CHAPTER 84 Percutaneous Discectomy
INTRODUCTION
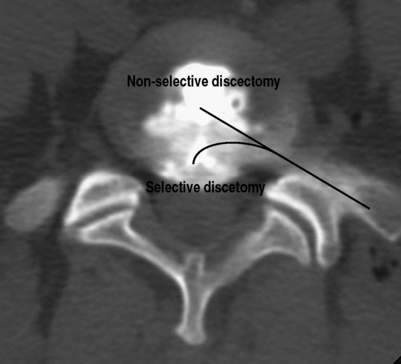
Percutaneous discectomy is a method for treating disc herniations. There are two basic types of percutaneous discectomy: selective and nonselective.1 The goal of a selective percutaneous discectomy is to remove disc from the posterior third of the disc space, where herniations reside.1 This is typically accomplished with surgical instruments and/or lasers which can be placed in the disc with an endoscope and then directed to the area of the herniation under direct vision. As such, it is a true surgical procedure, and will not be discussed further in this chapter. The goal of a nonselective discectomy is to remove nucleus from the center of the disc, relying on the accompanying changes in disc mechanics to effect a change in the herniation (Fig. 84.1).
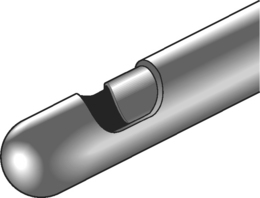
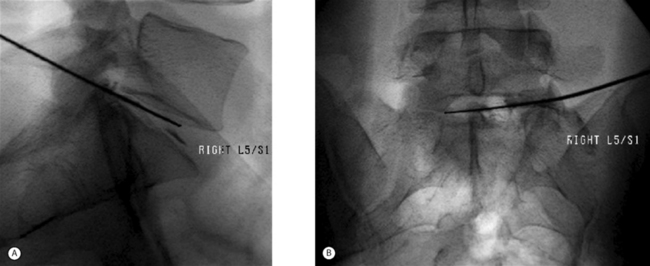
The original device used to perform a nonselective percutaneous discectomy was the nucleotome (Fig. 84.2). This device functions as a mechanical shaver which is placed into the center of the disc under fluoroscopic guidance, and nucleus is then aspirated. While other devices have largely supplanted the nucleotome, the objective of the procedure remains nonselective removal of nucleus from the disc. As all the devices used for nonselective discectomy are placed using fluoroscopy, without incisions or surgical instruments (Fig. 84.3), in contemporary practice the procedure is commonly done by interventional spine physicians and radiologists as well as by surgeons.
EVOLUTION OF PERCUTANEOUS DISCECTOMY
The driving force behind the development of percutaneous discectomy was the series of neurological catastrophes that occurred with the use of chymopapain in the United States in the 1970s.1 Chemonucleolysis with chymopapain was introduced into clinical practice in 1964 by Lyman Smith.2 In the years since, it has been demonstrated in randomized controlled trials to be an effective treatment for sciatica due to disc herniation.3 Unfortunately, due to technical errors by some practitioners, chymopapain was injected into the subarachnoid space of a number of patients who developed severe neurological deficits, leading it to be removed from the US market in 1985, although it continued to be available in other parts of the world. The prevailing theory on the mechanism of action of chymopapain at the time was that it relieved nerve root pressure due to disc herniation by ‘internally decompressing’ the disc; that is, by decreasing intradiscal pressure it reduced mechanical tension on a bulging anulus and relieved nerve root compression.4
Onik, who introduced the nucleotome, postulated that decreasing intradiscal pressure by mechanical means would have the same therapeutic effect as chymopapain, while avoiding the dangers associated with chymopapain.5 Subsequent biomechanical studies confirmed that percutaneous discectomy resulted in a decrease in intradiscal pressure.6 Percutaneous discectomy with the nucleotome, which became known as automated percutaneous lumbar discectomy (APLD), was adopted with considerable enthusiasm. This enthusiasm seemed justified by early, nonrandomized studies, which yielded 70–75% success rates in treating patients with sciatica due to a disc herniation with very low complication rates.7,8 However, controlled studies, one comparing APLD to chemonucleolysis and the other to microdiscectomy, demonstrated considerably lower success rates of 29–37%.9,10 By the latter part of the 1990s, APLD had fallen out of favor in much of the world, although there continue to be areas, most notably Italy, where it is still commonly performed.
