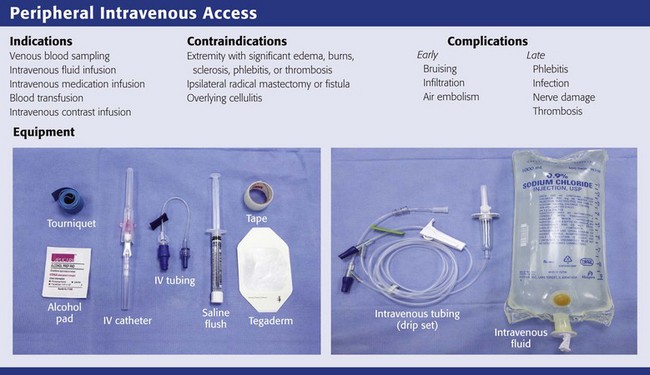
Peripheral Intravenous Access
Introduction
Intravenous (IV) access is a mainstay of modern medicine. IV cannulation is a procedure performed by a wide array of health care professionals, including physicians, nurses, physician assistants, phlebotomists, and emergency medical technicians. In the emergency department (ED), uncomplicated peripheral venous access is usually secured by a nurse or technician. In the United States, more than 25 million patients have peripheral IV catheters placed each year as vascular access for the administration of medications and fluids and sampling of blood for analysis. IV access can usually be accomplished in less than 5 minutes.1–4 Despite their growing number, dedicated IV teams are very costly and not always cost-effective.5,6 Moreover, in the ED setting multiple providers may be called on to obtain IV access, thus making it an essential skill for both emergency physicians and nurses to master. Subtleties in technique are important and can be improved with practice; this is why some providers are able to place IV lines in even the most challenging situations.
Historical Perspective
Bloodletting, or bleeding, dates to the time of Hippocrates. The ancient technique consisted of tying a bandage around the arm to distend the forearm veins, opening a vein with a sharp knife, and collecting the blood into a basin. In the Middle Ages this was performed by barber-surgeons. In 1656, Sir Christopher Wren injected opium into dogs intravenously with a quill and bladder, thereby becoming the father of modern IV therapy.7 Blood transfusions also date back to the mid-1600s. The French physician Jean Denis is credited with the first successful transfusion by giving lamb’s blood to a 15-year-old boy.8,9
Originally, 16- to 18-gauge indwelling steel needles were used for IV infusions. In the 1950s the Rochester needle was introduced, which was a resinous catheter on the outside of a steel introducer needle. Because of increased comfort and mobility, plastic catheters have replaced indwelling metal needles and are now almost universal.7,10
Indications and Contraindications
Obtaining timely and adequate vascular access is a major priority during any resuscitation. In patients with normal perfusion, differences in delivery times for injections centrally versus peripherally are minimal—within seconds.11 During cardiopulmonary resuscitation (CPR), however, medications have been shown to reach the central circulation faster with central access than with peripheral venous access.12 A change in outcome, though, has not been demonstrated with the central administration of advanced cardiac life support drugs; hence, peripheral IV cannulation is the procedure of choice even during CPR because of the speed, ease, and safety with which it can be accomplished. In less critically ill patients, the role of IV therapy is more often debated and access is ultimately unnecessary in a large proportion of patients in whom it is obtained.13 In broad terms, IV access or therapy is needed in patients for whom IV medications are required or when oral therapy is inadequate (e.g., severe shock states), contraindicated (e.g., surgical emergencies), or impossible (e.g., intractable vomiting).
Saline or heparin locks are preferable when IV medications are needed and there are limited foreseeable fluid requirements. Saline locks cost less than a full IV fluid and tubing assembly and are especially helpful when vascular access is needed suddenly.14,15 Access to the catheter requires irrigation with a separate syringe and flush.
A peripheral IV central catheter (PICC) shares the attributes of both central and peripheral venous IV lines (see Chapter 24). A PICC is composed of a thin tube of biocompatible material with an attachment hub. It is inserted percutaneously, under ultrasound guidance by a dedicated PICC team, into a peripheral vein and then advanced into a large central vein, followed by radiographic confirmation of placement. PICCs are suitable for long-term vascular access for blood sampling, infusion of antibiotics and hyperosmolar solutions such as total parenteral nutrition, and infusion of certain chemotherapeutic agents. Insert a PICC line as soon as long-term access is anticipated.3
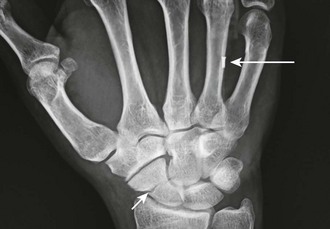
Peripheral IV lines should not be placed in extremities with massive edema, burns, sclerosis, phlebitis, or thrombosis due to risk for extravasation or suboptimal volume flow. When practical, avoid placing an IV line in extremities on the same side as radical mastectomies, though they can be used when an urgent condition exists and other peripheral access is not possible. When feasible, cannulation at infected sites, such as through an area of cellulitis, as well on extremities with shunts or fistulas, should be avoided because it may cause bacteremia or thrombosis. If possible, do not cannulate a vein over or distal to a recent fracture site on an extremity (Fig. 21-1). Veins that drain from an area affected by trauma or major vascular disruption (e.g., distal to a ruptured aorta) are also suboptimal because fluid or medications may not be delivered to the circulatory system.
Blood samples for laboratory analysis are usually drawn before IV cannulation to avoid contamination with IV fluid or medication. However, studies have shown that accurate basic electrolyte and hematologic values can be obtained with peripheral IV lines when infusions are shut off for at least 2 minutes, at least 5 mL of blood is wasted, and all tubes are filled to avoid inaccurate bicarbonate readings.16–18 By adopting this technique, one can reduce the number of peripheral needlesticks, minimize trauma or sclerosis of the vein, and improve patient satisfaction.
