CHAPTER 33 PERTINENT SURGICAL ANATOMY OF THE THORAX AND MEDIASTINUM
The thorax consists of the chest wall comprising the sternum, ribs, and thoracic vertebrae; the mediastinum containing the pericardium, heart, esophagus, trachea, great vessels, thoracic duct, and thymus; and the paired pleural cavities containing the lungs. This chapter will discuss the anatomy of these structures and spaces, as pertinent to trauma surgery and the surgical intensive care unit.
CHEST WALL
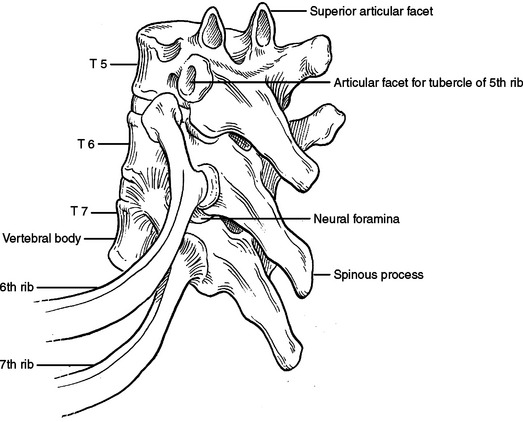
The bony structures of the chest wall include 12 ribs, 12 thoracic vertebrae, and the sternum. All ribs articulate posteriorly with the transverse processes and vertebral bodies of their respective thoracic vertebrae and the vertebral body directly superior (Figure 1). Ribs 1 through 7 are called true ribs because they articulate anteriorly directly with the sternum through their own costal cartilage. Ribs 8, 9, and 10 are called false ribs because they articulate anteriorly to the costal cartilage of the rib above. This creates a construct of stairstepping costal cartilages, which ultimately articulates with the sternum and creates the costal arch or costal margin. Ribs 11 and 12 are called floating ribs because they do not articulate with any structure anteriorly (Figure 2). Rather, they attach to the abdominal wall musculature, primarily the internal oblique muscle.
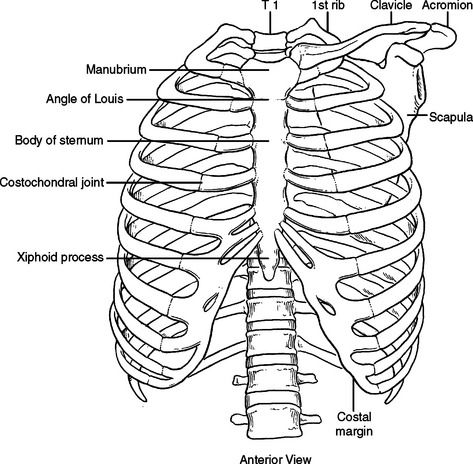
Figure 2 Bony chest wall. Anterior view.
(Redrawn from Grant’s Atlas of Anatomy, 11th ed., Philadelphia, Lippincott Williams & Wilkins, 2004, figure 1.8, p. 9.)
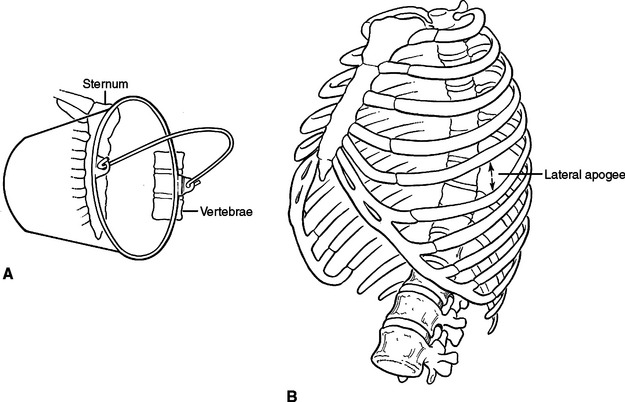
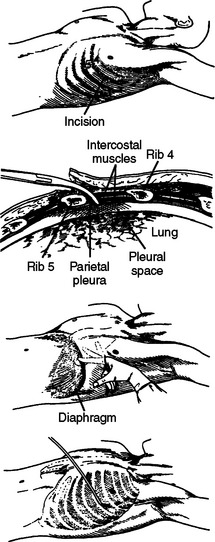
Because ribs 1 through 10 are fixed anteriorly and posteriorly, they function much like a bucket handle (Figure 3A). When performing a tube thoracostomy, as you approach the sternum anteriorly and the transverse processes posteriorly, the size of the interspace becomes fixed and narrow. Laterally, away from these points of attachment, the ribs separate and the interspace opens. The widest portion of the interspaces can be found at the lateral apogee or “keystone” of the rib. Tube thoracostomies placed laterally will be easier to place through the interspace and more comfortable for the patient (Figure 3B). Also, when creating a thoracotomy, division of the intercostal muscles far anterior and posterior will create a larger working space without tearing the intercostal muscle or fracturing a rib with placement of the rib spreader. The skin need only be divided over the working space, not over the entire intercostal incision.
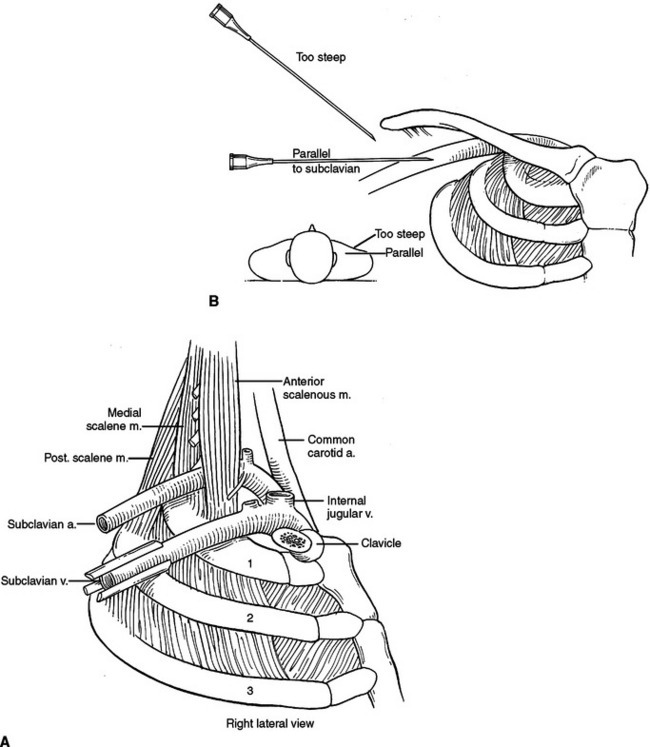
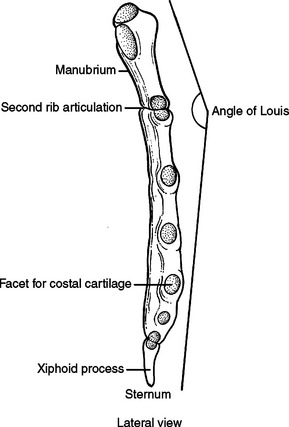
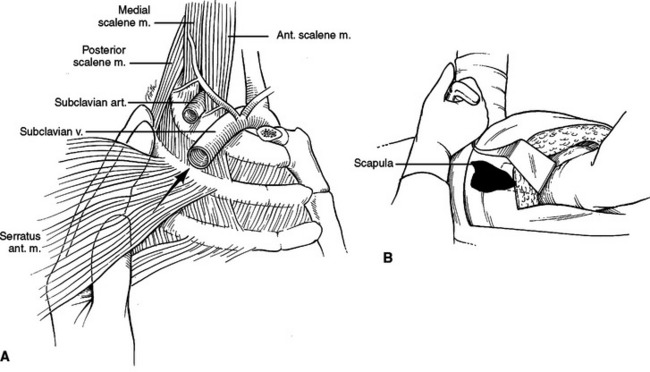
The sternum has three parts, the manubrium, the body, and the xiphoid process. The manubrium is thick and broad, articulating with the clavicle, first rib, and sharing the second rib articulation with the body of the sternum. The sternoclavicular articulation is the only bony articulation of the thorax to the shoulder girdle (see Figure 2). Understanding the angle of the clavicle, manubrium, and first rib is important in safe placement of central venous catheters into the subclavian vein. The subclavian vein and artery leave the arm and enter the thoracic inlet over the top of the first rib and under the clavicle. Once under the clavicle, a needle directed parallel to the clavicle and first rib will not enter the chest and cause a pneumothorax before finding the subclavian vein. A needle directed too steeply in its approach will quickly enter and exit the triangle where the subclavian vein is found, penetrate the intercostal space, and puncture the lung (Figure 4).
