CHAPTER 86 Percutaneous Vascular Interventions
The first known use of angiography was performed on a cadaver hand in 1896, a year after Roentgen developed the first x-ray. The progress of angiography was initially slow because of the lack of a suitable in vivo contrast medium. Eventually, a contrast agent was developed based on linking iodine to carbon, a formula fundamental to all current iodinated agents. Vascular access remained a problem until 1954, when Seldinger described the technique for percutaneous vascular access.1 This allowed safe vascular cannulation and resulted in the rapid development of techniques for diagnostic percutaneous angiography.
Percutaneous vascular therapeutic interventions evolved from the successes of diagnostic angiography. The first case of percutaneous revascularization was performed by Dotter and Judkins,2 who successfully dilated a superficial femoral artery stenosis in 1964 using serial dilation over a percutaneously inserted guide wire. Since then, many technologic innovations in hardware materials, such as balloons, metallic stents and guide wires, have led to the rapid progression of percutaneous vascular interventions. Advances in contrast media and imaging technology, such as digital subtraction angiography, CT, and MRI, have also ushered in new approaches and methods to identify and treat a wide array of vascular diseases through the percutaneous approach. One such example of great clinical usefulness is the use of carbon dioxide (CO2) as a contrast material for arteriography in digital subtraction angiography system.3 The knowledge gained through such arterial interventions has been applied for cancer therapy to achieve temporary tumor devascularization in preparation for surgery or to treat the tumor through a combination of chemotherapy and vascular occlusion materials.
EQUIPMENT AND TOOLS
Catheters
A catheter commonly serves as a delivery conduit for contrast materials, drugs and embolic devices. Catheters are long hollow tubes made of various materials, usually polyethylene or polyurethane. The size (diameter) refers to the outer diameter of a catheter, with typical sizes varying from 4F to 9F. The inner lumen of a diagnostic catheter is constant, and allows a 0.038-inch diameter guide wire. Within the shaft of a catheter, there is a layer of fine braided wire, resulting in a flexible, kink resistant, torqueable structure. The luminal surface is coated with Teflon or other low-friction substances to provide a low-friction surface for passage of the guide wire and other devices used for peripheral arterial interventions. The tip of the catheter is soft and often tapered. Some catheters have a preformed shape. This may help manipulate the catheter across a vessel or allow selective cannulation of a vessel. Various tip configurations are currently available. Catheter selection depends on the angle at which the target vessel arises from the parent vessel. Several catheters have a reverse curve (e.g., Simmons, SOS omni), which require reformation in a larger vessel to return to the original curve of the catheter.4 Other catheters have multiple side holes at the tip and allow injection of a large volume of contrast material at a high flow rate.
Balloons
Although the first percutaneous angioplasty was described by Dotter and Judkins2 in 1964, Gruentzig devised the first successful balloon angioplasty in 1976, and this method became widely accepted.5 The primary mechanism of balloon angioplasty is controlled tear and shearing of the atheromatous plaque, the intima, and the media beneath the plaque.
Compliant and Noncompliant Angioplasty Balloons
Cryoplasty Balloons
The PolarCath angioplasty system (Boston Scientific) simultaneously dilates and cools the plaque and vessel wall. Nitrous oxide gas is instilled into the balloon and chills the vessel wall to −10° C over a depth of 500 µm. It is believed that cooling to −10° C can induce cell apoptosis rather than necrosis in smooth muscle cells, resulting in decreased elastic recoil and reduced neointimal hyperplasia. This device is relatively new and more clinical studies are required to provide a robust evidence of the efficacy of this mode of treatment.6
Atherectomy Devices
Atherectomy devices (e.g., SilverHawk Plaque Excision System; U.S. Peripheral Products, Plymouth, MN) use a small rotating blade to cut and remove vessel wall atheroma. Theoretically, atherectomy offers the following advantages over conventional percutaneous transluminal angioplasty. It reduces focally and selectively the degree of stenosis by debulking the atheromatous mass, which increases immediate technical success, given the absence of subintimal dissection and local trauma. Although short-term results show a favorable trend toward decreased restenosis rates, long-term efficacy data of this technique is not yet available.7
Stents
The first endovascular stent (endovascular splint) was used by Dotter in 1969.8 Since then, stent technology has grown significantly and revolutionized the percutaneous management of vascular disease. Currently, there are two basic devices, balloon-mounted and self-expanding stents.
