Chapter 49 Percutaneous and Minimally Invasive Approaches to Decompression and Arthrodesis of the Cervical Spine
Dorsal Minimally Invasive Approaches for the Cervical Spine
Dorsal decompressive procedures are fundamental tools in the surgical treatment of symptomatic cervical degenerative spine disease.1–4 Even as ventral cervical procedures have gained prominence, posterior cervical laminoforaminotomy still provides symptomatic relief in 92% to 97% of patients with radiculopathy from foraminal stenosis or lateral herniated discs.3,5 Similarly, dorsal cervical decompression for cervical stenosis achieves neurologic improvement in 62.5% to 83% of myelopathic patients undergoing either laminectomy or laminoplasty.4,6–8 Moreover, these operations avoid the complications attendant to ventral approaches to the cervical spine, namely, esophageal injury, vascular injury, recurrent laryngeal nerve paralysis, dysphagia, and accelerated degeneration of adjacent motion segments after fusion.9–11
However, open dorsal approaches to the cervical spine require extensive subperiosteal stripping of the paraspinal musculature that leads to postoperative pain, spasm, and dysfunction and can be persistently disabling in 18% to 60% of patients.4,9,12,13 Furthermore, preoperative loss of lordosis and long segment decompressions increase the risk for postoperative sagittal plane deformity,14–17 a complication that frequently prompts instrumented arthrodesis at the time of laminectomy. Employing these extensive posterior fusion techniques increases operative risks, time, and blood loss; exacerbates early postoperative pain; and potentially contributes to adjacent-level degeneration.
The fundamental tenet of minimal access techniques is reduction of approach-related morbidity. To that end, the advent of muscle-splitting tubular retractor systems and the use of endoscopic technology or the microscope have allowed for the application of minimally invasive techniques to dorsal cervical decompressive procedures13,18–35 and fixation.36–40
Spurling, Scoville, and Frykholm were the first to describe the open cervical foraminal decompression between 1944 and 1947.41–43 In 1983 Williams reported the first microsurgical technique for dorsal cervical foraminotomy.44 Several minimally invasive dorsal cervical techniques were described after that.18–40 To avoid confusion, and to simplify the description of all these techniques, we divided them into two main approaches: (1) the minimally invasive midline cervical approach, and (2) the minimally invasive paramedian (transtubular or transmuscular) cervical approach. An endoscope or microscope could be used in either approach. These approaches are used to perform laminotomy/foraminotomy/discectomy, laminectomy, laminoplasty,34,35 and lateral mass fixation.36–40
Indications
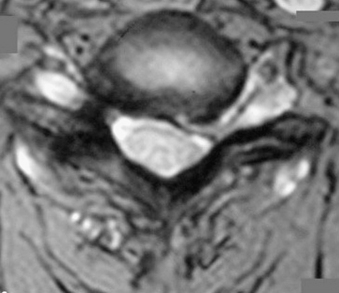
The operative indications for minimally invasive laminotomy/foraminotomy/discectomy are (1) unilateral single-root (Fig. 49-1) or multiple-root cervical radiculopathy from lateral disc herniations or foraminal stenosis (single-level or multilevel), without instability, significant kyphosis, or severe axial neck pain; (2) persistent or recurrent root symptoms following anterior cervical discectomy and fusion; (3) cervical disc disease in patients for whom anterior approaches are relatively contraindicated (e.g., ventral neck infection, tracheostomy, prior irradiation); and (4) cervicothoracic disc herniation and radiculopathy, to avoid ventral approach potential complications and when the ventral approach is not feasible (short neck or others). A ventral approach should be considered in case of same-level bilateral radiculopathy, central disc herniation, significant kyphosis, and severe axial neck pain.
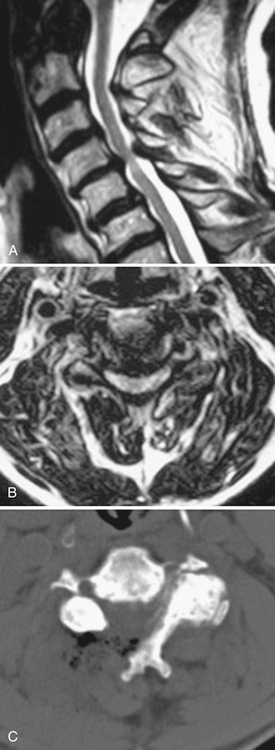
Most patients who are candidates for a noninstrumented, dorsal cervical decompression are also candidates for a minimally invasive cervical decompression. These are selected patients with clinical myelopathy or myeloradiculopathy, radiographic evidence of spinal cord compression from one to three adjacent cervical levels, and a lordotic cervical spine (Fig. 49-2). Contraindications include loss of the normal cervical lordosis, severe ventral disease (disease that extends for more than three levels), and segmental instability.
