CHAPTER 161 Percutaneous Procedures for Trigeminal Neuralgia
Historical Development of Percutaneous Procedures for Trigeminal Neuralgia
Percutaneous procedures for treatment of trigeminal neuralgia were first introduced in 1853 by Patruban, who divided the maxillary nerve behind the orbit by passing a tenotome along the floor of the orbit and cutting the nerve as far back as possible.1 In 1910, Harris applied a lateral approach to reach the foramen ovale and inject alcohol.2 In 1914, Härtel modified the approach to an anterior one similar to that currently practiced,3,4 and in 1930, Irger used an inferior approach (both approaches used alcohol injection).4,5 Fortuitous serendipity led to the application of glycerol to treat trigeminal neuralgia. In 1981, Häkanson,6 while using glycerol as a medium to introduce tantalum dust into the trigeminal cistern as part of a stereotactic radiosurgical technique for the treatment of trigeminal neuralgia, noticed that patients became pain free, immediately. Later, he used glycerol alone and confirmed the pain relief effect.
It was Rethi who first introduced the use of electrolytic techniques for treatment of trigeminal neuralgia.7 In 1960, radiofrequency lesion was introduced by Sweet, and it was applied to the treatment of trigeminal neuralgia in 1974.8,9 By applying Taarnhøj and Shelden’s10–12 principle of internal neurolysis of the root of the trigeminal nerve in the dural canal over the margin of the petrous ridge, Mullan and Lichtor,13,14 in 1983, introduced the technique of percutaneous balloon compression of the gasserian ganglion.
With the development of percutaneous techniques there was a need to ensure procedure safety. A first safety step was to ensure adequate needle placement to avoid extratrigeminal injury. This was first addressed in 1916 by Pollock and Potter15 in cadaveric studies, in which needle site during alcohol injection was confirmed using radiography. It was not until 1936 that Putnam and Hampton applied this procedure to patients, with good results.10,16 A second safety step was to ensure physiologically adequate placement and adequate neurolysis without causing excessive damage. To this end, in 1955, Silverstone designed an insulated needle with an exposed tip used as a nerve stimulator.10,17 Later, motor and sensory evoked potential monitoring was used in trigeminal destructive procedures by Karol and colleagues,18 and this was adopted by Sindou and coworkers.19,20 Most recently, neuronavigation has been used to assist with percutaneous trigeminal neurolysis.21,22
Trigeminal Anatomy
The foramen ovale is situated in the anterior part of the sphenoid bone. It lies lateral to the foramen lacerum, which is occluded at its base only by cartilage. It is also near the posterior margin of the lateral pterygoid plate. Posterolaterally lies the foramen spinosum, which transmits the middle meningeal artery and meningeal branch of the mandibular nerve. Rarely, the foramen spinosum and ovale may be confluent. Posteroinferiorly lies the jugular foramen, and posterolaterally, the carotid canal.23 The shape of the foramen ovale is typically oval, yet it can be almond shaped, round, or slit-like. The average length and width are 7.46 mm ± 1.41 mm and 3.21 mm ± 1.02 mm, respectively.24 The foramen may be divided into two or three components in 4.5% of cases.25
Several nerves, arteries, and veins pass through the foramen ovale: the mandibular nerve, lesser superficial petrosal nerve, accessory meningeal artery, and emissary veins (from the cavernous sinus to the pterygoid plexus). The optic ganglion is situated directly under the foramen but may also pass through the foramen ovale.23
The gasserian ganglion originates from the cranial neural crest and the overlying thickened ectoderm. The three processes of the ganglion can be identified at 6 weeks’ gestation; the ophthalmic division develops first, followed by the maxillary and mandibular divisions. Meckel’s cave attains its final shape at 12 weeks’ gestation, with the arachnoid ending around the ganglia.26
The gasserian ganglion is a sensory ganglion of the trigeminal nerve; it is crescent shaped with convexity directed forward. It is located at the apex of the petrous and may extend to the foramen lacerum, or the posterior lip or floor of the foramen ovale. The mean distance from the foramen ovale to the gasserian ganglion is 6 mm (range, 5.8 to 6.3 mm).27 The anterior border of the ganglion is usually within 2 mm in front of, or behind, the posterior lip of the foramen ovale.28 The ganglia’s dural covering forms Meckel’s cave and, medially, the cavernous sinus.28
Meckel’s cave is confined between the outer and inner layers of the dura but also contains a separate sleeve of meningeal dura formed by extension of the posterior fossa dura into the middle fossa. Surfaces are concave inferiorly and medially and more flat superiorly. The sensory branches of the trigeminal nerve and partially the gasserian ganglia and its three divisions occupy Meckel’s cave. The subarachnoid space within Meckel’s cave extends an average of 4.9 mm medially and 1.7 mm laterally beyond the posterior edge of the gasserian ganglia. This space may extend over the divisions of the trigeminal nerve. Mean length of Meckel’s cave ranges from 6 to 16 mm.29 The gasserian ganglion measures 4 to 5 mm wide and 15 to 25 mm long. The length of the sensory root varies from 5 to 15 mm; the length of the mandibular nerve from the anterosuperior margin of the foramen ovale to the gasserian ganglion is 0 to 10 mm.28–30
The ganglion has a posterior sensory root, which joins the brainstem about halfway between the lower and upper borders of the pons. It starts with an oblique run upward from the lateral part of the pons toward the petrous apex, exits the posterior fossa, enters the middle cranial fossa by passing forward beneath the tentorial attachment, and enters Meckel’s cave to join the ganglia through a deep hilum on its posterior aspect. The motor branch of the trigeminal nerve runs in front of and medial to the sensory root and passes beneath the ganglion, leaving the skull through the foramen ovale and, immediately below this foramen, joining the mandibular nerve.31
The sensory rootlets, between the gasserian ganglion and the root in the cerebellopontine angle, form a plexus at its entry into the ganglion. This retrogasserian triangular area has been named the triangular plexus and has been identified as the best place to create a lesion for the treatment of trigeminal neuralgia.32
The trigeminal ganglion is formed by union of the three divisions of the trigeminal system: the mandibular nerve, maxillary nerve, and ophthalmic nerve, also known as V3, V2, and V1, respectively. The ophthalmic nerve is the smallest of the three trigeminal divisions. It inclines upward as it passes forward near the medial surface of the dura, forming the lower part of the lateral wall of the cavernous sinus and reaching the superior orbital fissure. The maxillary nerve takes a more direct course and enters the foramen rotundum. The mandibular nerve occupies most of the gasserian ganglion and takes a caudolateral course from the ganglion and enters the foramen ovale.31,33
Diagnosis
The first known references to what is believed to be trigeminal neuralgia were by Aretaeus of Cappadocia34 and later by Avicenna, who described trigeminal neuralgia in his text Canon Medicinx.35,36
The International Association for the Study of Pain (IASP) defined the essential features of idiopathic trigeminal neuralgia as “sudden, transient, intense bouts of superficially located pain, strictly confined to the distribution of one or more divisions of the trigeminal nerve usually precipitated by light mechanical activation of a trigger point or area.”37 Penman, in a detailed description of trigeminal neuralgia, stated that the only constant feature is the symptom of pain that is unilateral, trigeminal, severe, paroxysmal, and precipitated.38
A diagnosis of trigeminal neuralgia is dependent on the patient’s history and analysis of the patient’s complaint. Pain is usually described as spreading outward from the trigger point to cover an area that roughly approximates the territory of distribution of one (or more) of the trigeminal divisions.39 Usually, there is no clinically evident sensory deficit, yet quantitative and qualitative tests have demonstrated deficits for tactile and warm sensations in the affected area, with no deficit for heat pain or for pinprick.39–42 There is also an absolute, followed by a relative, refractory period after an attack, during which further paroxysms cannot be elicited by trigger-point stimulation.43
A differential diagnosis of trigeminal neuralgia includes a large array of facial pains. Trigeminal neuralgia itself may be due to multiple causes and pathologic processes, which influence the mode of treatment and outcome. To assist in disease management, a trigeminal neuralgia classification system has been proposed by Burchiel.44,45
Diagnostic Tools
Trigeminal evoked potentials and electrophysiologic studies are not widely used but are complementary diagnostic tools.46,47 Magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) processed as three-dimensional images have been used to verify vascular compression.48–50 MRI may not be necessary in cases managed purely by a percutaneous procedure.