The second rib inserts into the sternomanubrial junction (angle of Louis). This can be easily palpated in most people as a horizontal ridge in the sternum or where the two planes that make up the sternum intersect (Figure 5). The interspace immediately below the angle of Louis is the second interspace. The angle of Louis serves as a landmark to rapidly locate the second rib and second interspace for placement of a catheter to decompress a tension pneumothorax or to place an anterior tube thoracostomy for an apical pneumothorax.
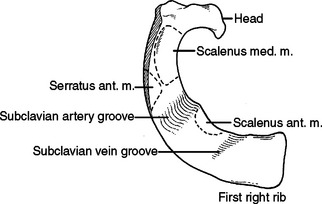
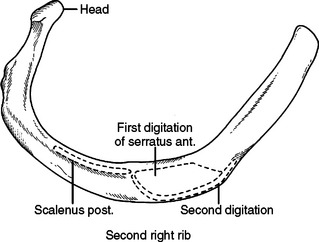
The first rib is short, broad, flat, and arches sharply from posterior to anterior (Figure 6). The second rib is longer than but very similar to the first rib (Figure 7). The first slip of the serratus anterior muscle attaches to the second rib approximately one-third of the arc from posterior to anterior—this slip also attaches to the inferior aspect of the first rib. Posterior to this attachment, the scalenus posterior attaches to the second rib.
When performing a thoracotomy, counting ribs can identify the correct interspace. Once the latissimus dorsi muscle has been divided and the serratus anterior muscle divided or swept anterior, the scapula is elevated. Thin fibrous attachments hold the undersurface of the scapula to the chest wall. A hand placed deep to the scapula, posterior near the spine, and apically can palpate ribs. The first rib is identified by its conspicuously broad and flat contour. Inferior to this, the second rib can be identified by the attachment of the scalenus posterior muscle. This muscle body is palpable by sweeping the finger from posterior to anterior along the second rib (Figure 8). Less distinct will be the third rib, which seems to “turn the corner” from the apex of the chest to the lateral chest wall (Figure 9). In a lateral decubitus position, the tip of the scapula overlies the sixth interspace. In a male, the nipple overlies the fourth interspace.
MUSCLES OF THE CHEST WALL
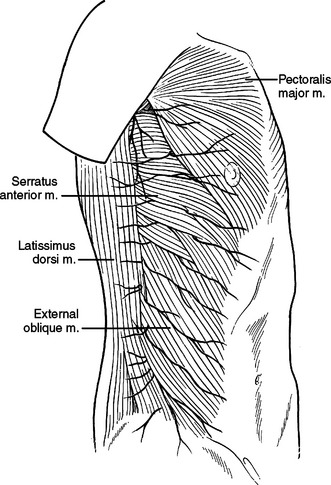
The paired pectoralis major muscles cover the majority of the anterior chest wall. The pectoralis major muscle originates from the clavicle and anterior aspects of ribs 1 through 6 inserting on the proximal humerus. Its origin from the chest wall is broad and an anterior thoracotomy will divide or separate its fibers. Inferiorly, the rectus abdominus muscle inserts onto the costal cartilages of ribs 5 through 7 and the xiphoid process. Lateral to this, the muscle fibers of the external oblique insert onto ribs 5 through 12. The external oblique muscle interdigitates with the serratus anterior muscle as it inserts on ribs 1 through 8 (Figure 10). Most thoracotomies do not traverse the interspaces guarded by the rectus abdominus and external oblique. These muscles will be encountered with thoracoabdominal incisions crossing the costal margin.
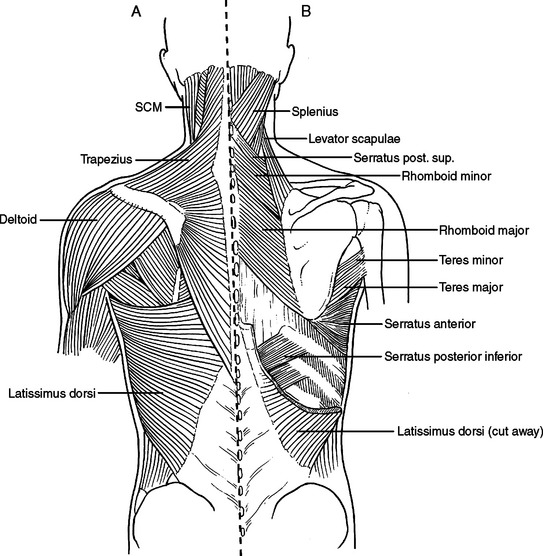
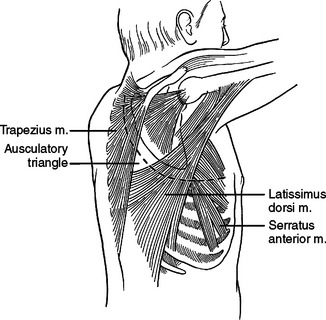
Laterally and posteriorly, two musculo-fascial layers guard the ribs. The more superficial layer contains the latissimus dorsi muscle laterally. Posteriorly, at the ausculatory triangle, or posterior border of the latissimus dorsi, this layer becomes a thin but tough layer of fascia, which more posteriorly envelopes the trapezius muscle. The second musculo-fascial layer contains the serratus anterior muscle laterally, becoming a broader sheet of thin but tough fibrous tissue posteriorly and then becoming the rhomboid major muscle then the rhomboid minor muscle posteriorly and superiorly (Figure 11). A tube thoracostomy will traverse these muscle layers to reach the ribs and interspaces. Knowing where you are in these layers allows precious time to be saved in traversing them and getting to where you need to be to complete the procedure.
A thoracotomy can be fashioned to divide or spare these muscles as needed in order to gain access to the rib cage. A full thoracotomy will divide the latissimus dorsi laterally and the trapezius posteriorly. The incision sweeps from horizontal across the lateral chest to vertical and parallel to the spine posteriorly (Figure 12). Deep to this layer, the serratus anterior can be swept anterior or divided. Posteriorly, the fascial layer coming off the serratus anterior is divided and then the rhomboid major and rhomboid minor muscles are divided. The innervation of the trapezius muscle and rhomboid muscles runs from medial to lateral. The more muscle body that is left medially, the more muscle function will be retained. Enough muscle needs to be left attached to the scapula to allow suture repair of the muscle, and the muscle should not be stripped from the scapula. The posterior and vertical aspect of this incision where the trapezius and rhomboids are divided is done to elevate the scapula off the chest wall, to access the interspaces underneath.
