CHAPTER 113 Peripheral Artery Disease
Peripheral arterial disease, most often a result of atherosclerosis, occurs in approximately 10% of the adult population and affects more than 10 million people in the United States alone.1 Most frequently, patients present with claudication. Less often, patients suffer from critical limb ischemia. Risk of illness or death from other cardiovascular causes in these patients with chronic limb ischemia is greatly increased, making peripheral arterial disease an important marker for atherosclerotic cardiovascular disease in general.2
ACUTE LIMB ISCHEMIA
Prevalence and Epidemiology
The incidence of acute limb ischemia is approximately 1.7/10,000 per year.3 Patients presenting with a pulseless extremity suffer amputation rates as high as 10% and mortality rates of 5% to 15%.4,5 These patients are characteristically at high risk for perioperative complication and are medically fragile (Table 113-1).
Etiology and Pathophysiology
Limb ischemia can result acutely from a variety of causes (Table 113-2). Sudden-onset acute pain is indicative of an embolic event; a history of chronic pain or claudication before the acute ischemic episode is suggestive of a thrombotic etiology. Graft occlusions are slightly more common than thromboses of native arteries. Thrombotic occlusions are approximately six times more common than embolic events.
| Embolism (often to an arterial bifurcation) |
Bypass graft thrombosis has become the most frequent cause of acute lower extremity ischemia. Intimal hyperplasia and valvular hyperplasia are the most common causes of thrombosis in native conduit bypasses. In prosthetic grafts, acute thrombosis is most commonly due to kinking across joints or to the thrombogenicity of the graft material itself.6
Manifestations of Disease
Clinical Presentation
Because of its acuity and thus the absence of adequate preformed collateral arteries, arterial embolization is the classic situation resulting in acute arterial occlusion and acute limb ischemia. The anatomic distribution of arterial embolism is depicted in Figure 113-1. The embolus most frequently lodges at an arterial bifurcation.
In the patient with bilateral lower extremity ischemia and absent femoral pulses, the most common clinical scenario is saddle embolus to the aortic bifurcation. The patient will complain of sudden onset of bilateral buttock and lower extremity pain. Mottling of the lower extremities and lower abdomen, sometimes up to the umbilicus, will be evident on physical examination. The diagnosis in these patients is too frequently missed, probably because of the bilateral nature of the ischemic insult. Even in the setting of absent femoral pulses, patients are often evaluated for possible neurologic or neurosurgical problems, resulting in a delay of diagnosis and appropriate therapy. This disease can carry with it a poor prognosis, with a 27% mortality in one modern series.7
The most common site of clinically significant embolism in the lower extremity is the common femoral artery bifurcation.8 Patients with common femoral artery embolism will experience foot and calf pain. Finally, an embolus to the popliteal artery will result in absent pedal pulses and an ischemic foot.
Approximately 90% of the time, arterial emboli arise in the heart.9 Atrial fibrillation results in a dilated, noncontractile left atrial appendage, which predisposes to thrombus formation and—on spontaneous or therapeutic cardioversion—thromboembolism. Left ventricular thrombus may also form, for instance, adjacent to a noncontractile left ventricular segment after myocardial infarction, in a left ventricular aneurysm, or in the setting of dilated cardiomyopathy. Saddle emboli, which lodge at the aortic bifurcation and cause bilateral lower extremity ischemia, are most commonly the result of left ventricular thrombus. Thromboembolism from heart valves are less common today than in the past owing to the relative decrease in prevalence of rheumatic heart disease. Bacterial endocarditis can result in septic emboli that may cause both acute ischemia and infection of the distal vessel wall, which in turn results in mycotic aneurysm. Atheroemboli may arise from either the thoracic or the abdominal aorta. Finally, in some 5% of cases, the source of embolism is never identified.