Drug-Eluting Stents
Following angioplasty and stent placement, smooth muscle cells in the arterial wall may undergo hyperplasia, resulting in neointimal hyperplasia and subsequent stenosis within the stent. Drug-eluting stents are designed to decrease stent restenosis rates. Drug-eluting stents contain three components, a metallic stent, a slow-release chemical coating, and a drug. The drug acts locally on the vascular smooth muscle cells, theoretically reducing neointimal hyperplasia that results in decreased stent restenosis. To date, the most promising drugs include sirolimus, zotorolimus, everolimus, and paclitaxel. Long-term data are lacking but multiple studies are underway to assess the efficacy of drug-eluting stents.9
Stent Grafts
Stent grafts are covered stents that serve as a vascular conduit. The covering material is generally a synthetic textile such as Dacron, extruded polytetrafluoroethylene (ePTFE), or polyethylene terephthalate (PET). Stent grafts may be balloon-mounted (e.g., Fluency stent, Bard Peripheral) or self-expanding (e.g., Viabahn, W.L. Gore, Flagstaff, Ariz). Common indications for stent grafts are treatment of ruptured or unruptured aneurysm, repair of an inadvertently ruptured vessel during angioplasty, and helping prevent restenosis.10
PERCUTANEOUS ARTERIOGRAPHY
Percutaneous Vascular Access
Vascular access techniques have evolved little since Seldinger described the technique of percutaneous access using a removable core or hollow needle, which allows the insertion of a guide wire.1 The choice of the vascular access site is based on the procedure being performed, the location of the target vessel, and the degree of focal atherosclerotic disease in the affected vascular region. The access vessel should be readily free of disease. Access may be performed using a single-wall or double-wall technique. Regardless of the technique, once the vessel is accessed, a nontraumatic wire is passed through the lumen of the needle into the vessel. The entrance site to the vessel should be located over a bone whenever possible. This provides a stable object against which to compress the vessel following completion of the procedure and removal of the catheters and sheaths.
Specific Regions of Interest
Arch Aortography
Arch aortography is commonly performed for the assessment of atherosclerotic vascular disease, such as stenosis of the origin of the great vessels—the innominate, carotid, and subclavian arteries (Fig. 86-1). It is also used in the evaluation of thoracic aortic aneurysms and dissections, post-traumatic vascular injuries and, in some cases, congenital anomalies of the aorta and/or great vessels, especially if therapeutic intervention is desired.
Lower Limb Angiography
Lower extremity arteriography is indicated for the diagnosis and endovascular management of limb ischemia (Fig. 86-2), trauma, vascular malformation, and tumors. Vascular access may be gained through a femoral, brachial, or radial route.
Renal Angiography
Renal angiography is indicated for the diagnosis and management of suspected renovascular hypertension (Fig. 86-3), hematuria of unknown origin, trauma, preoperative assessment of a donor kidney, and postoperative evaluation of a transplanted kidney. It may also be performed for the devascularization of renal tumors prior to surgery or as a palliative therapy.
Indications and Contraindications
Indications for percutaneous arteriography are as follows:
Outcomes and Complications
Complications of angiography are generally related to the arterial puncture site and nephrotoxic effects of the contrast material.11,12 The most common puncture site complication is local hemorrhage, which occurs in fewer than 3% of patients. Large hematomas may require transfusion or vascular surgical repair. The risk of hemorrhage is increased with large-caliber catheters, multiple sheath or catheter changes, and patients with bleeding diathesis, local aneurysmal or atherosclerotic arterial disease, or inadequate compression of the puncture artery. In addition to hematoma, false aneurysms (less than 0.5%) and, rarely, arteriovenous fistula formation (less than 0.1%) may occur.