Minimally invasive dorsal cervical fixation could be applied in case of facet dislocation or segmental instability or to support ventral instrumentation. Few clinical reports are available in the literature.36,37,39 Minimally invasive cervical laminoplasty is still undergoing investigation.
Operative Setup
General endotracheal anesthesia is induced on a standard electric operating table. A neurophysiologic monitoring array with capabilities for somatosensory evoked potentials (SSEPs), motor evoked potentials (MEPs), and free running electromyography (EMG) is put in place. In cases of myelopathy, a fiberoptic intubation may be elected and evoked potentials are compared before and after positioning to identify positioning-related cord ischemia. Maintenance of normotension to avoid spinal cord hypoperfusion is best directed with continuous blood pressure measurements afforded by an arterial line. Measures to detect and treat air embolism, such as a precordial Doppler and a central line, are options but have not yet proven necessary. Given the small exposure, the risk of air embolism is low. A urinary catheter is generally not necessary for one- or two-level procedures. Routine perioperative antibiotics are administered. Relaxants are minimized after induction to allow for effective neurophysiologic monitoring.
Posterior cervical approaches might be performed with the patient in the prone or sitting position. With the prone position, the head is held with a Mayfield pin-holder or a well-padded horseshoe-shaped headrest, with slight flexion. The operating table is tilted in a reverse Trendelenburg position to ensure that the cervical spine is parallel to the floor. The senior author (RGF) prefers the sitting position (Fig. 49-3) because it confers advantages of decreased epidural bleeding, decreased pooling of blood in the operative field, decreased anesthesia time, and gravity-dependent positioning of the shoulders for better lateral fluoroscopic images. The table is turned 180 degrees relative to the anesthesiologist. The patient’s head is fixed in a Mayfield head holder. The table is manipulated to place the patient in a semisitting position with the head flexed and the neck straight and perpendicular to the floor.
Paramedian Approach
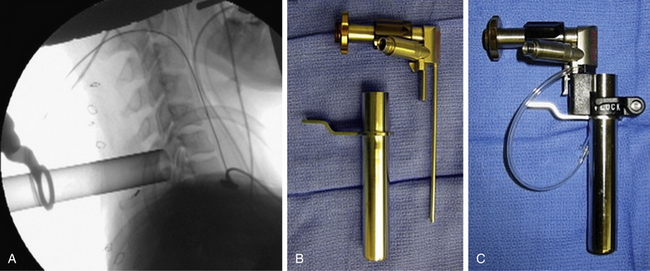
The operative level(s) and entry point are confirmed on lateral fluoroscopy with a K-wire. A 1.8-cm longitudinal incision is marked out approximately 1.5 cm off the midline on the operative side and injected with local anesthetic. For two-level procedures the incision should be placed midway between the targeted levels. After an initial stab incision, the K-wire is advanced slowly though the subcutaneous tissue and docked on the cervicodorsal fascia. Once an optimal trajectory is established, the fascia is incised with a scalpel to accommodate dilators. The fascia is retracted, and the smallest dilator is placed through the posterior cervical musculature under fluoroscopic guidance and docked at the lamina-facet junction of the level of interest. A slightly lateral trajectory is advised to avoid the spinal canal and ensure contact with the lateral mass. Successive tubular muscle dilators are carefully and gently inserted, remembering that the axial forces that are routinely applied during muscle dilation in the lumbar spine are hazardous in the cervical spine. After dilation, the final tubular retractor is placed and secured over the junction of the lamina and the facet with a table-mounted flexible retractor arm and the dilators are removed. The following steps are performed under microscopic magnification or using loupes or an endoscope. The endoscope is inserted and attached to the tubular retractor (Fig. 49-4). Monopolar cautery and pituitary rongeurs are used to clear the remaining soft tissue off of the lateral mass and lamina of interest, taking care to start the dissection over solid bone laterally.
