Chapter 55
Peripheral Arterial Disease
1. What are the key components of the vascular physical examination?
 Blood pressure measurements in both arms
Blood pressure measurements in both arms
 Carotid pulse palpation for upstroke and amplitude, and auscultation for bruits
Carotid pulse palpation for upstroke and amplitude, and auscultation for bruits
 Auscultation of the abdomen and flank for bruits
Auscultation of the abdomen and flank for bruits
 Palpation of the abdomen for aortic pulsation and its maximal diameter
Palpation of the abdomen for aortic pulsation and its maximal diameter
 Palpation of brachial, radial, ulnar, femoral, popliteal, dorsalis pedis, and posterior tibial pulses; pulse intensity is scored as follows: 0, absent; 1, diminished; 2, normal; 3, bounding
Palpation of brachial, radial, ulnar, femoral, popliteal, dorsalis pedis, and posterior tibial pulses; pulse intensity is scored as follows: 0, absent; 1, diminished; 2, normal; 3, bounding
 Performance of the Allen test when knowledge of hand perfusion is needed
Performance of the Allen test when knowledge of hand perfusion is needed
 Auscultation of the femoral arteries for the presence of bruits
Auscultation of the femoral arteries for the presence of bruits
 Inspection of the feet for color, temperature, and integrity of the skin, and for ulcers
Inspection of the feet for color, temperature, and integrity of the skin, and for ulcers
 Observation of other findings suggestive of severe PAD, including distal hair loss, trophic skin changes, and hypertrophic nails
Observation of other findings suggestive of severe PAD, including distal hair loss, trophic skin changes, and hypertrophic nails
2. Can the location of the patient’s lower extremity claudication help to localize the site of occlusive disease?
 Occlusive iliac artery disease may produce hip, buttock, and thigh pain, as well as calf pain.
Occlusive iliac artery disease may produce hip, buttock, and thigh pain, as well as calf pain.
 Occlusive femoral and popliteal artery disease usually produces calf pain.
Occlusive femoral and popliteal artery disease usually produces calf pain.
 Occlusive disease in the tibial arteries may produce calf pain or, more rarely, foot pain and numbness.
Occlusive disease in the tibial arteries may produce calf pain or, more rarely, foot pain and numbness.
3. What noninvasive tests are used in the assessment of lower limb claudication?
 Ankle-brachial index (ABI): The ankle-brachial index is the ankle systolic pressure (as determined by Doppler) divided by the brachial systolic pressure. An abnormal index is less than 0.90. The sensitivity is approximately 90% for diagnosis of PAD. (See Question 4 for further details.)
Ankle-brachial index (ABI): The ankle-brachial index is the ankle systolic pressure (as determined by Doppler) divided by the brachial systolic pressure. An abnormal index is less than 0.90. The sensitivity is approximately 90% for diagnosis of PAD. (See Question 4 for further details.)
 Pulse volume recordings (PVRs): Pulse volume recordings measure changes in volume of toes, fingers, or parts of limbs that occur with each pulse beat as blood flows into or out of the extremity. A toe-to-brachial index of less than 0.6 is abnormal, and values of less than 0.15 are seen in patients with rest pain (toe pressures of less than 20 mm Hg).
Pulse volume recordings (PVRs): Pulse volume recordings measure changes in volume of toes, fingers, or parts of limbs that occur with each pulse beat as blood flows into or out of the extremity. A toe-to-brachial index of less than 0.6 is abnormal, and values of less than 0.15 are seen in patients with rest pain (toe pressures of less than 20 mm Hg).
 Duplex ultrasonography: Duplex ultrasonography is a noninvasive method of evaluating arterial stenosis and blood flow. This method can localize and quantify the degree of stenosis. Ultrasonography is dependent on operator skill.
Duplex ultrasonography: Duplex ultrasonography is a noninvasive method of evaluating arterial stenosis and blood flow. This method can localize and quantify the degree of stenosis. Ultrasonography is dependent on operator skill.
