CHAPTER 39 Petroclival Meningiomas
Suboccipital Retrosigmoid Approach 
INTRODUCTION
The suboccipital retrosigmoid approach (SRA) is a safe and relatively simple approach to the cerebellopontine angle (CPA) and the petroclival area. It is related to a very low procedure-related morbidity rate1–3 and therefore is among the most frequently utilized operative routes in neurosurgery.4–6 The advantages of the approach are: it offers an excellent panoramic visualization to the whole CPA; it is a hearing-preserving technique that allows for a wide exposure of the tumor, whatever its size and pathologic type; and it offers increased safety when working in the vicinity of the brain stem. The dissection of the tumor at all stages is performed under direct visual control and the cranial nerves can be identified early both in their proximal and distal ends, thus increasing the chances for their preservation. If the SRA is combined with a resection of the petrous apex and incision of the tentorium, any petroclival lesions can be adequately exposed.5,7–9 This retrosigmoid–suprameatal approach (RSMA), which was developed and introduced by the senior author in 1982, provides access to meningiomas of Meckel’s cave, petroclival area, and even the posterior cavernous sinus.8,10 It avoids the risks related to alternative approaches, such as extensive petrous bone resection or retraction of the temporal lobe with the associated risks of damage to neural and vascular structures. Meningiomas extending to the foramen magnum level can be removed via a similar retrosigmoid craniectomy and a C1 hemilaminectomy/laminectomy.7
Potential drawbacks of the approach are the need of cerebellar retraction, as well as the relatively higher rate of postoperative headache. With current neuroanesthesia and some modifications of the original retrosigmoid approach, described in the chapter, these disadvantages are rather theoretical.3
SURGICAL APPROACH
Patient Positioning and Anesthesia Considerations
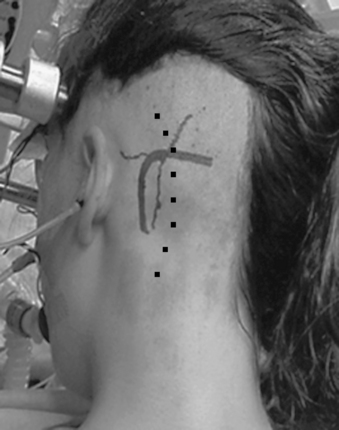
The position of the patient on the operating table—semi-sitting, lateral, or supine with the head rotated 90 degrees to the contralateral side1,2,6,11—is a matter of surgeon’s preference and institutional tradition. We prefer the semi-sitting position,3,12 which has several major advantages: (1) the surgeon can work bimanually because there is no need for constant suction and (2) the continuous irrigation of the operative field performed by the assistant obviates the need for frequent coagulation during tumor removal. The head is held with a three-point head fixation frame; then it is flexed and rotated approximately 30 degrees to the involved side, avoiding occlusion of venous jugular outflow or hyperflexion of the cervical spine (Fig. 39-1).
A drawback of this patient position is the risk of venous air embolism, paradoxic air embolism, tension pneumocephalus, or circulatory instability. However, in experienced hands these are not related to any lasting morbidity.13,14 Transesophageal echocardiography is the most specific and sensitive method in detection of air embolism. However, the combined monitoring of end-tidal carbon dioxide and precordial Doppler echocardiography yield similar results.13 If immediate measures are carried out at the first sign of venous air embolism, the related morbidity is insignificant. Further, some studies have shown that the morbidity due to venous air embolism is similar in both the semi-sitting and supine positions.
Neurophysiology
Continuous neurophysiologic monitoring should be performed throughout the surgery: from the positioning of the patient to the skin closure.3,15,16 Monitoring of somatosensory evoked potentials is especially important during patient positioning in order to prevent spinal cord compression. Preoperative functional radiographs of the cervical spine could determine the patients at risk, such as those with severe degenerative spine disease or spinal instability.
