration of action are dose related. After a typical IV dose of 0.1 mg/kg, paralysis occurs within 2 to 5 minutes and lasts approximately 60 minutes. Paralysis may be maintained safely by repeated bolus or drip infusion. Because the effects of the drug are cumulative, repeating the original dose significantly lengthens the duration of paralysis.
Relatively few adverse effects are associated with the use of pancuronium. Many patients experience an increase in heart rate, blood pressure, and cardiac output because of the vagolytic effect of the drug. Ventricular tachycardia and severe hypertension have been reported but are quite rare.160,161 Pancuronium may cause release of histamine resulting in bronchospasm or anaphylactic reactions.162 Prolonged paralysis may occur, primarily in patients with myasthenia gravis or with significant impairment in renal function. One consensus panel recommended pancuronium for maintaining paralysis, except in patients with cardiac disease or hemodynamic instability, for whom they recommended vecuronium.163
Intermediate-Acting Agents: Vecuronium, Atracurium, Mivacurium, and Rocuronium
Vecuronium and atracurium are intermediate-acting agents with an onset of action of approximately 3 minutes and a duration of action of 30 minutes. Mivacurium has an onset of action of 2 to 3 minutes and a duration of action of 15 to 20 minutes. Rocuronium has an onset of action within 1 to 1.5 minutes and a duration of action of 20 to 75 minutes (longer in geriatric patients). These drugs have minimal cardiovascular effects, cause little release of histamine (with the exception of mivacurium),132 and lack cumulative effects.164
The recommended doses of vecuronium, atracurium, mivacurium, and rocuronium are listed in Table 5-2. Use of larger doses hastens the onset of action but greatly prolongs the period of paralysis. For example, vecuronium at a dose of 0.25 mg/kg intravenously will cause paralysis in as little as 1 minute, but the period of paralysis will last 1 to 3 hours.165,166 Because a rapid onset of action comparable to that of succinylcholine is achieved at high doses of intermediate-acting agents, they may be used as the sole agents to facilitate intubation, particularly if a long period of paralysis is desired after intubation. The use of succinylcholine before intubation and an intermediate-acting agent at a normal dose after intubation provides rapid intubating conditions and better control over the duration of paralysis. The paralysis induced by vecuronium or atracurium may be maintained by repeated boluses or drip infusion. Unlike both pancuronium and succinylcholine, these agents have no side effects specifically related to repeated dosing in the ED. A repeated dose of 0.01 to 0.02 mg/kg of vecuronium will extend the period of paralysis 12 to 15 minutes.
Rocuronium, a structural analogue of vecuronium, is emerging as a desirable alternative agent for RSI when succinylcholine is contraindicated. At doses of 0.6 to 1.2 mg/kg, rocuronium consistently provides good to excellent intubating conditions within 1 minute of administration. Its duration of action is dose dependent and ranges from 20 to 75 minutes.167,168 Smaller anesthesia and ED-based studies have demonstrated its clinical utility and safety in RSI protocols.55,167–173 However, a 2003 Cochrane review174 found that except when used in conjunction with propofol, the intubation conditions produced by rocuronium tend to be inferior to those produced by succinylcholine for RSI.
Awake Intubation
Thomas175 likened standard laryngoscopy in an awake patient to the “mouth being held open with a wrench.” Awake nasotracheal intubation and fiberoptic intubation can also be an extremely unpleasant experience. The upper airway is richly innervated by sensory branches of the 5th, 7th, 9th, and 10th cranial nerves. In addition to pain fibers, there are stretch receptors that stimulate the coughing and gagging reflexes with even minor airway manipulation. It is essential that adequate analgesia be provided before intubation in all but the most extreme circumstances. Treatment options include topical application of anesthetic agents to the pharyngeal and tracheal mucosa and IV infusion of analgesic or sedative agents.
Direct Application
This procedure begins with spraying the tongue and pharynx with a topical agent. Use of atomization devices that attach to standard syringes (e.g., Mucosal Atomization Device [MAD], Wolfe Tory Medical, Inc., Salt Lake City) can provide effective drug dispersal without a forceful spray (Fig. 5-3). The more forceful pressurized canister sprays commonly provoke a cough reflex. After allowing at least 2 to 3 minutes to achieve numbing of the tongue and pharynx, the epiglottis and vocal cords can be sprayed with the MAD device. A malleable extension tube allows the tip of the MAD to pass around the base of the tongue, thereby permitting direct spraying of the epiglottis and vocal cords. This is generally well tolerated. An alternative method is to visualize the epiglottis and vocal cords with a laryngoscope and directly spray with the anesthetic agent. The use of a laryngoscope to visualize the vocal cords is much more stimulating to the patient and often not well tolerated. Another alternative is percutaneous injection of an anesthetic agent into the trachea at the level of the cricothyroid membrane.176,177
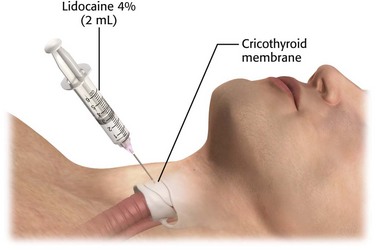
Cricothyroid Membrane Puncture
Direct application of topical anesthetics to the subglottic region can also be achieved through cricothyroid membrane puncture (Fig. 5-4). In this procedure, identify the cricothyroid membrane immediately below the thyroid cartilage. After antiseptic skin preparation, puncture the overlying tissue and membrane with a 22-gauge needle in the midline and just above the superior border of the cricoid cartilage. Take care to maintain the needle in the midline at all times to avoid injury to the recurrent laryngeal nerves. Advance the needle until air can be aspirated, which indicates placement of the tip in the trachea. Inject a 2-mL volume of 4% lidocaine rapidly. If the 4% concentration is not available, use 3 to 4 mL of 1% to 2% lidocaine. Typically, this will precipitate a cough and distribute the anesthetic over the upper part of the trachea, vocal cords, and epiglottis.
