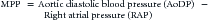Chapter 34 Performance of Cardiopulmonary Resuscitation in Infants and Children
Pediatric cardiac arrest is not a rare event. Approximately 16,000 American children (8-20/100,000 children/year) experience cardiopulmonary arrest each year.1–5 Approximately half of these cardiac arrests occur in-hospital, and about half outside the hospital.5,6 In times past, survival outcomes were not good and many children had severe neurological injury after their arrest event. With advances in resuscitation science and implementation techniques, survival from pediatric cardiac arrest has improved substantially over the past 25 years.7 This chapter focuses on pediatric cardiac arrest, cardiopulmonary resuscitation (CPR), and other therapeutic interventions that have been specifically designed to improve outcomes from pediatric cardiac arrest.
Four Phases of Cardiac Arrest
The four distinct phases of cardiac arrest and CPR interventions are (1) prearrest, (2) no flow (untreated cardiac arrest), (3) low flow (CPR), and (4) postresuscitation. Interventions to improve the outcome of pediatric cardiac arrest should optimize therapies targeted to the time and phase of CPR, as suggested in Table 34-1.
Table 34–1 Phases of Cardiac Arrest and Targeted Interventions
| Phase | Interventions |
|---|---|
| Prearrest phase: Protect |
Prearrest
The prearrest phase refers to relevant preexisting conditions of the child (e.g., neurologic, cardiac, respiratory, or metabolic problems) and precipitating events (e.g., respiratory failure or shock). It is known that pediatric patients who suffer an in-hospital cardiac arrest often have changes in their physiological status in the hours leading up to their arrest event.8,9 Therefore, interventions during the prearrest phase focus on preventing the cardiac arrest, with special attention to early recognition and treatment of respiratory failure and shock. Rapid-response teams or medical emergency teams (METs) are in-hospital emergency teams designed specifically for this purpose. These teams respond to patients on general inpatient units who are at high risk of clinical decompensation and transfer these children to more acute care areas, with the goal to prevent progression to full cardiac arrest. Implementation of pediatric METs has been moderately successful; decreased cardiac arrest frequency and mortality have been demonstrated.10–12 While METs cannot identify all children at risk for cardiac arrest, it seems reasonable to assume that transferring critically ill children to an intensive care unit (ICU) early in their disease process for better monitoring and more aggressive interventions can improve resuscitative care and clinical outcome.
No Flow/Low Flow
In order to improve outcomes from pediatric cardiac arrest, it is imperative to shorten the no-flow phase of untreated cardiac arrest. To that end, it is important to monitor high-risk patients to allow early recognition of the cardiac arrest and prompt initiation of basic and advanced life support. Effective CPR optimizes coronary perfusion pressure and cardiac output to critical organs to support vital organ viability during the low-flow phase. Important tenets of basic life support are push hard, push fast, allow full chest recoil between compressions, and minimize interruptions of chest compression. Achieving optimal coronary perfusion pressure, exhaled carbon dioxide concentration, and cardiac output during the low-flow phase of CPR is consistently associated with an improved chance for return of spontaneous circulation (ROSC) and improved short and long term outcome in both animal and human studies.13–20 For ventricular fibrillation (VF) and pulseless ventricular tachycardia (VT), rapid detection and prompt defibrillation are vital for successful resuscitation. For cardiac arrests resulting from asphyxia and/or ischemia, provision of adequate myocardial perfusion and myocardial oxygen delivery are most important.
Epidemiology of Pediatric Cardiac Arrest
Cardiovascular disease remains the most common cause of disease-related death in the United States, resulting in approximately 1 million deaths per year.21 It is estimated that more than 400,000 Americans will have a cardiac arrest each year, nearly 90% in prehospital settings. While data regarding the incidence of childhood cardiopulmonary arrest are less robust, the best data suggest that about 16,000 American children suffer a cardiac arrest each year (annual incidence: 8 to 20 per 100,000 children per year).1–522 For in-hospital arrests specifically, it is estimated that approximately 2% to 6% of all children admitted to pediatric intensive care units,1,2,23 and 4% to 6% of children admitted to cardiac units will suffer a cardiac arrest.24,25 In short, pediatric cardiac arrest is an important public health problem.
Outcomes from pediatric cardiac arrest have improved significantly over the past 20 years (Table 34-2). Nearly two thirds of children who have an in-hospital cardiac arrest are successfully resuscitated initially (i.e., attain sustained ROSC). Moreover, more than 25% of them will survive to hospital discharge, and many (nearly 75%) will have good neurologic function.1–4,7,25–37 Factors that influence outcome from pediatric cardiac arrest include (1) the preexisting condition of the child, (2) the initial electrocardiographic (ECG) rhythm detected, (3) the duration of no-flow time (the time during an arrest without spontaneous circulation or provision of CPR), and (4) the quality of the life-supporting therapies provided during the resuscitation. With this knowledge, it is no surprise then that out-of-hospital pediatric arrests have worse outcomes compared to in-hospital arrests.3,22,23,30,35,38–44 As many of these out-of-hospital events are not witnessed and bystander CPR is not common (less than 30% of children receive bystander CPR),3 the duration of no-flow time can be prolonged. As a result, less than 10% of these children survive their initial event, and in those that do survive, neurological injury is common. These findings are especially troublesome given that bystander CPR more than doubles patient survival rates.45
Table 34–2 Cardiac Arrest Outcomes for Both In-Hospital and Out-of-Hospital Pediatric Cardiac Arrest
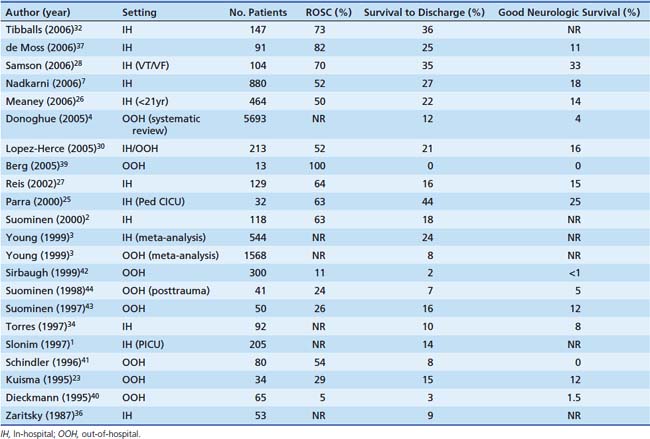
Compared to adults, superior survival rates are documented after pediatric cardiac arrest, specifically after in-hospital events; 27% of children survive to hospital discharge compared with only 17% of adults.7 These findings may be in part due to differences in the initial ECG rhythm detected. While pediatric arrests are less commonly caused by arrythmias, such as ventricular tachycardia or ventricular fibrillation—10% of pediatric arrests versus 25% of adult arrests—the superior pediatric survival rate reflects a substantially higher survival rate among children with asystole or pulseless electrical activity compared with adults (24% vs. 11%). Moreover, the higher survival rate seen in children is mostly attributable to a much better survival rate among infants and preschool age children compared with older children.26 Although this is speculative, the higher survival rates in children may be due to improved coronary and cerebral blood flow during CPR because of increased chest compliance in these younger arrest victims.46,47
Interventions During the Low-Flow Phase: Cardiopulmonary Resuscitation
Airway and Breathing
During the low-flow state of CPR, cardiac output and pulmonary blood flow are approximately 25% of that during normal sinus rhythm; therefore, much less ventilation is necessary for adequate gas exchange from the blood traversing the pulmonary circulation. Moreover, animal and adult data indicate that a rapid rate of assisted ventilation (“overventilation” from exuberant rescue breathing) during CPR is common and can substantially compromise venous return and cardiac output by increasing intrathoracic pressure.48–50 Moreover, these detrimental hemodynamic effects are compounded when one considers the effect of interruptions in CPR to provide airway management and rescue breathing.51–55 While overventilation is problematic, in light of the fact that most pediatric arrests are asphyxial in nature, provision of adequate ventilation is still important. The difference between arrythmogenic and asphyxial arrests lies in the physiology. In animal models of sudden VF cardiac arrest, acceptable PaO2 and PaCO2 persist for 4 to 8 minutes during chest compressions without rescue breathing.56 This is in part because aortic oxygen and carbon dioxide concentrations at the onset of the arrest do not vary much from the prearrest state. As a result, the lungs act as a reservoir of oxygen during CPR, and adequate oxygenation and ventilation can continue without rescue breathing. However, during asphyxial arrest, blood continues to flow to tissues in the prearrest state, resulting in significant arterial and venous oxygen desaturation, elevated lactate levels, and depletion of the pulmonary oxygen reserve. Therefore, at the onset of resuscitation, there is substantial arterial hypoxemia and acidemia. In this circumstance, rescue breathing with controlled ventilation can be lifesaving. In contrast, the adverse hemodynamic effects from overventilation during CPR combined with the interruptions in chest compressions to open the airway and deliver rescue breathing are a lethal combination in certain circumstances such as VT/VF arrests. In short, the resuscitation technique should be titrated to the physiology of the patient to optimize patient outcome.