INTERCOSTAL SPACE
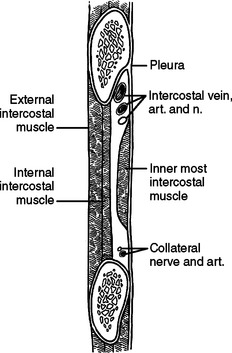
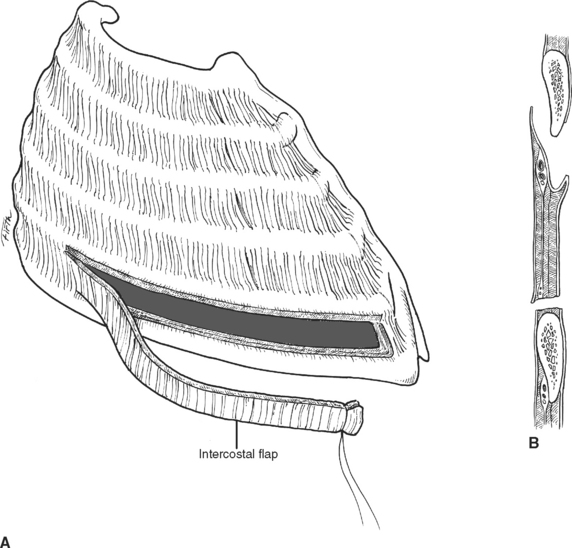
Each intercostal space from superficial to deep, has two layers of muscle; an artery, a vein, and a nerve; and a diminutive inner layer of muscle. The external intercostal muscles run obliquely with fibers in the same orientation as the external oblique muscle of the abdomen (fingers in pockets). Deep are the internal intercostal muscles running in the opposite direction. The intercostal artery, vein, and nerve run along the inferior aspect of each rib, occasionally running underneath a ledge in the costal groove. To avoid injury to these three structures, tube thoracostomies and thoracotomies are directed over the superior aspect of each rib or through the middle of the interspace, but not the inferior aspect of the rib (Figure 13). The innermost intercostal muscles are located deep to the neurovascular bundle and run in the same direction as the internal intercostal muscles. While mentioned in anatomy texts, surgically, the innermost intercostal muscles do not need to be considered separately from the internal intercostal muscle (Figure 14). The intercostal arteries originate as segmental branches off the descending aorta. The intercostal space, including the underlying pleura, can be harvested as a posteriorly based pedicled muscle flap (Figure 15). This flap is useful for reinforcing bronchial or esophageal repairs.
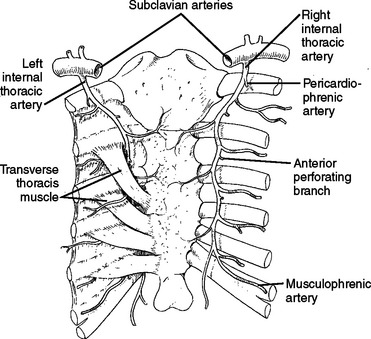
The internal mammary artery originates from the subclavian arteries bilaterally, and descends on the inside of the chest wall, approximately 1 cm lateral to the sternum bilaterally (Figure 16).
DIAPHRAGM
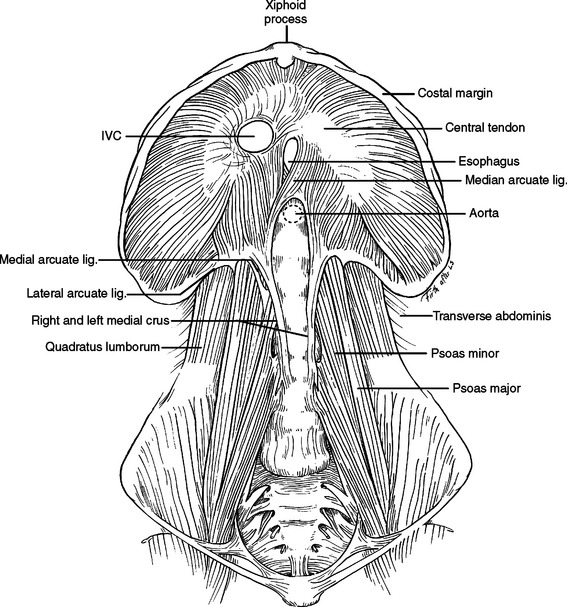
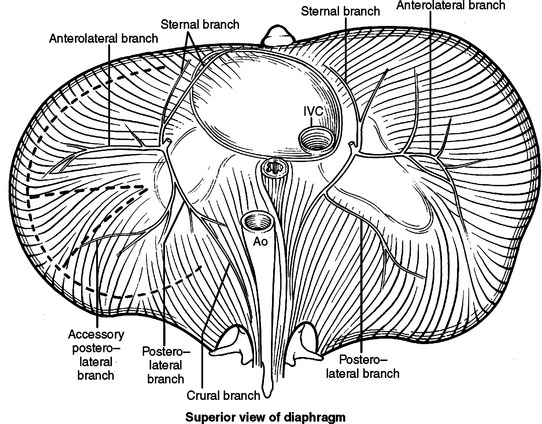
The diaphragm is the movable dome-shaped partition between the thoracic and abdominal cavities. With full exhalation, the dome of the diaphragm can rise to the level of the fourth interspace anteriorly (nipple level). With full inhalation, the diaphragm flattens, bringing the thoracic cavity down to the level of the costal margin anteriorly and the 12th rib posteriorly. The muscle fibers of the diaphragm originate from the sternum, the ribs, and the vertebral column. All three groups insert on a tough, fibrinous central tendon. Fibers of the sternal portion are short, arising as small slips from the back of the xiphoid process. Laterally on either side of the xiphoid, fibers originate from the inner surface of the lower six costal cartilages (costal margin). Posteriorly, fibers originate from a thick band arching over the quadratus lumborum (lateral arcuate ligament) and the psoas major (medial arcuate ligament). The paired lateral arcuate ligaments extend from the tip and lower margin of the 12th ribs and arch over the quadratus lumborum muscle to the transverse processes of L1. The paired medial arcuate ligaments complete the journey, arching over the psoas major from the tip of the transverse process of the first lumbar vertebrae to the tendinous portion of each diaphragmatic crus (Figure 17).
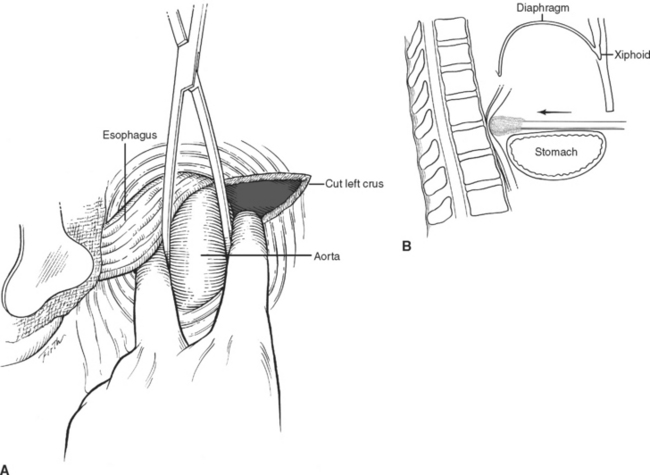
The posterior medial portion of the diaphragm is composed of two crura—an anatomic right crus originating from the upper three lumbar vertebral bodies and an anatomic left crus originating from the upper two lumbar vertebral bodies. Anterior to the aorta, the medial margins of the two crura form a poorly defined arch called the median arcuate ligament. Anterior to this arch, either the anatomic right crus (64%) or the anatomic left crus (2%) or both (34%) form the esophageal hiatus. While anatomists name the crura left or right by their origin from the left or right side of the vertebral bodies, surgeons name the crura left or right by their relationship to the esophagus. In the abdomen, visualization of the esophagus and division of the crus running to the left of the esophagus will expose the distal thoracic aorta above the level of the celiac artery and renal arteries. A clamp can be applied here to obtain vascular control. Alternatively, a retractor wrapped with a laparotomy pad can be used in this position to occlude the aorta by compressing it against the posteriorly located vertebral body (Figure 18).