Clinical Categorization of Acute Limb Ischemia
Since 1997, the Rutherford criteria have been used to grade the clinical severity of acute limb ischemia.10 These grades, as summarized in Table 113-3, are indicative both of whether emergent surgical intervention is indicated and of whether the limb is salvageable. Most commonly, category I represents an acute occlusion in a chronically narrowed artery, with well-formed collaterals. Category II represents a limb that is salvageable with immediate therapy or intervention. In the case of irreversible ischemia, category III, the patient will present with profound vascular and neurologic deficits; the limb may be in a state of rigor mortis and will require amputation.
Before imaging studies, in patients with acute limb ischemia, laboratory studies are indicated. In particular, and in anticipation of imaging studies with use of nephrotoxic contrast agents, a serum creatinine concentration should be obtained. If a hypercoagulable state is suspected, a hypercoagulable profile should be sent before institution of any anticoagulation. An electrocardiogram will aid in the diagnosis of atrial fibrillation and will provide some information as to the patient’s cardiac status. Assessment of the patient’s cardiac risk for general anesthesia by the Goldman index or other scale may be useful.11
Whereas some surgeons will elect to observe a transiently ischemic limb for signs of compartment syndrome postoperatively, in the setting of more than 6 hours of profound ischemia, many perform four-compartment fasciotomies prophylactically at the time of revascularization. If one elects to observe the patient’s leg, any increased pain, especially with passive plantar flexion of the foot, or loss of sensation in the first web space of the foot (sensory distribution of the deep peroneal nerve) should prompt reevaluation. Compartment pressures may be measured by a Stryker needle (Stryker Instruments, Kalamazoo, MI) or other device. Compartment pressure of greater than 30 mm Hg can result in tissue ischemia and necrosis. Patients demonstrating hypotension or shock, those requiring pressors, those with absent flow through the popliteal artery at presentation, and younger patients with greater muscle mass and fewer arterial collaterals are at increased risk for development of compartment syndrome. Four-compartment fasciotomy is usually performed through both a medial and a lateral incision (Fig. 113-2). The incisions are left open for subsequent delayed primary closure or skin grafting.
Differential Diagnosis
Acute arterial occlusion causing acute limb ischemia is difficult to confuse with other pathologic conditions. That said, there are some not uncommon pitfalls in patients presenting with pulseless, cool, painful extremities. In patients suffering from aortic bifurcation saddle embolus, the diagnosis is frequently missed, as discussed before. These patients are often evaluated for possible neurologic or neurosurgical problems owing to the bilateral paralysis on presentation, resulting in a delay of diagnosis and appropriate therapy. Aortic dissection can cause acute arterial insufficiency of one or both legs and must be considered in any patient presenting with a cold pulseless limb and abdominal, back, or chest pain.12 Finally, acute venous thrombosis causing phlegmasia cerulea dolens can manifest as a painful, cold, pulseless extremity; however, massive swelling should alert the physician to the correct diagnosis.13
Synopsis of Treatment Options
Medical
Acute limb ischemia is a surgical disease; however, attention to the patient’s medical and overall condition is essential. Maintenance intravenous fluids should be initiated and the patient kept nil per os. A Foley catheter should be inserted to monitor urine output. Consideration should be given to either oral acetylcysteine14 or bicarbonate infusion15 if the patient has chronic renal insufficiency or diabetes because diagnostic or completion angiography will likely be indicated.
Surgical/Interventional
Intra-arterial or catheter-directed thrombolysis or fibrinolysis has proved effective and beneficial in a variety of clinical scenarios involving acute limb ischemia.16–18 However, in a number of situations, thrombolytics are absolutely contraindicated; these include recent surgery or trauma, recent stroke, active bleeding diathesis, and recent history of gastrointestinal bleeding. In some cases, percutaneous aspiration or mechanical thrombectomy is used before and in conjunction with thrombolysis. It is essential that any stenoses revealed on arteriography after thrombolysis be treated with angioplasty, bypass graft revision, or revascularization. In the interim from completion of thrombolysis until intervention directed at flow-limiting lesions, heparin infusion must be continued.