Circulation
Optimizing Blood Flow During Low-Flow Cardiopulmonary Resuscitation: Push Hard, Push Fast
When the heart arrests and no blood flows to the aorta, coronary blood flow ceases immediately.57 At that point, provision of high-quality CPR (push hard, push fast) is necessary to reestablish flow. The goal during CPR is to maximize the myocardial perfusion pressure (MPP). Related by the following equation:
myocardial blood flow improves as the gradient between AoDP and RAP increases. During downward compression phase, aortic pressure rises at the same time as right atrial pressure with little change in the MPP. However, during the decompression phase of chest compressions, the right atrial pressure falls faster and lower than the aortic pressure, which generates a pressure gradient perfusing the heart with oxygenated blood during this artificial period of “diastole.” Several animal and human studies have demonstrated in both VT/VF and asphyxial models the importance of establishing MPP as a predictor for short term survival outcome (ROSC).19,58–61
Based on the equation above, MPP can be improved by strategies that increase the pressure gradient between the aorta and the right atrium. As an example, the inspiratory impedance threshold device (ITD) is a small, disposable valve that can be connected directly to the tracheal tube or face mask to augment negative intrathoracic pressure during the inspiratory phase of spontaneous breathing and the decompression phase of CPR by impeding airflow into the lungs. Application in animal and adult human trials of CPR has established the ability of the ITD to improve vital organ perfusion pressures and myocardial blood flow51,62–65; however, in the only randomized trial during adult CPR, mortality benefit was limited to the subgroup of patients with pulseless electrical activity.66 Additional evidence that augmentation of negative intrathoracic pressure can improve perfusion pressures during CPR comes from the active compression-decompression device (ACD). The ACD is a handheld device that is fixed to the anterior chest of the victim by means of suction—think household plunger—that can be used to apply active decompression forces during the release phase, thereby creating a vacuum within the thorax. By actively pulling during the decompression phase, blood is drawn back into the heart by the negative pressure.67 Animal and adult studies have demonstrated that the combination of ACD with ITD acts in concert to further improve perfusion pressures during CPR compared to ACD alone.63 In the end, while novel interventions such as the ITD and ACD are promising to improve blood flow during CPR, the basic tenants of “push hard, push fast, minimize interruptions, and don’t overventilate” are still the dominate factors to improve blood flow during CPR and chance of survival.
Chest Compression Depth
The pediatric chest compression depth recommendation of at least one-third anterior-posterior chest depth (approximately 4 cm in infants and 5 cm in children) is based largely upon expert clinical consensus, using data extrapolated from animal, adult, and limited pediatric data. Recently, Maher et al. published data from a case series of infants postcardiac surgery associating arterial blood pressure with qualitative chest compression depths. In this small study of 6 infants, chest compressions targeted to one-half anterior-posterior chest depth imparted improved systolic blood pressures compared to those at one-third anterior-posterior chest depth.68 While a small series with qualitatively estimated chest compression depths, this is the first study to collect actual data from children supporting the existing chest compression depth guidelines. On the contrary, two recent studies using computer-automated tomography69,70 suggest that depth recommendations based on a relative (%) anterior-posterior chest compression depth are deeper than those recommended for adults, and that a depth of one-half anterior-posterior chest depth is unattainable in most children. Future studies that collect data from actual children and that associate quantitatively measured chest compression depths with short- and long-term clinical outcomes (arterial blood pressure, end-tidal carbon dioxide, return of spontaneous circulation, survival) are needed.
Compression/Ventilation Ratios
The amount of ventilation provided during CPR should match, but not exceed, perfusion and should be titrated to the amount of circulation during the specific phase of resuscitation as well as the metabolic demand of the tissues. Therefore during the low-flow state of CPR when the amount of cardiac output is roughly 25% of normal, less ventilation is needed.71 However, the best ratio of compressions to ventilations in pediatric patients is largely unknown and depends on many factors including the compression rate, the tidal volume, the blood flow generated by compressions, and the time that compressions are interrupted to perform ventilations. Recent evidence demonstrated that a compression/ventilation ratio of 15:2 delivers the same minute ventilation and increases the number of delivered chest compressions by 48% compared to CPR at a compression/ventilation ratio of 5:1 in a simulated pediatric arrest model.72,73 This is important because when chest compressions cease, the aortic pressure rapidly decreases and coronary perfusion pressure falls rapidly.57 Increasing the ratio of compressions to ventilations minimizes these interruptions, thus increasing coronary blood flow. These findings are in part the reason the American Heart Association (AHA) now recommends a pediatric compression/ventilation ratio of 15:2.
Duty Cycle
In a model of human adult cardiac arrest, cardiac output and coronary blood flow are optimized when chest compressions last for 30% of the total cycle time (approximately 1:2 ratio of time in compression to time in relaxation).74 As the duration of CPR increases, the optimal duty cycle may increase to 50%. In a juvenile swine model, a relaxation period of 250 to 300 milliseconds (duty cycle of 40% to 50% at a compression rate of 120/min) correlates with improved cerebral perfusion pressures compared with shorter duty cycles of 30%.75
Circumferential Versus Focal Sternal Compressions
In adult and animal models of cardiac arrest, circumferential (vest) CPR has been demonstrated to improve CPR hemodynamics dramatically.76 In smaller infants, it is often possible to encircle the chest with both hands and depress the sternum with the thumbs, while compressing the thorax circumferentially (thoracic squeeze). In an infant animal model of CPR, this “two-thumb” method of compression with thoracic squeeze resulted in higher systolic and diastolic blood pressures and a higher pulse pressure than traditional two-finger compression of the sternum.77
Open-Chest Cardiopulmonary Resuscitation
Excellent standard closed-chest CPR generates cerebral blood flow that is approximately 50% of normal. By contrast, open-chest CPR can generate cerebral blood flow that approaches normal. Whereas open-chest massage improves coronary perfusion pressure and increases the chance of successful defibrillation in animals and humans,78–80 performing a thoracotomy to allow open-chest CPR is impractical in many situations. A retrospective review of 27 cases of CPR following pediatric blunt trauma (15 with open-chest CPR and 12 with closed-chest CPR) demonstrated that open-chest CPR increased hospital cost without altering rates of ROSC or survival to discharge. However, survival in both groups was 0%, indicating that the population may have been too severely injured or too late in the process to benefit from this aggressive therapy.81 Earlier institution of open-chest CPR may warrant reconsideration in selected special resuscitation circumstances.
Medications Used to Treat Cardiac Arrest
Vasopressors
Epinephrine (adrenaline) is an endogenous catecholamine with potent α- and β-adrenergic stimulating properties. The α-adrenergic action (vasoconstriction) increases systemic and pulmonary vascular resistance. The resultant higher aortic diastolic blood pressure improves coronary perfusion pressure and myocardial blood flow even though it reduces global cardiac output during CPR. Adequacy of myocardial blood flow is a critical determinant of ROSC. Epinephrine also increases cerebral blood flow during CPR because peripheral vasoconstriction directs a greater proportion of flow to the cerebral circulation.82–84 However, recent evidence suggests that epinephrine can decrease local cerebral microcirculatory blood flow at a time when global cerebral flow is increased.85 The β-adrenergic effect increases myocardial contractility and heart rate and relaxes smooth muscle in the skeletal muscle vascular bed and bronchi; however, the β-adrenergic effects are not observed in the peripheral vascular beds secondary to the high dose used in cardiac arrest. Epinephrine also increases the vigor and intensity of VF, increasing the likelihood of successful defibrillation.
High-dose epinephrine (0.05 to 0.2 mg/kg) improves myocardial and cerebral blood flow during CPR more than standard-dose epinephrine (0.01 to 0.02 mg/kg) in animal models of cardiac arrest and may increase the incidence of initial ROSC.86,87 Administration of high-dose epinephrine, however, can worsen a patient’s postresuscitation hemodynamic condition. Retrospective studies indicate that use of high-dose epinephrine in adults or children may be associated with a worse neurologic outcome.88,89 A randomized, controlled trial of rescue high-dose epinephrine versus standard-dose epinephrine following failed initial standard-dose epinephrine in pediatric in-hospital cardiac arrest demonstrated a worse 24-hour survival in the high-dose epinephrine group (1/27 vs. 6/23, P < .05).90 Based on these clinical data, high-dose epinephrine cannot be recommended routinely for either initial or rescue therapy.
