Chapter 85A Percutaneous methods for ablating liver tumors
Overview
Overview
The term liver tumor generally includes a variety of primary and metastatic tumors involving the hepatic parenchyma. More than 90% of the primary tumors are hepatocellular carcinomas (HCCs); the other 10% include cholangiocarcinomas and rare tumors such as hemangioendotheliomas, lymphomas, and sarcomas (McGlynn et al, 2001). Hepatic metastases (METs) can originate from all types of primary tumors, but excluding local and regional lymph nodes, the liver is usually the first organ targeted by tumors of the gastrointestinal tract (Wiess, 1986).
Background
Percutaneous Ablation of Hepatocellular Carcinoma
HCC is the fifth most common tumor throughout the world, and its incidence is increasing worldwide because of the spread of infections caused by the hepatitis B and C viruses (see Chapters 64 and 80). Surveillance programs have been developed for patients with cirrhosis, and this approach is associated with annual HCC detection rates ranging from 3% to 8% (Bruix & Llovet, 2002). Unfortunately, although advances in imaging technology have improved the early detection of HCC, these tumors are difficult to treat and are still associated with a poor prognosis.
Conventional approaches, such as systemic chemotherapy and radiation, have proved to be ineffective in HCC, although the results of some recent randomized trials suggest that sorafenib may be associated with slightly improved survival in patients with advanced HCC (Llovet et al, 2008; Peck-Radosavljevic et al, 2010; Vitale et al, 2009; see Chapter 88). Surgical resection can result in tumor eradication and improved survival in patients with small HCCs; however, few patients are candidates for potentially curative resection, which comes with an 80% risk of recurrence (Akriviadis et al, 1998; see Chapter 90B). Liver transplantation is the ideal approach for early HCC, but it is limited by age-related contraindications and a shortage of organs (Llovet et al, 1999; see Chapter 97D). For these reasons, a number of percutaneous methods have been developed for the chemical or thermal ablation of HCC, and all have displayed satisfactory efficacy in terms of local tumor control.
Percutaneous Ablation of Hepatic Metasteses
Percutaneous ablation has been used to treat hepatic METs from primary tumors in various organs, but its systematic use has been limited to those produced by colorectal cancers (CRCs). CRC is the fourth most common tumor worldwide (Greenlee et al, 2000), and the healthy liver is the first site of metastatic involvement in patients with CRC and a source of further spread (Lenhert, 1999). Twenty-five percent of these patients already have liver METs when the primary tumor is diagnosed, and another 50% develop them within 5 years of diagnosis (see Chapter 81A). In more than 30% of the patients with liver METs, the metastatic disease appears to be confined to the liver at the time of detection.
Throughout the world, resection is considered a potentially curative approach for the treatment of CRC liver METs, and it is associated with a 5-year survival rate of 40% (Ballantyne, 1993; Greenway, 1988; Nakamura, 1997). However, more than 70% of patients are ineligible for surgical resection because of the extent of their disease, serious comorbidities, or both. For those who do undergo resection, recurrence is frequent; repeat resections are difficult to propose, and in some cases, they are ineffective. Chemical or thermal ablation methods have been proposed for patients with no surgical prospects, for those with recurrence after resection or tumor progression after systemic or local chemotherapy, and for those who refuse surgery. Chemical ablation has displayed limited efficacy in the local control of liver METs (Livraghi et al, 1991), but thermal ablation has proved to be safe and effective for the treatment of small liver METs from CRC (Lencioni et al, 2004; Livraghi et al, 2003a; Solbiati et al, 1999). It has also produced promising results in the management of lung METs (Rossi et al, 2006). Furthermore, thermal ablation coupled with open surgery seems to increase the overall number of treatable patients (Abdalla et al, 2004).
Pretreatment Studies and Patient Selection
For HCC patients, treatment eligibility screening generally involves routine laboratory tests, abdominal ultrasonography (US), contrast-enhanced US, spiral computed tomography (CT) or magnetic resonance imaging (MRI), esophagogastroduodenoscopy, and other studies as indicated. Diagnosis of cirrhosis is generally based on histology or concordant laboratory and imaging findings. Most investigators believe that HCCs more than 2 cm in diameter can be reliably diagnosed on the basis of characteristic findings documented with at least two imaging modalities. One modality is sufficient if the patient also has an α-fetoprotein (AFP) level greater than 200 ng/mL. For HCC nodules less than 2 cm in diameter, diagnosis requires typical findings of two imaging techniques and AFP levels above 200 ng/mL. Otherwise, biopsy is mandatory (Bruix & Llovet, 2002). We and others believe that diagnosis of the original tumor should be based on histologic examination of US-guided fine needle biopsy (FNB) or laparoscopic findings (subcapsular tumors), although imaging findings can be useful for recurrences (Rossi et al, 1996, 1998). US-guided biopsy, cutting or aspirative, with an 18- to 21-gauge needle is always indicated when the AFP level is above 200 ng/mL.
Laboratory screening for CRC liver METs is similar to that described above, and diagnosis is generally based on imaging findings. In a patient with a history of CRC, a focal hepatic lesion is generally regarded as metastatic if it displays peripheral contrast enhancement in the arterial phase and complete washout in the portal and late phases on at least two types of imaging studies. US and FNB confirmation should be obtained if the diagnosis is in doubt, and it can also be used to identify the treatment-relevant receptor status of tumor cells (Midorikawa et al, 2009). Patients with CRC liver METs should always undergo colonoscopy before thermal ablation to exclude the possibility of local disease recurrence.
Candidates for percutaneous ablation have tumors with characteristics similar to those of patients who are eligible for surgical resection, but for a variety of reasons, they are not considered suitable for surgery. Thermal/chemical ablation is generally chosen to treat HCC on the basis of findings related to the tumor, residual liver function, and the patient’s general health. The criteria most commonly used include documented cirrhosis; no more than three HCC nodules, measuring no more than 3.0 to 3.5 cm each in diameter; no neoplastic thrombosis of the portal or hepatic veins; and no evidence of extrahepatic metastases. Residual liver function is classified according to the Child-Turcotte-Pugh (CTP) system, and patients with scores higher than B7 to B9 are usually considered unsuitable for ablation. Candidates must also have safe coagulation parameters—prothrombin time (PT) ratio of 50% or higher, international normalized ratio (INR) no higher than 1.7, and a platelet count of 50 to 70 x 109/L or more—and no esophageal varices at a high risk for bleeding (Ebara et al, 1995; Rossi et al, 1998).
Similar criteria are used to select patients with CRC liver METs for percutaneous thermal ablation (Rossi et al, 1996; Solbiati et al, 1999). The patient’s coagulation status still needs to be assessed, because liver disease is not the only cause of clotting defects. Compared with the criteria applied to HCCs, those used for METs are more restrictive, because metastatic tissue is more difficult to ablate than HCC tissue, so METs with diameters exceeding 2.5 to 3 cm are often considered ineligible for curative percutaneous ablation.
In terms of safety, one of the most important factors to consider is the location of the tumor. For nodules located superficially in segments III, IV, or V of the liver; on the subdiaphragmatic surface of the liver; and for exophytic nodules (surrounded by abdominal viscera) or those close to gallbladder, a laparoscopic or surgical approach is preferable, although in experienced hands, percutaneous ablation of these tumors is by no means impossible (Chopra et al, 2003). Caution must be used when the tumor is situated close to a major bile duct, because damage to these structures can lead to biliary stricture or fistula, which often requires additional interventional radiologic procedures or surgical correction (see Chapter 42A, Chapter 42B ).
For tumors located near a large hepatic blood vessel, risk of incomplete treatment and treatment failure is increased. Flow through these vessels can increase convectional heat loss near the tumor, thereby reducing the volume of the thermal lesion, and it can also result in more rapid clearance of the chemical ablative substances. It is often useful to devascularize these tumors before chemical or thermal ablation; this can be accomplished with selective transarterial embolization (TAE; see Chapter 83) or balloon-catheter occlusion of the vessels supplying or draining the tumor (Rossi et al, 2000).
Assessment of Local Tumor Control
Evaluation of local efficacy is based largely on evidence of complete tumor ablation that persists during the follow-up (lack of local recurrence). To achieve this, the entire tumor must be included in the volume of tissue that undergoes chemically or thermally induced coagulative necrosis, which renders it completely avascular. Shortly after the procedure, the necrotic area is surrounded by a halo of edematous, intensely hyperemic tissue. A few days later, this halo is replaced by an inflammatory halo, which persists for approximately 1 month. Thereafter, the mass of necrotic tissue shrinks and is replaced by fibrotic tissue (Rossi et al, 1990). The location, size, and evolution of this ablation zone are assessed with radiologic imaging techniques.
Evaluation of Immediate Treatment Response
The immediate response to any type of percutaneous ablation procedure is generally assessed with contrast-enhanced US (CEUS), enhanced spiral CT, or T2-weighted MRI—the gold standards for this purpose (Chen et al, 2007; Cioni et al, 2001). The presence at the ablation site of a well-defined, nonenhancing area as large or larger than the treated tumor itself is regarded as reliable evidence of a complete response, or complete radiologic necrosis. The timing of the imaging study is important. CEUS examinations performed too soon after ablation are characterized by numerous artifacts caused by gases produced during ablation or the diffusion of chemical substances within tissues surrounding tumor. Under these circumstances, it is impossible to distinguish the origin of the hyperechoic signals. If CEUS, CT, and MRI are performed in the days following the ablative procedures, it is impossible to determine whether the peripheral enhancement observed is due to residual tumor tissue or the hyperemic/inflammatory response of the liver parenchyma. This enhancement is a result of inflammatory tissue response, and it resolves within a month of tumor ablation. Therefore the persistence of an enhancing rim after this point must be considered evidence of residual tumor viability. For these reasons, the contrast-enhanced imaging studies should be scheduled at least 4 weeks after the procedure. If the results of this study are ambiguous, US-guided FNB can resolve the problem (Rossi et al, 1998).