Surgical Treatment
There are no specific surgery guidelines for patient selection or guidelines for which surgical method to select. A recent report details the diagnostic evaluation and treatment of trigeminal neuralgia as an evidence-based review and makes some recommendations.51
Surgery is generally offered to patients who fail medical treatment, have only partial relief of pain after 1 year, or according to Garvan and Siegfried,52 still require medication after consumption of more than 3000 tablets of a single drug. Microvascular decompression (MVD) remains the surgical option of choice for trigeminal neuralgia, with percutaneous procedures generally reserved for patients who experience recurrent pain after MVD, are a high surgical risk owing to medical comorbidity, and are older than 65 to 70 years of age, although this latter indication has been questioned.53,54 Percutaneous procedures may also be offered to patients with multiple sclerosis (MS).55–57 In a case of suspected symptomatic trigeminal neuralgia51 due to MS, further evaluation may be needed for surgical planning and prognostication.
Percutaneous Preoperative Care
All percutaneous procedures for the management of trigeminal neuralgia involve producing some injury to trigeminal afferents. This is achieved by using heat in the case of radiofrequency rhizotomy, chemical neurolysis in the case of glycerol rhizotomy, mechanical neurolysis in the case of balloon compression rhizotomy, and radiation-induced neural injury in the case of stereotactic radiosurgery (SRS). That so many percutaneous procedures are available points to the fact that none have proved ideal. That is, no one percutaneous procedure is applicable in all cases with a uniformly high rate of long-term success and with minimal possibility of complication or recurrence. Radiofrequency rhizotomy is perhaps the most commonly used procedure, and long-term case results are available.57–59
Patient Selection
Percutaneous procedures are usually offered to patients with trigeminal neuralgia who have failed medical management or have developed complications or side effects of medical management and have either already had or are not suitable candidates for MVD. Although there are no surgical age guidelines or limits, age older than 65 years can often lead to exclusion of an otherwise eligible patient from the benefits of MVD. Hence, some surgeons prefer percutaneous procedures for patients older than 65 years of age.14,59,60 One could argue that it is prudent to offer MVD to the most eligible patients: those who demonstrate vascular impingement of the trigeminal root on the affected side and are otherwise fit to undergo the procedure. Patients with MS, pontine infarction that affects the trigeminal root entry zone, or brainstem white matter lesions without MS, and those who have previously failed MVD should initially be considered for a percutaneous procedure.58,61,62
Preoperative Patient Preparation
We recommend preoperative MRI for all trigeminal neuralgia patients, with the exception of those with a confirmed MS diagnosis. Patients with trigeminal schwannoma and epidermoid tumor have been described as presenting with trigeminal pain, but this is not usually considered typical trigeminal neuralgia. MRI serves to begin to address primary pathology and to potentially exclude such patients from percutaneous procedures.63–66 Also, when a vascular compression is in doubt, high-resolution, three-dimensional, time-of-flight MRA may be performed to assess and demonstrate vascular impingement (if present) with greater than 90% accuracy.67 Improvement to imaging techniques for the evaluation of trigeminal neuralgia using balanced fast-field echo (BFFE) imaging techniques have also been reported.64
Percutaneous Surgical Procedures
Radiofrequency Rhizotomy
Radiofrequency (RF) rhizotomy was popularized by Sweet and Wepsic, as reported in 1974.9 The concept behind the technique is to use radiofrequency stimulation (alternating electric field with an oscillating frequency of 500,000 Hz) to cause a thermal lesion in the retrogasserian root, or ganglion.68 In an animal study, radiofrequency heating was reported to cause selective injury to small myelinated and unmyelinated fibers69; however, a subsequent neuropathologic clinical model study failed to substantiate this.70 It is now generally believed that radiofrequency heating causes a nonselective destruction of axons, regardless of fiber size.71
In the operating suite, the patient is placed supine on the operating table. After intravenous access is secured, anticholinergics (usually 0.4 mg of atropine) may be given to minimize oral secretions and blunt the vasovagal response during penetration of the needle through the foramen ovale. Some surgeons prefer to watch for the vagal response as a guide while engaging the foramen ovale and may not use preoperative anticholinergics. If anticholinergics are not used, an external pacemaker strapped to the chest wall, set to deliver 45 beats per minute, should bradycardia occur during electrode insertion or during stimulation, may be used.71
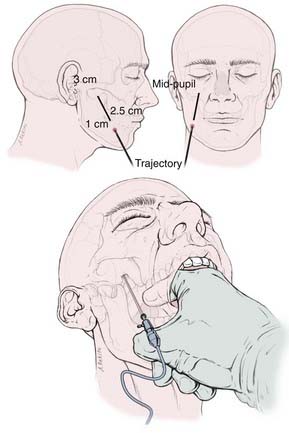
The skin of the cheek and perioral region on the ipsilateral side is painted with antimicrobial solution after the required depth of anesthesia has been obtained (eyelash reflex is lost). A nasal cannula or “trumpet” may assist in the maintenance of the airway. The surgeon stands on the same side of the patient’s pain, and cannulation of the foramen proceeds under fluoroscopic guidance using the Härtel technique with the RF electrode trochar.3,72 The needle enters the cheek through a point 2.5 cm lateral to the corner of the mouth and 1 cm inferior to the occlusal plane and advances through the cheek in the submucosal layer, between the pterygoid bone and the angle of the mandible, guided by the surgeon’s index finger of the other hand within the patient’s mouth (Fig. 161-1). The needle is directed along a line representing the intersection of a vertical plane passing through the lateral aspect of the ipsilateral pupil and a horizontal plane passing through a point 3 cm anterior to the external auditory meatus along the inferior border of the zygoma. The needle engages the foramen ovale about 6 to 8 cm from the skin surface. This can usually be evidenced by a contraction of the masseter muscle manifested as jaw jerk (see Fig. 161-1).