 Transcutaneous oxygen tension measurements: These measurements are useful in assessing tissue viability for wound healing. Measurements greater than 55 mm Hg are considered normal and less than 20 mm Hg are associated with nonhealing ulcers.
Transcutaneous oxygen tension measurements: These measurements are useful in assessing tissue viability for wound healing. Measurements greater than 55 mm Hg are considered normal and less than 20 mm Hg are associated with nonhealing ulcers.
 Exercise testing: This testing determines treadmill walking time and preexercise and postexercise ABI. In those without significant PAD, the ABI is unchanged after exercise. In patients with PAD, the ABI falls after exercise. This test is more sensitive for detecting disease than a resting ABI alone.
Exercise testing: This testing determines treadmill walking time and preexercise and postexercise ABI. In those without significant PAD, the ABI is unchanged after exercise. In patients with PAD, the ABI falls after exercise. This test is more sensitive for detecting disease than a resting ABI alone.
Experts emphasize that the ABI is a continuous variable below 0.90. Values of 0.41 to 0.90 are considered to be mildly to moderately diminished; values of 0.40 or less are considered to be severely decreased. An ABI of 0.40 or less is associated with an increased risk of rest pain, ischemic ulceration, or gangrene. Patients with long-standing diabetes or end-stage renal disease on dialysis and elderly patients may have noncompressible leg arterial segments caused by medial calcification, precluding assessment of the ABI. These patients are best evaluated using digital pressures and with assessment of the quality of the arterial waveform in the PVR studies. A system for interpretation of the ABI is given in Table 55-1.
TABLE 55-1
INTERPRETATION OF THE ANKLE-BRACHIAL INDEX
| ABI | Interpretation |
| >1.30 | Noncompressible |
| 1.00-1.29 | Normal |
| 0.91-0.99 | Borderline (equivocal) |
| 0.41-0.90 | Mild to moderate PAD |
| 0.00-0.40 | Severe PAD |
ABI, Ankle-brachial index; PAD, peripheral arterial disease.
Modified from Hiatt WR: Medical treatment of peripheral arterial disease and claudication, N Engl J Med 344:1608-1621, 2001.
5. What are the recommended medical therapies and lifestyle interventions in patients with lower extremity PAD?
A supervised exercise regimen is recommended as the initial treatment modality for patients with intermittent claudication. Supervised exercise training is recommended over unsupervised exercise training. Cilostazol treatment can lead to a modest increase in exercise capacity. Because agents with similar biologic effects have been shown to increase mortality in patients with heart failure, this drug should not be used in patients with heart failure. Smoking cessation must be strongly emphasized to the patient. Other measures include general secondary prevention interventions. Recommended medical therapies and lifestyle interventions in patients with lower extremity PAD are summarized in Box 55-1. An algorithm for the management of patients with suspected peripheral arterial disease is presented in Figure 55-1.
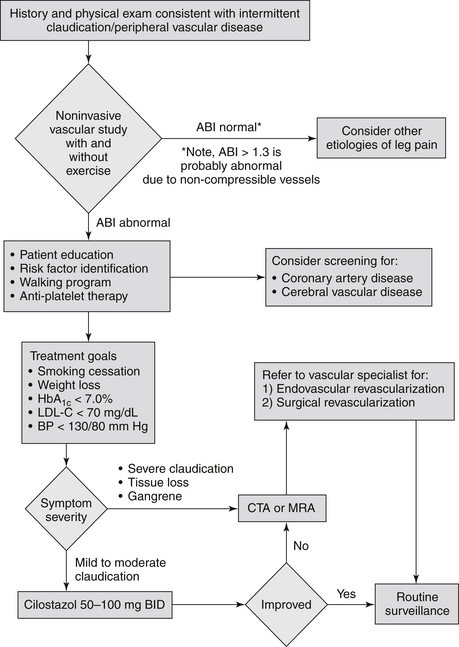
Figure 55-1 Algorithm for the evaluation and management of patients with suspected lower extremity peripheral artery disease. (From Toth PP, Shammas NW, Dippel EJ, et al: Cardiovascular disease. In Rakel RE, editor: Textbook of family medicine, ed 7, Philadelphia, 2007, Saunders.) ABI, Ankle-brachial index; BP, blood pressure; CTA, computed tomographic angiography; Hg, glycated hemoglobin; LDL, low-density lipoprotein; MRA, magnetic resonance angiography.