Nebulized Anesthesia
Nebulized anesthesia is a simple and painless technique that can be used to facilitate awake intubation when the patient’s condition is stable enough to permit a several-minute delay. Deliver the anesthetic via a standard nebulizer and face mask connected to an oxygen source that delivers 4 to 8 L/min. Nebulize a 4-mL volume of a 4% lidocaine solution over a period of about 5 minutes. Bourke and associates178 reported achieving consistently good topical anesthesia with this technique, although their patients were often premedicated with combinations of opioids and sedatives.
Sedation for Awake Intubation
Many patients can be intubated while awake with adequate topical anesthesia, but anxiolysis and mild to moderate sedation may be helpful for selected patients. The use of propofol in low doses (0.2 to 0.3 mg/kg) may be helpful. If time allows, anticholinergic agents such as glycopyrrolate may be helpful to reduce airway secretions. A new sedative agent, dexmedetomidine (an α2-adrenoreceptor agonist), has been described for use in awake intubation. Dexmedetomidine produces sedation and anxiolysis with minimal respiratory depression. Patients become sleepy but, if stimulated, can easily be aroused and are generally cooperative. These properties make it seem like an ideal agent for awake intubation, but its use is limited in emergencies by a requisite 10-minute loading dose followed by a maintenance infusion. Propofol may also cause hypertension with high doses because of direct stimulation of α2 receptors on the vasculature and can cause hypotension when given as a low-dose infusion179,180 because of inhibition of release of norepinephrine from sympathetic terminals. Future studies will be needed before this medication can be recommended for use in the ED.
References
1. Taryle, DA, Chandler, JE, Good, JT, et al. Emergency room intubation—complications and survival. Chest. 1979;75:541.
2. Tayal, VS, Riggs, RW, Marx, JA, et al. Rapid-sequence intubation at an emergency medicine residency: Success rate and adverse events during a two-year period. Acad Emerg Med. 1999;6:31.
3. Phelan, MP. Airway registry: a performance improvement surveillance project of emergency department airway management. Am J Med Qual. 2010;25:346–350.
4. Gambee, AM, Hertzka, RE, Fischer, DM. Preoxygenation techniques: comparison of three minute and four breaths. Anesth Analg. 1987;66:468.
5. Hedges, JR, Dronen, SC, Feero, S, et al. Succinylcholine-assisted intubations in prehospital care. Ann Emerg Med. 1988;17:469.
6. Pace, SA, Fuller, FP. Out-of-hospital succinylcholine-assisted endotracheal intubation by paramedics. Ann Emerg Med. 2000;35:568.
7. Sloan, C, Vilke, GM, Chan, TC, et al. Rapid sequence intubation in the field versus hospital in trauma patients. J Emerg Med. 2000;19:259.
8. Wayne, MA, Friedland, E. Prehospital use of succinylcholine: a 20-year review. Prehosp Emerg Care. 1999;3:107.
9. Gausche, M, Lewis, RJ, Stratton, SJ, et al. Effect of out-of-hospital pediatric endotracheal intubation on survival and neurological outcome: a controlled clinical trial. JAMA. 2000;283:783.
10. Fragen, RJ, Shanks, CA, Molteni, A. Effect on plasma cortisol concentrations of a single induction dose of etomidate or thiopentone. Lancet. 2005;2:625.
11. Katz, SH, Falk, JL. Misplaced endotracheal tubes by paramedics in an urban emergency medical services system. Ann Emerg Med. 2001;37:32.
12. Dunford, JV, Davis, DP, Ochs, M, et al. Incidence of transient hypoxia and pulse rate reactivity during paramedic rapid sequence intubation. Ann Emerg Med. 2003;42:721.
13. Davis, DP, Hoyt, DB, Ochs, M, et al. The effect of paramedic rapid sequence intubation on outcome in patients with severe traumatic brain injury. J Trauma. 2003;54:444.
14. Prys-Roberts, C, Meloche, R, Foex, P. Studies of anaesthesia in relation to hypertension: I. Cardiovascular responses of treated and untreated patients. Br J Anaesth. 1971;43:122.
15. Prys-Roberts, C, Meloche, R, Foex, P. Studies of anaesthesia in relation to hypertension: II. Haemodynamic consequences of induction and endotracheal intubation. Br J Anaesth. 1971;43:531.