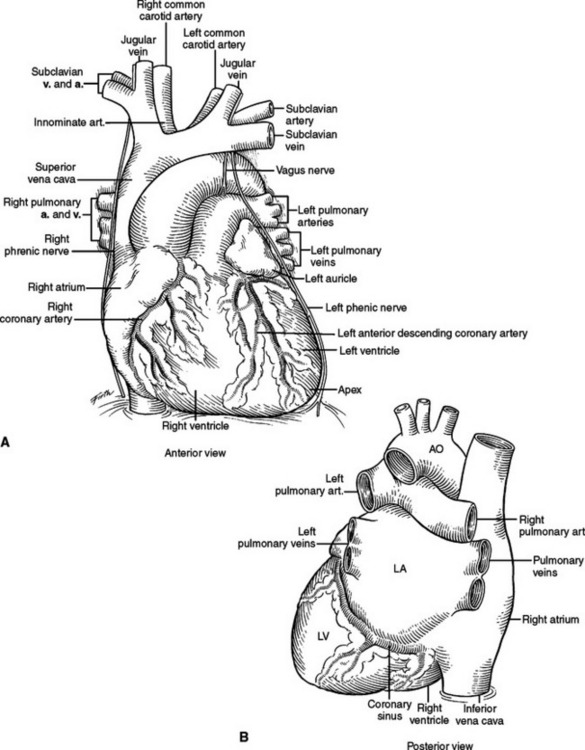
The phrenic nerve and twigs from the lower intercostal nerves innervate the diaphragm. The phrenic nerve originates primarily from the C4 nerve root, but receives innervation from C3 and C5 (C3, C4, and C5 keep the body alive). In the neck, the phrenic nerve originates lateral to the scalenus anterior muscle and descends from lateral to medial on the superficial surface of this muscle, deep to the sternocleidomastoid muscle. It enters the thoracic inlet and is found on the medial aspect of the mediastinum just deep to the pleura bilaterally. Superiorly, it is very anterior in the chest and vulnerable to injury, especially with a median sternotomy and dissection of the great vessels where it is often not readily visible in the wound, but very close to the dissection. On the left, it descends outside the pericardium, deep to the pleura, passing over the arch of the aorta, anterior to the hilum of the lung, and anterior to the inferior pulmonary ligament. As it nears the diaphragm, it is often invested in a veil of pericardial fat, hanging like a curtain between the pericardium and the diaphragm. The nerve reaches the diaphragm just lateral to the left border of the heart and in a plane slightly more anterior than the right phrenic nerve (see Figure 33). The right phrenic nerve descends along the right lateral border of the superior vena cava, passes anterior to the hilum of the lung, and anterior to the inferior pulmonary ligament. It is also invested in a veil of pericardial fat as it approaches the diaphragm. The right phrenic nerve enters the diaphragm just lateral to the inferior vena cava (see Figure 32).
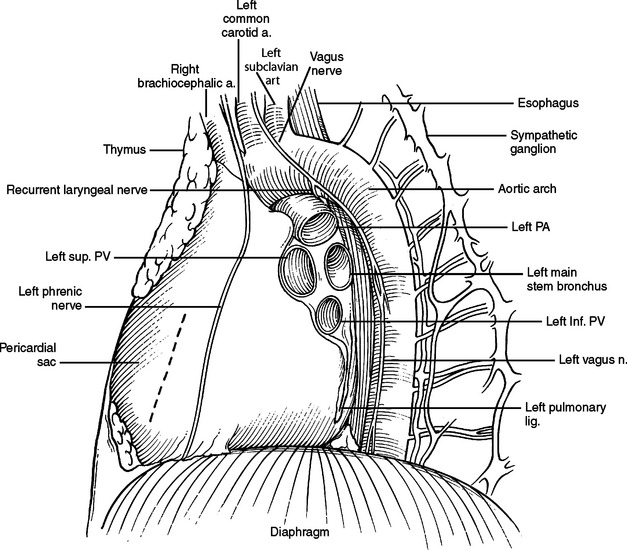
Figure 33 Hilum of left lung. Dotted line marks incision for pericardial window.
(Adapted from Grant’s Atlas of Anatomy, 11th ed., Philadelphia, Lippincott Williams & Wilkins, 2004, figure 1.44, p. 44.)
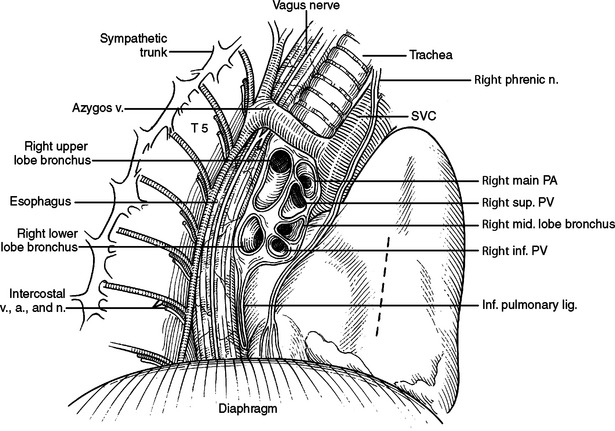
Figure 32 Hilum of right lung. Dotted line marks incision for pericardial window from right chest.
(Redrawn from Grant’s Atlas of Anatomy, 11th ed., Philadelphia, Lippincott Williams & Wilkins, 2004, figure 1.43, p. 40.)
Both left and right phrenic nerves immediately trifurcate into three muscular branches after entering the hemi-diaphragm. One is directed anterior-medially toward the sternum, one anterior-laterally, and a third posteriorly. The posterior branch bifurcates into a branch directed toward the 12th rib and one toward the crus. Safe incisions in the diaphragm are fashioned to avoid cutting major branches of the phrenic nerve (Figure 19). A peripheral and circumferential incision will avoid all but distal twigs of the phrenic nerve. Radial incision can be placed but must be done with care to avoid major branches of the phrenic nerve.
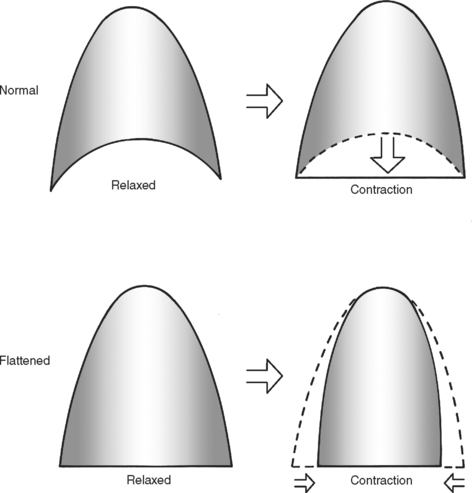
Because the primary innervation of the diaphragm, the phrenic nerve, enters centrally and spreads centrifugally, the diaphragm can be transposed to higher or lower origins from the thoracic cage while maintaining its innervation. This is occasionally required in repair of a diaphragmatic rupture when surface area of the diaphragm is lost or the chest wall has lost its rigidity and can no longer subserve its cylinder function. Care should be taken to maintain a dome shape to the diaphragm. A diaphragm that is flattened at rest will pull the walls of the thorax closer together when contracting. With contraction, instead of increasing intrathoracic volume, the diaphragm will now decrease intrathoracic volume and become a muscle of expiration (Figure 20).
PERICARDIUM
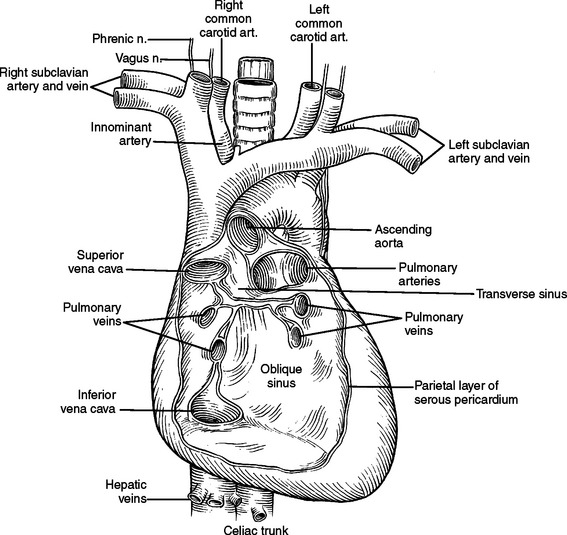
The pericardial space is considerably smaller than the pleural space and a small increase in the volume of fluid in this space can have a dramatic impact on cardiac function. The parietal pericardium is a thick, fibrous sac with an inner serosal surface containing the heart, the proximal ascending aorta, the distal superior vena cava, the distal inferior vena cava, the pulmonary trunk and bifurcation, proximal left and right main pulmonary arteries, and a short segment of all four distal pulmonary veins. From this description, it can be visualized that all vessels flowing into and out of the heart have short segments contained in the pericardial sac (Figure 21). It is also these vascular structures, which fix the heart in the pericardial sac. If the heart is allowed to rotate, these structures will be twisted or kinked, impeding blood flow. Because they are the lowest pressure conduits, the superior vena cava and inferior vena cava are the most vulnerable to kinking and impedance of flow. With decreased blood flow into the heart, there is decreased blood flow out of the heart, and systemic blood pressure falls. This is the physiology of hypotension associated with tension pneumothorax and with cardiac herniation.
