CHAPTER 105 PERIPHERAL NERVE INJURY
Peripheral nerve trauma is any injury to a nerve caused by an extrinsic agent; such injuries constitute a distinct category of peripheral nerve disorders. Peripheral nerve trauma is a commonly encountered problem in level I trauma centers after severe trauma, and residual manifestations of nerve dysfunction are a common reason for referral to a neuromuscular practice. In one population-based study, injury to the peripheral nerves was responsible for 2% of hospital admissions and 29% of surgical procedures after trauma.1 In the majority of cases, nerve dysfunction is the result of penetrating, stretch, or crush injury from violent accidents, although a variety of other extrinsic agents may also produce nerve injury. Appropriate early identification and evaluation of nerve trauma is important in guiding the treatment and the attempts to preserve or improve function of the nerve. The clinical features, pathophysiology, evaluation, and treatment of physical nerve injury are discussed in this chapter.
ANATOMY AND PHYSIOLOGY OF PERIPHERAL NERVE INJURY
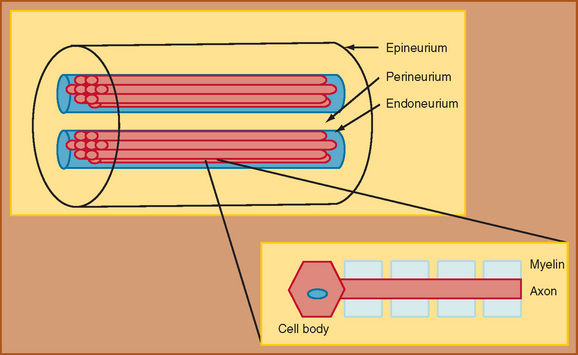
The foundation for understanding nerve injury begins with a brief discussion of nerve anatomy and physiology. This topic and its relation to nerve injury have been reviewed extensively.2,3 Through the classic work of Sunderland (1990), the details of the microstructure of nerve anatomy have been elucidated and have formed the foundation for the development of classification schemata of nerve injury.2 The peripheral nerve is composed of several components, each of which plays an integral role in nerve function (Fig. 105-1). The cell body, or cyton, is the metabolic center of the nerve and produces the structural elements and nutrients that are important for maintaining nerve function. The axon is the main transporting segment of the nerve and conducts action potentials and transports proteins from the cell body to the nerve terminal. Most nerves contain a myelin sheath that surrounds the axon and provides a mechanism for rapid conduction of the action potential along the axon.
The primary function of a nerve is to conduct information from one site to another in the nervous system. In the peripheral nervous system, the motor nerves conduct impulses from the brainstem or spinal cord to a muscle, whereas the sensory nerves conduct sensory stimulus from a peripheral receptor back to the spinal cord or brainstem. An inability to conduct these impulses produces weakness, sensory loss or disturbance, or autonomic dysfunction. The physiology of propagation of these impulses begins with an action potential generated at the cell body of the motor neuron or peripheral sensory receptor. The action potential then rapidly spreads from the point of origin over the entire axon membrane to the nerve terminal at the neuromuscular junction, in the case of a motor axon, or to the central nervous system, in the case of a sensory axon. Rapid propagation of the action potential along the nerve relies on the integrity of the axon, the transmembrane channels, and the myelin sheath. When nerve injury occurs, interference with action potential development and propagation may occur, leading to neurological symptoms.
CLASSIFICATION OF PERIPHERAL NERVE INJURY
Different classifications of stages of nerve injury have been developed.2,4 One scheme, proposed by Sunderland (1990), separates nerve injury into five stages on the basis of the extent of anatomical disruption of the nerve (Table 105-1).2 In this classification, the higher stages of nerve injury represent a greater degree of damage to the nerve and the surrounding structures, and therefore the potential for nerve regeneration and regrowth diminishes, leading to a poorer prognosis. The other classification scheme, developed by Seddon, consists of fewer stages that also reflect the degree of damage to the different components of the nerve and supporting structures. Seddon proposed three stages of nerve injury: neurapraxia, axonotmesis, and neurotmesis.4
Axonotmesis in Seddon’s classification scheme is a more severe stage of injury. In this stage, the continuity of the axon is disrupted and the portion of the axon separated from the anterior horn cell or dorsal root ganglia undergoes wallerian degeneration. For the first week after an axonotmetic injury, the distal portion of the axon that is separated from the cell body still has the ability to propagate an action potential when stimulated electrically. After 1 week, wallerian degeneration of the axon occurs, and the disconnected segment of the axon can no longer conduct an action potential when stimulated. Axonal regeneration and regrowth along the endoneurial tubes is possible. Sunderland divided axonotmetic lesions into three separate degrees, on the basis of the amount of damage to the connective tissue. In Sunderland’s stage 2 injuries, the axon is disrupted but the endoneurial tube, and remainder of the connective tissue is intact. In stage 3, the axon and endoneurial tube are disrupted, but the perineurium and epineurium are intact. In stage 4 lesions, the axon, endoneurial tube, and perineurium are damaged, and only the epineurium is preserved. The duration and extent of axonal regrowth and regeneration depend on the degree of disruption of the nerve and the distance required for the nerve to regrow. The amount and speed of recovery are worse with higher grades of injury in Sunderland’s classification.
EVALUATION OF NERVE TRAUMA
Electrophysiological Evaluation
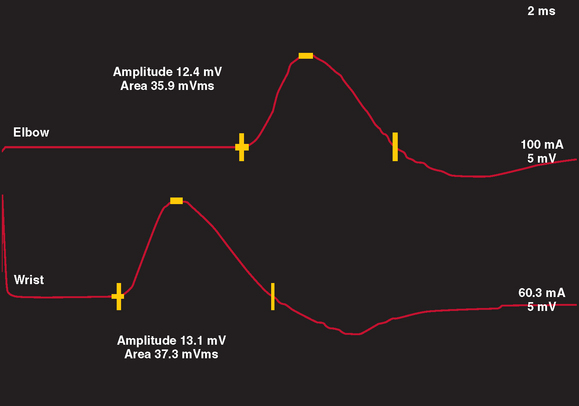
Motor NCS are often more useful than sensory NCS in identifying the degree and stage of nerve injury. In a normal nerve, supramaximal electrical stimulation of a nerve depolarizes all of the motor axons within the nerve, which subsequently produce action potentials within all muscle fibers innervated by that nerve. This produces a summated compound muscle action potential (CMAP), or M wave, recorded from a muscle innervated by the nerve. When the nerve is stimulated at a proximal and distal site, the CMAP amplitudes and areas of the two sites are similar, inasmuch as all axons within the nerve are similar in size and conduct at a similar rate. In most motor nerves, there is never more than a 20% reduction in amplitude and area between the two sites of stimulation (Fig. 105-2).
