CHAPTER 363 Perioperative Management of Subarachnoid Hemorrhage
Subarachnoid hemorrhage (SAH) is a pathologic condition that exists when blood enters the subarachnoid space. SAH accounts for 5% of strokes. The most common overall cause of SAH is head injury, and aneurysms are the most common cause of spontaneous SAH.1 The list of other causes of SAH is extensive (Table 363-1).
TABLE 363-1 Causes of Subarachnoid Hemorrhage
| CATEGORY | CAUSES |
|---|---|
| Idiopathic | Benign perimesencephalic subarachnoid hemorrhage |
| Infections | Bacterial, tuberculous, and fungal meningitis; syphilis; herpes simplex or other viral encephalitis; leptospirosis; listeriosis; brucellosis; yellow fever; typhoid fever; dengue; malaria; anthrax |
| Trauma | Closed head injury, electrical injury, gunshot wounds and other penetrating cranial trauma, heat injury, strangulation, high altitude, caisson disease, radiation, germinal matrix hemorrhage in neonates |
| Toxins | Amphetamines, cocaine, monoamine oxidase inhibitors, epinephrine, alcohol, ether, carbon monoxide, morphine, nicotine, lead, quinine, phosphorus, pentylenetetrazol, hydrocyanic acid, insulin, snake venom |
| Vascular | Atherosclerosis; rupture of hypertensive, amyloid, or other type of intracerebral hemorrhage into cerebrospinal fluid; hemorrhagic transformation of ischemic infarction; ruptured arteriovenous or other vascular malformation; vasculitis from systemic lupus erythematosus or polyarteritis nodosa; eclampsia; intracranial venous thrombosis secondary to pregnancy, oral contraceptives, volume depletion, hypercoagulable states, trauma, or infection |
| Blood diseases | Leukemia, hemophilia, sickle cell anemia, pernicious anemia, aplastic anemia, agranulocytosis, thrombocytopenic purpura, polycythemia vera, Waldenström’s macroglobulinemia, lymphoma, myeloma, hereditary spherocytosis, afibrinogenemia, liver diseases associated with coagulopathy, disseminated intravascular coagulation, acquired coagulopathies secondary to anticoagulant drugs |
| Neoplasm | Glioma, meningioma, hemangioblastoma, choroid plexus papilloma, chordoma, hemangioma, pituitary adenoma, sarcoma, osteochondroma, ependymoma, neurofibroma, bronchogenic carcinoma, choriocarcinoma, melanoma |
Modified from Weir B. Aneurysms Affecting the Nervous System. Baltimore: Williams & Wilkins; 1987.
The overall incidence of SAH is approximately 9 per 100,000 person-years. Reports exist of a higher incidence in Japan and Finland, and the incidence increases with age. Beginning in the sixth decade, the incidence in women seems to be higher. The decline in the incidence of SAH over the past 45 years is relatively moderate in comparison to the decline in stroke in general.2 SAH may exhibit a seasonal (winter and spring), diurnal (late morning), and daily (Sunday) peak pattern.3 Despite a relatively constant incidence, there has been a decrease in mortality. In Rochester, Minnesota, for example, mortality fell from 6.8 per 100,000 for patients managed between 1955 and 1964 to 4.3 per 100,000 for patients managed between 1975 and 1984.4 Hop and coworkers reviewed 25 study periods between 1960 and 1992.5 Case fatality rates varied between 32% and 67% and decreased by 0.5%/yr, with the decline being steeper after adjustment for age and sex (0.9%/yr). They concluded that the case fatality rate after SAH has decreased during the past 3 decades. The annual mortality from SAH in Sweden decreased significantly in both sexes by nearly 4% between the years 1985 and 2000, and currently the case fatality rate is estimated to be 50%,5,6 including the 10% to 15% of patients who die before receiving medical care.7 The cause of this decrease in mortality is multifactorial but includes improvements in the medical management of patients with SAH.
Pathophysiology
Intracranial Pressure Response
The ICP response to rebleeding of aneurysms is known in the subset of patients with SAH who have ICP monitors in place during their rebleeding, but these changes may not be representative of those that occur with the first hemorrhage. During rebleeding, ICP rises to diastolic blood pressure and CBF occurs only during systole.8,9 Temporary circulatory arrest may help stop the aneurysmal bleeding but may also be associated with transient global ischemia, which would cause loss of consciousness. Because many patients do not lose consciousness, however, normal clotting probably also contributes to arrest of the hemorrhage. In addition, animal experiments have shown that the rate of blood flowing into the subarachnoid space determines the volume of SAH.10 High flow rates, which would theoretically occur with large aneurysm tears, produce large-volume SAH in a short time, whereas low flow rates, which might result from a small hole in the aneurysm, lead to slow accumulation of a small volume of SAH.
The results of ICP monitoring in 52 patients for a mean of 8 days after SAH showed that mean ICP rose as clinical grade worsened.11 Mean ICP was 10 mm Hg in patients with clinical grades 1 and 2, 18 mm Hg in patients with clinical grades 2 and 3, and 29 mm Hg in those with clinical grades 3 to 5. Vasospasm, which was more common in patients with a poor clinical grade and larger SAH, was associated with a significant rise in ICP from a mean of 16 mm Hg in those without vasospasm to 29 mm Hg in those with vasospasm. This fact should be considered carefully in patients experiencing deterioration from vasospasm days after SAH. Substantial improvement in cerebral perfusion pressure can be achieved by ventricular drainage. Cerebral perfusion pressure is equal to mean arterial blood pressure minus ICP. There are no specific data regarding optimal cerebral perfusion pressure in patients with aneurysmal SAH, but guidelines for head injury suggest that it be maintained at values higher than 70 mm Hg. ICP is related to outcome; 80% of patients with pressure below 15 mm Hg do well, as opposed to good outcomes in just 15% of patients with ICP in excess of 15 mm Hg.12
Cerebral Blood Flow, Volume, and Metabolism
Numerous studies of CBF, cerebral blood volume, and cerebral metabolism have been conducted in patients with ruptured aneurysms. Almost all studies agree that CBF is globally decreased after SAH.12 For example, in 30 patients with SAH, regional CBF decreased from a mean of 54 mL/100 g per minute in normal individuals to 42 mL/100 g per minute in patients with grade 1 to 2 and no vasospasm, 35 mL/100 g per minute in patients with grade 3 to 4 and no vasospasm, 36 mL/100 g per minute in patients with grade 1 to 2 and vasospasm, and 33 mL/100 g per minute in patients with grade 3 to 4 and vasospasm.13 The cerebral metabolic rate of oxygen (CMRO2) showed a similar pattern, with progressive reductions being associated with deteriorating clinical grade and worsening vasospasm. Cerebral blood volume was markedly increased in patients with severe neurological deficits associated with severe vasospasm. It was concluded that vasospasm produces narrowing of the large, angiographically visible arteries at the base of the brain and that this narrowing is accompanied by compensatory dilation of the distal, intracerebral arterioles. Other studies have shown a lack of increased cerebral blood volume after SAH, thus suggesting impaired autoregulatory vasodilation.14
Mean CBF decreases with time after SAH; it reaches a nadir in 10 to 14 days, after which it slowly increases toward normal.12,15 A relative hyperemia in relation to the reduced CMRO2 occurs immediately after SAH.16,17 In patients with poor grades, CBF and cerebral metabolism may remain depressed for weeks. In addition to global reductions in CBF and CMRO2, regional perfusion defects can develop after SAH and can be correlated with areas of angiographically demonstrated severe vasospasm and ventricular dilation. Areas of low CBF are also present around intracerebral hematomas, although their size and importance may be overestimated.18 Regions of brain irrigated by vasospastic arteries have elevated oxygen extraction fractions. Positron emission tomography shows that as long as the area is ischemic and infarction has not developed, CMRO2 remains normal but flow is reduced. The development of infarction is heralded by a fall in CMRO2 with relatively increased CBF (relative hyperemia).16 The degree to which the reduced blood flow after SAH is due to hypovolemia and hypotension is unclear, but there is some evidence that the alterations in flow can be prevented by maintenance of normovolemia or hypervolemia with or without induced hypertension.19,20
Little information is available on the pathogenesis of CBF and the metabolic changes that occur after SAH. In patients without vasospasm, intracerebral clots, or hydrocephalus studied in the first 4 days after SAH, CMRO2 is decreased without accompanying changes in the oxygen extraction fraction, thus suggesting that the primary alteration is a reduction in CMRO2 and that CBF falls because of decreased demand.16 This is usually attributed to a toxic effect of the subarachnoid blood, but a neural mechanism or an effect of global ischemia may be important. A relative hyperemia is usually present and is postulated to be due to intracranial circulatory arrest, transient global cerebral ischemia, and lactic acidosis occurring at the time of rupture. Mitochondrial respiration, sodium-potassium adenosine triphosphatase activity, and extracellular potassium and calcium levels are altered in the brain tissue of experimental animals exposed to subarachnoid blood, although the relationship of these changes to CBF and CMRO2 has not been fully clarified.21–23
The relationship of CBF to blood pressure and PaCO2 is also altered after SAH. The response of CBF to changes in blood pressure at different times after SAH was studied in 38 patients.24 Autoregulation was intact in good-grade patients but became progressively impaired in poor-grade patients and with development of vasospasm. Autoregulation is not lost in an all-or-none fashion. The degree of impairment tends to be worse as consciousness is more impaired, as vasospasm becomes more severe, and 5 to 10 days after SAH. Loss of the CBF response to changes in PaCO2 occurs with more severe brain damage than that required to disturb autoregulation, and the combination of loss of autoregulation and variation in CBF with changes in PaCO2 is termed vasomotor paralysis. After SAH, vasomotor paralysis may be observed in patients with clinical grades 4 and 5, usually with severe vasospasm. Measurements of CBF with intra-arterial xenon 133 in 38 patients with aneurysmal SAH found that responses to alterations in PaCO2 were generally preserved, although they were reduced.24 Impaired CO2 reactivity was associated with increased ICP and high lactate levels in CSF, and poor clinical grade and vasospasm were associated with impaired CO2 responsiveness. Transcranial Doppler studies have demonstrated impairment in CO2 reactivity after SAH, even in patients with a good clinical grade.25 This tends to occur during vasospasm and then subsequently resolves. Hyperventilation should be used with caution in patients with SAH. It may be useful for reducing increased ICP and increasing CMRO2, but it may also increase the risk for ischemia by causing vasoconstriction.26
Patient Evaluation
Symptoms and Signs of Subarachnoid Hemorrhage
The hallmark of SAH is a sudden, usually severe, headache, although at most about 80% of patients who can give a history will recount such a headache.12 Challenges arise in assessing the minority of patients in whom an atypical manifestation occurs, and for this reason clinicians must remain vigilant for the possibility of SAH because a missed diagnosis can result in catastrophic sequelae. The classically described cardinal symptom of SAH as a sudden, severe headache or the “worst headache of my life” may be overemphasized as a reliable initial diagnostic symptom, but all patients with headaches that are unusually severe or sudden in onset should be investigated for SAH. Byyny and coauthors reported that 4 of 27 patients seen in an emergency department with a sudden severe headache were found to have SAH on computed tomography (CT).27 An additional 19% had SAH detectable only by lumbar puncture. The positive predictive value of a sudden severe headache was just 93%. Linn and colleagues reported that it may be the initial symptom in only a third of patients with SAH. In the same series, just half of the patients reported their headache to reach maximum severity instantaneously, with a fifth of the patients reporting it to escalate over a period of 1 to 5 minutes and the remainder over a period greater than 5 minutes.28 Other ominous features include vomiting, onset with exertion, altered level of consciousness, meningismus, or focal neurological deficit. Absence of these clinical findings, however, does not rule out SAH.
Premonitory symptoms (warning leaks or sentinel hemorrhages), typically consisting of an unusually severe headache of sudden onset, sometimes associated with nausea, vomiting, and dizziness, are usually attributed to small hemorrhages from the aneurysm. In 1752 patients with ruptured aneurysms from three series, 20% had a history of a sudden, severe headache before the event leading to admission.29–31 Other possible mechanisms for these headaches include hemorrhage into the aneurysm wall, acute expansion of the aneurysm sac, or ischemia. The importance of recognizing warning leaks has repeatedly been emphasized because the diagnosis may be delayed until catastrophic SAH occurs, which almost certainly makes the outcome worse than if the diagnosis had been made promptly and correctly.30 Some authors discourage the use of terminology such as “sentinel bleed” or ““warning leak” and instead encourage clinicians to have a high index of suspicion in the pursuit of an accurate diagnosis of SAH to foster the mindset that the patient either has or has not experienced SAH.28
Many other symptoms and signs can develop before rupture of an aneurysm, including hemiparesis, dysphasia, extraocular muscle impairment, visual loss, visual field defects, and localized headache. They depend on the size and anatomic location of the aneurysm.12
Ruptured aneurysms may produce distinct clinical features at specific sites. Transient bilateral lower extremity weakness may be due to rupture of an anterior cerebral artery aneurysm. SAH from a middle cerebral artery aneurysm is more likely to produce hemiparesis, paresthesia, hemianopia, and dysphasia. Sarner and Rose did not find that any particular aneurysm site had a higher propensity to induce coma.32 Seizures occur more commonly with aneurysms of the anterior circulation and probably with middle cerebral artery aneurysms. Third nerve palsy or unilateral retro-orbital pain suggests an aneurysm arising at the junction of the internal carotid and posterior communicating artery. Third nerve lesions also occur with aneurysms at the origin of the superior cerebellar artery. Carotid-ophthalmic artery aneurysms may produce unilateral visual loss or visual field defects. Focal neurological deficits after SAH may be due to a mass effect from the aneurysm, vasospasm, seizures, or hematomas in the brain or subdural spaces.
Terson reported vitreous hemorrhage and hemiparesis in association with SAH.12 Vitreous and other intraocular hemorrhages may be seen on ophthalmoscopic examination in 3% to 13% of patients with SAH and are associated with poor clinical grades.33,34 Because the prognosis for visual recovery is good with no treatment, vitrectomy is generally reserved for patients who fail to improve after months.
Numerous exertional activities have been temporally associated with aneurysm rupture. A cooperative study reported that a third of 2288 aneurysm ruptures occurred during sleep, a third during unspecified circumstances, and a third during various exertional activities, including lifting, emotional strain, defecation, coitus, coughing, and parturition.35 Schievink and associates found that SAH occurred during stressful events in 43% of patients, nonstressful events in 34%, rest or sleep in 12%, and uncertain circumstances in 11%.36 If one takes into account the fact that exertional activities probably occupy a small percentage of one’s lifetime, it seems likely that the fluctuations in blood pressure and changes in venous and CSF pressure that may occur with these activities increase the risk for rupture of an aneurysm. Head injury has only rarely been associated with aneurysmal SAH.
