Chapter 10 Peripheral Applications of Ultrasonography for Chronic Pain
 Applying ultrasonography for injection of peripheral structures in chronic pain is a very useful technique because it offers an affordable and portable device that provides visualization of the target structures.
Applying ultrasonography for injection of peripheral structures in chronic pain is a very useful technique because it offers an affordable and portable device that provides visualization of the target structures. In contrast, fluoroscopy is not designed to visualize various soft tissues in the injection of the peripheral structures.
In contrast, fluoroscopy is not designed to visualize various soft tissues in the injection of the peripheral structures. Ultrasound-guided injections of peripheral structures have mostly been validated in the literature, showing with high accuracy in reaching the target structures, in contrast to the poor location of target structures with conventional landmark-based techniques.
Ultrasound-guided injections of peripheral structures have mostly been validated in the literature, showing with high accuracy in reaching the target structures, in contrast to the poor location of target structures with conventional landmark-based techniques. The ideal injection site for the SSN is along the course of the nerve between the suprascapular and spinoglenoid notches, where the nerve is contained in a compartment.
The ideal injection site for the SSN is along the course of the nerve between the suprascapular and spinoglenoid notches, where the nerve is contained in a compartment. The risk of pneumothorax with intercostal nerve injection is real because the distance between the intercostal nerve and pleura is usually less than 0.5 cm. The ideal injection site is at the costal angle where the nerve is in between two layers of muscle in the subcostal groove.
The risk of pneumothorax with intercostal nerve injection is real because the distance between the intercostal nerve and pleura is usually less than 0.5 cm. The ideal injection site is at the costal angle where the nerve is in between two layers of muscle in the subcostal groove. Although it is abundantly clear that the course of the IL and IH nerves is highly variable medial to anterior superior iliac spine (ASIS) and is very constant lateral to ASIS, all landmark-based techniques proposed the needle insertion site medial to ASIS with a high failure rate. Ultrasonography allows accurate injection of the IL and IH nerves lateral to the ASIS.
Although it is abundantly clear that the course of the IL and IH nerves is highly variable medial to anterior superior iliac spine (ASIS) and is very constant lateral to ASIS, all landmark-based techniques proposed the needle insertion site medial to ASIS with a high failure rate. Ultrasonography allows accurate injection of the IL and IH nerves lateral to the ASIS. The course of GF nerve is highly variable, but the genital branch consistently transverses the inguinal canal. Ultrasonography can accurately locate the inguinal canal and the spermatic cord, allowing blockade of the genital branch of GF nerve.
The course of GF nerve is highly variable, but the genital branch consistently transverses the inguinal canal. Ultrasonography can accurately locate the inguinal canal and the spermatic cord, allowing blockade of the genital branch of GF nerve. The lateral femoral cutaneous nerve is a small peripheral nerve with a variable course in the infrainguinal region. Experience is required to locate this nerve with ultrasonography.
The lateral femoral cutaneous nerve is a small peripheral nerve with a variable course in the infrainguinal region. Experience is required to locate this nerve with ultrasonography.Introduction
Application of ultrasonography in pain medicine (USPM) is a rapidly growing medical field in interventional pain management. It is evidenced by the remarkable increase in the publication of literature on ultrasound-guided injection1 and the remarkable increase in the number of USPM workshops held in the past 3 years in North America, Europe, and Asia.
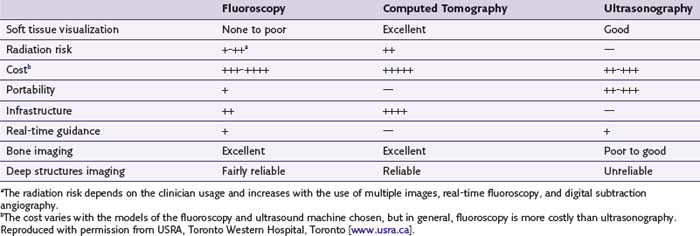
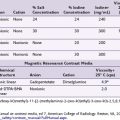
Traditionally, interventional procedures for pain management are performed either by a landmark-based technique or with imaging guidance such as fluoroscopy and computed tomography (CT) scanning. A comparison of the advantages and disadvantages of various imaging equipment is summarized in Table 10-1. Based on the target structures, the application of USPM can be classified into three areas: peripheral, axial, and musculoskeletal (Table 10-2). Applying ultrasonography for the injection of peripheral structures is particularly useful because it allows the visualization of various soft tissues (nerve, muscle, tendon, or vessels), and most of the interventional pain procedures for peripheral structures are performed without imaging guidance. This chapter reviews the relevant anatomy, sonoanatomy, and the injection techniques of a few peripheral structures.
Suprascapular Nerve
First described in 1941,2 suprascapular nerve (SSN) block has been performed over the years by anesthesiologists, rheumatologists, and pain specialists to manage the pain that follows trauma3 or shoulder surgery,4,5 to ameliorate the pain associated with various chronic shoulder pain syndromes (adhesive capsulitis, frozen shoulder, rotator cuff tear, and glenohumeral arthritis),6–9 and to aid in the diagnosis of suprascapular neuropathy.10
Anatomy and Sonoanatomy
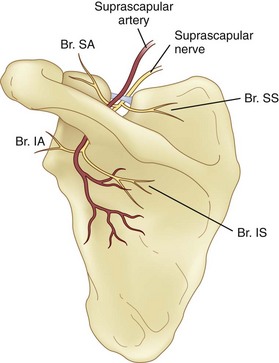
The SSN originates from the superior trunk of the brachial plexus (formed by the union of the fifth and sixth cervical nerves). It then runs parallel to the omohyoid muscle and courses under the trapezius before it passes under the transverse scapular ligament in the suprascapular notch (Fig. 10-1). Entering the suprascapular fossa, the SSN passes beneath the supraspinatus and curves around the lateral border of the spine of the scapula (spinoglenoid notch) to the infraspinatus fossa (Fig. 10-2). In the supraspinatus fossa, it gives off two branches: one to the supraspinatus muscle and another as an articular branch to the shoulder joint; and in the infraspinatus fossa, it gives off branches to the infraspinatus muscle and to the shoulder joint and scapula. The sensory component of the SSN provides fibers to about 70% of the shoulder joint. There is no significant cutaneous branch of this nerve.
The suprascapular notch is located on the superior margin of the scapula and medial to the coracoid process. The size and shape of the notch are highly variable and even absent in up to 8% of cadavers.11 Above the transverse scapular ligament run the suprascapular artery and vein, although rarely, the artery travels along with the SSN through the notch. The suprascapular fossa is bordered by the spine of the scapula dorsally, by the plate of the scapula ventrally, and by the supraspinatus fascia superiorly, forming a classic compartment, the only exit through which is the suprascapular notch.
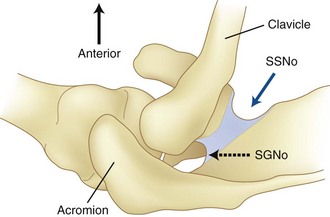
The ideal site to perform the SSN injection is at the floor of the scapular spine between the suprascapular notch and spinoglenoid notch (Fig. 10-2).12 First, this technique is independent of the notch as a target. Thus, it avoids the risk of pneumothorax if one considers the direction of the needle. This technique is also feasible in individuals without a suprascapular notch (8% of the population). Second, the suprascapular fossa forms a compartment and retains the local anesthetic around the nerve with a small volume.13 In contrast, depositing local anesthetic at the notch level will potentially result in the spread of local anesthetic to the brachial plexus.13 Third, although imaging the suprascapular notch is possible, advancing the needle perfectly in plane in this orientation is very challenging (Fig. 10-3). A slight deviation in the anterior direction will direct the needle toward the thorax.
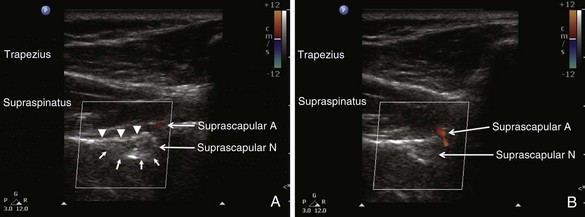
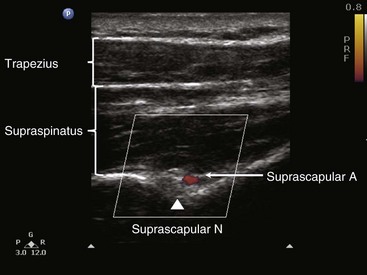
When imaging the suprascapular fossa with ultrasonography, the two key muscles in the scan are trapezius and supraspinatus muscles (Fig. 10-4). The SSN is often seen accompanied by the suprascapular artery on the floor of the scapular spine between the suprascapular notch and spinoglenoid notch (Fig. 10-5). The scapula spine forms an angle (39.5 degrees ± 5.8 degrees) to the axis of the scapula blade,14 so the orientation of the ultrasonography probe should be closer to the coronal plane to visualize the contents of the suprascapular fossa (Fig. 10-6).
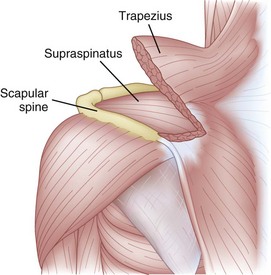
Fig. 10-4 Left shoulder showing the muscle layers in the suprascapular fossa.