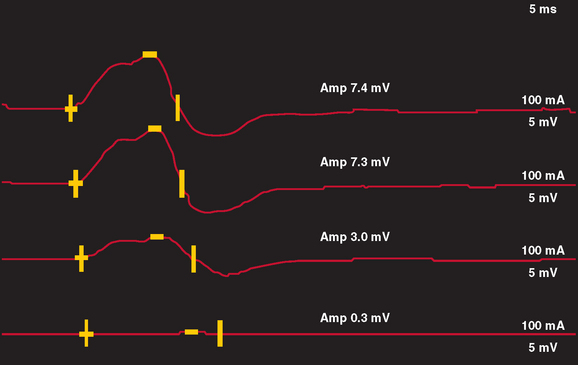
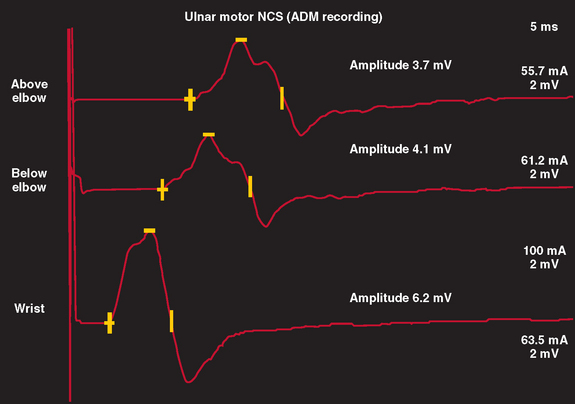
In nerve trauma characterized by neurapraxia or early axonotmesis, the evoked action potential initiated at a site proximal to the injury is unable to propagate through the lesion. Thus, the lesion produces a “conduction block” of the action potential. However, because the axonal segment and supporting structures distal to the injury remain intact, stimulation distal to the lesion produces a normal response. In the most severe cases in which all axons within a nerve are affected, such as with complete nerve transection or a neurapractic lesion involving all motor axons, no response is obtained with proximal stimulation (complete conduction block) (Fig. 105-3). Stimulation distal to the site of nerve injury produces a propagated action potential and a normal CMAP. In cases of incomplete nerve injury, only some axons are affected, and a partial conduction block may occur (Fig. 105-4). Stimulation proximal to the lesion produces action potentials in all the motor axons. Some percentage of these action potentials are propagated by motor axons that are damaged, and these action potentials do not spread through the lesion. There, action potential spread is blocked. Other motor axons in the nerve may be spared by the lesion, and these spread across the lesion to reach the muscle and produce a CMAP. The amplitude and area of the CMAP are a rough guide to the number of axons spared in an acute to subacute lesion. In more superficial nerves, or with near-nerve stimulation with a monopolar needle, the precise site of the nerve injury can be localized to within 2 cm by short-segment incremental stimulation (“inching”) studies.
In neurapractic lesions, the conduction block persists until the underlying process is reversed. However, in lesions characterized by axonotmesis or neurotmesis, subsequent events produce further changes on NCS. Within the first 5 to 7 days after injury, the NCS simulates a focal conduction block that is indistinguishable from a neurapractic lesion, with normal conduction distal to the injury but block of conduction with stimulation proximal to the lesion. However, after 5 to 7 days, wallerian degeneration of the axons in the distal segment of the nerve occurs, leading to inexcitability of the distal nerve. This is manifest on NCS by a low or absent CMAP response with stimulation proximal and distal to the site of the lesion. Therefore, within the first week after injury, NCS is useful in localizing the lesion but not in determining severity or prognosis. However, after approximately 2 weeks, NCS can help the clinician distinguish neurapractic from axonotmetic lesions and better determine severity, pathophysiology, and prognosis, but in the case of axonotmetic lesions, it may be less helpful in precisely localizing the injury.
Electromyographic assessment of voluntary motor unit potentials is helpful in identifying whether reinnervation by regrowth or collateral spouting is occurring. In pure neurapractic lesions, the needle examination reveals a reduced number of voluntary motor unit potentials recruited when the patient attempts to produce a more forceful activation of the muscle under examination. There are no fibrillation potentials or change in the amplitude, duration, or structure of the motor unit potentials. In axonotmetic or neurotmetic lesions, the disintegration of the motor axons extends to the nerve terminal at the neuromuscular junction, and the muscle fiber is separated from its innervation. Immediately after the injury, the needle examination reveals reduced recruitment if the lesion is partial or no motor unit activity if the lesion is complete. In partial lesions, the structure of the motor unit potentials should be normal. Fibrillation potentials appear in the denervated muscles after 2 to 3 weeks. In partial or incomplete axonotmetic lesions, the nerve terminals of surviving motor axons begin to produce axonal sprouts 4 days after the injury. These effectively reinnervate some denervated muscle fibers after 3 weeks. At that time, the structure of the surviving motor unit potentials begins to change. There is an increased number of turns or phases on the motor unit potential. As more and more collateral sprouts from a motor neuron reinnervate muscle fibers, the motor unit potentials become more complex, larger in amplitude, and longer in duration.