16. Fox, EJ, Sklar, GS, Hill, CH, et al. Complications related to the pressor response to endotracheal intubation. Anesthesiology. 1977;47:524.
17. Chaemmer-Jorgensen, B, Hoilund-Carlsen, PF, Marving, J, et al. Lack of effect of intravenous lidocaine on hemodynamic responses to rapid sequence induction of general anesthesia: a double-blind controlled clinical trial. Anesth Analg. 1986;65:1037.
18. Kautto, UM. Attenuation of the circulatory response to laryngoscopy and intubation by fentanyl. Acta Anaesthesiol Scand. 1982;26:217.
19. Laurito, CE, Baughman, VL, Becker, GL, et al. Effects of aerosolized and/or intravenous lidocaine on hemodynamic responses to laryngoscopy and intubation in outpatients. Anesth Analg. 1988;67:389.
20. Denlinger, JK, Ellison, N, Omnisky, AJ. Effects on intratracheal lidocaine on circulatory responses to tracheal intubation. Anesthesiology. 1974;41:409.
21. Derbyshire, DR, Smith, G, Achola, KJ. Effect of topical lignocaine on the sympathoadrenal responses to tracheal intubation. Br J Anaesth. 1987;59:300.
22. Abou-Madi, MN, Keszler, H, Yacoub, JM. Cardiovascular reaction to laryngoscopy and tracheal intubation following small and large intravenous doses of lidocaine. Can Anaesth Soc J. 1977;24:12.
23. Youngberg, JA, Graybar, G, Hutchings, D. Comparison of intravenous and topical lidocaine in attenuating the cardiovascular responses to endotracheal intubation. South Med J. 1983;76:1122.
24. Boralessa, H, Senior, DF, Whitwam, JG. Cardiovascular response to intubation. Anaesthesia. 1983;38:623.
25. Lehtinen, AM, Hovorka, J, Widholm, O. Modification of aspects of the endocrine response to tracheal intubation by lignocaine, halothane and thiopentone. Br J Anaesth. 1984;56:239.
26. Davies, MJ, Cronin, KD, Cowie, RW. The prevention of hypertension at intubation. A controlled study of intravenous hydralazine on patients undergoing intracranial surgery. Anaesthesia. 1981;36:147.
27. Forbes, AM, Dally, FG. Acute hypertension during induction of anaesthesia and endotracheal intubation in normotensive man. Br J Anaesth. 1970;42:618.
28. Fassoulaki, A, Kaniaris, P. Does atropine premedication affect the cardiovascular response to laryngoscopy and intubation? Br J Anaesth. 1982;545:1065.
29. Martin, DE, Rosenberg, H, Aukburg, SJ, et al. Low-dose fentanyl blunts circulatory responses to tracheal intubation. Anesth Analg. 1982;61:680.
30. Harris, CE, Murray, AM, Anderson, JM, et al. Effects of thiopentone, etomidate and propofol on the hemodynamic response to tracheal intubation. Anaesthesia. 1988;43:32.
31. Price, ML, Millar, B, Grounds, M, et al. Changes in cardiac index and estimated systemic vascular resistance during induction of anesthesia with thiopentone, methohexitone, propofol, and etomidate. Br J Anaesth. 1992;69:172.
32. Weiss-Bloom, LJ, Reich, DL. Haemodynamic responses to tracheal intubation following etomidate and fentanyl for anaesthetic induction. Can J Anaesth. 1992;39:780.
33. Levitt, MA, Dresden, GM. The efficacy of esmolol versus lidocaine to attenuate hemodynamic response to intubation in isolated head trauma patients. Acad Emerg Med. 2001;8:19.
34. Butterworth, JF, Bean, VE, Royster, RL. Sufentanil is preferable to etomidate during rapid-sequence anesthesia induction for aortocoronary bypass surgery. J Cardiothorac Anesth. 1989;3:396.
35. Lunn, JK, Stanley, TH, Webster, L, et al. High dose fentanyl anesthesia for coronary artery surgery: plasma fentanyl concentrations and influence of nitrous oxide on cardiovascular responses. Anesth Analg. 1979;58:390.
36. Chung, KS, Sinatra, RS, Halevy, JD, et al. A comparison of fentanyl, esmolol, and their combination for blunting the haemodynamic responses during rapid-sequence induction. Can J Anaesth. 1992;39:774.
37. Dahlgren, N, Messeter, K. Treatment of stress response to laryngoscopy and intubation with fentanyl. Anaesthesia. 1981;36:1022.
38. Silivotti, ML, Ducharme, J. Randomized double-blind study on sedatives and hemodynamics during rapid-sequence intubation in the emergency department: the SHRED study. Ann Emerg Med. 1998;31:313.
39. Adachi, YU, Satomoto, M, Higuchi, H, et al. Fentanyl attenuates the hemodynamic response to endotracheal intubation more than the response to laryngoscopy. Anesth Analg. 2002;95:233.