(Modified with permission from USRA, Toronto Western Hospital, Toronto [www.usra.ca].)
Existing Techniques
Various approaches have been described. In general, the targets for the SSN are either at the suprascapular notch itself or in the suprascapular fossa.1 To direct the needle to these targets, various methods have been used, including a “blind” insertion using various landmarks,2 a peripheral nerve stimulator3 or electromyography,7 and a direct insertion using fluoroscopic15 or CT scan guidance.16
There are a few disadvantages to targeting the SSN at the notch using the blind or landmark-guided approach, including the risk of pneumothorax, intravascular injection, and nerve injury.17 In an attempt to evaluate needle tip placement radiologically following a “blind” needle placement, Brown et al18 demonstrated that the proximity of the “needle tip-to-notch” was poor. The precision of the needle tip location can be improved by fluoroscopy or CT scan guidance. Placing the needle into the suprascapular fossa is a popular alternative.13 The technique is easy to perform and further minimizes the risk of pneumothorax because of the direction of the needle. To ensure that the SSN is blocked, an adequate volume of solution is injected into the suprascapular fossa compartment. A recent CT scan study showed that 10-mL injectate spread to the brachial plexus in the axilla in three of 33 cadavers.13 They also showed that blind injection could lead to placement of the needle in or even above the supraspinatus muscle.
Four articles described the sonoanatomy of the SSN relevant to the nerve injection.12,19–21 Most of them19–21 described the visualization of the suprascapular notch, transverse scapular ligament, and suprascapular artery and nerve. The ultrasound probe was placed in the suprascapular fossa in the long axis of supraspinatus muscle. According to some authors19,20 the ultrasound image showed the suprascapular notch (as a curved continuous hyperechoic line), transverse scapular ligament, and SSN. A correlation study with fluoroscopy and cadaver dissection suggested the site of injection was actually at the suprascapular fossa between the suprascapular notch and spinoglenoid notch (Fig. 10-2).12
Ultrasound-Guided Injection Technique
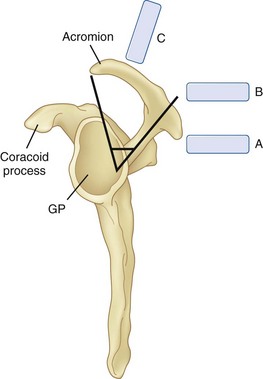
With the patient in sitting or prone position, ultrasound scanning is performed with a linear ultrasound probe (7-13 MHz) placed in a coronal plane over the suprascapular fossa with a slight anterior tilt. The probe is place in an orientation such that it is in the short axis to the line joining medial aspect of the coracoid process (approximating the suprascapular notch) and posterior aspect of the acromion (reflecting the position of the spinoglenoid notch). The supraspinatus and trapezius muscles and the bony fossa underneath them should come into view (Fig. 10-5). By adjusting the angle of the ultrasound probe in a cephalocaudad direction, the SSN and artery should be brought into view in the trough of the floor (Fig. 10-5). The nerve can sometimes be difficult to visualize because of the orientation. A 22-gauge, 80-mm needle is inserted in plane from the medial aspect of the probe because of the presence of the acromion process on the lateral side. Because of the proximity of the nerve, an injectate volume of 5 to 8 mL is usually sufficient.
Clinical Pearls and Pitfalls
Intercostal Nerve
The intercostal nerves (ICNs) supply skin and musculature of chest and abdominal wall. Blockade of ICNs has been in clinical use for decades for treatment of acute and chronic pain conditions affecting the thorax and upper abdomen.22 ICN blockade provides excellent analgesia for pain from rib fractures23 and from chest and upper abdominal surgery.24 Neuroablation of ICN may be used to manage chronic pain conditions such as postmastectomy and postthoracotomy pain.25–28
Anatomy and Sonoanatomy
The ICNs are the ventral rami of the 12 thoracic nerves (Fig. 10-7, A). After exiting from the spine, the ICNs are located between the pleura and the internal intercostal membrane and subsequently traverse the membrane. Between costal tubercles and costal angles, the ICNs lie between the external intercostal muscle and internal intercostal membrane, which are immediately adjacent to the parietal pleura. The course of ICNs for that distance really deserves their name “intercostal” nerve (or “in between ribs”). From the costal angle onward, the ICNs run deep to the subcostal groove within the internal intercostal muscles, thus creating an innermost muscular layer known as the innermost intercostals muscle (Fig. 10-7, B).29,30 Readers should be aware that in some anatomy textbooks, the ICNs are described to situate in between the two layers of internal intercostal muscle. The ICNs travel in the costal groove accompanied by intercostal vein and artery and the neurovascular bundle is arranged from above downwards in the manner: “V-A-N” (Fig. 10-8). At a distance about 5 to 8 cm anterior to the angle of the rib, the groove ends and blends into the surface of the lower edge of the rib. The lateral cutaneous branch of the ICN, which supplies the skin of the chest, branches off and pierces the external intercostal muscle in the region between the posterior and mid-axillary line. As the ICN approaches the midline anteriorly, it pierces the overlying muscles and skin to terminate as the anterior cutaneous branch (Fig. 10-7, A).
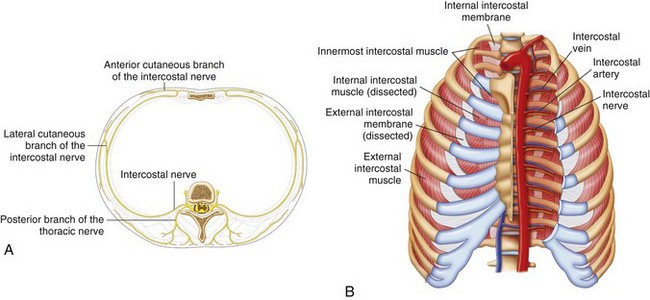
Fig. 10-7 A, Branches of the typical intercostal nerves. B, Intercostal muscles in the chest wall.
(Reprinted with permission from USRA, Toronto Western Hospital, Toronto [www.usra.ca].)
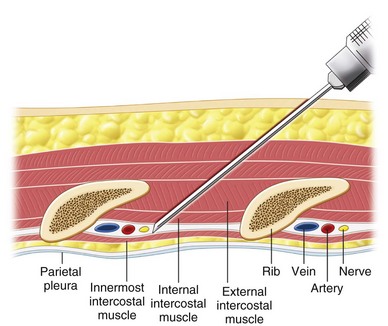
Fig 10-8 Cross section of chest wall showing intercostal muscles and neurovascular bundles.
(Reprinted with permission from USRA, Toronto Western Hospital, Toronto [www.usra.ca].)
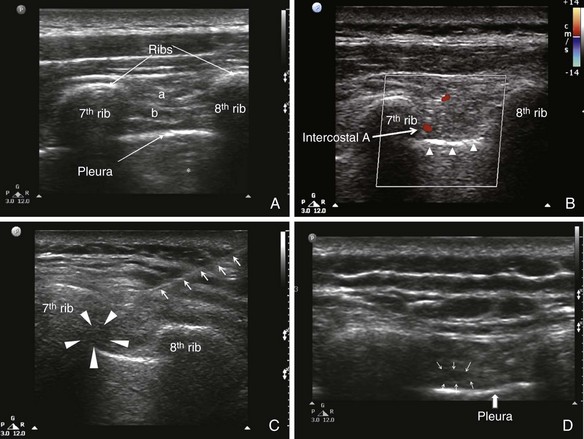
Under ultrasound scanning, different layers of the intercostal muscles and the pleura can be visualized (Fig. 10-9). The pleura appear as a definite hyperechogenic line that glides with respiratory movement. Normally, two types of artifacts can be visualized: reverberation artifacts appearing as a series of horizontal lines parallel to the pleural interface and vertical comet tail artifacts. The neurovascular bundle cannot be visualized under normal circumstances because it is covered by the rib except in the region between costal tubercles to costal angle. However, the location of the ICN can be estimated by directing the needle in between the internal and innermost intercostal muscle.
Existing Techniques
The classic landmark-based technique is performed with the patient in the sitting position. The ICN block is usually performed at the angle of the rib to ensure that the tissues innervated by the lateral cutaneous nerve are blocked. The most feared complication with the existing “blind” technique is pneumothorax. The distance between the neurovascular bundles to the pleura in thin patients is usually within 0.5 cm.26 The injection is performed after negative aspiration for air and blood, but this maneuver cannot reliably prevent pneumothorax or hemothorax . The incidence of pneumothorax ranges anywhere from 0.09% to 8.7%.22,31,32
The fluoroscopic technique is performed with the patient in the prone position. The appropriate rib is identified under fluoroscopic anteroposterior (AP) view, and the needle is introduced in the inferior margin of the rib. After negative aspiration, a contrast injection is performed to ensure appropriate spread before injection.27 This technique does not theoretically minimize the risk of pneumothorax because the pleura cannot be visualized with fluoroscopy.