Ultrasound Guidance and Transillumination
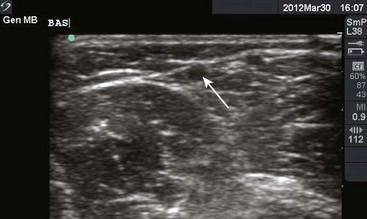
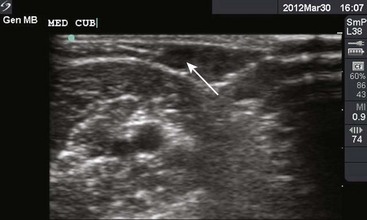
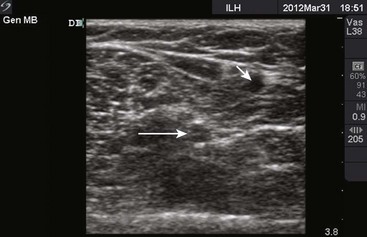
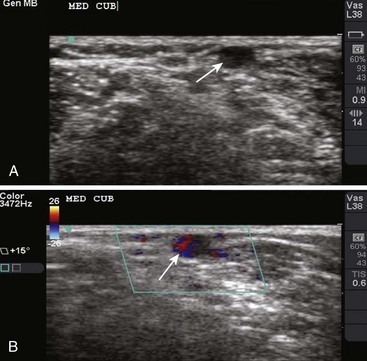
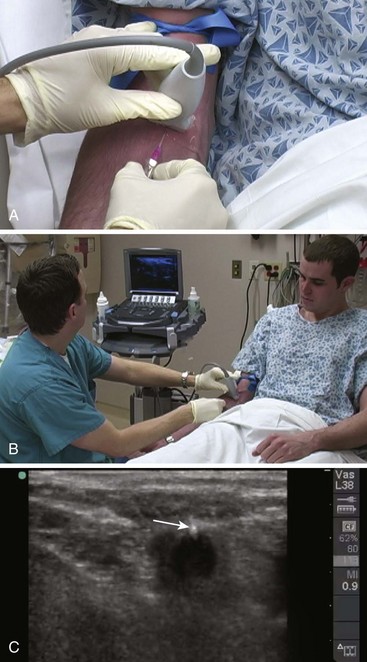
Though more commonly used with central venous access, ultrasound can also assist in the placement of peripheral lines. For IV placements that have been designated “difficult” after a certain number of attempts by nursing staff, use of ultrasound guidance increases the success rate and decreases the number of attempts necessary for successful cannulation in both adult and pediatric patients.19–22 One recent prospective randomized control study, however, did not show a benefit; conflicting evidence may ultimately stem from differences in the experience of ultrasound operators.23 The caliber of the vein identified on ultrasound is predictive of its ability to be cannulated. If no vessel is identified, cannulation is not usually possible.24,25 An additional issue with ultrasound-guided peripheral IV lines is their longevity. One study has brought attention to the high premature failure rate of ultrasound-guided peripheral lines.26 This is probably related to the depth of the veins being cannulated, the length and type of catheter used, and the angle of the catheter through soft tissue.
Anatomy
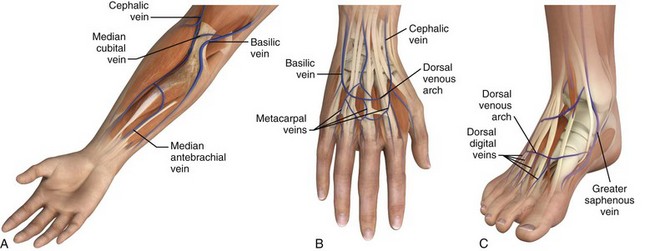
The success of cannulation depends on familiarity with the vascular anatomy of the extremities. In the upper extremity, the veins of the hands are drained by the metacarpal and dorsal veins, which connect to form the dorsal venous arch (Fig. 21-2). These sites are excellent for IV therapy and comfortably accommodate 22- and 20-gauge catheters. The venous supply of the wrist and forearm consists of the basilic vein, which courses along the ulnar portion of the posterior aspect of the forearm. It is often ignored because of its location but can easily be accessed if the patient’s forearm is flexed and the clinician stands at the head of the patient.27 On the radial side of the forearm, the cephalic is best known as the “intern vein.” Readily accessible, this vein can accommodate 22- to 16-gauge catheters. The median veins of the forearm course through the middle of the forearm, and the accessory cephalic veins on the radial aspect of the forearm are easily stabilized and accessible.
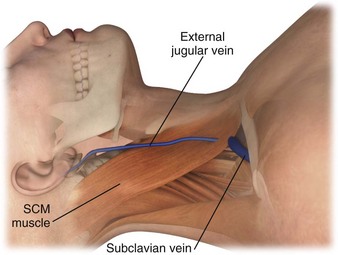
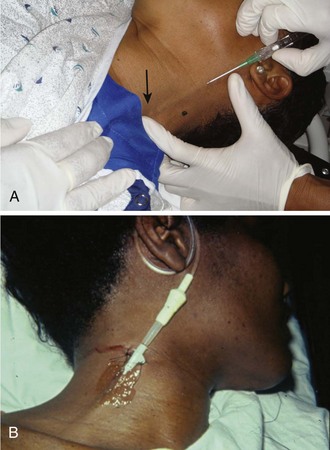
The external jugular vein is formed below the ear and behind the angle of the mandible (Fig. 21-3). It then passes downward and obliquely across the sternocleidomastoid and under the middle of the clavicle to join the subclavian vein. It is important to note the presence of valves in the external jugular, usually about 4 cm above the clavicle, because they can significantly impede IV function.28
Preparation
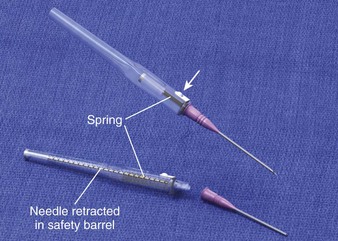
Universal precautions must be applied to all patients, especially in emergency care settings, in which the risk for exposure to blood is increased and the infection status of patients is largely unknown.29 One study showed that 11% of all hospital IV catheter injuries to health care workers occurred in the ED.30 Newer catheter devices have emerged that prevent inadvertent needle injuries (Fig. 21-4). The Protectiv IV Catheter Safety System has a protective sleeve that encases the sharp stylet as it is retracted from the catheter. The needle of the Insyte Autoguard Shielded IV Catheter is instantly encased inside a tamper-resistant safety barrel by pressing an activation button. The Saf-T-Intima IV catheter, Punctur-Guard Safety Winged Set, Vacutainer Safety-Lok, Shamrock safety winged needle, and Angel Wing Safety Needle systems are all types of winged safety devices with shields that advance over the needle to prevent exposure of the needle.7
Choosing the Catheter Gauge
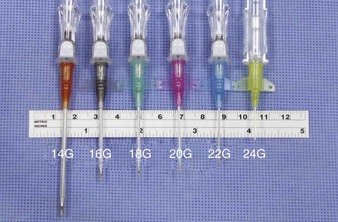
The specific gauge of catheter to use depends on the clinical scenario (Fig. 21-5). The narrowest catheter typically used in adults is a 22 gauge, which is sufficient for the routine administration of maintenance fluids and antibiotics. A 20 or 18 gauge is necessary for the administration of blood products, and a 16-gauge needle is preferred in resuscitation settings when large amounts of fluid must be given quickly.27 A second IV line at a different location allows additional IV therapy and also acts as a backup line in critical resuscitations. An 18-gauge catheter in the antecubital fossa is the standard device for IV contrast–enhanced computed tomography (CT) studies such as pulmonary CT angiogram.