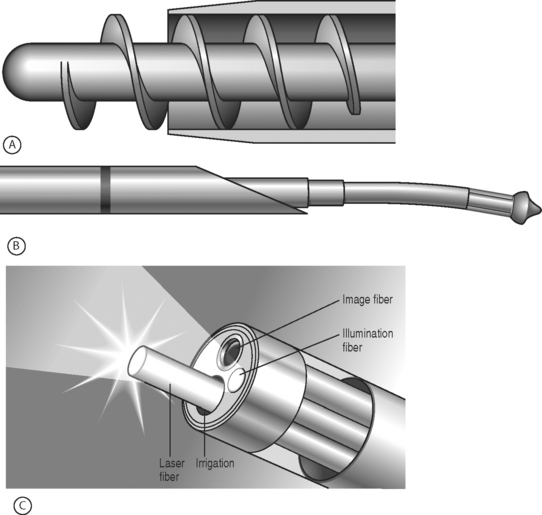
Recently, there has been a resurgence of interest in percutaneous discectomy, coinciding with the introduction of new devices for doing the procedure (Fig. 84.4). These devices are all considerably smaller than the nucleotome and are therefore easier to insert. The Dekompressor device is similar to the nucleotome in that it removes tissue mechanically, while both the Lase device and the Arthrocare spine wand vaporize tissue, the Lase device with laser and the Arthrocare device with a low-temperature process known as coblation. As there are no controlled studies available on the efficacy of these devices, at this point it is not possible to determine whether they represent an advance over percutaneous discectomy done with the nucleotome.
INDICATIONS
The original indication for percutaneous discectomy, patients with sciatica due to a contained disc herniation, was based on a logical analysis of the effects of the procedure on the disc. Early clinical studies showed good results in that patient population as well as high failure rates in patients with noncontained disc herniations,8 stenosis, or loss of more than 25% of disc height.11 While these observational studies supported the proposed indication for percutaneous discectomy, subsequent comparative studies did not. The comparative study that is most relevant is from Chatterjee et al., who compared the results from percutaneous discectomy against microdiscectomy, which has become the gold standard treatment for disc herniations.10 All patients in that study had a primary complaint of radicular pain and a contained HNP at a single level, the height of which was less than 30% of the sagittal canal size. Patients were randomized to either microdiscectomy or APLD, with the results demonstrating 80% good or excellent outcomes in the microdiscectomy group and 29% in the APLD group.
Based upon these results, at present there is no evidence-based justification for the use of APLD over microdiscectomy for the treatment of contained disc herniations. Despite this, there may be a subpopulation of patients with contained disc herniations that will do better with percutaneous discectomy than surgery. Chatterjee et al. noted that the success rate from surgery in their patients, while much better than APLD, was worse than studies on noncontained disc herniations, which have success rates in 90% range.10 They speculated that patients with small contained disc protrusions are different from the overall population of patients with herniated discs, that inflammatory changes may be particularly important in those discs, and that nonsurgical remedies should be sought.10 More recently, Carragee et al. demonstrated that, based on intraoperative findings, contained disc herniations could be classified into two types: those with an associated subanular fragment and those without.12
Of the types of contained disc herniation defined by Carragee, the one without a fragment would seem to be the most likely to respond to percutaneous discectomy. If there is a fragment of nucleus trapped in the outer annular fibers it would not be expected to shift towards the center in response to a debulking procedure in the center of the disc.1 Debulking the center of the disc may, however, lead to a reduction in the size of a herniation, if pressure equilibrates between the center of the disc and the herniated portion. This latter contention is supported by Castro et al., who found an 80% success rate with percutaneous discectomy when a broad-based protrusion was identified on CT-discography and a 53% success rate with a narrow based herniation.6 In contrast, Dullerud and Johansen did not find any correlation between outcomes following percutaneous discectomy and CT-discography findings.11 Although appealing on theoretical grounds, additional study is needed to determine if the specific subset of contained disc herniation without a subanular fragment constitutes an appropriate indication for percutaneous discectomy.