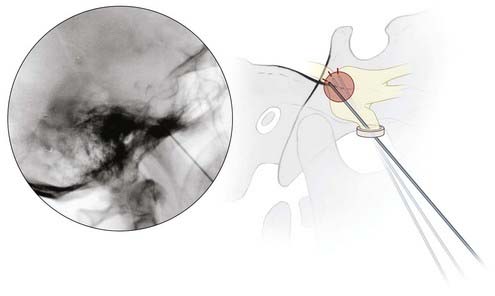
Lateral fluoroscopy is used thereafter, and the needle is advanced to a point just superior to the intersection of petrous bone and the clivus on a true lateral view. The patient is now awakened from anesthesia, and the stylet of the trochar is withdrawn and replaced with the RF electrode. A curved-tip electrode (Tew) may be necessary to target V1 and V2, but typically a straight electrode suffices to produce a lesion in V3 (Fig. 161-2).
In 98% to 99% of the patients, pain relief is obtained immediately after surgery.73,74 A recurrence rate of 20% at 9 years has been described, with an incidence of 9% for mild dysesthesia, 2% for major dysesthesia, and 0.2% for anesthesia dolorosa.73 Because hypoesthesia is the end point of the procedure, numbness is present in 100% of patients after surgery.73 The irreversible loss of long latency trigeminal root evoked potentials has been documented as a means of objective assessment of the effects of the rhizotomy,75 and a recently described multiarray electrode method for mapping the trigeminal nerve may help in producing more selective lesions.76
Glycerol Rhizotomy
The procedure for injecting anhydrous glycerol (99.5%), a mild neurolytic, can be performed both in an operating room with fluoroscopic facilities and in a radiology suite setting with anesthetic support.77 Because the procedure is largely anatomic (does not require intraoperative patient response to guide the surgeon during the lesioning), the patient can be sedated throughout the procedure, although some surgeons prefer that the patient be awakened after the needle has been placed through the foramen ovale.77 Patients often receive a short-acting barbiturate, such as methohexital, or another short-acting agent, such as propofol. Alternatively, an external pacemaker may be used, set to trigger at 45 beats per minute, should bradycardia occur during needle insertion or during the glycerol injection.
The patient is positioned supine with the neck extended, similar to the RF procedure, and a 20-gauge, 3.5-inch spinal needle is inserted into the foramen ovale using the Härtel technique, as described earlier. To ensure access to the trigeminal cistern, the foramen ovale should be entered through the medial third. The position of the needle is confirmed by fluoroscopy and is manipulated, usually in 1-mm increments under guidance, to obtain a free flow of cerebrospinal fluid (CSF) on removing the stylet. When a free flow of CSF is obtained on removing the stylet, the stylet is reinserted into the needle, and the patient is carefully moved into a sitting position with the neck flexed forward such that the orbital-meatal line is past horizontal. A cisternogram is then performed using a water-soluble contrast medium such as iohexol (Omnipaque). Using a tuberculin syringe, the contrast medium is injected in 0.05-mL boluses, and under continuous lateral fluoroscopy, the filling of the trigeminal cistern is noted. A typical pear-shaped appearance of the cistern is visualized. The volume of the cistern is recorded as the amount of contrast required to fill up the cistern, and then the contrast is allowed to drain out either through the needle by removing the syringe or into the posterior fossa by tilting the head backward. The average volume of the cistern is 0.25 to 0.30 mL, and it uncommonly exceeds 0.5 mL (range, 0.1 to 1 mL).71,78 Some surgeons have reported successful procedures without using the cisternogram for localization, instead relying on fluoroscopy and CSF backflow,79 although this introduces an element of uncertainty as to the destination of the injected glycerol.
Glycerol is then slowly injected into the trigeminal cistern in a similar manner, under continuous fluoroscopy, with the patient sitting upright and the head flexed forward. Although many surgeons simply fill the cistern with glycerol,80 others attempt to produce a more selective lesion.71 Calculating the volume of glycerol required for a more “selective” neurolytic effect relies on the “floating glycerol” technique, which depends on the assumption that glycerol has a lower specific gravity than the contrast medium and therefore “floats” on its surface. The ratio of glycerol to contrast medium can be varied for directing treatment against specific targeted fibers; ratios of 30 : 70 for V1, 50 : 50 for V2, and 70 : 30 for V3 are used to achieve the purpose. The emphasis is that it is more important to drain the cistern completely of contrast medium before injecting glycerol in the case of V3 pain than in the case of V1 pain.
After withdrawal of the spinal needle, the glycerol should be kept in contact with the nerve for at least 1 hour (up to 2 hours has been recommended), by keeping the patient in a sitting position with the head flexed.71,74,77,78 The patient may then be discharged home.
Most patients experience relief immediately or within 1 to 2 days after surgery, although it can take up to 2 weeks to achieve pain relief in some cases.80 Liu and Apfelbaum77 have recommended a repeat procedure if pain relief does not occur within 7 days of surgery.
Balloon Compression
Percutaneous balloon microcompression of the trigeminal ganglion using a balloon catheter was introduced by Mullan and Lichtor.14 This technique was purportedly derived from the open technique of mechanical injury to the trigeminal ganglion performed through temporal craniotomy by Shelden and Pudenz in 1955.74,81,82 Skirving and Dan have reported performing a similar technique since 1980, following an introduction to the method by Mullan and before the detailed method published by Mullan in 1983.14,83 Based on a rabbit experimental study, Brown and colleagues have postulated that balloon compression has the advantage of selectively avoiding injury to the small, unmyelinated fibers that mediate corneal reflex, providing relative protection of the corneal sensation.84 Microcompression of the trigeminal ganglion takes place during the procedure; it has also been documented in anatomic study on cadavers that when the balloon is fully inflated, there is stretching of the dura, relieving what is called the dural compression of the trigeminal ganglion and its root.85 This lends some support to the hypothesis of Taarnhøj, who postulated that trigeminal neuralgia may, in part, be caused by the compression and angulation of the nerve root where it crosses the apex of the petrous ridge.86,87
The patient is positioned supine with the head extended about 15 degrees. Using fluoroscopic guidance and Härtel guidelines, as discussed under “Radiofrequency Rhizotomy,” a 14-gauge needle with a blunt obturator is introduced into the foramen ovale of the affected side.3 When the cannula has engaged the foramen ovale, the blunt obturator is removed, and a straight guiding stylet is inserted. Under fluoroscopic guidance, the stylet is then directed toward the porus trigeminus (proximal entrance to Meckel’s cave), radiographically denoted by the medial dip in the petrous bone on an anteroposterior image (with the petrous ridge positioned in the radiographic center of the orbital cavity). This brings the tip of the stylet between 17 and 22 mm beyond the foramen ovale, within 5 mm beyond the clival line. In this location, the tip of the stylet abuts the maxillary fibers. Manipulations (1 mm) of the stylet medially or laterally bring the tip closer to V1 or V3 fibers, respectively.