The use of ultrasound guidance for intercostal block was recommended from the personal experiences in two reviews.1,33 Either the in-plane or out-of-plane technique was recommended. However, both reviews emphasized hydrodissection to ensure the visualization of the needle tip. A small case series also confirmed the feasibility and technical advantages of ultrasound-guided cryoablation of the ICNs in four patients with postthoracotomy pain syndrome.26
Ultrasound-Guided Injection Technique
The patient is in the prone position, and a linear probe (7-13 MHz) is ideal for the superficial structures. The site is at the costal angle (≈7 cm from the midline). Both the in-plane and out-of-plane technique can be used. The authors prefer the in-plane technique because the direction of the needle is similar to the classical technique described for ICN block, and the complete needle path can be traced.1 The needle entry site is the upper margin of the rib one level caudal to the targeted ICNs. After the needle penetrates the skin, the ultrasound probe can be rolled over the needle, which is advanced deep to the internal intercostal muscle. One of the drawbacks for the in-plane technique is that when the probe is not perfectly in line with the needle, the practitioner may have a false impression of the location of the needle tip. Because the distance between the costal groove and the pleura is in the dimension of 0.5 cm, it is advisable to inject a small amount of injectate when the needle is in the external intercostal muscle to confirm the needle tip position. Under real-time injection, intravascular injection should be suspected if the spread of the medication is not visualized. After the needle tip is confirmed deep to the internal intercostal muscle, the local anesthetic can be injected, and spread of medication can be seen.
Clinical Pearls and Pitfalls
 The course of ICNs is “intercostal” from the costal tubercle to costal angle (i.e., between the ribs). Although one can locate the intercostal artery and thus the neurovascular bundle, the ICNs are adjacent to the parietal pleura. Injection at this site is recommended only for very experienced practitioners. The author recommends the injection site at the costal angle.
The course of ICNs is “intercostal” from the costal tubercle to costal angle (i.e., between the ribs). Although one can locate the intercostal artery and thus the neurovascular bundle, the ICNs are adjacent to the parietal pleura. Injection at this site is recommended only for very experienced practitioners. The author recommends the injection site at the costal angle.Ilioinguinal, Iliohypogastric, and Genitofemoral Nerves (Border Nerves)
The ilioinguinal (IL), iliohypogastric (IH), and genitofemoral (GF) nerves are the primary nerves providing sensory innervation to the skin bordering between the thigh and abdomen. Therefore, they are also called border nerves collectively.1 Injury to the IL and IH nerves is a known risk in open appendectomy incisions, postinguinal herniorrhaphy, low transverse incisions (e.g., Pfannenstiel incision), and during trocar insertion for laparoscopic surgery of the abdomen and pelvis.34–38 Patients with neuropathy after injury to these nerves present with groin pain that may extend to the scrotum or the testicle in men, the labia majora in women, and the medial aspect of the thigh. Accurate diagnostic block of those nerves is important in understanding the etiology of the clinical problem.
Anatomy and Sonoanatomy
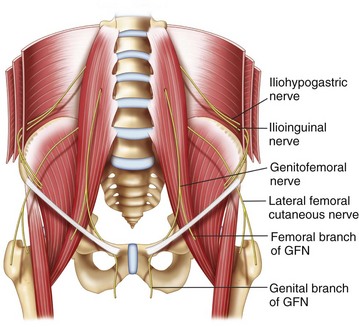
The IL and IH nerves originate from the T12 and L1 nerve roots. Emerging near the lateral border of the psoas major muscle, the nerves extend diagonally toward the crest of the ilium (Fig. 10-10).
The IH nerve pierces the transversus abdominis muscle above the iliac crest midway between the iliac crest and the twelfth rib. The IL nerve runs caudally and parallel to the IH nerve. Here, both nerves can be found consistently (90%) between the transversus abdominis and internal oblique muscles.39 Terminal branches of the IH nerve perforate the external oblique muscle aponeurosis 4 cm lateral to the midline to supply the skin over the lower portion of the rectus abdominis.40 The IH nerve also provides sensory innervation to the skin above the tensor fasciae lata through a lateral cutaneous branch. Terminal branches of the IL nerve enter the inguinal canal through the deep inguinal ring and may lie upon the cremasteric muscle and fascial layer of the spermatic cord in men or round ligament in women.41,42 This terminal branch is often accompanied by the genital branch of the GF nerve, and wide variations in the course of these nerves within the inguinal canal have been documented.4,8,10,12–14 The terminal sensory branches may innervate the skin of the mons pubis, inner thigh, inguinal crease, and anterior surface of the scrotum or anterior third of the labia.
The GF nerve originates from the first and second lumbar nerve roots (Fig. 10-10). It emerges on the anterior surface of the psoas muscle either a single trunk or separate genital and femoral branches.43 The femoral branch passes laterally over the external iliac artery and then penetrates the fascia lata to enter the femoral sheath. Terminal branches provide cutaneous innervation to the femoral triangle.44 The genital branch also crosses in front of the external iliac artery but then passes through the ventral aspect of the internal ring of the inguinal canal. Anatomical studies describe this branch running between the cremaster and internal spermatic fascia, incorporating with the cremasteric fascia, or lying outside of the spermatic cord.42,44,45 Terminal sensory branches may innervate the scrotum and possibly the upper, inner, and medial thigh.42
Anatomical studies highlight the variability of the IL, IH, and GF nerves. Variations from conventional descriptions have been reported with respect to communication among nerves, penetration of fascial layers, branching patterns, and dominance patterns.1 The most consistent anatomical location for the IL and IH nerves to perforate the abdominal muscular layers is lateral and superior to the anterior superior iliac spine (ASIS), where they run between the transversus abdominis and internal oblique muscular layers.
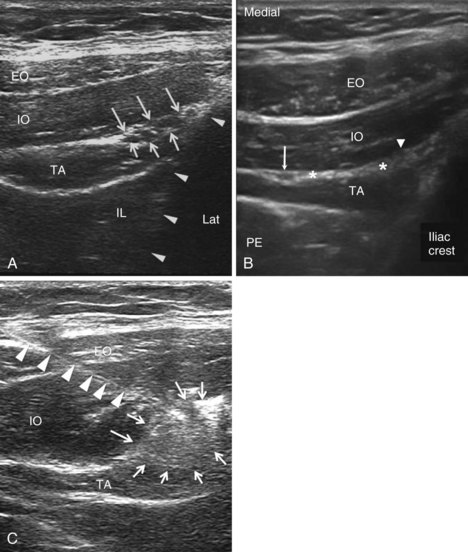
The recommended area for ultrasound scanning of IL and IH nerves is lateral and superior to the ASIS (Fig. 10-11). At this position, the iliac crest appears as a hyperechoic structure adjacent to which appear the three muscular layers of the abdominal wall (Fig. 10-12). Below the transversus abdominis, peristaltic movements of the bowel may be detected. After the muscular layers have been identified, the II and IH nerves will be found in the split fascial plane between the internal oblique and transversus abdominis muscle layers. Both nerves should be within 1.5 cm of the iliac crest at this site, with the II nerve closer to the iliac crest.46 The nerves are usually in close proximity to each other47 and located on the “upsloping” split fascia close to the iliac crest. In some cases, the nerves may run approximately 1 cm apart. The deep circumflex iliac artery that is close to the two nerves in the same fascial layer can be revealed with the use of color-flow Doppler (Fig. 10-13). A neural structure within the fascial split may also be seen medial and on the flat part of the internal oblique and transversus abdominis muscle junction. This is the subcostal nerve, and if mistaken for the IL and IH nerves, the nerve blockade will result in aberrant distribution of anesthesia.
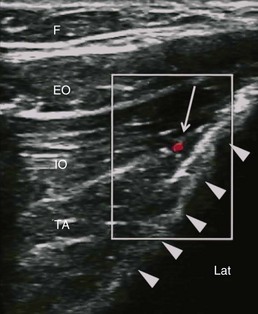
Fig. 10-13 Similar view as in Fig. 10-12, A with color-flow Doppler. A branch of the deep circumflex iliac artery is shown in red. Solid arrows outline the iliac crest. EO, external oblique muscle; F, subcutaneous fat; IO, internal oblique muscle; Lat, lateral; TA, transverse abdominis muscle.
(Reprinted with permission from USRA, Toronto Western Hospital, Toronto [www.usra.ca].)
Existing Technique
As discussed previously, there is a high degree of anatomical variability in not only the course of the nerves but also their branching patterns, areas of penetration of the fascial layers, and dominance patterns.1 The sites at which the IL and IH nerves pierce the abdominal wall muscle layers are significantly variable.36 Unfortunately, virtually all injection techniques describe the landmark for injection medial to the ASIS.1,48 By far the most consistent location of the II and IH nerves is lateral and superior to the ASIS where the nerves are found between the transverses abdominis and internal oblique muscular layers (90%).39,40
Because the existing landmark-based techniques rely on blind infiltration of local anesthetic through different layers, the risk of complications are well understandable. These include inadvertent femoral nerve block,49,50 colonic puncture,51,52 and vascular injury.53 More importantly, the failure rate of the blind technique lies in the range of 10% to 40%.54,55 This failure rate is attributed to the potential for injecting local anesthetic into the wrong abdominal plane.55
The use of ultrasonography in IL and IH nerves has been evaluated in clinical practice in adults and validated in a cadaver study.46,56–58 All three clinical studies or reports used ASIS as landmark, and the position of the probe was above ASIS and moved along the line joining the ASIS and umbilicus. When the probe is placed too near to ASIS, the external oblique muscle layer may be missed. When the probe is too far away from the ASIS (the “bone shadow of iliac crest” not in the scan), the nerve seen in between the transverses abdominis and internal oblique muscular layers is likely the twelfth intercostal nerve instead.