FUTURE DIRECTIONS
The therapeutic effect associated with percutaneous discectomy may be a result of changes in pressure on the nerve that accompany removal of disc tissue. However, clinical studies suggest that there may be other therapeutic mechanisms, as outcome following percutaneous discectomy is independent of changes in disc height and removed disc volume.13 It has been postulated that percutaneous discectomy may lead to pain relief by drainage of inflammatory substances from the disc.14,15 Another potential mechanism is alterations in expression of inflammatory cytokines associated with pain and tissue healing. This latter hypothesis is supported by findings from a large animal model demonstrating that percutaneous discectomy using coblation technology led to significant changes in interleukin-1 and interleukin-8 in degenerated discs.16 Better understanding of the therapeutic effects of percutaneous discectomy may lead to more valid indications for the procedure, as well as optimized treatment protocols, potentially including co-interventions such as pharmacologic manipulation of cytokine networks.16
At present, the gold standard surgical treatment for patients with low back pain secondary to a contained disc herniation is a spinal fusion, which has a considerably lower success rate than a microdiscectomy for sciatica. A number of observational studies have examined the effect of percutaneous discectomy on patients with a primary complaint of low back pain,8,11,14 with success rates ranging from 0%14 to 70%.11 Given the limited options available to patients with discogenic pain, the existing evidence would seem to suggest that a controlled trial is indicated.
1 Mayer HM. Spine update. Percutaneous lumbar disc surgery. Spine. 1994;19(23):2719-2723.
2 Smith L. Preliminary communication: enzyme dissolution of the nucleus pulposus in humans. JAMA. 1964;187(2):177-180.
3 Gibson JN, Grant IC, et al. The Cochrane review of surgery for lumbar disc prolapse and degenerative lumbar spondylosis [In process citation]. Spine. 1999;24(17):1820-1832.
4 Spencer DL, Miller JA, et al. The effect of intervertebral disc space narrowing on the contact force between the nerve root and a simulated disc protrusion. Spine. 1984;9(4):422-426.
5 Onik G, Helms CA, et al. Percutaneous lumbar diskectomy using a new aspiration probe: porcine and cadaver model. Radiology. 1985;155(1):251-252.
6 Castro WH, Jerosch J, et al. Restriction of indication for automated percutaneous lumbar discectomy based on computed tomographic discography. Spine. 1992;17(10):1239-1243.
7 Grevitt MP, McLaren A, et al. Automated percutaneous lumbar discectomy. An outcome study. J Bone Joint Surg [Br]. 1995;77(4):626-629.
8 Onik G, Mooney V, et al. Automated percutaneous discectomy: a prospective multi-institutional study. Neurosurgery. 1990;26(2):228-232. discussion 232–233
9 Revel M, Payan C, et al. Automated percutaneous lumbar discectomy versus chemonucleolysis in the treatment of sciatica. A randomized multicenter trial. Spine. 1993;18(1):1-7.
10 Chatterjee S, Foy PM, et al. Report of a controlled clinical trial comparing automated percutaneous lumbar discectomy and microdiscectomy in the treatment of contained lumbar disc herniation. Spine. 1995;20(6):734-738.
11 Dullerud R, Johansen JG. CT-diskography in patients with sciatica. Comparison with plain CT and MR imaging. Acta Radiol. 1995;36(5):497-504.
12 Carragee EJ, Han MY, et al. Clinical outcomes after lumbar discectomy for sciatica: the effects of fragment type and annular competence. J Bone Joint Surg [Am]. 2003;85A(1):102-108.
13 Dullerud R, Amundsen T, et al. Clinical results after percutaneous automated lumbar nucleotomy. A follow-up study. Acta Radiol. 1995;36(4):418-424.
14 Kornberg M. Automated percutaneous lumbar discectomy as treatment for lumbar disc disruption. Spine. 1993;18(3):395-397.
15 Gunzburg R, Fraser RD, et al. An experimental study comparing percutaneous discectomy with chemonucleolysis. Spine. 1993;18(2):218-226.
16 O’Neill CW, Liu JJ, et al. Percutaneous plasma decompression alters cytokine expression in injured porcine intervertebral discs. Spine J. 2004;4(1):88-98.