A No. 4 Fogarty balloon catheter is test-flatted with contrast, the stylet is withdrawn, and the balloon with inner stylet is introduced. This is the standard apparatus used for this procedure, although the use of variably sized balloon catheters has also been reported.88 A pressure transducer attached to an insufflation syringe or a tuberculin syringe is screwed to the proximal end of the catheter using a three-way stopcock. The balloon is then inflated using about 1 mL of contrast agent. If accurately positioned, the balloon assumes a pear shape on lateral fluoroscopy. The thin neck of the pear is the portion of the balloon within the porus trigeminus. Adequate compression can be verified by measuring the intraluminal pressure changes and is limited by lifting the dura off the trigeminal ganglion. An intraluminal pressure of 1200 to 1500 mm Hg (1.3 to 1.5 atmospheres) is considered adequate, signifying a resultant tissue compression pressure of 650 to 950 mm Hg.71,74,89,90
The balloon is kept inflated for 1 to 1.5 minutes if it is a first surgery and for 1.5 to 2 minutes in cases of recurrent pain to minimize the risk for dysesthesia. Compression times of up to 10 minutes have been reported.83,91 Limiting the compression time to less than 2 minutes has resulted in reduction of the incidence of masseter muscle weakness and severe numbness.90 After compression is completed, the balloon is deflated, and the balloon and catheter are removed, with pressure applied to the cheek for about 5 minutes to prevent hematoma formation. The patient can be discharged home after about 4 hours of observation. Most patients obtain pain relief immediately after surgery. The remaining patients experience pain relief by postoperative day 2.
Postoperative Management
After all percutaneous procedures, the entry point on the cheek is cleaned and dressed, if necessary. Corneal sensation should be tested, and the patient should be counseled as to how to recognize signs of corneal irritation. If substantial loss of corneal sensation occurs or corneal irritation occurs, artificial tears may be advised for use every 2 to 4 hours, and an ophthalmologic evaluation may be warranted. Dietary restrictions are not necessary; however, many patients prefer to restrict their intake to soft foods for a few days. Jaw opening exercises may be necessary, especially in patients undergoing the balloon compression procedure. The inner cheek on the affected side may feel numb, and the patient should be advised to avoid biting the tongue and cheek on that side. Some patients may develop a maceration of the buccal mucosa, requiring irrigation of the mouth with warm saline solution every 4 hours. Some patients may develop “cold sores” a few days after surgery, which need to be treated with a topical application of acyclovir.71 In cases of headache after glycerol rhizotomy, it is necessary to differentiate between chemical meningitis, which will resolve over time, and infective meningitis. Anticonvulsant medications need to be continued immediately after surgery but can be tapered off and discontinued depending on the degree of pain relief obtained.
Radiosurgery
Stereotactic surgery (SRS) was first used by Leksell for the treatment of trigeminal neuralgia in 1971.92 Since then, several groups have demonstrated the efficacy of various radiosurgical techniques in the management of the condition.93–96 The mechanism of action of radiosurgery is presumed to be axonal degeneration as result of radiation. This was postulated by Kondziolka and colleagues from their work on a primate model97 and was later confirmed by histologic analysis of the cisternal segment of the trigeminal nerve in a patient with recurrent trigeminal neuralgia who underwent partial sectioning of the nerve.98 Arteriolar thickening that occurs after radiation insult to the vessel in contact with the nerve has been postulated to have a possible therapeutic effect.99
The procedure involves placing a stereotactic head frame on a patient who is under local anesthesia or with the help of a brief general anesthetic. Thin-section (1 to 1.4 mm) axial MRI is obtained. Constructive interference in steady state (CISS) images are of great value in identifying the nerve as it passes through the trigeminal cistern. Various radiosurgical targets, including the gasserian ganglion, the root entry zone at the pons, and the retrogasserian portion of the nerve (4 to 6 mm long, 2 to 3 mm anterior to the root entry zone), have been used. Most centers now employ the posterior target (root entry zone).77,100–105 The patient is positioned in the radiosurgery device (linear particle accelerator or Gamma Knife), and the target is radiated using a small collimator (4 mm) at a maximal dose of between 60 and 90 Gy for initial treatment and of about 50 Gy in cases undergoing repeat treatment. A 50% isodose line is used at the nerve boundary.
Results and Complications
Of the available percutaneous procedures for trigeminal neuralgia, RF rhizotomy has been reported to provide the highest rate of pain relief. In some series, results are comparable to those of MVD.71 Long-term pain relief (average, 6 years; range, 1 to 9 years) has been reported in 75% of patients (range, 63% to 89%) after RF rhizotomy.71 Pain relief after glycerol rhizotomy (average, 3 years; range, 0.5 to 5.5 years) has been reported in 55% of patients (range, 22% to 70%),71 whereas in the case of balloon compression, pain relief was reported in 73% of patients (range, 62% to 83%) at 4 years’ follow-up (range, 0.5 to 10.7 years).71 Pain relief obtained after SRS has been reported to be as high as 83.1% after the first year,106 with a decrease to 55.8% (complete or partial pain relief) at 5 years.107
Although graded lesions involving the different trigeminal nerve distributions are best obtained using RF rhizotomy, in the case of trigeminal neuralgia affecting the V1 segment, corneal hypoesthesia is a significant complication in up to 16% of cases.108 The initial theory that glycerol could relieve neuralgia without causing sensory loss has largely been disproved. Sensory loss and dysesthesia continue to be significant complications of the procedure.109–111 Damage to the motor rootlets affects the results of balloon compression and has been shown to occur in up to 12% of cases.112
The most common complications of RF rhizotomy are severe dysesthesia in 6% to 9% of cases, corneal hypoesthesia in 1% to 17% (average, 6%), and transient motor weakness in about 19%. After glycerol rhizotomy, the most common complications are corneal hypoesthesia in about 5% (range, 0% to 10%) of cases and significant dysesthesia in about 4% (range, 0% to 13%). Although permanent motor root weakness is a major complication of balloon rhizotomy, occurring in 3% of cases (range, 0% to 12%), significant dysesthesia is also common, occurring in about 5% (range, 0% to 10.6%). The most significant complication of SRS is numbness, which has been reported to occur in up to 21% of cases106 and is considered as bothersome or painful in 5% to 10% of patients.106,113 Significant dysesthesia has been reported in about 3.2% of patients after radiosurgery for trigeminal neuralgia.114
Other potential, but rare, complications resulting from percutaneous needle procedures through the foramen ovale include the risks for stroke, hemorrhage, pseudomeningocele, permanent hearing loss or facial weakness, diplopia due to trochlear nerve or abducens nerve palsy, and CSF rhinnorhea.71,115–118
Special Considerations
Multiple Sclerosis
About 2% of patients with MS suffer from trigeminal neuralgia.119 Although the possibility of concomitant vascular impingement on the trigeminal nerve in cases of MS with trigeminal neuralgia has been well documented120 and anecdotal reports of treatment with decompression published,121 the procedure of MVD is not routinely recommended for such cases even by staunchest of advocates,122,123 primarily because of high failure rate. In this instance, failures may be due to MS patients having extension of demyelination into the brainstem, central to the area generally treated by MVD.124,125 MS patients generally present at a younger age and are somewhat more likely to have bilateral facial pain.55,124 Percutaneous techniques, including glycerol rhizotomy, RF lesioning, and radiosurgery, have been advocated for management.58,125–127 Reported results indicate that 63% of patients treated with SRS required retreatment, compared with 71% of patients treated with RF rhizotomy and 75% of those treated with glycerol rhizotomy. Mean elapsed time periods before retreatment was required were 35 months, 29 months, and 18 months for SRS, RF, and glycerol rhizotomy, respectively.128
Recurrent Treatment
In patients in whom pain has returned after MVD, a second MVD procedure is unlikely to be of any benefit unless the offending vessels from the first MVD were reported to be veins.129,130 In recurrent cases, glycerol rhizotomy or RF rhizotomy has been advocated as the procedure of choice.131 Because the trigeminal cistern may become scarred after a prior glycerol rhizotomy, a repeat glycerol injection has a higher chance of technical failure.132 It may be prudent in such cases to undertake an RF procedure. There is no evidence that RF rhizotomy and balloon compression are associated with greater technical failure rates when repeated.71
Conclusion
At present, there is no ideal surgical procedure for trigeminal neuralgia—one that is minimally invasive, uniformly effective, lacking complications, and without failures or recurrences. MVD still remains the standard by which all other contemporary procedures are measured. MVD provides the longest pain-free interval, yet it is not free of morbidity and mortality. Stereotactic radiosurgery provides a reasonable noninvasive option, but it has delayed onset and a recurrence interval (a few years). Finding an answer to what procedure to use, and when to use it, is difficult because there is no class I or II evidence, in part owing to a lack of uniformity in terms of both the outcome measures and the reporting methods used by most reported case series. Clearly, randomized controlled studies are needed that employ standardized outcomes measures to answer these questions.133–135
Bowsher D. Trigeminal neuralgia: an anatomically oriented review. Clin Anat. 1997;10:409-415.