The transverse pericardial sinus allows a finger or clamp to be placed along the right side of the ascending aorta, behind the aorta and pulmonary trunk, and be visualized to the left of the pulmonary trunk and superior to the left superior pulmonary vein in the vicinity of the left atrial appendage (see Figure 21).
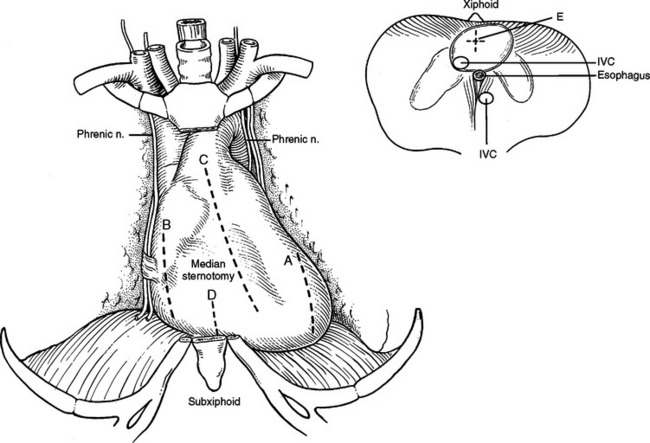
The pericardium can be drained through a median sternotomy, left or right thoracotomy, subxiphoid approach, or laparotomy. From a left or right thoracotomy, an incision is made anterior or posterior to and parallel to the phrenic nerve. From the left chest the left ventricle and from the right chest the right atrium will be encountered in the pericardial space behind these incisions (Figure 22).
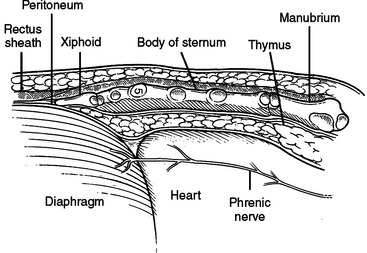
Subxiphoid Space
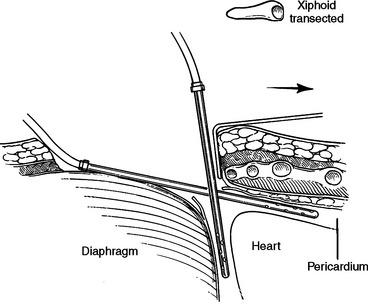
The subxiphoid space is a favored access to the pericardium for diagnosis and treatment of pericardial effusions. Both the linea alba and the diaphragm attach to the xiphoid. The peritoneum on the diaphragm is continuous with the peritoneum on the deep surface of the posterior fasciae of the anterior abdominal wall. An incision from above the xiphoid process to 4 cm below will pass through skin, fat, and linea alba. Incising the linea alba will reveal the xiphoid superiorly and the peritoneum inferiorly. The diaphragmatic attachments to the xiphoid can be divided flush with the xiphoid and the xiphoid resected to the level of the sternal body/costal margin. A large vein is routinely encountered at the angle between the xiphoid, costal margin, and sternal body. Posterior retraction of the diaphragm and superior retraction of the sternum will reveal the pericardial reflection on the diaphragm. Incising the pericardium will enter the pericardial space. The acute margin of the right ventricle will be visible through this incision (Figure 23). Since this incision is at the corner where two perpendicular planes meet, fluid can be aspirated in two directions. First, straight posterior, parallel to the diaphragm, along the inferior border of the heart, and second, superior, parallel to the sternal body, anterior to the anterior surface of the heart (Figure 24).
HEART
Body Surface Markings for Heart
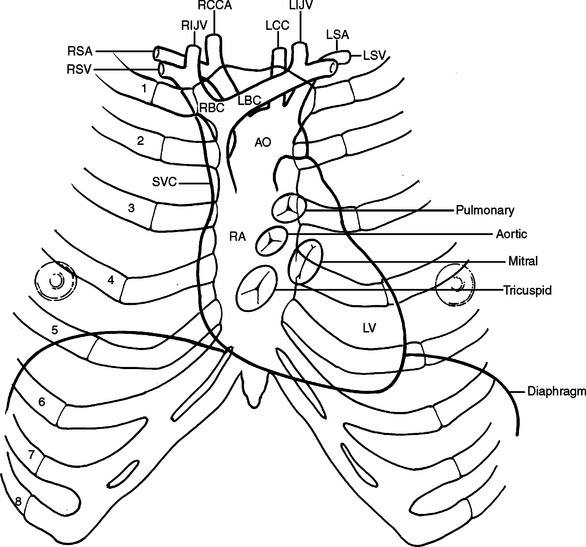
The surface projection of the superior border of the heart is a line joining a point 2 cm lateral to the sternum in the left second intercostal space to a point just to the right of the sternum in the same space. This marks the line of the main pulmonary arteries. The right border extends inferiorly to the sixth costal cartilage adjacent to the sternum. This is formed by the right atrium. The inferior border extends from the right sixth costal cartilage to the point of maximum cardiac impulse, which is usually in the left fifth intercostal space just medial to the mid-clavicular line. The right ventricle forms the inferior border. The left border extends superiorly to the second intercostal space 2 cm lateral to the sternal edge. The left ventricle forms the left border (Figure 25).
External Features
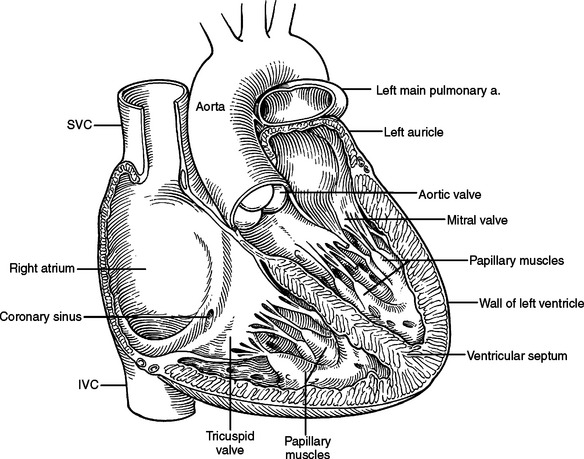
The heart consists of four chambers divided by three grooves. The atrioventricular groove contains the coronary sinus, which is the largest vein of the heart and lies posteriorly opening into the right atrium. The interatrial groove is covered anteriorly by the ascending aorta and the main pulmonary artery. The interatrial groove is visible to the right of the heart as a fatty line between the superior vena cava and right superior pulmonary vein. The interventricular groove runs anteriorly toward the apex and contains the great cardiac vein. Posteriorly, it continues along the inferior surface of the heart toward the right margin and contains the middle cardiac vein. The heart has five surfaces, anterior, posterior, inferior, and a right and left. The anterior surface is formed primarily by the right ventricle and the right atrium, and is therefore at risk from any frontal injury. Two-thirds of the right atrium and ventricle face anteriorly. The posterior surface or the base of the heart is formed by the left and right atria. The two pulmonary veins on either side, inferior and superior, open into the left atrium at this posterior location. The superior and inferior vena cava open into the right atrium. The posterior surface of the heart is related to the sixth through the ninth thoracic vertebrae being separated from them only by the pericardium, right pulmonary veins, esophagus, and aorta (from right to left). One-third of the right ventricle and two-thirds of the left ventricle form the inferior or diaphragmatic surface of the heart. This part of the heart is in contact with the central portion of the diaphragm. The right atrium and the right ventricle form the right surface of the heart. They are related to the pericardium, the right lung, and the right phrenic nerve just anterior to the hilum. The left ventricle and the left atrium form the left surface. They are related to the same structures as on the right but the phrenic nerve runs across the middle of the surface (Figure 26).