Before 1963 and the introduction of Thomas J. Fogarty’s balloon embolectomy catheter (Fig. 113-3), only 23% of patients suffering embolic vascular occlusion were treated surgically with embolectomy. Subsequent to the introduction of this catheter and continuing today, most of these patients undergo surgical embolectomy, which now—along with systemic anticoagulation—is the mainstay of therapy.
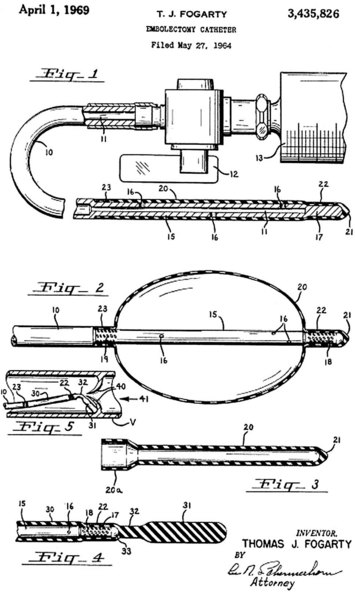
 FIGURE 113-3 Original patent application for Fogarty embolectomy catheter.
FIGURE 113-3 Original patent application for Fogarty embolectomy catheter.
(From archives of U.S. Patent and Trademark Office, with permission.)
Acute unilateral iliac occlusion can occur in patients having undergone prior aortic aneurysmorrhaphy with a bifurcated graft, prior aortobifemoral reconstruction, or prior abdominal aortic stent grafting. In these situations, the surgeon may be able to restore patency of the graft limb with balloon thrombectomy performed from a groin incision, but a technical problem in the affected limb and graft stenosis must be suspected and subsequently addressed (Fig. 113-4).19 If limb patency cannot be reestablished, femoral-femoral or axillofemoral bypass is indicated.
Symptomatic popliteal aneurysms generally present with limb ischemia—from aneurysm thrombosis or distal thromboembolism—rather than with rupture. The diagnosis should be suspected in any patient with acute or chronic ischemia of the leg and a palpable firm pulseless (in the case of thrombosis) or pulsatile mass behind the knee. Duplex ultrasound examination can confirm the diagnosis. Angiography, MRA, and CTA are useful for operative planning. Treatment of a popliteal aneurysm presenting with acute limb ischemia consists of emergent catheter-directed thrombolysis to reestablish distal arterial patency20 and subsequent surgical exclusion and bypass; resection of the aneurysm is not necessary. There is also an emerging experience with covered stents for treatment of popliteal aneurysms.21 Half of patients will have bilateral popliteal aneurysms, and one third of patients with a popliteal aneurysm will have a coincident abdominal aortic aneurysm; therefore, these patients should be screened for coexisting aneurysmal disease.
In patients suffering from thrombotic arterial occlusions and in those whose thromboembolism cannot be extracted or lysed, arterial bypass grafting may be necessary to treat stenotic or occluded vascular segments. Surgical arterial bypass and other revascularization procedures are detailed in Chapter 114.
CHRONIC LIMB ISCHEMIA
Definition
Atherosclerosis resulting in peripheral arterial disease is the most common cause of chronic limb ischemia. Chronic limb ischemia presents as a spectrum of disease from asymptomatic to claudication to critical limb ischemia. This spectrum is encapsulated in two similar staging systems for peripheral arterial disease: Fontaine stages and Rutherford categories (Table 113-4).