The description of a GF nerve block is mainly a “blind” technique59,60 and relies on the pubic tubercle, inguinal ligament, inguinal crease, and femoral artery as landmarks. One involves infiltration of local anesthetic immediately lateral to the pubic tubercle caudad to the inguinal ligament.60 In another method, a needle is inserted into the inguinal canal to block the genital branch.59 The blind techniques described are essentially infiltration techniques and rely on high volumes of local anesthetic for consistent results. Although the basis of this landmark is not clear, the needle is likely directed toward the spermatic cord, and important structures of the spermatic cord (testicular artery and vas deferens) or the peritoneum are at risk.
Ultrasound-guided blockade of the genital branch of the GF nerve has been described in several review articles.1,48,61 The genital nerve is difficult to visualize, and blockade is achieved by identification of the inguinal canal. In males, the GF nerve may travel within or outside the spermatic cord. Thus, the local anesthetic and steroid is deposited both outside and within the spermatic cord. Ultrasound-guided blockade of the femoral branch of the GF nerve has also been described, and cryoanalgesia of the femoral branch of GF nerve was performed.62
Ultrasound-Guided Injection Technique
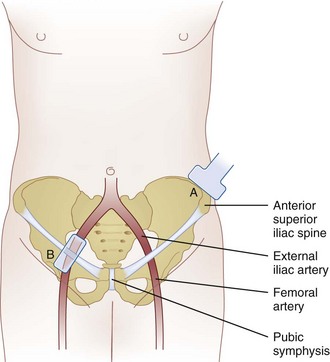
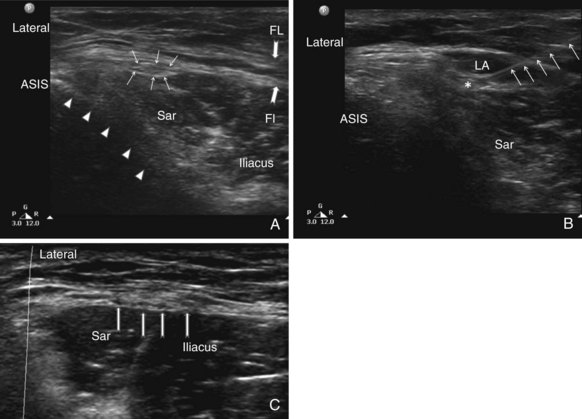
The IH and IL nerves are superficial nerves that are best viewed with a linear probe of high frequency (6-13 MHz). In very obese patients, a linear medium frequency probe may be useful. The probe is placed cranial and three fingerbreadths lateral to the ASIS with the transducer perpendicular to the inguinal ligament and its lateral end in contact with the iliac crest (Fig. 10-11). The hyperechoic shadow of the iliac crest can be visualized, and the probe is then tilted until all three layers of the muscles are brought into view. The peritoneum can be seen as the fascia layer underneath the transversus abdominis. Between the layers of the transversus abdominis and the internal oblique muscles, the IL and IH nerves can be visualized within the splitting of the fascia layer (Fig. 10-12). With Doppler ultrasonography, branches of the deep circumflex iliac artery can be identified within this fascia split (Fig. 10-13). In some patients (those with previous surgery or multiple childbirth), either the IIH and IIN or the fascia split is difficult to visualize; the target will be the fascia plane between the internal oblique and transverse abdominal muscles. Both the out-of-plane and in-plane technique can be used, and the volume of injectate is between 6 and 8 mL.
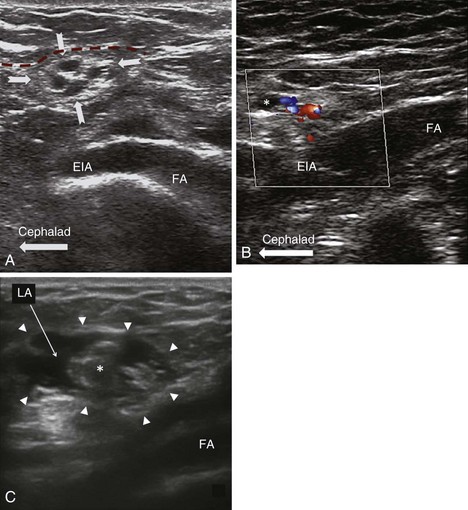
The genital branch is difficult to visualize directly unless a high-frequency probe (≤18 MHz) is used. Furthermore, the GF nerve can be found within or outside the spermatic cord. Thus, the most reliable method is to infiltrate the inguinal canal and deposit the injectate both inside and outside the spermatic cord.1 The patient is placed in a supine position, and the surface anatomy of the ASIS, inguinal ligament, and femoral artery is established. A linear ultrasound transducer of high frequency (6-13 MHz) is placed over the femoral artery along its long axis (Fig. 10-11). The transducer is moved cephalad over the artery, which starts to descend at a steep angle toward the inguinal ligament. At this point, the femoral artery transitions to become the external iliac artery and runs in a retroperitoneal plane (Fig. 10-14). After the external iliac artery is identified, the inguinal canal can be viewed superficial to the vessel as an oval soft tissue structure (Fig. 10-14). This contains the spermatic cord in men and the round ligament in women. The canal is then scanned medially so the final ultrasound probe position is approximately one fingerbreadth away from the pubic tubercle. In males, testicular arteries may be identified in the spermatic cord as pulsatile structures and confirmed with the use of color-flow Doppler (Fig. 10-14). Vessels may be accentuated by asking the patient to cough or perform a Valsalva maneuver, which increases blood flow through the pampiniform plexus. The vas deferens may also be identified as a thick, tubular structure.
Clinical Pearls and Pitfalls
 The IL and IH nerves should always be located two to three fingerbreadths lateral to the ASIS. In patients who are obese, with a history of ventral hernia, multiple childbirths, or abdominal surgeries, the nerves may even be impossible to visualize. However, identification of the three layers of muscles is always possible.
The IL and IH nerves should always be located two to three fingerbreadths lateral to the ASIS. In patients who are obese, with a history of ventral hernia, multiple childbirths, or abdominal surgeries, the nerves may even be impossible to visualize. However, identification of the three layers of muscles is always possible. The practitioner should avoid scanning to medial to the ASIS because the external oblique may be missed.
The practitioner should avoid scanning to medial to the ASIS because the external oblique may be missed. Sometimes a fascia appears in the subcutaneous fat that creates an illusion of two layers with the deeper layer mistaken as the external oblique muscle. Therefore, the practitioner should scan medially and laterally to trace the layer. The fascia in the subcutaneous fat is inconsistent, but the external oblique muscle gets thicker when traced laterally.
Sometimes a fascia appears in the subcutaneous fat that creates an illusion of two layers with the deeper layer mistaken as the external oblique muscle. Therefore, the practitioner should scan medially and laterally to trace the layer. The fascia in the subcutaneous fat is inconsistent, but the external oblique muscle gets thicker when traced laterally. When a nerve is discovered along with an artery in the plane between the internal oblique and the transversus abdominis muscles, it is not necessarily the IL or IH nerve. Both nerves should be within 1.5 inches from the iliac crest. A nerve discovered in a scan farther away from the iliac crest is likely the twelfth ICN or subcostal nerve.
When a nerve is discovered along with an artery in the plane between the internal oblique and the transversus abdominis muscles, it is not necessarily the IL or IH nerve. Both nerves should be within 1.5 inches from the iliac crest. A nerve discovered in a scan farther away from the iliac crest is likely the twelfth ICN or subcostal nerve.Lateral Femoral Cutaneous Nerve
The lateral femoral cutaneous nerve (LFCN) provides sensory innervation to the skin of the anterior and lateral parts of the thigh as far as the knee. Regional block of the LFCN is performed for acute pain relief after surgical procedures and for the diagnosis and treatment of meralgia paresthetica.1 The latter condition is a painful mononeuropathy of the LFCN, which presents with unpleasant paresthesia, pain, and numbness in the anterolateral aspect of the thigh.63 The incidence in a primary care setting was estimated at 4.3 per 10,000 person-years.64 Anesthesiologists are commonly involved in the management of this group of patients because of their expertise in the interventional procedures.1,65
Anatomy and Sonoanatomy
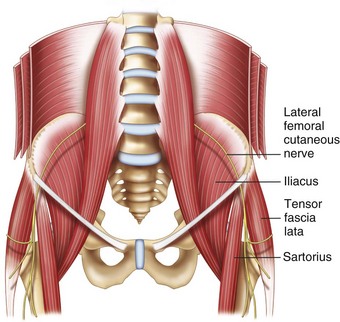
The LFCN is a purely sensory nerve that arises from branches of dorsal divisions of the second and third lumbar nerves (Fig. 10-15). It emerges from the lateral border of the psoas major and crosses the iliacus muscle obliquely toward the ASIS.66 The nerve then passes under the inguinal ligament medial and close to the ASIS (at a distance of 3.6 ± 2.0 cm). Entering the thigh, the LFCN turns laterally and downward, where it typically divides into the anterior and posterior branches (Fig. 10-15).67 The course and location of the LFCN as it crosses the inguinal ligament has been found to be quite variable, such as far medial to ASIS, over the iliac crest, or through the sartorius muscle. Although the nerve courses medial to the ASIS most of the time, it can pass over or even posterior to the ASIS in up to 25% of patients.66–68 Although the LFCN commonly enters the thigh superficial to the sartorius muscle beneath the fascia lata, the LFCN passes through the muscle itself in 22% of cases.69 The LFCN has been shown to cross under the inguinal ligament as far as 4.6 to 7.3 cm medial to the ASIS.67,70,71 The LFCN divides into an anterior and a posterior branch in the thigh. The anterior branch becomes superficial at a variable distance below the inguinal ligament and divides into branches that are distributed to the skin of the anterior and lateral parts of the thigh as far as the knee. The posterior branch pierces the fascia lata and subdivides into filaments that pass backward across the lateral and posterior surfaces of the thigh, supplying the skin from the level of the greater trochanter to the middle of the thigh.