Embolization into the distal arterial tree occurs most frequently in the lower limbs, given the overwhelming predominance of femoral arterial access. Embolization is caused by thrombus stripping off the guide wire or catheter or by dislodgment of an atheroma (cholesterol emboli) during catheter manipulation. The administration of systemic anticoagulation during the procedure helps reduce the incidence of distal embolization. The use of embolic protection devices during lower extremity interventions also may reduce the incidence of clinically significant distal embolization.13
ANGIOPLASTY
Angioplasty was initially described by Dotter and Judkins,2 who successfully carried out angioplasty of a superficial femoral arterial stenosis using serial dilation. Later, balloon technology was developed by Gruentzig in 1976. Since then, balloon angioplasty has become the standard for revascularization procedures. Successful angioplasty requires careful attention to patient work-up, high-quality angiography, selection of proper hardware, and careful postprocedure management. In general, short-segment stenoses and occlusions respond well to angioplasty.
In addition to routine laboratory work-up and noninvasive vascular studies prior to a planned revascularization procedure, patients should ideally be treated with antiplatelet drugs (e.g., clopidogrel and/or aspirin). Depending on the location of the stenosis or occlusion, an antegrade or retrograde approach is undertaken. In general, a guide catheter or large sheath is placed at the arterial access site to allow angiography of the segment being treated and a balloon catheter to be used. Heparin (70 to 100 U/kg) is administered intravenously prior to crossing the stenosis. The stenosis or occlusion is crossed first with a hydrophilic wire and then with a catheter. The intravascular location of the catheter distal to the stenotic segment is confirmed by contrast material administration and then a stiff working wire (e.g., Rosen or Amplatz wire) is positioned across the stenosis. The catheter is exchanged for a properly sized balloon. The diameter of the balloon is determined by the vascular segment being treated and the size of the normal adjacent vessel. The balloon is inflated, deflated, and removed. Inflation devices are helpful to treat the lesion adequately. A postangioplasty diagnostic arteriogram is obtained (Fig. 86-4) to confirm the therapeutic result. A successful angioplasty is revealed by the absence of any significant residual stenosis (i.e., residual stenosis less than 30%), normalization of intravascular pressures across the stenosis (pressure gradient less than 10 mm Hg), and disappearance of collaterals. Postprocedure, heparin may be infused intravenously for 24 hours.
Special Considerations
Indications and Contraindications
The main indications for angioplasty vary based on the specific arterial region. They include life- limiting claudication and chronic critical limb or organ ischemia (e.g., rest pain, ulcer, gangrene), bypass graft stenosis, and clinically significant arterial stenoses in the renal artery, mesenteric circulation, and other arterial territories. Angioplasty may also be performed to treat in-stent stenosis (Fig. 86-5). Specific indications for each vascular territory are discussed in subsequent chapters.
Imaging Findings
Preprocedural Planning
It is paramount to review previous noninvasive studies (e.g., CTA, MRA, Doppler, ankle-brachial index [ABI] testing) and any prior arteriograms. These are reviewed to assess the extent of the steno-occlusive disease, disease at the access site, anatomy, and disease affecting the distal arterial bed. Imaging studies such as Doppler provide hemodynamic information about the severity of the stenosis. Careful clinical evaluation and recent clinical laboratory studies, in particular renal function, are essential. Administration of antiplatelet medications prior to a planned angioplasty may also improve clinical outcome.14
Postprocedural Surveillance
Antiplatelet therapy with aspirin and/or clopidogrel is generally recommended following angioplasty.14 The duration of antiplatelet therapy is debatable and many interventional interpreting physicians prefer to treat the patient for at least 6 months following angioplasty. It is essential to carry out a postprocedure assessment such as a Doppler ultrasound examination or ABI testing at 24 to 48 hours postangioplasty. Long-term follow-up (e.g., at 1, 3, 6, and 12 months) is also important to monitor patient outcome. Imaging studies such as Doppler or CTA may be performed if there is suspicion of residual or recurrent disease. Secondary interventions with repeat angioplasty or stent placement may be performed to increase the assisted patency following angioplasty.