Coronary Arteries and Veins
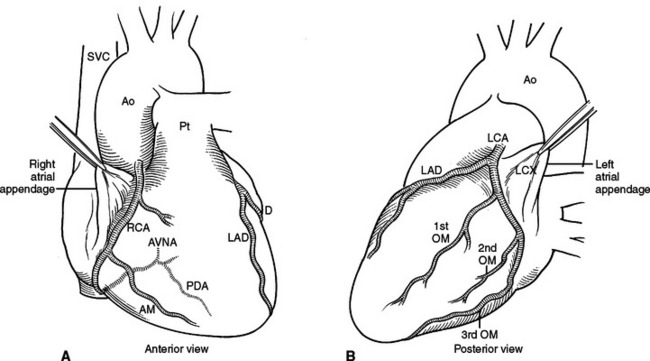
Right and left coronary arteries arise from the ascending aorta. The right coronary artery supplies the right atrium, the right ventricle, the posterior one-third of the interventricular septum and the inferior portion of the septum. The left coronary artery supplies the left atrium, the left ventricle and the anterior two-thirds of the interventricular septum. Collateral circulation in the heart is minimal therefore occlusion of a coronary artery results in a specific area of myocardial infarction and dysfunction (Figure 27).
The named coronary arteries travel just under the epicardium, superficial to the myocardium. Lacerations close to a coronary artery, but not including the artery, can be repaired with unpledgeted horizontal mattress sutures of Halsted. Alternatively, pledgeted horizontal mattress sutures may also be used, placed under the coronary bed, effectively repairing the myocardium but not occluding the coronary artery (Figure 28). Care should be taken in placing and tying the suture so as not to kink the coronary by incorporating too much myocardium. If the left anterior descending artery is the adjacent vessel being avoided, it is possible with this suture to occlude a major septal perforator diving deep to the vessel.
The venous system of the heart is centered on the coronary sinus, which receives the tributaries from the different areas of the heart and drains into the posterior aspect of the right atrium just superior to the tricuspid valve (see Figure 26B).
Conduction System
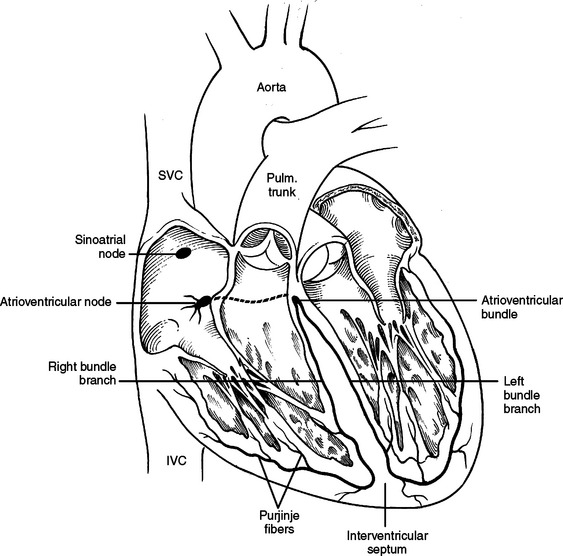
The sinoatrial node is the pacemaker of the heart and is located just to the right and anterior to the opening of the superior vena cava. The impulses are transmitted to the atrioventricular node through the wall of the atrium. The atrioventricular node is situated in the posteroinferior portion of the interatrial septum just superior to the opening of the coronary sinus. From here the impulses travel through the bundle of His (atrioventricular bundle) along the posterosuperior edge of the muscular interventricular septum to the right and left bundle branches. These run through the interventricular septum to the papillary muscles in the left and right ventricle and then form a subendocardial network (Purkinje fibers) (Figure 29).
Internal Features of Heart Chambers
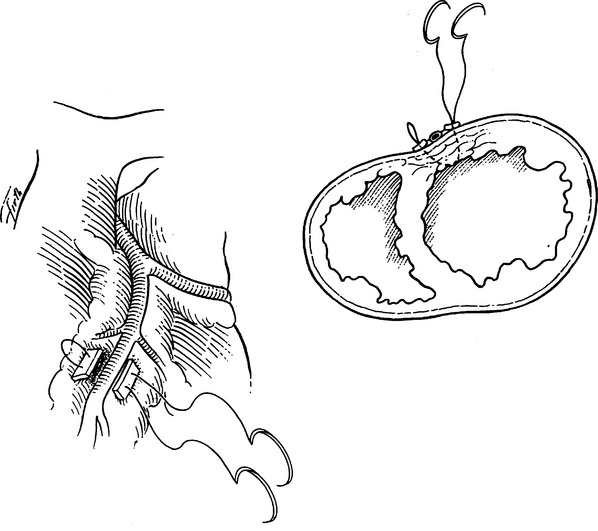
The left atrium is quadrangular in shape and has smooth walls. The left atrial appendage projects to the left and is the only portion of the atrium that can be seen anteriorly. The openings of the veins lie posteriorly on the left and right. The interatrial septum lies to the right and slopes posteriorly making the left atrium lie behind the right atrium. The mitral valve orifice lies in the anteroinferior part of the atrium (Figure 30).
The left ventricle is muscular and has an inflow area from the mitral orifice and an outflow area to the aortic root. The ventricular wall gives rise to anterior and posterior papillary muscles that have chordae tendineae that attach to the anterior and posterior mitral valve leaflets (cusps). The anterior leaflet separates the inflow of the mitral valve orifice from the outflow of the aortic root. The aortic orifice is therefore positioned anterior to the mitral orifice. The interventricular septum is present to the right and anteriorly (see Figure 30).
ANATOMY OF PULMONARY ARTERY/SWAN-GANZ CATHETER PLACEMENT
A pulmonary artery catheter is usually introduced via the subclavian or internal jugular vein but the femoral vein can also be used. The catheter passes through these veins into the superior or inferior vena cava and then into the right atrium. The flow of blood carries the tip through the tricuspid valve orifice into the right ventricle and then through the right ventricular outflow tract and the pulmonary valve into the main pulmonary artery. Due to the orientation of the right main pulmonary artery to the pulmonary trunk the catheter tends to pass to the right preferentially and lodge in the distal pulmonary artery.
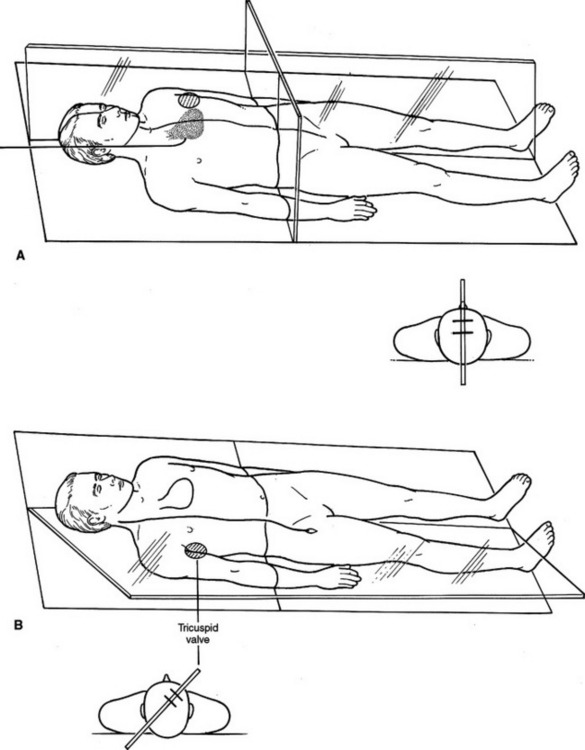
Traditional instruction on pulmonary artery catheter placement includes orienting the coil of the catheter such that it enters the atrium from the SVC and is directed toward the tricuspid valve. The coil of the catheter is oriented on a coronal plane with the tricuspid valve perceived to be a hole in a sagittal plane (Figure 31A). The tricuspid valve, however, truly exists on a plane rotated 40–50 degrees off the sagittal plane (Figure 31B). In a patient lying supine, blood flows from a right posterior position in the right atrium, through the tricuspid valve diagonally anterior and to the left. The coil of the catheter should therefore have its tip directed 40–50 degrees toward the ceiling rather than straight toward the left wall. The right ventricular outflow tract, however, is in the sagittal plane. Once the pressure tracing indicates the tip of the catheter is in the right ventricle, it should be rotated counterclockwise such that the coil is directed toward the left wall.