Burchiel KJ. A new classification for facial pain. Neurosurgery. 2003;53:1164-1166.
Cruccu G, Gronseth G, Alksne J, et al. AAN-EFNS guidelines on trigeminal neuralgia management. Eur J Neurol. 2008;15:1013-1028.
Hakanson S. Trigeminal neuralgia treated by the injection of glycerol into the trigeminal cistern. Neurosurgery. 1981;9:638-646.
Burchiel K, editor. Surgical Management of Pain. New York: Thieme. 2002:228-301.
Kanpolat Y, Berk C, Savas A, et al. Percutaneous controlled radiofrequency rhizotomy in the management of patients with trigeminal neuralgia due to multiple sclerosis. Acta Neurochir (Wien). 2000;142:685-689.
Leksell L. Sterotaxic radiosurgery in trigeminal neuralgia. Acta Chir Scand. 1971;137:311-314.
Lopez BC, Hamlyn PJ, Zakrzewska JM. Systematic review of ablative neurosurgical techniques for the treatment of trigeminal neuralgia. Neurosurgery. 2004;54:973-982.
Mullan S, Lichtor T. Percutaneous microcompression of the trigeminal ganglion for trigeminal neuralgia. J Neurosurg. 1983;59:1007-1012.
Resnick DK, Jannetta PJ, Lunsford LD, et al. Microvascular decompression for trigeminal neuralgia in patients with multiple sclerosis. Surg Neurol. 1996;46:358-361.
Sweet WH, Wepsic JG. Controlled thermocoagulation of trigeminal ganglion and rootlets for differential destruction of pain fibers. 1. Trigeminal neuralgia. J Neurosurg. 1974;40:143-156.
Taha J. Trigeminal neuralgia: percutaneous procedures. Semin Neurosurg. 2004;15:115-134.
Tatli M, Satici O, Kanpolat Y, et al. Various surgical modalities for trigeminal neuralgia: literature study of respective long-term outcomes. Acta Neurochir (Wien). 2008;150:243-255.
1 Harris W. A history of the treatment of trigeminal neuralgia. Postgrad Med J. 1951;27:18-21.
2 Harris W. An analysis of 1,433 cases of paroxysmal trigeminal neuralgia (trigeminal-tic) and the end-results of gasserian alcohol injection. Brain. 1940;63:209-224.
3 Härtel F. Über die intracranielle Injektionsbehandlung der Trigeminusneuralgie. Med Klinik. 1914;10:582-584.
4 Vaughan GT. Injection of the gasserian ganglion for neuralgia of the fifth cranial nerve. Ann Surg. 1917;66:287-289.
5 Irger JM. Penetrating to the gasserian ganglion. Ann Surg. 1930;92:984-992.
6 Häkanson S. Trigeminal neuralgia treated by the injection of glycerol into the trigeminal cistern. Neurosurgery. 1981;9:638-646.
7 Wilkins RH. Trigeminal neuralgia: historical overview, with emphasis on surgical treatment. In: Burchiel K, editor. Surgical Management of Pain. New York: Thieme; 2002:228-301.
8 Sweet WH, Mark VH, Hamlin H. Radiofrequency lesions in the central nervous system of man and cat: including case reports of eight bulbar pain-tract interruptions. J Neurosurg. 1960;17:213-225.
9 Sweet WH, Wepsic JG. Controlled thermocoagulation of trigeminal ganglion and rootlets for differential destruction of pain fibers. 1. Trigeminal neuralgia. J Neurosurg. 1974;40:143-156.
10 Cole CD, Liu JK, Apfelbaum RI. Historical perspectives on the diagnosis and treatment of trigeminal neuralgia. Neurosurg Focus. 2005;18:E4.
11 Taarnhøj P. Decompression of the trigeminal root and the posterior part of the ganglion as treatment in trigeminal neuralgia; preliminary communication. J Neurosurg. 1952;9:288-290.
12 Taarnhøj P. Trigeminal neuralgia and decompression of the trigeminal root. Surg Clin North Am. 1956:1145-1157.
13 Mullan S, Duda EE, Patronas NJ. Some examples of balloon technology in neurosurgery. J Neurosurg. 1980;52:321-329.
14 Mullan S, Lichtor T. Percutaneous microcompression of the trigeminal ganglion for trigeminal neuralgia. J Neurosurg. 1983;59:1007-1012.
15 Pollock LJ, Potter HE. Experimental studies of injection of the gasserian ganglion controlled by fluoroscopy. JAMA. 1916;67:1357-1361.
16 Putnam TJ, Hampton AO. A technique of injection into the gasserian ganglion under roentgenographic control. Arch Neurol Psychiat. 1936;35:92-98.
17 Stookey BP, Ransohoff J. Trigeminal neuralgia: its history and treatment. Springfield, Ill.: Thomas; 1959.
18 Karol EA, Sanz OP, Rey RD. Sensory and motor trigeminal evoked potentials to localize the position of trigeminal electrodes. Acta Neurochir (Wien). 1991;108:110-115.
19 Sindou M, Fobe JL, Berthier E, et al. Facial motor responses evoked by direct electrical stimulation of the trigeminal root. Localizing value for radiofrequency thermorhizotomy. Acta Neurochir (Wien). 1994;128:57-67.
20 Sindou MP. Neurophysiological navigation in the trigeminal nerve: use of masticatory responses and facial motor responses evoked by electrical stimulation of the trigeminal rootlets for RF-thermorhizotomy guidance. Stereotact Funct Neurosurg. 1999;73:117-121.