Laminotomy/Foraminotomy/Discectomy
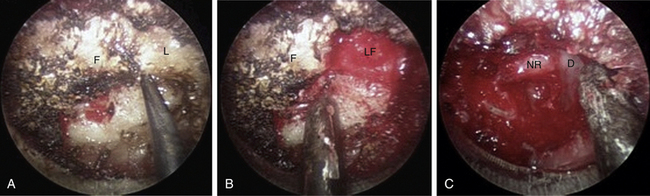
The medial facet/interlaminar space junction is identified. Using a high-speed drill, a partial laminotomy-facetectomy is performed beginning at the medial facet/interlaminar space and going laterally, without exceeding 50% facet removal, to maintain biomechanical integrity. The dorsolateral portion of the superior lamina and the medial part of the inferior articular facet are removed first. This will permit the removal of the lateral corner of the inferior lamina and the medial part of the superior articular facet, exposing the medial border of the caudal pedicle. The nerve root is located directly above the caudal pedicle and anterior to the superior articular facet. The ligamentum flavum can be removed medially after the foraminotomy to expose the lateral edge of the dura and proximal portion of the nerve root. Progressive lateral dissection can then proceed along the root as it enters the foramen. The venous plexus overlying the nerve root should be carefully coagulated with bipolar cautery and incised. With the root well visualized, a fine-angled dissector can be used to palpate ventrally to the nerve root for osteophytes or disc fragments. Should an osteophyte be present, a down-angled curette may be used to tamp the material further ventrally into the disc space or fragment it for subsequent removal. In the case of a soft disc herniation, a nerve hook may be passed ventrally and inferiorly to the root to gently tease the fragment away from the nerve for ultimate removal with a pituitary rongeur. In either case, additional drilling of the superomedial quadrant of the caudal pedicle allows greater access to the ventral pathology and obviates the need for excessive nerve root retraction superiorly (Fig. 49-5).
Decompression for Stenosis
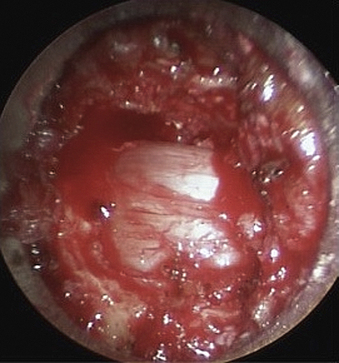
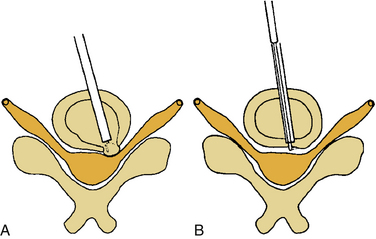
In this case, ipsilateral laminotomy of the levels of interest is performed and the ligamentum flavum is left in place to protect the dura. The tube is then angled about 45 degrees off the midline such that the tube is oriented to visualize the contralateral side. A plane between the ligament and undersurface of the spinous process is gently dissected with a fine curette. The drill with guard sleeve extended is then used to progressively drill the undersurface of the spinous process and contralateral lamina all the way to the contralateral facet. This initial decompression allows greater working space within which to remove hypertrophied ligament while avoiding downward pressure on the dura and spinal cord. Dissection and removal of the ligament with curettes and Kerrison rongeurs may now proceed safely. Any compressive elements of the contralateral facet or the superior edge of the caudal lamina may also be drilled off or removed with Kerrison rongeurs at this time because their impact on the dura is more apparent with the ligament removed. After gently confirming decompression over to the contralateral foramen with a fine probe, the tube is returned to its original position to complete the ipsilateral removal of ligament and bone. This should then reveal completely decompressed and pulsatile dura (Fig. 49-6). If indicated, ipsilateral foraminotomy, as described earlier, also may be performed at this time.
Lateral Mass Fixation
After exposure of the facet joint, a hand drill is used to create a pilot hole. The starting point is 1 mm medial to the midpoint of the lateral mass, and the trajectory will be 25 degrees lateral and parallel to the facet joint. Appropriate dimension screws are inserted after taping, and a rod is fixed with set screws. A midline incision is preferred in this case for the transmuscular approach. The same incision could be used for both sides. Also, fluoroscopic guidance is preferred.