40. Singh, H, Vichitvejpaisal, P, Gaines, GY, et al. Comparative effects of lidocaine, esmolol, and nitroglycerin in modifying the hemodynamic response to laryngoscopy and intubation. J Clin Anesth. 1995;7:5.
41. Kuschner, WG. Massive esophageal variceal hemorrhage triggered by complicated endotracheal intubation. J Emerg Med. 2000;18:317.
42. Clancy, M, Halford, S, Walls, R, et al. In patients with head injuries who undergo rapid sequence intubation using succinylcholine, does pretreatment with a competitive neuromuscular blocking agent improve outcome? A literature review. J Emerg Med. 2001;18:373.
43. Shapiro, HM, Gallindo, A, Wyte, SR, et al. Rapid intraoperative reduction of intracranial pressure with thiopentone. Br J Anaesth. 1973;45:1057.
44. Hunter, AR. Methohexitone as a supplement to nitrous oxide during intracranial surgery. Br J Anaesth. 1972;44:1188.
45. Ravussin, P, Guinard, JP, Ralley, F, et al. Effect of propofol on cerebrospinal fluid pressure and cerebral perfusion in patients undergoing craniotomy. Anaesthesia. 1988;43(suppl):37.
46. Modica, PA, Tempelhoff, R. Intracranial pressure during induction of anaesthesia and tracheal intubation with etomidate-induced EEG burst suppression. Can J Anaesth. 1992;39:236.
47. Harvey, SC. Hypnotics and sedatives. In: Gilman AG, Goodman LS, Rall TW, et al, eds. The Pharmacologic Basis of Therapeutics. New York: Macmillan, 1985.
48. Dripps, RD, Eckenhoff, JE, Vandam, LD. Intravenous anesthetics. In Dripps RD, Eckenhoff JE, Vandam LD, eds.: Introduction of Anesthesia, 7th ed, Philadelphia: WB Saunders, 1988.
49. Bedford, RF, Persing, JA, Pobereskin, L, et al. Lidocaine or thiopental for rapid control of intracranial hypertension? Anesth Analg. 1980;59:435.
50. Shapiro, HM, Wyte, SR, Loeser, J. Barbiturate-augmented hypothermia for reduction of persistent intracranial hypertension. J Neurosurg. 1974;40:90.
51. Sivilotti, ML, Fibrin, MR, Murray, HE, et al. Does the sedative agent facilitate emergency rapid sequence intubation? Acad Emerg Med. 2003;10:612.
52. Gooding, JM, Weng, JT, Smith, RA, et al. Cardiovascular and pulmonary responses following etomidate induction of anesthesia in patients with demonstrated cardiac disease. Anesth Analg. 1979;58:40.
53. Sprung, J, Ogletree-Hughes, ML, Moravec, CS. The effects of etomidate on the contractility of failing and nonfailing human heart muscle. Anesth Analg. 2000;91:68.
54. Bergen, JM, Smith, DC. A review of etomidate for rapid sequence intubation in the emergency department. J Emerg Med. 1998;16:485.
55. Laurin, EG, Sakles, JD, Panacek, EA, et al. A comparison of succinylcholine and rocuronium for rapid sequence intubation of emergency department patients. Acad Emerg Med. 2000;7:1362.
56. Schenarts, CL, Burton, JH, Riker, RR. Adrenocortical dysfunction following etomidate induction in emergency department patients. Acad Emerg Med. 2001;8:1.
57. Smith, DC, Bergen, JM, Smithline, H, et al. A trial of etomidate for rapid sequence intubation in the emergency department. J Emerg Med. 2000;18:13.
58. Sokolove, PE, Price, DD, Okada, P. The safety of etomidate for emergency rapid sequence intubation of pediatric patients. Pediatr Emerg Care. 2001;16:18.
59. Guldner, G, Schultz, J, Sexton, P, et al. Etomidate for rapid sequence intubation in young children: hemodynamic effects and adverse events. Acad Emerg Med. 2003;10:134.
60. Giese, JL, Stanley, TH. Etomidate: a new intravenous anesthetic induction agent. Pharmacotherapy. 1983;3:251.
61. Wadbrook, PS. Advances in airway pharmacology: emerging trends and evolving controversy. Emerg Med Clin North Am. 2000;18:767.
62. Zed, PJ, Abu-Laban, RB, Harrison, DW. Intubating conditions and hemodynamic effects of etomidate for rapid sequence intubation in the emergency department: an observational cohort study. Acad Emerg Med. 2006;13:378.
63. Famewo, CE. The safety of etomidate: a new intravenous anaesthetic induction agent. Afr J Med Med Sci. 1983;12:95.
64. Holdcroft, A, Morgan, M, Whitwam, JG, et al. Effect of dose and premedication on induction complications with etomidate. Br J Anaesth. 1976;48:199.
65. DeJong, H, Mallios, C, Janse, C, et al. Etomidate suppresses adrenocortical function by inhibition of 11-B hydroxylation. J Clin Endocrinol Metab. 1984;59:1143.
66. Wagner, RL, White, PF, Kan, KB, et al. Inhibition of adrenal steroidogenesis by the anesthetic etomidate. N Engl J Med. 1984;310:1415.