Imaging of Peripheral Nerve Trauma
Historically, the sensitivity of imaging studies for identifying the anatomy of the peripheral nerve in detail was low, and imaging studies had low utility in the evaluation of peripheral nerve trauma. However, as the quality of imaging has improved, these studies are becoming increasingly useful adjunctive measures for assessing peripheral nerve trauma. Imaging studies can provide important information regarding the continuity of a nerve after trauma. Magnetic resonance imaging with short-time inversion recovery sequences can demonstrate denervated skeletal muscle, which is high in extracellular water content and thereby produces increased signal on short-time inversion recovery and T2-weighted images.5 The distribution of muscle involvement can be extrapolated to determine which nerve or nerves are affected by trauma. More recently, magnetic resonance neurography has evolved to allow for visualization of large peripheral nerves.6,7 Indications for magnetic resonance neurography include the need to visualize roots and nerves for entrapments, adhesions, or the effects of trauma.8 In some cases, magnetic resonance neurography can identify discontinuity of the nerve and therefore is helpful in determining prognosis of recovery.8
Ultrasonography provides less detail of nerve anatomy than does magnetic resonance neurography, but it has been evaluated for its utility in monitoring patients who have undergone direct nerve repair after peripheral nerve injury.9 In a group of 11 patients, reliable identification of the nerve was made with ultrasonography, and neuroma formation, scarring with compression of underlying nerve, and partial discontinuity of nerve fascicles could be identified.9
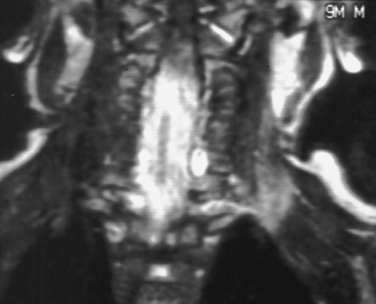
In cases of injury to the brachial plexus in which root avulsion may have occurred, myelography and computed tomographic myelography are the imaging modalities of choice.10 The myelogram may demonstrate several findings to indicate root avulsion, including pseudomeningocele, poor root sleeve filling, and spinal cord edema or atrophy (Fig. 105-5). Although magnetic resonance imaging may also demonstrate findings suggestive of root avulsion, the findings on computed tomographic myelogram were correlated better with such injury at surgery than were the findings on magnetic resonance imaging.11
CAUSES OF PERIPHERAL NERVE TRAUMA
There are many different causes of nerve trauma, each of which presents distinct issues related to nerve function, recovery, and treatment (Table 105-2). Much of the early information related to nerve trauma was derived from wartime injuries during the American Civil War and the World Wars. Currently, the most commonly encountered cause is blunt or stretch injury related to high-speed motor vehicle accidents, followed by falls, penetrating trauma, and industrial accidents.12 Population studies have estimated that 5% of patients admitted to level I trauma centers have sustained peripheral nerve injuries.13 In many instances of injury related to major trauma, the precise mechanism of nerve injury may not be identified, or several mechanisms may have contributed. For example, in a severe motor vehicle accident, compression, crush, and stretch injury may occur, as may ischemia from arterial damage related to the injury.
| Type of Trauma | Examples |
|---|---|
| Acute transection | Glass or knife wound, surgically induced |
| Chronic compression | Carpal tunnel syndrome |
| Stretch injury (stretch, rupture, avulsion) | Traction from motor vehicle accidents |
| Injection | Intraneural injections |
| Ischemic | Compartment syndromes |
| Radiation | Post-irradiation plexopathy |
| Temperature (cold) | Frostbite, trench foot |
Acute Nerve Transection or Laceration
Acute transection of the nerve is less common than blunt or compressive injury and occurs with clean, sharp lacerations, such as wounds inflicted by glass or knife injuries. Nerve transactions may also occur as a result of fracture of an adjacent bone or as an iatrogenic injury after inadvertent nerve transection during surgical procedures. In a study of 722 surgically treated cases of nerve trauma, approximately 17% were iatrogenic, the majority occurring during orthopedic procedures or minor surgery.14 The nerve most commonly affected was the spinal accessory nerve (after lymph node biopsy), followed by the common peroneal, radial, and genitofemoral nerves.
In most cases of nerve laceration, neurotmesis or complete transection of the entire axon and surrounding connective tissue structures occurs, producing a distinct separation of the two ends of the nerve. Depending on the length of the nerve separation, potential for regrowth or reinnervation is low. After clean nerve laceration, early surgical repair is usually necessary to align the two ends of the nerve. With lacerations related to blunt injuries, such as chainsaw or propeller blades, a delay of several weeks before surgical intervention may allow for identification and resection of damaged nerve tissue before realignment.15 In iatrogenic surgically induced nerve lacerations, the outcome after surgical intervention was good in approximately half of the patients, with some improvement in sensory, motor, or pain.
Chronic Nerve Compression
The pathological changes that occur with chronic or repeated compression include mechanical and vascular changes. One of the first changes is focal demyelination at the site of compression. Experimental studies with tourniquet compression have been performed to produce a model of compressive lesions.16 In these studies, compression of the myelin with invagination and intussusception of paranodal regions was identified most prominently at the edge of the tourniquet. Over time, focal demyelination is followed by reduction in the axon diameter and, ultimately, axonal degeneration. However, if the cause is removed, reinnervation and remodeling of myelin may lead to recovery of nerve function and resolution of symptoms within weeks to months. In addition to the mechanical changes in the nerve, mechanical compression produces increased intraneural pressure, which in turn produces intraneural venous obstruction and capillary congestion.
Stretch Injury
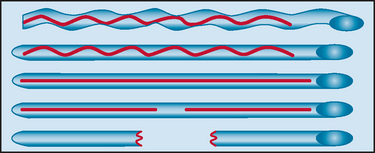
As discussed earlier, nerve fibers travel in a plexiform configuration within the fascicles, and supporting connective tissue structures, which assist in maintaining the structure and integrity of the nerve, surround the axons. Although the epineurium protects the nerve fibers from external compression, the perineurial tissue is most important in the protection against stretch injury, because it provides more of the elasticity and resistance than do other structures. Several features of nerves protect them from stretch injury: (1) the undulating course of the nerve fibers within the fascicle, (2) the anatomical course of nerves across flexor aspects of joints (with the exception of the ulnar nerve at the elbow and the sciatic nerve at the hip), and (3) the elasticity of the nerve trunks.17
When the nerve is stretched, stepwise alterations of nerve anatomy occur (Fig. 105-6). First, the undulations of the epineurial connective tissue disappear, although partial laxity of the nerve fibers remains unaffected. Next, with continued stretching, the nerve fibers become straightened, the cross-sectional area of the nerve is reduced, and the intraneural pressure is increased. Finally, with continued stretch, the nerve fibers become stretched and ultimately may rupture if continued tension is present.2,17 Experimental studies with rabbit nerves have shown that with mild, gradual stretch to 30% of the initial nerve length, minimal macroscopic changes occurred and the epineurium, perineurium, and endoneurium remained intact; however, the cross-sectional area of the nerve was reduced, and the myelin became swollen. With more severe gradual stretch, epineurium rupture occurred. The mean elongation to the point of limit of elasticity was 56%. No significant difference was seen in the pattern of damage with gradual or sudden stretching.18