Diagnosis
Computed Tomography
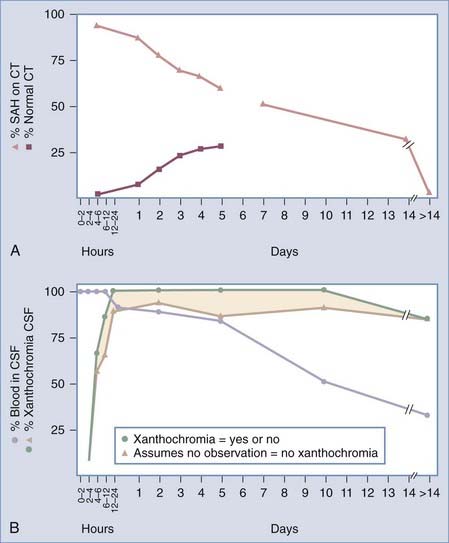
Non–contrast-enhanced cranial CT is the first investigation in patients with suspected SAH. The probability of detecting the hemorrhage is proportional to the volume of blood in the subarachnoid space, the time after hemorrhage, and the quality of the scan (Fig. 363-1). In a cooperative study, 3% of 1553 patients were found to have normal scans within 24 hours of confirmed SAH.37 The incidence is probably lower with current CT scanners, but CT still has only about 93% sensitivity for SAH, thus highlighting the need for lumbar puncture when clinically indicated in the context of normal CT findings.27 CT on the day of the ictus showed SAH in 92%, intraventricular hemorrhage in 20%, intracerebral hemorrhage in 19%, hydrocephalus in 16%, mass effect in 8%, aneurysm in 5%, subdural hemorrhage in 2%, and hypodense areas in 1%. With time, the incidence of normal CT scans increases, as well as the presence of areas of hypodensity, whereas hydrocephalus and hemorrhage decrease. By 5 days after SAH, 27% of the scans were normal and 58% showed hemorrhage. Intracerebral hemorrhage takes longer to resolve than SAH. Alert patients are significantly more likely than drowsy patients to have a normal scan or a thin, local collection of blood, and all other abnormalities evident on CT are more common in patients with a poor clinical grade. Additional stigmata of SAH include enlargement of the temporal horns in the absence of increases in other parts of the ventricular system.38
The volume and location of subarachnoid blood on CT give prognostic information about the risk for vasospasm and outcome after SAH. A widely used system of grading SAH on CT was proposed by Fisher and coworkers (Fig. 363-2).39,40 In a prospective study, these authors reported good correlation between the location and volume of the blood and the subsequent development of vasospasm. The degree of SAH on CT was an independent risk factor for death and disability in the International Cooperative Study on the Timing of Aneurysm Surgery.37 Subsequent studies have suggested that initial clot volume and percentage of clot cleared per day are significant predictors of vasospasm whereas Fisher grade and initial clot density are less important.41 The Fisher scale may not correlate well with vasospasm because the categories are broad and not defined with modern imaging techniques.
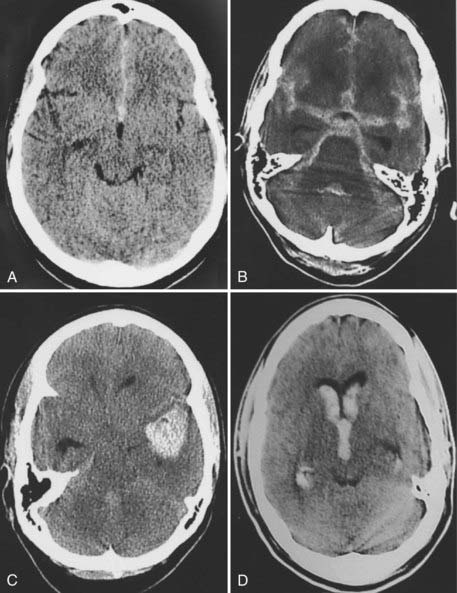
FIGURE 363-2 Computed tomography shows different grades of subarachnoid hemorrhage (SAH) according to the scale of Fisher and colleagues.39 Grade 1 is a scan with no SAH. A, Grade 2 is a scan showing a thin layer of subarachnoid blood less than 1 mm thick. This measurement, however, was taken from scans printed at different magnification, so it is not actually 1 mm. B, Grade 3 is a scan showing focal or diffuse subarachnoid blood thicker than 3 mm. Grade 4 is a scan showing intracerebral (C) or intraventricular (D) blood with or without subarachnoid blood.
Studies evaluating CT angiography (CTA) for detection of intracranial aneurysms report sensitivities of 77% to 97% and specificities of 87% to 100%. Sensitivity diminishes significantly with aneurysms measuring less than 3 mm in maximal diameter, and in such cases sensitivities have been reported in the range of 40% to 91%.42–47 CTA has been demonstrated to be useful in determining suitability of the aneurysm for endovascular treatment in more than 95% of cases.48
Lumbar Puncture
Lumbar puncture is indicated for the diagnosis of SAH when findings on CT are normal. CT may show normal results if the SAH is very small or an inordinate amount of time has elapsed between hemorrhage and the CT scan. Contraindications to lumbar puncture include abnormal blood clotting, increased ICP as a result of a space-occupying lesion, suspected spinal arteriovenous malformation, and infection at the lumbar puncture site. When bedside lumbar puncture is not feasible, fluoroscopically assisted lumbar puncture is recommended. Risks associated with lumbar puncture include neurological deterioration from rebleeding of the aneurysm or cerebral herniation. Data from two studies reported that 17 (10%) of 165 patients with SAH who underwent lumbar puncture experienced deterioration within 24 hours.49,50 It was thus recommended that CT always be performed before lumbar puncture in the setting of suspected SAH. Limited accessibility to a CT scanner and a high level of suspicion for infectious meningitis may make lumbar puncture the initial diagnostic test in patients who do not have focal neurological deficits or a depressed level of consciousness. A review of the literature on lumbar puncture and meningitis concluded that there was no evidence to recommend CT before lumbar puncture in patients with suspected acute meningitis unless atypical features or focal neurological findings were present.51
Interpretation of CSF results can sometimes be challenging. Theoretically, any erythrocytes in CSF represent hemorrhage. It is possible that a small hemorrhage from an aneurysm could occur directly into the brain parenchyma or into a loculated CSF space and that erythrocytes would not be detected in lumbar CSF, although this is very rare. During CSF access, however, erythrocytes may be introduced artifactually into the CSF sample (traumatic tap). There are many criteria for differentiating a traumatic tap from SAH (Table 363-2), but none are very reliable. A declining erythrocyte count in subsequent tubes is an unreliable indicator of a traumatic tap.52 Xanthochromia is a yellow discoloration in a centrifuged CSF sample caused by release of hemoglobin and its breakdown products from hemolysis of erythrocytes. It is a very reliable sign of SAH in CSF obtained more than 12 hours after SAH. The most sensitive test for detection of xanthochromia, however, is spectrophotometry, but most laboratories only visually inspect uncentrifuged CSF. If the CSF is not centrifuged, it may be discolored by erythrocytes that are present as a result of either SAH or a traumatic tap. Furthermore, when erythrocytes are introduced into the CSF sample during a traumatic tap, they will eventually lyse and produce xanthochromia. Therefore, CSF samples should be kept at 4°C, centrifuged immediately, and examined for xanthochromia in timely fashion. The time for xanthochromia to appear after SAH is variable, but it persists longer than intact erythrocytes do (see Fig. 363-1). The sensitivity of visual inspection for detecting xanthochromia in 81 patients more than 12 hours after SAH was less than 50%, thus highlighting the utility of spectrophotometry.53 If spectrophotometry for hemoglobin and bilirubin is negative on CSF obtained more than a few hours after the onset of symptoms, angiography is probably not necessary except under unusual circumstances. If findings on CT are normal, erythrocytes are present but not xanthochromia, and 12 or more hours has elapsed after the ictus, we generally perform at least CTA or magnetic resonance angiography (MRA) and occasionally catheter angiography. About 70% of patients seen 3 or more weeks after suspected SAH have xanthochromia. CT will usually be normal, and if CSF is clear at this time, CTA or catheter-based angiography should be performed.
TABLE 363-2 Characteristics of Cerebrospinal Fluid after Subarachnoid Hemorrhage and Traumatic Lumbar Puncture*
| FEATURE | TRAUMATIC TAP | SUBARACHNOID HEMORRHAGE |
|---|---|---|
| Erythrocyte count | Decreasing with sequential tubes | Constant count between tubes, usually thousands per cubic millimeter but may be as few as 350 cells/mm3 |
| Clotting | Clots | Does not clot |
| Xanthochromia | None | Xanthochromia present on spectrophotometry of centrifuged sample |
| Erythrocyte-to-leukocyte ratio | Normal | May be decreased |
| Protein | Normal or increased in direct relation to the number of erythrocytes | May be increased |
| Hemosiderin-laden macrophages | Absent | Present within days of SAH |
| Cerebrospinal fluid pressure | Normal | Elevated |
| Repeat tap at another level | Normal | Consistent with SAH |
* The presence of any erythrocytes or xanthochromia should lead to the tentative diagnosis of a ruptured aneurysm.
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI; fluid-attenuated inversion recovery [FLAIR] and proton density sequences) has been shown to be as sensitive as CT in detecting acute SAH, but MRI remains impractical in most cases as an initial investigation.54 It is, however, useful for investigating atypical hemorrhage patterns (e.g., craniocervical junction, parenchymal hemorrhage) or when there has been a delay from the onset of symptoms to initial imaging. For the detection of subacute hemorrhage, gradient echo T2 is the most sensitive sequence, with sensitivities of 94% in the acute phase and 100% in the subacute phase. The next most sensitive sequence is FLAIR, with values of 81% and 87% for the acute and subacute phases, respectively.55
There is concern about performing MRI postoperatively in patients with aneurysm clips, and a fatality has been reported.56 Modern aneurysm clips are alloys of cobalt, nickel, molybdenum, and chromium, with or without small amounts of iron.57 They are not ferromagnetic and should not move in the magnet. Titanium clips are either pure titanium or alloys of titanium, vanadium, and aluminum and are not ferromagnetic. Some institutions test the clips by checking whether they move in the magnet before implantation. Stainless steel clips should not be used.
Catheter Cerebral Angiography
In a review of 2899 procedures, the rate of neurological complications with catheter angiography was 1.3%; 0.7% were transient, 0.2% were reversible, and 0.5% were permanent. Neurological complications were significantly more common in patients 55 years or older, in patients with cardiovascular disease, and when fluoroscopic times were 10 minutes or longer.58 Allergic reactions to contrast medium occur in less than 1 in 50,000 studies, and about 1 in a million patients die as a result of such a reaction.59
Rupture of an aneurysm during angiography is uncommon. In the first cooperative study, in which 5484 patients were studied by angiography, 7 patients (0.13%) rebled during angiography and 12 (0.22%) 10 minutes to 24 hours later.35 Based on subsequent studies, it is estimated that 3% of patients show extravasation of dye when angiography is performed for investigation of SAH.60 If extravasation was observed on an angiogram, the mortality was 70%. It has been suggested that the rate of rebleeding associated with cerebral angiography can be reduced by avoiding catheter angiography within the first 6 hours after SAH, but this remains unproven.60,61 Because rebleeding is probably most common immediately after the first hemorrhage, whether angiography causes the rebleeding is unknown. We do not delay angiography because a patient is seen immediately after SAH has occurred.
Twenty percent to 30% of patients have multiple aneurysms.12 A combination of clinical and radiologic features can identify the ruptured aneurysm in 90% to 95% of cases.62–65 A review of 69 patients with multiple aneurysms generated the following algorithm to predict which aneurysms would bleed: (1) exclude extradural aneurysms, (2) study CT for the presence of focal SAH, (3) look for focal spasm or a mass effect on the angiogram, (4) pick the larger or more irregularly shaped aneurysm, (5) examine the patient for focal neurological signs, (6) consider repeating the angiogram at a later date to look for change in aneurysm size or for focal angiographic signs, and (7) choose the aneurysm that has the highest chance of rupture (anterior communicating artery aneurysm).66 Overall, the most proximal and largest aneurysm usually ruptures. If there are two aneurysms on the same artery, it is generally the proximal one that is ruptured. In some cases, MRI has provided additional evidence of localizing value. In about two thirds of patients with multiple aneurysms, all lesions will be able to be clipped through a single craniotomy, and this may be advisable depending on the age and condition of the patient and the location of the aneurysms. In exceptional circumstances and despite the best diagnostic aids, it may not be possible to determine preoperatively which aneurysm bled. The literature contains cases in which recurrent SAH developed in patients with multiple aneurysms after the unruptured one was clipped.
No cause of SAH will be found in 9% to 30% of patients undergoing angiography for SAH, but there are numerous other causes of SAH (see Table 363-1). We perform cranial MRI and MRA in such patients, although the yield is low. In 15 series published between 1978 and 1988, 253 of 1218 patients underwent repeat angiography after an initially negative study, and an aneurysm was found in 11%.67 The aneurysm may be missed initially if it thromboses totally after bleeding. Review of the initial studies by another neuroradiologist may be helpful. The anterior communicating artery complex probably harbors the most missed aneurysms. Studies have shown that there is a subgroup of patients with angiogram-negative SAH in whom blood is located predominately in the prepontine and perimesencephalic cisterns. Repeat angiography is probably unnecessary in this situation if a good-quality initial angiogram does not show a posterior circulation aneurysm.68
Residual aneurysm was detected on 8% of postoperative angiograms obtained within days of surgery on 2416 patients reported in nine series.69 Residual aneurysms hemorrhage at a rate of about 0.02%/yr, although the natural history of these rests is not known with certainty.70,71 The advantage of knowing about an unexpected residual aneurysm intraoperatively is that an attempt can be made to obliterate it at the time. This must be weighed against the risk associated with further clip manipulation and angiography itself. The incidence of unexpected major arterial occlusion was about 6% in the series just reviewed.69 Occlusion is also best detected by intraoperative angiography before permanent ischemia develops. Several series have identified characteristics that increase the yield of intraoperative angiography, such as giant aneurysms and those arising at the ophthalmic, anterior communicating, or middle cerebral arteries or at the basilar bifurcation.69,72,73 Angiography is virtually always performed at the completion of endovascular aneurysm treatment, and residual aneurysms are seen frequently. Follow-up of residual aneurysms after surgery or endovascular treatment, usually with MRI/MRA, is indicated if treatment would be undertaken should growth occur.