67. Fraser, GL, Riker, RR. The uncertain risk of single-dose etomidate in the critically ill. Hosp Pharm. 2005;8:658.
68. Nestor, NB. ED use of etomidate for rapid sequence induction. Am J Emerg Med. 2008;26:946.
69. Schenarts, CL, Burton, JH, Raker, RR. Adrenocortical dysfunction following etomidate induction in emergency department patients. Acad Emerg Med. 2001;8:1.
70. Absalom, A, Pledger, D, Kong, A, et al. Adrenocortical function in critically ill patients 24 hours after a single dose of etomidate. Anaesthesia. 1999;54:861.
71. Annane, D, Sébille, V, Troché, G, et al. A 3-level prognostic classification in septic shock based on cortisol levels and cortisol response to corticotropin. JAMA. 2000;282:1038.
72. Annane, D, Sébille, V, Charpentier, C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862.
73. De Jong, M, Beishuizen, A, Spijkstra, J, et al. Relative adrenal insufficiency as a predictor of disease severity, mortality, and beneficial effects of corticosteroid treatment in septic shock. Crit Care Med. 2007;35:8.
74. Cotton, BA, Guillamondegui, OD, Fleming, SB, et al. Increased risk of adrenal insufficiency following etomidate exposure in critically injured patients. Arch Surg. 2008;143:62.
75. Lundy, JB, Slane, ML, Frizzi, JD. Acute adrenal insufficiency after a single dose of etomidate. J Intensive Care Med. 2007;22:111.
76. den Brinker, M, Joosten, KF, Liem, O, et al. Adrenal insufficiency in meningococcal sepsis: Bioavailable cortisol levels and impact of interleukin-6 levels and intubation with etomidate on adrenal function and mortality. J Clin Endocrinol Metab. 2005;90:5110.
77. Lipiner-Friedman, D, Sprung, CL, Laterre, PF, et al. For the Corticus Study Group. Adrenal function in sepsis: the retrospective CORTICUS cohort study. Crit Care Med. 2007;35:1012.
78. Malerba, G, Romano-Girard, F, Cravoisy, A, et al. Risk factors of relative adrenocortical deficiency in intensive care patients needing mechanical ventilation. Intensive Care Med. 2005;31:388.
79. Mohammad, Z, Afessa, B, Finkielman, JD. The incidence of relative adrenal insufficiency in patients with septic shock after the administration of etomidate. Crit Care. 2006;10:R105.
80. Bloomfield, R, Noble, DW. Etomidate, pharmacological adrenalectomy and the critically ill: a matter of vital importance. Crit Care. 2006;10(4):161.
81. Tekwani, KL, Watts, HF, Sweis, RT, et al. A comparison of the effects of etomidate and midazolam on hospital length of stay in patients with suspected sepsis: a prospective, randomized study. Ann Emerg Med. 2010;56:481.
82. Dmello, D, Taylor, S, O’Brien, J, et al. Outcomes of etomidate in severe sepsis and septic shock. Chest. 2010;138:1327.
83. Jabre, P, Combes, X, Lapostolle, F, et al. Etomidate versus ketamine for rapid sequence intubation in acutely ill patients: a multicentre randomised controlled trial. Lancet. 2009;374:293.
84. Hohl, CM, Kelly-Smith, CH, Yeung, TC, et al. The effect of a bolus dose of etomidate on cortisol levels, mortality, and health services utilization: a systematic review. Ann Emerg Med. 2010;56:105.
85. Bourgoin, A, Albanèse, J, Léone, M, et al. Effects of sufentanil or ketamine administered in target-controlled infusion on the cerebral hemodynamics of severely brain-injured patients. Crit Care Med. 2005;33:1109.
86. Långsjö, JW, Salmi, E, Kaisti, KK, et al. Effects of subanesthetic ketamine on regional cerebral glucose metabolism in humans. Anesthesiology. 2004;100:1065.
87. Dmello, D, Taylor, S, O’Brien, J, et al. Outcomes of etomidate in severe sepsis and septic shock. Chest. 2010;138:1327.
88. White, PF, Way, WL, Trevor, AJ. Ketamine—its pharmacology and therapeutic uses. Anesthesiology. 1982;56:119.
89. Wong, DHW, Jenkins, LCP. An experimental study of the mechanism of action of ketamine on the central nervous system. Can Anaesth Soc J. 1974;21:57.
90. Schwartz, DA, Horwitz, LD. Effects of ketamine on left ventricular performance. J Pharmacol Exp Ther. 1975;194:410.
91. Albanèse, J, Arnaud, S, Rey, M, et al. Ketamine decreases intracranial pressure and electroencephalographic activity in traumatic brain injury patients during propofol sedation. Anesthesiology. 1997;87:1328.
92. Bourgoin, A, Albanèse, J, Léone, M, et al. Effects of sufentanil or ketamine administered in target-controlled infusion on the cerebral hemodynamics of severely brain-injured patients. Crit Care Med. 2005;33:1109.