Appropriate Site
Site selection depends largely on the expected duration of IV therapy, the patient’s activity level, and the condition of the extremities. When choosing a location to initiate IV access, the best place to start is the hand and then advance cephalad as necessary. Hand veins are appropriate for 22-gauge catheters. Cephalic, accessory, or basilic veins are ideal for larger-bore IV lines. Avoid veins that are not resilient and feel hard or cordlike because they are often thrombosed.7 Deep, percutaneous antecubital venipuncture and external jugular vein cannulation are also options in patients with difficult veins or those who may need IV access quickly.7 The lower extremities veins can also be useful locations, especially in pediatric patients. In patients who have undergone radical mastectomy, avoid the arm on the same side as the surgery because circulation may be impaired, flow may be affected, and edema and other complications such as thrombosis could result.7, 22 Scalp veins are commonly used in neonates.3,31
Adjuncts for Finding a Vein
Patients often have nonvisible and nonpalpable veins. A common method of increasing venous distention is to ask patients to open and close their fist. Lowering the arm below the level of the heart can also increase venous distention. Light tapping can likewise be effective, although heavy tapping may cause the vein to spasm. If these methods are inadequate, heat packs can be applied for 10 to 20 minutes to increase venous engorgement. This is particularly useful in the pediatric population.7
Nitroglycerin ointment applied to the hands of patients with small-caliber veins has been shown to increase the diameter of the vein by two to six times and increase the rate of successful first-attempt cannulation. Once the tourniquet is applied to the wrist, a quarter inch of 2% nitroglycerin is applied to a 2.5-cm2 area, left on for 2 minutes, and then rubbed off.32 Nitroglycerin has been found to be useful and safe in the pediatric population as well.33 This technique is contraindicated in hypotensive patients.
In the late 1980s, several small studies demonstrated the potential use of a venous distention device—a cardboard mailing tube that was placed over the forearm with a sealed bulb at one end that would cause a vacuum within the tube. Of the patients predetermined to be difficult to access, 90% were cannulated when this device was used. Reported complications were few and included petechiae and discomfort.34,35
Anesthesia
Though somewhat time-consuming, local anesthesia at the site should be considered part of routine care. Local anesthesia significantly decreases pain before cannulation.36–38 Anesthetics such as lidocaine or bupivacaine may be instilled just beneath the skin at the site of planned cannulation through a tuberculin (1-mL) syringe equipped with a 27- or smaller-gauge needle. Adding bicarbonate (e.g., buffered lidocaine), warming the solution to room temperature, instilling the solution slowly, and distracting the patient during injection all contribute to reducing pain.39 In the pediatric population, 2.5 g of EMLA (eutectic mixture of local anesthetics) can be applied topically over the site.7,40 Its main disadvantage is slow onset, with as long as an hour needed for induction of anesthesia before cannulation.41 Other options include ethyl chloride topical spray,42 which temporarily numbs the skin, and oral sucrose in infants.43
IV Assembly
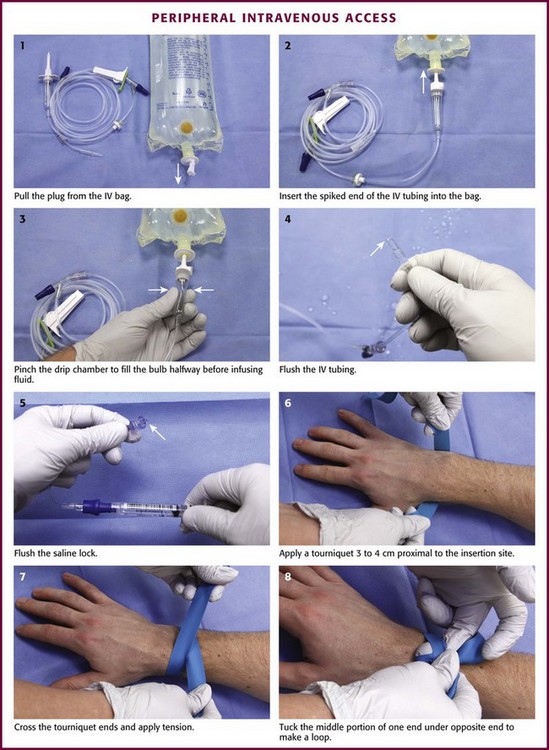
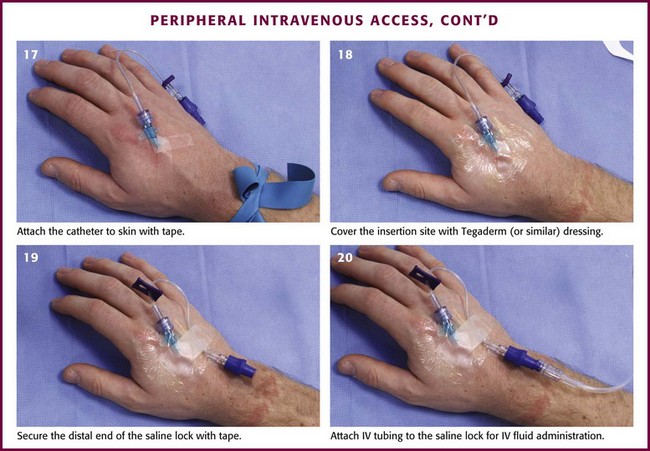
Review Box 21-1 itemizes the materials necessary for IV cannulation. The procedure is detailed in Figure 21-6. The first step is to prepare the IV fluids and tubing. Remove the cap from the IV tubing and the tab from the IV bag. Clamp the IV tubing shut and insert the spiked end into the IV fluid bag. Pinch the drip chamber and fill it halfway. Open the clamp slightly to flush the IV tubing. If saline locks are being used, flush them similarly before cannulation. To do this, attach the lock to a saline-filled syringe and push saline through it.