Clinical Grading
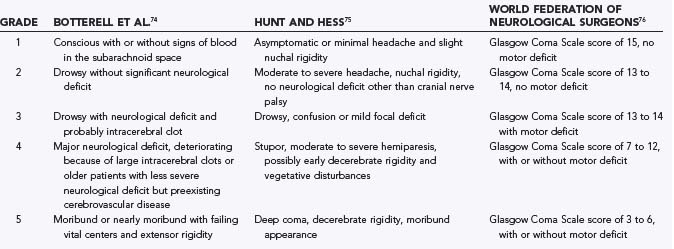
Several clinical grading scales have been developed, including the Botterell, Hunt and Hess, and World Federation of Neurosurgical Societies (WFNS) scales (Table 363-3).74–76 None are universally accepted despite numerous analyses.77–79 Challenges in the development of a universal grading scale include significant interobserver and intraobserver variability and omission of important additional features that may be predictive of outcome but are too complex to include in a clinical grading scale.79
Clinical grading is useful for estimating prognosis, for standardizing assessment to facilitate communication between physicians, and possibly for improving outcome measures in multicenter studies. Finally, repeated standardized assessment with some type of semiquantitative neurological scale is essential to detect deterioration in the patient’s condition. The neurological grade may best be determined after the patient is resuscitated and has undergone ventricular drainage if necessary. Neurological grade is an independent predictor of outcome after aneurysmal SAH.37,80 Assessment of level of consciousness with the Glasgow Coma Scale (GCS), which is the basis of the WFNS scale, has less interobserver variability.81,82 The WFNS scale was based on the observation that in a large clinical trial, the clinical features that best predicted outcome were the level of consciousness and the presence of a focal neurological deficit. The GCS is probably the most useful aspect of the grading scales.79
General Management
General Care
Typical admitting orders are shown in Table 363-4. Most patients are admitted to an intensive care or high-intensity observation unit. Bed rest in a dark room, limited visitors, and minimal stimulation are advocated by some practitioners but have not been proved to reduce rerupture rates. Once the aneurysm is repaired, early mobilization is encouraged as tolerated in an effort to minimize the complications associated with bed rest.83 Adequate analgesia should be ensured and excessive painful stimuli avoided because pain can increase cerebral oxygen use by up to 30%.84 Intermittent pneumatic compression devices are used routinely, as well as low-molecular-weight heparin prophylaxis, usually beginning 24 hours after aneurysm repair once a postoperative CT scan confirms no unexpected findings.
TABLE 363-4 Admitting Orders for Patients with Aneurysmal Subarachnoid Hemorrhage
Daily fluid intake should be approximately 3 L. Fluid intake of less than 2 L/day combined with the use of antihypertensive drugs increases the risk for cerebral ischemia in patients with SAH in comparison to those not treated with antihypertensive drugs who receive more than 3 L of fluid per day.85 The optimal hematocrit to maintain after SAH is not known.86,87 Trials of hemodilution for ischemic stroke have not shown benefit over untreated controls, even when hemodilution was instituted within 6 hours of the onset of symptoms.86 Other important principles in the management of patients with vasospasm are the administration of nimodipine and avoidance of hyperthermia, hypotension, hypovolemia, increased ICP, hypomagnesemia, and hyponatremia. Nimodipine is usually administered enterally (60 mg every 4 hours) to all patients with SAH. The recommended duration of treatment is 21 days, although delayed cerebral ischemia occurring more than 14 days after SAH is rare. Side effects include headache, hypotension, and intestinal pseudo-obstruction. Hypotension can be avoided by giving 30 mg every 2 hours or by reducing the dose. Patients with liver failure may also require a lower dose.
The use of corticosteroids remains controversial.88 Even a short course is associated with hyperglycemia and increased risk for infection.89 Hydrocortisone prevents excessive natriuresis and hyponatremia after SAH but does not affect outcome.90 Morbid long-term complications such as avascular necrosis of the femur have been reported even after short-term steroid use.91
Management of Blood Pressure
The target blood pressure in patients with SAH depends on many factors, including the time after SAH, whether the aneurysm has been repaired, ICP, and the patient’s premorbid blood pressure. The optimal target blood pressure requires balancing brain perfusion and the transmural pressure gradient across the aneurysm. In a patient with premorbid uncontrolled hypertension, reducing blood pressure to below “normal” levels may compromise cerebral perfusion. Detection of rapid variations in blood pressure may be more important than absolute blood pressure measurements.92 Treating elevated blood pressure after SAH has been shown to increase the risk for cerebral ischemia and to have no effect on outcome, so patients with SAH should have their blood pressure management individualized.93 Hypertension should be avoided, particularly during transport and performance of angiography in the early hours after SAH. In general, before repair of an aneurysm, blood pressure should be maintained in the “normotensive” range for that individual patient, with an arbitrary upper limit of 160 to 180 mm Hg systolic and a lower limit of 100 mm Hg systolic. After aneurysm repair, elevated blood pressure is not treated unless it is extremely elevated or when infarction has already occurred because CBF may be dependent on pressure as a result of loss of autoregulation. Any time after SAH, blood pressure may be elevated as a homeostatic response to increased ICP or vasospasm. In patients with unsecured aneurysms at the time when vasospasm develops (4 to 14 days after SAH), a more conservative approach to blood pressure reduction should be taken because the risk for rebleeding is lower during this period than it is in the first 24 hours after hemorrhage and a reduction in blood pressure may precipitate ischemia and infarction from vasospasm.
Complications Specific to Subarachnoid Hemorrhage
Rebleeding
Aneurysm rebleeding has historically been an important cause of mortality after SAH. In the International Cooperative Study on the Timing of Aneurysm Surgery, the peak risk for rebleeding was within the first 24 hours of SAH.94 Four percent of patients rebled within 24 hours of SAH. The rate declined thereafter to 1.5% per day, with a cumulative risk of 19% in the first 2 weeks. In another series, 17% of patients rebled in the first 24 hours after the initial SAH despite maintenance of systolic blood pressure below 150 mm Hg.95 Rebleeding risk was maximal immediately after the SAH, with 39% of patients who rebled within 24 hours rebleeding within 2 hours. Rebleeding was documented in 7% of 574 patients registered in the Columbia University SAH Outcomes Project between 1996 and 2002. Seventy-three percent occurred within 3 days of ictus. The Hunt-Hess grade on admission and maximum aneurysm diameter were independent predictors of rebleeding.96 Other series found an increased risk for rebleeding in patients with poor clinical grade, high blood pressure, short interval from SAH to admission, large aneurysm size, advanced age, and female sex.95,97 Rebleeding was associated with a mortality in excess of 75% in several series.96,98
Ruptured aneurysms should be obliterated by endovascular treatment or clipping as soon as feasible. Early aneurysm occlusion reduces the risk for rebleeding, facilitates treatment of vasospasm by increasing the safety of hemodynamic manipulations, and allows the patient to be mobilized more quickly, thereby avoiding the complications related to bed rest. For surgery, there is evidence that early clipping is associated with a better outcome.37,99,100 Endovascular treatment of ruptured aneurysms in good-grade patients with relatively small, narrow-necked, anterior circulation aneurysms resulted in better 1-year outcomes than did neurosurgical clipping in one study.71,101 Endovascular treatment is associated with a lower incidence of epilepsy.71 However, the incidence of early rebleeding after coiling of a ruptured aneurysm is higher than after surgery and is about 1.4% in the acute phase, with mortality approaching 100%. Independent risk factors for rebleeding after endovascular treatment were intracerebral hematoma and small aneurysm size.102 Late rebleeding, defined as rebleeding from a coiled aneurysm less than 1 month after coiling, has been reported to occur in approximately 0.3%/yr with a mortality of 0.2%/yr.103 Rebleeding after surgical clipping is less frequent and varied from 0.14% of 715 patients monitored for an average of 8 years to about 1% in Yasargil and colleagues’ series of more than 1350 aneurysms observed for an unspecified time.104,105
Antifibrinolytic drugs for the treatment of patients with SAH had been abandoned, but there is renewed interest. Randomized trials have shown that antifibrinolytic drugs reduce the risk for rebleeding but increase the risk for cerebral infarction and as a result had no overall effect on outcome.106,107 A Cochrane review of the use of antifibrinolytics in patients with SAH concluded that they were of no benefit.108 Antifibrinolytics increase mortality even in good-grade patients with small-volume SAH,109 and they have been found to be harmful even when given for as little as 4 days.110 Recent observational studies suggest that short-term antifibrinolytic treatment, which reduces rebleeding, could improve outcome when combined with modern SAH treatment, but randomized, blinded trials are needed to prove the efficacy of such treatment.111,112
Hydrocephalus
The frequency of acute ventricular dilation after SAH is 20%.37,113 Ventricular drainage may be required on an emergency basis as a lifesaving measure to relieve acute hydrocephalus and decrease ICP in patients with a depressed level of consciousness. Factors associated in several studies with clinically important hydrocephalus within the first days after SAH include increasing age, preexisting or postoperative hypertension, intraventricular hemorrhage, additional SAH, posterior circulation aneurysms, use of antifibrinolytic drugs, hyponatremia, and depressed level of consciousness (equivalent to worsening clinical grade).114,115 The pathogenesis of ventricular dilation is probably multifactorial and related to blockade of CSF circulation either within the ventricular system (aqueduct of Sylvius, outlets of the fourth ventricle) or in the subarachnoid space (tentorial incisura or basal cisterns) or related to increased resistance to outflow of CSF at the arachnoid granulations. Acutely, such blockages must be due to blood clots, which leads to proliferation of macrophages, arachnoid cells, and fibroblasts after several weeks.
It is worthwhile quantifying the degree of hydrocephalus by measuring the ventriculocranial ratio and determining whether the ventricles are larger than the 95th percentile for age (Fig. 363-3 and Table 363-5).116 Insertion of a ventricular drain is usually indicated for patients with a ventriculocranial ratio 20% to 25% greater than the 95th percentile for age and a depressed level of consciousness. This applies to most grade 4 and 5 patients.117 Risks associated with ventricular drainage include rebleeding, infection, and intracerebral hematoma along the catheter track. Fountas and colleagues reviewed the literature on ventricular drainage and rebleeding and concluded that studies had not controlled adequately for factors in addition to ventricular drainage that might affect rebleeding, so whether ventricular drainage increased the risk for rebleeding could not be determined.118 If ventricular drainage is needed, it is probably best to lower ICP just enough to maintain adequate cerebral perfusion pressure.
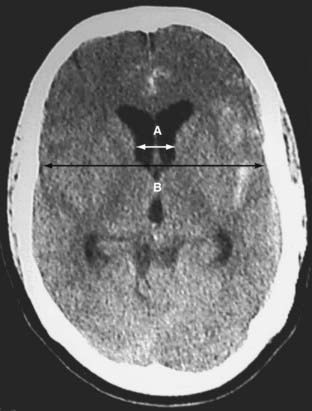
FIGURE 363-3 Computed tomography showing ventriculomegaly. The ventriculocranial ratio (A/B) is calculated by taking the ratio of the width of the ventricles and the width of the inner diameter of the skull at the level of the foramen of Monro at a point behind the head of the caudate nuclei where the lateral walls of the ventricles are parallel.116
TABLE 363-5 Upper 95% Confidence Value for Ventriculocranial Ratio by Age
| AGE (yr) | UPPER 95% CONFIDENCE VALUE |
|---|---|
| <30 | 0.16 |
| <50 | 0.18 |
| <60 | 0.19 |
| <80 | 0.21 |
| <100 | 0.25 |
Data from van Gijn J, Hijdra A, Wijdicks EFM, et al. Acute hydrocephalus after aneurysmal subarachnoid hemorrhage. J Neurosurg. 1985;63:355-362.
If a drain is not in place preoperatively, we usually insert one at the time of surgery to achieve optimal brain relaxation.119 The catheter is left in place postoperatively to monitor ICP, drain CSF as necessary, and administer fibrinolytic agents if indicated. In addition, if an endovascular approach that requires antiplatelet agents is being considered, a ventricular drain may best be placed before endovascular treatment. Some physicians change the catheter or convert it to a permanent shunt if it cannot be removed within 5 to 7 days, unless substantial intraventricular blood is present. A randomized trial showed no difference in the need for permanent CSF diversion or the length of intensive care or hospital stay between gradually decreasing versus immediate closure and discontinuation of external ventricular drainage.120
Chronic hydrocephalus develops in 10% to 21% of patients surviving aneurysmal SAH.115 Factors associated with shunt-dependent hydrocephalus after aneurysmal SAH were increasing age, female sex, poor Hunt and Hess grade on admission, thick SAH on initial CT scans, intraventricular hemorrhage, radiologically confirmed hydrocephalus at the time of admission, location of the ruptured aneurysm in the distal posterior circulation, clinical vasospasm, and endovascular treatment.115 Data on rates of hydrocephalus and vasospasm after clipping and coiling are conflicting and would best be examined in studies in which patients are randomized to the treatment methods.71
Intraventricular Hemorrhage and Increased Intracranial Pressure
A major intraventricular hemorrhage complicates aneurysm rupture in 13% to 28% of patients in clinical series and 37% to 54% of subjects in autopsy series.37,117 Small amounts of intraventricular blood, such as blood layered in the occipital horns, are seen more commonly. In 91 cases of intraventricular hemorrhage, the aneurysm involved the anterior cerebral artery in 40%, the internal carotid artery in 25%, and the middle cerebral artery in 21%.117 Aneurysms of the anterior communicating artery and the basilar termination are the ones that most commonly cause large, primarily intraventricular hemorrhage. Intraventricular hemorrhage is an independent risk factor for death, disability, vasospasm, and acute and chronic hydrocephalus after SAH.114,115,121–123
More than 50% of patients with large intraventricular hemorrhages are admitted with poor grades, and the mortality in such cases exceeds 64%.117 Ventricular size predicts survival, in addition to known prognostic factors such as age, clinical grade, and hypertension. A fourth ventricle that is dilated and packed with clot is a particularly ominous sign.124 Intraventricular thrombolysis with recombinant tissue plasminogen activator seems to assist in the acute management of patients with large aneurysmal intraventricular hemorrhages by speeding clearance of aneurysmal intraventricular hemorrhage, normalizing ICP, and reducing ventricular catheter obstruction.125–127 A randomized trial is needed to confirm these findings, establish the safety of treatment, and determine whether treatment affects outcome. A ruptured aneurysm should be repaired before administering tissue plasminogen activator; otherwise, there would be potential for lysis of the clot in the ruptured aneurysm with catastrophic rebleeding.
Some patients with acute hydrocephalus have markedly increased ICP and require ventricular drainage as a lifesaving measure.113 Increased ICP may occur in the absence of ventricular dilation.128 The infection rate after ventricular drain placement is about 10%.129
Causes of increased ICP acutely after SAH include acute hydrocephalus, intraventricular or intracerebral hemorrhage, brain swelling, ischemic brain edema, and increased resistance to CSF outflow, probably from blockade of the arachnoid villi by blood. There is some evidence that the blood-brain barrier is disrupted after SAH, and this could contribute to the edema and brain swelling.130
Intracerebral Hemorrhage
Aneurysms arising from the distal anterior cerebral arteries are the most likely to produce intracerebral hematomas. Because of the relative rarity of these aneurysms, however, intracerebral hematomas are more commonly seen with aneurysms of the middle and anterior communicating arteries, which are associated with clots in about 67% and 62% of cases in autopsy series, respectively. These percentages are lower in patients surviving their ruptured aneurysms. Intracerebral hematomas complicated 34% of the aneurysm cases reported by Pasqualin and associates.131 The sites of hemorrhage vary depending on the location of the aneurysm and the direction of rupture of the aneurysm into the brain parenchyma. The pattern is usually distinctive but does not always differ sufficiently from that of hypertensive intracerebral hemorrhage to allow an accurate diagnosis based on CT or MRI. Indications for angiography must be based on clinical suspicion.