93. Lundy, PA, Gowdey, DW, Calhoun, EH. Tracheal smooth muscle relaxant effect of ketamine. Br J Anaesth. 1974;46:333.
94. Betts, EK, Parkin, CE. Use of ketamine in an asthmatic child: a case report. Anesth Analg. 1971;50:420.
95. Grace, RF. The effect of variable-dose diazepam on dreaming and emergence phenomena in 400 cases of ketamine-fentanyl anaesthesia. Anaesthesia. 2003;58:904.
96. Chudnofsky, CR, Weber, JE, Stoyanoff, PJ, et al. A combination of midazolam and ketamine for procedural sedation and analgesia in adult emergency department patients. Acad Emerg Med. 2000;7:278.
97. Sherwin, TS, Green, SM, Khan, A. Does adjunctive midazolam reduce recovery agitation after ketamine sedation for pediatric procedures? A randomized, double-blind, placebo-controlled trial. Ann Emerg Med. 2000;35:239.
98. Wathen, JE, Roback, MG, Mackenzie, T. Does midazolam alter the clinical effects of intravenous ketamine sedation in children? A double-blind, randomized, controlled emergency department trial. Ann Emerg Med. 2000;36:579.
99. Taylor, PA, Towey, RM. Depression of laryngeal reflexes during ketamine anesthesia. BMJ. 1971;2:688.
100. Penrose, BH. Aspiration pneumonitis following ketamine induction for a general anesthetic. Anesth Analg. 1972;51:41.
101. Swanson, ER, Seaberg, DC, Mathias, S. The use of propofol in the emergency department. Acad Emerg Med. 1996;3:234.
102. Burton, JH, Miner, JR, Shipley, ER, et al. Propofol for emergency department procedural sedation and analgesia: a tale of three centers. Acad Emerg Med. 2006;13:24.
103. Langley, MS, Heel, RC. Propofol: a review of its pharmacodynamic and pharmacokinetic properties and use as an intravenous anaesthetic. Drugs. 1988;35:334.
104. White, PF. Propofol: pharmacokinetics and pharmacodynamics. Semin Anesth. 1988;7(suppl 1):4.
105. Whitwam, JG, Al-Khudhairi, D, McCloy, RF. Comparison of midazolam and diazepam in doses of comparable potency during gastroscopy. Br J Anaesth. 1983;55:773.
106. Baker, TJ, Gordon, HL. Midazolam (Versed) in ambulatory surgery. Plast Reconstr Surg. 1987;82:244.
107. Kanto, J, Analtonen, L, Himberg, JJ, et al. Midazolam as an intravenous induction agent in the elderly: a clinical and pharmacokinetic study. Anesth Analg. 1986;65:15.
108. Westphal, LM, Cheng, EY, White, PF, et al. Use of midazolam infusion for sedation following cardiac surgery. Anesthesiology. 1987;67:257.
109. Marty, J, Nitenberg, J, Blanchet, S, et al. Effects of midazolam on the coronary circulation in patients with coronary artery disease. Anesthesiology. 1986;64:206.
110. Reves, JG, Fragen, RJ, Vinik, HR, et al. Midazolam: pharmacology and uses. Anesthesiology. 1985;62:310.
111. Choi, YF, Wong, TW, Lau, CC, et al. Midazolam is more likely to cause hypotension than etomidate in emergency department rapid sequence intubation. Emerg Med J. 2004;21:700.
112. Adams, P, Gelman, S, Reves, JG, et al. Midazolam pharmacodynamics and pharmacokinetics during acute hypovolemia. Anesthesiology. 1985;63:140.
113. Davis, DP, Kimbro, TA, Vilke, GM. The use of midazolam for prehospital rapid-sequence intubation may be associated with a dose-related increase in hypotension. Prehosp Emerg Care. 2001;5:163.
114. Mostert, JW, Trudnowski, RJ, Seniff, AM, et al. Clinical comparison of fentanyl with meperidine. J Clin Pharmacol. 1968;8:382.
115. Rosow, DE, Philbin, DM, Keegan, CR, et al. Hemodynamics and histamine release during induction with sufentanil or fentanyl. Anesthesiology. 1984;60:489.
116. Rosow, CE, Moss, J, Philbin, DM, et al. Histamine release during morphine and fentanyl anesthesia. Anesthesiology. 1982;56:93.
117. Flack, JW, Flack, WE, Bloor, BC, et al. Histamine release by four narcotics: a double-blind study in humans. Anesth Analg. 1987;66:723.
118. Schleimer, R, Benjamini, E, Eisele, J. Pharmacokinetics of fentanyl as determined by radioimmunoassay. Clin Pharmacol Ther. 1978;23:188.
119. McClain, DA, Hug, CC. Intravenous fentanyl kinetics. Clin Pharmacol Ther. 1980;28:106.
120. Finch, JS, DeKornfeld, TJ. Clinical investigation of the analgesic potency and respiratory depressant activity of fentanyl, a new narcotic analgesic. J Clin Pharmacol. 1967;7:46.
121. Stanley, TH, Webster, LR. Anesthetic requirements and cardiovascular effects of fentanyl-oxygen and fentanyl-diazepam-oxygen anesthesia in man. Anesth Analg. 1978;57:411.