Inspection and Positioning
After collecting and preparing the equipment and supplies, palpate the veins. Position the patient comfortably on a flat surface. Place a 1-inch-wide tourniquet on the upper part of the patient’s arm or forearm and pull it sufficiently tight to impede venous flow but not tight enough to compromise arterial flow. Place the tourniquet under the arm. Fold both ends of it above the arm and cross the ends. Pull the overlying end taut and tuck the middle portion below the underlying end to create a loop. After placement of the tourniquet, palpate the veins with the index and middle finger of one’s nondominant hand. The veins are soft, elastic, resilient, and pulseless.27
Cannulation
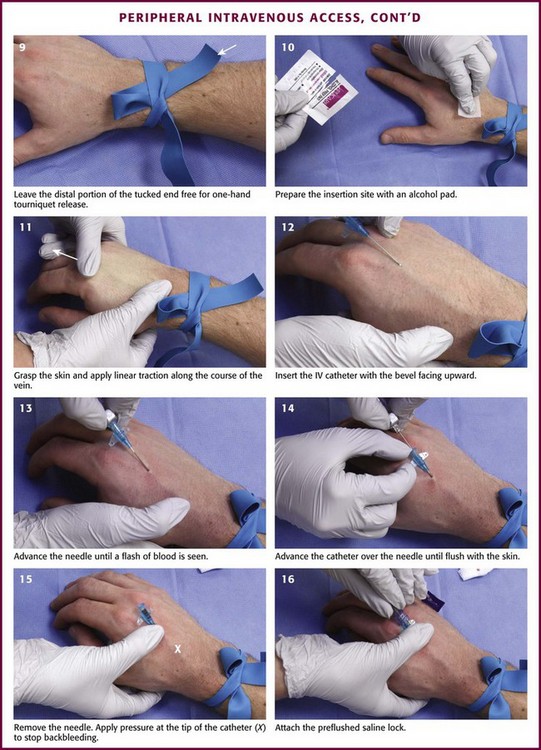
Wash your hands, don gloves (nonsterile is adequate), and clean the injection site with iodine, alcohol, or both. Iodine is a better antiseptic than alcohol and results in fewer infections.44 If using alcohol, allow it to dry on the surface of the skin. Stabilize the vein without contaminating the prepared site. One method is to position one’s thumb alongside the vein and pull down while the index finger is positioned more cephalad and pulls upward. Take the angiocatheter between the thumb and forefinger of the dominant hand. With the bevel up, angle the angiocatheter 10 to 30 degrees between the catheter and the vein and parallel to the vein. Puncture the vein. Once a flash of blood is seen, advance the catheter several millimeters more to ensure that the catheter has entered the vein and not just the wall. Avoid advancing too far and puncturing the posterior wall. Loosen the stylet and advance only the catheter. Use the fingers that were anchoring the vein to occlude the vein at the tip of the catheter to prevent extravasation of blood from the angiocatheter. Remove the needle; connect the saline lock, IV lining, or syringe for phlebotomy; and release the tourniquet.27
Cannulation of the external jugular vein deserves a special note (Fig. 21-7). In patients with otherwise limited peripheral access, it can be cannulated as follows. Place patient in the Trendelenburg position to fill the external jugular vein. Rotate the head to the opposite side and prepare the area as above. Take the cannula and align it in the direction of the vein with the point aiming toward the ipsilateral shoulder. Puncture midway between the angle of the jaw and the midclavicular line while lightly compressing the vein with the free finger above the clavicle. Proceed as described previously for cannulation.
Anchoring the Device
After the IV cannula has been connected to the saline lock or IV tubing, anchor the device (see Fig. 21-6). Use a half-inch-wide strip of tape, adhesive side up, under the hub of the catheter and fold it over in a bow-shaped manner. This will secure the catheter and prevent lateral movement. Clear polyurethane dressings can also be used with or instead of tape. Saline locks can be connected to needleless hubs to prevent accidental needle injury. Then secure the loose saline lock or IV tubing with tape to prevent accidental dislodgement. Connect the IV tubing to the angiocatheter and anchor it. Alternatively, use a commercially available securing device. Sign and date the dressings to ensure timely dressing changes.7 As an option, topical antibiotics or iodophor ointment may be applied to the insertion site to prevent infection, though the efficacy of doing so is unproven.45
Maintaining Patency
An important component of IV care is maintaining patency with frequent flushing. Until recently, heparin solutions were used to flush catheters and maintain patency, but heparin can cause problems such as hemorrhage. Saline flushes are as effective as heparin in maintaining patency and preventing phlebitis but do not carry the risks of bleeding or heparin-induced thrombocytopenia.46–48
Dressing
It is not cost-effective to continually re-dress peripheral venous catheters at periodic intervals. Sterile gauze or transparent, semipermeable, polyurethane dressings can be left in place until removal of the catheter without increasing infection rates, as long as the site is regularly evaluated.49 Securing techniques that use proprietary devices, such as the StatLock IV, a sterile, adhesive-backed anchor, and distal male Luer-tip extensions, may reduce complications by decreasing mobility and risk of dislodgement.50 Commonly used topical antimicrobial ointments have not been consistently proved to reduce the rate of peripheral catheter–related infection but have been associated with increased rates of antimicrobial resistance and Candida colonization. Such ointments are not harmful in the ED, but their routine use is not supported.