REVASCULARIZATION WITH STENTS AND STENT GRAFTS
Primary stenting—direct placement of a stent without prior angioplasty—is often practiced for the treatment of iliac, renal, and subclavian artery disease when the stenosis affects the ostium or is a result of an eccentric ulcerated plaque (Fig. 86-6). Secondary stenting following failed angioplasty or a complication of angioplasty is more commonly done.
Outcomes and Complications
In general, stents provide better patency rates compared with angioplasty in the treatment of renal artery disease, subclavian stenosis, and iliac artery disease. The role of stenting has been debated for the treatment of femoropopliteal and infrapopliteal disease. It appears that stents provide better short-term patency rates in the treatment of superficial femoral artery disease.15 The role of stent grafts in the treatment of aneurysms is now well established for abdominal aortic and iliac artery aneurysms.
THROMBOLYSIS
Special Considerations
Mechanical Thrombolysis
As its name suggests, the ultrasound thrombolytic infusion catheter (EKOS, Bothell, Wash) combines the use of catheter-directed pharmacologic thrombolysis with ultrasound. Ultrasound functions to alter the structure of the thrombus by temporarily increasing its permeability while providing an acoustic pressure gradient that helps move the drug into the thrombus to speed its dissolution (Fig. 86-7).
Indications and Contraindications
Indications for thrombolysis are as follows:
Outcomes and Complications
The technical success rate is around 80% with urokinases or rt-PA for acute thrombotic occlusions.16 The success rate decreases with the age of the occlusion. In general, infusion duration is much shorter with rt-PA.
Complications include the following:
ATHERECTOMY
The concept of removing an obstructive plaque by a catheter-based excision technique was first introduced by Höfling and colleagues.17 There are several different types of atherectomy devices available, including directional atherectomy devices (e.g., SilverHawk), orbital atherectomy devices (e.g., Diamondback 360, Cardiovascular Systems, Minneapolis), rotational atherectomy devices (e.g., Pathway Jetstream, Pathway Medical Technologies, Kirkland, Wash; Rotablator, Boston Scientific), and laser atherectomy devices (e.g., Excimer laser wire).18 At present, no specific recommendations have been established for the treatment of lower limb disease with atherectomy devices and there are no long-term results of peripheral application of atherectomy. The procedure involves crossing of a stenotic lesion with a wire and repeatedly passing the atherectomy catheter over the wire to remove the plaque. The size of the atherectomy device depends on the target vessel diameter.
Outcomes and Complications
Few large studies exist evaluating the efficacy of atherectomy compared with balloon angioplasty.19,20 However, there are several theoretic advantages. Physical debulking of the atheromatous plaque compared with fracturing of the media would seem to provide better long-term patency. However, studies have demonstrated that the long-term patency obtained with atherectomy catheters is no better than that achieved with traditional balloon angioplasty.20,21
EMBOLIZATION
Embolic Agents and Devices
A variety of embolic agents are available, including the following:
Principles of Embolization
A detailed description of various embolization procedures is beyond the scope of this chapter. Here we will briefly discuss the principles of embolization.22 When distal arteriolar or capillary embolization (as in tumor embolization) is desired, particulate or liquid embolizing agents are used. It is important to occlude all collateral supply to the tissue to achieve complete devascularization. When a distal arterial bed needs to be reperfused through collaterals (as in splenic artery embolization for splenic trauma), proximal embolization with mechanical devices is carried out. During therapy of aneurysms and pseudoaneurysms, the arterial inflow and outflow should be occluded to prevent persistent perfusion of the aneurysm. Another option is to completely pack the aneurysm sac with mechanical coils. In the treatment of arteriovenous malformations, all the branch vessels supplying the nidus or the entire nidus should be completely embolized. Arteriovenous fistulas can be treated with coil embolization of the communication or by excluding the communication with a stent graft.