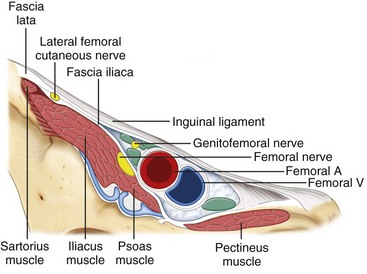
Fig. 10-16 Nerves at the inguinal area. A, artery; V, vein.
(Reprinted with permission from USRA, Toronto Western Hospital, Toronto [www.usra.ca].)
Ultrasound-Guided Injection Technique
With the patient in the supine position, the ASIS and the inguinal ligament are marked on the skin. Using a high-frequency linear array transducer (6-13 MHz), the ASIS is visualized as a hyperechoic structure with posterior acoustic shadowing (Fig. 10-17). The ultrasound probe is placed over the ASIS initially with the long-axis view of inguinal ligament and is then moved distally. The sartorius muscle will be seen as an inverted triangular-shape structure. Attention is paid to the orientation of the probe to the course of the nerve. The LFCN will appear as one or more hyperechoic or hypoechoic structures in the short-axis view. If the nerve cannot be identified, the alternative is to look for the LFCN in the plane between the tensor of the fascia lata and the sartorius muscle. After the LFCN has been identified, a needle is advanced in plane with the ultrasound probe. Alternatively, the needle can be advanced out of plane using a nerve-stimulating needle to confirm placement.
When the LFCN cannot be identified by the above methods, two methods can be tried. One is to inject dextrose 5% solution to hydrodissect the plane between fascia lata and fascia over the iliacus muscle. The other is to locate the proximity of the nerve with a transdermal nerve stimulator to locate the nerve percutaneously.74
Clinical Pearls and Pitfalls
 One common practice for an inexperienced practitioner is to scan close to the ASIS at the inguinal line. Knowing the highly variable course, it may be easier to scan at either (1) two to three fingerbreadths below the ASIS and look for the LFCN in the anterior surface of the sartorius muscle or (2) the fat-containing plane between the sartorius and tensor fascia lata muscle.
One common practice for an inexperienced practitioner is to scan close to the ASIS at the inguinal line. Knowing the highly variable course, it may be easier to scan at either (1) two to three fingerbreadths below the ASIS and look for the LFCN in the anterior surface of the sartorius muscle or (2) the fat-containing plane between the sartorius and tensor fascia lata muscle.Pudendal Nerve
The pudendal nerve supplies the anterior and posterior urogenital areas (clitoris, penis, vulva, and perianal area). Chronic pelvic pain involving the sensory distribution of the pudendal nerve is termed pudendal neuralgia.2 The classical presentation includes pain that is worse with sitting and relieved or diminished on standing, lying on the nonpainful side, or sitting on a toilet seat.2 A pudendal nerve block serves both diagnostic and therapeutic purposes.48
Anatomy and Sonoanatomy
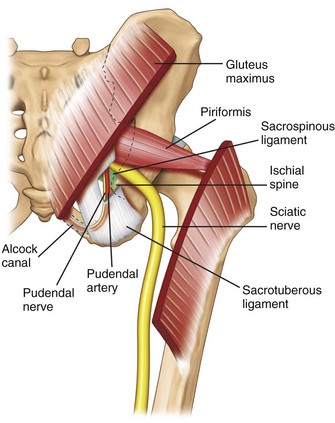
The pudendal nerve is formed from the anterior rami of the second, third, and fourth sacral nerves (S2, S3, and S4). Exiting the pelvis through the greater sciatic notch, the pudendal nerve is accompanied by the internal pudendal artery on its medial side and travels dorsal to the sacrospinous ligament abutting the attachment of the latter to the ischial spine (Fig. 10-18). At this level, the nerve is situated between the sacrospinous and sacrotuberous ligaments (interligamentous plane).76–78 The nerve then swings ventrally to enter the lesser sciatic foramen and then Alcock’s canal,78,79 which is the fascial tunnel formed by the duplication of the obturator internus muscle under the plane of the levator ani muscle on the lateral wall of ischiorectal fossa (Fig. 10-18).80 The pudendal nerve subsequently gives off three terminal branches: the dorsal nerve of the penis (or clitoris); the inferior rectal nerve; and the perineal nerve, providing the sensory branches to the skin of penis (or clitoris), perianal area, and posterior surface of the scrotum or labia majora. They also innervate the external anal sphincter (inferior rectal nerve) and deep muscles of the urogenital triangle (perineal nerve).
The path of the pudendal nerve either in between the sacrotuberous and the sacrospinous ligaments or through Alcock’s canal makes it susceptible to entrapment.81 In Alcock’s canal, possible cause of entrapment can be the falciform process of the sacrotuberous ligament or fascia of the obturator internus muscle.
To date, the best imaging technique for Alcock’s canal is CT scan. Ultrasonography is useful in imaging the interligamentous plane at the ischial spine level. The pudendal nerve is a thin nerve, measuring 4 to 6 mm at the level of the ischial spine.82 Nerves of this size can be difficult to detect with ultrasonography at the ischial spine, which is on average 5 cm deep to the skin.82 These circumstances may account for why the pudendal nerve is visualized with ultrasonography as little as 47.2% of the time.82
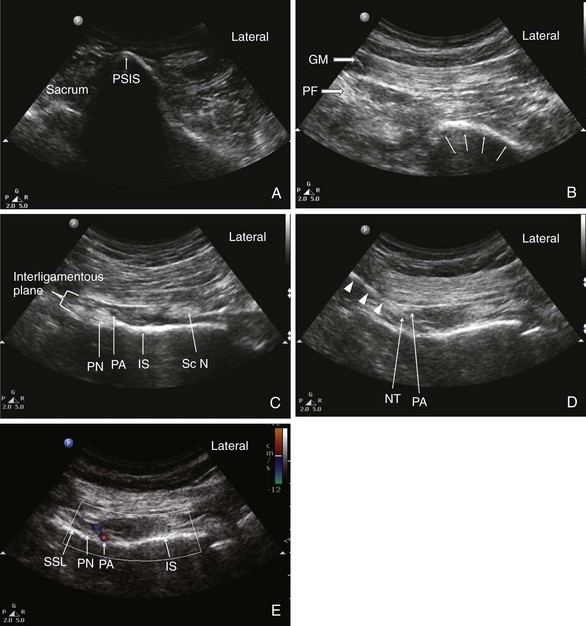
To reveal the structures at this level, the key is the recognition of the ischial spine (Fig. 10-19, probe position C). A few hints can be very helpful in identifying the ischial spine: (1) the spine appears as a straight hyperechoic line, and the ischium cephalad is seen as a curved line because it forms the posterior aspect of the acetabulum (Fig. 10-20); (2) the sacrospinous ligament appears as a hyperechoic line in continuity with the medial end of the ischial spine, with lower echogenicity than bone; (3) the sacrotuberous ligament is seen as a light hyperechoic line ventral to the gluteus maximus muscle overlapping the fascia ventral to this muscle and appears parallel and dorsal to the sacrospinous ligament; and (4) the internal pudendal artery can be localized with the use of color-flow Doppler in close proximity to the ischial spine (Fig. 10-20). Another arterial pulsation is often seen lateral to the tip of the ischial spine and is accompanied by the sciatic nerve. This is the descending branch of the inferior gluteal artery. Mistaking this artery for the pudendal artery will result in sciatic nerve block.