Percutaneous Brachial Vein Cannulation
To cannulate the brachial vein, palpate the brachial artery in the antecubital fossa. Prepare the site in the usual manner and apply a tourniquet above the antecubital space. At a point immediately medial or lateral to the pulse, insert an angiocatheter with an attached syringe and advance it at a 45-degree angle while maintaining suction on the syringe. After entrance into the vein, continue 2 to 3 mm further to ensure cannulation. Advance the catheter and remove the needle as usual.26,42
Complications
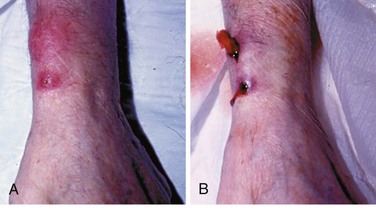
Phlebitis is a common complication after IV cannulation and is described as the presence of a palpable cord accompanied by warmth, erythema, tenderness, and induration over the involved vein (Fig. 21-8). Phlebitis necessitates removal of the catheter and replacement on another extremity. Avoiding IV placement in the lower extremities (where there is more often stagnant blood flow) and across joints (where motion traumatizes the venous wall) minimizes the incidence of IV catheter–related phlebitis.7 Other causes of phlebitis include IV infusion of potassium chloride, certain antibiotics (vancomycin, erythromycin), many cytotoxic chemotherapy agents, phenytoin, and any hyperosmolar solution (e.g., 50% dextrose solution).51,52 The role of in-line filters to prevent phlebitis is controversial. Particulates from reconstituted medications, degradation products, precipitates, glass from vials, and other foreign debris may all play a part in postinfusion phlebitis. In-line filters may therefore play a role in preventing phlebitis, but given their cost, risk of clogging, and paucity of evidence that they improve outcomes, these filters have not become routine.53
Even with the most pristine technique, there is about a 0.5% incidence of catheter-related bloodstream infection with peripheral IV catheters. IV devices facilitate infection by damaging epithelial barriers and thereby providing microorganisms direct access to the bloodstream.54 The risk for infection with peripheral venous catheters is higher in the lower extremity than in the upper extremity and higher in the wrist or upper part of the arm than in the hand. The most common infectious complication of peripheral IV access is self-limited cellulitis.55 The safety of maintaining peripheral IV lines for up to 72 hours before they are relocated has been established in a large, prospective study.49 With rates of clinically significant bacteremia lower than 0.5%, some argue that routine replacement of catheters is now no longer needed.56 Nonetheless, infection can be a costly and potentially devastating complication of IV therapy. Suppurative thrombophlebitis is extremely rare. It most frequently occurs in patients with thermal injury and long-term or lower extremity cannulation.54 Local signs of inflammation or suppuration are often absent and can occur 2 to 10 days after removal of the catheter.57 Treatment is immediate surgical excision of the entire length of the involved vein and tributaries. Though rare with peripheral IV catheters, intravascular device–related bloodstream infection may be an unrecognized cause of nosocomial infection. Peripheral IV catheters are most often associated with staphylococcal and candidal species.58 Infectious complications can be reduced significantly by hand washing, wearing gloves, preparing the site with iodine, and monitoring the site for signs of infection.7
Bruising is a common complication of IV therapy. Contrary to popular belief, flexing of the elbow after venipuncture does not prevent bruising in the antecubital site.59 Applying direct pressure immediately after decannulation is the most useful technique to prevent bruising.
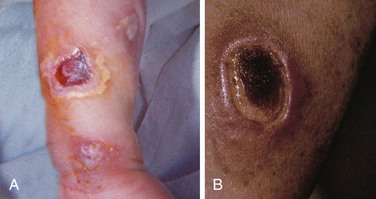
Tissue or interstitial infiltration occurs when the catheter is dislodged from the vein during infusion. It is a common and usually relatively minor complication of IV therapy. Extravasation of certain infusions, such as hypertonic solutions, vasopressors, or chemotherapeutic agents, however, poses a significant risk for necrosis and skin sloughing when infiltration and extravasation occur. In extreme cases, skin grafting may be required.7 For extravasation of dopamine or epinephrine, local injection of antidotes such as phentolamine may be used to reverse the tissue damage.60
Nerve injury is rare after IV cannulation. Any peripheral nerve is potentially vulnerable to a needle-induced injury, and sequelae can range from a minor motor or sensory abnormality to complete paralysis. Nerve damage may be due to direct injury by the needle, intraneural microvascular damage from hematomas, or toxic effects of the agent injected.61 The first symptoms are usually pain, numbness, or paresthesia. Pain may persist for years and can be debilitating. Fortunately, most simple procedures do not result in nerve injury because nerves tend to roll or slide away from a needle. Like all procedures, knowledge of the relevant anatomy is essential. Should a patient complain of numbness or severe pain after a needle puncture, stop the injection immediately.62,63
Thrombosis and subsequent pulmonary embolism (PE) are commonly associated with centrally placed IV catheters.7 Though rare, thrombosis followed by clinically significant PE may occur in patients with peripheral IV lines if saline locks are not flushed or IV fluids run dry. Should this occur, aspirate the line. If the return fluid appears bloody, discard the syringe and then gently flush the saline lock and resume the infusion. If there is no bloody aspirate, use 2 to 3 mL of saline to gently flush the line. If resistance is encountered, stop flushing immediately because there is a risk for development of an embolism. Attempt IV insertion at another site.64
Venous air embolism is another significant, though exceedingly rare complication of peripheral IV access. Symptoms include chest pain, shortness of breath, sudden vascular collapse, cyanosis, and hypotension. If air embolism is suspected, place the patient in the left lateral decubitus Trendelenburg position. Invasive maneuvers include aspiration of air through a central venous catheter and even thoracotomy with direct aspiration from the heart (see Chapter 18). This complication can be prevented by eliminating air from the IV tubing before initiating therapy and not allowing IV lines to run dry.7 If air bubbles are present in an IV line, tap the tubing while holding it taut to allow the air to escape to the top. Similarly, curl the tubing around a pen or syringe to accomplish the same goal. If the air is near a Y connector, one can use a needle and syringe to directly remove it. If all else fails and there is air between the Y connector and the patient, disconnect the tubing and flush it.65