A retrospective review of patients from 11 medical centers identified 132 patients with intracerebral hematoma secondary to ruptured aneurysms.132 Discriminant function analysis showed that in order of importance, size and location of the hematoma, aneurysm location, and extent of the midline shift were factors contributing to prediction of survival. Hematoma size was also a strong predictor of clinical grade. About 40% of hematomas were frontal and 40% were temporal. Patients with temporal lobe clots have the greatest capacity for clinical recovery.
Craniotomy for evacuation of hematoma is generally indicated in patients with a depressed or deteriorating level of consciousness, with or without signs of herniation. The aneurysm should be obliterated at the time of clot removal. An alternative to aneurysm clipping at the time of hematoma evacuation is endovascular coiling followed by clot evacuation.133 In the majority of patients we consider this a less desirable approach because of the need for two procedures and the risk for rebleeding after clot removal before endovascular repair.
Seizures
Seizures occur at or around the time of SAH in up to 20% of patients.134 They are not usually witnessed by medical personnel, so it is difficult to differentiate these episodes from posturing or movements precipitated by acutely increased ICP. In-hospital seizures or abnormal movements within hours of SAH are probably associated with rather than the cause of rebleeding.135 Patients who have seizures should undergo investigations to rule out an underlying new event, such as rebleeding, cerebral ischemia, hydrocephalus, or metabolic disturbances such as hyponatremia. There are several reasons to treat seizures in patients with SAH. Seizures are associated with brain swelling secondary to increased cerebral blood volume, which may be poorly tolerated after SAH, when intracranial compliance may be low. Seizures increase cerebral oxygen consumption and may cause hypoxemia, hypercapnia, acidosis, aspiration, and pneumonia. The rise in blood pressure that can accompany a seizure may increase the risk for rebleeding. Early prevention of seizures might reduce the risk for late epilepsy by preventing kindling, although this has not generally been demonstrable clinically after SAH 136,137 or head injury.138 Early seizures within a week of SAH do not seem to be a risk factor for late epilepsy.135,136,139,140
The incidence of epilepsy, defined as two or more seizures at least 1 week after SAH, in patients who survived after surgery for ruptured aneurysms is 3% to 10%.136,141–143 In one large series, 72% of seizures began within 1 year and 94% within 2 years.139 Risk factors for the late development of epilepsy have been determined in several series and commonly include younger age, middle cerebral artery aneurysms, intracerebral or subdural hematoma, poor initial grade, postoperative focal neurological deficit with cortical infarction, medial temporal lobe retraction, history of seizures, and shunt-dependent hydrocephalus.136,139,142
Prophylactic antiepileptic drug administration after SAH is used very arbitrarily in neurosurgical centers and is associated with increased in-hospital complications and worse outcome.80 Our current practice is to administer antiepileptic drugs to patients who have had seizures before or associated with SAH and to poor-grade patients. Endovascular treatment of ruptured aneurysms is associated with a lower incidence of epilepsy than surgical clipping is; our only indication for prophylaxis in these cases is previous seizures.71
Medical Complications
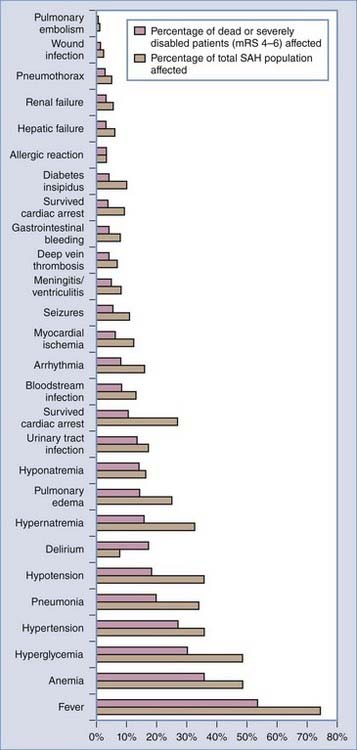
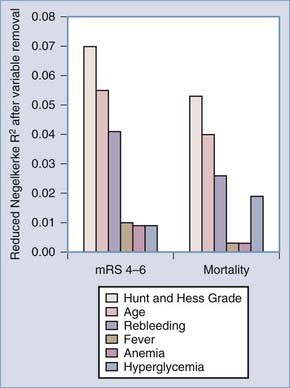
Medical complications are common after SAH and contribute substantially to morbidity and mortality. In 457 patients of the placebo group of the Multicenter Cooperative Aneurysm Study, almost every patient suffered at least one complication. Forty percent had at least one life-threatening complication, and a fourth of the deaths were due to medical complications (Table 363-6).144,145 The most common medical complications were anemia, hypertension, cardiac arrhythmias, fever, and electrolyte disturbances. Additional complications included elevated liver enzymes, pulmonary edema, pneumonia, and atelectasis. Medical complications were significantly more common in poor-grade patients and in those with diffuse, thick SAH.144 Deaths attributable to medical complications equaled those caused by neurological complications, with about a fifth of patients dying of vasospasm, rebleeding, direct effects of SAH, and medical complications (Table 363-7; also see Table 363-10). This study excluded poor-grade patients and did not control for other predictors of poor outcome when evaluating medical complications.145 In 580 patients admitted to one medical center between 1996 and 2002, the medical complications with the most impact on outcome were fever, anemia treated by transfusion, and hyperglycemia (Figs. 363-4 and 363-5).145
TABLE 363-6 Frequency of Causes of Morbidity and Mortality in Patients with Aneurysmal Subarachnoid Hemorrhage*
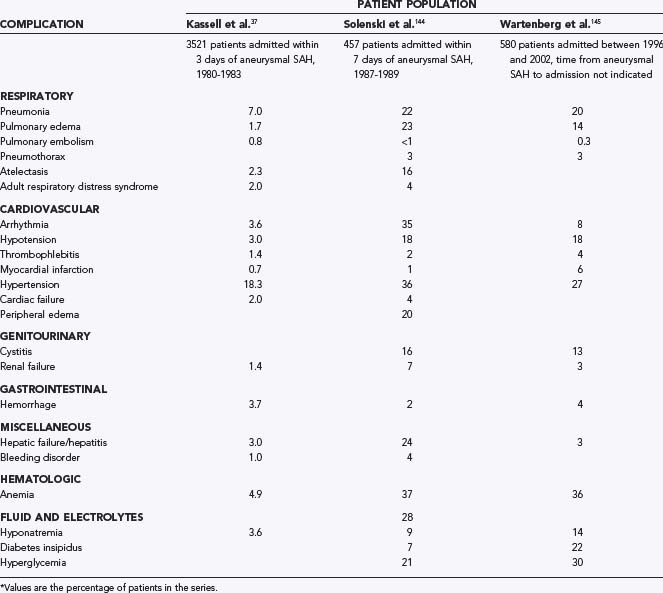
TABLE 363-7 Neurological Complications in a Series of Patients with Aneurysmal Subarachnoid Hemorrhage*
| COMPLICATION | RESULT | |
|---|---|---|
| Death (%) | Disability (%) | |
| Rebleeding | 6.7 | 0.8 |
| Vasospasm | 7.2 | 6.3 |
| Hydrocephalus | 0.3 | 1.4 |
| Direct effect of subarachnoid hemorrhage | 7.0 | 3.6 |
| Intracerebral hemorrhage | 1.0 | 1.0 |
| Complications of intracranial surgery | 1.7 | 2.3 |
| Other | 2.0 | 1.2 |
* Patient population: 3521 patients with aneurysmal subarachnoid hemorrhage admitted within 3 days.37
Data from Kassell NF, Torner JC, Haley ECJ, et al. The international cooperative study on the timing of aneurysm surgery: I. Overall management results. J Neurosurg. 1990;73:18-36.
Fever
Fever (temperature >38.3°C) occurred in 54% of 580 patients in one series (Fig. 363-4).141 Other studies found temperatures higher than 38.5°C 8 days after SAH, cumulative fever burden, and fever unresponsive to treatment to be associated with a poor outcome.146–148 Mechanisms by which fever alters the physiology of patients with SAH include increases in cerebral edema and ICP,149,150 exacerbation of ischemia,151 decreasing arteriojugular difference in oxygen content,149 and alteration in the level of consciousness.145 Infection can be identified in 75% of febrile SAH patients.152 The remainder of fevers may be central or neurogenic in origin. Standard fever therapy with a target of normothermia includes acetaminophen, ibuprofen, and cooling blankets. Fever refractory to conventional therapy may be considered for core temperature–controlled surface or endovascular cooling therapies.153–155 Antishivering therapies may also be used concomitantly. No randomized, controlled trials of treatment of fever after SAH have been conducted, but sufficient evidence exists at present to warrant treatment.
Anemia
Anemia was the second most common medical complication after SAH (see Fig. 363-4).145 Hemoglobin levels lower than 9 mg/dL developed in 36% of 580 patients and led to transfusion. Anemia after SAH is usually attributed to the combined effects of an SAH-related reduction in red blood cell mass, immobility, phlebotomy, and hemodilution.156 Conflicting data have been published on what hemoglobin level is appropriate for patients with SAH, and no randomized clinical trials have been performed. Anemia was associated with a poor outcome145 and higher hemoglobin levels with a better outcome in some studies,157 and transfusion was associated with vasospasm and a poor outcome in others.158 These observational data are correlative, and it remains unclear whether the need for blood transfusion reflects more severe illness or whether blood transfusions themselves contribute to poorer outcomes. A transfusion threshold of 7 g/dL was at least as effective in critically ill patients as a threshold of 10 g/dL, although few patients with brain injuries were included.159
There are many potential mechanisms to explain the detrimental effects of red blood cell transfusions, including alterations in vasoconstriction caused by a depleted supply of nitric oxide,160 proinflammatory effects of stored erythrocytes affecting immune function,161 reduced deformability of transfused erythrocytes, and alterations in the ability of stored erythrocytes to bind and release oxygen, both of which lead to ischemia.162 Transfused erythrocytes are generally depleted of leukocytes to decrease the generation of interleukin-1 (IL-1), IL-6, IL-8, and tumor necrosis factor-α.163
Until further studies evaluating the optimum transfusion threshold are conducted, decisions to transfuse should take into account such factors as vasospasm and concomitant coronary heart disease. We currently consider transfusing patients when the hemoglobin concentration is 8 mg/dL or less in the absence of symptomatic vasospasm and when the hemoglobin is less than 10 mg/dL in the presence of symptomatic vasospasm. Alternative strategies such as erythropoietin, which has been reported to possess neuroprotective properties, may be considered in the future.164
Hyperglycemia
Hyperglycemia (glucose >200 g/dL) occurred in 30% of SAH patients and was a predictor of poor functional outcome and mortality at 3 months (see Figs. 363-4 and 363-5).145 Predictors of hyperglycemia in one study were higher Hunt and Hess grade, elevated admission Acute Physiology, Age, and Chronic Health Evaluation (APACHE) II physiologic subscores, older age, and history of diabetes mellitus.165 Strict glucose control has been associated with reductions in ICP, duration of mechanical ventilation, length of hospital stay, use of vasopressors, and frequency of seizures and diabetes insipidus in critically ill neurological patients.166 Conversely, hypoglycemia is obviously potentially detrimental.167 No prospective trials are available to guide treatment of patients with SAH. At present, tight glucose control (5 to 7 mmol/L) is a reasonable goal.
Respiratory Complications
Respiratory complications are more common with advancing age and poor clinical grade. They have been reported to cause almost 50% of deaths from medical causes,144 although more recent studies have suggested that respiratory complications had no independent impact on neurological outcome 3 months after SAH.145 The most common respiratory complications are pulmonary edema, pneumonia, atelectasis, aspiration, pneumothorax, asthma, and pulmonary emboli.
A small number of patients with SAH suffer immediate respiratory arrest or life-threatening arrhythmias from which they die unless emergency life support is instituted. In a series of 245 patients with SAH, 15% required emergency intubation and ventilation.168
Pulmonary edema may complicate the course of patients with aneurysmal SAH at any time and may be cardiogenic (increased pulmonary venous pressure secondary to left-sided heart failure) or noncardiogenic (neurogenic or secondary to pulmonary insults such as aspiration and shock). Delayed cases are usually cardiogenic and caused by fluid overload during hemodynamic therapy for vasospasm.169 In patients dying of SAH, pulmonary edema is more common and was diagnosed clinically in 34% and at autopsy in 71%.170 Eight percent of 477 patients in one series had acute neurogenic pulmonary edema on admission.171 Clinical grade and volume of SAH were significantly higher than in patients without pulmonary edema, and the outcome was worse.172,173 Survival can be achieved in about 50% of cases. A sudden increase in ICP is probably a prerequisite in all cases. SAH may be associated with marked sympathetic hyperactivity that causes systemic hypertension and pulmonary vasoconstriction. If the constriction extends to the pulmonary veins, pressure in the pulmonary capillaries will be increased and, by a hydrostatic mechanism, cause transudation of low-protein fluid into the lungs.170,174 A second theory is that a primary increase in lung capillary permeability allows protein-rich plasma to leak into the lungs. The cause of increased capillary permeability may involve neural pathways from the central nervous system or pressure changes in the lung, thus creating some overlap with the hydrostatic theory. In any case, neurogenic pulmonary edema is characterized by rapid onset, association with severe neurological injury often involving the hypothalamus, suppression by adrenergic blockers, high protein content in the edema fluid, and resemblance to epinephrine-induced pulmonary edema. Treatment includes immediate intubation and ventilation, adequate oxygenation, positive end-expiratory pressure, furosemide, and measures to reduce increased ICP.
Most pneumonia occurs in intubated patients. Prevention of pneumonia in these cases should include removal of nasogastric and endotracheal tubes as soon as indicated, avoidance of unanticipated extubation and gastric overdistention, strict hand washing, semirecumbent positioning of the patient, maintenance of adequate nutrition, oral intubation, and proper ventilator care.175 Gastrointestinal stress ulcer prophylaxis with sucralfate does not eliminate the protective antibacterial effect of acidic gastric acid secretions. Such prophylaxis may decrease gastric bacterial growth and reduce the risk for pneumonia. Exposure to antibiotics is a risk factor for ventilator-associated pneumonia because it results in colonization of the patient with antibiotic-resistant bacteria. The use of antibiotics for inappropriate or unproven indications or for prophylactic purposes should be considered carefully.