Vasopressin is a long-acting endogenous hormone that acts at specific receptors to mediate systemic vasoconstriction (V1 receptor) and reabsorption of water in the renal tubule (V2 receptor). The vasoconstriction is most intense in the skeletal muscle and skin vascular beds. Unlike epinephrine, vasopressin is not a pulmonary vasoconstrictor. In experimental models of cardiac arrest, vasopressin increases blood flow to the heart and brain and improves long term survival compared with epinephrine. However, vasopressin can decrease splanchnic blood flow during and following CPR and can increase afterload in the postresuscitation period.91–95 Adult randomized controlled trials suggest that outcomes are similar after use of vasopressin or epinephrine during CPR.96,97 During pediatric arrest, a case series of four children who received vasopressin during six prolonged cardiac arrest events suggested that the use of bolus vasopressin may result in ROSC when standard medications have failed.98 However, a more recent retrospective study of 1293 consecutive pediatric arrests from the National Registry of CPR (NPCRP) found that vasopressin use, while infrequent (administered in only 5% of events), was associated with a lower likelihood of ROSC. Therefore, it is unlikely that vasopressin will replace epinephrine as a first-line agent in pediatric cardiac arrest. However, the available data suggest that its use in conjunction with epinephrine may deserve further investigation.
Calcium
Calcium is used frequently in cases of cardiac arrest, despite the lack of evidence for efficacy when it is administered routinely during resuscitation attempts. In the absence of a documented clinical indication (i.e., hypocalcemia, calcium channel blocker overdose, hypermagnesemia, or hyperkalemia), administration of calcium does not improve outcome from cardiac arrest.37,99–107 To the contrary, three pediatric studies have suggested a potential for harm, as routine calcium administration was associated with decreased survival rates and/or worse neurological outcomes.37,99,100
Buffer Solutions
There are no randomized controlled studies in children examining the use of sodium bicarbonate for management of pediatric cardiac arrest. Two randomized controlled studies have examined the value of sodium bicarbonate in the management of adult cardiac arrest108 and in neonates with respiratory arrest in the delivery room.109 Neither was associated with improved survival. One multicenter retrospective in-hospital pediatric study found that sodium bicarbonate administered during cardiac arrest was associated with decreased survival, even after controlling for age, gender, and first documented cardiac rhythm.99 Therefore, during pediatric cardiac arrest resuscitation, the routine use of sodium bicarbonate is NOT recommended.
Clinical trials involving critically ill adults with severe metabolic acidosis did not demonstrate a beneficial effect of sodium bicarbonate on hemodynamics despite correction of acidosis.110,111 However, the presence of severe acidosis may depress the action of catecholamines, so the use of sodium bicarbonate may be considered in an acidemic child who is refractory to catecholamine administration.112,113 Acidosis may increase the threshold for myocardial stimulation in a patient with an artificial cardiac pacemaker114; therefore administration of bicarbonate or another buffer is appropriate for management of severe documented acidosis in these children. Administration of sodium bicarbonate also is indicated in the patient with a tricyclic antidepressant overdose, hyperkalemia, hypermagnesemia, or sodium channel blocker poisoning. The buffering action of bicarbonate occurs when a hydrogen cation and a bicarbonate anion combine to form carbon dioxide and water. If carbon dioxide is not effectively cleared through ventilation, its buildup counterbalances the buffering effect of bicarbonate. Because carbon dioxide readily penetrates cell membranes, intracellular acidosis may increase without adequate ventilation. Therefore, bicarbonate should not be used for management of respiratory acidosis.
Unlike sodium bicarbonate, tromethamine (THAM) buffers excess protons without generating carbon dioxide. Carbon dioxide is consumed following THAM administration. In a patient with limited ventilation, tromethamine may be preferable when buffering is necessary. Tromethamine undergoes renal elimination, and renal insufficiency may be a relative contraindication to its use. Carbicarb, an equimolar combination of sodium bicarbonate and sodium carbonate, is another buffering solution that generates less carbon dioxide than sodium bicarbonate. In a canine model of cardiac arrest comparing animals given normal saline, sodium bicarbonate, THAM, or Carbicarb, the animals given any buffer solution had a higher rate of ROSC than the animals given normal saline. In the animals given sodium bicarbonate or Carbicarb, the interval to ROSC was significantly shorter than in animals given normal saline. However, at the end of the 6-hour study period, all resuscitated animals were in a deep coma, so no inferences regarding meaningful survival can be drawn.115 It is premature to recommend either THAM or Carbicarb during CPR at this time.
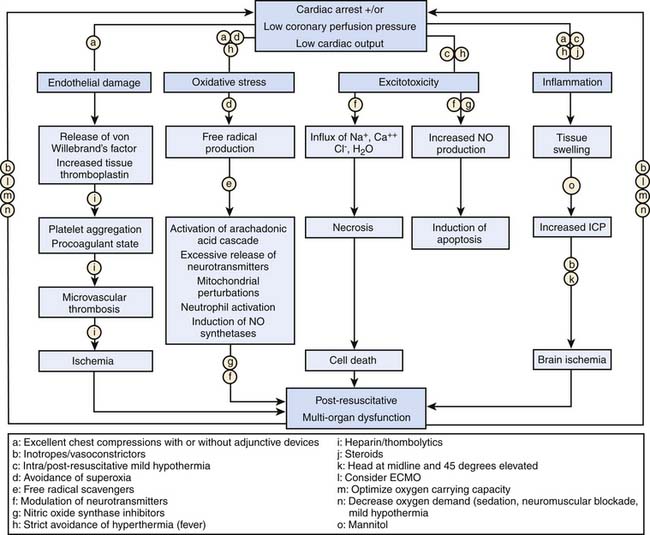
Postresuscitation Interventions
Temperature Management
Hyperthermia following cardiac arrest is common in children, and fever following cardiac arrest is associated with poor neurologic outcome.116,117 Two seminal articles addressing adult out-of-hospital VF cardiac arrest have established that mild induced hypothermia (32° C to 34° C) is a clinically promising recent goal-directed postresuscitation therapy. In these randomized studies of comatose patients older than 18 years after VF cardiac arrest, outcomes were improved.118,119 However, extrapolation of these findings to the pediatric arrest victim is difficult, as fever, trauma, stroke, and other ischemic conditions, common in pediatric cardiac arrest, are associated with poor neurologic outcome. Emerging neonatal trials of selective brain cooling and systemic cooling show promise in neonatal hypoxic-ischemic encephalopathy, suggesting that induced hypothermia may improve outcomes.120,121 At a minimum, it is advisable to avoid hyperthermia in children following CPR. Using an approach of “therapeutic normothermia” with scheduled administration of antipyretic medications and the use of external cooling devices may be necessary to prevent hyperthermia in this population.
Glucose Control
Both hyperglycemia and hypoglycemia following cardiac arrest are associated with worse neurologic outcome.122–125 While it seems intuitive that hypoglycemia would be associated with worse neurologic outcome, whether hyperglycemia per se is harmful or is simply a marker of the severity of the stress hormone response from prolonged ischemia is not clear. In critically ill adult patients, tight glucose control using an insulin infusion was associated with improved survival.126,127 However, subsequent studies of nonsurgical adult populations and neonatal/pediatric trials have demonstrated no survival benefit and/or the potential for harm when rates of inadvertent hypoglycemia were high during treatment.125,128–135 Using the available data, there is insufficient evidence to formulate a strong recommendation on the management of hyperglycemia in children with ROSC following cardiac arrest. If hyperglycemia is treated following ROSC in pediatric patients, blood glucose concentrations should be carefully monitored to avoid hypoglycemia.
Blood Pressure Management
Compared with healthy volunteers, adults resuscitated from cardiac arrest have impaired autoregulation of cerebral blood flow.136 Hence they may not maintain adequate cerebral blood flow in the context of low systemic pressure and, likewise, may not be able to protect the brain from excessive blood flow and microvascular perfusion pressure in the context of systemic hypertension. However, in animal models, brief induced hypertension following resuscitation results in improved neurologic outcome compared with normotensive reperfusion.137,138 Therefore, a practical approach to blood pressure management following cardiac arrest is to attempt to minimize blood pressure variability in this high-risk period following resuscitation.