Indications and Contraindications
There are three main therapeutic indications for embolization:
Outcomes and Complications
Imaging Findings
Postprocedural Surveillance
KEY POINTS
 Angiography remains the gold standard for the diagnosis of vascular disorders and surveillance of various endovascular therapies.
Angiography remains the gold standard for the diagnosis of vascular disorders and surveillance of various endovascular therapies.Allaqaband S, Solis J, Kazemi S, Bajwa T. Endovascular treatment of peripheral vascular disease. Curr Probl Cardiol. 2006;31:711-760.
Aronow H. Peripheral arterial disease in the elderly: recognition and management. Am J Cardiovasc Drugs. 2008;8:353-364.
Beard JD. Which is the best revascularization for critical limb ischemia: endovascular or open surgery? J Vasc Surg. 2008;48:11S-16S.
Mannava K, Money SR. Current management of peripheral arterial occlusive disease: a review of pharmacologic agents and other interventions. Am J Cardiovasc Drugs. 2007;7:59-66.
Stoyioglou A, Jaff MR. Medical treatment of peripheral arterial disease: a comprehensive review. J Vasc Interv Radiol. 2004;15:1197-1207.
1 Berneus B, Carlsten A, Holmgren A, Seldinger Si. Percutaneous catheterization of peripheral arteries as a method for blood sampling. Scand J Clin Lab Invest. 1954;6:217-221.
2 Dotter CT, Judkins MP. Transluminal treatment of arteriosclerotic obstruction. Description of a new technic and a preliminary report of its application. Circulation. 1964;30:654-670.
3 Hawkins IF. Carbon dioxide digital subtraction arteriography. AJR Am J Roentgenol. 1982;139:19-24.
4 Berman HL, Cornell TJ. A technique for reforming the Simmons curved cerebral catheters. AJR Am J Roentgenol. 1982;139:824.
5 Jerie P. [Thirty years of the balloon catheter—A. Gruentzig and percutaneous balloon angioplasty.]. Cas Lek Cesk. 2004;143:866-871.
6 Fava M, Loyola S, Polydorou A, et al. Cryoplasty for femoropopliteal arterial disease: late angiographic results of initial human experience. J Vasc Interv Radiol. 2004;15:1239-1243.
7 Zeller T, Rastan A, Schwarzwalder U, et al. Percutaneous peripheral atherectomy of femoropopliteal stenoses using a new-generation device: six-month results from a single-center experience. J Endovasc Ther. 2004;11:676-685.
8 Dotter CT. Transluminally-placed coilspring endarterial tube grafts. Long-term patency in canine popliteal artery. Invest Radiol. 1969;4:329-332.
9 Schmehl J, Tepe G. Current status of bare and drug-eluting stents in infrainguinal peripheral vascular disease. Expert Rev Cardiovasc Ther. 2008;6:531-538.
10 Marin ML, Hollier LH. Endovascular grafts. Semin Vasc Surg. 1999;12:64-73.
11 Halpern M. Percutaneous transfemoral arteriography: an analysis of the complications in 1,000 consecutive cases. Am J Roentgenol Radium Ther Nucl Med. 1964;92:918-934.
12 Nunn DB. Complications of peripheral arteriography. Am Surg. 1978;44:664-669.
13 Lam RC, Shah S, Faries PL, et al. Incidence and clinical significance of distal embolization during percutaneous interventions involving the superficial femoral artery. J Vasc Surg. 2007;46:1155-1159.
14 Dorffler-Melly J, Koopman MM, Prins MH, Buller HR. Antiplatelet and anticoagulant drugs for prevention of restenosis/reocclusion following peripheral endovascular treatment. Cochrane Database Syst Rev 2005; (1):CD002071.
15 Ruef J, Hofmann M, Haase J. Endovascular interventions in iliac and infrainguinal occlusive artery disease. J Interv Cardiol. 2004;17:427-435.