Evaluation of Long-Term Results
Abdominal US/CEUS studies and tumor marker assays are performed every 4 to 6 months, or even more frequently if needed. Local recurrence is diagnosed when enhancement reappears within the ablation zone or within 2 cm, 1 cm according to some authors, from its margins, or when there is histologic evidence of tumor viability in this area (Berber et al, 2005). If the ablation zone remains unenhanced but fails to shrink during follow-up, or when levels of tumor markers are elevated in the absence of other intrahepatic or extrahepatic lesions, focused US-guided biopsy of the ablation zone is indicated. Nonlocal recurrence comprises extrahepatic metastases and all new intrahepatic regrowth located more than 2 cm from the ablation zone.
Assessment of Complications
Immediate and late complications have been reported after all types of percutaneous tumor ablation. They should be evaluated according to previously described guidelines (Sacks et al, 2003). Major complications are those that, if left untreated, could threaten the patient’s life, produce substantial morbidity, and/or prolong the hospital stay. All other events are considered minor complications. In general, patients treated with percutaneous ethanol injection (PEI)/percutaneous acetic acid injection (PAI) are monitored for 2 to 3 hours in a dedicated recovery room and are then discharged after a postablation abdominal US examination (Ebara et al, 1995; Shiina et al, 1991). Laboratory tests and other studies are indicated only when clinical or US evidence of complications are apparent. The 24-hour study is also accompanied by measurements of hemoglobin, lactic dehydrogenase and aminotransferases levels, and blood studies to assess hepatic function.
Chemical Ablation
Chemical ablation is based on the cytotoxic properties displayed by certain chemical substances when directly injected into a tissue. The most widely used cytotoxic substance is ethanol, but in some centers, acetic acid has recently been used as an alternative in the treatment of HCCs (Huo et al, 2003), and sodium hydroxide is under evaluation in a tumor model after evaluation in rats (Lin et al, 2001). Chemical ablation has been extensively used to treat HCCs. It has also been evaluated for the treatment of METs, but this experience was abandoned based on unsatisfactory results (Livraghi et al, 1991).
Percutaneous Ethanol Injection
Physical Principles
Direct injection of ethanol into a tumor results in its distribution in the tissue by diffusion and convection. Diffusion is favored by the existence of a concentration gradient between the injection point and the surrounding tissues, by the low molecular weight of the ethanol, by the increase in local pressure determined by the injection, and by the hypervascularity of the tumor (Ho et al, 2007). Ethanol spreads through the tumor tissue, exerting cytotoxic effects that include cytoplasmic dehydration and denaturation of cellular proteins. The endothelial cell necrosis and platelet aggregation it causes occlude tumor vessels and produce ischemic damage in the neoplastic tissue (Shiina et al, 1991; Ebara et al, 1995). The final result is coagulative necrosis within the tissue around the site of injection.
Technique
The procedure is performed under real-time US monitoring. The ethanol is usually injected with a 20- to 22-gauge endhole needle (Chiba or Chiba-like) or a conical-tip needle with multiple sideholes (Livraghi et al, 1995). The tip is positioned at the desired point in the HCC nodule with the aid of real-time US guidance, and the predetermined amount of ethanol is infused slowly into the tumor. After few seconds, the HCC nodule becomes completely hyperechoic. Light aspiration is applied during withdrawal to prevent excess ethanol from leaking into the peritoneum through the insertion tract. If all goes well, the patient is kept under observation for about 2 hours.
The volume of ethanol required to ablate a tumor nodule depends on the size of the tumor, but the final decision on the total dose is based on the results of imaging studies performed at the end of each session (Livraghi et al, 1995; Shiina et al, 1991).
An alternative to this multiple-session approach is one-shot PEI. As the term implies, the total amount of ethanol required for the ablation, based on the calculations reported above, is injected during a single session (Giorgio et al, 1996; Livraghi et al, 1993). This technique has been proposed for HCCs with diameters exceeding 3 cm. It is more painful than conventional PEI and is therefore performed under general anesthesia. In one study, the amounts of ethanol administered with this technique ranged from 20 to 165 mL (mean, 62 mL).
Percutaneous Acetic Acid Injection
Physical Principles and Technique
After ethanol, the most widely used agent for chemical ablation is acetic acid. In vitro studies have shown that it dissolves lipids and extracts collagen from various kinds of tissues (Ho et al, 2007). Compared with ethanol, acetic acid offers certain advantages, including more effective destruction of tumor septa and capsules. In addition, the same degree of cell kill can be obtained with a smaller infusion volume (Ohnishi, 1998).
The technique of PAI is quite similar to that of PEI. The infusion consists of a 50% solution of acetic acid and sterile water injected with the same technique and needles used for ethanol. Observations in animal models and clinical explants indicate that the volume of acetic acid needed to ablate a tumor nodule is roughly one third of the volume calculated with the formula used for ethanol: ablation of a nodule measuring 3 cm in diameter would require 34 mL of ethanol but only 11.3 mL of acetic acid (Giorgio et al, 2000).
Complications of Percutaneous Ethanol/Acetic Acid Injection
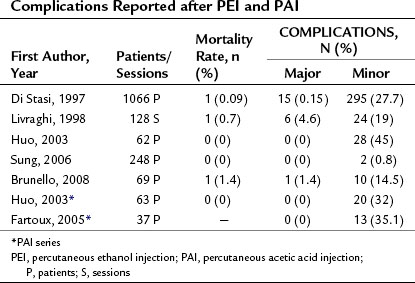
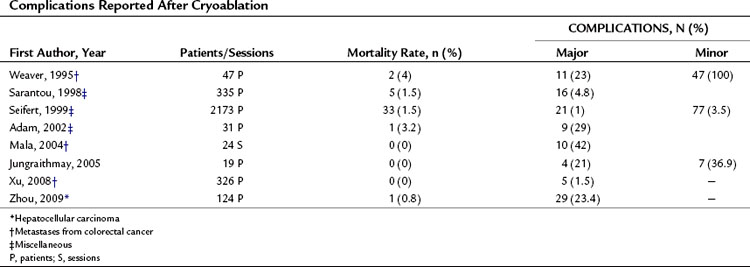
Although PEI and PAI are both low-risk procedures, in rare cases they can produce severe complications. Table 85A.1 shows the complication rates reported after these procedures. The mortality rates associated with PEI/PAI are very low, ranging from 0% to 1.4%. Major complications, which ranged from 0% to 4.6%, include hemoperitoneum, hemobilia, subcapsular or intraparenchymal hematoma, hepatic abscess, cholangitis, intestinal perforation, hepatic venous thrombosis, pneumothorax or pleural effusion, hepatic infarction, and tumor seeding along the needle insertion tract.
Local Tumor Control of Chemical Ablation
Several authors have analyzed the risk factors for local recurrence of HCC after PEI, and tumor size seems to be the most accurate predictor of this outcome. The 2-year local HCC recurrence rates reported after PEI are 10% for nodules smaller than 2 cm in diameter, 18% for those between 2.1 and 3 cm, and 30% for those larger than 3 cm (Ishii et al, 1996). In other studies, local recurrence rates ranged from 33% to 38% for HCCs smaller than 3 cm and from 43% to 68% for those exceeding 3 cm in diameter (Gaiani et al, 2003; Khan et al, 2000). Thus far, complete response rates observed after PAI seem to be similar to those obtained with PEI, but very few studies are available for comparison.
Long-Term Results
Several investigators have reported 5-year survival rates close to 50% after PEI for HCCs up to 3 cm in diameter in patients with cirrhosis; in other words, results are similar to those achieved with resection (Bruix & Llovet, 2002). In a consecutive series of 685 patients with HCC nodules measuring 3 cm or less, initial treatment with PEI was reportedly associated with a cumulative 5-year survival rate of 49% (Shiina et al, 2009). The Seventeenth Nationwide Follow-up Survey conducted by the Liver Cancer Study Group of Japan revealed survival rates of 91.3%, 77.5%, 63%, 50.2%, and 39.4% at 1, 2, 3, 4, and 5 years, respectively, for 14,726 patients whose HCCs were treated with ethanol injection (Ikai et al, 2007). A randomized trial failed to detect any significant difference in survival between HCC patients treated with PEI or surgical resection (Huang et al, 2005); however, other randomized trials have also shown that radiofrequency ablation (RFA) is superior to all types of chemical ablation for small HCCs, and the difference involved treatment responses, local tumor control, and survival rates (Bouza et al, 2009; Lin et al, 2005; Orlando et al, 2009; Shiina et al, 2005). For this reason, thermal ablation techniques are now preferred over chemical ablation for treatment of HCC. The chemical approach is still the treatment of choice when thermal ablation cannot be performed safely, such as when tumors form adhesions to the gastrointestinal tract, or when tumors are located in difficult sites or close to a bile duct.
Thermal Ablation
The goal of thermal ablation methods is to completely destroy a tumor without damaging the surrounding liver tissue. This can be achieved by generating killing temperatures, those exceeding 45° C or less than −40° C, within the mass of tissue that has to be ablated. The temperature reached and the duration of exposure determine how rapidly cell death occurs. Hyperthermia can be produced by delivering electromagnetic (radiofrequency, microwave), light (laser; see Chapters 85C and 85D), or mechanical (high-intensity focused ultrasound) energy into the tissue by means of dedicated probes. Hypothermia is created with cryoprobes (see Chapter 85B), which subtract heat from the tumor tissue by convection. RFA is the thermal technique most widely used for HCCs and liver METs and the one supported by the largest body of published evidence. Microwave ablation (MWA) and laser thermal ablation (LTA) have also been used in these settings, but these experiences are more limited. To date, experiences with high-intensity focused ultrasound (HIFU) have been confined to experimental studies or preliminary clinical investigations.