Postresuscitation Myocardial Dysfunction
Postarrest myocardial stunning and arterial hypotension occur commonly after successful resuscitation in both animals and humans.118,119,139–146 Animal studies demonstrate that postarrest myocardial stunning is a global phenomenon with biventricular systolic and diastolic dysfunction. This postarrest myocardial stunning is pathophysiologically and physiologically similar to sepsis-related myocardial dysfunction and post–cardiopulmonary bypass myocardial dysfunction, including increases in inflammatory mediators and nitric oxide production.139,141,142,145 Because cardiac function is essential to reperfusion following cardiac arrest, management of postarrest myocardial dysfunction may be important to improving survival. The classes of agents used to maintain circulatory function (i.e., inotropes, vasopressors, and vasodilators) must be carefully titrated during the postresuscitation phase to the patient’s cardiovascular physiology. Trials in animal models have shown that various vasoactive medications can effectively ameliorate postarrest myocardial dysfunction (e.g., dobutamine, milrinone, levosimendan).147–151 Similarly, in human observational studies, fluid resuscitation and various vasoactive medications (i.e., epinephrine, dobutamine, and dopamine) have been provided for myocardial dysfunction syndrome.118,119,140–144 In the end, optimal use of these agents involves close goal-directed titration, and the use of invasive hemodynamic monitoring may be appropriate.
Other Considerations
Quality of CPR
The quality of healthcare provider CPR during adult resuscitations typically does not comply with American Heart Association clinical practice guidelines. Long CPR-free intervals, shallow chest compressions, incorrect chest compression rates, and overventilation are common.152–155 Unfortunately, the quality of CPR performed during the resuscitation attempt is directly related to patient outcome.52,152,156 Studies have shown in adults and in children that patients with a witnessed cardiac arrest4 and those who receive bystander CPR157 have an increased chance of survival. Those that suffer their in-hospital cardiac arrest at night or during weekends (presumably when the quality of resuscitation is not as good as in the daytime or on weekdays) have higher mortality.158 Furthermore, pediatric outcomes are improved in hospitals staffed with highly trained pediatric specific providers.22 Taken all together, these findings establish that the quality of resuscitative care, specifically early high-quality CPR, is an important determinate of patient survival.
In an effort to improve CPR quality, CPR-monitoring defibrillators with audiovisual feedback have been used during adult resuscitation, and improvements in CPR quality and clinical outcomes have been achieved.156,159 In a recent pediatric article, the combination of focused bedside training and automated feedback defibrillators improved CPR guideline compliance of in-hospital providers.154 However, there were still significant portions of the resuscitation that suffered from substandard resuscitative care. Future studies should continue to focus on novel ways to improve pediatric CPR during resuscitation attempts.
Extracorporeal Membrane Oxygenation Cardiopulmonary Resuscitation
Venoarterial extracorporeal membrane oxygenation (ECMO) has been increasingly used as a rescue therapy during CPR, especially for potentially reversible acute postoperative myocardial dysfunction or arrhythmias. Studies of extracorporeal CPR (E-CPR) have demonstrated favorable early survival outcomes in children with primary cardiac disease when E-CPR protocols were in place at the time of the arrest.31,160–170 Interestingly, data has been mixed regarding the relationship between outcome and CPR duration before ECMO cannulation. CPR and ECMO are not curative treatments. They are simply cardiopulmonary supportive measures that restore tissue perfusion until recovery from the precipitating disease process is achieved. As such, they can be powerful tools. Thus E-CPR should be considered for children with cardiac arrest who have heart disease amenable to recovery or transplantation, if the arrest occurs in a highly supervised environment such as an intensive care unit with existing clinical protocols and available expertise and equipment to rapidly initiate extracorporeal life support (ECLS).
Ventricular Fibrillation and Ventricular Tachycardia in Children
Pediatric VF or VT has been an underappreciated pediatric problem. Recent studies indicate that VF and VT (i.e., shockable rhythms) occur in 27% of in-hospital cardiac arrests at some time during the resuscitation.28 In a population of pediatric cardiac intensive care unit patients, as many as 41% of arrests were associated with VF or VT.24 According to the National Registry of Cardiopulmonary Resuscitation (NRCPR) database, during in-hospital arrest, 10% of children had an initial rhythm of VF/VT. In all, 27% of the children had VF/VT at some time during the resuscitation.28 The incidence of VF varies by setting and age.171 In special circumstances, such as tricyclic antidepressant overdose, cardiomyopathy, status post–cardiac surgery, and prolonged QT syndromes, VF and pulseless VT are more likely.
Antiarrhythmic Medications: Lidocaine and Amiodarone
Lidocaine traditionally has been recommended for shock-resistant VF in adults and children. However, only amiodarone improved survival to hospital admission in the setting of shock-resistant VF compared with placebo.172 In another study of shock-resistant out-of-hospital VF, patients receiving amiodarone had a higher rate of survival to hospital admission than patients receiving lidocaine.173 Neither study included children. Because there is moderate experience with amiodarone use as an antiarrhythmic agent in children and because of the adult studies, it is rational to use amiodarone similarly in children with shock-resistant VF/VT. The recommended dosage is 5 mg/kg by rapid intravenous bolus. There are no published comparisons of antiarrhythmic medications for pediatric refractory VF. Although extrapolation of adult data and electrophysiologic mechanistic information suggest that amiodarone may be preferable for pediatric shock-resistant VF, the optimal choice is not clear.
Pediatric Automated External Defibrillators
Automated external defibrillators (AEDs) have improved adult survival from VF.174,175 AEDs are recommended for use in children 8 years or older with cardiac arrest.176,177 The available data suggest that some AEDs can accurately diagnose VF in children of all ages, but many AEDs are limited because the defibrillation pads and energy dosage are geared for adults. Adapters having smaller defibrillation pads that dampen the amount of energy delivered have been developed as attachments to adult AEDs, allowing their use in children. However, it is important that the AED diagnostic algorithm is sensitive and specific for pediatric VF and VT. The diagnostic algorithms from several AED manufacturers have been tested for such sensitivity and specificity and therefore can be reasonably used in younger children.
1. Slonim A.D., Patel K.M., Ruttimann U.E., Pollack M.M. Cardiopulmonary resuscitation in pediatric intensive care units. Crit Care Med. 1997;25(12):1951-1955.
2. Suominen P., Olkkola K.T., Voipio V. Utstein style reporting of in-hospital paediatric cardiopulmonary resuscitation. Resuscitation. 2000;45(1):17-25.
3. Young K.D., Seidel J.S. Pediatric cardiopulmonary resuscitation: A collective review. Ann Emerg Med. 1999;33(2):195-205.
4. Donoghue A.J., Nadkarni V., Berg R.A., et al. Out-of-hospital pediatric cardiac arrest: an epidemiologic review and assessment of current knowledge. Ann Emerg Med. 2005;46(6):512-522.
5. Atkins D.L., Everson-Stewart S., Sears G.K., et al. Epidemiology and outcomes from out-of-hospital cardiac arrest in children. The resuscitation outcomes consortium epistry of cardiac arrest. Circulation. 2009;119:1484-1491.
6. Berg RA: Personal communication of up-to-date data from the National Registry of CPR.
7. Nadkarni V.M., Larkin G.L., Peberdy M.A., et al. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA. 2006;295(1):50-57.
8. Buist M.D., Jarmolowski E., Burton P.R. Recognising clinical instability in hospital patients before cardiac arrest or unplanned admission to intensive care. A pilot study in a tertiary-care hospital. Med J Aust. 1999;171(1):22-25.
9. Chaplik S., Neafsey P.J. Pre-existing variables and outcome of cardiac arrest resuscitation in hospitalized patients. Dccn. 1998;17(4):200-207.
10. Brilli R.J., Gibson R., Luria J.W., et al. Implementation of a medical emergency team in a large pediatric teaching hospital prevents respiratory and cardiopulmonary arrests outside the intensive care unit. Pediatr Crit Care Med. 2007;8(3):236-246.
11. Sharek P.J., Parast L.M., Leong K., et al. Effect of a rapid response team on hospital-wide mortality and code rates outside the ICU in a children’s hospital. JAMA. 2007;298(19):2267-2274.
12. Tibballs J., Kinney S. Reduction of hospital mortality and of preventable cardiac arrest and death on introduction of a pediatric medical emergency team. Pediatr Crit Care Med. 2009;10(3):306-312.
13. Kern K.B., Carter A.B., Showen R.L., et al. Twenty-four hour survival in a canine model of cardiac arrest comparing three methods of manual cardiopulmonary resuscitation. J Am Coll Cardiol. 1986;7(4):859-867.