The functional integrity of the facial nerve is monitored continuously by electromyography transferred by loudspeakers. Bipolar recording needle electrodes are fixed at the eyebrow for the orbicularis oculi muscle and at the mouth angle for the orbicularis oris muscle. Electrical activation by 1 to 4 mA is applied in the course of surgery in case of difficult nerve identification or for testing the reactivity of the nerve to mechanical stimulation. Monitoring of the brain stem auditory evoked potentials allows for control and prediction of auditory function during tumor resections. It provides instant feedback information on the functional status of the cochlear nerve. Waves I, III, and V are functional correlates of the cochlea, the nucleus cochlearis, and the colliculus inferior; the main parameters that are observed are the waves’ latencies, amplitudes, and losses. The loss of a wave V is the parameter most definitely associated with deafness.15 The loss of any wave may be prevented by early recognition of its deterioration, and the surgeon’s actions should be modified accordingly. Monitoring of the abducent and of the caudal cranial nerves is performed if needed according to the particular tumor extension and clinical presentation.
Skin Incision and Craniotomy
A slightly curved skin incision 1.5 to 2 cm medial to the mastoid process is performed (see Fig. 39-1) and the underlying muscles are incised in line with the skin. Traditionally the position of the burr hole was determined in relation to the asterion.17 However, some recent studies have shown that the asterion is not an absolutely reliable anatomic landmark and is variable both in the cranial–caudal plane and in the anterior–posterior plane.18–20 The superior nuchal line is more reliable and overlies the transverse sinus in all cases.21 The course of the sigmoid sinus is less variable. It descends along an axis defined by the mastoid tip and the squamosal–parietomastoid suture junction or over the mastoid groove.21 We prefer to place the burr hole 2 to 2.5 cm below the superior nuchal line, two thirds behind and one third in front of the occipitomastoid suture.
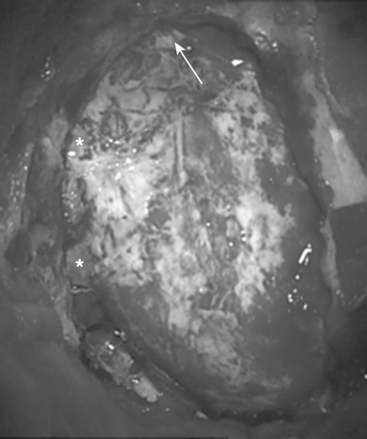
The craniectomy is performed using bone rongeurs, exposing the borders of the sigmoid and transverse sinuses (Fig. 39-2). More extensive exposure of these sinuses is unnecessary and might lead to their laceration or desiccation, with the risk of subsequent thrombosis. The issue of whether to make a craniectomy or craniotomy is a matter of individual preference.22,23 Our experience, however, showed that the craniotomy might be more dangerous procedure. Therefore, we avoid making one-piece craniotomy, although we performed it earlier, due to the related high risk of injury to the underlying sinuses and tearing the dura by the craniotome.
The bone opening should extend to the floor of the posterior fossa, thus allowing access to the lateral cerebellomedullary cistern. In case of caudally extending or craniocervical meningiomas, it might be necessary to open the foramen magnum. The dura is incised in a curvilinear manner just 1.5 to 2 mm medial to the sigmoid and inferior to the transverse sinus (Fig. 39-3). This allows for a primary watertight dural closure and avoids the need of using dural substitute in almost all cases. The lateral cerebellomedullary cistern is then opened and CSF is drained. Thus, the cerebellum relaxes away from the petrous bone and the self- retaining retractor supports and protects the cerebellar hemisphere, instead of compressing it.