1. Record and date the time of catheter insertion in an obvious location near the insertion site.
2. Do not palpate the insertion site after the skin has been cleansed with antiseptic.
3. Palpate the insertion site for tenderness daily through an intact dressing.
4. Visually inspect the site if the patient reports tenderness.
5. Wash hands before and after palpating, inserting, replacing, or dressing any intravascular access site.
6. Replace dressings when they are damp, loose, or soiled.55
Extravasation of Medications and Vasopressors
Usually, infiltration of a vein is a relatively minor and common complication of IV therapy if only sterile fluid extravasates, even in large amounts. This often occurs when the catheter is dislodged from the vein during infusion. However, if the infusions consist of hypertonic substances, vasopressors, or chemotherapeutics, there is a significant risk for skin sloughing if infiltration and extravasation occur (see Box 21-1). Pain at the infusion site or the alarm sounding on an infusion pump device requires inspection of the infusion site for extravasation. In extreme cases, grafting may be required for skin sloughing (Fig. 21-9).5 If dopamine, phenylephrine (Neo-Synephrine), or norepinephrine extravasate, phentolamine may be used as an antidote to prevent ischemia locally; its use is encouraged as soon as extravasation is identified. Reversal of ischemia with phentolamine is a common technique, but its ability to totally reverse or prevent skin sloughing is not guaranteed. However, if infiltration of these vasopressors occurs, the authors suggest that it be used routinely. There are few downsides to this intervention, although hypotension is a theoretical side effect because phentolamine is an α-adrenergic antagonist. To inject phentolamine, place 5 mg in a vial and dilute with equal parts of saline (final form: 5 mg in 2 mL). For large areas, use two vials with the contents of each vial injected 10 minutes apart through a 25- to 27-gauge needle or a tuberculin syringe. If the IV line is still in place, inject about 1 mL of phentolamine through the catheter before it is removed; however, the IV line is often removed before this can be done. The entire area of skin blanching—or suspected area of extravasation—is injected with multiple small aliquots of the solution, about 0.25 to 0.5 mL each. The procedure may be repeated in 2 to 4 hours. Hyaluronidase is probably benign and has been suggested in the past to ameliorate some effects of extravasation of other solutions. Though it has been a common suggestion, its efficacy has not been well established, and the product is not readily available. Ice and heat have varying effects in counteracting fluid extravasation. Extravasation of IV contrast material is discussed in Chapter 36.
Extravasation of chemotherapy solutions is particularly common and can produce full-thickness tissue sloughing. The patient may complain of pain and burning at the time of infusion, but skin sloughing may be delayed for many days. Table 21-1 lists possible antidotes and dosages for chemotherapy-induced extravasation injury. Results of these interventions vary.
TABLE 21-1
Possible Antidotes for Extravasated Chemotherapeutic Agents*
| CHEMOTHERAPEUTIC AGENT | ANTIDOTE | DOSE |
| Anthracycline | Dexrazoxane hydrochloride† | First dose, inject the equivalent of 500 mg dexrazoxane intravenously over 1-2 hr, second dose at 24 hr, and third dose at 48 hr |
| Mechlorethamine | Sodium thiosulfate | Multiple subcutaneous injections in and around the area of extravasation with a 25-gauge needle: 4 mL of 10% sodium thiosulfate + 6 mL water |
| Vinca alkaloids (vincristine, vinblastine, and vinorelbine) | Hyaluronidase | Inject subcutaneously in and around the area of extravasation with a 25-gauge needle: 150 units (1 mL) For vinca alkaloids, apply local hot compresses |
| Doxorubicin | Granulocyte-macrophage colony-stimulating factor‡ | Inject subcutaneously in and around the area of extravasation with a 25-gauge needle |
| Doxorubicin, daunorubicin, and mitomycin | Dimethyl sulfoxide (free radical scavenger) | Apply a 50-70% solution topically qid for 14 days. Leave uncovered |
| Mitomycin | Pyridoxine‡ | Inject subcutaneously in and around the area of extravasation with a 25-gauge needle |
| Nonspecific | Saline | Inject subcutaneously in and around the area of extravasation with a 25-gauge needle |
| Nonspecific | Corticosteroids§ | Inject subcutaneously in and around the area of extravasation with a 25-gauge needle: hydrocortisone, 500 mg diluted in 500 mL saline |
*Many of these interventions are anecdotal and none are guaranteed to reverse or ameliorate tissue injury. Controversy surrounds the actual benefit, and no randomized prospective trials have been conducted for many of the suggested regimens. Also consider elevation and surgical débridement when necessary.
†Approved by U.S. Food and Drug Administration for this indication.
‡Not well studied, theoretical benefit.
§Results are variable; injury is not an inflammatory reaction.