14. Kern K.B., Ewy G.A., Voorhees W.D. Myocardial perfusion pressure: a predictor of 24-hour survival during prolonged cardiac arrest in dogs. Resuscitation. 1988;16(4):241-250.
15. Kern K.B., Lancaster L., Goldman S., Ewy G.A. The effect of coronary artery lesions on the relationship between coronary perfusion pressure and myocardial blood flow during cardiopulmonary resuscitation in pigs. Am Heart J. 1990;120(2):324-333.
16. Sanders A.B., Ewy G.A., Taft T.V. Prognostic and therapeutic importance of the aortic diastolic pressure in resuscitation from cardiac arrest. Crit Care Med. 1984;12(10):871-873.
17. Sanders A.B., Kern K.B., Atlas M. Importance of the duration of inadequate coronary perfusion pressure on resuscitation from cardiac arrest. J Am Coll Cardiol. 1985;6(1):113-118.
18. Sanders A.B., Kern K.B., Otto C.W. End-tidal carbon dioxide monitoring during cardiopulmonary resuscitation. A prognostic indicator for survival. JAMA. 1989;262(10):1347-1351.
19. Paradis N.A., Martin G.B., Rivers E.P., et al. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA. 1990;263(8):1106-1113.
20. Ornato J.P., Levine R.L., Young D.S. The effect of applied chest compression force on systemic arterial pressure and end-tidal carbon dioxide concentration during CPR in human beings. Ann Emerg Med. 1989;18(7):732-737.
21. Zheng Z.J., Croft J.B., Giles W.H., Mensah G.A. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104(18):2158-2163.
22. Donoghue A.J., Nadkarni V.M., Elliott M., Durbin D. American Heart Association National Registry of Cardiopulmonary Resuscitation Investigators. Effect of hospital characteristics on outcomes from pediatric cardiopulmonary resuscitation: a report from the national registry of cardiopulmonary resuscitation. Pediatrics. 2006;118(3):995-1001.
23. Kuisma M., Suominen P., Korpela R. Paediatric out-of-hospital cardiac arrests–epidemiology and outcome. Resuscitation. 1995;30(2):141-150.
24. Rhodes J.F., Blaufox A.D., Seiden H.S., et al. Cardiac arrest in infants after congenital heart surgery. Circulation. 1999;100(Suppl 19):194-199.
25. Parra D.A., Totapally B.R., Zahn E., et al. Outcome of cardiopulmonary resuscitation in a pediatric cardiac intensive care unit. Crit Care Med. 2000;28(9):3296-3300.
26. Meaney P.A., Nadkarni V.M., Cook E.F., et al. Higher survival rates among younger patients after pediatric intensive care unit cardiac arrests. Pediatrics. 2006;118(6):2424-2433.
27. Reis A.G., Nadkarni V., Perondi M.B. A prospective investigation into the epidemiology of in-hospital pediatric cardiopulmonary resuscitation using the international Utstein reporting style. Pediatrics. 2002;109(2):200-209.
28. Samson R.A., Nadkarni V.M., Meaney P.A., et al. Outcomes of in-hospital ventricular fibrillation in children. N Engl J Med. 2006;354(22):2328-2339.
29. Hintz S.R., Benitz W.E., Colby C.E., et al. Utilization and outcomes of neonatal cardiac extracorporeal life support: 1996-2000. Pediatr Crit Care Med. 2005;6(1):33-38.
30. Lopez-Herce J., Garcia C., Dominguez P., et al. Outcome of out-of-hospital cardiorespiratory arrest in children. Pediatr Emerg Care. 2005;21(12):807-815.
31. Thiagarajan R.R., Laussen P.C., Rycus P.T. Extracorporeal membrane oxygenation to aid cardiopulmonary resuscitation in infants and children. Circulation. 2007;116(15):1693-1700.
32. Tibballs J., Kinney S. A prospective study of outcome of in-patient paediatric cardiopulmonary arrest. Resuscitation. 2006;71(3):310-318.
33. Chamnanvanakij S., Perlman J.M. Outcome following cardiopulmonary resuscitation in the neonate requiring ventilatory assistance. Resuscitation. 2000;45(3):173-180.
34. Torres A.Jr., Pickert C.B., Firestone J. Long-term functional outcome of inpatient pediatric cardiopulmonary resuscitation. Pediatr Emerg Care. 1997;13(6):369-373.
35. Tunstall-Pedoe H., Bailey L., Chamberlain D.A. Survey of 3765 cardiopulmonary resuscitations in British hospitals (the BRESUS Study): methods and overall results. BMJ. 1992;304(6838):1347-1351.
36. Zaritsky A., Nadkarni V., Getson P., Kuehl K. CPR in children. Ann Emerg Med. 1987;16(10):1107-1111.
37. de Mos N., van Litsenburg R.R., McCrindle B. Pediatric in-intensive-care-unit cardiac arrest: incidence, survival, and predictive factors. Crit Care Med. 2006;34(4):1209-1215.
38. Gerein R.B., Osmond M.H., Stiell I.G. What are the etiology and epidemiology of out-of-hospital pediatric cardiopulmonary arrest in Ontario, Canada? Acad Emerg Med. 2006;13(6):653-658.
39. Berg M.D., Samson R.A., Meyer R.J. Pediatric defibrillation doses often fail to terminate prolonged out-of-hospital ventricular fibrillation in children. Resuscitation. 2005;67(1):63-67.
40. Dieckmann R.A., Vardis R. High-dose epinephrine in pediatric out-of-hospital cardiopulmonary arrest. Pediatrics. 1995;95(6):901-913.
41. Schindler M.B., Bohn D., Cox P.N., et al. Outcome of out-of-hospital cardiac or respiratory arrest in children. N Engl J Med. 1996;335(20):1473-1479.
42. Sirbaugh P.E., Pepe P.E., Shook J.E., et al. A prospective, population-based study of the demographics, epidemiology, management, and outcome of out-of-hospital pediatric cardiopulmonary arrest. Ann Emerg Med. 1999;33(2):174-184.
43. Suominen P., Korpela R., Kuisma M. Paediatric cardiac arrest and resuscitation provided by physician-staffed emergency care units. Acta Anaesthesiol Scand. 1997;41(2):260-265.
44. Suominen P., Rasanen J., Kivioja A. Efficacy of cardiopulmonary resuscitation in pulseless paediatric trauma patients. Resuscitation. 1998;36(1):9-13.
45. Holmberg M., Holmberg S., Herlitz J. Effect of bystander cardiopulmonary resuscitation in out-of-hospital cardiac arrest patients in Sweden. Resuscitation. 2000;47(1):59-70.
46. Dean J.M., Koehler R.C., Schleien C.L., et al. Age-related changes in chest geometry during cardiopulmonary resuscitation. J Appl Physiol. 1987;62(6):2212-2219.
47. Kouwenhoven W.B., Jude J.R., Knickerbocker G.G. Closed-chest cardiac massage. JAMA. 1960;173:1064-1067.
48. Aufderheide T.P., Sigurdsson G., Pirrallo R.G., et al. Hyperventilation-induced hypotension during cardiopulmonary resuscitation. Circulation. 2004;109(16):1960-1965.
49. Aufderheide T.P., Lurie K.G. Death by hyperventilation: a common and life-threatening problem during cardiopulmonary resuscitation. Crit Care Med. 2004;32(Suppl 9):S345-S351.
50. Milander M.M., Hiscok P.S., Sanders A.B. Chest compression and ventilation rates during cardiopulmonary resuscitation: the effects of audible tone guidance. Acad Emerg Med. 1995;2(8):708-713.
51. Yannopoulos D., Aufderheide T.P., Gabrielli A., et al. Clinical and hemodynamic comparison of 15:2 and 30:2 compression-to-ventilation ratios for cardiopulmonary resuscitation. Crit Care Med. 2006;34(5):1444-1449.
52. Edelson D.P., Abella B.S., Kramer-Johansen J., et al. Effects of compression depth and pre-shock pauses predict defibrillation failure during cardiac arrest. Resuscitation. 2006;71(2):137-145.
53. Valenzuela T.D., Kern K.B., Clark L.L., et al. Interruptions of chest compressions during emergency medical systems resuscitation. Circulation. 2005;112(9):1259-1265.
54. Ewy G.A. Continuous-chest-compression cardiopulmonary resuscitation for cardiac arrest. Circulation. 2007;116(25):2894-2896.