Symptomatic patients with peripheral arterial disease generally present with either intermittent claudication or critical limb ischemia. Intermittent claudication is defined as muscle discomfort in the lower limb reproducibly produced by exercise and relieved by rest within 10 minutes. Critical limb ischemia is defined as persistent, recurring ischemic rest pain requiring opiate analgesia for at least 2 weeks with ulceration or gangrene of the foot or toes. Hence, Rutherford categories 4 through 6 comprise those patients with critical limb ischemia.1
Prevalence and Epidemiology
Intermittent claudication has a prevalence of approximately 3% in 40-year-olds and about 6% in 60-year-olds. Claudication often is not progressive; revascularization is required in less than one quarter of patients at 10 years, and amputation is required in approximately 2% of patients at 5 years. However, continued smoking or coexistent diabetes portends worse clinical outcomes for claudicants, including more frequent eventual amputation. The outcome of intermittent claudication at 5 years is summarized in Figure 113-5.
Critical limb ischemia has an incidence in the United States of approximately 1000 new cases per 1 million population. Figure 113-6 summarizes risk factors for the development of critical limb ischemia in patients with peripheral arterial disease. More than half of patients with critical limb ischemia undergo attempts at revascularization as definitive treatment; regardless, significant numbers of patients lose their limbs or die within 1 year (Fig. 113-7).
Manifestations of Disease
Clinical Presentation
Critical Limb Ischemia
The location and appearance of the ulcer or gangrene can provide the astute clinician a suggestion as to the etiology of the wound. For example, as indicated in Figure 113-8, calf ulcers are more often reflective of venous disease than of peripheral arterial disease.
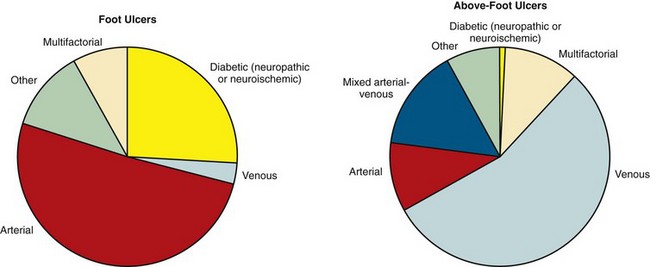
 FIGURE 113-8 The causes and frequencies of lower limb ulcers.
FIGURE 113-8 The causes and frequencies of lower limb ulcers.
(From Norgren L, Hiatt WR, Dormandy JA, et al; TASC II Working Group. Inter-Society Consensus for the Management of Peripheral Arterial Disease [TASC II]. J Vasc Surg 2007; 45[Suppl S]:S5-S67.)
Subsequently, pulse volume recordings may be performed by use of a plethysmograph in a vascular laboratory. This device measures volume changes in the limb at various levels with multiple blood pressure cuffs. Pulse volume recordings can localize arterial occlusive disease with up to 85% accuracy compared with angiography. Figure 113-9 demonstrates pulse volume recordings in a patient with unilateral superficial femoral artery occlusion before and after angioplasty and stenting of the superficial femoral artery.
Imaging Techniques and Findings
Ultrasonography
Duplex ultrasonography is noninvasive and does not require the administration of contrast material or other medication. It is highly sensitive and specific for identification of stenoses in bypass grafts. It is sometimes less effective at imaging calcified vessels and tibial arteries. Ultrasound studies are somewhat dynamic and technologist dependent by their very nature, and thus objective results and images are not as portable or reproducible as in other imaging modalities. Nevertheless, Duplex ultrasonography is greater than 80% sensitive and specific for iliac, femoropopliteal, and infrapopliteal disease.22
Differential Diagnosis
In the case of critical limb ischemia with tissue loss, other causes of or factors contributing to the ulcer or gangrene must be considered. These include diabetes, venous insufficiency, neuropathies, musculoskeletal abnormalities causing disturbance in gait or mobility, infection, conventional trauma, calciphylaxis, and debility of any cause contributing to pressure or decubitus ulceration.23
Synopsis of Treatment Options
Surgical/Interventional
In the case of severe intermittent claudication that has an impact on a patient’s livelihood or ability to perform activities of daily living, revascularization is sometimes indicated. Recent advances in and widespread adoption of endovascular therapy for peripheral arterial disease have lowered the threshold—in some practices—for attempted revascularization in patients with intermittent claudication. Meanwhile, revascularization is indicated and necessary in those patients with critical limb ischemia. Specific approaches and techniques for revascularization are discussed in Chapter 114.