Radiofrequency Ablation (see Chapter 85C)
Physical Principles
Radiofrequency (RF) energy generates heat in the tissue that is in direct contact with the noninsulated tip of the needle electrode (McGahan et al, 1990; Rossi et al, 1990). The heat is the result of ionic and molecular friction, and it spreads into the surrounding tissues by a process of conduction. The highest temperatures are created in the tissue immediately adjacent to the electrode tip, and the heat rapidly decreases as the distance from the tip increases. Permanent tissue destruction occurs at temperatures of 45° C or higher (Cosman et al, 1983; Organ, 1976). Temperatures ranging from 46° C to 60° C produce irreversible cellular damage only after relatively long periods of exposure. In contrast, temperatures between 60° C and 100° C cause almost instantaneous protein coagulation with irreversible damage to mitochondria and cytosolic cell enzymes. When temperatures exceed 100° C, tissue fluids undergo boiling, vaporization, and ultimately carbonization. The final size of the thermal lesion, an area of coagulative necrosis that is gradually replaced by fibrotic tissue, depends on the total amount of heat deposited in the tissue, the thermal and electrical conductivity of the tissue, and the amount of heat lost through convection; specifically, it depends on heat lost through local blood flow, which acts as a heat sink. Tissue impedance limits the amount of heat that can be introduced into a tissue. It is inversely related to tissue hydration, which in turn reflects the ion content of the tissue (Djavan et al, 1997). During RFA the ions are quickly destroyed, and the tissue undergoes desiccation and charring, and the resultant increases in impedance ultimately prevent further delivery of RF energy.
Conventional RF electrodes used in the monopolar mode produce thermal lesions with a maximum diameter of about 1.8 cm (Rossi et al, 1996). Consequently, the ablation of a relatively small HCC nodule requires the creation of multiple, overlapping lesions. This used to mean multiple needle insertions and multiple treatment sessions; however, second-generation RF electrodes produced thermal lesions with diameters as large as 3 cm, and the volume of tissue that could be ablated with a single pulse of energy increased exponentially. These advances were achieved by increasing the active surface area of the electrode with a set of retractable hooks or prongs (expandable-tip electrodes; Rossi et al, 1998) or by cooling the electrode tip by means of an internal water-circulation system (cooled-tip electrodes) (Goldberg et al, 1998b). The expandable electrodes allow the delivery of larger amounts of RF energy, because rapid charring is less likely when the volume of tissue to be dehydrated is large. Cooled-tip electrodes achieve the same result by preventing temperatures rising above 100° C in tissues adjacent to the active surface of the electrode. The heat dissipation prevents charring and keeps impedance low, thereby allowing the delivery of larger amounts of RF energy. Larger thermal lesions, those 4 cm or larger, have also been produced with multiple cooled electrodes combined in a cluster (Head & Dodd, 2004), cooled catheter electrodes, and expandable spiral electrodes (Rossi et al, 2006).
Other investigators have attempted to increase thermal lesion volumes by reducing convectional heat loss during the RF procedure (Goldberg, 1998a; Patterson et al, 1998; Rossi, 1999). HCCs are supplied almost exclusively by vessels arising from the hepatic artery (Breedis & Young, 1954); however, when RFA was performed after balloon-catheter occlusion of the hepatic artery, the thermal lesions produced were large as expected on the basis of experimental studies; when RFA was performed after embolization of the feeding arteries with gelatin sponge particles, the thermal lesions produced were larger than expected (Rossi et al, 2000). With this approach, in fact, HCC nodules more than 6 cm in diameter could be treated in a single RF session.
Low impedance during the procedure explains the unexpectedly large thermal lesions. Occlusion of the tumor’s arterial supply with gelatin sponge particles reduces impedance in at least two ways: First, it increases the hydrostatic pressure within the HCC (Rossi et al, 2007). As a result, the boiling point of tissue fluid is higher during the thermal ablation procedure, which prolongs the delivery time and allows more energy to be deposited in the tissue, before desiccation and charring occur. Second and more importantly, a gelatin sponge is a hydrogel capable of modifying the electrical and thermal conductivity of the tissue; its presence in the ablation area markedly increases the amount of RF energy that can be delivered.
Technique
Patients undergoing RFA are hospitalized and treated after an overnight fast. In our department, most RFA procedures are performed without general anesthesia or conscious sedation, but some investigators prefer to perform the procedure under general anesthesia or deep sedation. A grounding pad is attached to the patient’s back and connected to the RF generator to close the electrical circuit. A local anesthetic (1% lidocaine) is injected along the predefined electrode insertion line, from the skin to the peritoneum. The skin is nicked with a small lancet to facilitate insertion of the electrode, and the tip is then advanced into the HCC nodule under real-time US guidance. The RF generator is activated, and the predefined amount of energy is delivered for 8 to 12 minutes. On US, the nodule becomes hyperechoic with a posterior acoustic shadow (Rossi et al, 1996). The pull-back technique can be used to create multiple thermal lesions along the major electrode axis (Rossi et al, 2000). At the end of the procedure, the electrode is withdrawn, but the generator remains on during this phase; this way, the electrode tract itself also undergoes coagulation, which diminishes the risks of bleeding and tumor seeding.
This technique can be used for HCCs up to 3.5 cm in diameter and for CRC liver METs with diameters of 2.5 cm or less. For larger tumors, other treatment strategies have been adopted. For HCC nodules whose diameters exceed 3.5 cm, RFA has been performed with a multiple-insertion technique (Livraghi et al, 2000), an expandable triple-spiral electrode (Rossi et al, 2006), clusters of cooled electrodes (Cheng et al, 2008), and combination of RFA with other techniques (Murakami et al, 2007). As discussed above, a larger volume of necrotic tissue can also be achieved by preablation embolization of the tumor with gelatin sponge particles. Some operators perform selective transarterial chemoembolization (TACE; see Chapter 83) of larger tumors after RFA debulking. The rationale of this approach is that the amount of embolic agent will be greater in the tumor tissue if its volume was previously reduced with RFA.
Complications
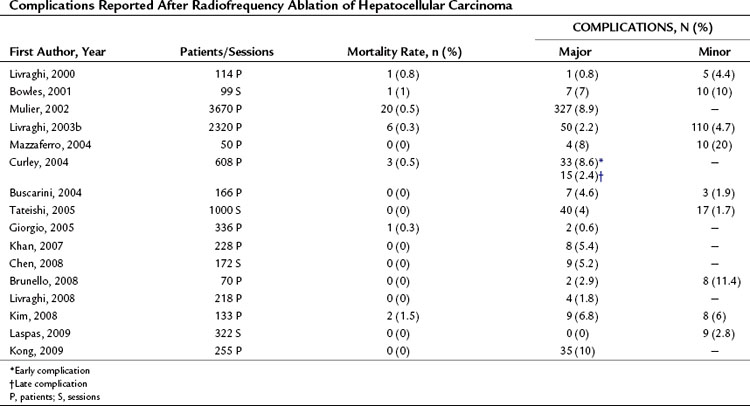
RFA is a safe procedure with very low rates of death and major complications. Tables 85A.2, 85A.3, and 85A.4 show the post-RFA complication rates observed in HCC or MET series reported by our group and others. Mortality rates ranged from 0% to 3.7%, whereas major complication rates were reported in 0% to 22%. Most of the adverse outcomes reported occurred in the initial clinical experiences, and fatal and nonfatal complications have become progressively less common with the increasing experience of the investigators. Indeed, in reports published during the last 5 years, mortality rates are consistently close to 0%, and major complication rates range from 0% to 10%. The complications described include abdominal hemorrhage, hepatic abscess, pleural effusion, hepatic infarction, bronchobiliary fistula, bile peritonitis, biloma, hemobilia, thrombosis of vessels in the hepatic venous system, skin burn, and perforation of the stomach, intestine, or diaphragm. Table 85A.4 shows the complications observed by our group over the last 10 years. This series includes 706 patients with 859 HCC nodules and no procedure-related deaths, which is consistent with previous reports. Less than 1% of the procedures were associated with major complications, a percentage similar of those reported after percutaneous injection therapies. The most serious adverse effect in our series involved shearing of a branch of the hepatic artery; however, all cases were promptly detected by Doppler US studies and controlled by selective embolization of the damaged vessel. Our patients receive neither general anesthesia nor sedation, and the most frequent complication is therefore mild to moderate pain during the procedure that occurs in about 30% of all patients. The pain is easily managed with intravenous analgesia and disappears entirely shortly after the procedure. Use of local, rather than general, anesthesia has several advantages, and this approach is now used by most operators. For one thing, it simplifies the procedure and reduces the cost. More importantly perhaps, it reduces the risk of extrahepatic complications such as perforations of intestinal loops, diaphragm, and gallbladder. The frequency of these events obviously depends on the operator’s skill and experience, but conscious patients can report severe pain, which almost inevitably reflects incorrect electrode placement; indeed, a conscious patient can provide important feedback that can prevent major organ damage.
Table 85A.4 Complications in Patients with Hepatocellular Carcinoma after 1921 Radiofrequency Ablation Sessions
| Complication | Number (%) |
|---|---|
| Major | |
| Hemoperitoneum | 6 (0.3) |
| Hemothorax requiring drainage | 2 (0.1) |
| Self-limiting hemobilia | 1 (0.05) |
| Subcutaneous seeding* | 2 (0.1) |
| Skin burn | 1 (0.05) |
| Total | 12 (0.6) |
| Minor | |
| Postoperative pain | 18 (0.9) |
| Self-limiting pleural effusion | 32 (1.6) |
| Transient worsening of liver function | 54 (2.8) |
| Low-grade fever | 76 (3.9) |
| Total | 180 (9.3) |
* It is not possible to define the origin of seeding (radiofrequency ablation vs. ultrasound-guided biopsy).