Injection Injury
Patients with injection nerve injury typically develop neurological symptoms immediately or within minutes after the injection.19 Sharp stabbing or radiating pain in the distribution of the nerve is the initial symptom. Motor and sensory loss is common and occurs to a variable degree, ranging from severe weakness and sensory loss in the distribution of the injured nerve to only mild deficits. Spontaneous recovery is variable. Early reviews suggested that residual deficits persist in the majority of cases, with recovery in only 14%.19 Experimental studies of injection injury have demonstrated that regeneration of the injured nerve does occur, beginning 2 to 3 weeks after injection.20
A number of mechanisms of nerve injury after injections have been proposed. The most supported mechanism is a direct toxic affect of the offending agent on the nerve. Several factors may be important in the development of nerve injury after injection. First, the nature of the offending agent may affect the degree of nerve injury. When injected intraneurally, many different pharmacological agents, including antibiotics, paraldehyde, and magnesium sulfate, produce significant nerve damage. In one animal study, injections of nine different agents produced a variable degree of pathological changes within the nerve. Meperidine and cephalothin resulted in minimal loss of myelinated and unmyelinated fibers, whereas severe nerve injury occurred after injections of penicillin, diazepam, and chlorpromazine, even when administered in the extrafascicular region.20 Comparison of the degree of injury with dose of several medications indicated that higher doses resulted in more severe injury. Because most active components of medications are contained in a buffered mixture with other agents, it is difficult to determine whether the nerve response was a reaction to the active ingredient or to the buffering agent. Control studies with intrafascicular or extrafascicular saline injection produces no pathological change.20
Second, the site of injection in relation to the nerve anatomy plays an important role in the development of nerve injury. Early studies have shown that injection in the epineurial region produces little no pathological change, although some medications such as paraldehyde may produce circumferential fibrosis. On the other hand, intraneural injections consistently produce inflammatory cell infiltration.19–21 In addition to direct toxic effect of the infused agent, nerve ischemia caused by thrombosis or spasm of a nutrient artery may occur as a reaction to the toxic effect of the drug infused, or mechanical trauma from the needle may also injure the nerve.22,23 The effect of the length of the bevel tip on the degree of nerve injury has been studied in mechanical nerve “impalement” and has demonstrated that shorter bevel tips produce more severe damage than do long bevel tips.22
Treatment of injection injury is largely symptomatic. However, exploratory surgery with nerve irrigation or neurolysis has been proposed.20
Ischemic Nerve Injury
Peripheral nerves are relatively resistant to ischemia, inasmuch as they are supplied by a network of longitudinally arranged arterioles and veins, with feeding arteries entering the nerve at several sites along the nerves. Therefore, pure ischemia of nerve after trauma is unlikely to occur without concomitant nerve compression, stretch, or crush. Experimental studies of ischemia in the nerve are sparse, because ligation of a feeding artery does not produce significant ischemia.24,25 In a study on rat sciatic nerve, conduction failure occurred after 30 minutes of ischemia and was reversible if the ischemia persisted for less than 1 hour.26 Dyck and associates found a patchy distribution of nerve fiber degeneration that began in a central fascicular location in watershed zones in the mid–upper arm and in the midthigh for the lower extremity after nerve ischemia in humans.27
In many cases of peripheral nerve trauma, direct injury to the nerve from laceration, crush, or stretch produces nerve dysfunction. However, with these mechanisms of injury, interruption of the vascular supply surrounding the nerve may also play a role in the mechanism of nerve injury. Ischemic nerve injuries may occur after thrombosis that follows soft tissue trauma or bone fractures. This seems typically to result from bone and arterial lesions around the distal humerus, distal femur, and proximal tibia, where the arterial blood supply to nerves is more vulnerable because long sections of these nerves are dependent on a single nutrient artery. Embolization or graft thrombosis after aortofemoral bypass or peripheral vascular surgeries may produce ischemic damage to the distal tibial, distal peroneal, sciatic, or femoral nerves.28 Similar complications have been reported after transfemoral intraaortic balloon assist pumps.29 Therapeutic or accidental intra-arterial injection of drugs may result in severe arterial spasm and thrombosis with soft tissue and nerve damage.23,30 Upper limb ischemia associated with artificial arteriovenous shunts for dialysis has produced ischemic damage to nerves.31
Radiation Injury
Radiationinduced nerve injury is a delayed manifestation of radiation therapy. This entity most commonly occurs in the brachial plexus in patients receiving radiation to the breast or axilla, but it may affect the lumbosacral plexus or individual distal nerves coursing through in other fields of radiation. The incidence of development of brachial plexus injury after radiation usually ranges from 1.8% to 5% but has been reported as high as 9%.32–34 Clinical manifestations of radiation neuropathy include gradually progressive paresthesias and sensory loss, weakness and atrophy, and pain in the distribution of the nerve or nerves affected. Symptom onset occurs 1 month to 18 years after radiation exposure.34,35 Several factors have been associated with an increased risk of development of brachial plexopathy, including a higher dose of radiation (greater than 5000 cGy), increased number of ports of radiation administration, the use of adjunctive chemotherapy, and the extent of axillary node dissection.32–34,36 Although some authors have reported more selective involvement of the upper trunk of the brachial plexus, others have shown that the upper, lower, or entire plexus may be involved.32,36–38
The underlying pathophysiology of radiationinduced injury has not been completely established. Pathological studies have demonstrated loss of myelin, the presence of fibrosis and thickening of the neurolemma sheath, and hyalinization and obliteration of the vasa nervorum to the brachial plexus, which are suggestive of either focal compression of the plexus by fibrosis or chronic nerve ischemia as possible underlying mechanisms.32
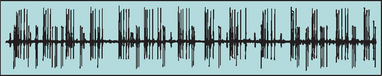
Electrophysiological studies demonstrate abnormal motor and sensory findings and large motor unit potentials with reduced recruitment in the majority of patients. Myokymia is the hallmark clinical feature of radiation neuropathy and is seen as an undulation, wormlike rippling of the muscle. Myokymic discharges, spontaneously recurring bursts of motor unit potentials, are the electrophysiological correlate of myokymia and are found in up to 63% of patients with radiationinduced brachial plexopathies35 (Fig. 105-7). The presence of myokymic discharges is helpful in distinguishing radiationinduced plexopathies from neoplastic plexopathies.35–37 Neuroimaging studies may show increased or decreased signal and fibrosis in the region of the brachial plexus.39