21 Yang Y, Shao Y, Wang H, et al. Neuronavigation-assisted percutaneous radiofrequency thermocoagulation therapy in trigeminal neuralgia. Clin J Pain. 2007;23:159-164.
22 Xu SJ, Zhang WH, Chen T, et al. Neuronavigator-guided percutaneous radiofrequency thermocoagulation in the treatment of intractable trigeminal neuralgia. Chin Med J (Engl). 2006;119:1528-1535.
23 Gray H, Standring S, Ellis H, et al. Gray’s Anatomy: the Anatomical Basis of Clinical Practice, 39th ed. Edinburgh: Elsevier Churchill Livingstone; 2005.
24 Ray B, Gupta N, Ghose S. Anatomic variations of foramen ovale. Kathmandu Univ Med J. 2005;3:64-68.
25 Reymond J, Charuta A, Wysocki J. The morphology and morphometry of the foramina of the greater wing of the human sphenoid bone. Folia Morphol (Warsz). 2005;64:188-193.
26 Kehrli P, Maillot C, Wolff MJ. Anatomy and embryology of the trigeminal nerve and its branches in the parasellar area. Neurol Res. 1997;19:57-65.
27 Kaplan M, Erol FS, Ozveren MF, et al. Review of complications due to foramen ovale puncture. J Clin Neurosci. 2007;14:563-568.
28 Henderson WR. The anatomy of the gasserian ganglion and the distribution of pain in relation to injections and operations for trigeminal neuralgia. Ann R Coll Surg Engl. 1965;37:346-373.
29 Janjua RM, Al-Mefty O, Densler DW, et al. Dural relationships of Meckel cave and lateral wall of the cavernous sinus. Neurosurg Focus. 2008;25:E2.
30 Soeira G, Abd el-Bary TH, Dujovny M, et al. Microsurgical anatomy of the trigeminal nerve. Neurol Res. 1994;16:273-283.
31 Rhoton ALJr. The cavernous sinus, the cavernous venous plexus, and the carotid collar. Neurosurgery. 2002;51:S375-S410.
32 Sindou M, Keravel Y. [Trigeminal neuralgia. Percutaneous thermocoagulation of the trigeminal nerve (author’s transl)]. Neurochirurgie. 1979;25:166-172.
33 Rhoton ALJr. The anterior and middle cranial base. Neurosurgery. 2002;51:S273-S302.
34 Rose FC. Trigeminal neuralgia. Arch Neurol. 1999;56:1163-1164.
35 Aciduman A, Arda B, Gunaydin A, et al. Laqve (wry mouth) considered in Avicenna’s renowned treatise the Canon of Medicine. Neurocirugia (Astur). 2008;19:267-271.
36 Ameli NO. Avicenna and trigeminal neuralgia. J Neurol Sci. 1965;2:105-107.
37 Mersky H, Bogduk N. Classification of Chronic Pain. 2nd ed. Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. Seattle: IASP Press. 1994:59-71.
38 Penman J. The differential diagnosis and treatment of tic douloureux. Postgrad Med J. 1950;26:627-636.
39 Bowsher D. Trigeminal neuralgia: an anatomically oriented review. Clin Anat. 1997;10:409-415.
40 Chan AW, MacFarlane IA, Bowsher D, et al. Weighted needle pinprick sensory thresholds: a simple test of sensory function in diabetic peripheral neuropathy. J Neurol Neurosurg Psychiatry. 1992;55:56-59.
41 Fruhstorfer H, Lindblom U, Schmidt WC. Method for quantitative estimation of thermal thresholds in patients. J Neurol Neurosurg Psychiatry. 1976;39:1071-1075.
42 Nurmikko TJ. Altered cutaneous sensation in trigeminal neuralgia. Arch Neurol. 1991;48:523-527.
43 Kugelberg E, Lindblom U. The mechanism of the pain in trigeminal neuralgia. J Neurol Neurosurg Psychiatry. 1959;22:36-43.
44 Burchiel KJ. A new classification for facial pain. Neurosurgery. 2003;53:1164-1166.
45 Eller JL, Raslan AM, Burchiel KJ. Trigeminal neuralgia: definition and classification. Neurosurg Focus. 2005;18:E3.
46 Lunsford LD, Bennett MH. Percutaneous retrogasserian glycerol rhizotomy for tic douloureux: Part 1. Technique and results in 112 patients. Neurosurgery. 1984;14:424-430.
47 Szapiro JJr, Sindou M, Szapiro J. Prognostic factors in microvascular decompression for trigeminal neuralgia. Neurosurgery. 1985;17:920-929.
48 Fukuda H, Ishikawa M, Okumura R. Demonstration of neurovascular compression in trigeminal neuralgia and hemifacial spasm with magnetic resonance imaging: comparison with surgical findings in 60 consecutive cases. Surg Neurol. 2003;59:93-99.
49 Yoshino N, Akimoto H, Yamada I, et al. Trigeminal neuralgia: evaluation of neuralgic manifestation and site of neurovascular compression with 3D CISS MR imaging and MR angiography. Radiology. 2003;228:539-545.
50 Miller JP, Acar F, Burchiel KJ. Trigeminal neuralgia and vascular compression in patients with trigeminal schwannomas: case report. Neurosurgery. 2008;62:E974-E975.
51 Gronseth G, Cruccu G, Alksne J, et al. Practice parameter: the diagnostic evaluation and treatment of trigeminal neuralgia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the European Federation of Neurological Societies. Neurology. 2008;71:1183-1190.
52 Garvan NJ, Siegfried J. Trigeminal neuralgia—earlier referral for surgery. Postgrad Med J. 1983;59:435-437.
53 Ashkan K, Marsh H. Microvascular decompression for trigeminal neuralgia in the elderly: a review of the safety and efficacy. Neurosurgery. 2004;55:840-848.
54 Sekula RF, Marchan EM, Fletcher LH, et al. Microvascular decompression for trigeminal neuralgia in elderly patients. J Neurosurg. 2008;108:689-691.
55 Brisman R. Trigeminal neuralgia and multiple sclerosis. Arch Neurol. 1987;44:379-381.
56 Brown JA, McDaniel MD, Weaver MT. Percutaneous trigeminal nerve compression for treatment of trigeminal neuralgia: results in 50 patients. Neurosurgery. 1993;32:570-573.
57 Kanpolat Y, Berk C, Savas A, et al. Percutaneous controlled radiofrequency rhizotomy in the management of patients with trigeminal neuralgia due to multiple sclerosis. Acta Neurochir (Wien). 2000;142:685-689.
58 Berk C, Constantoyannis C, Honey CR. The treatment of trigeminal neuralgia in patients with multiple sclerosis using percutaneous radiofrequency rhizotomy. Can J Neurol Sci. 2003;30:220-223.
59 Nugent GR. Radiofrequency treatment of trigeminal neuralgia using a cordotomy-type electrode. A method. Neurosurg Clin N Am. 1997;8:41-52.
60 Broggi G, Franzini A, Giorgi C, et al. Trigeminal neuralgia: new surgical strategies. Acta Neurochir Suppl (Wien). 1993;58:171-173.
61 Golby AJ, Norbash A, Silverberg GD. Trigeminal neuralgia resulting from infarction of the root entry zone of the trigeminal nerve: case report. Neurosurgery. 1998;43:620-622.