HILUM OF LUNG
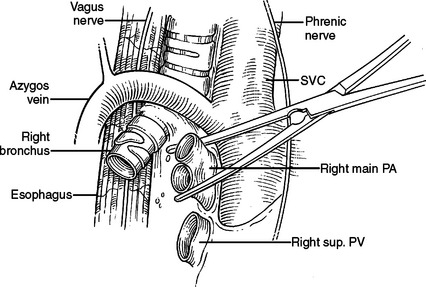
Much thoracic surgery is done through exposures retracting the lung anterior or posterior or looking directly at the anterior or posterior surface of the hilum. Bilaterally, the respective phrenic nerves run anterior to the hilum. On the right (Figure 32), the esophagus, vagus nerve, thoracic duct, and azygous veins are posterior. On the left (Figure 33), the descending aorta, esophagus, and vagus nerve are posterior.
Right Hilum
At the superior aspect of the right hilum is the azygous vein coursing posterior to anterior to join the backside of the superior vena cava. Deep to the azygous vein the trachea bifurcates. Traveling under or medial to the azygous vein is anteriorly the right main stem bronchus and posteriorly the esophagus. The right main pulmonary artery enters the right chest underneath the superior vena cava just inferior to the azygous vein and anterior to the trachea and right mainstem bronchus. The right main pulmonary artery travels further than the left main pulmonary artery before reaching the pleural space and before branching. After entering the right chest, the pulmonary artery takes an abrupt turn inferior into the deepest part of the horizontal and oblique fissures. It gives off branches to the right upper lobe, right middle lobe, and right lower lobe, respectively. It should be remembered that the pulmonary artery branches distally into the lung like a deciduous tree. Larger vessels will be found close to the hilum and in the horizontal and oblique fissures. Progressively smaller vessels will be found as you approach the outer surface of the lung (Figure 34). The first branch of the right main pulmonary artery goes to the right upper lobe. This branch may come off the pulmonary artery very proximal and course under the superior vena cava separate from the main pulmonary artery. This branch is often located just anterior to the right upper lobe bronchus, and just inferior to the azygous vein as it arches over the hilum. In this location, it is very susceptible to iatrogenic traction injury by too vigorously pulling the lung inferior.
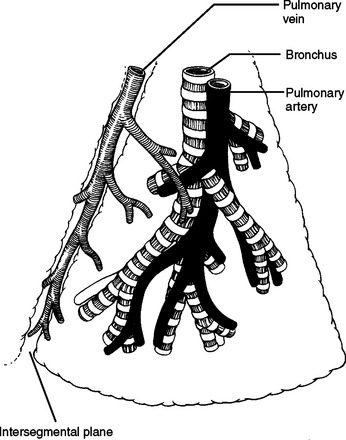
Figure 34 Generic bronchopulmonary segment.
(From Pearson FG, editor: Thoracic Surgery, 2nd ed. Philadelphia, Churchill Livingstone, 2002, figure 20-1, p. 428.)
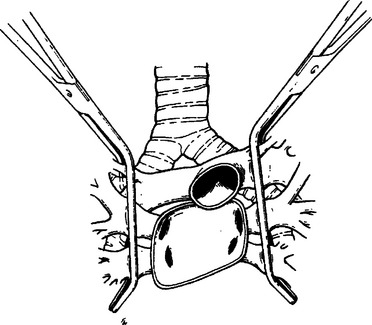
Proximal vascular control of the right pulmonary artery can be obtained as it courses under the superior vena cava either by encircling the vessel with a vessel loop, careful vascular clamping (Figure 35), or if needed, applying a nonselective clamp across the entire hilum (Figure 36). A nonselective clamp is sometimes referred to as “dirty” clamping because the immediate need is control of hemorrhage and structures other than the offending vessel may initially be included in the clamp.
Left Hilum
As with the right lung, the left inferior lobe is tethered to the mediastinum by the left inferior pulmonary ligament. The superior most aspect of this ligament is the left inferior pulmonary vein, often with a lymph node in the ligament, just inferior to the vein. The superior aspect of the left hilum has the arch of the aorta crossing from the right to the left and from anterior to posterior. The superior most structure in the hilum proper is the left main pulmonary artery. The left main pulmonary artery is shorter than the right main pulmonary artery. Its first branch is to the left upper lobe and is often buried in the medial substance of the lung parenchyma. This branch is also vulnerable to injury during inferior retraction of the lung. The vagus nerve descends in the left chest anterior to the left subclavian artery, crossing the lateral surface of the arch of the aorta and diving anterior to the descending aorta to join and travel next to the more medially placed esophagus. The vagus nerve gives off the left recurrent laryngeal nerve just below the arch of the aorta. The left recurrent laryngeal nerve will dive around the ligamentum arteriosum and also join the esophagus, but travel superiorly in the tracheoesophageal groove back into the neck to innervate the larynx. The ligamentum arteriosum is the vestigial ductus arteriosum. It is fibrous, possibly calcified, and connects the top of the bifurcation of the pulmonary trunk to the arch of the aorta. It is visible in the left chest and emphasizes the proximity of the bifurcation of the pulmonary trunk to the left hilum of the lung. Minimizing the use of electrocautery in this region and keeping dissections close to the pulmonary artery and away from the aorta and ligamentum arteriosum can avoid injury to the recurrent laryngeal nerve. Proximal control of the left pulmonary artery can be obtained by encircling the pulmonary artery, application of vascular clamps, or by hilar clamping. Because the left pulmonary artery is so short, it is sometimes necessary to incise the pericardium anterior to the left main pulmonary artery, taking care not to injure the phrenic nerve. The pulmonary trunk and intrapericardial course of the left main pulmonary artery can then be visualized and the left main pulmonary artery clamped. Immediate vascular collapse after placement of this clamp may mean blood flow to the right pulmonary artery was occluded as well and the clamp should be reapplied. The left atrial appendage will be present in this location. It is mobile and often an unwelcome companion. It is susceptible to injury, hemorrhage, and air embolism by clamping and retraction and should be treated with respect.
The left hilum can also be nonselectively clamped to obtain vascular control (see Figure 36).
LUNG ANATOMY
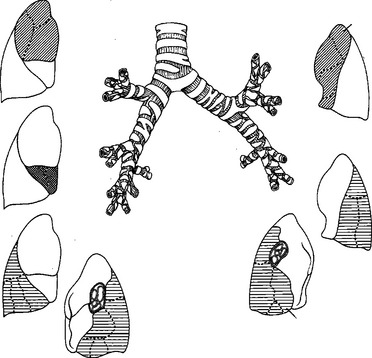
The right lung has three lobes, the right upper, right middle, and right lower. The left lung has two lobes, the left upper and left lower. The lingula of the left upper lobe is analogous to the middle lobe on the right. Fissures of the lung are usually present, but variably complete. On the right, the horizontal fissure, usually incomplete, divides the right upper lobe from the right middle lobe. The horizontal fissure joins the oblique fissure posteriorly. The oblique fissure divides the right upper lobe from the superior segment of the right lower lobe and the right middle lobe from the right lower lobe. The right lower lobe rests on the diaphragm posteriorly. The right middle lobe will often rest on the diaphragm anteriorly and is mistaken for the right lower lobe in this position. On the left, the oblique fissure divides the left upper lobe from the left lower lobe. The left lower lobe rests on the diaphragm posteriorly. The lingula of the left upper lobe can extend down to the diaphragm anteriorly.
Figure 37 shows the lung segments. Pulmonary arteries and the airway will enter the middle of their respective lung segment and bifurcate toward the periphery. Pulmonary veins travel in the border zones between lung segments and along the walls of the horizontal and oblique fissures (see Figure 34).