The course of radiationinduced neuropathy is usually one of steady progression or stabilization in 90% of patients, although cases of improvement have been reported.36,40,41 There is no treatment established to reverse or improve the nerve injury, although surgical intervention, such as neurolysis or neurolysis with omental grafting, has been performed in some patients with variable improvement in symptoms.42,43
Cold-Induced Nerve Injury
Clinical manifestations of cold-induced peripheral nerve injury begin an average of 6 days after exposure and initially consist of hyperemia and skin edema. With more severe or prolonged exposure, vesicle and blister formation occurs, followed by skin necrosis and loss of tissue. Symptoms may persist for months to years after the initial injury.44 Blair and colleagues reviewed the manifestations of cold-induced injuries in 100 patients 4 years after exposure during the Korean War.45 The most commonly experienced residual symptoms included (1) neuropathic symptoms (recurrent pain, sensitivity to cold, numbness) and (2) signs of sympathetic overactivity (vascular changes, hyperhidrosis).44,45 Pain is a prominent symptom; it may be described as aching, sharp, or burning and is aggravated by prolonged standing and cold. Electrophysiological studies in cold-induced peripheral nerve injury have demonstrated decreased sensory nerve action potential with slowed conduction velocity in the affected foot 6 months to 1 year after exposure.46,47
Several mechanisms of cold-induced nerve injury have been proposed, including primary vascular constriction or venous stasis in the vasa nervorum, vascular occlusion caused by thrombus formation, and cellular changes produced directly by cold.44 Early studies in cold-induced peripheral nerve injury demonstrated abnormalities in large myelinated fibers after cold exposure.48 More recent experiments on rat sciatic nerves have also demonstrated degeneration of large myelinated fibers, occurring within 48 hours after exposure.49 Although the duration of exposure is important in the development of nerve injury, repeated exposure with rewarming plays a crucial role in extending the degree of nerve injury. Experimental studies on rat sciatic nerves demonstrated that the amplitudes of compound nerve fiber action potentials and recovery of nerve blood flow to nerves that were exposed to intermittent cooling and rewarming were significantly reduced in comparison with those of nerves exposed to continuous cold for the same duration.50 Furthermore, pathological analysis demonstrated preservation of myelinated and unmyelinated fibers after continuous cold injury but axonal degeneration with intermitted cooling.50 These findings suggest that initial insult to the nerve after cold exposure results from factors such as prolonged vascular stasis, endoneurial anoxia, and direct injury. However, rewarming produces further nerve damage, possibly from free radical injury.50
Treatment of cold-induced nerve injury is largely symptomatic. Agents previously used include anticoagulants, sympathetic blocking agents, and sympathetic blocks but have not been systematically studied.44
Electrical Nerve Injury
Electrical injuries to peripheral nerves are uncommon and usually occur in the context of more severe or diffuse electrical injury of an extremity. In an early study of electrical injuries in 64 patients, 13% of patients developed peripheral nerve complications.51 The site of nerve injury is correlated with the site of electrical contact. Extensive tissue destruction and burn are often present.51 The anatomical distribution of nerve injury follows the path that the electrical current takes. Factors that affect the extent of nerve injury include the duration of contact, the voltage of the exposure, and the current density within the tissue.52
Peripheral nerves are very sensitive to electrical forces and have a lower resistance to current flow than blood, muscle, skin, tendon, or bone.53 The pathway of electrical current typically follows the course of neurovascular bundles because of its lower resistance to electrical current. Several mechanisms have been implicated in the pathogenesis of electrical nerve injury. The immediate and acute injuries to motor and sensory fibers are the direct result of the electrical current.54 The mechanism of electrical nerve injury is related to the propagating electrical current through the skin and deeper tissues to the nerves. The electrical current results in heating of the tissue and the buildup of electrical charge along the nerve membrane. In most instances, only a few seconds of exposure are necessary for the high-energy electrical trauma to occur. The current is denser at the closer proximity to the contact point; however there is often nonuniform exposure along the electrical field, which makes the pattern of tissue involvement variable. Pathological studies have suggested that permanent changes in the nerve do not extend beyond the area of local destruction.53,54 Persistent or delayed damage results from thermal or mechanical injury, fibrosis of the perineural tissues, or thrombosis of nutrient blood vessels.53
The clinical manifestations of electrical nerve injury most commonly include sensory disturbances, such as paresthesias, dysesthesias, and sensory loss, which may resolve within hours or days. The nerves most commonly affected are the median and ulnar nerves.51,53 In addition, sympathetic dysfunction may begin within hours, producing pain, burning sensation, and allodynia in the distribution of the nerve. Months after the injury, other sudomotor autonomic signs may manifest, including muscle atrophy, hair loss, and osteoporosis. Weakness occurs in more severe exposure but may be related to direct skeletal muscle injury, as well as neurogenic injury. Muscle swelling, myonecrosis, and compartment syndromes may also occur.
Treatment of electrical nerve injury is predominantly supportive, rehabilitative, and focused on pain management. Early surgical intervention with fasciotomy and escharotomies may be effective for reducing increasing pressure from compartment syndromes. Neurological manifestations may persist for years after exposure, and the degree of recovery is variable.55 In one study of more than 2000 patients with electrical injuries over a 20-year period, approximately 8% sustained long-term peripheral nerve manifestations, including pain, sensory loss, and weakness.56
TREATMENT OF NERVE TRAUMA
Surgical Treatment
The surgical treatment of traumatic nerve injury is dependent on the etiology, type, and pathophysiology of injury and the timing of evaluation in relationship to the injury. In many cases, surgical intervention provides an opportunity for more rapid and significant recovery of function, although one of the most difficult treatment decisions is the determination of the need for surgical intervention and the appropriate timing of surgery. Spinner and Kline (2000) reviewed the management of peripheral nerve injuries.10 When the decision to surgically intervene is being considered, electrodiagnostic studies can be helpful in the determining how many axons are injured and whether the lesion is neurapractic or axonotmetic. Neurapractic lesions are more likely to recover spontaneously and are generally treated nonsurgically unless they persist longer than expected. In contrast, minimal and protracted recovery is expected with axonotmetic lesions, and therefore surgical intervention may help improve the chance and rapidity of recovery.