KEY POINTS
 Peripheral arterial disease is the most common etiology of both chronic limb ischemia and acute limb ischemia.
Peripheral arterial disease is the most common etiology of both chronic limb ischemia and acute limb ischemia. Acute limb ischemia is a surgical emergency and is most often due to thromboembolus or acute thrombotic occlusion of a native artery or bypass graft.
Acute limb ischemia is a surgical emergency and is most often due to thromboembolus or acute thrombotic occlusion of a native artery or bypass graft.Kasirajan K, Ouriel K. Acute limb ischemia. In: Rutherford R, editor. Vascular Surgery. 6th ed. Philadelphia: Elsevier; 2005:959-971.
Norgren L, Hiatt WR, Dormandy JA, et al. TASC II Working Group. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg. 2007;45(Suppl S):S5-S67.
Perera GB, Lyden SP. Current trends in lower extremity revascularization. Surg Clin North Am. 2007;87:1135-1147. x
White C. Intermittent claudication. N Engl J Med. 2007;356:1241-1250.
1 Norgren L, Hiatt WR, Dormandy JA, et al. TASC II Working Group. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg. 2007;45(Suppl S):S5-S67.
2 Golomb BA, Dang TT, Criqui MH. Peripheral arterial disease: morbidity and mortality implications. Circulation. 2006;114:688-699.
3 Davies B, Braithwaite BD, Birch PA, et al. Acute leg ischaemia in Gloucestershire. Br J Surg. 1997;84:504-508.
4 Dormandy J, Heeck L, Vig S. Acute limb ischemia. Semin Vasc Surg. 1999;12:148.
5 Kasirajan K, Ouriel K. Acute limb ischemia. In: Rutherford R, editor. Vascular Surgery. 6th ed. Philadelphia: Elsevier; 2005:959-971.
6 Ouriel K, Shortell CK, Green RM, et al. Differential mechanisms of failure of autogenous and non-autogenous bypass conduits: an assessment following successful graft thrombolysis. Cardiovasc Surg. 1995;3:469.
7 Woratyla S, Darling RCIII, Lloyd W, et al. Acute and chronic aortic occlusion: analysis of outcome. Proceedings of the Eastern Vascular Society. 1998;12:82.
8 Pfeiffer RBIII, O’Mara CS. Peripheral arterial embolus. In: Cameron JL, editor. Current Surgical Therapy. 8th ed. Philadelphia: Elsevier; 2004:817-820.
9 Abbott W, Maloney R, McCabe C, et al. Arterial embolism: a 44 year perspective. Am J Surg. 1982;143:460.
10 Rutherford RB, Baer JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26:517.
11 Goldman L, Caldera DL, Nussbaum SR, et al. Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med. 1977;297:845-850.
12 Ince H, Nienaber CA. Diagnosis and management of patients with aortic dissection. Heart. 2007;93:266-270.
13 Perkins JM, Magee TR, Galland RB. Phlegmasia caerulea dolens and venous gangrene. Br J Surg. 1996;83:19-23.
14 Tepel M, van der Giet M, Schwarzfeld C, et al. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med. 2000;343:180-184.
15 Merten GJ, Burgess WP, Gray LV, et al. Prevention of contrast-induced nephropathy with sodium bicarbonate: a randomized controlled trial. JAMA. 2004;291:2328-2334.
16 Ouriel K, Shortell C, DeWeese JA, et al. A comparison of thrombolytic therapy with operative revascularization in the initial treatment of acute peripheral arterial ischemia. J Vasc Surg. 1994;19:1021.