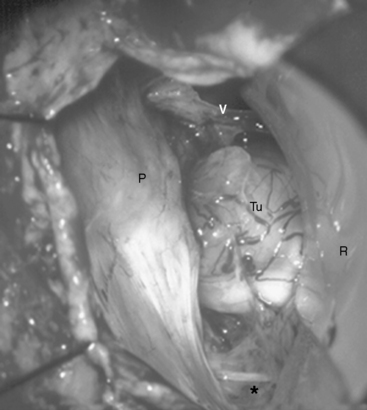
Petroclival meningiomas are located medially to the Vth, VIIth, and VIIIth cranial nerves and their removal should be performed through one of the CPA levels: the upper level (defined by the tentorium and the trigeminal nerve), the second level (between the trigeminal and the VIIth–VIIIth nerve complex), the third level (between the VIIth and VIIIth nerves and the caudal nerves), or the lowest level (between the caudal nerves and foramen magnum).8,9 The anatomic space between the cranial nerves may be relatively narrow; however, the tumor usually widens it sufficiently to allow safe manipulations. The removal should start at the level that is most widely expanded by the meningioma (see Fig. 39-3). Initial internal decompression is performed using an ultrasonic surgical aspirator at the appropriate levels. Importantly, the dissection of the tumor capsule should be performed only after sufficient internal decompression has been achieved. The extent of tumor removal is determined by its relation to neural structures: in the 10% to 20% of cases in which the pia mater of brain stem is infiltrated, a small part of the tumor capsule may be left.
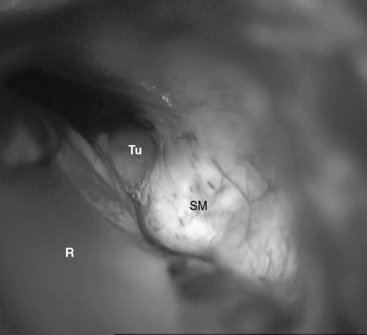
In case of large meningiomas, the brain stem is displaced so that adequate access even to the contralateral side or supratentorially is provided. If necessary, however, the petrous apex may be resected, as soon as the tumor portion in the CPA is removed. The apex of the pyramid is resected by intradural drilling of the suprameatal tubercle, which is a bone eminence located above and anterior to the internal auditory meatus (Figs. 39-4 and 39-5). The extent of bone removal is determined by the individual anatomic characteristics and tumor expansion. Some studies have shown that the retrosigmoid approach may thus be extended anterior as far as 13.0 mm.10 The opening of Meckel’s cave allows for mobilization of the trigeminal nerve, which further increases the working space (Fig. 39-6). The structures at risk in the area are the internal carotid artery anterolaterally, the petrosal sinus superiorly, and the superior and posterior semicircular canals laterally.9 The abducens nerve may be identified early at its brain stem exit zone and followed during tumor removal up to Dorello’s canal, which allows for its preservation. The exposure of the rostral tumor portion can be further enlarged by resection of the tentorium. Thus, the RSMA provides access to Meckel’s cave, the petroclival area, and the middle fossa including the posterior cavernous sinus.
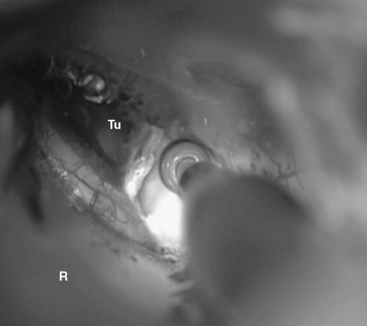
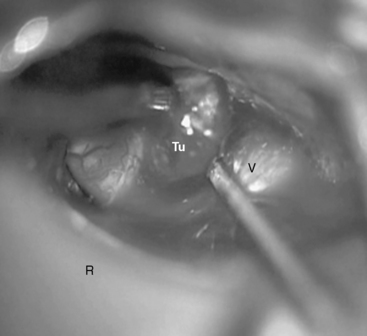
FIGURE 39-5 The suprameatal tubercle is drilled off with a high-speed diamond drill. R, retractor; Tu, tumor.