122. Comstock, MK, Carter, JG, Moyers, JR. Rigidity and hypercarbia associated with high dose fentanyl induction of anesthesia [letter]. Anesth Analg. 1981;60:362.
123. Hill, AB, Nahrwold, ML, De Rasayro, AM. Prevention of rigidity during fentanyl-oxygen induction of anesthesia. Anesthesiology. 1981;55:451.
124. Chudnofsky, CR, Wright, SW, Dronen, SC, et al. Safety of fentanyl use in the emergency department. Ann Emerg Med. 1988;17:881.
125. Li, J, Murphy-Lavoie, H, Bugas, C, et al. Complications of emergency intubation with and without paralysis. Am J Emerg Med. 1999;17:141.
126. Perry, JJ, Lee, JS, Sillberg, VA, et al. Rocuronium versus succinylcholine for rapid sequence induction intubation. Cochrane Database Syst Rev. 2, 2008. [CD002788].
127. Taylor, P. Neuromuscular blocking agents. In: Gilman AG, Goodman LS, Rall TW, et al, eds. The Pharmacologic Basis of Therapeutics. New York: Macmillan, 1985.
128. Leigh, MD, McCoy, DD, Belton, MK, et al. Bradycardia following intravenous administration of succinylcholine chloride to infants and children. Anesthesiology. 1957;18:699.
129. Katz, RL, Ryan, JF. The neuromuscular effects of suxamethonium in man. Br J Anaesth. 1969;41:381.
130. Galindo, A. Depolarizing neuromuscular block. J Pharmacol Exp Ther. 1971;178:339.
131. Rose, JB, Theroux, MC, Katz, MS. The potency of succinylcholine in obese adolescents. Anesth Analg. 2000;90:576.
132. Orebaugh, SL. Succinylcholine: adverse effects and alternatives in emergency medicine. Am J Emerg Med. 2000;18:637.
133. Bennike, KA, Neilson, E. Muscle pain following suxamethonium. Dan Med Bull. 1964;11:122.
134. Schreiber, JU, Lysakowski, C, Fuchs-Buder, T, et al. Prevention of succinylcholine-induced fasciculations and myalgia: a meta-analysis of randomized trials. Anesthesiology. 2005;103:877.
135. Clancy, M, Halford, S, Walls, R, et al. In patients with head injuries who undergo rapid sequence intubation using succinylcholine, does pretreatment with a competitive neuromuscular locking agent improve outcome? A literature review. Emerg Med J. 2001;18:373–375.
136. Zink, BJ, Snyder, HS, Raccio-Robak, N. Lack of a hyperkalemic response in emergency department patients receiving succinylcholine. Acad Emerg Med. 1995;2:974.
137. Tomie, JD, Joyce, TH, Mitchell, GD. Succinylcholine danger in the burned patient. Anesthesiology. 1969;31:540.
138. Mazze, RI, Escue, HM, Houston, JB. Hyperkalemia and cardiovascular collapse following administration of succinylcholine to the traumatized patient. Anesthesiology. 1975;43:89.
139. Smith, RB, Grenvik, A. Cardiac arrest following succinylcholine in patients with central nervous system injuries. Anesthesiology. 1970;33:558.
140. Gronert, GA, Theye, RA. Pathophysiology of hyperkalemia induced by succinylcholine. Anesthesiology. 1978;49:298.
141. Thapa, S, Brull, SJ. Succinylcholine-induced hyperkalemia in patients with renal failure: an old question revisited. Anesth Analg. 2000;91:237.
142. Schow, AJ, Lubarsky, DA, Olson, RP, et al. Can succinylcholine be used safely in hyperkalemic patients? Anesth Analg. 2002;95:119.
143. Donlon, JV, Newfield, P, Sreter, F, et al. Implications of masseter spasm after succinylcholine. Anesthesiology. 1978;49:298.
144. Tsang, HS, Frederick, GS. Malignant hyperthermia. Ill Med J. 1976;149:471.
145. May, DC, Morris, SW, Stewart, RM, et al. Neuroleptic malignant syndrome: response to dantrolene sodium. Ann Intern Med. 1983;938:183.
146. Barnes, PK. Masseter spasm following intravenous suxamethonium. Br J Anaesth. 1973;45:759.
147. Gill, M, Graeme, K, Gueterberg, K. Masseter spasm after succinylcholine administration. J Emerg Med. 2005;29:167.
148. McStravog, LJ. Dangers of succinylcholine in anesthesia. Laryngoscope. 1974;84:929.
149. Halldin, M, Wahlin, H. Effect of succinylcholine on intraspinal fluid pressure. Acta Anaesthesiol Scand. 1959;38:155.
150. Burney, R, Winn, HR. Increased cerebrospinal fluid pressure during laryngoscopy and intubation for induction of anesthesia. Anesth Analg. 1975;54:687.
151. Shapiro, HM, Wyte, S, Harris, A, et al. Acute intraoperative intracranial hypertension in neurosurgical patients: mechanical and pharmacological factors. Anesthesiology. 1972;37:399.