62 Arrese I, Lagares A, Alday R, et al. Typical trigeminal neuralgia associated with brainstem white matter lesions on MRI in patients without criteria of multiple sclerosis. Acta Neurochir (Wien). 2008;150:1157-1161.
63 de Benedittis G, Bernasconi V, Ettorre G. Tumours of the fifth cranial nerve. Acta Neurochir (Wien). 1977;38:37-64.
64 Miller J, Acar F, Hamilton B, et al. Preoperative visualization of neurovascular anatomy in trigeminal neuralgia. J Neurosurg. 2008;108:477-482.
65 Guttal KS, Naikmasur VG, Joshi SK, et al. Trigeminal neuralgia secondary to epidermoid cyst at the cerebellopontine angle: case report and brief overview. Odontology. 2009;97:54-56.
66 Klieb HB, Freeman BV. Trigeminal neuralgia caused by intracranial epidermoid tumour: report of a case. J Can Dent Assoc. 2008;74:63-65.
67 Anderson VC, Berryhill PC, Sandquist MA, et al. High-resolution three-dimensional magnetic resonance angiography and three-dimensional spoiled gradient-recalled imaging in the evaluation of neurovascular compression in patients with trigeminal neuralgia: a double-blind pilot study. Neurosurgery. 2006;58:666-673.
68 Sluijter M, Racz G. Technical aspects of radiofrequency. Pain Pract. 2002;2:195-200.
69 Letcher FS, Goldring S. The effect of radiofrequency current and heat on peripheral nerve action potential in the cat. J Neurosurg. 1968;29:42-47.
70 Smith HP, McWhorter JM, Challa VR. Radiofrequency neurolysis in a clinical model. Neuropathological correlation. J Neurosurg. 1981;55:246-253.
71 Taha J. Trigeminal neuralgia: percutaneous procedures. Semin Neurosurg. 2004;15:115-134.
72 Gokalp HZ, Kanpolat Y, Tumer B. Carotid-cavernous fistula following percutaneous trigeminal ganglion approach. Clin Neurol Neurosurg. 1980;82:269-272.
73 Taha JM, Tew JMJr. Comparison of surgical treatments for trigeminal neuralgia: reevaluation of radiofrequency rhizotomy. Neurosurgery. 1996;38:865-871.
74 Brown JA. Percutaneous techniques, 5th ed. Winn HR, Youmans JR, editors. Youmans Neurological Surgery, Vol 3. Philadelphia: Saunders. 2004:2996-3004.
75 Macon JB, Poletti CE. Human trigeminal root evoked potentials during differential retrogasserian thermal and chemical rhizotomy. Pain. 1987;31:307-316.
76 Karol EA, Karol MN. A multiarray electrode mapping method for percutaneous thermocoagulation as treatment of trigeminal neuralgia. Technical note on a series of 178 consecutive procedures. Surg Neurol. 2009;71:11-17.
77 Liu JK, Apfelbaum RI. Treatment of trigeminal neuralgia. Neurosurg Clin N Am. 2004;15:319-334.
78 Kondziolka D, Lunsford LD. Percutaneous retrogasserian glycerol rhizotomy for trigeminal neuralgia: technique and expectations. Neurosurg Focus. 2005;18:E7.
79 Pickett GE, Bisnaire D, Ferguson GG. Percutaneous retrogasserian glycerol rhizotomy in the treatment of tic douloureux associated with multiple sclerosis. Neurosurgery. 2005;56:537-545.
80 Jho HD, Lunsford LD. Percutaneous retrogasserian glycerol rhizotomy. Current technique and results. Neurosurg Clin N Am. 1997;8:63-74.
81 Park SS, Lee MK, Kim JW, et al. Percutaneous balloon compression of trigeminal ganglion for the treatment of idiopathic trigeminal neuralgia: experience in 50 patients. J Korean Neurosurg Soc. 2008;43:186-189.
82 Shelden CH, Pudenz RH, Freshwater DB, et al. Compression rather than decompression for trigeminal neuralgia. J Neurosurg. 1955;12:123-126.
83 Skirving DJ, Dan NG. A 20-year review of percutaneous balloon compression of the trigeminal ganglion. J Neurosurg. 2001;94:913-917.
84 Brown JA, Chittum CJ, Sabol D, et al. Percutaneous balloon compression of the trigeminal nerve for treatment of trigeminal neuralgia. Neurosurg Focus. 1996;1:e4.
85 Urculo E, Martinez L, Arrazola M, et al. Macroscopic effects of percutaneous trigeminal ganglion compression (Mullan’s technique): an anatomic study. Neurosurgery. 1995;36:776-779.
86 Taarnhøj P. Decompression of the posterior trigeminal root in trigeminal neuralgia. A 30-year follow-up review. J Neurosurg. 1982;57:14-17.
87 Taarnhøj P. Decompression of the trigeminal root. J Neurosurg. 1954;11:299-305.
88 Goerss SJ, Atkinson JL, Kallmes DF. Variable size percutaneous balloon compression of the gasserian ganglion for trigeminal neuralgia. Surg Neurol. 2008;70:672.
89 Bale RJ, Laimer I, Martin A, et al. Frameless stereotactic cannulation of the foramen ovale for ablative treatment of trigeminal neuralgia. Neurosurgery. 2006;59:ONS394-ONS401.
90 Brown JA, Pilitsis JG. Percutaneous balloon compression for the treatment of trigeminal neuralgia: results in 56 patients based on balloon compression pressure monitoring. Neurosurg Focus. 2005;18:E10.
91 Liu HB, Ma Y, Zou JJ, et al. Percutaneous microballoon compression for trigeminal neuralgia. Chin Med J (Engl). 2007;120:228-230.
92 Leksell L. Sterotaxic radiosurgery in trigeminal neuralgia. Acta Chir Scand. 1971;137:311-314.
93 Chen JC, Girvigian M, Greathouse H, et al. Treatment of trigeminal neuralgia with linear accelerator radiosurgery: initial results. J Neurosurg. 2004;101(suppl 3):346-350.
94 Guo S, Chao ST, Reuther AM, et al. Review of the treatment of trigeminal neuralgia with Gamma Knife radiosurgery. Stereotact Funct Neurosurg. 2008;86:135-146.
95 Sheehan J, Pan HC, Stroila M, et al. Gamma knife surgery for trigeminal neuralgia: outcomes and prognostic factors. J Neurosurg. 2005;102:434-441.
96 Villavicencio AT, Lim M, Burneikiene S, et al. Cyberknife radiosurgery for trigeminal neuralgia treatment: a preliminary multicenter experience. Neurosurgery. 2008;62:647-655.
97 Kondziolka D, Lacomis D, Niranjan A, et al. Histological effects of trigeminal nerve radiosurgery in a primate model: implications for trigeminal neuralgia radiosurgery. Neurosurgery. 2000;46:971-976.
98 Foy AB, Parisi JE, Pollock BE. Histologic analysis of a human trigeminal nerve after failed stereotactic radiosurgery: case report. Surg Neurol. 2007;68:655-658.