A 12% seeding rate was reported by a Spanish group during their initial experience with RFA (Llovet et al, 2001). The results contrast with those of other studies, and they may be a reflection of poor technique. Seeding along the electrode tract is virtually impossible, if the electrode is activated before and during each withdrawal. If the recommendations made in the Technique section are followed, the electrode tract itself undergoes coagulation, produced by the gases that develop during the procedure or by the heat generated by the activated electrode during its withdrawal. Our group, in fact, has observed only two cases of subcutaneous tumor seeding after a total of 1921 RFA sessions, and in both cases, it is impossible to say whether the seeding was actually caused by the RFA or by previous US-guided biopsies.
When RFA is performed during or after TAE, other complications are also possible (Rossi et al, 2000). Sporadic cases have been reported of chemical cholecystitis and intimal dissection of the hepatic artery. All patients experience transient increases in serum aminotransferase levels, but baseline values are generally restored within a week. Some patients also display transient declines in liver function reflected by CTP scores, but these changes rarely last more than 2 weeks.
Local Tumor Control
Histologic examination of whole livers from transplant recipients who had previously been treated with RFA shows that more than 80% of HCCs measuring less than 3 cm are completely destroyed with a single RF electrode insertion (Mazzaferro et al, 2004). Complete response rates ranging from 95% to 98% have been documented in several studies; however, in numerous series of cirrhotic patients with small HCCs treated with RFA, the local recurrence rates range from 0% to 45%, as shown in Table 85A.5. In our series of 859 HCC nodules with maximum diameters of 3.5 cm, the overall local recurrence rate after a 10-year follow-up was 15.7%. The recurrence rate was 5.2% for nodules up to 2 cm in diameter, 12.7% for those with diameters of 2.1 to 3 cm, and 21% for those measuring 3.1 to 3.5 cm; however, in more than 70% of these cases, complete and persistent ablation of the recurrent disease was achieved with a single additional “clean-up” RFA session. This brought the overall local control rate close to 94%, obtained with one treatment in 82% of the cases and with two treatments in 12%.
Table 85A.5 Local Recurrence and Survival in Patients Treated with Radiofrequency Ablation for Hepatocellular Carcinoma
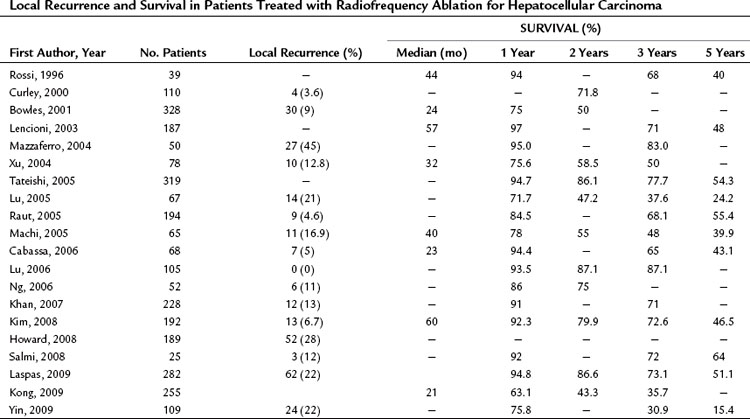
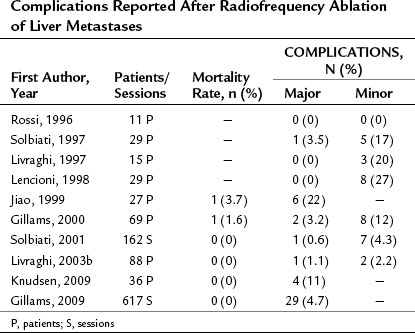
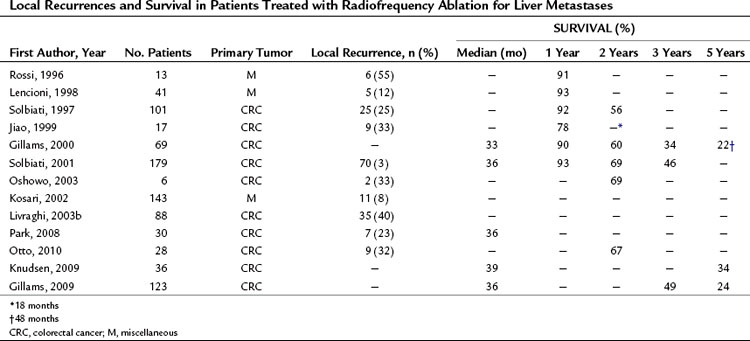
As shown in Tables 85A.5 and 85A.6, similar complete response rates have been reported for RFA of CRC liver METs, but the recurrence rates in these cases are higher, ranging between 8% and 40%. These results are probably due in part to the use of RFA to treat METs that are too large for this type of ablation. Given the characteristics of metastatic tissue discussed above, percutaneous RFA with curative intent should probably be reserved for METs with diameters no larger than 2.5 cm. If larger METs are treated with this method, local recurrence must be expected in more than 30% of the cases.
Long-Term Results in Hepatocellular Carcinoma Patients
The long-term results reported after RFA treatment of HCCs are summarized in Tables 85A.5 and 85A.6. In our own RFA series, cumulative disease-free and overall survival was similar to those observed by others after RFA, percutaneous injection therapy, or surgical resection. Meaningful comparison of the mortality data and survival curves reported after surgical and nonsurgical treatment of HCC is virtually impossible, because only perfunctory descriptions are provided of recurrences occurring after the initial treatment. Most reports simply do not mention the characteristics of first recurrences (location, extension, etc.), how this recurrence was managed (with the same method used to treat the original tumor or with one or more other techniques), or the outcome of the treatment; even fewer details are provided on subsequent episodes of recurrence. Comparison of results reported for surgical and nonsurgical series is further complicated by the fact that cases of tumor understaging and/or positive resection margins are generally excluded from analyses of survival in surgical series, whereas cases of this type—understaged disease, tumors with persistent viability after two treatments—are usually included in the nonsurgical series (Hanazaki et al, 2000; Helling & Woodall, 2007; McCormack et al, 2005; Shimozawa & Hanazaki, 2004; Torzilli et al, 1999).
Randomized controlled studies have demonstrated that RFA is superior to chemical ablation methods in terms of both local tumor control and survival (Bouza et al, 2009; Brunello et al, 2008; Orlando et al, 2009). These findings suggest that, when possible, RFA should be the first choice for nonsurgical ablation of HCCs. RFA was also directly compared with surgical resection in two randomized clinical trials, and neither revealed any significant differences between the local tumor control or survival rates associated with the two approaches (Chen et al, 2006; Lu et al, 2006).
Recently, HCC nodules with diameters larger than 3 cm have been treated with a cluster of cooled RF needle electrodes and a multiple-insertion technique (Goldberg, 1998a). Complete responses were observed in 61% of the nodules up to 5.0 cm in diameter and in 24% of larger nodules. These results were to be expected, because tumor volume increases exponentially with its diameter, and larger diameters are also associated with higher frequencies of daughter nodules, capsule infiltration, and neoplastic thrombosis of the portal vessels close to the tumor. Better results have been obtained with more conventional RFA electrodes during gelatin-sponge occlusion of the hepatic artery flow (see Physical Principles below). A complete response rate of 82% was achieved with this approach in 62 HCC nodules, with a mean diameter of 5.6 cm. The local recurrence rate was 19%, and the overall intrahepatic recurrence rate was 45% at 1 year (Rossi et al, 2000). RFA is now recognized as a potentially curative treatment for HCC nodules up to 3 cm in diameter, and some investigators maintain that it should be considered the treatment of choice for these cases (Choi et al, 2007; Livraghi et al, 2008; Shiina, 2009).
Long-Term Results in Patients with Metastases
Table 85A.6 shows the long-term results of RFA for resectable and unresectable CRC liver METs. Survival rates range from 33% to 46% at 3 years and from 22% to 30% at 5 years (Abdalla et al, 2004). These rates are worse than those reported after surgical resection, but it is important to recall that surgery is used in a more select population with fewer risk factors for mortality. RFA can now be considered an approach capable of improving survival in patients with limited but inoperable metastatic liver disease (Otto et al, 2010; Seidenfeld et al, 2002). RFA alone or combined with resection has also proved to be superior to other nonsurgical therapies in patients with multiple liver METs (Abdalla et al, 2004). Finally, some investigators suggest that RFA can be used as a part of a “test of time” approach. Use of RFA as the first option for limited hepatic METs would reduce the need for surgical resections by producing complete and persistent tumor necrosis in some patients. In others, RFA would be followed by new intrahepatic and/or extrahepatic METs, which means that surgery would have been a useless ordeal for the patient. And in a few, post-RFA recurrence will be limited to local regrowth at the site of the ablated MET, and in these cases, surgical resection may prove worthwhile (Livraghi et al, 2003a).
Microwave Ablation (See Chapter 85D)
Physical Principles
The term microwave (MW) refers to electromagnetic radiation with frequencies ranging from 900 to 2450 MHz. MW radiation is emitted by the tip of a bipolar antenna electrode placed within the tissue. Microwaves cause rapid vibration and rotation of water molecules within the tissue, which immediately generates frictional heat that persists for the duration of energy delivery. The uniform distribution of this heat in all directions results in coagulative necrosis, and charring near the tip of electrode limits further heat diffusion in the tissue. The thermal lesions produced are spherical to elliptical in shape. Their diameters vary with the power delivered, the electrode caliber, and exposure time. With a 14-gauge electrode, a power output of 60 W, and an exposure time of 120 seconds, the maximum and minimum diameters of the thermal lesions were 2.4 ± 0.4 cm (length) and 1.6 ± 0.3 cm (width). Corresponding diameters achieved with 30 minutes at 40 W were 3.6 cm (length) and 2.6 cm (width) (Seki et al, 1994).