16 Ouriel K, Veith FJ, Sasahara AA. A comparison of recombinant urokinase with vascular surgery as initial treatment for acute arterial occlusion of the legs. Thrombolysis or Peripheral Arterial Surgery (TOPAS) Investigators. N Engl J Med. 1998;338:1105-1111.
17 Höfling B, Pölnitz AV, Backa D, et al. Percutaneous removal of atheromatous plaques in peripheral arteries. Lancet. 1988;1:384-386.
18 Shrikhande GV, McKinsey JF. Use and abuse of atherectomy: where should it be used? Semin Vasc Surg. 2008;21:204-209.
19 McKinsey JF, Goldstein L, Khan HU, et al. Novel treatment of patients with lower extremity ischemia: use of percutaneous atherectomy in 579 lesions. Ann Surg. 2008;248:519-528.
20 Biskup NI, Ihnat DM, Leon LR, et al. Infrainguinal atherectomy: a retrospective review of a single-center experience. Ann Vasc Surg. 2008;22:776-782.
21 Chung SW, Sharafuddin MJ, Chigurupati R, Hoballah JJ. Midterm patency following atherectomy for infrainguinal occlusive disease: a word of caution. Ann Vasc Surg. 2008;22:358-365.
22 Osuga K, Mikami K, Higashihara H, et al. Principles and techniques of transcatheter embolotherapy for peripheral vascular lesions. Radiat Med. 2006;24:309-314.

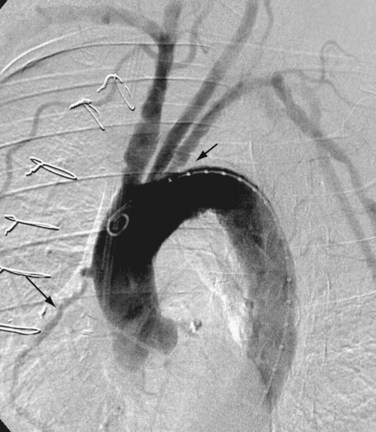
 FIGURE 86-1
FIGURE 86-1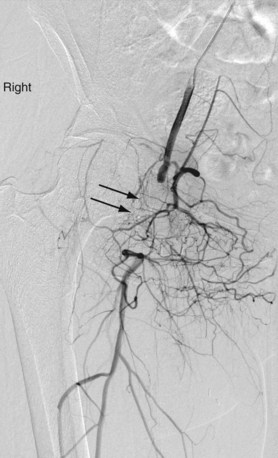
 FIGURE 86-2
FIGURE 86-2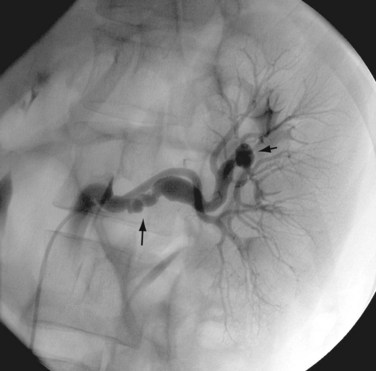
 FIGURE 86-3
FIGURE 86-3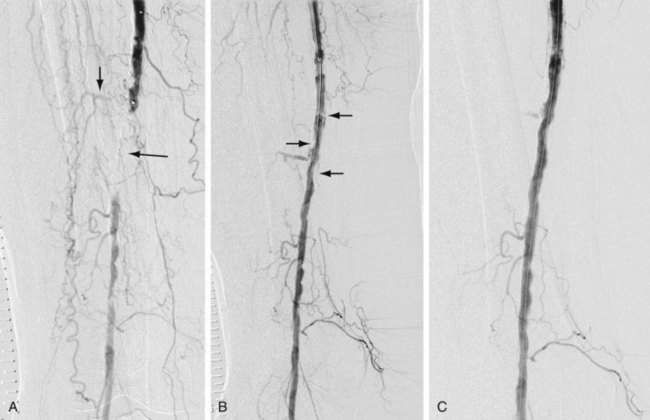
 FIGURE 86-4
FIGURE 86-4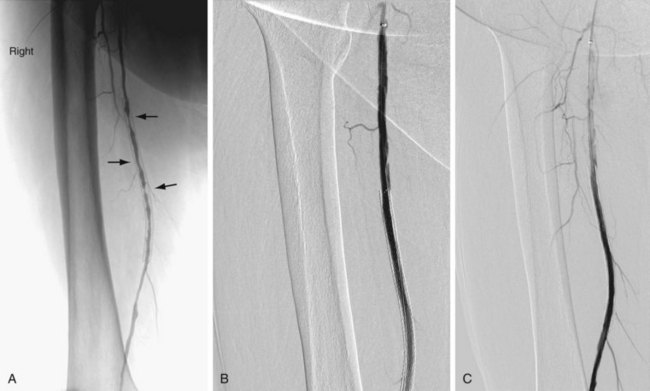
 FIGURE 86-5
FIGURE 86-5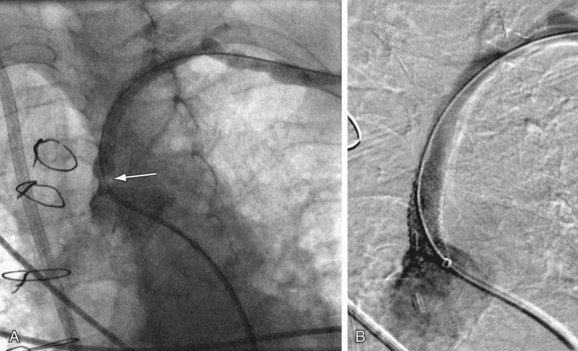
 FIGURE 86-6
FIGURE 86-6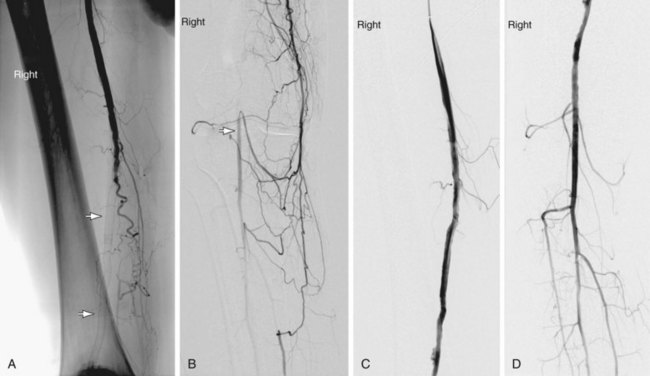
 FIGURE 86-7
FIGURE 86-7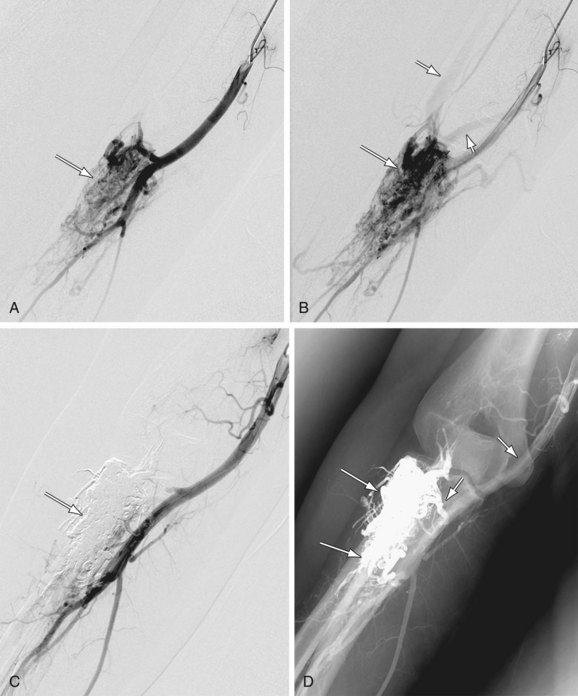
 FIGURE 86-8
FIGURE 86-8