Early surgical intervention may be considered immediately after the injury as primary repair or after a short delay of 3 to 4 days as delayed primary repair. Immediate primary repair is usually recommended when there has been a clean laceration of the nerve by a sharp object and where the nerve endings are not injured by crush or stretch. When a clean division of the nerve has occurred, end-to-end suture repair within 72 hours is recommended.10
In most other situations, it is often necessary to delay surgery by weeks to months. Secondary repairs may be performed as early as 2 to 4 weeks after the injury or 3 to 6 months after the injury. In some cases, surgery may be performed as late secondary repairs 1 to 2 years after the injury. Secondary early surgical repair after 2 to 4 weeks is generally recommended for blunt injuries or injuries with extensive soft tissue damage in which the nerve injury appears to be complete or very severe. In this situation, neuroma and scarring may develop within several weeks after trauma. Delaying surgery by several weeks allows for better determination of neuroma formation and scarring, and resection of the neuroma and scarring may improve recovery. When these scarred nerve stumps are resected, the growth cones of the new nerve endings may grow more rapidly.57 However, in many cases, surgery is usually delayed between several weeks and 6 months in order to determine the degree of spontaneous improvement. If the nerves remain in continuity and nerve regeneration is evident clinically or electrophysiologically during this time, surgical intervention is unlikely to be more beneficial than physiological recovery and is not recommended, because approximately 90% of patients in these cases recover.10 In one series, secondary repair 3 weeks to 3 months after sciatic nerve injury resulted in useful motor recovery in the tibial nerve in 83% of patients and the peroneal nerve in 39%.58 Useful recovery was achieved in 71% with thigh lesions and in only 31% with buttock-level lesions. Late surgery 1 to 2 years after the injury has not generally resulted in good recovery of motor function but may be necessary for pain control or to resect a neuroma.
A variety of operative methods can be used to attempt to improve nerve function after nerve trauma10,59 (Table 105-3). Details of each method are beyond the scope of this chapter, but the neurosurgical literature contains more extensive reviews of this subject.10,59 One of the simplest procedures is internal neurolysis, which consists of opening the epineurium and separating out the different fascicles under magnification. The surgeon then inspects the individual fascicles and stimulates and records from them to determine which fascicles are intact. After internal neurolysis, the surgeon separates the fascicles within the nerve, and if some fascicles are interrupted or not functional, the surgeon attempts to identify the distal and proximal stumps, match up the fascicles, and suture together the distal and proximal stumps of the matching fascicles. If there is no evidence of viable axons crossing the injured area of the fascicle, the injured segment is resected back to normal-appearing nerve, and an end-to-end repair or graft repair is performed. These procedures risk damage to the blood supply of the nerve and damage to branches of the fascicles either leaving the nerve or passing from one fascicle to another, as well as scar and neuroma formation.
| Method | Technique | Indication |
|---|---|---|
| External neurolysis | Removal of circumferential scar | Lesion in continuity |
| Internal neurolysis/split repair | Resection of scar around a portion of the nerve fascicle | Asymmetrical nerve injury; lesion in continuity |
| End-to-end repair | Aligning two ends of transected nerve | Transected nerve with short gap between ends |
| Graft repair | Grafting from sural nerve or antebrachial nerve | Transected nerve with large gap between ends |
| Retracted stumps of transected nerve | ||
| Nerve transfer | Transfer functioning nerve to denervated muscle | Irreparable nerve injury (e.g., root avulsion) |
Intraoperative electrophysiological monitoring can be useful to determine the precise borders of the nerve injury and determine whether nerve action potentials can be conducted through the injured segment.3 The prognosis after surgical intervention varies according to the procedure performed. Direct suture repair carries the best prognosis, with approximately 79% of patients demonstrating recovery, whereas only 50% experience improvement with graft repair.10 Furthermore, in brachial plexus injuries, there is a better chance of recovery with upper trunk/lateral cord lesions than with lower trunk/medial cord lesions. Contraindication to surgical intervention include delayed evaluation (more than 1 year), C8-T1 root avulsion, or other comorbid conditions.10
Spinner and Kline (2000) gave some general guidelines based on their experience.10 They found that injured nerves with a measurable nerve action potential transmitted across the lesion during intraoperative nerve stimulation have a good recovery of function in 90% of cases. Good functional results can be expected in about 70% of end-to-end repairs and in 50% of graft repairs. Characteristics with a better outcome include (1) shorter nerve grafts, (2) early surgical intervention, and (3) young age of patients. Proximal lesions do worse than distal lesions, and lesions in the lower trunk brachial plexus do worse than those in the upper trunk.
If a long segment of nerve is damaged or a long gap is present between the two retracted ends of the nerve, it may not be possible to perform an end-to-end repair of the nerve, and a nerve graft may be necessary to bridge the gap. With this technique, the axonal sprouts must cross two gaps, which slows growth, increases the risk of neuroma formation, and increases the risk that the axonal sprouts will not reach the proper end organ. However, grafts up to 20 cm in length have been used successfully. The survival and success of the graft depend on the quality of the operative bed, the vascular supply of the graft, and the thickness of the graft. Nerve grafts may be autologous (from the same patient), allografts (from another human), xenografts (from another species), or artificial or synthetic.60 Autologous nerve grafts from a remote donor site are the most commonly used technique for bridging a nerve gap. Various nerve guides have been used to direct axonal sprouts toward the distal stump, including silicone tubes, collagen-based nerve conduits, and a biodegradable polyglycolic acid-collagen tube filled with laminin-coated collagen fibers.59,61 Vanderhooft62 and Frykman and Gramyk63 reviewed the results of nerve grafting. In combining results from multiple centers, grafting of digital nerves produced good or better results in 50%. Even with a delay between injury and repair of up to 6 months, 90% of patients achieved useful recovery. MacKinnon and Dellon performed grafts by using polyglycolic tubes in carefully selected patients and reported good results in 86%.64
Other procedures, such as end-to-side nerve anastomosis have been used.65,66 The distal segment of the injured nerve is sutured in an end-to-side manner to an adjacent nerve trunk, usually after an epineurial window and sometimes a perineurial window are formed in the donor nerve. The distal degenerated nerve segment attracts collateral sprouts from the healthy intact axons. The collateral sprouts from the donor nerve theoretically reinnervate the distal segment of the injured nerve. In the future, peripheral nerve repair may employ tissue engineering, neurotrophic factors, immunosuppression, and bioimplants.
In cases in which the nerve injury is so severe that primary repair or grafting is impossible, such as root avulsion or proximal nerve injuries, then neurotization or nerve-to-nerve anastomosis may be employed. They are also useful in cases of destruction of a very long segment of nerve or cases of a long time between the injury and repair.67 For example, if the musculocutaneous axons to the biceps are destroyed very proximally, a portion of the median nerve can be divided and connected to the musculocutaneous nerve near the point where the musculocutaneous nerve enters the biceps. Nath and MacKinnon reported excellent recovery of biceps strength with this technique in 20 of 22 patients.67 Various neural structures such as the cervical plexus, contralateral spinal nerves, or components of the brachial plexus, spinal accessory nerve, phrenic nerve, pectoral nerves, and intercostal nerves have been employed. These nerves are severed, and the ends are connected primarily or by graft to a distal nerve such as the musculocutaneous, radial, or median nerve. The interposing nerve graft is usually taken from the sural nerve. More recently, muscles such as the gracilis have been removed from the lower extremity with particular care to preserve the nerve and vascular supply to the muscle. Then the muscle is transferred to the upper extremity and the nerve connected, usually to one of the intercostal nerves. Such neurotization procedures have resulted in effective elbow flexion in 25% to 50% of cases. A clinically useful outcome of such reinnervation requires training to produce central motor reorganization. As knowledge of the neurotrophic and neurotropic effects of various growth factors expands, it is possible that systemic or local administration of these agents may be used to enhance neuronal survival and promote regeneration.