In patients with head injury, tracheostomy and percutaneous feeding gastrostomy shorten the duration of mechanical ventilation.176 Tracheostomy should be considered when an SAH patient has been intubated for 10 to 14 days and extubation is not imminent.
Cardiovascular Complications
Some type of cardiovascular abnormality develops in most patients with SAH. Hypertension (systolic blood pressure >160 mm Hg) and hypotension (systolic blood pressure <100 mm Hg) are common and occur in 27% and 18%, respectively.145 Other cardiovascular events include life-threatening arrhythmias (8%), myocardial ischemia (6%), and successful resuscitation from cardiac arrest (4%).
Electrocardiographic (ECG) abnormalities are very common after SAH. The spectrum of ECG changes includes ST-segment alteration, T-wave changes, prominent U waves, QT prolongation, conduction abnormalities, and sinus bradycardia and tachycardia.177,178 These ECG changes were not associated with overall morbidity and mortality in one study.177
Echocardiography shows left ventricular wall motion abnormalities with moderate to severely reduced ejection fractions in 8% of patients studied within 6 days of SAH.179 Echocardiographic dysfunction is more common in patients who are poor grade, have had episodes of pulmonary edema and hypotension requiring intravenous pressors, and have elevated cardiac enzymes.179–181 Symmetrical T-wave inversion and severe QT prolongation may be specifically associated with the subpopulation of SAH patients with ventricular dysfunction.179,182 Echocardiography is recommended for SAH patients with hypotension, pulmonary edema, acute ECG changes (Q waves, ST-segment elevation, or other changes of acute ischemia), symmetrical T-wave inversion, or severe QT prolongation. Some patients with SAH and left ventricular dysfunction have tako-tsubo, or apical ballooning syndrome, which shares some features with the general population of SAH patients with ventricular dysfunction.183
Elevated troponin I has been reported in up to 68% of SAH patients.180,184 Levels peak about 2 days after SAH. Left ventricular wall motion abnormalities seen on echocardiography are more common in patients with elevated troponin, and elevated troponin levels are associated with disability and death after SAH, as well as worse neurological grade, systolic and diastolic cardiac dysfunction, and pulmonary congestion.180,184
Patients with SAH should have continuous monitoring of cardiac rhythm, as well as baseline and follow-up ECG studies and frequent monitoring of electrolytes, particularly potassium. Atrial flutter and fibrillation occur in up to 4% of patients and are associated with poor outcome.185 Hypokalemia should be avoided because it may aggravate arrhythmias and has been associated with a prolonged QT interval, ventricular fibrillation, and torsades de pointes,186,187 and echocardiography should be performed in high-risk patients to optimize care.
Venous Thromboembolism
Symptomatic deep venous thrombosis (DVT) develops in about 2% of patients with SAH, although a negative impact on outcome was not shown in one study.145 Asymptomatic DVT occurs in 19% to 50% of patients undergoing neurosurgical procedures. Risk factors for venous thromboembolism include increased age, heart failure, previous venous thromboembolism, direct trauma to the lower limbs, varicose veins, use of oral contraceptives, pregnancy and the puerperium, obesity, malignancy, infection, duration of surgery longer than 4 hours, and limb weakness or paralysis.188,189 Patients with deficiencies in antithrombin III, protein C, or protein S and with various genetic clotting factor abnormalities, such as factor V Leiden, are also at risk for venous thromboembolism.189 Graduated compression stockings and pneumatic compression devices decrease the incidence of DVT in neurosurgical patients, as detected by the radiolabeled fibrinogen technique, and are recommended for all patients.190,191 They work by intermittently squeezing the lower limbs, compressing the veins, and preventing thrombosis from venous stasis. There is evidence that they induce a systemic fibrinolytic state. Other proven methods of prophylaxis include low-dose subcutaneous heparin, adjusted-dose subcutaneous heparin, low-molecular-weight heparin, and oral anticoagulants. It is reasonable to assume that these drugs have a low therapeutic index in patients undergoing craniotomy. As the dose is increased, the risk for venous thromboembolism will decrease, but the risk for catastrophic intracranial bleeding will increase. We routinely place patients on a regimen of low-molecular-weight heparin 24 hours after aneurysm obliteration.
If DVT or pulmonary embolism develops, options include anticoagulation or placement of an inferior vena cava filter. Anticoagulation is usually considered to be safe when a week or more has passed since the craniotomy, although this is based on a very limited number of patients described in the medical literature.192 A filter may still be a better temporizing measure if the need for other invasive procedures, such as further angiography or shunting, has not been determined. Filters reduce the risk for pulmonary embolism, although whether they reduce mortality is less certain.193
Fluid and Electrolyte Disturbances
Euvolemia should be the goal in a patient with SAH in the absence of vasospasm. Daily fluid balance should not be greater than 500 mL positive after correcting for hypovolemia. Normal saline or lactated Ringer’s solution administered at a rate of up to 1.5 mL/kg per hour is recommended. Fluid management in patients with vasospasm is discussed in Chapter 364.
The most common electrolyte disturbances reported after aneurysmal SAH are hyponatremia (14% to 40%), hypomagnesemia (37%), hypokalemia (27%), and hypernatremia (20%).145,166,194,195 Hyponatremia may result from the syndrome of inappropriate antidiuretic hormone (SIADH), cerebral salt wasting, or a combination of the two.196 Opinions differ on the relative frequency of each, and in a series of 316 patients with spontaneous SAH, it was reported that of the 57% in whom hyponatremia developed, the cause was SIADH in 70%, salt wasting in 7%, and hypovolemic hyponatremia (some probably had salt wasting) in 21%.196 Hyponatremia is not thought to have major prognostic significance.145,195 The symptoms and signs of hyponatremia include deterioration in the level of consciousness, onset of or exacerbation of focal neurological deficits, seizures, and asterixis.
The first principle of fluid and sodium management is to make an accurate diagnosis (Table 363-8). The key differentiating features between SIADH and salt wasting is volume status. Acute change in body weight is a good measure of volume status, but there are numerous others, including changes in hematocrit, the blood urea nitrogen–to-creatinine ratio, and cardiac filling pressure.197 The primary treatment of salt wasting is adequate water and sodium replacement to maintain at least normovolemia and normal serum sodium. Natriuresis can be prevented by administering mineralocorticoids such as fludrocortisone acetate, but a randomized, controlled trial showed that this had no effect on the risk for cerebral ischemia or outcome, probably because administering large quantities of sodium and water is equally effective.198,199 Volume replacement should be guided by measures of total body water such as weight. Ventricular filling pressures are less reliable. In patients with SIADH, fluid restriction with careful management during the vasospastic period may be appropriate. An alternative approach when fluid restriction is potentially detrimental includes the judicious use of hypertonic saline and salt supplements. SIADH should be reversed slowly because rapid salt and water replacement may lead to fatal osmotic demyelination.200
Diabetes insipidus complicates the course of about 0.04% of patients with aneurysmal SAH.201 Most cases are associated with ruptured anterior communicating artery aneurysms. Treatment consists of replacement of fluid losses with hypotonic solutions such as 5% dextrose in water or 0.45% sodium chloride. In the chronic phase, exogenous replacement of antidiuretic hormone may be required.
Magnesium is a potentially neuroprotective agent that acts as a glutamate receptor antagonist and calcium channel blocker. Magnesium showed a trend toward a reduced risk for delayed cerebral ischemia in 283 patients with SAH randomized to magnesium or placebo administration.202 Another randomized, placebo-controlled, dose-adapted trial failed to demonstrate a difference in the incidence of delayed ischemic neurological deficits or secondary ischemia.167 Larger phase III clinical trials are ongoing.203
Gastrointestinal Complications
There is a marked increase in urinary catecholamine excretion within the first 3 days after SAH that is probably due to sympathetic hyperactivity. This, as well as increased cortisol, glucagon, and cytokine release, probably contributes to the increased oxygen consumption, carbon dioxide production, and metabolic expenditure noted in SAH patients.204 In addition to a hypermetabolic state, patients with SAH have a catabolic response marked by negative nitrogen balance and impaired ability to use exogenous nitrogen. These changes are similar to those found in patients with other acute, severe neurological illnesses such as head injury.205 This response causes weight loss and may increase the risk for infection and impair wound healing.206 The primary therapy to counteract it is early nutritional support. Few studies have investigated this treatment in patients with SAH, but after stroke there is evidence that early institution of nutritional support improves survival.207 In patients who cannot swallow because of impaired consciousness or focal neurological deficits, nutritional support is best achieved by gastrostomy or jejunostomy tubes rather than by nasogastric tubes.207 It is probably preferable to feed enterally instead of parenterally because parenteral nutrition may be associated with loss of the intestinal mucosal bacterial barrier and thus increased risk for sepsis. Enteral feeding avoids the risk for sepsis from central venous catheters, reduces the incidence of hyperglycemia, and is less expensive. Parenteral nutrition is indicated for the occasional patients who cannot meet their nutritional needs with enteral feeding and in whom this situation is likely to persist for at least 5 days.
Mucosal ulceration (stress ulceration) in the stomach and upper gastrointestinal tract may occur after SAH and can cause serious and sometimes fatal bleeding. Studies specific to patients with SAH are limited but suggest that such lesions occur after SAH just as they do after other serious neurological injuries. Accepted treatment includes neutralization of gastric acid secretion with antacids or drugs that block acid production, such as histamine receptor antagonists (cimetidine, ranitidine) or proton pump inhibitors (omeprazole).208 The use of drugs to prevent gastrointestinal bleeding remains controversial, and recent reviews suggest that prophylaxis can be limited to patients at increased risk, such as those ventilated for more than 48 hours or those with coagulopathy.208 These studies may not apply to brain-injured patients, so withholding prophylaxis is controversial.
Stool softeners are administered routinely to prevent straining, impaction, and abdominal pain. Nimodipine may be associated with gastrointestinal pseudo-obstruction and ileus.209 This usually resolves with conservative measures but may progress to perforation and require surgery.
Postoperative Deterioration
Peerless listed 39 causes of deterioration in patients after SAH (Table 363-9).210 Multiple causes were often found for each episode of worsening. Although he determined that vasospasm accounted for 30% of cases of deterioration, systemic abnormalities and neurological complications must be detected before neurological decline can be attributed to vasospasm. Any factor that disrupts perfusion of brain tissue with well-oxygenated, glucose-rich blood can cause deterioration in patients with aneurysmal SAH. Particularly important systemic factors were hyponatremia, hypoxemia, hypercapnia, hypotension, and cardiac arrhythmias.
TABLE 363-9 Causes of Neurological Deterioration after Subarachnoid Hemorrhage
| Neurological |
SIADH, syndrome of inappropriate diuretic hormone.
Data from Peerless SJ. Pre- and postoperative management of cerebral aneurysms. Clin Neurosurg. 1979;26:209-231.
Special Considerations
Special considerations in the management of SAH include cocaine use, sickle cell disease, and SAH in children and adolescents. Cocaine use is associated with aneurysm rupture, although about half the cocaine-positive patients with intracranial hemorrhage have no underlying vascular anomalies and 15% of those with SAH have no detectable aneurysm. The mean age of such individuals is younger than the SAH population as a whole, but the distribution of aneurysms is similar. The pathophysiology is believed to involve hypertension induced by sympathetic hyperactivity secondary to blockade of norepinephrine reuptake into neurons. When clinically relevant, urine may be tested for cocaine metabolites, which remain detectable for up to 72 hours after use, depending on the frequency and doses used. Outcomes tend to be worse than in patients who have not used cocaine.211 Cocaine use is also associated with myocardial ischemia and arrhythmias, and the clinician should remain vigilant for these conditions.
Sickle cell anemia may be associated with aneurysmal SAH, although it is a less common neurological complication of sickle cell anemia than cerebral infarction or intracerebral hemorrhage.212,213 The pathogenesis of aneurysm formation may involve repeated sickle cell–induced endothelial injury with eventual damage to and destruction of the internal elastic lamina, degeneration of the tunica media, and aneurysm formation. Aneurysms are more likely to be multiple (up to 40% to 60% of reported cases) and tend to be small. Numerous stimuli can induce sickling and should be avoided, including hypoxia, acidosis, infection, volume depletion, hypothermia, and use of contrast media. Recommended management is exchange transfusion until the level of hemoglobin S is below 30% to 40% and the hematocrit is higher than 30% before angiography and then throughout the hospital stay. Supplemental oxygen should be used liberally. Preanesthetic sedation carries a risk of causing respiratory acidosis and hypoxemia and should be avoided. Mannitol and furosemide increase the risk and must therefore be used cautiously and only when brain relaxation cannot be achieved by other measures such as ventricular drainage. Mild hyperventilation is acceptable but carries a risk of excessive vasoconstriction that may precipitate sickling. The patient should not be allowed to become hypothermic. Dehydration and hemoconcentration are to be avoided. During anesthesia, adequate oxygenation, prevention of respiratory acidosis (hypercapnia), maintenance of adequate circulating blood volume, and prevention of hypothermia and venous stasis must be ensured.
Aneurysmal SAH is progressively less common with decreasing age. The clinical findings in older children are no different from those in adults, although neonates and infants may have irritability or other vague symptoms that suggest a diagnosis of meningitis.84 Aneurysms in children may be more likely to be associated with an underlying disorder predisposing to or causing the aneurysm,214 although in most series no cause of the aneurysm was found in the majority of patients. Few studies have specifically addressed the treatment of children with aneurysmal SAH, and at this time, management of pediatric patients with SAH should follow the same general guidelines as those for adults.
Clinical Outcome after Subarachnoid Hemorrhage
A review of 21 studies of SAH published between 1960 and 1992 found that the case fatality rate decreased by 0.5%/yr, which represents a 15% cumulative decrease over a 30-year period.5 The confidence intervals, however, included 0% improvement. Estimates are that 12% of patients die of aneurysmal SAH before reaching medical attention and that 40% of those reaching the hospital die.215 Recent clinical trials, which are probably biased in favor of good-grade patients, show that 31% of patients undergoing surgical clipping and 24% undergoing endovascular coiling of a ruptured aneurysm will be dead or dependent at 1 year.101
Factors associated with poor outcome after SAH include age, neurological grade, extent of SAH on CT, preoperative hypertension, vasospasm, presence of intracerebral or intraventricular hemorrhage or both, and the location and size of the aneurysm (Table 363-10; also see Fig. 363-5).216,217
Bederson JB, Awad IA, Wiebers DO, et al. Recommendations for the management of patients with unruptured intracranial aneurysms: a statement for healthcare professionals from the Stroke Council of the American Heart Association. Stroke. 2000;31:2742-2750.
Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980;6:1-9.
Fountas KN, Kapsalaki EZ, Machinis T, et al. Review of the literature regarding the relationship of rebleeding and external ventricular drainage in patients with subarachnoid hemorrhage of aneurysmal origin. Neurosurg Rev. 2006;29:14-18.