Outcomes and Results
Favorable outcomes were reported in the literature for posterior cervical foraminotomy with a range between 75% and 100%.1,3,9,44–48 Krupp et al. separated the outcomes by soft, hard, and mixed pathology, with favorable outcomes of 98%, 84%, and 91%, respectively.45 Jodicke et al. reported a significantly better outcome for soft discs compared to hard discs in early follow-up, but no difference was found at long-term follow-up.47
The reports of minimally invasive, microscopic, and microendoscopic posterior cervical formainotomy have demonstrated equivalent efficacy to the open technique, but the blood loss, length of stay, and postoperative pain medication usage were reduced with the minimally invasive techniques.9,13,20,21,25,26,48 The senior author and Khoo prospectively used cervical microendoscopic posterior foraminotomy in 25 patients and compared the results with another 26 patients treated via open cervical laminoforaminotomy.9 The microendoscopic group had a lower overall operative time (115 vs. 171 minutes), less blood loss (138 vs. 246 mL), shorter postoperative hospital stay (20 vs. 68 hours), and fewer postoperative narcotic medications (11 vs. 40 equivalents) when compared with the open technique group.
Ruetten et al. conducted a prospective, randomized, controlled study with lateral cervical herniations, operated either in a full endoscopic posterior foraminotomy (89 patients) or conventional microsurgical anterior technique with fusion/plating (86 patients), with 2 years of follow-up.20 There was no significant difference between the groups in the clinical outcome, revision, or complication rates. Preservation of motion was conserved in the full endoscopic posterior group.
Perez-Cruet and the senior author have reported on five patients undergoing cervical microendoscopic decompression for stenosis at one, two, or three levels.16 All patients demonstrated improvement in their myelopathy and returned to work with the only complication being one unintended durotomy that sealed spontaneously. Yabuki et al. performed endoscopic partial laminectomy in 10 patients with degenerative cervical compressive myelopathy.28 All patients experienced symptomatic improvement with slight postoperative wound pain. The mean operative duration was 164 ± 35 minutes and the intraoperative blood loss was 45.5 ± 27 mL. Boehm et al. reported the results of 13 patients who were operated on under the microscope through a transmuscular tubular approach.30 Nine of these patients suffered from cervical myelopathy. The average follow-up was 17 months. The neck disability index score and visual analogue scale score were significantly improved. Thirty-three percent showed complete recovery of the preoperative neurologic deficit, while 67% had improvement.
Wang et al. reviewed retrospectively 18 patients treated with lateral mass screws placed in a minimally invasive fashion.37 In two cases, the minimally invasive technique was converted to the standard open technique because of inability to visualize anatomic landmarks on fluoroscopy (bulky shoulders). Successful fusion was documented in all cases, and there were no hardware failures during the minimum 2 years of follow-up. Two patients were lost to follow-up after 6 months.
Complications
The posterior cervical foraminotomy is a safe procedure associated with a low rate of complications (1–15%),1,3,9,13,20,21,25,26,44–48 with wound infection and dural tear contributing to the majority of them. The senior author has no infection to date in his microendoscopic series, and the unintended durotomy rate has dropped from 8% in the initial series of patients9 to around 1% more recently. Direct suture repair of durotomy is difficult through the narrow-diameter tubes or small incisions. Therefore one technique for handling small defects is to simply cover the durotomy with muscle, fat, hemostatic gelatin (Gelfoam, Baxter Healthcare, Glendale, CA), or dural susbstitute followed by fibrin glue or synthetic sealant such as Coseal (Baxter Healthcare, Glendale, CA). Using this approach, overnight bedrest is usually sufficient to seal the defect. For larger dural tears that cannot be primarily closed, 2 to 3 days of lumbar cerebrospinal fluid (CSF) drainage may prevent a leak. Ultimately, the small opening and relative lack of dead space after minimally invasive procedures have made the incidence of postoperative pseudomeningoceles and CSF49-cutaneous fistulae negligible.
Ventral Minimally Invasive Approaches for the Cervical Spine
Anterior cervical discectomy and fusion (ACDF) was first described by Smith and Robinson and then by Cloward in the 1950s.49–51 Orozco introduced anterior plating in 1970 as adjunctive treatment in cervical fractures.52 Since then, several types of plates and grafts were developed. ACDF has now developed as a standard procedure in the treatment of cervical radiculomyelopathy. It is described as a safe and efficacious procedure with good fusion rates. However, several problems can occur, mainly because of access complications and adjacent segment degeneration as a disadvantage of fusion. Hilibrand et al. postulated that up to 25% of the patients who undergo a ventral cervical fusion could require treatment for degenerative changes of the adjacent segments within 10 years.53 With the fascination of minimally invasive techniques and the intent to preserve motion and prevent adjacent segment disease, several anterior alternative approaches have been reported: (1) ventral cervical foraminotomy (microsurgical and endoscopic); (2) percutaneous ventral cervical procedures for discectomy, anuloplasty, and stabilization; and (3) cervical arthroplasty. The final approach is not discussed in this chapter.