152. Cottrell, JE, Hartung, J, Giffin, JP, et al. Intracranial and hemodynamic changes after succinylcholine administration in cats. Anesth Analg. 1983;62:1006.
153. Lam, AM, Gelb, AW. Succinylcholine and intracranial pressure—a cause for pause. Anesth Analg. 1984;63:620.
154. Brown, MM, Parr, MJ, Manara, AR. The effect of suxamethonium on intracranial pressure and cerebral perfusion pressure in patients with severe head injuries following blunt trauma. Eur J Anaesthesiol. 1996;13:474.
155. Kovarik, WD, Mayberg, TS, Lam, AM, et al. Succinylcholine does not change intracranial pressure, cerebral blood flow velocity or the electroencephalogram in patients with neurologic injury. Anesth Analg. 1994;78:469.
156. Foldes, FF. The rational use of neuromuscular blocking agents: the role of pancuronium. Drugs. 1972;4:153.
157. Rupp, SM, McChristian, JW, Miller, RD, et al. Neostigmine and edrophonium antagonism of varying intensity. Neuromuscular blockade induced by atracurium, pancuronium, or vecuronium. Anesthesiology. 1986;64:711.
158. Breen, PJ, Doherty, WG, Donati, F, et al. The potencies of edrophonium and neostigmine as antagonists of pancuronium. Anaesthesia. 1985;40:844.
159. Welliver, M. New drug sugammadex: a selective relaxant binding agent. AANA J. 2006;74:357.
160. Anderson, EF, Rosenthal, MH. Pancuronium bromide and tachyarrhythmias. Crit Care Med. 1975;3:13.
161. Fraley, DS, Lemoncelli, GL, Coleman, A. Severe hypertension associated with pancuronium bromide. Anesth Analg. 1978;57:265.
162. Bodman, RI. Pancuronium and histamine release. Can Anaesth Soc J. 1978;25:40.
163. Shapiro, BA, Warren, J, Egol, AB, et al. Practice parameters for sustained neuromuscular blockade in the adult critically ill patient: an executive summary. Crit Care Med. 1995;23:1601.
164. Sohn, YJ, Bencini, AF, Scaf, AHJ, et al. Comparative pharmacokinetics and dynamics of vecuronium and pancuronium in anesthetized patients. Anesth Analg. 1986;65:233.
165. Schwarz, S, Ilias, W, Lackner, F, et al. Rapid tracheal intubation with vecuronium: the priming principle. Anesthesiology. 1985;62:388.
166. Kunjappan, VE, Brown, EM, Alexander, GD. Rapid sequence induction using vecuronium. Anesth Analg. 1986;65:503.
167. Fuchs-Buder, T, Tassony, E. Intubating conditions and time course of rocuronium-induced neuromuscular block in children. Br J Anaesth. 1996;77:335.
168. Magorian, T, Flannery, KB, Miller, RD. Comparison of rocuronium, succinylcholine and vecuronium for rapid-sequence induction of anesthesia in adult patients. Anesthesiology. 1993;79:913.
169. Dobson, AP, McCluskey, A, Meakin, G, et al. Effective time to satisfactory intubation conditions after administration of rocuronium in adults: comparison of propofol and thiopentone for rapid sequence induction of anesthesia. Anaesthesia. 1999;54:172.
170. Mazurek, AJ, Rae, B, Hann, S, et al. Rocuronium versus succinylcholine: are they equally effective during rapid-sequence induction of anesthesia? Anesth Analg. 1998;87:1259.
171. Skinner, HJ, Biswas, A, Mahajan, RP. Evaluation of intubating conditions with rocuronium and either propofol or etomidate for rapid sequence induction. Anaesthesia. 1998;53:702.
172. Sakles, JC, Laurin, EG, Rantappa, AA, et al. Rocuronium for rapid sequence intubation of emergency department patients. J Emerg Med. 1999;17:611.
173. Perry, JJ, Lee, J, Wells, G. Are intubation conditions using rocuronium equivalent to those using succinylcholine? Acad Emerg Med. 2002;9:813.
174. Perry, J, Lee, J, Wells, G, et al. Rocuronium versus succinylcholine for rapid sequence induction intubation. Cochrane Database Syst Rev. 1, 2003. [CS002788].
175. Thomas, JF. Awake intubation. Anaesthesia. 1969;24:28.
176. Danzl, DF, Thomas, DM. Nasotracheal intubations in the emergency department. Crit Care Med. 1980;8:677.
177. Boster, SR, Danzl, DF, Madden, RJ, et al. Translaryngeal absorption of lidocaine. Ann Emerg Med. 1982;11:461.
178. Bourke, DL, Katz, J, Tonneson, A. Nebulized anesthesia for awake endotracheal intubation. Anesthesiology. 1985;63:690.
179. Abdelmalak, B, Makary, L, Hoban, J, et al. Dexmedetomidine as sole sedative for awake intubation in management of the critical airway. J Clin Anesth. 2007;19:370.
180. Bergese, SD, Bender, P, McSweeney, TD, et al. A comparative study of dexmedetomidine with midazolam and midazolam alone for sedation during elective away fiberoptic intubations. J Clin Anesth. 2010;22:35.