Hillman J, Fridriksson S, Nilsson O, et al. Immediate administration of tranexamic acid and reduced incidence of early rebleeding after aneurysmal subarachnoid hemorrhage: a prospective randomized study. J Neurosurg. 2002;97:771-778.
Hop JW, Rinkel GJ, Algra A, et al. Case-fatality rates and functional outcome after subarachnoid hemorrhage: a systematic review. Stroke. 1997;28:660-664.
Kassell NF, Torner JC, Haley ECJr, et al. The International Cooperative Study on the Timing of Aneurysm Surgery. Part 1: Overall management results. J Neurosurg. 1990;73:18-36.
Klebl FH, Scholmerich J. Therapy insight: prophylaxis of stress-induced gastrointestinal bleeding in critically ill patients. Nat Clin Pract Gastroenterol Hepatol. 2007;4:562-570.
Leal-Noval SR, Munoz-Gomez M, Murillo-Cabezas F. Optimal hemoglobin concentration in patients with subarachnoid hemorrhage, acute ischemic stroke and traumatic brain injury. Curr Opin Crit Care. 2008;14:156-162.
Lee VH, Oh JK, Mulvagh SL, et al. Mechanisms in neurogenic stress cardiomyopathy after aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2006;5:243-249.
Lin CL, Dumont AS, Lieu AS, et al. Characterization of perioperative seizures and epilepsy following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2003;99:978-985.
Linn FH, Rinkel GJ, Algra A, et al. Headache characteristics in subarachnoid haemorrhage and benign thunderclap headache. J Neurol Neurosurg Psychiatry. 1998;65:791-793.
Macdonald RL, Rosengart A, Huo D, et al. Factors associated with the development of vasospasm after planned surgical treatment of aneurysmal subarachnoid hemorrhage. J Neurosurg. 2003;99:644-652.
McCarron MO, Alberts MJ, McCarron P. A systematic review of Terson’s syndrome: frequency and prognosis after subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 2004;75:491-493.
Molyneux A, Kerr R, Stratton I, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360:1267-1274.
Molyneux AJ, Kerr RS, Yu LM, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366:809-817.
Rinkel GJE, Wijdicks EFM, Vermeulen M, et al. Nonaneurysmal perimesencephalic subarachnoid hemorrhage: CT and MR patterns that differ from aneurysmal rupture. Am J Neuroradiol. 1991:157829-157834.
Rosen DS, Macdonald RL. Subarachnoid hemorrhage grading scales: a systematic review. Neurocrit Care. 2005;2:110-118.
Rosengart AJ, Schultheiss KE, Tolentino J, et al. Prognostic factors for outcome in patients with aneurysmal subarachnoid hemorrhage. Stroke. 2007;38:2315-2321.
Sahs AL, Perret GE, Locksley HB, et al. Intracranial Aneurysms and Subarachnoid Hemorrhage. Philadelphia: JB Lippincott; 1969. 1-296
Sherlock M, O’Sullivan E, Agha A, et al. The incidence and pathophysiology of hyponatraemia after subarachnoid haemorrhage. Clin Endocrinol (Oxf). 2006;64:250-254.
Teasdale G, Jennett B. Assessment of impaired consciousness and coma: a practical scale. Lancet. 1974;2:81-84.
van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet. 2007;369:306-318.
Wartenberg KE, Schmidt JM, Claassen J, et al. Impact of medical complications on outcome after subarachnoid hemorrhage. Crit Care Med. 2006;34:617-623.
Weir B. Aneurysms Affecting the Nervous System. Baltimore: Williams & Wilkins; 1987. 1-671
Whitfield PC, Kirkpatrick PJ. Timing of surgery for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. 2001;1:CD001697.
Willinsky RA, Taylor SM, Terbrugge K, et al. Neurologic complications of cerebral angiography: prospective analysis of 2,899 procedures and review of the literature. Radiology. 2003;227:522-528.
1 van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet. 2007;369:306-318.
2 de Rooij NK, Linn FH, van der Plas JA, et al. Incidence of subarachnoid haemorrhage: a systematic review with emphasis on region, age, gender and time trends. J Neurol Neurosurg Psychiatry. 2007;78:1365-1372.
3 Feigin VL, Anderson CS, Rodgers A, et al. Subarachnoid haemorrhage occurrence exhibits a temporal pattern—evidence from meta-analysis. Eur J Neurol. 2002;9:511-516.
4 Ingall TJ, Whisnant JP, Wiebers DO, et al. Has there been a decline in subarachnoid hemorrhage mortality? Stroke. 1989;20:718-724.
5 Hop JW, Rinkel GJ, Algra A, et al. Case-fatality rates and functional outcome after subarachnoid hemorrhage: a systematic review. Stroke. 1997;28:660-664.
6 Stegmayr B, Eriksson M, Asplund K. Declining mortality from subarachnoid hemorrhage: changes in incidence and case fatality from 1985 through 2000. Stroke. 2004;35:2059-2063.
7 Huang FP, Xi G, Keep RF, et al. Brain edema after experimental intracerebral hemorrhage: role of hemoglobin degradation products. J Neurosurg. 2002;96:287-293.
8 Hassler W, Steinmetz H, Gawlowski J. Transcranial Doppler ultrasonography in raised intracranial pressure and in intracranial circulatory arrest. J Neurosurg. 1988;68:745-751.
9 Nornes H. The role of intracranial pressure in the arrest of hemorrhage in patients with ruptured intracranial aneurysm. J Neurosurg. 1973;39:226-234.
10 McCormick PW, McCormick J, Zabramski JM, et al. Hemodynamics of subarachnoid hemorrhage arrest. J Neurosurg. 1994;80:710-715.
11 Kaye AH, Brownbill D. Postoperative intracranial pressure in patients operated on for cerebral aneurysms following subarachnoid hemorrhage. J Neurosurg. 1981;54:726-732.
12 Weir B. Aneurysms Affecting the Nervous System. Baltimore: Williams & Wilkins; 1987. 1-671
13 Grubb RLJ, Raichle ME, Eichling JO, et al. Effects of subarachnoid hemorrhage on cerebral blood volume, blood flow, and oxygen utilization in humans. J Neurosurg. 1977;46:446-453.
14 Yundt KD, Grubb RLJr, Diringer MN, et al. Autoregulatory vasodilation of parenchymal vessels is impaired during cerebral vasospasm. J Cereb Blood Flow Metab. 1998;18:419-424.
15 Meyer CH, Lowe D, Meyer M, et al. Progressive change in cerebral blood flow during the first three weeks after subarachnoid hemorrhage. Neurosurgery. 1983;12:58-76.
16 Carpenter DA, Grubb RLJ, Tempel LW, et al. Cerebral oxygen metabolism after aneurysmal subarachnoid hemorrhage. J Cereb Blood Flow Metab. 1991;11:837-844.
17 Voldby B, Enevoldsen EM, Jensen FT. Regional CBF, intraventricular pressure, and cerebral metabolism in patients with ruptured intracranial aneurysms. J Neurosurg. 1985;62:48-58.
18 Videen TO, Dunford-Shore JE, Diringer MN, et al. Correction for partial volume effects in regional blood flow measurements adjacent to hematomas in humans with intracerebral hemorrhage: implementation and validation. J Comput Assist Tomogr. 1999;23:248-256.
19 Origitano TC, Wascher TM, Reichman OH, et al. Sustained increased cerebral blood flow with prophylactic hypertensive hypervolemic hemodilution (“triple-H” therapy) after subarachnoid hemorrhage. Neurosurgery. 1990;27:729-739.
20 Rosenstein J, Suzuki M, Symon L, et al. Clinical use of a portable bedside cerebral blood flow machine in the management of aneurysmal subarachnoid hemorrhage. Neurosurgery. 1984;15:519-525.
21 Fein JM. Brain energetics and circulatory control after subarachnoid hemorrhage. J Neurosurg. 1976;45:498-507.
22 Hubschmann OR, Nathanson DC. The role of calcium and cellular membrane dysfunction in experimental trauma and subarachnoid hemorrhage. J Neurosurg. 1985;62:698-703.
23 Marzatico F, Gaetani P, Rodriguez y Baena R. Experimental subarachnoid hemorrhage. Lipid peroxidation and Na+,K+-ATPase in different rat brain areas. Mol Chem Neuropathol. 1989;11:99-107.
24 Voldby B, Enevoldsen EM, Jensen FT. Cerebrovascular reactivity in patients with ruptured intracranial aneurysms. J Neurosurg. 1985;62:59-67.
25 Dernbach PD, Little JR, Jones SC, et al. Altered cerebral autoregulation and CO2 reactivity after aneurysmal subarachnoid hemorrhage. Neurosurgery. 1988;22:822-826.
26 Voldby B, Enevoldsen EM. Intracranial pressure changes following aneurysm rupture. Part 1: clinical and angiographic correlations. J Neurosurg. 1982;56:186-196.
27 Byyny RL, Mower WR, Shum N, et al. Sensitivity of noncontrast cranial computed tomography for the emergency department diagnosis of subarachnoid hemorrhage. Ann Emerg Med. 2008;51:697-703.
28 Linn FH, Rinkel GJ, Algra A, et al. Headache characteristics in subarachnoid haemorrhage and benign thunderclap headache. J Neurol Neurosurg Psychiatry. 1998;65:791-793.
29 Bassi P, Bandera R, Loiero M, et al. Warning signs in subarachnoid hemorrhage: a cooperative study. Acta Neurol Scand. 1991;84:277-281.
30 Hauerberg J, Andersen BB, Eskesen V, et al. Importance of the recognition of a warning leak as a sign of a ruptured intracranial aneurysm. Acta Neurol Scand. 1991;83:61-64.
31 Juvela S. Minor leak before rupture of an intracranial aneurysm and subarachnoid hemorrhage of unknown etiology. Neurosurgery. 1992;30:7-11.
32 Sarner M, Rose FC. Clinical presentation of ruptured intracranial aneurysm. J Neurol Neurosurg Psychiatry. 1967;30:67-70.
33 McCarron MO, Alberts MJ, McCarron P. A systematic review of Terson’s syndrome: frequency and prognosis after subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 2004;75:491-493.
34 Fountas KN, Kapsalaki EZ, Lee GP, et al. Terson hemorrhage in patients suffering aneurysmal subarachnoid hemorrhage: predisposing factors and prognostic significance. J Neurosurg. 2008;109:439-444.
35 Sahs AL, Perret GE, Locksley HB, et al. Intracranial Aneurysms and Subarachnoid Hemorrhage. Philadelphia: JB Lippincott; 1969. 1-296
36 Schievink WI, Karemaker JM, Hageman LM. Circumstances surrounding aneurysmal subarachnoid hemorrhage. Surg Neurol. 1989;32:266-272.
37 Kassell NF, Torner JC, Haley ECJr, et al. The International Cooperative Study on the Timing of Aneurysm Surgery. Part 1: Overall management results. J Neurosurg. 1990;73:18-36.
38 Johansson I, Bolander HG, Kourtopoulos H. CT showing early ventricular dilatation after subarachnoidal hemorrhage. Acta Radiol. 1992;33:333-337.
39 Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980;6:1-9.
40 Kistler JP, Crowell RM, Davis KR, et al. The relation of cerebral vasospasm to the extent and location of subarachnoid blood visualized by CT scan: a prospective study. Neurology. 1983;33:424-436.
41 Reilly C, Amidei C, Tolentino J, et al. Clot volume and clearance rate as independent predictors of vasospasm after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2004;101:255-261.
42 Bederson JB, Awad IA, Wiebers DO, et al. Recommendations for the management of patients with unruptured intracranial aneurysms: a statement for healthcare professionals from the Stroke Council of the American Heart Association. Stroke. 2000;31:2742-2750.
43 Dammert S, Krings T, Moller-Hartmann W, et al. Detection of intracranial aneurysms with multislice CT: comparison with conventional angiography. Neuroradiology. 2004;46:427-434.
44 Chappell ET, Moure FC, Good MC. Comparison of computed tomographic angiography with digital subtraction angiography in the diagnosis of cerebral aneurysms: a meta-analysis. Neurosurgery. 2003;52:624-631.
45 White PM, Teasdale EM, Wardlaw JM, et al. Intracranial aneurysms: CT angiography and MR angiography for detection—prospective blinded comparison in a large patient cohort. Radiology. 2001;219:739-749.
46 Harrison MJ, Johnson BA, Gardner GM, et al. Preliminary results on the management of unruptured intracranial aneurysms with magnetic resonance angiography and computed tomographic angiography. Neurosurgery. 1997;40:947-955.
47 Kangasniemi M, Makela T, Koskinen S, et al. Detection of intracranial aneurysms with two-dimensional and three-dimensional multislice helical computed tomographic angiography. Neurosurgery. 2004;54:336-340.
48 Agid R, Lee SK, Willinsky RA, et al. Acute subarachnoid hemorrhage: using 64-slice multidetector CT angiography to “triage” patients’ treatment. Neuroradiology. 2006;48:787-794.
49 Patel MK, Clarke MA. Lumbar puncture and subarachnoid haemorrhage. Postgrad Med J. 1986;62:1021-1024.
50 Duffy GP. Lumbar puncture in spontaneous subarachnoid haemorrhage. BMJ. 1982;285:1163-1164.
51 Archer BD. Computed tomography before lumbar puncture in acute meningitis: a review of the risks and benefits. CMAJ. 1993;148:961-965.
52 Vermeulen M, van Gijn J. The diagnosis of subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 1990;53:365-372.
53 Arora S, Swadron SP, Dissanayake V. Evaluating the sensitivity of visual xanthochromia in patients with subarachnoid hemorrhage. J Emerg Med. 2010;39:13-16.
54 Fiebach JB, Schellinger PD. [Modern magnetic resonance techniques in stroke.]. Radiologe. 2003;43:251-263.
55 Mitchell P, Wilkinson ID, Hoggard N, et al. Detection of subarachnoid haemorrhage with magnetic resonance imaging. J Neurol Neurosurg Psychiatry. 2001;70:205-211.
56 Klucznik RP, Carrier DA, Pyka R, et al. Placement of a ferromagnetic intracerebral aneurysm clip in a magnetic field with a fatal outcome. Radiology. 1993;187:855-856.
57 Payner TD, Tew JMJr, Steiger HJ. Aneurysm clips, 2nd ed. Wilkins RH, Rengachary SS, editors. Neurosurgery, Vol 2. New York: Williams & Wilkins. 1996:2271-2276.
58 Willinsky RA, Taylor SM, Terbrugge K, et al. Neurologic complications of cerebral angiography: prospective analysis of 2,899 procedures and review of the literature. Radiology. 2003;227:522-528.
59 Dion JE, Gates PC, Fox AJ, et al. Clinical events following neuroangiography: a prospective study. Stroke. 1987;18:997-1004.