6. What are the interventional treatment options for patients with claudication?
Claudication that severely interferes with quality of life or employment should be treated. Endovascular and open surgical reconstruction have both been extensively used for this purpose. Endovascular options are less invasive, typically performed on an outpatient basis, and are associated with lower complication rates. Open surgical options are more durable and best suited for good risk or young patients. Outcomes of either type of intervention are vascular-bed dependent. Iliac stenting has been associated with 5-year patency rates that in most cases are only slightly inferior to that of their open counterparts. Endovascular intervention in the infrainguinal segment, however, is associated with inferior patency, particularly when compared to open bypass using venous conduit.
7. What is critical limb ischemia (CLI) and how is it graded clinically?
Whereas claudication is produced by decreased perfusion to the muscles upon increased demand, CLI refers to inadequate tissue perfusion at rest and is manifested as rest pain or tissue loss. Patients with CLI have multilevel disease that typically involves iliac, femoral, and tibial arteries. Due to extent of the disease and the coexistent comorbidities, the management of the patient with CLI involves substantial judgment. Hybrid procedures that include simultaneous open and endovascular components, multiple debridements, and extensive rehabilitation therapy programs are fairly typical. Best results are achieved with multidisciplinary approaches that involve interventionalists, surgeons, internists, podiatrists, and infectious disease and endocrine specialists, among others. Isolated vessel-based intervention in the absence of a grand plan for overall patient management should be discouraged, as most of these patients benefit from coordinated treatment in centers familiar with the intricacies and the issues surrounding the management of CLI. One widely used scheme for classifying limb ischemia is given in Table 55-2.
TABLE 55-2
CLINICAL CATEGORIES OF CHRONIC LIMB ISCHEMIA
| Grade | Category | Clinical Description |
| 0 | Asymptomatic, not hemodynamically correct | |
| I | 1 | Mild claudication |
| 2 | Moderate claudication | |
| 3 | Severe claudication | |
| II | 4 | Ischemic rest pain |
| 5 | Minor tissue loss: nonhealing ulcer, focal gangrene with diffuse pedal ulcer | |
| III | 6 | Major tissue loss extending above transmetatarsal level, functional foot no longer salvageable |
From Rutherford RB, Baker JD, Ernst C, et al: Recommended standards for reports dealing with lower extremity ischemia: revised version, J Vasc Surg 26:517, 1997.
8. What are the main complications of open and endovascular infrainguinal interventions?
Complications of open interventions include cardiac events, respiratory complications, bleeding, wound infection, hernias, and graft failure. Complications associated with endovascular procedures include access site hematoma, bleeding or pseudoaneurysm, vessel rupture, contrast-induced nephropathy or anaphylactic reactions, recurrent stenosis or occlusion, and radiation-related patient injury. Thrombolytic treatment in particular is associated with increased risk of intracavitary, extremity, or intracranial bleeding, which is heavily dependent on the thrombolytic dose and duration of administration.