55. Ewy G.A., Zuercher M., Hilwig R.W., et al. Improved neurological outcome with continuous chest compressions compared with 30:2 compressions-to-ventilations cardiopulmonary resuscitation in a realistic swine model of out-of-hospital cardiac arrest. Circulation. 2007;116(22):2525-2530.
56. Chandra N.C., Gruben K.G., Tsitlik J.E., et al. Observations of ventilation during resuscitation in a canine model. Circulation. 1994;90(6):3070-3075.
57. Berg R.A., Sanders A.B., Kern K.B., et al. Adverse hemodynamic effects of interrupting chest compressions for rescue breathing during cardiopulmonary resuscitation for ventricular fibrillation cardiac arrest. Circulation. 2001;104(20):2465-2470.
58. Voorhees W.D., Babbs C.F., Tacker W.A.Jr. Regional blood flow during cardiopulmonary resuscitation in dogs. Crit Care Med. 1980;8(3):134-136.
59. Michael J.R., Guerci A.D., Koehler R.C., et al. Mechanisms by which epinephrine augments cerebral and myocardial perfusion during cardiopulmonary resuscitation in dogs. Circulation. 1984;69(4):822-835.
60. Halperin H.R., Tsitlik J.E., Guerci A.D., et al. Determinants of blood flow to vital organs during cardiopulmonary resuscitation in dogs. Circulation. 1986;73(3):539-550.
61. Schleien C.L., Dean J.M., Koehler R.C., et al. Effect of epinephrine on cerebral and myocardial perfusion in an infant animal preparation of cardiopulmonary resuscitation. Circulation. 1986;73(4):809-817.
62. Plaisance P., Lurie K.G., Vicaut E., et al. Evaluation of an impedance threshold device in patients receiving active compression-decompression cardiopulmonary resuscitation for out of hospital cardiac arrest. Resuscitation. 2004;61(3):265-271.
63. Lurie K.G., Coffeen P., Shultz J. Improving active compression-decompression cardiopulmonary resuscitation with an inspiratory impedance valve. Circulation. 1995;91(6):1629-1632.
64. Lurie K., Zielinski T., McKnite S., Sukhum P. Improving the efficiency of cardiopulmonary resuscitation with an inspiratory impedance threshold valve. Crit Care Med. 2000;28(Suppl 11):N207-N209.
65. Yannopoulos D., Metzger A., McKnite S., et al. Intrathoracic pressure regulation improves vital organ perfusion pressures in normovolemic and hypovolemic pigs. Resuscitation. 2006;70(3):445-453.
66. Aufderheide T.P., Pirrallo R.G., Provo T.A., Lurie K.G. Clinical evaluation of an inspiratory impedance threshold device during standard cardiopulmonary resuscitation in patients with out-of-hospital cardiac arrest. Crit Care Med. 2005;33(4):734-740.
67. Wolcke B.B., Mauer D.K., Schoefmann M.F., et al. Comparison of standard cardiopulmonary resuscitation versus the combination of active compression-decompression cardiopulmonary resuscitation and an inspiratory impedance threshold device for out-of-hospital cardiac arrest. Circulation. 2003;108(18):2201-2205.
68. Maher K.O., Berg R.A., Lindsey C.W. Depth of sternal compression and intra-arterial blood pressure during CPR in infants following cardiac surgery. Resuscitation. 2009;80(6):662-664.
69. Braga M.S., Dominguez T.E., Pollock A.N., et al. Estimation of optimal CPR chest compression depth in children by using computer tomography. Pediatrics. 2009;124(1):e69-e74.
70. Kao P.C., Chiang W.C., Yang C.W., et al. What is the correct depth of chest compression for infants and children? A radiological study. Pediatrics. 2009;124(1):49-55.
71. Idris A.H., Staples E.D., O’Brien D.J., et al. Effect of ventilation on acid-base balance and oxygenation in low blood-flow states. Crit Care Med. 1994;22(11):1827-1834.
72. Kinney S.B., Tibballs J. An analysis of the efficacy of bag-valve-mask ventilation and chest compression during different compression-ventilation ratios in manikin-simulated paediatric resuscitation. Resuscitation. 2000;43(2):115-120.
73. Srikantan S.K., Berg R.A., Cox T. Effect of one-rescuer compression/ventilation ratios on cardiopulmonary resuscitation in infant, pediatric, and adult manikins. Pediatr Crit Care Med. 2005;6(3):293-297.
74. Babbs C.F., Thelander K. Theoretically optimal duty cycles for chest and abdominal compression during external cardiopulmonary resuscitation. Acad Emerg Med. 1995;2(8):698-707.
75. Dean J.M., Koehler R.C., Schleien C.L., et al. Age-related effects of compression rate and duration in cardiopulmonary resuscitation. J Appl Physiol. 1990;68(2):554-560.
76. Halperin H.R., Tsitlik J.E., Gelfand M., et al. A preliminary study of cardiopulmonary resuscitation by circumferential compression of the chest with use of a pneumatic vest. N Engl J Med. 1993;329(11):762-768.
77. Dorfsman M.L., Menegazzi J.J., Wadas R.J., Auble T.E. Two-thumb vs. two-finger chest compression in an infant model of prolonged cardiopulmonary resuscitation. Acad Emerg Med. 2000;7(10):1077-1082.
78. Sanders A.B., Kern K.B., Ewy G.A. Improved resuscitation from cardiac arrest with open-chest massage. Ann Emerg Med. 1984;13(9 Pt 1):672-675.
79. Boczar M.E., Howard M.A., Rivers E.P., et al. A technique revisited: hemodynamic comparison of closed- and open-chest cardiac massage during human cardiopulmonary resuscitation. Crit Care Med. 1995;23(3):498-503.
80. Fleisher G., Sagy M., Swedlow D.B., Belani K. Open- versus closed-chest cardiac compressions in a canine model of pediatric cardiopulmonary resuscitation. Am J Emerg Med. 1985;3(4):305-310.
81. Sheikh A., Brogan T. Outcome and cost of open- and closed-chest cardiopulmonary resuscitation in pediatric cardiac arrests. Pediatrics. 1994;93(3):392-398.
82. Berkowitz I.D., Gervais H., Schleien C.L. Epinephrine dosage effects on cerebral and myocardial blood flow in an infant swine model of cardiopulmonary resuscitation. Anesthesiology. 1991;75(6):1041-1050.
83. Koehler R.C., Michael J.R., Guerci A.D., et al. Beneficial effect of epinephrine infusion on cerebral and myocardial blood flows during CPR. Ann Emerg Med. 1985;14(8):744-749.
84. Lindner K.H., Ahnefeld F.W., Bowdler I.M., Prengel A.W. Influence of epinephrine on systemic, myocardial, and cerebral acid-base status during cardiopulmonary resuscitation. Anesthesiology. 1991;74(2):333-339.
85. Ristagno G., Sun S., Tang W. Effects of epinephrine and vasopressin on cerebral microcirculatory flows during and after cardiopulmonary resuscitation. Crit Care Med. 2007;35(9):2145-2149.
86. Brown C.G., Martin D.R., Pepe P.E., et al. A comparison of standard-dose and high-dose epinephrine in cardiac arrest outside the hospital. The Multicenter High-Dose Epinephrine Study Group. N Engl J Med. 1992;327(15):1051-1055.
87. Lindner K.H., Ahnefeld F.W., Bowdler I.M. Comparison of different doses of epinephrine on myocardial perfusion and resuscitation success during cardiopulmonary resuscitation in a pig model. Am J Emerg Med. 1991;9(1):27-31.
88. Behringer W., Kittler H., Sterz F., et al. Cumulative epinephrine dose during cardiopulmonary resuscitation and neurologic outcome. Ann Intern Med. 1998;129(6):450-456.
89. Callaham M., Madsen C.D., Barton C.W. A randomized clinical trial of high-dose epinephrine and norepinephrine vs standard-dose epinephrine in prehospital cardiac arrest. JAMA. 1992;268(19):2667-2672.
90. Perondi M.B., Reis A.G., Paiva E.F. A comparison of high-dose and standard-dose epinephrine in children with cardiac arrest. N Engl J Med. 2004;350(17):1722-1730.
91. Lindner K.H., Prengel A.W., Pfenninger E.G., et al. Vasopressin improves vital organ blood flow during closed-chest cardiopulmonary resuscitation in pigs. Circulation. 1995;91(1):215-221.
92. Prengel A.W., Lindner K.H., Keller A. Cerebral oxygenation during cardiopulmonary resuscitation with epinephrine and vasopressin in pigs. Stroke. 1996;27(7):1241-1248.