99 Kondziolka D, Lunsford LD, Claassen D, et al. Radiobiology of radiosurgery: part I. The normal rat brain model. Neurosurgery. 1992;31:271-279.
100 Fariselli L, Marras C, De Santis M, et al. CyberKnife radiosurgery as a first treatment for idiopathic trigeminal neuralgia. Neurosurgery. 2009;64:A96-A101.
101 Adler JRJr, Bower R, Gupta G, et al. Nonisocentric radiosurgical rhizotomy for trigeminal neuralgia. Neurosurgery. 2009;64:A84-A90.
102 Huang CF, Tu HT, Liu WS, et al. Gamma Knife surgery used as primary and repeated treatment for idiopathic trigeminal neuralgia. J Neurosurg. 2008;109(suppl):179-184.
103 Longhi M, Rizzo P, Nicolato A, et al. Gamma Knife radiosurgery for trigeminal neuralgia: results and potentially predictive parameters—part I: Idiopathic trigeminal neuralgia. Neurosurgery. 2007;61:1254-1260.
104 Kondziolka D, Lunsford LD, Flickinger JC, et al. Stereotactic radiosurgery for trigeminal neuralgia: a multiinstitutional study using the gamma unit. J Neurosurg. 1996;84:940-945.
105 Matsuda S, Serizawa T, Nagano O, et al. Comparison of the results of 2 targeting methods in Gamma Knife surgery for trigeminal neuralgia. J Neurosurg. 2008;109(suppl):185-189.
106 Dellaretti M, Reyns N, Touzet G, et al. Clinical outcomes after Gamma Knife surgery for idiopathic trigeminal neuralgia: review of 76 consecutive cases. J Neurosurg. 2008;109(suppl):173-178.
107 Kondziolka D, Lunsford LD, Flickinger JC. Stereotactic radiosurgery for the treatment of trigeminal neuralgia. Clin J Pain. 2002;18:42-47.
108 Steiger HJ. Prognostic factors in the treatment of trigeminal neuralgia: analysis of a differential therapeutic approach. Acta Neurochir (Wien). 1991;113:11-17.
109 Saini SS. Reterogasserian anhydrous glycerol injection therapy in trigeminal neuralgia: observations in 552 patients. J Neurol Neurosurg Psychiatry. 1987;50:1536-1538.
110 Burchiel KJ. Percutaneous retrogasserian glycerol rhizolysis in the management of trigeminal neuralgia. J Neurosurg. 1988;69:361-366.
111 Young RF. Glycerol rhizolysis for treatment of trigeminal neuralgia. J Neurosurg. 1988;69:39-45.
112 Lobato RD, Rivas JJ, Sarabia R, et al. Percutaneous microcompression of the gasserian ganglion for trigeminal neuralgia. J Neurosurg. 1990;72:546-553.
113 Little AS, Shetter AG, Shetter ME, et al. Long-term pain response and quality of life in patients with typical trigeminal neuralgia treated with Gamma Knife stereotactic radiosurgery. Neurosurgery. 2008;63:915-923.
114 Brisman R. Gamma Knife surgery with a dose of 75 to 76.8 Gray for trigeminal neuralgia. J Neurosurg. 2004;100:848-854.
115 Urculo E, Alfaro R, Arrazola M, et al. Trochlear nerve palsy after repeated percutaneous balloon compression for recurrent trigeminal neuralgia: case report and pathogenic considerations. Neurosurgery. 2004;54:505-508.
116 Ugur HC, Savas A, Elhan A, et al. Unanticipated complication of percutaneous radiofrequency trigeminal rhizotomy: rhinorrhea. Report of three cases and a cadaver study. Neurosurgery. 2004;54:1522-1524.
117 Harrigan MR, Chandler WF. Abducens nerve palsy after radiofrequency rhizolysis for trigeminal neuralgia: case report. Neurosurgery. 1998;43:623-625.
118 Rath GP, Dash HH, Bithal PK, et al. Intracranial hemorrhage after percutaneous radiofrequency trigeminal rhizotomy. Pain Pract. 2009;9:82-84.
119 Solaro C, Brichetto G, Amato MP, et al. The prevalence of pain in multiple sclerosis: a multicenter cross-sectional study. Neurology. 2004;63:919-921.
120 Broggi G, Ferroli P, Franzini A, et al. Microvascular decompression for trigeminal neuralgia: comments on a series of 250 cases, including 10 patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2000;68:59-64.
121 Rasche D, Kress B, Schwark C, et al. Treatment of trigeminal neuralgia associated with multiple sclerosis: case report. Neurology. 2004;63:1714-1715.
122 Resnick DK, Jannetta PJ, Lunsford LD, et al. Microvascular decompression for trigeminal neuralgia in patients with multiple sclerosis. Surg Neurol. 1996;46:358-361.
123 Eldridge PR, Sinha AK, Javadpour M, et al. Microvascular decompression for trigeminal neuralgia in patients with multiple sclerosis. Stereotact Funct Neurosurg. 2003;81:57-64.
124 Hooge JP, Redekop WK. Trigeminal neuralgia in multiple sclerosis. Neurology. 1995;45:1294-1296.
125 Kondziolka D, Lunsford LD, Bissonette DJ. Long-term results after glycerol rhizotomy for multiple sclerosis–related trigeminal neuralgia. Can J Neurol Sci. 1994;21:137-140.
126 Rogers CL, Shetter AG, Ponce FA, et al. Gamma Knife radiosurgery for trigeminal neuralgia associated with multiple sclerosis. J Neurosurg. 2002;97:529-532.
127 Huang E, Teh BS, Zeck O, et al. Gamma knife radiosurgery for treatment of trigeminal neuralgia in multiple sclerosis patients. Stereotact Funct Neurosurg. 2002;79:44-50.
128 Cheng JS, Sanchez-Mejia RO, Limbo M, et al. Management of medically refractory trigeminal neuralgia in patients with multiple sclerosis. Neurosurg Focus. 2005;18:e13.
129 Yamaki T, Hashi K, Niwa J, et al. Results of reoperation for failed microvascular decompression. Acta Neurochir (Wien). 1992;115:1-7.
130 Lee SH, Levy EI, Scarrow AM, et al. Recurrent trigeminal neuralgia attributable to veins after microvascular decompression. Neurosurgery. 2000;46:356-361.
131 Rath SA, Klein HJ, Richter HP. Findings and long-term results of subsequent operations after failed microvascular decompression for trigeminal neuralgia. Neurosurgery. 1996;39:933-938.
132 Rappaport ZH, Gomori JM. Recurrent trigeminal cistern glycerol injections for tic douloureux. Acta Neurochir (Wien). 1988;90:31-34.
133 Cruccu G, Gronseth G, Alksne J, et al. AAN-EFNS guidelines on trigeminal neuralgia management. Eur J Neurol. 2008;15:1013-1028.
134 Lopez BC, Hamlyn PJ, Zakrzewska JM. Systematic review of ablative neurosurgical techniques for the treatment of trigeminal neuralgia. Neurosurgery. 2004;54:973-982.
135 Tatli M, Satici O, Kanpolat Y, et al. Various surgical modalities for trigeminal neuralgia: literature study of respective long-term outcomes. Acta Neurochir (Wien). 2008;150:243-255.