9. What are the causes of renal artery stenosis (RAS)?
10. What are ACC/AHA class I indications for the referral for diagnostic study to identify clinically significant RAS?
 Onset of hypertension before age 30
Onset of hypertension before age 30
 Onset of hypertension after age 55
Onset of hypertension after age 55
 Accelerated hypertension (sudden and persistent worsening of previously controlled hypertension), resistant hypertension (failure to achieve blood pressure goal with full adherences to full doses of an appropriate three-drug regimen that includes a diuretic), and malignant hypertension (hypertension with acute end-organ damage)
Accelerated hypertension (sudden and persistent worsening of previously controlled hypertension), resistant hypertension (failure to achieve blood pressure goal with full adherences to full doses of an appropriate three-drug regimen that includes a diuretic), and malignant hypertension (hypertension with acute end-organ damage)
 New azotemia or worsening renal function after angiotensin-converting enzyme (ACE) inhibitor or angiotensin-receptor blocker (ARB) treatment
New azotemia or worsening renal function after angiotensin-converting enzyme (ACE) inhibitor or angiotensin-receptor blocker (ARB) treatment
 Unexplained atrophic kidney or discrepancy in size between the two kidneys of more than 1.5 cm
Unexplained atrophic kidney or discrepancy in size between the two kidneys of more than 1.5 cm
 Sudden, unexplained pulmonary edema (especially in azotemic patients)
Sudden, unexplained pulmonary edema (especially in azotemic patients)
11. What are the main indications for RAS percutaneous revascularization?
Most ACC/AHA indications for percutaneous revascularization are class IIa recommendations, meaning the procedure is reasonable.
 Hemodynamically significant RAS and recurrent, unexplained congestive heart failure or sudden, unexplained pulmonary edema
Hemodynamically significant RAS and recurrent, unexplained congestive heart failure or sudden, unexplained pulmonary edema
 Hemodynamically significant RAS and accelerated hypertension, resistant hypertension, malignant hypertension, hypertension with an unexplained unilateral small kidney, and hypertension with intolerance to medication
Hemodynamically significant RAS and accelerated hypertension, resistant hypertension, malignant hypertension, hypertension with an unexplained unilateral small kidney, and hypertension with intolerance to medication
 Hemodynamically significant RAS and unstable angina
Hemodynamically significant RAS and unstable angina
 RAS and progressive chronic kidney disease with bilateral RAS or a RAS to a solitary functioning kidney
RAS and progressive chronic kidney disease with bilateral RAS or a RAS to a solitary functioning kidney
 Although percutaneous intervention is generally the procedure of choice for lesions and disease involving the proximal and main renal artery, surgical revascularization is generally recommended for those cases with complex disease involving branching or segmental arteries or when aortic surgery is also indicated.
Although percutaneous intervention is generally the procedure of choice for lesions and disease involving the proximal and main renal artery, surgical revascularization is generally recommended for those cases with complex disease involving branching or segmental arteries or when aortic surgery is also indicated.
12. What are the most common types of visceral artery aneurysms?
13. In general, when should patients with an infrarenal or juxtarenal abdominal aortic aneurysm (AAA) undergo repair?
14. What are the relative pros and cons of the treatment options for patients with infrarenal AAA that meets size criteria for repair?
15. What are the anatomic eligibility criteria for endovascular infrarenal AAA repair?
 Proximal aortic neck of at least 15 mm in length and no more than 32 mm in diameter
Proximal aortic neck of at least 15 mm in length and no more than 32 mm in diameter
 Angulation of aortic neck less than 60 degrees
Angulation of aortic neck less than 60 degrees
 Iliac vessels of adequate diameter to accommodate the delivery device
Iliac vessels of adequate diameter to accommodate the delivery device
16. What are the primary indications for treatment of extracranial carotid artery occlusive disease?
In very general terms, indications for intervention are as follows:
 Symptomatic stenosis 50% to 99% diameter if the risk of perioperative stroke or death is less than 6%
Symptomatic stenosis 50% to 99% diameter if the risk of perioperative stroke or death is less than 6%
 Asymptomatic stenosis greater than 60% in diameter if the expected perioperative stroke rate is less than 3% and if life expectancy is greater than 2 years
Asymptomatic stenosis greater than 60% in diameter if the expected perioperative stroke rate is less than 3% and if life expectancy is greater than 2 years