17 Weaver FA, Camerota AJ, Youngblood M, et al. Surgical revascularization versus thrombolysis for non-embolic lower extremity native artery occlusions: results of a prospective randomized trial. The STILE Investigators: Surgery versus Thrombolysis for Ischemia of the Lower Extremity. J Vasc Surg. 1996;24:513.
18 Ouriel K, Veith FJ, Sasahara AA. Thrombolysis or peripheral arterial surgery: phase I results. TOPAS Investigators. J Vasc Surg. 1996;23:64.
19 Milner R, Golden MA, Velazquez OC, et al. A new endovascular approach to treatment of acute iliac limb occlusions of bifurcated aortic stent grafts with an exoskeleton. J Vasc Surg. 2003;37:1329-1331.
20 Carpenter JP, Barker CF, Roberts B, et al. Popliteal artery aneurysms: current management and outcome. J Vasc Surg. 1994;19:65-72.
21 Mohan IV, Bray PJ, Harris JP, et al. Endovascular popliteal aneurysm repair: are the results comparable to open surgery? Eur J Vasc Endovasc Surg. 2006;32:149-154.
22 Koelemay MJ, den Hartog D, Prins MH, et al. Diagnosis of arterial disease of the lower extremities with duplex ultrasonography. Br J Surg. 1996;83:404-409.

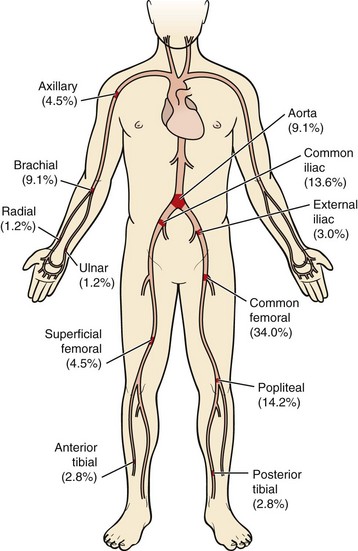
 FIGURE 113-1
FIGURE 113-1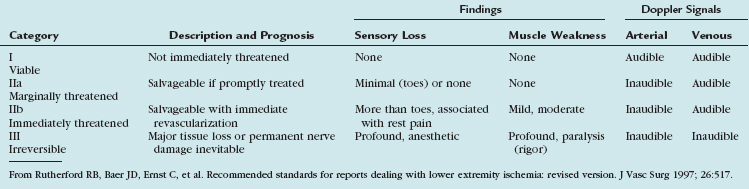
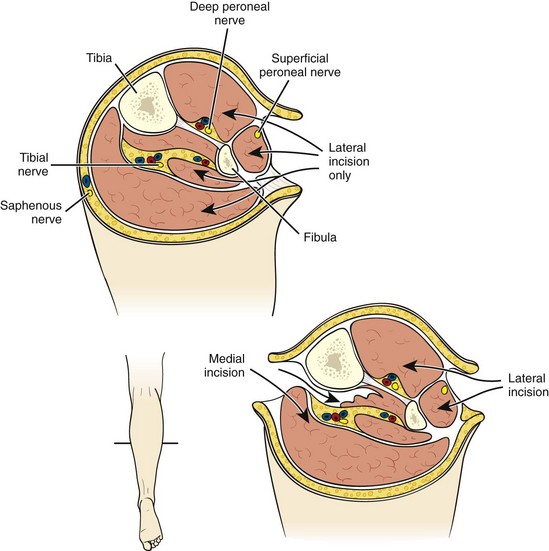
 FIGURE 113-2
FIGURE 113-2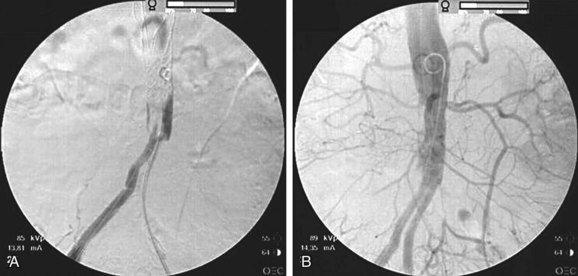
 FIGURE 113-4
FIGURE 113-4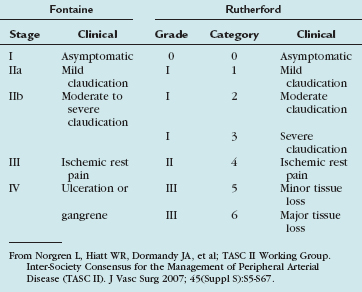
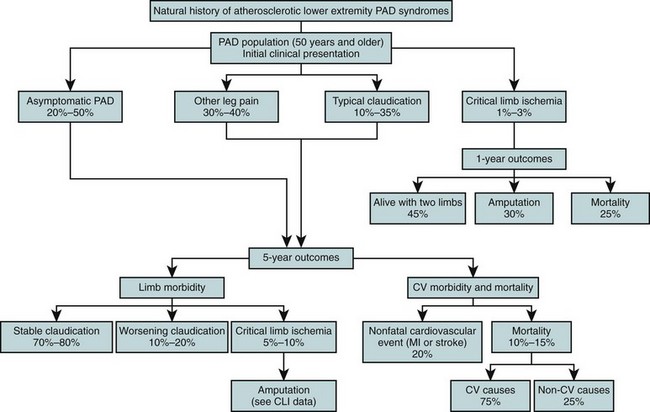
 FIGURE 113-5
FIGURE 113-5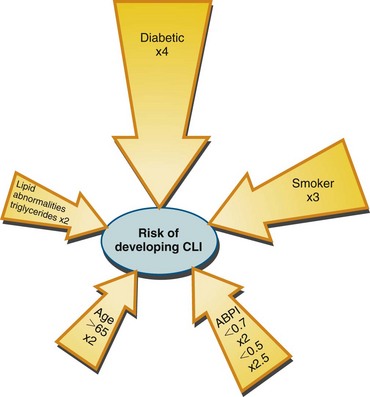
 FIGURE 113-6
FIGURE 113-6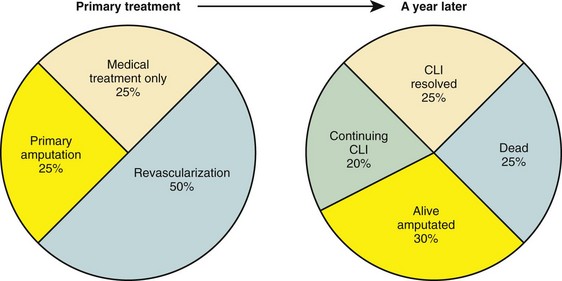
 FIGURE 113-7
FIGURE 113-7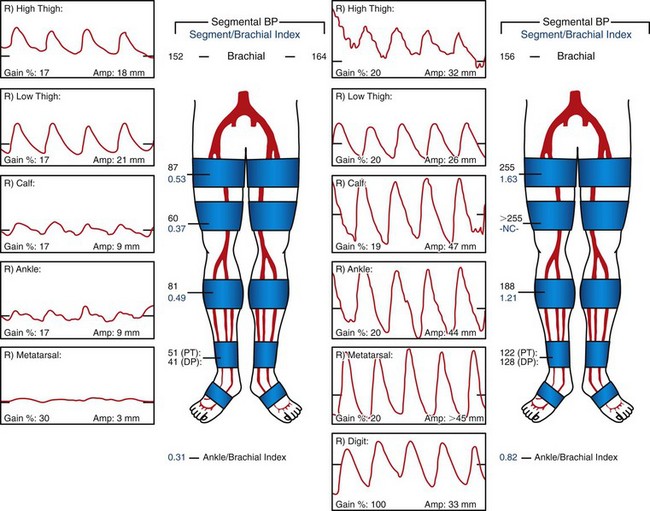
 FIGURE 113-9
FIGURE 113-9