CONCLUSION AND RECOMMENDATIONS
Filler AG, Maravilla KR, Tsuruda JS. MR neurography and muscle MR imaging for image diagnosis of disorders affecting the peripheral nerves and musculature. Neurol Clin. 2004;22:643-682.
Harper CM, Thomas JE, Cascino TL, et al. Distinction between neoplastic and radiationinduced brachial plexopathy with emphasis on the role of EMG. Neurology. 1989;39:502-506.
Robinson LR. Traumatic injury to peripheral nerves. Muscle Nerve. 2000;23:863-873.
Spinner RJ, Kline DG. Surgery for peripheral nerve and brachial plexus injuries or other nerve lesions. Muscle Nerve. 2000;23:680-695.
Sunderland S. The anatomy and physiology of nerve injury. Muscle Nerve. 1990;13:771-784.
1 Selecki BR, Ring IT, Simpson DA, et al. Trauma to the central and peripheral nervous systems: Part II, a statistical profile of surgical treatment in New South Wales. Aust N Z J Surg. 1982;52:111-116.
2 Sunderland S. The anatomy and physiology of nerve injury. Muscle Nerve. 1990;13:771-784.
3 Robinson LR. Traumatic injury to peripheral nerves. Muscle Nerve. 2000;23:863-873.
4 Seddon HJ. Surgical Disorders of the Peripheral Nerves, 2nd ed. Edinburgh: Churchill Livingstone, 1975.
5 West GA, Haynor DR, Goodkin R, et al. Magnetic resonance imaging signal changes in denervated muscles after peripheral nerve injury. Neurosurgery. 1994;35:1077-1086.
6 Filler AG, Kliot M, Howe FA, et al. Application of magnetic resonance neurography in the evaluation of patients with peripheral nerve injury. J Neurosurg. 1996;85:229-303.
7 Maravilla KR, Aagaard BD, Kliot M. MR neurography. MR imaging of peripheral nerve. Magn Reson Imaging Clin North Am. 1998;6:179-194.
8 Filler AG, Maravilla KR, Tsuruda JS. MR neurography and muscle MR imaging for image diagnosis of disorders affecting the peripheral nerves and musculature. Neurol Clin. 2004;22:643-682.
9 Peer S, Harpf C, Willeit J, et al. Sonographic evaluation of primary peripheral nerve repair. J Ultrasound Med. 2003;22:1317-1322.
10 Spinner RJ, Kline DG. Surgery for peripheral nerve and brachial plexus injuries or other nerve lesions. Muscle Nerve. 2000;23:680-695.
11 Carvalho GA, Nikkhah G, Matthis C, et al. Diagnosis of root avulsion in traumatic brachial plexus injuries. Value of computerized tomography, myelography, and magnetic resonance imaging. J Neurosurgery. 1997;86:69-76.
12 Hill C, Riaz M, Mozzam A, et al. A regional audit of hand and wrist injuries. A study of 4873 injuries. J Hand Surg [Br]. 1998;23:196-200.
13 Noble J, Munro CA, Prasad VSSV, et al. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J Trauma. 1998;45:116-122.
14 Kretschmer T, Antoniadis G, Braun V, et al. Evaluation of iatrogenic lesions in 722 surgically treated cases of peripheral nerve trauma. J Neurosurg. 2001;94:905-912.
15 Klein DG. Timing for exploration of nerve lesions and evaluation of the neuroma in continuity. Clin Orthop. 1982;163:42-49.
16 Ochoa J, Fowler TJ, Gilliatt RW. Anatomical changes in peripheral nerves compressed by a pneumatic tourniquet. J Anat. 1972;113:433-455.
17 Sunderland S. Stretch-compression neuropathy. Clin Exp Neurol. 1981;18:1-13.
18 Haftek J. Stretch injury of peripheral nerve. Acute effects of stretching on rabbit nerve. J Bone Joint Surg Br. 1970;52:354-365.
19 Clark K, Williams PE, Willis W, et al. Injection injury of the sciatic nerve. Clin Neurosurg. 1970;17:111-125.
20 Gentili F, Hudson A, Kline DG, et al. Peripheral nerve injection injury: an experimental study. Neurosurgery. 1979;4:244-253.
21 Tarlov IM, Perlmutter I, Berman AJ. Paralysis caused by penicillin injection; mechanism of complication—a warning. J Neuropath Exp Neurol. 1951;10:158-176.
22 Rice ASC, McMahon SB. Peripheral nerve injury caused by injection needles used in regional anaesthesia: influence of bevel configuration, studied in a rat model. Br J Anaesth. 1992;69:433-438.
23 Stohr M, Dichgans J, Dorstelmann D. Ischaemic neuropathy of the lumbosacral plexus following intragluteal injection. J Neurol Neurosurg Psychiatry. 1980;43:489-494.
24 Chalk CH, Dyck PJ. Ischemic neuropathy. In: Dyck PJ, Thomas PK, editors. Peripheral Neuropathy. 3rd ed. Philadelphia: WB Saunders; 1993:980-989.
25 Hess K, Eames R, Darveniza P, et al. Acute ischaemic neuropathy in the rabbit. J Neurol Sci. 1979;44:19-43.
26 Schmelzer JD, Zochodne D, Low PA. Ischemic and reperfusion injury of rat peripheral nerve. Proc Natl Acad Sci U S A. 1989;86:1639-1642.
27 Dyck PJ, Conn DL, Okazaki H. Necrotizing angiopathic neuropathy. Three-dimensional morphology of fiber degeneration related to sites of occluded vessels. Mayo Clin Proc. 1972;47:461-475.
28 Boontje AH, Haaxma R. Femoral neuropathy as a complication of aortic surgery. J Cardiovasc Surg (Torino). 1987;28:286-289.
29 Honet JC, Wajszczuk WJ, Rubenfire M, et al. Neurologic abnormalities in the leg(s) after use of intraaortic balloon pump: report of 6 cases. Arch Phys Med Rehabil. 1975;56:346-352.