60 Inagawa T. Effect of ultra-early referral on management outcome in subarachnoid haemorrhage. Acta Neurochir. 1995;136:51-61.
61 Inagawa T. Timing of admission and management outcome in patients with subarachnoid hemorrhage. Surg Neurol. 1994;41:268-276.
62 Almaani WS, Richardson AE. Multiple intracranial aneurysms: identifying the ruptured lesion. Surg Neurol. 1978;9:303-305.
63 Marttila I, Heiskanen O. Value of neurological and angiographic signs as indicators of the ruptured aneurysm in patients with multiple intracranial aneurysms. Acta Neurochir. 1970;23:95-102.
64 Sakamoto T, Kwak R, Mizoi K, et al. [Angiographical study of ruptured aneurysm in the multiple aneurysm patients (author’s transl).]. No Shinkei Geka. 1978;6:549-553.
65 Wood EH. Angiographic identification of the ruptured lesion in patients with multiple cerebral aneurysms. J Neurosurg. 1964;21:182-198.
66 Nehls DG, Flom RA, Carter LP, et al. Multiple intracranial aneurysms: determining the site of rupture. J Neurosurg. 1985;63:342-348.
67 Friedman AH. Subarachnoid hemorrhage of unknown etiology. In: Wilkins RH, Rengachary SS, editors. Neurosurgery Update II. New York: McGraw-Hill; 1991:73-77.
68 Rinkel GJE, Wijdicks EFM, Vermeulen M, et al. Nonaneurysmal perimesencephalic subarachnoid hemorrhage: CT and MR patterns that differ from aneurysmal rupture. Am J Neuroradiol. 1991;157:829-834.
69 Alexander TD, Macdonald RL, Weir B, et al. Intraoperative angiography in cerebral aneurysm surgery: a prospective study of 100 craniotomies. Neurosurgery. 1996;39:10-17.
70 Rates of delayed rebleeding from intracranial aneurysms are low after surgical and endovascular treatment. Stroke. 2006;37:1437-1442.
71 Molyneux AJ, Kerr RS, Yu LM, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366:809-817.
72 Tang G, Cawley CM, Dion JE, et al. Intraoperative angiography during aneurysm surgery: a prospective evaluation of efficacy. J Neurosurg. 2002;96:993-999.
73 Klopfenstein JD, Spetzler RF, Kim LJ, et al. Comparison of routine and selective use of intraoperative angiography during aneurysm surgery: a prospective assessment. J Neurosurg. 2004;100:230-235.
74 Botterell EH, Lougheed WM, Scott JW, et al. Hypothermia, and interruption of carotid, or carotid and vertebral circulation, in the surgical management of intracranial aneurysms. J Neurosurg. 1956;1:31-42.
75 Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968;28:14-20.
76 Drake CG, Hunt WE, Sano K, et al. Report of World Federation of Neurological Surgeons committee on a universal subarachnoid hemorrhage grading scale. J Neurosurg. 1988;68:985-986.
77 Oshiro EM, Walter KA, Piantadosi S, et al. A new subarachnoid hemorrhage grading system based on the Glasgow Coma Scale: a comparison with the Hunt and Hess and World Federation of Neurological Surgeons Scales in a clinical series. Neurosurgery. 1997;41:140-147.
78 Ogilvy CS, Cheung AC, Mitha AP, et al. Outcomes for surgical and endovascular management of intracranial aneurysms using a comprehensive grading system. Neurosurgery. 2006;59:1037-1042.
79 Rosen DS, Macdonald RL. Subarachnoid hemorrhage grading scales: a systematic review. Neurocrit Care. 2005;2:110-118.
80 Rosengart AJ, Huo JD, Tolentino J, et al. Outcome in patients with subarachnoid hemorrhage treated with antiepileptic drugs. J Neurosurg. 2007;107:253-260.
81 Teasdale G, Jennett B. Assessment of impaired consciousness and coma: a practical scale. Lancet. 1974;2:81-84.
82 Lindsay KW, Teasdale G, Knill-Jones RP, et al. Observer variability in grading patients with subarachnoid hemorrhage. J Neurosurg. 1982;56:628-633.
83 Vroomen PCAJ, De Krom MCTFM, Wilmink JT, et al. Lack of effectiveness of bed rest for sciatica. N Engl J Med. 1999;340:418-423.
84 Weir B. Subarachnoid Hemorrhage: Causes and Cures (Contemporary Neurology, vol 52). New York: Oxford University Press; 1998. 1-301
85 Hasan D, Vermeulen M, Wijdicks EFM, et al. Effect of fluid intake and antihypertensive treatment on cerebral ischemia after subarachnoid hemorrhage. Stroke. 1989;20:1511-1515.
86 Asplund K, Israelsson K, Schampi I. Haemodilution for acute ischaemic stroke (Cochrane Review). The Cochrane Library. Oxford, Update Software, 1999.
87 Leal-Noval SR, Munoz-Gomez M, Murillo-Cabezas F. Optimal hemoglobin concentration in patients with subarachnoid hemorrhage, acute ischemic stroke and traumatic brain injury. Curr Opin Crit Care. 2008;14:156-162.
88 Schurkamper M, Medele R, Zausinger S, et al. Dexamethasone in the treatment of subarachnoid hemorrhage revisited: a comparative analysis of the effect of the total dose on complications and outcome. J Clin Neurosci. 2004;11:20-24.
89 DeMaria EJ, Reichman W, Kenney PR, et al. Septic complications of corticosteroid administration after central nervous system trauma. Ann Surg. 1985;202:248-252.
90 Katayama Y, Haraoka J, Hirabayashi H, et al. A randomized controlled trial of hydrocortisone against hyponatremia in patients with aneurysmal subarachnoid hemorrhage. Stroke. 2007;38:2373-2375.
91 Pfeiffer M, Griss P. Craniocerebral trauma and aseptic osteonecrosis. Steroid-induced sequelae after therapy of brain edema. Unfallchirurg. 1992;95:284-287.
92 Stornelli SA, French JD. Subarachnoid hemorrhage factors in prognosis and management. J Neurosurg. 1964;21:769-781.
93 Wijdicks EF, Vermeulen M, Murray GD, et al. The effects of treating hypertension following aneurysmal subarachnoid hemorrhage. Clin Neurol Neurosurg. 1990;92:111-117.
94 Kassell NF, Torner JC. Aneurysmal rebleeding: a preliminary report from the Cooperative Aneurysm Study. Neurosurgery. 1983;13:479-481.
95 Fujii Y, Takeuchi S, Sasaki O, et al. Ultra-early rebleeding in spontaneous subarachnoid hemorrhage. J Neurosurg. 1996;84:35-42.
96 Naidech AM, Janjua N, Kreiter KT, et al. Predictors and impact of aneurysm rebleeding after subarachnoid hemorrhage. Arch Neurol. 2005;62:410-416.
97 Torner JC, Kassell NF, Wallace RB, et al. Preoperative prognostic factors for rebleeding and survival in aneurysm patients receiving antifibrinolytic therapy: report of the cooperative aneurysm study. Neurosurgery. 1981;9:506-513.
98 Weir BK, Kongable GL, Kassell NF, et al. Cigarette smoking as a cause of aneurysmal subarachnoid hemorrhage and risk for vasospasm: a report of the Cooperative Aneurysm Study. J Neurosurg. 1998;89:405-411.
99 Öhman J, Heiskanen O. Timing of operation for ruptured supratentorial aneurysms; a prospective randomized study. J Neurosurg. 1989;70:55-60.
100 Whitfield PC, Kirkpatrick PJ. Timing of surgery for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. 2001;1:CD001697.
101 Molyneux A, Kerr R, Stratton I, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360:1267-1274.
102 Sluzewski M, van Rooij WJ. Early rebleeding after coiling of ruptured cerebral aneurysms: incidence, morbidity, and risk factors. AJNR Am J Neuroradiol. 2005;26:1739-1743.
103 Sluzewski M, van Rooij WJ, Beute GN, et al. Late rebleeding of ruptured intracranial aneurysms treated with detachable coils. AJNR Am J Neuroradiol. 2005;26:2542-2549.
104 Feuerberg I, Lindquist C, Lindqvist M, et al. Natural history of postoperative aneurysm rests. J Neurosurg. 1987;66:30-34.
105 Yasargil MG, Smith RD, Young PH, et al. Microneurosurgery II. Clinical Considerations, Surgery of the Intracranial Aneurysms and Results. Stuttgart: Thieme; 1984. 1-386
106 Roos EJ, Rinkel GJ, Velthuis BK, et al. The relation between aneurysm size and outcome in patients with subarachnoid hemorrhage. Neurology. 2000;54:2334-2336.
107 Tsementzis SA, Hitchcock ER, Meyer CH. Benefits and risks of antifibrinolytic therapy in the management of ruptured intracranial aneurysms. A double-blind placebo-controlled study. Acta Neurochir. 1990;102:1-10.
108 Roos Y, Rinkel G, Vermeulen M, et al. Antifibrinolytic therapy for aneurysmal subarachnoid hemorrhage: a major update of a Cochrane review. Stroke. 2003;34:2308-2309.
109 Beguelin C, Seiler R. Subarachnoid hemorrhage with normal cerebral panangiography. Neurosurgery. 1983;13:409-411.
110 Wijdicks EF, Hasan D, Lindsay KW, et al. Short-term tranexamic acid treatment in aneurysmal subarachnoid hemorrhage. Stroke. 1989;20:1674-1679.
111 Hillman J, Fridriksson S, Nilsson O, et al. Immediate administration of tranexamic acid and reduced incidence of early rebleeding after aneurysmal subarachnoid hemorrhage: a prospective randomized study. J Neurosurg. 2002;97:771-778.
112 Starke RM, Kim GH, Fernandez A, et al. Impact of a protocol for acute antifibrinolytic therapy on aneurysm rebleeding after subarachnoid hemorrhage. Stroke. 2008;39:2617-2621.
113 Hasan D, Vermeulen M, Wijdicks EF, et al. Management problems in acute hydrocephalus after subarachnoid hemorrhage. Stroke. 1989;20:747-753.
114 Graff-Radford NF, Torner J, Adams HPJr, et al. Factors associated with hydrocephalus after subarachnoid hemorrhage. A report of the cooperative aneurysm study. Arch Neurol. 1989;46:744-752.
115 Dorai Z, Hynan LS, Kopitnik TA, et al. Factors related to hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2003;52:763-769.
116 van Gijn J, Hijdra A, Wijdicks EF, et al. Acute hydrocephalus after aneurysmal subarachnoid hemorrhage. J Neurosurg. 1985;63:355-362.
117 Mohr G, Ferguson G, Khan M, et al. Intraventricular hemorrhage from ruptured aneurysm. Retrospective analysis of 91 cases. J Neurosurg. 1983;58:482-487.
118 Fountas KN, Kapsalaki EZ, Machinis T, et al. Review of the literature regarding the relationship of rebleeding and external ventricular drainage in patients with subarachnoid hemorrhage of aneurysmal origin. Neurosurg Rev. 2006;29:14-18.
119 Paine JT, Batjer HH, Samson D. Intraoperative ventricular puncture. Neurosurgery. 1988;22:1107-1109.
120 Klopfenstein JD, Kim LJ, Feiz-Erfan I, et al. Comparison of rapid and gradual weaning from external ventricular drainage in patients with aneurysmal subarachnoid hemorrhage: a prospective randomized trial. J Neurosurg. 2004;100:225-229.
121 Hasan D, Tanghe HL. Distribution of cisternal blood in patients with acute hydrocephalus after subarachnoid hemorrhage. Ann Neurol. 1992;31:374-378.
122 Rosen DS, Macdonald RL, Huo D, et al. Intraventricular hemorrhage from ruptured aneurysm: clinical characteristics, complications, and outcomes in a large, prospective, multicenter study population. J Neurosurg. 2007;107:261-265.
123 Macdonald RL, Rosengart A, Huo D, et al. Factors associated with the development of vasospasm after planned surgical treatment of aneurysmal subarachnoid hemorrhage. J Neurosurg. 2003;99:644-652.
124 Shapiro SA, Campbell RL, Scully T. Hemorrhagic dilation of the fourth ventricle: an ominous predictor. J Neurosurg. 1994;80:805-809.
125 Vereecken KK, Van Havenbergh T, De Beuckelaar W, et al. Treatment of intraventricular hemorrhage with intraventricular administration of recombinant tissue plasminogen activator: a clinical study of 18 cases. Clin Neurol Neurosurg. 2006;108:451-455.
126 Varelas PN, Rickert KL, Cusick J, et al. Intraventricular hemorrhage after aneurysmal subarachnoid hemorrhage: pilot study of treatment with intraventricular tissue plasminogen activator. Neurosurgery. 2005;56:205-213.
127 Findlay JM, Jacka MJ. Cohort study of intraventricular thrombolysis with recombinant tissue plasminogen activator for aneurysmal intraventricular hemorrhage. Neurosurgery. 2004;55:532-537.
128 Bailes JE, Spetzler RF, Hadley MN, et al. Management morbidity and mortality of poor-grade aneurysm patients. J Neurosurg. 1990;72:559-566.
129 Mack WJ, King RG, Hoh DJ, et al. An improved functional neurological examination for use in nonhuman primate studies of focal reperfused cerebral ischemia. Neurol Res. 2003;25:280-284.
130 Mocco J, Prickett CS, Komotar RJ, et al. Potential mechanisms and clinical significance of global cerebral edema following aneurysmal subarachnoid hemorrhage. Neurosurg Focus. 2007;22(5):E7.
131 Pasqualin A, Bazzan A, Cavanazzi P, et al. Intracranial hematomas following aneurysmal rupture: experience with 309 cases. Surg Neurol. 1986;25:6-17.
132 Benoit RG, Cochrane DD, Durity F, et al. Clinical-radiological correlates in intracerebral hematomas due to aneurysmal rupture. Can J Neurol Sci. 1982;9:409-414.
133 Jeong JH, Koh JS, Kim EJ. A less invasive approach for ruptured aneurysm with intracranial hematoma: coil embolization followed by clot evacuation. Korean J Radiol. 2007;8:2-8.
134 Hart RG, Byer JA, Slaughter JR, et al. Occurrence and implications of seizures in subarachnoid hemorrhage due to ruptured intracranial aneurysms. Neurosurgery. 1981;8:417-421.
135 Hasan D, Schonck RS, Avezaat CJ, et al. Epileptic seizures after subarachnoid hemorrhage. Ann Neurol. 1993;33:286-291.
136 Sbeih I, Tamas LB, O’Laoire SA. Epilepsy after operation for aneurysms. Neurosurgery. 1986;19:784-788.
137 Baker CJ, Prestigiacomo CJ, Solomon RA. Short-term perioperative anticonvulsant prophylaxis for the surgical treatment of low-risk patients with intracranial aneurysms. Neurosurgery. 1995;37:863-870.