Existing Technique
The transgluteal approach is the most popular route of injection for pudendal neuralgia, allowing blockade at the ischial spine and Alcock’s canal. At the level of ischial spine, both ultrasonography and CT scan are ideal for visualizing the interligamentous plane because they identify all of the important landmarks, which are the ischial spine, sacrotuberous ligament, sacrospinous ligament, pudendal artery, and pudendal nerve.1 Traditionally, fluoroscopy has been used to guide needle placement using the ischial spine as a surrogate landmark.76 The needle is placed medial to the ischial spine, which corresponds to the course of the pudendal nerve at this level.76 The major limitation of fluoroscopy is that it cannot accurately demonstrate the interligamentous plane.1,61 At the level of the ischial spine, the pudendal artery lies between the pudendal nerve and the pudendal artery in the majority of cases (76% to 100%). Therefore, injectate may not spread to the pudendal nerve using this landmark. In addition, the potential proximity of the sciatic nerve at this level makes it susceptible to the anesthetic if spread of the injectate is not visualized in real time. Furthermore, the depth for needle insertion cannot be assessed with fluoroscopy.83
Two reports described the ultrasound visualization of the pudendal nerve.84,85 Validation of the ultrasound-guided procedure in a clinical setting was performed recently. The study demonstrated the reliability of blocking the pudendal nerve in the interligamentous plane at the ischial spine level.82
Ultrasound-Guided Injection Technique
The patient is placed in the prone position. A curvilinear probe with a low frequency (2-5 MHz) is required to visualize the structures given its location deep to the gluteus maximus muscle. The ultrasound transducer should be first placed transversely over the posterior superior iliac spine (PSIS) to visualize the sacroiliac joint. After this is seen, the transducer is moved laterally to view the ilium (Fig. 10-20). The posterior aspect of the iliac wing should be clearly visualized as a hyperechoic line descending laterally. After the iliac wing has been identified, the angle of the probe is rotated such that it would be in line with the long axis of the piriformis muscle as it runs from the sacrum to the greater trochanter. As scanning continues caudally, the hyperechoic line of the ilium starts to disappear in the medial aspect of the image at the level of the sciatic notch. The lateral aspect of the ultrasound image continues to display a curved hyperechoic line representing the ischium. At this level, two separate muscular layers are identified: the gluteus maximus and the piriformis muscles. Scanning slightly more caudally, the curved line of the ischium becomes straighter as it transitions to the ischial spine. At this point, the sacrospinous ligament should be seen as a slight hyperechoic structure extending from the tip of the ischial spine, projecting toward the sacrum (Fig. 10-20). The sacrotuberous ligament may also be seen at this level deep to the gluteus maximus muscle. The piriformis muscle will no longer be visualized. The pudendal artery lies slightly medial to the tip of the ischial spine and the pudendal nerve situated medial to the artery. It may not be clearly visualized under ultrasonography given its small diameter and depth in the gluteal region.
Clinical Pearls and Pitfalls
 Always make sure one can visualize and insert the needle when the sacrospinous ligament is in view and the spread of the injectate is between the sacrospinous and sacrotuberous ligaments. This is important because it ensures that the injection is into the interligamentous plane.
Always make sure one can visualize and insert the needle when the sacrospinous ligament is in view and the spread of the injectate is between the sacrospinous and sacrotuberous ligaments. This is important because it ensures that the injection is into the interligamentous plane. When the needle is inserted as in-plane technique, the needle is usually poorly visualized because of the steep angle. One can minimize this by having the needle entry point inserted in a far medial location. In some patients, the sacrum may prevent the advancement of the needle. In that situation, the needle should be inserted from lateral to medial.
When the needle is inserted as in-plane technique, the needle is usually poorly visualized because of the steep angle. One can minimize this by having the needle entry point inserted in a far medial location. In some patients, the sacrum may prevent the advancement of the needle. In that situation, the needle should be inserted from lateral to medial. The pudendal nerve can be easier to visualize with Doppler ultrasonography because one will appreciate the displacement of the artery by a hyperechoic structure.
The pudendal nerve can be easier to visualize with Doppler ultrasonography because one will appreciate the displacement of the artery by a hyperechoic structure. Injection is medial to the pudendal artery, which is usually found medial to the ischial spine. If an artery is seen lateral to the ischial spine, the practitioner should suspect that it is the descending branch of the inferior gluteal artery that accompanies sciatic nerve. A few hints will suggest that is not pudendal artery: (1) scan farther medially and caudally and the pudendal artery will appear on the medial aspect, (2) the visualization of the sciatic nerve adjacent to the artery, and (3) the artery is seen more than 1 cm lateral to the ischial spine. However, the pudendal artery can be found lateral to the ischial spine or in a mistaken scan when the level of scan is above the ischial spine. In the latter case, the sacrospinous ligament cannot be seen.
Injection is medial to the pudendal artery, which is usually found medial to the ischial spine. If an artery is seen lateral to the ischial spine, the practitioner should suspect that it is the descending branch of the inferior gluteal artery that accompanies sciatic nerve. A few hints will suggest that is not pudendal artery: (1) scan farther medially and caudally and the pudendal artery will appear on the medial aspect, (2) the visualization of the sciatic nerve adjacent to the artery, and (3) the artery is seen more than 1 cm lateral to the ischial spine. However, the pudendal artery can be found lateral to the ischial spine or in a mistaken scan when the level of scan is above the ischial spine. In the latter case, the sacrospinous ligament cannot be seen.Piriformis Injection
Piriformis syndrome is an uncommon and often underdiagnosed cause of buttock and leg pain. The clinical presentation of this syndrome has been well described elsewhere in the literature.86,87 The management of piriformis syndrome includes the injection of the piriformis muscle with local anesthetic and steroids87 or the injection of botulinum toxin.88
Anatomy
The piriformis muscle originates from the anterior surface of the second, third, and fourth sacral vertebrae and the capsule of the sacroiliac joint. Running laterally anterior to the sacroiliac joint, the piriformis muscle exits the pelvis through the greater sciatic foramen (Fig. 10-18). At this point, the muscle becomes tendinous, inserting into the upper border of the greater trochanter as a round tendon. The piriformis functions as an external rotator of the lower limb in the erect position, an abductor when supine, and a weak hip flexor when walking.86
All neurovascular structures exiting the pelvis to the buttock pass through the greater sciatic foramen, and the sciatic nerve is one of them. There are six possible anatomical relationships between the sciatic nerve and the piriformis muscle. The most common arrangement is found when the undivided nerve passes below the piriformis muscle (78% to 84%).89,90 The second most common arrangement is found when the divided nerve passes through and below the muscle (12% to 21%). The aberrant course of the sciatic nerve through the piriformis muscle can cause sciatica and may suggest the important role of a nerve stimulator in the injection of the piriformis muscle.
Existing Techniques
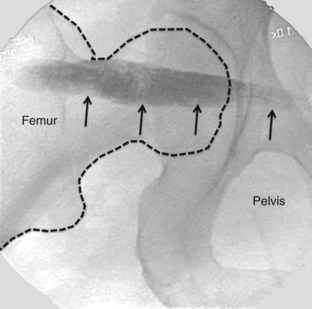
Various image-guided techniques used to inject the piriformis muscle have been described, including fluoroscopy,91 CT,92 and magnetic resonance imaging (MRI).93 Electrophysiological guidance has been used alone and in conjunction with the above modalities.94 Contrast injection is commonly used in fluoroscopic-guided technique to confirm needle placement within the piriformis muscle (Fig. 10-21), which has been shown to be unreliable.95 A validation study with cadavers suggested that fluoroscopically-guided, contrast-controlled injection was only accurate in guiding an intrapiriformis injection in 30% of the injections.95 In cases in which the needle was incorrectly placed, the usual final position of the needle was within the gluteus maximus muscle, which overlies the piriformis.
In contrast, ultrasonography is seen as an attractive imaging technique because it provides visualization of the soft tissue and neurovascular structures and allows real-time imaging of the needle insertion toward the target. Furthermore, the success of injection can be assessed by the spread of injectate under ultrasonography.1 Multiple reports of ultrasound-guided piriformis muscle injection have been published with similar techniques described.96,97 The accuracy of needle placement with ultrasonography was recently validated in a cadaveric study suggesting an accuracy of 95%.95
The use of ultrasonography allows for direct visualization of the piriformis muscle.45,46 Using this technique, one may see the needle insert within the substance of the muscle and visualize the spread of injectate within or around the muscle. Adjacent anatomical structures such as the sciatic nerve are also visualized. This technique has proven accurate and reproducible.45,47 Furthermore, the cost is significantly lower than MRI or CT scanning, and ultrasonography is available to a greater number of physicians.
Ultrasound-Guided Injection Technique
The patient is placed in the prone position. A curvilinear probe with a low frequency (2-5 MHz) is required to visualize the muscle given its location deep to the gluteus maximus muscle. Similar to the technique for pudendal nerve injection, the ultrasound transducer should be first placed transversely over the PSIS. After the iliac wing is identified, the angle of the probe is rotated such that it would be in line with the long axis of the piriformis muscle as it runs from the sacrum to the greater trochanter. As scanning continues caudally, the hyperechoic line of the ilium starts to recede from the medial aspect of the image at the level of the sciatic notch. The lateral aspect of the ultrasound image continues to display a curved hyperechoic line representing the ischium. At this level, two separate muscular layers are identified: the gluteus maximus and the piriformis muscles (Fig. 10-20, B). By internal and external rotation of the patient’s hip with the knee flexed, the piriformis muscle can be seen gliding underneath the gluteus maximus muscle. The sequence of scanning the PSIS first and then moving the probe caudally is important because the ultrasound image of the lesser sciatic notch may be confused with that of the greater sciatic notch.
Clinical Pearls and Pitfalls
 Although internal and external rotation of the piriformis muscle is a very useful maneuver to differentiate between the gluteus maximus and piriformis muscle, the image can be similar when the probe is scanning the lesser sciatic notch. Therefore, the practitioner needs to make sure the scanning starts from the ilium and the probe is then moved caudally.
Although internal and external rotation of the piriformis muscle is a very useful maneuver to differentiate between the gluteus maximus and piriformis muscle, the image can be similar when the probe is scanning the lesser sciatic notch. Therefore, the practitioner needs to make sure the scanning starts from the ilium and the probe is then moved caudally.1 Peng P, Narouze S. Ultrasound-guided interventional procedures in pain Medicine: A review of anatomy, sonoanatomy and procedures. Part I: non-axial structures. Reg Anesth Pain Med. 2009;34:458-474.