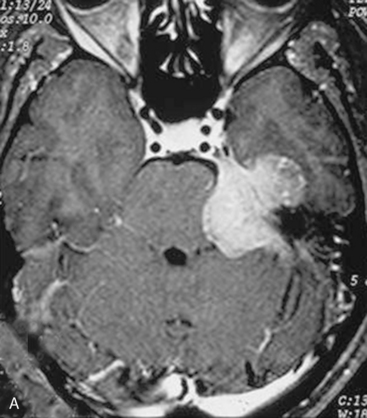
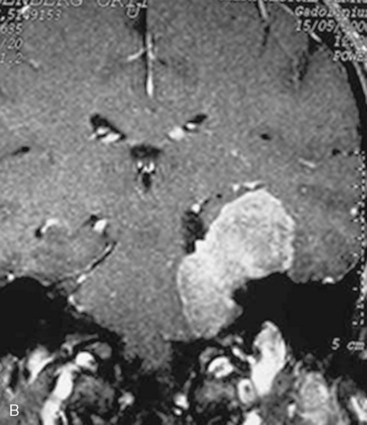
For several years, in some particularly extensive meningiomas that not only extend supratentorially but also engulf the carotid artery or the optic nerve, we have favored staged surgeries. The tumor portion in the CPA, clivus, and/or Meckel’s cave is removed via a retrosigmoid–suprameatal approach, the brain stem is decompressed, and the risk of severe neurologic deterioration is thus avoided. The supratentorial portion is removed at a second stage via the frontotemporal approach. If, however, the supratentorial extension is limited to the middle cranial fossa, the resection of the tentorium generally provides sufficient access and allows for complete removal of the tumor at the same surgery (Fig. 39-7).
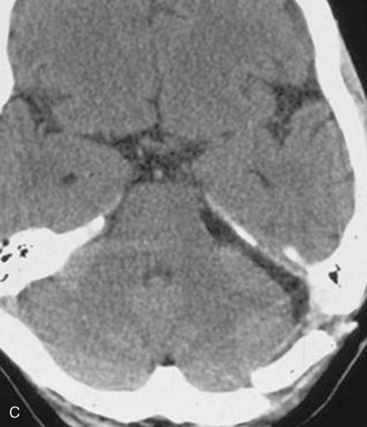
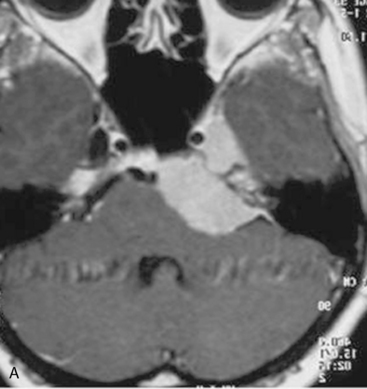
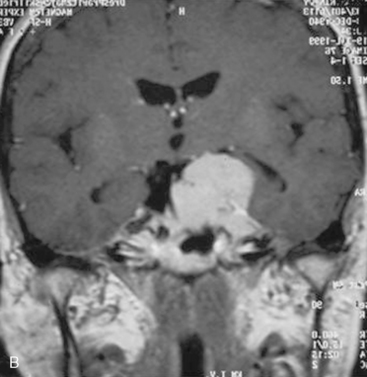
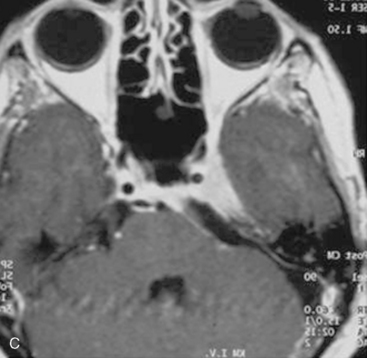
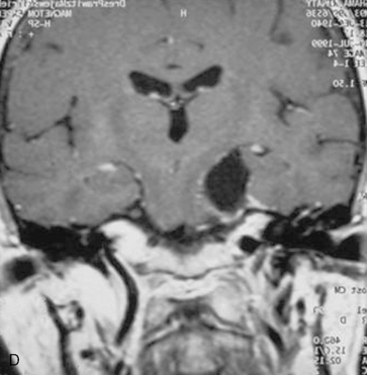
In case of incomplete resection, follow-up magnetic resonance imaging (MRI) studies are performed 3 to 6 months after surgery, and radiosurgery/radiotherapy or follow-up of the residual tumor is recommended accordingly (Fig. 39-8).
[1] Ciric I., Zhao J.C., Rosenblatt S., et al. Suboccipital retrosigmoid approach for vestibular schwannomas: facial nerve function and hearing preservation. Neurosurgery. 2005;56:560-570.
[2] Giannotta S.L. Surgical approaches to acoustic neuromas. In: Barrow D.L., editor. Surgery of the Cranial Nerves of the Posterior Fossa. Park Ridge, IL: American Association of Neurological Surgeons; 1993:275-287.