Ventral Cervical Foraminotomy (Microsurgical and Endoscopic)
In 1968, Verbiest described a ventrolateral approach to the cervical neuroforamen, which involved sectioning of the longus colli muscle, exposure of the transverse process, mobilization of the vertebral artery, and performing discectomy with and without fusion.54 In 1976, Hakuba described the transuncodiscal approach. In his approach the vertebral artery was not displaced, and a complete discectomy was performed with and without fusion.55 In 1989, Snyder and Bernhardt published a new anterior cervical fractional interspace decompression technique, which consisted of a 6-mm-wide cylindric bur hole in the lateral third of the intervertebral disc, and fragmentectomy. They reported minimal disc space collapse and a 4% rate of spontaneous fusion.56 In 1996, Jho described the anterior cervical foraminotomy with resection of the uncus process, lateral portion of the rostral end plate, and lateral portion of the intervertebral disc. This technique required cutting of the longus colli muscle and exposure of the vertebral artery along its medial surface.57 In 2002, Saringer proposed a modification of Jho’s technique by preserving a thin piece of cortical bone of the lateral wall of the uncinate process, avoiding exposure of the vertebral artery,58 and he added the endoscope to his procedure in 2003.59 Again in 2002, Jho reported an upper vertebral transcorporeal foraminotomy technique. The hole in this technique was drilled at the most lateral and inferior 4 to 5 mm of the upper vertebral body.60 The cartilage end plate was exposed and entered in its posterior third. In 2007, Choi et al. described a modification of the upper vertebral transcorporeal Jho’s approach, made by drilling the hole more medially, to avoid cutting the longus colli muscle and exposing the vertebral artery.61 The evolution of this technique has led to (1) having less disc disruption, (2) avoiding exposure of the vertebral artery, and (3) avoiding cutting the longus colli muscle, which could result in the injury of the sympathetic chain.
Surgical Technique
After the skin incision, the platysma muscle is sectioned along the same line. The superficial cervical fascia is opened medial to the sternocleidomastoid muscle and blunt dissection is directed deeply to the spinal column, with the vascular structures retracted laterally and the trachea and esophagus medially. The prevertebral fascia is opened, and the anterior part of the vertebral bodies with intervertebral discs and longus colli muscles are exposed. The level of concern is again checked with fluoroscopy. As mentioned earlier, several techniques and modifications have been reported. These can be divided into two main approaches: (1) the transuncal approach and (2) the transcorporeal approach.
Transuncal Approach
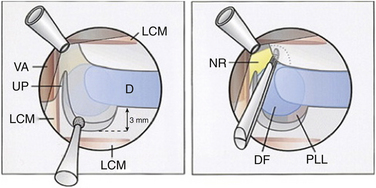
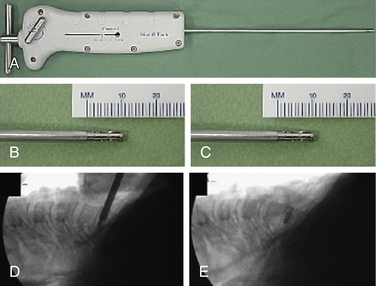
The medial border of the longus colli muscle is cut perpendicularly and retracted or excised to expose the medial aspect of the transverse process and the uncinate process of the lower vertebrae. Care should be taken when exposing the C6-7 level because the vertebral artery lies between the transverse process of C7 and the longus colli muscle. The next steps are performed under microscopic magnification or using the endoscope as described by Saringer in his 2003 technique modification.59 According to Jho, in his first description,57 the uncinate process is undertaken with a 2-mm high-speed drill, leaving a thin dorsolateral rim of cortical bone that limits the vertebral artery laterally and the nerve root dorsally. The thin cortical bone is removed with a curette and a Kerrison rongeur. The posterior longitudinal ligament is exposed, which covers the nerve root posteriorly. At this point, the foraminotomy is completed and the uncovertebral osteophyte is removed. In case of a herniated disc, the posterior longitudinal ligament should be excised with a curette and Kerrison rongeur and the disc fragment removed. Epidural bleeding could be troublesome and should be controlled with bipolar cauterization or by application of Gelfoam and cottonoid. Saringer, in his microscopic58 and endoscopic59 techniques (Figs. 49-7 and 49-8), described two essential modifications to Jho’s transuncal approach: (1) leaving the lateral cortical rim of the uncinate process to protect the vertebral artery, and (2) extending the foraminotomy by removing more bone from the caudal dorsolateral aspect of the rostral vertebrae.