Technique
The technique of MWA is quite similar to that described above for RFA (Seki et al, 1994). Percutaneous MWA is usually performed as an inpatient procedure after an overnight fast. General anesthesia or deep sedation is induced, and a thin (14- to 16-gauge) MW antenna is advanced directly into the tumor nodule under US guidance. The antenna is connected to the MW generator, which is activated to deliver 60 to 90 W of power. Delivery times vary from 2 to 30 minutes, depending on tumor size. In general, tumors less than 2 cm in diameter can be ablated with one or (more commonly) two electrode insertions; for larger tumors, multiple insertions are necessary. The same precautions described for RFA apply to MWA. The changes revealed by US consist of the appearance around the tip of the electrode of a hyperechoic area with a posterior acoustic shadow. This zone increases in size with exposure time and ultimately involves the entire tumor. The hyperechogenicity diminishes rapidly after the generator is switched off and disappears completely within 8 hours of the procedure.
Complications
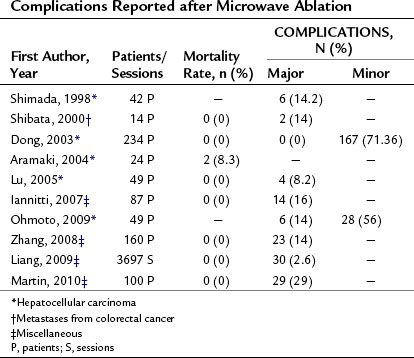
The complications reported after MWA are quite similar to those listed above for RFA. Table 85A.7 shows the mortality and complication rates observed in the various published series. Mortality ranged from 0% to 8.3%, and complication rates from 2.6% to 14% were reported.
Local Tumor Control
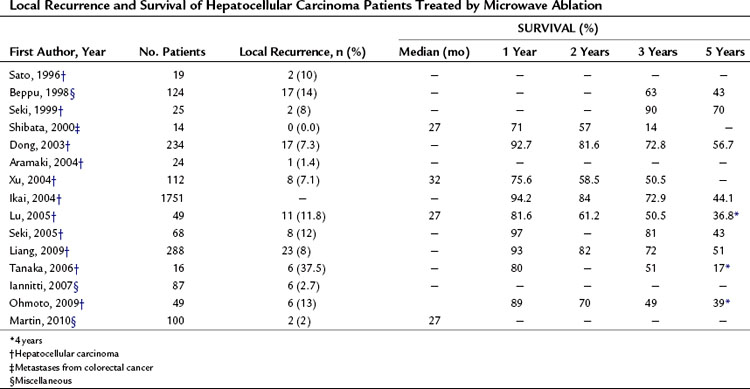
MWA can produce complete tumor necrosis. Histologic examination revealed no viable tumor in 180 (92.3%) of 192 treated HCC nodules that were biopsied after MWA and in 5 (90%) of 6 HCC nodules that were surgically resected after MWA (Seki et al, 1999). None of these nodules were more than 3 cm in diameter. For HCCs of this size, the complete ablation rate is reportedly quite good, more than 90%, but it is significantly lower for larger tumors. The local recurrence rates reported by several investigators after MWA of hepatic tumors ranged from 2% to 37%, as shown in Table 85A.8.
Long-Term Results
Several clinical studies have investigated the usefulness of MWA in the treatment of HCC. The long-term results observed in these studies are summarized in Table 85A.8. The overall survival rates 3 years after the procedure ranged from 50.5% to 90%, and at 5 years from 17% to 70%. The Fifteenth Nationwide Follow-up Survey conducted by the Liver Cancer Study Group of Japan found 1-, 2-, 3, and 5-year survival rates of 94.2%, 84%, 72.9%, and 44.1%, respectively, in 1751 patients whose HCC had been treated with MWA (Ikai et al, 2004). The effectiveness of MWA and that of RFA have also been compared in randomized trials (Ohmoto et al, 2009; Shibata et al, 2002). No statistically significant differences were observed between the two treatment methods in terms of local efficacy, but RFA tended to be associated with slightly better local recurrence and complication rates; however, since RFA was introduced in 1999, use of MWA has steadily decreased, even in centers where it was once widely used (Shiina et al, 2002).
Limited data have been reported so far concerning the long-term results of MWA of liver METs (see Table 85A.8). Some investigators have reported promising results in their initial experience with several types of primary tumors (Abe et al, 2005; Liang et al, 2003). In another randomized study comparing MWA and surgical resection in patients with multiple CRC liver METs, no statistically significant differences were observed in the survival rates of the two groups (Tanaka et al, 2006).
Laser Thermal Ablation (See Chapter 85D)
Physical Principles
Laser light, which has a wavelength of 800 to 1064 nm, is delivered to the tissue through the tip of a bare, flexible, quartz laser fiber (300 to 600 µm in diameter) where it is scattered, reflected, and absorbed to varying degrees, depending on its specific wavelength and the specific optical properties of the tissue. The production of heat stems from interaction between the photons of low-intensity laser energy and molecular chromophores—hemoglobin, myoglobin, bilirubin, the cytochrome pigments in mitochondria, and so on—structures ubiquitous in human tissues. This distinguishes laser thermal ablation (LTA) from RFA and MWA, which produce heat by inducing ionic and molecular friction, but the results are the same: lethal thermal injury. Temperatures between 45° C and 55° C ultimately lead to cell death if the heating is persistent. In contrast, temperatures that exceed 60° C cause immediate and irreversible cellular death with protein denaturation, breakage of chemical bonds in DNA and RNA molecules, and loss of integrity in the lipid layers (Head & Dodd, 2004; Izzo, 2002).
Use of high-power laser energy results in vaporization and rapid tissue charring around the tip of the fiber. Although temperatures near the light-emitting portion of the laser fiber can range from 300° C to 1000° C, the presence of coagulated tissue around the tip drastically reduces the optical penetration of the light into adjacent tissues and thereby limits heat diffusion. As a consequence of overheating and charring, the areas of thermal necrosis produced with LTA are often less than 1 cm in diameter. If low-power laser energy is used, tissue heating occurs slowly, which decreases vaporization and tissue carbonization around the tip of the laser fiber. This approach produces a zone of radiant and conductive tissue heating that enlarges progressively, and the area of thermal necrosis ranges from 1.0 to 1.5 cm in diameter (Germer et al, 1998).
Obviously, the relatively small volume of LTA thermal lesions limits the clinical applicability of this thermal ablation technique. To overcome this problem, a variety of strategies have been proposed. The use of sapphire-tipped laser fibers significantly reduces the occurrence of carbonization at the fiber tip, but it does not significantly increase the size of the thermal lesions (Vogl et al, 2002). The use of interstitial fibers, quartz fibers with flat or cylindrical diffusers at the tip, reduces tissue carbonization and produces thermal lesions ranging from 2.3 to 5 cm in diameter (Khan, 2008). Cooled laser application sheaths, designed to reduce temperatures around the laser fiber tip, allow the use of higher power output without carbonization and produce larger thermal lesions (Vogl et al, 2002); however, diffusers increase the caliber of the laser fiber, and water-cooled sheaths require insertion of a coaxial dilation system introduced through an 18-gauge puncture. The beam-splitting multiple-fiber LTA device offers further improvement in thermal lesion size. Most of these systems use four parallel fibers positioned 1.5 to 2 cm from each other. The simultaneous activation of all four fibers produces a thermal lesion that is larger than the sum of the individual lesions produced by each fiber, because killing temperatures are also reached in the overlapping peripheral zones. This laser device can produce thermal lesions from 4 to 7 cm in diameter; however, positioning the multiple fibers in the tumor nodule is a highly complex procedure that requires training and a great deal of practice. In addition, even for skilled investigators, it is almost impossible to place the fiber tips correctly within the three-dimensional space, and in most cases, the thermal lesion produced will be irregularly shaped.
Technique
The appearance of the thermal lesions on US is the same as that described for other methods of thermal ablation. The technique used with the beam-splitting multiple-fiber laser device is basically the same, but it involves the placement four Chiba-like needles as guides for the four laser fibers. With single and multifiber techniques, the pullback technique can be used to create multiple lesions (Pacella et al, 2009).
Unlike other thermal ablation methods, LTA can be performed under the guidance of MRI. This approach allows real-time monitoring of the temperatures reached in the tissue undergoing ablation. Several MRI sequences have been evaluated for this purpose (Botnar, 1998; Matsumoto et al, 1994; Vogl et al, 1995). The simplest and most widely used is the T1-weighted sequence. The T1 values of a tissue decreases linearly with increasing temperatures (up to about 55° C). However, conventional MRI significantly limits access to the patient, so percutaneous insertion and placement of laser fibers is often done under US or CT guidance, and the patient is then transferred into the MRI scanner to monitor the treatment. This limitation may be overcome by the use of open MRI systems, which are becoming more and more common (Izzo, 2002).
Complications
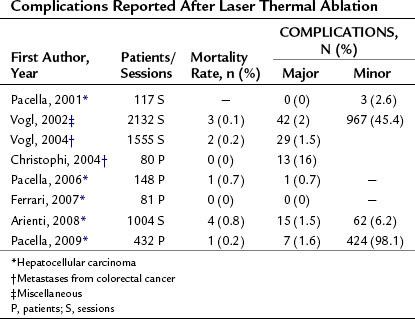
The complications reported after LTA are also quite similar to those observed after the other percutaneous methods of thermal ablation. Table 85A.9 shows the LTA mortality and complication rates reported by several investigators. Mortality ranged between 0% and 0.8%, and complication rates were between 0% and 2%.
Local Tumor Control
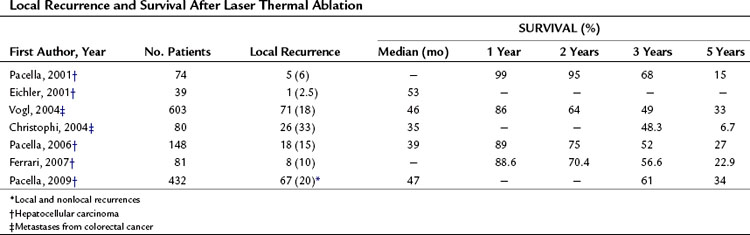
LTA has been shown to be effective in producing complete ablation of tumor nodules, and as expected the probability of success depends on the size of the tumor. Increasing size is associated with decreasing rates of complete ablation; therefore all three major methods for thermal ablation can produce complete ablation in more than 90% of HCC nodules, as long as their diameters do not exceed 3 cm. As shown in Table 85A.10, the local recurrence rates reported by several investigators vary widely after LTA of hepatic tumors, from 2% to 37% (Eichler et al, 2001).