The bottom line is that most extravasated chemotherapy and other agents have no specific antidote or reversal agents to alter the final outcome. At most extravasation sites it may be best to avoid the empirical use of suggested treatments such as sodium bicarbonate, sodium thiosulfate, heparin, calcium gluconate, magnesium sulfate, lidocaine, cimetidine, diphenhydramine, and other chemical substances that are believed to inactivate drugs and reduce toxic effects on cells. In some experimental settings these substances have made the necrosis and ulceration worse.53–56
References
1. Lawrence, DW, Lauro, AJ. Complications from i.v. therapy: results from field-started and emergency department–started i.v.’s compared. Ann Emerg Med. 1988;17:314–317.
2. Spaite, DW, Valenzuela, TD, Meislin, HW, et al. Prospective validation of a new model for evaluating emergency medical services systems by in-field observation of specific time intervals in prehospital care. Ann Emerg Med. 1993;22:638–645.
3. Stovroff, M, Teague, WG. Intravenous access in infants and children. Pediatr Clin North Am. 1998;45:1373–1393. [viii].
4. Jones, SE, Nesper, TP, Alcouloumre, E. Prehospital intravenous line placement: a prospective study. Ann Emerg Med. 1989;18:244–246.
5. Meier, PA, Fredrickson, M, Catney, M, et al. Impact of a dedicated intravenous therapy team on nosocomial bloodstream infection rates. Am J Infect Control. 1998;26:388–392.
6. Soifer, NE, Borzak, S, Edlin, BR, et al. Prevention of peripheral venous catheter complications with an intravenous therapy team: a randomized controlled trial. Arch Intern Med. 1998;158:473–477.
7. Weinstein, S. Plumer’s Principles & Practice of Intravenous Therapy, 8th ed. Philadelphia: Lippincott; 2006.
8. McGrew, RE, McGrew, MP. Encyclopedia of Medical History. New York: McGraw-Hill; 1985.
9. Farr, AD. The first human blood transfusion. Med Hist. 1980;24:143–162.
10. Anderson, L. Venous catheterization for fluid therapy: a technique and results. J Lab Clin Med. 1950;36:645.
11. Barsan, WG, Hedges, JR, Nishiyama, H, et al. Differences in drug delivery with peripheral and central venous injections: normal perfusion. Am J Emerg Med. 1986;4:1–3.
12. Hedges, JR, Barsan, WB, Doan, LA, et al. Central versus peripheral intravenous routes in cardiopulmonary resuscitation. Am J Emerg Med. 1984;2:385–390.
13. Kuzma, K, Sporer, KA, Michael, GE, et al. When are prehospital intravenous catheters used for treatment? J Emerg Med. 2009;36:357.
14. Gausche, M, Tadeo, RE, Zane, MC, et al. Out-of-hospital intravenous access: unnecessary procedures and excessive cost. Acad Emerg Med. 1998;5:878–882.
15. Schwarzman, P, Rottman, SJ. Prehospital use of heparin locks: a cost-effective method for intravenous access. Am J Emerg Med. 1987;5:475–477.
16. Himberger, JR, Himberger, LC. Accuracy of drawing blood through infusing intravenous lines. Heart Lung. 2001;30:66–73.
17. Herr, RD, Bossart, PJ, Blaylock, RC, et al. Intravenous catheter aspiration for obtaining basic analytes during intravenous infusion. Ann Emerg Med. 1990;19:789–792.
18. Herr, RD, Swanson, T. Pseudometabolic acidosis caused by underfill of Vacutainer tubes. Ann Emerg Med. 1992;21:177–180.
19. Brannam, L, Blaivas, M, Lyon, M, et al. Emergency nurses’ utilization of ultrasound guidance for placement of peripheral intravenous lines in difficult-access patients. Acad Emerg Med. 2004;11:1361–1363.
20. Keyes, LE, Frazee, BW, Snoey, ER, et al. Ultrasound-guided brachial and basilic vein cannulation in emergency department patients with difficult intravenous access. Ann Emerg Med. 1999;34:711–714.
21. Costantino, TG, Parikh, AK, Satz, WA, et al. Ultrasonography-guided peripheral intravenous access versus traditional approaches in patients with difficult intravenous access. Ann Emerg Med. 2005;46:456–461.
22. Doniger, SJ, Ishimine, P, Fox, JC, et al. Randomized controlled trial of ultrasound-guided peripheral intravenous catheter placement versus traditional techniques in difficult-access pediatric patients. Pediatr Emerg Care. 2009;25:154–159.
23. Stein, J, George, B, River, G, et al. Ultrasonographically guided peripheral intravenous cannulation in emergency department patients with difficult intravenous access: a randomized trial. Ann Emerg Med. 2009;54:33.
24. Panebianco, NL, Fredette, JM, Szyid, D, et al. What you see (sonographically) is what you get: vein and patient characteristics associated with successful ultrasound-guided peripheral intravenous placement in patients with difficult access. Acad Emerg Med. 2009;16:1298.
25. Schnadower, D, Lin, S, Perera, P, et al. A pilot study of ultrasound analysis before pediatric peripheral vein cannulation attempt. Acad Emerg Med. 2007;14:483.
26. Dargin, JM, Rebholz, CM, Lowenstein, RA, et al. Ultrasonography-guided peripheral intravenous catheter survival in ED patients with difficult access. Am J Emerg Med. 2010;28:1.
27. Ellenberger, A. An expert answers questions about starting an i.v. line. Nursing. 1999;29:56–59.
28. Field, JM, Gonzales, L, Hazinski, MF, et al. Advanced Cardiovascular Life Support Provider Manual. Dallas: American Heart Association; 2006.
29. Recommendations for prevention of HIV transmission in health-care settings. MMWR Morb Mortal Wkly Rep. 1987;36(suppl 2):1S–18S.
30. Jagger, J, Bentley, M, Perry, J. Protecting yourself from high-risk i.v. devices. Nursing. 1999;29:20.
31. Steward, DJ. Venous cannulation in small infants: a simple method to improve success. Anesthesiology. 1999;90:930–931.
32. Roberge, RJ, Kelly, M, Evans, TC, et al. Facilitated intravenous access through local application of nitroglycerin ointment. Ann Emerg Med. 1987;16:546–549.