30 Castellanos AM, Glass JP, Yung WKA. Regional nerve injury after intra-arterial chemotherapy. Neurology. 1987;37:834-837.
31 Wilbourn AJ, Levin KH. Ischemic neuropathy. In: Brown WF, Bolton CF, editors. Clinical Electromyography. 2nd ed. Boston: Butterworth-Heinemann; 1993:369-390.
32 Pierce SM, Recht A, Lingos TI, et al. Long-term radiation complications following conservative surgery and radiation therapy in patients with early stage breast cancer. Int J Radiat Oncol Biol Phys. 1992;23:915-923.
33 Olsen NK, Pfeiffer P, Johannsen L, et al. Radiationinduced brachial plexopathy: neurological followup in 116 recurrencefree breast cancer patients. J Radiat Oncol Biol Phys. 1993;26:43-49.
34 Stoll RA, Andrews JT. Radiationinduced peripheral neuropathy. BMJ. 1966;1:834-837.
35 Harper CM, Thomas JE, Cascino TL, et al. Distinction between neoplastic and radiationinduced brachial plexopathy with emphasis on the role of EMG. Neurology. 1989;39:502-506.
36 Mondrup K, Olsen NK, Pfeiffer P, et al. Clinical and electrodiagnostic findings in breast cancer patients with radiationinduced brachial plexus neuropathy. Acta Neurol Scand. 1990;81:153-158.
37 Kori AH, Foley KM, Posner JB. Brachial plexus lesions in patients with cancer: 100 cases. Neurology. 1981;31:45-50.
38 Killer HE, Hess K. Natural history of radiationinduced brachial plexopathy compared with surgically treated patients. J Neurol. 1990;237:247-250.
39 Mumenthaler M, Narakas A, Gilliat RW. Brachial plexus disorders. Dyck JP, Thomas PK, Lambert EH, et al, editors. Peripheral Neuropathy, 2nd ed., vol 2. Philadelphia: WB Saunders, 1987;1384-1424.
40 Salner AL, Botnick LE, Herzog AG, et al. Reversible brachial plexopathy following primary radiation therapy for breast cancer. Cancer Treatment Rep. 1981;65:797-802.
41 Bowen BC, Verma A, Brandon AH, et al. Radiationinduced brachial plexopathy: MR and clinical findings. AJNR Am J Neuroradiol. 1996;17:1932-1936.
42 Wouter van Es H, Engelen AM, Witkamp TD, et al. Radiationinduced brachial plexopathy: MR imaging. Skeletal Radiol. 1997;26:284-288.
43 Gerard JM, Franck N, Moussa Z, et al. Acute ischemic brachial plexus neuropathy following radiation therapy. Neurology. 1989;39:450-451.
44 Shafer JC, Thompson AW. Local cold injury: a report of sequelae. Arch Dermatol. 1955;72:335-347.
45 Blair JR, Schatzki R, Orr KD. Sequelae to cold injury in one hundred patients: followup study four years after occurrence of cold injury. JAMA. 1957;163:1203-1208.
46 Hanifin JM, Cuetter AC. In patients with immersion foot type of cold injury diminished nerve conduction velocity. Electromyogr Clin Neurophysiol. 1974;14:173-178.
47 Carter JL, Shefner JM, Krarup C. Cold-induced peripheral nerve damage: involvement of touch receptors of the foot. Muscle Nerve. 1988;11:1065-1069.
48 Denny-Brown D, Adams RD, Brenner C, et al. The pathology of injury to the nerve induced by cold. J Neuropathol Exp Neurol. 1945;4:305-323.
49 Kennett RP, Gilliatt RW. Nerve conduction studies in experimental nonfreezing cold injury: II. Generalized nerve cooling by limb immersion. Muscle Nerve. 1991;14:960-967.
50 Jia J, Pollock M. Cold nerve injury is enhanced by intermittent cooling. Muscle Nerve. 1999;22:1644-1652.
51 Solem L, Fischer RP, Strate RG. The natural history of electrical injury. J Trauma. 1977;17:487-492.
52 Danielson JR, Capelli-Schellpfeffer M, Lee RC. Upper extremity electrical injury. Hand Clin. 2000;16:225-234.
53 DiVincenti FC, Moncrief JA, Pruitt BA. Electrical injuries: a review of 65 cases. J Trauma. 1969;9:497-507.
54 Skoog T. Electrical injuries. J Trauma. 1970;10:816-830.
55 Silversides J. The neurologic sequelae of electrical injury. CMAJ. 1964;91:195-204.
56 Gourbiere E, Corbut J-P, Bazin Y. Functional consequences of electrical injury. N Y Acad Sci. 1994;720:259-271.
57 McQuarrie IG. Perpheral nerve surgery. Neurol Clin. 1985;3:453-466.
58 Taha A, Taha J. Results of suture of the sciatic nerve after missile injuries. J Trauma. 1998;45:340-344.
59 Ijkema-Paassen J, Jansen K, Gramsbergen A, et al. Transection of peripheral nerves, bridging strategies and effect evaluation. Biomaterials. 2004;25:1583-1592.
60 Millesi H. Techniques for nerve grafting. Hand Clin. 2000;16:73-91.
61 Matsumoto K, Ohnishi K, Kiyotani T, et al. Peripheral nerve regeneration across an 80-mm gap bridged by a polyglycolic acid (PGA)–collagen tube filled with laminin-coated collagen fibers: a histological and electrophysiological evaluation of regenerated nerves. Brain Res. 2000;868:315-328.
62 Vanderhooft E. Functional outcomes of nerve grafts for the upper and lower extremities. Hand Clin. 2000;16:93-103.
63 Frykman GK, Gramyk K. Results of nerve grafting. In: Gelberman RH, editor. Operative Nerve Repair and Reconstruction. Philadelphia: JB Lippincott; 1991:553-567.
64 MacKinnon SE, Dellon AL. Clinical nerve reconstruction with a bioabsorbable polyglycolic acid tube. Plast Reconstr Surg. 1990;85:419-424.
65 Giovanoli P, Koller R, Meuli-Simmen C, et al. Functional and morphometric evaluation of end-to-side neurorrhaphy for muscle reinnervation. Plast Reconstr Surg. 2000;106:383-392.
66 Rowan PR, Chen LE, Urbaniak JR. End-to-side nerve repair. A review. Hand Clin. 2000;16:151-159.
67 Nath RK, MacKinnon SE. Nerve transfer in the upper extremity. Hand Clin. 2000;16:131-139.