Long-Term Results
The long-term results of LTA of HCC and METs are summarized in Table 85A.10. In terms of survival, the best results were those of Vogl and coworkers (2004), who reported 3- and 5-year survival rates of 49% and 33% after LTA treatment of liver METs; however, the wide variability of the results and the limited number of reports make these data of limited value. Some investigators have reported promising 3- and 5-year survival rates after LTA of HCC (61% and 34%, respectively). On the whole, LTA is an effective method for treating hepatic tumors, but it has two major limitations: First, the small size of the thermal lesions obtained with a single laser fiber reduces the applicability of the technique and necessitates multiple treatment sessions to ablate most tumors. Second, the multiple-fiber technique is a much more complex procedure that requires a high degree of skill and experience. It also tends to produce irregularly shaped thermal lesions, therefore LTA technology needs to be improved before it gains worldwide use. At present, the available data on local recurrences and long-term survival are inadequate to allow any meaningful conclusions on the future of LTA.
Cryoablation (see Chapter 85B)
Technique
Cryoablation has been practiced during open surgery for more than 40 years. Recently, needle-like cryoprobes 3 mm in caliber have been developed that can be used for percutaneous tumor ablation, which is generally performed under US guidance (Mala, 2006); general anesthesia is typically used, because the procedure lasts longer than 30 minutes. The tip of the cryoprobe is placed in the deepest part of the tumor under US guidance, and the freeze-thaw cycle begins. On US, the ice ball appears as an increasing hyperechoic rim with a posterior acoustic shadow. Multiple cryoprobes must be inserted to ablate tumors larger than 3 cm in diameter. In this way, ablation areas larger than 5 cm in diameter can be created (Bilchik et al, 2000).
Complications
Cryoablation is associated with a higher rate of complications than other thermal ablation techniques (Seifert & Morris, 1999). One event that is peculiar to this type of ablation is cracking of the liver parenchyma, resulting in hemorrhage. Another typical complication is a syndrome known as cryoshock, which involves pulmonary, renal, and coagulation abnormalities in a variety of combinations (Head & Dodd, 2004). The severity varies, and the syndrome may include diffuse intravascular coagulopathy. Severe hypothermia can occur with either intraoperative or percutaneous cryoablation, and this can be lethal when the tumor being treated is large (Sarantou et al, 1998). Other complications are similar to those reported for the other thermal ablative methods. Table 85A.11 shows the mortality and complication rates reported by several investigators after cryoablation of hepatic tumors; they range from 0% to 4% and from 1.5 % to 29%, respectively.
Local Tumor Control
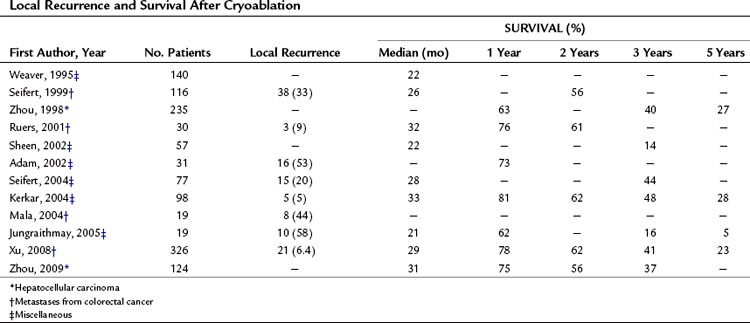
Cryoablation can completely destroy hepatic tumors, but the complete response rates, as shown in Table 85A.12, are lower than those reported for RFA or MWA. Local recurrence rates (6.4% to 58%) also seem to be higher than those observed after RFA.
Long-Term Results
In several centers, cryoablation has been used to destroy liver tumors, for the most part liver metastases from CRC. Only limited initial experiences have been reported for HCC, but for these tumors, RFA has surpassed cryoablation in terms of local tumor control (Adam et al, 2002). The long-term results documented in several studies are shown in Table 85A.12, but these data are characterized by a degree of variability that precludes any meaningful assessment of the efficacy of cryoablation.
Abdalla EK, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818-825.
Abe H, et al. Open-configuration MR-guided microwave thermocoagulation therapy for metastatic liver tumors from breast cancer. Breast Cancer. 2005;12:26-31.
Adam R, et al. A comparison of percutaneous cryosurgery and percutaneous radiofrequency for unresectable hepatic malignancies. Arch Surg. 2002;137:1332-1339.
Akriviadis EA, et al. Hepatocellular carcinoma. Br J Surg. 1998;85:1319-1331.
Aramaki M, et al. Microwave coagulation therapy for unresectable hepatocellular carcinoma. Hepatogastroenterology. 2004;51:1784-1787.
Arienti V, et al. Complications of laser ablation for hepatocellular carcinoma: a multicenter study. Radiology. 2008;246:947-955.
Ballantyne GH. Surgical treatment of liver metastases in patients with colorectal cancer. Cancer. 1993;71:4252-4266.
Beppu T, et al. Efficacy of microwave coagulation therapy (MCT) in patients with liver tumors. Gan To Kagaku Ryoho. 1998;25:1358-1361.
Berber E, et al. Predictors of survival after radiofrequency thermal ablation of colorectal cancer metastases to the liver: a prospective study. J Clin Oncol. 2005;23:1358-1364.
Bilchik AJ, et al. Cryosurgical ablation and radiofrequency ablation for unresectable hepatic malignant neoplasm: a proposed algorithm. Arch Surg. 2000;135:657-662.
Botnar R. Temperature-sensitive MR sequences. In: Debatin, J, Adam, G. Interventional Magnetic Resonance Imaging. Heidelberg: Springer, 1998.
Bouza C, et al. Meta-analysis of percutaneous radiofrequency ablation versus ethanol injection in hepatocellular carcinoma. BMC Gastroenterology. 2009;9:31-39.
Bowles BJ, et al. Safety and efficacy of radiofrequency thermal ablation in advanced liver tumors. Arch Surg. 2001;136:864-886.
Breedis C, Young G. The blood supply of neoplasm in the liver. Am J Pathol. 1954;30:969-985.
Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519-524.
Brunello F, et al. Radiofrequency ablation versus ethanol injection for early hepatocellular carcinoma: a randomized controlled trial. Scan J Gastroenterol. 2008;43:727-735.
Buscarini E, Buscarini L. Radiofrequency thermal ablation with expandable needle of focal liver malignancies: complications report. Eur Radiol. 2004;14:31-37.
Cabassa P, et al. Radiofrequency ablation of hepatocellular carcinoma: long-term experience with expandable needle electrodes. AJR Am J Roentgenol. 2006;186:316-321.
Chen MH, et al. The role of contrast-enhanced ultrasound in planning treatment protocols for hepatocellular carcinoma before radiofrequency ablation. Clin Radiol. 2007;62:752-760.
Chen MS, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321-328.
Chen TM, et al. Major complications of ultrasound-guided percutaneous radiofrequency ablations for liver malignancies: single center experience. Hepatology. 2008;23:445-450.
Cheng BQ, et al. Chemoembolization combined with radiofrequency ablation for patients with hepatocellular carcinoma larger than 3 cm: a randomized controlled trial. JAMA. 2008;299:1669-1677.
Choi D, et al. Percutaneous radiofrequency ablation for early-stage hepatocellular carcinoma as a first-line treatment: long-term results and prognostic factors in a large single-institution series. Eur Radiol. 2007;17:684-692.
Chopra S, et al. Radiofrequency ablation of hepatic tumors adjacent to the gallbladder: feasibility and safety. AJR Am J Roentgenol. 2003;180:697-701.
Christophi C, et al. Long-term survival of patients with unresectable colorectal liver metastases treated by percutaneous interstitial laser thermotherapy. World J Surg. 2004;28:987-994.
Cioni D, et al. Radiofrequency thermal ablation of hepatocellular carcinoma: using contrast-enhanced harmonic power Doppler sonography to assess treatment outcome. AJR Am J Roentgenol. 2001;177:783-788.
Cosman ER, et al. Stereotactic radiofrequency lesion making. Appl Neurophysiol. 1983;46:160-166.
Curley SA, et al. Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg. 2000;232:381-391.
Curley SA, et al. Early and late complications after radiofrequency ablation of malignant liver tumors in 608 patients. Ann Surg. 2004;239:450-458.
Di Stasi M, et al. Percutaneous ethanol injection in the treatment of hepatocellular carcinoma: a multicenter survey of evaluation practices and complication rates. Scand J Gastroenterol. 1997;32:1168-1173.
Djavan B, et al. Transperineal radiofrequency interstitial tumor ablation of the prostate: correlation magnetic resonance imaging with histopathologic examination. Urology. 1997;50:986-993.
Dong B, et al. Percutaneous sonographically guided microwave coagulation therapy for hepatocellular carcinoma: results in 234 patients. AJR Am J Roentgenol. 2003;180:1547-1555.
Ebara M, et al. Therapeutic effect of percutaneous ethanol injection on small hepatocellular carcinoma: evaluation with CT. Radiology. 1995;195:371-377.
Eichler K, et al. Oligonodular hepatocellular carcinoma (HCC): MR-controlled laser-induced thermotherapy. Radiologie. 2001;41:915-922.
Fartoux L, et al. Treatment of small hepatocellular carcinoma with acetic acid percutaneous injection. Gastroenterol Clin Biol. 2005;29:1213-1219.
Ferrari FS, et al. Treatment of small HCC through radiofrequency ablation and laser ablation: comparison of techniques and long-term. results. Radiol Med. 2007;112:377-393.
Gaiani S, et al. Review article: percutaneous treatment of hepatocellular carcinoma. Aliment Pharmacol Ther. 2003;17(Suppl 2):103-110.