[3] Samii M., Gerganov V., Samii A. Improved preservation of hearing and facial nerve function in vestibular schwannoma surgery via the retrosigmoid approach in a series of 200 patients. J Neurosurg. 2006;105:527-535.
[4] Ebersold M.J., Harner S.G., Beatty C.W., et al. Current results of the retrosigmoid approach to acoustic neuroma. J Neurosurg. 1992;76:901-909.
[5] Rhoton A.L.Jr. The cerebellopontine angle and posterior fossa cranial nerves by the retrosigmoid approach. Neurosurgery. 2000;47:S93-129.
[6] Ojemann R.G. Retrosigmoid approach to acoustic neuromas/vestibular schwannoma. Neurosurgery. 2001;48:553-558.
[7] Samii M., Klekamp J., Carvalho G. Surgical results for meningiomas of the craniocervical junction. Neurosurgery. 1996;39:1086-1095.
[8] Samii M., Carvalho G.A., Tatagiba M., et al. Surgical management of meningiomas originating in Meckel’s cave. Neurosurgery. 1997;41:767-775.
[9] Samii M., Tatagiba M., Carvalho G.A. Retrosigmoid intradural suprameatal approach to Meckel’s cave and the middle fossa: surgical technique and outcome. J Neurosurg. 2000;92:235-241.
[10] Chanda A., Nanda A. Retrosigmoid intradural suprameatal approach: advantages and disadvantages from an anatomical perspective. Neurosurgery. 2006;59:ONS1-6.
[11] Yasargil M.G., Smith R.D., Gasser J.C. The microsurgical approach to acoustic neuromas. Adv Tech Stand Neurosurg. 1977;4:93-128.
[12] Samii M., Matthies C. Management of 1000 vestibular schwannomas (acoustic neuromas): surgical management and results with an emphasis on complications and how to avoid them. Neurosurgery. 1997;40:11-21.
[13] Von Goesseln H.H., Samii M., Sur D., et al. The lounging position for posterior fossa surgery: anesthesiological considerations regarding air embolism. Childs Nerv Syst. 1991;7:368-374.
[14] Duke D.A., Lynch J.J., Harner S.G., et al. Venous air embolism in sitting and supine patients undergoing vestibular schwannoma resection. Neurosurgery. 1998;42:1282-1287.
[15] Samii M., Matthies C. Management of 1000 vestibular schwannomas (acoustic neuromas): hearing function in 1000 tumor resections. Neurosurgery. 1997;40:248-262.
[16] Romstock J., Strauss C., Fahlbush R. Continuous electromyography monitoring of motor cranial nerves during cerebellopontine angle surgery. J Neurosurg. 2000;93:586-593.
[17] Al-Mefty O., Fox J.L., Smith R.L. Petrosal approach for petroclival meningiomas. Neurosurgery. 1998;22:510-517.
[18] Day J.D., Kellogg J.X., Tschabitscher M., et al. Surface and superficial surgical anatomy of the posterolateral cranial base: significance for surgical planning and approach. Neurosurgery. 1996;38:1079-1084.
[19] Day J.D., Tschabitscher M. Anatomic position of the asterion. Neurosurgery. 1998;42:198-199.
[20] Bozbuga M., Boran B.O., Sahinoglu K. Surface anatomy of the posterolateral cranium regarding the localization of the initial burr-hole for a retrosigmoid approach. Neurosurg Rev. 2006;29:61-63.
[21] Avci E., Kocaogullar Y., Fossett D., et al. Lateral posterior fossa venous sinus relationships to surface landmarks. Surg Neurol. 2003;59:392-397.
[22] Bassiouni H., Hunold A., Asgari S., et al. Meningiomas of the posterior petrous bone: functional outcome after microsurgery. J Neurosurg. 2004;100:1014-1024.
[23] Schaller B., Baumann A. Headache after removal of vestibular schwannoma via the retrosigmoid approach: a long-term follow-up-study. Otolaryngol Head Neck Surg. 2003;128:387-395.