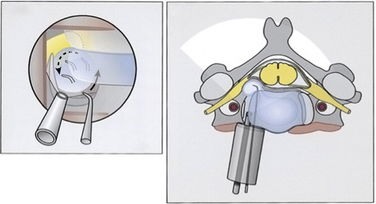
FIGURE 49-8 Left and right, Transtubular and axial depiction of the last step of the operation shown in Figure 49-7. To improve visualization, the endoscope is positioned with its tip inside the drilled canal. Herniated disc fragments are mobilized with a microhook.
(Modified from Saringer WF, Reddy B, Nöbauer-Huhmann I, et al: Endoscopic anterior cervical foraminotomy for unilateral radiculopathy: anatomical morphometric analysis and preliminary clinical experience. J Neurosurg 98(2 Suppl):171–180, 2003.)
Transcorporeal Approach
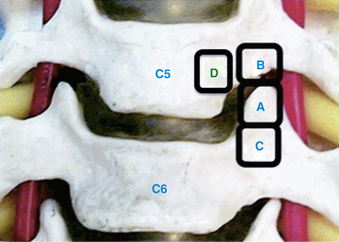
In his technique modification of the transcorporeal approach, Jho describes drilling a hole through the ventral caudolateral portion of the rostral vertebrae60 that extends dorsally to reach the dorsal portion of the uncinate process. Only this dorsal portion is removed. The uncovertebral osteophyte is removed, and as described earlier, the posterior longitudinal ligament should be opened if a disc fragment has to be removed. This approach reduced the disc disruption comparatively with the transuncal approach. Choi et al. more recently described a modification of this technique made by starting the hole more medially on the upper vertebrae, to avoid cutting the longus colli muscle and its related possible complications.61 They also stated that a transcorporeal approach through the inferior vertebrae could be feasible, especially for the upper cervical levels (Fig. 49-9).
Once the decompression is completed, the platysma is closed as usual with 3-0 absorbable sutures. The skin is closed with a subcuticular suture. No cervical collar is necessary postoperatively, and activity is advanced as tolerated.
Outcomes and Results
The microscopic or endoscopic ventral cervical foraminotomy for unilateral cervical radiculopathy has been shown to be an effective and safe procedure. Johnson et al. reported their series of 21 patients operated on by Jho’s original technique and followed between 6 and 36 months.62 Nineteen patients (91%) had improved or resolved radicular symptoms, and two (9%) had persistent radicular symptoms necessitating further surgery. No patients had evidence of instability or loss of disc height on lateral radiographs at 3 months postoperation. Tascioglu et al. reported their result of the same technique for three patients with long-term follow-up.63 The three patients were symptom free in the immediate postoperative period, and their follow-up examinations demonstrated findings within normal limits at 37, 36, and 36 months, respectively, after surgery. Saringer et al. reported in 2002 his results of 34 patients operated with microscopic uncoforaminotomy.58 The follow-up period varied from 2 to 17 months with a mean of 8.2 months. The large majority (97%) of patients were pleased with the results of their operation. The relief of neck pain and redicular pain in the affected dermatome was immediate in all patients. Motor weakness and sensory deficit improved dramatically immediately postoperatively, and improved to normal in the majority of patients within 3 to 6 months. One of the patients had a repeat herniation on the second postoperative day but recovered completely after reoperation and continued to do well at the 6-month follow-up. In 2003, Saringer et al. reported their results from 16 patients operated on with endoscopic uncoforaminotomy.59 During a mean follow-up period of 13.8 months, an average absolute improvement of 44% (P > .05) in the neck disability index score and of 96% (P > .05) in the visual analogue scale score for radicular pain (compared with the preoperative score) was observed. Jho et al. reported their results for 104 patients.60 Ninety-nine percent experienced excellent or good results. One patient developed discitis, which resulted in bony fusion. All other patients maintained their motion segments. Lee et al. reported their results for 13 patients operated on with the transuncal approach64; the mean follow-up was 19 months. All patients experienced complete relief of their radiating pain. The mobility was conserved, and no instability was detected on neuroimaging follow-up. Choi et al. also reported their results from 20 patients operated on by their technique.61 The maximum follow-up was 1 year. All patients experienced immediate postoperative relief of their radicular symptoms and recovery of their neurologic symptoms. The percentage change in disc height was only 6% from the baseline value, but the difference was statistically significant (P = .005). They noted that the loss in disc height seemed to stabilize after 3 months postoperation. Balasubramanian et al. also reported 94% good to excellent results in 34 patients.65
Hacker and Miller are the only researchers to report a significant number of poor outcomes (poor 35% and fair 13%) and a high reoperation rate (30%).66
Hong et al. compared the results of the transuncal approach (40 patients) and the transcorporeal approach (20 patients).67 The mean follow-up period was 9.5 months. They analyzed postoperative changes of disc height, the spinal instability, the average length of hospital stay, the degree of patients’ satisfaction, and complications from each approach. They stated that the transcorporeal approach is a better surgical technique than the transuncal approach, considering the preservation of disc height, spinal stability, length of hospital stay, degree of satisfaction, and complications.