93. Wenzel V., Lindner K.H., Krismer A.C., et al. Survival with full neurologic recovery and no cerebral pathology after prolonged cardiopulmonary resuscitation with vasopressin in pigs. J Am Coll Cardiol. 2000;35(2):527-533.
94. Prengel A.W., Lindner K.H., Wenzel V. Splanchnic and renal blood flow after cardiopulmonary resuscitation with epinephrine and vasopressin in pigs. Resuscitation. 1998;38(1):19-24.
95. Voelckel W.G., Lindner K.H., Wenzel V., et al. Effects of vasopressin and epinephrine on splanchnic blood flow and renal function during and after cardiopulmonary resuscitation in pigs. Crit Care Med. 2000;28(4):1083-1088.
96. Stiell I.G., Hebert P.C., Wells G.A., et al. Vasopressin versus epinephrine for in-hospital cardiac arrest: a randomised controlled trial. Lancet. 2001;358(9276):105-109.
97. Wenzel V., Krismer A.C., Arntz H.R., et al. A comparison of vasopressin and epinephrine for out-of-hospital cardiopulmonary resuscitation. N Engl J Med. 2004;350(2):105-113.
98. Mann K., Berg R.A., Nadkarni V. Beneficial effects of vasopressin in prolonged pediatric cardiac arrest: a case series. Resuscitation. 2002;52(2):149-156.
99. Meert K.L., Donaldson A., Nadkarni V., et al. Multicenter cohort study of in-hospital pediatric cardiac arrest. Pediatr Crit Care Med. 2009;10(5):544-553.
100. Srinivasan V., Morris M.C., Helfaer M.A., et al. Calcium use during in-hospital pediatric cardiopulmonary resuscitation: a report from the National Registry of Cardiopulmonary Resuscitation. Pediatrics. 2008;121(5):e1144-e1151.
101. Blecic S., De Backer D., Huynh C.H., et al. Calcium chloride in experimental electromechanical dissociation: a placebo-controlled trial in dogs. Crit Care Med. 1987;15(4):324-327.
102. Niemann J.T., Criley J.M., Rosborough J.P. Predictive indices of successful cardiac resuscitation after prolonged arrest and experimental cardiopulmonary resuscitation. Ann Emerg Med. 1985;14(6):521-528.
103. Redding J.S., Haynes R.R., Thomas J.D. Drug therapy in resuscitation from electromechanical dissociation. Crit Care Med. 1983;11(9):681-684.
104. Redding J.S., Pearson J.W. Evaluation of drugs for cardiac resuscitation. Anesthesiology. 1963;24:203-207.
105. Stueven H., Thompson B.M., Aprahamian C., Darin J.C. Use of calcium in prehospital cardiac arrest. Ann Emerg Med. 1983;12(3):136-139.
106. Stueven H.A., Thompson B., Aprahamian C. Lack of effectiveness of calcium chloride in refractory asystole. Ann Emerg Med. 1985;14(7):630-632.
107. Stueven H.A., Thompson B., Aprahamian C., Tonsfeldt D.J., Kastenson E.H. The effectiveness of calcium chloride in refractory electromechanical dissociation. Ann Emerg Med. 1985;14(7):626-629.
108. Vukmir R.B., Katz L. Sodium Bicarbonate Study G. Sodium bicarbonate improves outcome in prolonged prehospital cardiac arrest. Am J Emerg Med. 2006;24(2):156-161.
109. Lokesh L., Kumar P., Murki S., Narang A. A randomized controlled trial of sodium bicarbonate in neonatal resuscitation-effect on immediate outcome. Resuscitation. 2004;60(2):219-223.
110. Cooper D.J., Walley K.R., Wiggs B.R., Russell J.A. Bicarbonate does not improve hemodynamics in critically ill patients who have lactic acidosis. A prospective, controlled clinical study. Ann Intern Med. 1990;112(7):492-498.
111. Mathieu D., Neviere R., Billard V. Effects of bicarbonate therapy on hemodynamics and tissue oxygenation in patients with lactic acidosis: a prospective, controlled clinical study. Crit Care Med. 1991;19(11):1352-1356.
112. Huang Y.G., Wong K.C., Yip W.H. Cardiovascular responses to graded doses of three catecholamines during lactic and hydrochloric acidosis in dogs. Br J Anaesth. 1995;74(5):583-590.
113. Preziosi M.P., Roig J.C., Hargrove N. Metabolic acidemia with hypoxia attenuates the hemodynamic responses to epinephrine during resuscitation in lambs. Crit Care Med. 1993;21(12):1901-1907.
114. Dohrmann M.L., Goldschlager N.F. Myocardial stimulation threshold in patients with cardiac pacemakers: effect of physiologic variables, pharmacologic agents, and lead electrodes. Cardiol Clin. 1985;3(4):527-537.
115. Bar-Joseph G., Weinberger T., Castel T., et al. Comparison of sodium bicarbonate, Carbicarb, and THAM during cardiopulmonary resuscitation in dogs. Crit Care Med. 1998;26(8):1397-1408.
116. Hickey R.W., Kochanek P.M., Ferimer H. Hypothermia and hyperthermia in children after resuscitation from cardiac arrest. Pediatrics. 2000;106(1 Pt 1):118-122.
117. Zeiner A., Holzer M., Sterz F., et al. Hyperthermia after cardiac arrest is associated with an unfavorable neurologic outcome. Arch Intern Med. 2001;161(16):2007-2012.
118. Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549-556.
119. Bernard S.A., Gray T.W., Buist M.D., et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557-563.
120. Gluckman P.D., Wyatt J.S., Azzopardi D., et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365(9460):663-670.
121. Shankaran S., Laptook A., Wright L.L., et al. Whole-body hypothermia for neonatal encephalopathy: animal observations as a basis for a randomized, controlled pilot study in term infants. Pediatrics. 2002;110(2 Pt 1):377-385.
122. Langhelle A., Tyvold S.S., Lexow K. In-hospital factors associated with improved outcome after out-of-hospital cardiac arrest. A comparison between four regions in Norway. Resuscitation. 2003;56(3):247-263.
123. Ulate K.P., Lima Falcao G.C., Bielefeld M.R. Strict glycemic targets need not be so strict: a more permissive glycemic range for critically ill children. Pediatrics. 2008;122(4):e898-e904.
124. Beiser D.G., Carr G.E., Edelson D.P. Derangements in blood glucose following initial resuscitation from in-hospital cardiac arrest: a report from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2009;80(6):624-630.
125. Oksanen T., Skrifvars M.B., Varpula T., et al. Strict versus moderate glucose control after resuscitation from ventricular fibrillation. Intensive Care Med. 2007;33(12):2093-2100.
126. van den Berghe G., Wouters P., Weekers F., et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345(19):1359-1367.
127. Gandhi G.Y., Murad M.H., Flynn D.N., et al. Effect of peri-operative insulin infusion on surgical morbidity and mortality: systematic review and meta-analysis of randomized trials. Mayo Clin Proc. 2008;83(4):418-430.
128. Losert H., Sterz F., Roine R.O., et al. Strict normoglycaemic blood glucose levels in the therapeutic management of patients within 12h after cardiac arrest might not be necessary. Resuscitation. 2008;76(2):214-220.
129. Griesdale D.E., de Souza R.J., van Dam R.M., et al. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ. 2009;180(8):821-827.
130. Van den Berghe G., Wilmer A., Milants I., et al. Intensive insulin therapy in mixed medical/surgical intensive care units: benefit versus harm. Diabetes. 2006;55(11):3151-3159.
131. Wiener R.S., Wiener D.C., Larson R.J. Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA. 2008;300(8):933-944.
132. Treggiari M.M., Karir V., Yanez N.D. Intensive insulin therapy and mortality in critically ill patients. Crit Care. 2008;12(1):R29.
133. Gandhi G.Y., Nuttall G.A., Abel M.D., et al. Intensive intraoperative insulin therapy versus conventional glucose management during cardiac surgery: a randomized trial. Ann Intern Med. 2007;146(4):233-243.
134. NICE-SUGAR Study Investigators. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283-1297.
135. Beardsall K., Vanhaesebrouck S., Ogilvy-Stuart A.L., et al. Early insulin therapy in very-low-birth-weight infants. N Engl J Med. 2008;359(18):1873-1884.
136. Sundgreen C., Larsen F.S., Herzog T.M. Autoregulation of cerebral blood flow in patients resuscitated from cardiac arrest. Stroke. 2001;32(1):128-132.