33. Vaksmann, G, Rey, C, Breviere, GM, et al. Nitroglycerine ointment as aid to venous cannulation in children. J Pediatr. 1987;111:89–91.
34. Amsterdam, JT, Hedges, JR, Weinshenker, E, et al. Evaluation of venous distension device: phase II: cannulation of nonemergent patients. Am J Emerg Med. 1988;6:224–227.
35. Hedges, JR, Weinshenker, E, Dirksing, R. Evaluation of venous distension device: potential aid for intravenous cannulation. Ann Emerg Med. 1986;15:540–543.
36. Burgher, SW, McGuirk, TD. Subcutaneous buffered lidocaine for intravenous cannulation: is there a role in emergency medicine? Acad Emerg Med. 1998;5:1057–1063.
37. Brown, J, Larson, M. Pain during insertion of peripheral intravenous catheters with and without intradermal lidocaine. Clin Nurse Spec. 1999;13:283–285. [quiz 286-288].
38. Wightman, MA, Vaughan, RW. Comparison of compounds used for intradermal anesthesia. Anesthesiology. 1976;45:687–689.
39. Ong, EL, Lim, NL, Koay, CK. Towards a pain-free venipuncture. Anaesthesia. 2000;55:260.
40. Young, SS, Schwartz, R, Sheridan, MJ. EMLA cream as a topical anesthetic before office phlebotomy in children. South Med J. 1996;89:1184.
41. Hallen, B, Uppfeldt, A. Does lidocaine-prilocaine cream permit painfree insertion of IV catheters in children? Anesthesiology. 1982;57:340–342.
42. Selby, IR, Bowles, BJ. Analgesia for venous cannulation: a comparison of EMLA (5 minutes application), lignocaine, ethyl chloride, and nothing. J R Soc Med. 1995;88:264.
43. Akcam, M, Ormeci, AR. Oral hypertonic glucose spray: a practical alternative for analgesia in the newborn. Acta Paediatr. 2004;93:1330.
44. Jakobsen, CJ, Grabe, N, Damm, MD. A trial of povidone-iodine for prevention of contamination of intravenous cannulae. Acta Anaesthesiol Scand. 1986;30:447–449.
45. Maki, DG, Band, JD. A comparative study of polyantibiotic and iodophor ointments in prevention of vascular catheter–related infection. Am J Med. 1981;70:739–744.
46. Dougherty, L. Reducing the risk of complications in i.v. therapy. Nurs Stand. 1997;12:40–42.
47. Goode, CJ, Titler, M, Rakel, B, et al. A meta-analysis of effects of heparin flush and saline flush: quality and cost implications. Nurs Res. 1991;40:324–330.
48. Dunn, DL, Lenihan, SF. The case for the saline flush. Am J Nurs. 1987;87:798–799.
49. Maki, DG, Botticelli, JT, LeRoy, ML, et al. Prospective study of replacing administration sets for intravenous therapy at 48- vs 72-hour intervals. 72 hours is safe and cost-effective. JAMA. 1987;258:1777–1781.
50. Wood, D. A comparative study of two securement techniques for short peripheral intravenous catheters. J Intraven Nurs. 1997;20:280–285.
51. Hershey, CO, Tomford, JW, McLaren, CE, et al. The natural history of intravenous catheter–associated phlebitis. Arch Intern Med. 1984;144:1373–1375.
52. Lodge, JP, Chisholm, EM, Brennan, TG, et al. Insertion technique, the key to avoiding infusion phlebitis: a prospective clinical trial. Br J Clin Pract. 1987;41:816–819.
53. Friedland, G. Infusion-related phlebitis—is the in-line filter the solution? N Engl J Med. 1985;312:113–115.
54. Stamm, WE. Infections related to medical devices. Ann Intern Med. 1978;89(5 Pt 2 suppl):764–769.
55. Shreve, WS, Knotts, FB. Quality improvement with prehospital-placed intravenous catheters in trauma patients. J Emerg Nurs. 1999;25:285–289.
56. Monreal, M, Oller, B, Rodriguez, N, et al. Infusion phlebitis in post-operative patients: when and why. Haemostasis. 1999;29:247–254.
57. Maki, DG, Goldman, DA, Rhame, FS. Infection control in intravenous therapy. Ann Intern Med. 1973;79:867–887.
58. Tully, JL, Friedland, GH, Baldini, LM, et al. Complications of intravenous therapy with steel needles and Teflon catheters. A comparative study. Am J Med. 1981;70:702–706.
59. Dyson, A, Bogod, D. Minimising bruising in the antecubital fossa after venepuncture. Br Med J (Clin Res Ed). 1987;294:1659.
60. Bey, D, El-Chaar, GM, Bierman, F, et al. The use of phentolamine in the prevention of dopamine-induced tissue extravasation. J Crit Care. 1998;13:13–20.
61. Selander, D, Dhuner, KG, Lundborg, G. Peripheral nerve injury due to injection needles used for regional anesthesia. An experimental study of the acute effects of needle point trauma. Acta Anaesthesiol Scand. 1977;21:182–188.
62. Preston, D, Logigian, E. Iatrogenic needle-induced peroneal neuropathy in the foot. Ann Intern Med. 1988;109:921–922.
63. Yuan, RT, Cohen, MJ. Lateral antebrachial cutaneous nerve injury as a complication of phlebotomy. Plast Reconstr Surg. 1985;76:299–300.
64. Barrus, DH, Danek, G. Should you irrigate an occluded i.v. line? Nursing. 1987;17:63–64.
65. Boykoff, SL, Boxwell, AO, Boxwell, JJ. 6 ways to clear the air from an i.v. line. Nursing. 1988;18:46–48.

 -inch Arrow twin catheter or the longer catheter from the Arrow arterial line kit) may help reduce this problem.
-inch Arrow twin catheter or the longer catheter from the Arrow arterial line kit) may help reduce this problem.