2 Wertheim HM, Rovenstine EA. Suprascapular nerve block. Anesthesiology. 1941;2:541-545.
3 Gleeson AP, Graham CA, Jones I, et al. Comparison of intra-articular lignocaine and a suprascapular nerve block for acute anterior shoulder dislocation. Injury. 1997;28:141-142.
4 Ritchie ED, Tong D, Chung F, et al. Suprascapular nerve block for pain relief in arthroscopic shoulder surgery: a new modality? Anesth Analg. 1997;84:1306-1312.
5 Neal J, McDonald S, Larkin K, et al. Suprascapular nerve block prolongs analgesia after nonarthroscopic shoulder surgery but does not improve outcome. Anesth Analg. 2003;96:982-986.
6 Emery P, Bowman S, Wedderburn L, Grahame R. Suprascapular nerve block for chronic shoulder pain in rheumatoid arthritis. BMJ. 1989;299:1079-1080.
7 Jones DS, Chattopadhyay C. Suprascapular nerve block for the treatment of frozen shoulder in primary care: a randomized trial. Br J Gen Pract. 1999;49:39-41.
8 Karatas GK, Meray J. Suprascapular nerve block for pain relief in adhesive capsulitis: comparison of 2 different techniques. Arch Phys Med Rehabil. 2002;83:593-597.
9 Shanahan EM, Ahern M, Smith M, et al. Suprascapular nerve block (using bupivacaine and methylprednisolone acetate) in chronic shoulder pain. Ann Rheum Diseases. 2003;62:400-406.
10 Romeo AA, Rotenberg DD, Bach BRJr. Suprascapular neuropathy. J Am Acad Orthop Surg. 1999;7:358-367.
11 Natsis K, Totlis T, Tsikaras P, et al. Proposal for classification of the suprascapular notch: a study on 423 dried scapulas. Clin Anat. 2007;20:135-139.
12 Peng P, Wiley MJ, Liang J, Bellingham G. Ultrasound-guided suprascapular nerve block: a correlation with fluoroscopic and cadaveric findings. Can J Anesth. 2010;57:143-148.
13 Feigl GC, Anderhuber F, Dorn C, et al. Modified lateral block of the suprascapular nerve: a safe approach and how much to inject? A morphological study. Reg Anesth Pain Med. 2007;32:488-494.
14 Mallon WJ, Brown HR, Vogler JB, Martinez S. Radiographic and geometric anatomy of the scapula. Clin Orthop Relat Res. 1992;277:142-154.
15 Shah RV, Racz GB. Pulsed mode radiofrequency lesioning of the suprascapular nerve for the treatment of chronic shoulder pain. A case report. Pain Physician. 2003;6:503-506.
16 Schneider-Kolsky ME, Pike J, Connell DA. CT-guided suprascapular nerve blocks: a pilot study. Skeletal Radiol. 2004;33:277-282.
17 Moore DC. Block of the suprascapular nerve. Springfield, IL. Thomas CC, editor. Regional nerve block, ed 4. 1979:300-303.
18 Brown DE, James DC, Roy S. Pain relief by suprascapular nerve block in gleno-humeral arthritis. Scand J Rheumatol. 1988;17:411-415.
19 Gofeld M. Ultrasonography in pain medicine: a critical review. Pain Pract. 2008;8:226-240.
20 Harmon D, Hearty C. Ultrasound-guided suprascapular nerve block technique. Pain Physician. 2007;10:743-746.
21 Yucesoy C, Akkaya T, Ozel O, et al. Ultrasonographic evaluation and morphometric measurements of the suprascapular notch. Surg Radiol Anat. 2009;31:409-414.
22 Moore DC, Bridenbaugh LD. Intercostal nerve block in 4333 patients: indications, technique and complications. Anesth Analg. 1962;41:1-10.
23 Karmakar MK, Ho AMH. Acute pain management of patients with multiple fractured ribs. J Trauma. 2003;54:612-615.
24 Kopacz DJ, Thompson GE. Intercostal blocks for thoracic and abdominal surgery. Tech Reg Anesth Pain Manage. 1998;2:25-29.
25 Green CR, de Rosayro M, Tait AR. The role of cryoanalgesia for chronic thoracic pain: results of a long-term follow up. J Natl Med Assoc. 2002;94:716-720.
26 Byas-Smith MG, Gulati A. Ultrasound-guided intercostal nerve cryoablation. Anesth Analg. 2006;103:1033-1035.
27 Cohen SP, Sireci A, Wu CL, et al. Pulsed radiofrequency of the dorsal root ganglia is superior to pharmacotherapy or pulsed radiofrequency of the intercostal nerves in the treatment of chronic postsurgical thoracic pain. Pain Physician. 2006;9:227-235.
28 Stolker RJ, Vervest AC, Groen GJ. The treatment of chronic thoracic segmental pain by radiofrequency percutaneous partial rhizotomy. J Neurosurg. 1994;80:986-992.
29 Rendina EA, Ciccone AM. The intercostal space. Thorac Surg Clin. 2007;17:491-501.
30 Graeber GM, Nazim M. The anatomy of the ribs and the sternum and their relationship to chest wall structure and function. Thorac Surg Clin. 2007;17:473-489.
31 Knowles P, Hancox D, Letheren M, et al. An evaluation of intercostal nerve blockade for analgesia following renal transplantation. Eur J Anaesthesiol. 1998;15:457-461.
32 Shanti CM, Carlin AM, Tyburski JG. Incidence of pneumothorax from intercostal nerve block for analgesia in rib fractures. J Trauma. 2001;51:536-539.
33 Curatolo M, Eichenberger U. Ultrasound-guided blocks for the treatment of chronic pain. Tech Reg Anesth Pain Manage. 2007;11:95-102.
34 Cardosi RJ, Cox CS, Hoffman MS. Postoperative neuropathies after major pelvic surgery. Obstet Gynecol. 2002;100:240-244.
35 Luijendijk RW, Jekel J, Storm RK, et al. The low transverse Pfannenstiel incision and the prevalence of incisional hernia and nerve entrapment. Ann Surg. 1997;225:365-369.
36 Whiteside JL, Barber MD, Walters MD, Falcone T. Anatomy of the ilioinguinal and iliohypogastric nerves in relation to trocar placement and low transverse incisions. Am J Obstet Gynecol. 2003;189:1574-1578.
37 Sippo WC, Burghardt A, Gomez AC. Nerve entrapment after Pfannenstiel incision. Am J Obstet Gynecol. 1987;157:420-421.
38 Choi PD, Nath R, Mackinnon SE. Iatrogenic injury to the ilioinguinal and iliohypogastric nerves in the groin: case report, diagnosis, and management. Ann Plast Surg. 1996;37:60-65.
39 Jamieson RW, Swigart LL, Anson BJ. Points of parietal perforation of the ilioinguinal and iliohypogastric nerves in relation to optimal sites for local anaesthesia. Q Bull Northwest Univ Med Sch. 1952;26:22-26.
40 Mandelkow H, Loeweneck H. The iliohypogastric and ilioinguinal nerves. Distribution in the abdominal wall, danger areas in surgical incisions in the inguinal and pubic regions and reflected visceral pain in their dermatomes. Surg Radiol Anat. 1988;10:145-149.
41 Oelrich TM, Moosman DA. The aberrant course of the cutaneous component of the ilioinguinal nerve. Anat Rec. 1977;189:233-236.
42 Ducic I, Dellon AL. Testicular pain after inguinal hernia repair: an approach to resection of the genital branch of genitofemoral nerve. J Am Coll Surg. 2004;198:181-184.
43 Rab M, Ebmer And J, Dellon AL. Anatomic variability of the ilioinguinal and genitofemoral nerve: implications for the treatment of groin pain. Plast Reconstr Surg. 2001;108:1618-1623.
44 Liu WC, Chen TH, Shyu JF, et al. Applied anatomy of the genital branch of the genitofemoral nerve in open inguinal herniorrhaphy. Eur J Surg. 2002;168:145-149.
45 Lichtenstein IL, Shulman AG, Amid PK, et al. Cause and prevention of postherniorrhaphy neuralgia: a proposed protocol for treatment. Am J Surg. 1988;155:786-790.
46 Eichenberger U, Greher M, Kirchmair L, et al. Ultrasound-guided blocks of the ilioinguinal and iliohypogastric nerve: accuracy of a selective new technique confirmed by anatomical dissection. Br J Anaesth. 2006;97:238-243.
47 al-Dabbagh AK. Anatomical variations of the inguinal nerve and risks of injury in 110 hernia repairs. Surg Radiol Anat. 2002;24:102-107.
48 Peng PWH, Tumber PS. Ultrasound-guided interventional procedures for patients with chronic pelvic pain—a description of techniques and review of literature. Pain Physician. 2008;11:215-224.
49 Rosario DJ, Jacob S, Luntley J, et al. Mechanism of femoral nerve palsy complication percutaneous ilioinguinal field block. Br J Anaesth. 1997;78:314-316.
50 Van Schoor AN, Boon JM, Bosenberg AT, et al. Anatomical considerations of the pediatric ilioinguinal/iliohypogastric nerve block. Paediatr Anaesth. 2005;15:371-377.