138 Temkin NR, Dikmen SS, Wilensky AJ, et al. A randomized, double-blind study of phenytoin for the prevention of post-traumatic seizures. N Engl J Med. 1990;323:497-502.
139 Rose FC, Sarner M. Epilepsy after ruptured intracranial aneurysm. BMJ. 1965;1:18-21.
140 Lin CL, Dumont AS, Lieu AS, et al. Characterization of perioperative seizures and epilepsy following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2003;99:978-985.
141 Fabinyi GCA, Artiola-Fortuny L. Epilepsy after craniotomy for intracranial aneurysm. Lancet. 1980;1:1299-1300.
142 Ohman J. Hypertension as a risk factor for epilepsy after aneurysmal subarachnoid hemorrhage and surgery. Neurosurgery. 1990;27:578-581.
143 Claassen J, Peery S, Kreiter KT, et al. Predictors and clinical impact of epilepsy after subarachnoid hemorrhage. Neurology. 2003;60:208-214.
144 Solenski NJ, Haley ECJr, Kassell NF, et al. Medical complications of aneurysmal subarachnoid hemorrhage: a report of the multicenter, cooperative aneurysm study. Participants of the Multicenter Cooperative Aneurysm Study. Crit Care Med. 1995;23:1007-1017.
145 Wartenberg KE, Schmidt JM, Claassen J, et al. Impact of medical complications on outcome after subarachnoid hemorrhage. Crit Care Med. 2006;34:617-623.
146 Fernandez A, Schmidt JM, Claassen J, et al. Fever after subarachnoid hemorrhage: risk factors and impact on outcome. Neurology. 2007;68:1013-1019.
147 Rosengart AJ, Schultheiss KE, Tolentino J, et al. Prognostic factors for outcome in patients with aneurysmal subarachnoid hemorrhage. Stroke. 2007;38:2315-2321.
148 Naidech AM, Bendok BR, Bernstein RA, et al. Fever burden and functional recovery after subarachnoid hemorrhage. Neurosurgery. 2008;63:212-217.
149 Stocchetti N, Protti A, Lattuada M, et al. Impact of pyrexia on neurochemistry and cerebral oxygenation after acute brain injury. J Neurol Neurosurg Psychiatry. 2005;76:1135-1139.
150 Rossi S, Colombo A, Magnoni S, et al. Cerebral veno-arterial pCO2 difference as an estimator of uncompensated cerebral hypoperfusion. Acta Neurochir Suppl. 2002;81:201-204.
151 Ginsberg MD, Busto R. Combating hyperthermia in acute stroke. A significant clinical concern. Stroke. 1998;29:529-534.
152 Oliveira-Filho J, Ezzeddine MA, Segal AZ, et al. Fever in subarachnoid hemorrhage: relationship to vasospasm and outcome. Neurology. 2001;56:1299-1304.
153 Badjatia N, O’Donnell J, Baker JR, et al. Achieving normothermia in patients with febrile subarachnoid hemorrhage: feasibility and safety of a novel intravascular cooling catheter. Neurocrit Care. 2004;1:145-156.
154 Mayer SA, Kowalski RG, Presciutti M, et al. Clinical trial of a novel surface cooling system for fever control in neurocritical care patients. Crit Care Med. 2004;32:2508-2515.
155 Diringer MN. Treatment of fever in the neurologic intensive care unit with a catheter-based heat exchange system. Crit Care Med. 2004;32:559-564.
156 Solomon RA, Post KD, McMurtry JGIII. Depression of circulating blood volume in patients after subarachnoid hemorrhage: implications for the management of symptomatic vasospasm. Neurosurgery. 1984;15:354-361.
157 Naidech AM, Jovanovic B, Wartenberg KE, et al. Higher hemoglobin is associated with improved outcome after subarachnoid hemorrhage. Crit Care Med. 2007;35:2383-2389.
158 Smith MJ, Le Roux PD, Elliott JP, et al. Blood transfusion and increased risk for vasospasm and poor outcome after subarachnoid hemorrhage. J Neurosurg. 2004;101:1-7.
159 Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409-417.
160 McMahon TJ, Moon RE, Luschinger BP, et al. Nitric oxide in the human respiratory cycle. Nat Med. 2002;8:711-717.
161 Moore EE. Blood substitutes: the future is now. J Am Coll Surg. 2003;196:1-17.
162 Berezina TL, Zaets SB, Morgan C, et al. Influence of storage on red blood cell rheological properties. J Surg Res. 2002;102:6-12.
163 Shanwell A, Kristiansson M, Remberger M, et al. Generation of cytokines in red cell concentrates during storage is prevented by prestorage white cell reduction. Transfusion. 1997;37:678-684.
164 Siren AL, Fratelli M, Brines M, et al. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci U S A. 2001;98:4044-4049.
165 Frontera JA, Fernandez A, Claassen J, et al. Hyperglycemia after SAH: predictors, associated complications, and impact on outcome. Stroke. 2006;37:199-203.
166 Van den Berghe G, Schoonheydt K, Becx P, et al. Insulin therapy protects the central and peripheral nervous system of intensive care patients. Neurology. 2005;64:1348-1353.
167 Prakash A, Matta BF. Hyperglycaemia and neurological injury. Curr Opin Anaesthesiol. 2008;21:565-569.
168 Hijdra A, Vermeulen M, van Gijn J, et al. Respiratory arrest in subarachnoid hemorrhage. Neurology. 1984;34:1501-1503.
169 Medlock MD, Dulebohn SC, Elwood PW. Prophylactic hypervolemia without calcium channel blockers in early aneurysm surgery. Neurosurgery. 1992;30:12-16.
170 Weir BK. Pulmonary edema following fatal aneurysm rupture. J Neurosurg. 1978;49:502-507.
171 Muroi C, Terzic A, Fortunati M, et al. Magnesium sulfate in the management of patients with aneurysmal subarachnoid hemorrhage: a randomized, placebo-controlled, dose-adapted trial. Surg Neurol. 2008;69:33-39.
172 Kondoh T, Kuwamura K, Miyata M, et al. [Early surgery of ruptured intracranial aneurysms associated with neurogenic pulmonary edema. Report of two cases.]. Neurol Med Chir (Tokyo). 1988;28:1107-1112.
173 Niikawa S, Nokura H, Uno T, et al. [Neurogenic pulmonary edema following aneurysmal subarachnoid hemorrhage. Report of 9 cases.]. Neurol Med Chir (Tokyo). 1988;28:157-163.
174 Smith WS, Matthay MA. Evidence for a hydrostatic mechanism in human neurogenic pulmonary edema. Chest. 1997;111:1326-1333.
175 Kollef MH. The prevention of ventilator-associated pneumonia. N Engl J Med. 1999;340:627-634.
176 D’Amelio LF, Hammond JS, Spain DA, et al. Tracheostomy and percutaneous endoscopic gastrostomy in the management of the head-injured trauma patient. Am Surg. 1994;60:180-185.
177 Sakr YL, Lim N, Amaral AC, et al. Relation of ECG changes to neurological outcome in patients with aneurysmal subarachnoid hemorrhage. Int J Cardiol. 2004;96:369-373.
178 van den Bergh WM, Algra A, Rinkel GJ. Electrocardiographic abnormalities and serum magnesium in patients with subarachnoid hemorrhage. Stroke. 2004;35:644-648.
179 Mayer SA, LiMandri G, Sherman D, et al. Electrocardiographic markers of abnormal left ventricular wall motion in acute subarachnoid hemorrhage. J Neurosurg. 1995;83:889-896.
180 Tanabe M, Crago EA, Suffoletto MS, et al. Relation of elevation in cardiac troponin I to clinical severity, cardiac dysfunction, and pulmonary congestion in patients with subarachnoid hemorrhage. Am J Cardiol. 2008;102:1545-1550.
181 Lee VH, Oh JK, Mulvagh SL, et al. Mechanisms in neurogenic stress cardiomyopathy after aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2006;5:243-249.
182 Pollick C, Cujec B, Parker S, et al. Left ventricular wall motion abnormalities in subarachnoid hemorrhage: an echocardiographic study. J Am Coll Cardiol. 1988;12:600-605.
183 Lee VH, Connolly HM, Fulgham JR, et al. Tako-tsubo cardiomyopathy in aneurysmal subarachnoid hemorrhage: an underappreciated ventricular dysfunction. J Neurosurg. 2006;105:264-270.
184 Naidech A, Du Y, Kreiter KT, et al. Dobutamine versus milrinone after subarachnoid hemorrhage. Neurosurgery. 2005;56:21-27.
185 Frontera JA, Parra A, Shimbo D, et al. Cardiac arrhythmias after subarachnoid hemorrhage: risk factors and impact on outcome. Cerebrovasc Dis. 2008;26:71-78.
186 Estanol VB, Badui DE, Cesarman E, et al. Cardiac arrhythmias associated with subarachnoid hemorrhage: prospective study. Neurosurgery. 1979;5:675-680.
187 Brouwers PJAM, Wijdicks EFM, Hasan D, et al. Serial electrocardiographic recording in aneurysmal subarachnoid hemorrhage. Stroke. 1989;20:1162-1167.
188 Collins R, Scrimgeour A, Yusuf S, et al. Reduction in fatal pulmonary embolism and venous thrombosis by perioperative administration of subcutaneous heparin: Overview of results of randomized clinical trials in general, orthopedic, and urologic surgery. N Engl J Med. 1988;318:1162-1173.
189 Hirsh J. Comparison of the relative efficacy and safety of low molecular weight heparin and unfractionated heparin for the treatment of venous thrombosis. Haemostasis. 1996;26(suppl 4):189-198.
190 Bucci MN, Papadopoulos SM, Chen JC, et al. Mechanical prophylaxis of venous thrombosis in patients undergoing craniotomy: a randomized trial. Surg Neurol. 1989;32:285-288.
191 Turpie AG, Hirsh J, Gent M, et al. Prevention of deep vein thrombosis in potential neurosurgical patients. A randomized trial comparing graduated compression stockings alone or graduated compression stockings plus intermittent pneumatic compression with control. Arch Intern Med. 1989;149:679-681.
192 Lazio BE, Simard JM. Anticoagulation in neurosurgical patients. Neurosurgery. 1999;45:838-847.
193 Decousus H, Leizorovicz A, Parent F, et al. A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. N Engl J Med. 1998;338:409-415.
194 McGirt MJ, Blessing R, Nimjee SM, et al. Correlation of serum brain natriuretic peptide with hyponatremia and delayed ischemic neurological deficits after subarachnoid hemorrhage. Neurosurgery. 2004;54:1369-1373.
195 Qureshi AI, Suri MF, Sung GY, et al. Prognostic significance of hypernatremia and hyponatremia among patients with aneurysmal subarachnoid hemorrhage. Neurosurgery. 2002;50:749-755.
196 Sherlock M, O’Sullivan E, Agha A, et al. The incidence and pathophysiology of hyponatraemia after subarachnoid haemorrhage. Clin Endocrinol (Oxf). 2006;64:250-254.
197 Cole CD, Gottfried ON, Liu JK, et al. Hyponatremia in the neurosurgical patient: diagnosis and management. Neurosurg Focus. 2004;16(4):E9.
198 Diringer MN, Wu KC, Verbalis JG, et al. Hypervolemic therapy prevents volume contraction but not hyponatremia following subarachnoid hemorrhage. Ann Neurol. 1992;31:543-550.
199 Hasan D, Lindsay KW, Wijdicks EFM, et al. Effect of fludrocortisone acetate in patients with subarachnoid hemorrhage. Stroke. 1989;20:1156-1161.
200 Berendes E, Walter M, Cullen P, et al. Secretion of brain natriuretic peptide in patients with aneurysmal subarachnoid haemorrhage. Lancet. 1997;349:245-249.
201 Takaku A, Shindo K, Tanaka S, et al. Fluid and electrolyte disturbances in patients with intracranial aneurysms. Surg Neurol. 1979;11:349-356.
202 van den Bergh WM, Algra A, van Kooten F, et al. Magnesium sulfate in aneurysmal subarachnoid hemorrhage: a randomized controlled trial. Stroke. 2005;36:1011-1015.
203 Dorhout Mees SM. Magnesium in Aneurysmal Subarachnoid Hemorrhage (MASH II) phase III clinical trial. MASH-II study group. Int J Stroke. 2008;3:63-65.
204 Toho H, Sawada T, Karasawa J, et al. [Metabolic response to hypertensive intracerebral hemorrhage and ruptured intracranial aneurysm.]. Neurol Med Chir (Tokyo). 1986;26:683-688.
205 Gadisseux P, Ward JD, Young HF, et al. Nutrition and the neurosurgical patient. J Neurosurg. 1984;60:219-232.
206 Touho H, Karasawa J, Shishido H, et al. Hypermetabolism in the acute stage of hemorrhagic cerebrovascular disease. J Neurosurg. 1990;72:710-714.
207 Norton B, Homer-Ward M, Donnelly MT, et al. A randomised prospective comparison of percutaneous endoscopic gastrostomy and nasogastric tube feeding after acute dysphagic stroke. BMJ. 1996;312:13-21.
208 Klebl FH, Scholmerich J. Therapy insight: prophylaxis of stress-induced gastrointestinal bleeding in critically ill patients. Nat Clin Pract Gastroenterol Hepatol. 2007;4:562-570.
209 Wadworth AN, McTavish D. Nimodipine. A review of its pharmacological properties, and therapeutic efficacy in cerebral disorders. Drugs Aging. 1992;2:262-286.
210 Peerless SJ. Pre- and postoperative management of cerebral aneurysms. Clin Neurosurg. 1979;26:209-231.
211 Oyesiku NM, Colohan AR, Barrow DL, et al. Cocaine-induced aneurysmal rupture: an emergent negative factor in the natural history of intracranial aneurysms? Neurosurgery. 1993;32:518-525.
212 Oyesiku NM, Barrow DL, Eckman JR, et al. Intracranial aneurysms in sickle-cell anemia: clinical features and pathogenesis. J Neurosurg. 1991;75:356-363.
213 Batjer HH, Adamson TE, Bowman GW. Sickle cell disease and aneurysmal subarachnoid hemorrhage. Surg Neurol. 1991;36:145-149.
214 Meyer FB, Sundt TMJr, Fode NC, et al. Cerebral aneurysms in childhood and adolescence. J Neurosurg. 1989;70:420-425.
215 Schievink WI. Intracranial aneurysms. N Engl J Med. 1997;336:28-40.
216 Fortuny L, Prieto-Valiente L. Long-term prognosis in surgically treated intracranial aneurysms. Part 2: Morbidity. J Neurosurg. 1981;54:35-43.
217 Disney L, Weir B, Grace M. Factors influencing the outcome of aneurysm rupture in poor grade patients: a prospective series. Neurosurgery. 1988;23:1-9.