Germer CT, et al. Optical properties of native and coagulated human liver tissue and liver metastases in the near infrared range. Lasers Surg Med. 1998;23:194-203.
Gillams AR, Lees WR. Survival after percutaneous, image-guided thermal ablation of hepatic metastases from colorectal cancer. Dis Colon Rectum. 2000;43:656-661.
Gillams AR, Lees WR. Five-year survival in 309 patients with colorectal liver metastases treated with radiofrequency ablation. Eur Radiol. 2009;19:1206-1213.
Giorgio A, et al. One-shot percutaneous ethanol injection of liver tumors under general anaesthesia: preliminary data on efficacy and complications. Cardiovasc Intervent Radiol. 19, 1996. 27–23
Giorgio A, et al. Interstitial laser photocoagulation under ultrasound guidance of liver tumors: results in 104 treated patients. Eur J Ultrasound. 2000;11:181-188.
Giorgio A, et al. Complications after percutaneous saline-enhanced radiofrequency ablation of liver tumors: 3-year experience with 336 patients at a single center. AJR Am J Roentgenol. 2005;184:207-211.
Goldberg SN, et al. Large volume tissue ablation with radiofrequency by using a clustered, internally cooled electrode technique: laboratory and clinical experience in liver metastases. Radiology. 1998;209:371-379.
Goldberg SN, et al. Percutaneous radiofrequency tissue ablation: does perfusion-mediated tissue cooling limit coagulation necrosis? J Vasc Interv Radiol. 1998;9:101-111.
Greenlee RT, et al. Cancer statistics. CA Cancer J Clin. 2000;50:7-33.
Greenway B. Hepatic metastases from colorectal cancer: resection or not. Br J Surg. 1988;75:513-519.
Hanazaki K, et al. Survival and recurrence after hepatic resection of 386 consecutive patients with hepatocellular carcinoma. J Am Coll Surg. 2000;191:381-388.
Head HW, Dodd GD3rd. Thermal ablation for hepatocellular carcinoma. Gastroenterology. 2004;127:167-178.
Helling TS, Woodall CE. Referrals for surgical therapy in patients with hepatocellular carcinoma: a community experience. J Gastrointest Surg. 2007;11:76-81.
Ho CS, et al. Percutaneous ethanol injection of unresectable medium-to-large-sized hepatomas using a multipronged needle: efficacy and safety. Cardiovasc Intervent Radiol. 2007;30:241-247.
Howard JH, et al. Radiofrequency ablation for unresectable tumors of the liver. Am Surg. 2008;74:594-600.
Huang GT, et al. Percutaneous ethanol injection versus surgical resection for the treatment of small hepatocellular carcinoma: a prospective study. Ann Surg. 2005;242:36-42.
Huo TI, et al. Comparison of percutaneous acetic acid injection and percutaneous ethanol injection for hepatocellular carcinoma in cirrhotic patients: a prospective study. Scand J Gastroenterol. 2003;38:770-778.
Iannitti D, et al. Hepatic tumor ablation with clustered microwave antennae: the US phase II trial. HPB. 2007;9:120-124.
Ikai I, et al. Report of the 15th follow-up survey of primary liver cancer. Hepatol Res. 2004;28:21-29.
Ikai I, et al. Report of the 17th nationwide follow-up survey of primary liver cancer in Japan. Hepatol Res. 2007;37:676-691.
Ishii H, et al. Local recurrence of hepatocellular carcinoma after percutaneous ethanol injection. Cancer. 1996;77:1792-1796.
Izzo F. New approaches to the treatment of hepatic malignancies: other thermal ablation techniques—microwave and interstitial laser ablation of liver tumors. Ann Surg Oncol. 2002;10:492-497.
Jiao LR. Percutaneous radiofrequency thermal ablation for liver tumours. Lancet. 1999;354:427-428.
Jungraithmay W, et al. Cryoablation of malignant liver tumors: results of a single-center study. Hepatobiliary Pancreat Dis Int. 2005;4:554-560.
Kerkar S, et al. Long-term follow-up and prognostic factors for cryotherapy of malignant liver tumors. Surgery. 2004;136:770-779.
Khan KN, et al. Prospective analysis of risk factors for early intrahepatic recurrence of hepatocellular carcinoma following ethanol injection. J Hepatol. 2000;32:269-278.
Khan MR, et al. Comparison of percutaneous and surgical approaches for radiofrequency ablation of small and medium hepatocellular carcinoma. Arch Surg. 2007;142:1136-1143.
Khan S. Laser ablation of hepatocellular carcinoma: a review. World J Gastroenterol. 2008;14:7170-7174.
Kim Y, et al. Intraoperative radiofrequency ablation for hepatocellular carcinoma: long-term results in a large series. Ann Surg Oncol. 2008;15:1862-1870.
Knudsen AR, et al. Radiofrequency ablation of colorectal liver metastases downstaged by chemotherapy. Acta Radiol. 2009;50:716-721.
Kong WT, et al. Major complications after radiofrequency ablation for liver tumors: analysis of 255 patients. World J Gastroenterol. 2009;15:2651-2656.
Kosari K, et al. Local, intrahepatic, and systemic recurrence patterns after radiofrequency ablation of hepatic malignancies. J Gastrointest Surg. 2002;6:255-263.
Laspas F, et al. Computed tomography–guided radiofrequency ablation of hepatocellular carcinoma: treatment efficacy and complications. J Gastrointestin Liver Dis. 2009;18:323-328.
Lencioni R, et al. Radio-frequency thermal ablation of liver metastases with a cooled-tip electrode needle: results of a pilot clinical trial. Eur Radiol. 1998;8:1205-1211.
Lencioni R, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radiofrequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003;228:235-240.
Lencioni R, et al. Percutaneous radiofrequency ablation of hepatic colorectal metastases: technique, indications, results, and new promises. Invest Radiol. 2004;39:689-697.
Lenhert T. Sequential hepatic and pulmonary resection for metastatic colorectal cancer. Br J Surg. 1999;86:241-243.
Liang P, et al. Prognostic factors for percutaneous microwave coagulation therapy of hepatic metastases. AJR Am J Roentgenol. 2003;181:1319-1325.
Liang P, et al. Malignant liver tumors: treatment with percutaneous microwave ablation complications among cohort of 1136 patients. Radiology. 2009;251:933-940.
Lin SM, et al. Randomized controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut. 2005;54:1151-1156.
Lin XZ, et al. Electrochemical therapy: comparison with other local treatment methods on rat model. Hepatogastroenterology. 2001;48:91-94.
Livraghi T, et al. Liver metastases: results of percutaneous ethanol injection in 14 patients. Radiology. 1991;179:709-712.
Livraghi T, et al. Percutaneous ethanol injection of hepatic tumors: single-session therapy with general anaesthesia. AJR Am J Roentgenol. 1993;161:1065-1069.
Livraghi T, et al. Hepatocellular carcinoma and cirrhosis in 746 patients: long-term results of percutaneous ethanol injection. Radiology. 1995;197:101-108.
Livraghi T, et al. Saline-enhanced radio-frequency tissue ablation in the treatment of liver metastases. Radiology. 1997;202:205-210.
Livraghi T, et al. Long-term results of single-session percutaneous ethanol injection in patients with large hepatocellular carcinoma. Cancer. 1998;83:48-57.
Livraghi T, et al. Hepatocellular carcinoma: radio-frequency ablation on medium and large lesions. Radiology. 2000;214:761-768.
Livraghi T, et al. Percutaneous radiofrequency ablation of liver metastases in potential candidates for resection: the “test-of-time approach.”. Cancer. 2003;97:3027-3035.
Livraghi T, et al. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441-451.
Livraghi T, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology. 2008;47:82-89.
Llovet JM, et al. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434-1440.
Llovet JM, et al. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology. 2001;33:1124-1129.
Llovet JM, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390.
Lu MD, et al. Surgical resection versus percutaneous thermal ablation for early-stage hepatocellular carcinoma: a randomized clinical trial. Zhonghua Yi Xue Za Zhi. 2006;86:801-805.
Lu MD, et al. Percutaneous microwave and radiofrequency ablation for hepatocellular carcinoma: a retrospective comparative study. J Gastroenterol. 2005;40:1054-1060.
Machi J, et al. Long-term follow-up outcome of patients undergoing radiofrequency ablation for unresectable hepatocellular carcinoma. World J Surg. 2005;29:1364-1373.
Mala T, et al. Cryoablation of colorectal liver metastases: minimally invasive tumour control. Scand J Gastroenterol. 2004;6:571-578.
Mala T. Cryoablation for liver tumors: a review of mechanisms, techniques and clinical outcome. Minim Invasive Ther Allied Technol. 2006;15:9-17.
Martin R, Scoggins CR, McMasters KM. Safety and efficacy of microwave ablation of hepatic tumors: a prospective review of 5-year experience. Ann Surg Oncol. 2010;17:171-178.
Matsumoto R, et al. Tissue temperature monitoring for thermal interventional therapy: comparison of T1-weighted MR sequences. J Magn Reson Imaging. 1994;4:65-70.
Mazzaferro V, et al. Radiofrequency ablation of small hepatocellular carcinoma in cirrhotic patients awaiting liver transplantation: a prospective study. Ann Surg. 2004;240:900-909.
McCormack L, et al. Surgical therapy of hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2005;17:497-503.
McGahan JP, et al. Hepatic ablation using radiofrequency electrocautery. Invest Radiol. 1990;25:267-270.
McGlynn KA, et al. International trends and patterns of primary liver cancer. Int J Cancer. 2001;94:290-296.
Midorikawa Y, et al. Molecular targets for liver cancer therapy: from screening of target genes to clinical trials. Hepatol Res. 2009;40:49-60.
Mulier S, et al. Complications of radiofrequency coagulations of liver tumours. Br J Surg. 2002;89:1206-1222.