51 Johr M, Sossai R. Colonic puncture during ilioinguinal nerve block in a child. Anesth Analg. 1999;88:1051-1052.
52 Amory C, Mariscal A, Guyot E, et al. Is ilioinguinal/iliohypogastric nerve block always totally safe in children? Pediatr Anaesth. 2003;13:164-166.
53 Vaisman J. Pelvic hematoma after an ilioinguinal nerve block for orchialgia. Anesth Analg. 2001;92:1048-1049.
54 van Schoor AN, Boon JM, Bosenberg AT, et al. Anatomical considerations of the pediatric ilioinguinal/iliohypogastric nerve block. Paediatr Anaesth. 2005;15:371-377.
55 Weintraud M, Marhofer P, Bosenberg A, et al. Ilioinguinal/iliohypogastric blocks in children: where do we administer the local anesthetic without direct visualization? Anesth Analg. 2008;106:89-93.
56 Hu P, Harmon D, Frizelle H. Ultrasound guidance for ilioinguinal/iliohypogastric nerve block: a pilot study. Ir J Med Sci. 2007;176:111-115.
57 Gofeld M, Christakis M. Sonographically guided ilioinguinal nerve block. J Ultrasound Med. 2006;25:1571-1575.
58 Gucev G, Yasui GM, Chang TY, Lee J. Bilateral ultrasound-guided continuous ilioinguinal-iliohypogastric block for pain relief after Cesarean delivery. Anesth Analg. 2008;106:1220-1222.
59 Broadman L. Ilioinguinal, iliohypogastric, and genitofemoral nerves. In: Gay SG, editor. Regional anesthesia. An atlas of anatomy and techniques. St. Louis: Mosby; 1996:247-254.
60 Conn D, Nicholls B. Regional anaesthesia. In: Wilson IH, Allman KG, editors. Oxford handbook of anaesthesia. ed 2. New York: Oxford University Press; 2006:1055-1104.
61 Bellingham GA, Peng PWH. Ultrasound-guided interventional procedures for chronic pelvic pain. Tech Reg Anesth Pain Manag. 2009;13:171-178.
62 Campos NA, Chiles JH, Plunkett AR. Ultrasound-guided cryoablation of genitofemoral nerve for chronic inguinal pain. Pain Physician. 2009;12:997-1000.
63 Park JW, Kim DH, Hwang M, Bun HR. Meralgia paresthetica caused by hip-huggers in a patient with aberrant course of the lateral femoral cutaneous nerve. Muscle Nerve. 2007;35:678-680.
64 van Slobbe AM, Bohnen AM, Bernsen RM, et al. Incidence rates and determinants in meralgia paresthetica in general practice. J Neurol. 2004;251:294-297.
65 Grossman MG, Ducey SA, Nadler SS, et al. Meralgia paresthetica: diagnosis and treatment. J Am Acad Orthop Surg. 2001;9:336-344.
66 de Ridder VA, de Lange S, Popta J. Anatomical variations of the lateral femoral cutaneous nerve and the consequences for surgery. J Orthop Trauma. 1999;13:207-211.
67 Grothaus MC, Holt M, Mekhail AO, et al. Lateral femoral cutaneous nerve: an anatomic study. Clin Orthop Relat Res. 2005;437:164-168.
68 Murata Y, Takahashi K, Yamagata M, et al. The anatomy of the lateral femoral cutaneous nerve, with special reference to the harvesting of iliac bone graft. J Bone Joint Surg Am. 2000;82:746-747.
69 Dias Filho LC, Valença MM, Guimarães Filho FAV, et al. Lateral femoral cutaneous neuralgia: an anatomical insight. Clin Anat. 2003;16:309-316.
70 Hospodar PP, Ashman ES, Traub JA. Anatomic study of the lateral femoral cutaneous nerve with respect to the ilioinguinal surgical dissection. J Orthop Trauma. 1999;13:17-19.
71 Ropars M, Morandi X, Huten D, et al. Anatomical study of the lateral femoral cutaneous nerve with special reference to minimally invasive anterior approach for total hip replacement. Surg Radio Anat. 2009;31:199-204.
72 Bodner G, Bernathova M, Galiano K, et al. Ultrasound of the lateral femoral cutaneous nerve. Normal findings in a cadaver and in volunteers. Reg Anesth Pain Med. 2009;34:265-268.
73 Hurdle MF, Weingarten TN, Crisostomo RA, et al. Ultrasound-guided blockade of the lateral femoral cutaneous nerve: technical description and review of 10 cases. Arch Phys Med Rehabil. 2007;88:1362-1364.
74 Ng I, Vaghadia H, Choi PT, Helmy N. Ultrasound imaging accurately identifies the lateral femoral cutaneous nerve. Anesth Analg. 2008;107:1070-1074.
75 Damarey B, Demondion X, Boutry N, et al. Sonographic assessment of the lateral femoral cutaneous nerve. J Clin Ultrasound. 2009;37:89-95.
76 Robert R, Prat-Pradal D, Labat JJ, et al. Anatomic basis of chronic perineal pain: role of the pudendal nerve. Surg Radiol Anat. 1998;20:93-98.
77 Peng PWH, Antolak SJ, Gordon AS. Pudnedal Neuralgia. In: Goldstein I, Pukall C, Goldstein A, editors. Female sexual pain disorders: evaluation and management. ed 1. Oxford UK: Wiley-Blackwell Publisher; 2009:112-118.
78 Mahakkanukrauh P, Surin P, Vaidhayakarn P. Anatomical study of the pudendal nerve adjacent to the sacrospinous ligament. Clin Anat. 2005;18:200-205.
79 Shafik A, Doss SH. Pudendal canal: surgical anatomy and clinical implications. Am Surg. 1999;65:176-180.
80 Amareno G, Lanoe Y, Ghnassia RT, et al. Alcock’s canal syndrome and perineal neuralgia. Rev Neurol. 1988;144:523-526.
81 Warrick R, Williams PL. Myology: fascia of the truck. In: Warrick R, Williams PL, editors. Gray’s anatomy. ed 36. Edinburgh, UK: Churchill; 1980:542-564.
82 Rofaeel A, Peng P, Louis I, et al. Feasibility of real-time ultrasound for pudendal nerve block in patients with chronic perineal pain. Reg Anesth Pain Med. 2008;33:139-145.
83 Hough DM, Wittenberg KH, Pawlina W, et al. Chronic perineal pain caused by pudendal nerve entrapment: anatomy and CT-guided perineural injection technique. Am J Roentgenol. 2003;181:561-567. 2
84 Kovacs P, Gruber H, Piegger J, Bodner G. New, simple, ultrasound-guided infiltration of the pudendal nerve: ultrasonographic technique. Dis Colon Rectum. 2001;44:1381-1385. 9
85 Gruber H, Kovacs P, Piegger J, Brenner E. New, simple, ultrasound-guided infiltration of the pudendal nerve: topographic basics. Dis Colon Rectum. 2001;44:1376-1380. 9
86 Papadopoulos EC, Khan SN. Piriformis syndrome and low back pain: a new classification and review of the literature. Orthop Clin North Am. 2004;35:65-71.
87 Benzon HT, Katz JA, Benzon HA, Iqbal MS. Piriformis syndrome: anatomic considerations, a new injection technique, and a review of the literature. Anesthesiology. 2003;98:1442-1448.
88 Fishman LM, Anderson C, Rosner B. Botox and physical therapy in the treatment of piriformis syndrome. Am J Phys Med Rehabil. 2002;81:936-942.
89 Beason LE, Anson BJ. The relation of the sciatic nerve and its subdivisions to the piriformis muscle. Anat Rec. 1937;70:1-5.
90 Pecina M. Contribution to the etiological explanation of the piriformis syndrome. Acta Anat. 1979;105:181-187.
91 Fishman S, Caneris O, Bandman T, et al. Injection of the piriformis muscle by fluoroscopic and electromyographic guidance. Reg Anesth Pain Med. 1998;23(6):554-559.
92 Fanucci E, Masala S, Sodani G, et al. CT-guided injection of botulinic toxin for percutaneous therapy of piriformis muscle syndrome with preliminary MRI results about denervative process. Eur Radiol. 2001;11:2543-2548.
93 Filler A, Haynes J, Jordan S, et al. Sciatic pain of non-disc origin and piriformis syndrome: diagnosis by magnetic resonance neurography and interventional magnetic resonance imaging with outcome of resulting treatment. J Neurosurg Spine. 2005;2:99-115.
94 Fishman L, Konnoth C, Rozner B. Botulinum neurotoxin type B and physical therapy in the treatment of piriformis syndrome: a dose finding study. Am J Phys Med. 2004;83:42-50.
95 Finoff JT, Hurdle MFB, Smith J. Accuracy of ultrasound-guided versus fluoroscopically guided contrast controlled piriformis injections. A cadaveric study. J Ultrasound Med. 2008;27:1157-1163. 8
96 Huerto AP, Yeo SN, Ho KY. Piriformis muscle injection using ultrasonography and motor stimulation-report of a technique. Pain Physician. 2007;10:687-690. 5
97 Smith J, Hurdle M-F, Locketz AJ, Wisniewski SJ. Ultrasound-guided piriformis injection: technique description and verification. Arch Phys Med Rehabil. 2006;87:1664-1667. 12