17. What are the relative indications for carotid endarterectomy (CEA) and carotid artery stenting (CAS)?
Carotid stenting indications include the following:
 High carotid lesion not approachable via neck incision using standard techniques
High carotid lesion not approachable via neck incision using standard techniques
18. What are possible causes of lower limb arterial disease and ischemia or claudication in young patients?
19. What is fibromuscular dysplasia (FMD)?
 Multifocal type, with multiple stenoses and the “string-of-beads” appearance that is classic for medial fibroplasias
Multifocal type, with multiple stenoses and the “string-of-beads” appearance that is classic for medial fibroplasias
21. What is Takayasu arteritis?
Bibliography, Suggested Readings, and Websites
1. Blankensteijn, J.D., de Jong, S.E., Prinssen, M., et al. Two-year outcomes after conventional or endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2005;352:2398–2405.
2. Brott, T.G., Halperin, J.L., Abbara, S., et al, JASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. Circulation 2011;124:489–532 Stroke 42:e4120–e463, 2011; and J Am Coll Cardiol 57:1002–1044, 2011
3. Brott, T.G., Hobson, R.W., 2nd., Howard, G., et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. New Engl J Med. 2010;363:11–23.
4. Eckstein, H.H., Ringleb, P., Allenberg, J.R., et al. Results of the stent-protected angioplasty versus carotid endarterectomy (space) study to treat symptomatic stenoses at 2 years: a multinational, prospective, randomised trial. Lancet Neurol. 2008;7:893–902.
5. Greenhalgh, R.M., Brown, L.C., Kwong, G.P., Powell, J.T., Thompson, S.G. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: Randomised controlled trial. Lancet. 2004;364:843–848.
6. Hiatt, W.R. Medical treatment of peripheral arterial disease and claudication. N Engl J Med. 2001;344:1608–1621.
7. Hirsch, A.T., Haskal, Z.J., Hertzer, N.R., et al. ACC/AHA guidelines for the management of patients with peripheral arterial disease. J Am Coll Cardiol. 2006;47:1239–1312.
8. Norgren, L., Hiatt, W.R., Dormandy, J.A., et al. Inter-society consensus for the management of peripheral arterial disease (tasc ii). Eur J Vasc Endovasc Surg. 2007;33(Suppl 1):S1–S5.
9. Lederle, F.A., Freischlag, J.A., Kyriakides, T.C., et al. Outcomes following endovascular vs open repair of abdominal aortic aneurysm: A randomized trial. JAMA. 2009;302:1535–1542.
10. Mas, J.L., Trinquart, L., Leys, D., et al. Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial: results up to 4 years from a randomised, multicentre trial. Lancet Neurol. 2008;7:885–892.
11. Murad, M.H., Shahrour, A., Shah, N.D., et al. A systematic review and meta-analysis of randomized trials of carotid endarterectomy vs stenting. J Vasc Surg. 2011;53:792–797.
12. Norgren, L., Hiatt, W.R., Dormandy, J.A., et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Eur J Vasc Endovasc Surg. 2007;33(Suppl 1):S1–S75.
13. Ricotta, J., AbuRahma, A., Ascher, E., et al. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease. J Vasc Surg. 2011;54:832–836.
14. Ringleb, P.A., Allenberg, J., Bruckmann, H., et al. 30 day results from the space trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet. 2006;368:1239–1247.
15. Sacco, R.L., Adams, R., Albers, G., et al. AHA/ASA guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack. Stroke. 2006;37:577–617.
16. White, C.J., Jaff, M.R., Haskal, Z.J., et al. Indications for renal arteriography at the time of coronary arteriography: a science advisory from the American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology, and the Councils on Cardiovascular Radiology and Intervention and on Kidney in Cardiovascular Disease. Circulation. 2006;114:1892–1895.
17. Yadav, J.S., Wholey, M.H., Kuntz, R.E., et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. New Engl J Med. 2004;351:1493–1501.