Murakami T, et al. Percutaneous radiofrequency ablation and transcatheter arterial chemoembolization for hypervascular hepatocellular carcinoma: rate and risk factors for local recurrence. Cardiovasc Intervent Radiol. 2007;30:696-704.
Nakamura S. Resection of liver metastases of colorectal carcinoma. World J Surg. 1997;21:741-747.
Ng KK, et al. Efficacy and safety of radiofrequency ablation for perivascular hepatocellular carcinoma without hepatic inflow occlusion. Br J Surg. 2006;93:440-447.
Ohmoto K, et al. Comparison of therapeutic effects between radiofrequency ablation and percutaneous microwave coagulation therapy for small hepatocellular carcinomas. J Gastroenterol Hepatol. 2009;24:223-227.
Ohnishi K. Comparison of percutaneous acetic acid injection and percutaneous ethanol injection for hepatocellular carcinoma. Hepatogastroenterology. 1998;45:1254-1258.
Organ LW. Electrophysiologic principles of radiofrequency lesion making. Appl Neurophysiol. 1976;39:69-76.
Orlando A, et al. Radiofrequency thermal ablation vs percutaneous ethanol injection for small hepatocellular carcinoma in cirrhosis: meta-analysis of randomized controlled trials. Am J Gastroenterol. 2009;104:514-524.
Oshowo A, et al. Radiofrequency ablation extends the scope of surgery in colorectal liver metastases. EJSO. 2003;29:244-247.
Otto G, et al. Radiofrequency ablation as first-line treatment in patients with early colorectal liver metastases amenable to surgery. Ann Surg. 2010;251:796-803.
Pacella C, et al. Laser thermal ablation in the treatment of small hepatocellular carcinoma: results in 74 patients. Radiology. 2001;221:712-720.
Pacella C, et al. Analysis of factors predicting survival in patients with hepatocellular carcinoma treated with percutaneous laser ablation. J Hepatol. 2006;44:902-909.
Pacella C, et al. Long-term outcome of cirrhotic patients with early hepatocellular carcinoma treated with ultrasound-guided percutaneous laser ablation: a retrospective analysis. J Clin Oncol. 2009;27:2615-2621.
Park IJ, et al. Radiofrequency ablation for metachronous liver metastasis from colorectal cancer after curative surgery. Ann Surg Oncol. 2008;15:227-232.
Patterson EJ, et al. Radiofrequency ablation of porcine liver in vivo: effects of blood flow and treatment time on lesion size. Ann Surg. 1998;227:559-565.
Peck-Radosavljevic M, et al. Consensus on the current use of sorafenib for the treatment of hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2010;22:391-398.
Raut CP, et al. Significant long-term survival after radiofrequency ablation of unresectable hepatocellular carcinoma in patients with cirrhosis. Ann Surg Oncol. 2005;12:616-628.
Rossi S, et al. Thermal lesions induced by 480 KHz localized current field in guinea pig and pig liver. Tumori. 1990;76:54-57.
Rossi S, et al. Percutaneous RF interstitial thermal ablation in the treatment of hepatic cancer. AJR Am J Roentgenol. 1996;167:759-768.
Rossi S, et al. Percutaneous treatment of small hepatic tumors by an expandable RF needle electrode. AJR Am J Roentgenol. 1998;170:1015-1022.
Rossi S, et al. Relationship between the shape and size of radiofrequency induced thermal lesions and hepatic vascularization. Tumori. 1999;85(2):128-132.
Rossi S, et al. Percutaneous radio-frequency thermal ablation of nonresectable hepatocellular carcinoma after occlusion of tumor blood supply. Radiology. 2000;217:119-126.
Rossi S, et al. Percutaneous computed tomography–guided radiofrequency thermal ablation of small unresectable lung tumours. Eur Respir J. 2006;27:556-563.
Rossi S, et al. Effect of hyperbarism on radiofrequency ablation outcome. AJR Am J Roentgenol. 2007;189:876-882.
Ruers TJ, et al. Long-term results of treating hepatic colorectal metastases with cryosurgery. Br J Surg. 2001;88:844-849.
Sacks D, et al. Society of Interventional Radiology Clinical Practice Guidelines. JVIR. 2003;14:199-202.
Salmi A, et al. Efficacy of radiofrequency ablation of hepatocellular carcinoma associated with chronic liver disease without cirrhosis. Int J Med Sci. 2008;5:327-332.
Sarantou T, et al. Complications of hepatic cryosurgery. Semin Surg Oncol. 1998;14:156-162.
Sato M, et al. Microwave coagulation therapy for hepatocellular carcinoma. Gastroenterology. 1996;110:1507-1514.
Seidenfeld J, et al. Radiofrequency ablation of unresectable liver metastases. J Am Coll Surg. 2002;195:378-386.
Seifert JK, Junginger T. Cryotherapy for liver tumours: current status, perspectives, clinical results, and review of literature. Technol Cancer Res Treat. 2004;3:151-163.
Seifert JK, Morris D. World survey on the complications of hepatic and prostate cryotherapy. World J Surg. 1999;23:109-113.
Seki T, et al. Ultrasonically guided percutaneous microwave coagulation therapy for small hepatocellular carcinoma. Cancer. 1994;74:817-825.
Seki T, et al. Percutaneous microwave coagulation therapy for patients with small hepatocellular carcinoma: comparison with percutaneous ethanol injection therapy. Cancer. 1999;85:1694-1702.
Seki T, et al. Five-year survival of patients with hepatocellular carcinoma treated with laparoscopic microwave coagulation therapy. Endoscopy. 2005;37:1220-1225.
Sheen AJ, et al. Cryotherapeutic ablation of liver tumours. Br J Surg. 2002;89:1396-1401.
Shibata T, et al. Microwave coagulation therapy for multiple hepatic metastases from colorectal carcinoma. Cancer. 2000;89:276-284.
Shibata T, et al. Small hepatocellular carcinoma: comparison of radio-frequency ablation and percutaneous microwave coagulation therapy. Radiology. 2002;223:331-337.
Shiina S. Image-guided percutaneous ablation therapies for hepatocellular carcinoma. J Gastroenterol. 2009;44:122-131.
Shiina S, et al. Percutaneous ethanol injection therapy for hepatocellular carcinoma: a histopathologic study. Cancer. 1991;68:1524-1530.
Shiina S, et al. Nonsurgical treatment of hepatocellular carcinoma: from percutaneous ethanol injection therapy and percutaneous microwave coagulation therapy to radiofrequency ablation. Oncology. 2002;62:64-68.
Shiina S, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129:122-130.
Shimada S, et al. Complications and management of microwave coagulation therapy for primary and metastatic liver tumors. Jpn J Surg. 1998;28:1130-1137.
Shimozawa N, Hanazaki K. Long-term prognosis after hepatic resection for small hepatocellular carcinoma. J Am Coll Surg. 2004;198:356-365.
Solbiati L, et al. Hepatic metastases: percutaneous radio-frequency ablation with cooled-tip electrodes. Radiology. 1997;205:367-373.
Solbiati L, et al. Radiofrequency thermal ablation of liver metatstases. In: Bartolozzi, C, Lencioni, R. Liver Malignancies: Diagnostic and Interventional Radiology. Berlin: Springer, 1999.
Solbiati L, et al. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology. 2001;221:159-166.
Sung YM, et al. Long-term results of percutaneous ethanol injection for the treatment of hepatocellular carcinoma in Korea. Korean J Radiol. 2006;7:187-192.
Tanaka K, et al. Outcome after hepatic resection versus combined resection and microwave ablation for multiple bilobar colorectal metastases to the liver. Surgery. 2006;139:263-273.
Tateishi R, et al. Percutaneous radiofrequency ablation for hepatocellular carcinoma: an analysis of 1000 cases. Cancer. 2005;103:1201-1209.
Torzilli G, et al. No-mortality liver resection for hepatocellular carcinoma in cirrhotic and noncirrhotic patients. Arch Surg. 1999;134:984-992.
Vitale A, et al. Use of sorafenib in patients with hepatocellular carcinoma before liver transplantation: a cost-benefit analysis while awaiting data on sorafenib safety. Hepatology. 2009;51:165-173.
Vogl TJ, et al. Malignant liver tumors treated with MR imaging-guided laser-induced thermotherapy: technique and prospective results. Radiology. 1995;196:257-265.
Vogl TJ, et al. Malignant liver tumors treated with MR imaging-guided laser-induced thermotherapy: experience with complications in 899 patients (2,520 lesions). Radiology. 2002;225:367-377.
Vogl TJ, et al. Colorectal carcinoma metastases in liver: laser-induced interstitial thermotherapy-local tumor control rate and survival data. Radiology. 2004;230:450-458.
Weaver ML, et al. Hepatic cryosurgery in the treatment of unresectable metastases. Surg Oncol. 1995;4:231-236.
Wiess L. Haematogenous metastatic patterns in colonic carcinoma: an analysis of 1541 necropsies. J Pathol. 1986;150:195-203.
Xu H, et al. Ultrasound-guided percutaneous thermal ablation of hepatocellular carcinoma using microwave and radiofrequency ablation. Clin Radiol. 2004;59:53-61.
Xu KC, et al. Percutaneous cryosurgery for the treatment of hepatic colorectal metastases. World J Gastroenterol. 2008;14:1430-1436.
Yin XY, et al. Percutaneous thermal ablation of medium and large hepatocellular carcinoma: long-term outcome and prognostic factors. Cancer. 2009;115:1914-1923.
Zhang X, et al. Microwave ablation with cooled-tip electrode for liver cancer: an analysis of 160 cases. Hepatogastroenterology. 2008;55:2184-2187.
Zhou L, et al. Efficacy of argon-helium cryosurgical ablation on primary hepatocellular carcinoma. Chinese J Cancer. 2009;28:45-48.
Zhou XD, Tang ZY. Cryotherapy for primary liver cancer. Semin Surg Oncol. 1998;14:171-174.