137. Safar P., Xiao F., Radovsky A., et al. Improved cerebral resuscitation from cardiac arrest in dogs with mild hypothermia plus blood flow promotion. Stroke. 1996;27(1):105-113.
138. Sterz F., Leonov Y., Safar P. Hypertension with or without hemodilution after cardiac arrest in dogs. Stroke. 1990;21(8):1178-1184.
139. Laurent I., Monchi M., Chiche J.D., et al. Reversible myocardial dysfunction in survivors of out-of-hospital cardiac arrest. J Am Coll Cardiol. 2002;40(12):2110-2116.
140. Mullner M., Domanovits H., Sterz F., et al. Measurement of myocardial contractility following successful resuscitation: quantitated left ventricular systolic function utilising non-invasive wall stress analysis. Resuscitation. 1998;39(1-2):51-59.
141. Adrie C., Adib-Conquy M., Laurent I., et al. Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome. Circulation. 2002;106(5):562-568.
142. Laurent I., Adrie C., Vinsonneau C., et al. High-volume hemofiltration after out-of-hospital cardiac arrest: a randomized study. J Am Coll Cardiol. 2005;46(3):432-437.
142. Ruiz-Bailen M., Aguayo de Hoyos E., Ruiz-Navarro S., et al. Reversible myocardial dysfunction after cardiopulmonary resuscitation. Resuscitation. 2005;66(2):175-181.
144. Sunde K., Pytte M., Jacobsen D., et al. Implementation of a standardised treatment protocol for post resuscitation care after out-of-hospital cardiac arrest. Resuscitation. 2007;73(1):29-39.
145. Niemann J.T., Garner D., Lewis R.J. Tumor necrosis factor-alpha is associated with early post-resuscitation myocardial dysfunction. Crit Care Med. 2004;32(8):1753-1758.
146. Trzeciak S., Jones A.E., Kilgannon J.H., et al. Significance of arterial hypotension after resuscitation from cardiac arrest. Crit Care Med. 2009;37(11):2895-2903.
147. Kern K.B., Hilwig R.W., Berg R.A., et al. Post-resuscitation left ventricular systolic and diastolic dysfunction. Treatment with dobutamine. Circulation. 1997;95(12):2610-2613.
148. Meyer R.J., Kern K.B., Berg R.A. Post-resuscitation right ventricular dysfunction: delineation and treatment with dobutamine. Resuscitation. 2002;55(2):187-191.
149. Niemann J.T., Garner D., Khaleeli E., Lewis R.J. Milrinone facilitates resuscitation from cardiac arrest and attenuates post-resuscitation myocardial dysfunction. Circulation. 2003;108(24):3031-3035.
150. Vasquez A., Kern K.B., Hilwig R.W. Optimal dosing of dobutamine for treating post-resuscitation left ventricular dysfunction. Resuscitation. 2004;61(2):199-207.
151. Huang L., Weil M.H., Tang W. Comparison between dobutamine and levosimendan for management of post-resuscitation myocardial dysfunction. Crit Care Med. 2005;33(3):487-491.
152. Abella B.S., Sandbo N., Vassilatos P., et al. Chest compression rates during cardiopulmonary resuscitation are suboptimal: a prospective study during in-hospital cardiac arrest. Circulation. 2005;111(4):428-434.
153. Abella B.S., Alvarado J.P., Myklebust H., et al. Quality of cardiopulmonary resuscitation during in-hospital cardiac arrest. JAMA. 2005;293(3):305-310.
154. Sutton R.M., Niles D., Nysaether J., et al. Quantitative analysis of CPR quality during in-hospital resuscitation of older children and adolescents. Pediatrics. 2009;124(2):494-499.
155. Wik L., Kramer-Johansen J., Myklebust H., et al. Quality of cardiopulmonary resuscitation during out-of-hospital cardiac arrest. JAMA. 2005;293(3):299-304.
156. Kramer-Johansen J., Myklebust H., Wik L., et al. Quality of out-of-hospital cardiopulmonary resuscitation with real time automated feedback: a prospective interventional study. Resuscitation. 2006;71(3):283-292.
157. Stiell I.G., Nesbitt L., Berg R.A. Epidemiology of pediatric cardiac and respiratory arrest in the cities of the CanAm Pediatric Study Group. Can J Emerg Med. 2005;6(4):192.
158. Peberdy M.A., Ornato J.P., Larkin G.L., et al. Survival from in-hospital cardiac arrest during nights and weekends. JAMA. 2008;299(7):785-792.
159. Abella B.S., Edelson D.P., Kim S., et al. CPR quality improvement during in-hospital cardiac arrest using a real-time audiovisual feedback system. Resuscitation. 2007;73(1):54-61.
160. Dalton H.J., Siewers R.D., Fuhrman B.P., et al. Extracorporeal membrane oxygenation for cardiac rescue in children with severe myocardial dysfunction. Crit Care Med. 1993;21(7):1020-1028.
161. del Nido P.J., Dalton H.J., Thompson A.E., Siewers R.D. Extracorporeal membrane oxygenator rescue in children during cardiac arrest after cardiac surgery. Circulation. 1992;86(Suppl 5):300-304.
162. Tecklenburg F.W., Thomas N.J., Webb S.A. Pediatric ECMO for severe quinidine cardiotoxicity. Pediatr Emerg Care. 1997;13(2):111-113.
163. Thalmann M., Trampitsch E., Haberfellner N. Resuscitation in near drowning with extracorporeal membrane oxygenation. Ann Thorac Surg. 2001;72(2):607-608.
164. Morris M.C., Wernovsky G., Nadkarni V.M. Survival outcomes after extracorporeal cardiopulmonary resuscitation instituted during active chest compressions following refractory in-hospital pediatric cardiac arrest. Pediatr Crit Care Med. 2004;5(5):440-446.
165. Ravishankar C., Dominguez T.E., Kreutzer J., et al. Extracorporeal membrane oxygenation after stage I reconstruction for hypoplastic left heart syndrome. Pediatr Crit Care Med. 2006;7(4):319-323.
166. Hoskote A., Bohn D., Gruenwald C., et al. Extracorporeal life support after staged palliation of a functional single ventricle: subsequent morbidity and survival. J Thorac Cardiovasc Surg. 2006;131(5):1114-1121.
167. Prodhan P., Fiser R.T., Dyamenahalli U., et al. Outcomes after extracorporeal cardiopulmonary resuscitation (ECPR) following refractory pediatric cardiac arrest in the intensive care unit. Resuscitation. 2009;80(10):1124-1129.
168. Lequier L., Joffe A.R., Robertson C.M., et al. Two-year survival, mental, and motor outcomes after cardiac extracorporeal life support at less than five years of age. J Thorac Cardiovasc Surg. 2008;136(4):976-983. e3
169. del Nido P.J. Extracorporeal membrane oxygenation for cardiac support in children. Ann Thorac Surg. 1996;61(1):336-339.
170. Alsoufi B., Al-Radi O.O., Nazer R.I., et al. Survival outcomes after rescue extracorporeal cardiopulmonary resuscitation in pediatric patients with refractory cardiac arrest. J Thorac Cardiovasc Surg. 2007;134(4):952-959.
171. Appleton G.O., Cummins R.O., Larson M.P., Graves J.R. CPR and the single rescuer: at what age should you “call first” rather than “call fast”? Ann Emerg Med. 1995;25(4):492-494.
172. Kudenchuk P.J., Cobb L.A., Copass M.K., et al. Amiodarone for resuscitation after out-of-hospital cardiac arrest due to ventricular fibrillation. N Engl J Med. 1999;341(12):871-878.
173. Dorian P., Cass D., Schwartz B. Amiodarone as compared with lidocaine for shock-resistant ventricular fibrillation. N Engl J Med. 2002;346(12):884-890.
174. Caffrey S.L., Willoughby P.J., Pepe P.E., Becker L.B. Public use of automated external defibrillators. N Engl J Med. 2002;347(16):1242-1247.
175. Valenzuela T.D., Roe D.J., Nichol G. Outcomes of rapid defibrillation by security officers after cardiac arrest in casinos. N Engl J Med. 2000;343(17):1206-1209.
176. Samson R.A., Berg R.A., Bingham R., et al. Use of automated external defibrillators for children: an advisory statement from the pediatric advanced life support task force. Circulation. 2003;107(25):3250-3255.
177. American Heart Association. 2005 American Heart Association (AHA) guidelines for cardiopulmonary resuscitation (CPR) and Emergency Cardiovascular Care (ECC) of pediatric and neonatal patients: pediatric basic life support. Pediatrics. 2006;117(5):e989-e1004.