AORTA, TRACHEA, ESOPHAGUS, AND THORACIC DUCT
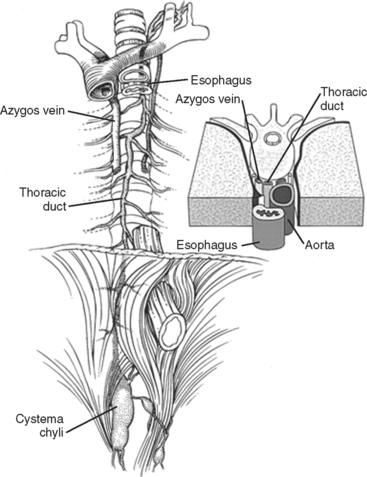
Posterior to the heart, outside the pericardium, several tubular structures travel parallel to each other. They are the aorta, trachea, esophagus, and thoracic duct (Figure 38).
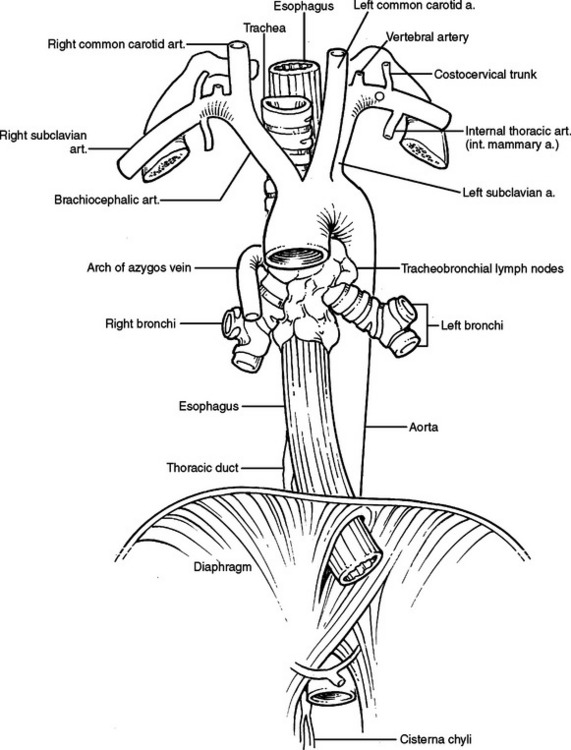
Figure 38 Aorta, trachea, esophagus, and thoracic duct.
(Adapted from Grant’s Atlas of Anatomy, 11th ed., Philadelphia, Lippincott Williams & Wilkins, 2004, figure 1.78, p. 68.)
TRACHEA
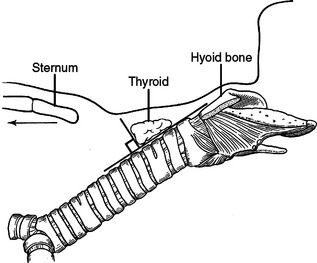
The trachea begins in the neck at the cricoid cartilage, enters the thorax anterior to the esophagus and posterior to the great vessels, including posterior to the arch and ascending aorta and the pulmonary arteries. Distally, near the carina, the arch of the aorta crosses to the left of the trachea. The trachea bifurcates into the right and left mainstem bronchi at the carina. The carina is at the level of the angle of Louis anteriorly and T4/T5 posteriorly. The average adult trachea is 11 cm in length and varies according to the height of the person. In a young person, hyperextension of the neck can bring 50% of the trachea out of the chest and into the neck. Conversely, in a kyphotic elderly patient, the cricoid cartilage can be at the level of the sternal notch. In the neck, the trachea is anterior and subcutaneous. As it enters the chest, it travels obliquely posterior to the posterior mediastinum. The shortest distance from a point to a line is a perpendicular from that line, intersecting the point. If the trachea is a line, obliquely posterior, the shortest distance from a point on the skin to the trachea will be in a trajectory slightly superior. Visualizing this relationship aids in tracheostomy and cricothyroidotomy incisions (Figure 39). The trachea is composed anteriorly of cartilaginous arches with fibrous tissue in between. The posterior wall of the trachea is membranous. The blood supply to the trachea is segmental, superiorly primarily from the inferior thyroidal arteries and inferiorly from the bronchial arteries. The subclavian artery, highest intercostal artery, internal thoracic arteries, and innominate artery also supply it. These vessels also supply the esophagus. The blood supply enters the trachea laterally at 3 and 9 o’clock.
ESOPHAGUS
The esophagus travels through the posterior mediastinum anterior to the vertebral bodies, to the right of the descending aorta, and to the left of the azygous vein (see Figure 38). Its blood supply is from segmental branches of the descending aorta, draining into intercostal veins. Above the level of the carina, the esophagus is posterior to the trachea and immediately abuts the membranous trachea. Above the level of the ligamentum arteriosum, the recurrent laryngeal nerve travels in the left tracheoesophageal groove. Above the level of the right brachiocephalic artery the right recurrent laryngeal nerve travels in the right tracheoesophageal groove. Below the carina, the esophagus directly abuts the posterior pericardium. To its left and right from superior to inferior are the superior pulmonary veins, the inferior pulmonary veins, and the inferior pulmonary ligaments. The esophagus enters the diaphragm through the esophageal hiatus at the level of T10 or T11. The area to the left and right of the esophagus at and below the inferior pulmonary ligament and before entering the diaphragm represents an anatomic weak spot. The wall of the esophagus is not buttressed by other firm mediastinal structures and is exposed to the negative pressure of the pleural space. It is for this reason that increased intraesophageal pressure causing a perforation of the esophagus most commonly occurs here (Boerhaave’s syndrome).
THORACIC DUCT
The thoracic duct originates from the cisterna chyli (Figure 40). The cisterna chyli is located in the abdomen, at the level of the celiac axis, anterior to the vertebral body and to the right of the aorta. The thoracic duct travels superiorly, entering the thorax through the aortic hiatus of the diaphragm. It ascends in the posterior mediastinum between the aorta and the azygous vein. Above the arch of the aorta, it travels posterior to the esophagus, arches behind the internal jugular vein to join the venous system at the junction of the internal jugular vein and subclavian vein. The thoracic duct is thin walled and often invisible to the naked eye if not distended with lymph. Injury to the duct is visible as a pooling of lymph in the vicinity of the leak. Fat delivered to the small bowel will within 10–20 minutes turn this lymph milky white, enhancing visualization. Ligation of the thoracic duct is accomplished by ligating all fatty material and lymphatics bounded by four walls, consisting of the azygous vein, the parietal pleura, the esophagus, and the aorta below the level of the suspected leak.
Agur AM R. Grant’s Atlas of Anatomy, 9th ed. Baltimore: Williams and Wilkins, 1991.
Christensen JB, Telford IR. Synopsis of Gross Anatomy, 5th ed. Philadelphia: J.B. Lippincott, 1988.
Collis JL, Kelly TD, Wiley AM. Anatomy of the crura of the diaphragm and the surgery of hiatus hernia. Thorax. 1954;9:175-189.
Collis JL. Surgical control of reflux in hiatus hernia. Am J Surg. 1968;115:465-471.
Graeber GM, Szwerc MP. Anatomy and physiology of the chest wall and sternum. In: Pearson FG, editor. Thoracic Surgery. 2nd ed. Philadelphia: Churchill Livingstone; 2002:1325-1335.
Mafhisen DJ, Grillo H. The trachea. Baue A, et al, editors. Glenn’s Thoracic and Cardiovascular Surgery, 6th ed., I. Stamford, CT: Appleton & Lange, 1996;665-690.
Mehran RJ, Deslauriers J. Anatomy and physiology of the pleural space. In: Pearson FG, editor. Thoracic Surgery. 2nd ed. Philadelphia: Churchill Livingstone; 2002:1133-1139.
Plestis KA, Fell SC. Anatomy, embryology, pathophysiology, and surgery of the phrenic nerve and diaphragm. In: Pearson FG, editor. Thoracic Surgery. 2nd ed. Philadelphia: Churchill Livingstone; 2002:1499-1507.
Rice T. Anatomy of the lung. In: Pearson FG, editor. Thoracic Surgery. 2nd ed. Philadelphia: Churchill Livingstone; 2002:427-441.