Complications
Possible complications are mostly the same as for a standard anterior cervical approach. As in some of these techniques when the longus colli muscle is cut, risk of injury of the sympathetic chain could be higher. This outcome is true especially at the lower cervical levels, where the sympathetic chain becomes more medial on the longus colli muscle. Jho reported 2 transient Horner syndromes in his 104-patient series.60 With Choi’s technique, this complication could be avoided. Injury of the vertebral artery is another concern of these techniques. Meticulous review of the anatomy of the vertebral artery on the imaging studies and good knowledge of the anatomy of this region should help to avoid this complication.
Percutaneous Ventral Cervical Procedures for Discectomy, Nucleoplasty, and Stabilization
In 1963 to 1964, Smith introduced the chemonucleolysis with chymopapain to treat herniated discs.68,69 In 1975, Hijikata developed the percutaneous lumbar discectomy and reported his series of 136 cases 12 years later.70 In 1986, Ascher reported the laser discectomy.71
The percutaneous ventral cervical approach was first described by Smith and Nicole in 1957, as the cervical discographic technique for the diagnosis of discogenic pain.72 Despite its low complication rates (0.16–2.48%),73–75 its use was limited due to the catastrophic consequences that can occur if a complication developed. Although controversies still exist, several reports of related percutaneous cervical discectomy (PCD) procedures have been reported: (1) PCD with chemonucleolysis,76 (2) automated/coblation PCD,77–82 (3) PCD with laser,83–90 (4) PCD with endoscopic manual and laser,83,91–95 and (5) PCD with stabilization.83
Indications and Contraindications
Patients who present with new onset of cervicobrachial neuralgia, due mainly to recent soft disc herniation (contained), and who are nonresponsive to conservative treatment and without severe neurologic deficit could be considered for PCD with laser, coblation, or chemonucleolysis. In case of the same scenario, but with noncontained herniated disc, PCD with endoscopic manual and laser is recommended. Percutaneous stabilization is indicated when axial symptoms predominate, in case of angular instability (kyphosis), and when cervicoencephalic pain is reproduced by discography.83
These procedures are contraindicated in patients with migrated or calcified discs, advanced spondylosis, significant anterior bony spurs that could block the entry into the disc, cervical canal stenosis, myelopathy, or evidence of instability, and in those who had previous neck surgery.83,92,94
Surgical Technique
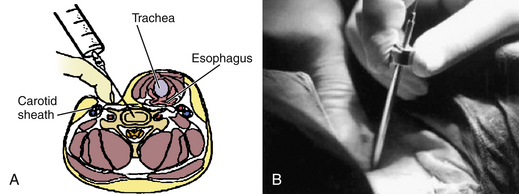
The procedures could be performed under local or general anesthesia. The patent is placed in the supine position, as for a conventional ventral cervical approach. A roll is placed under the shoulders, and the shoulders are taped down for better visualization of the lower cervical levels as needed. Preparation and draping are completed as usual. The choice of the side of approach depends on the surgeon’s preference, but an approach contralateral to the side of the lateral herniated disc is preferable. Fluoroscopic guidance is used through the procedure for anatomic orientation and avoidance of complications. The level of work is identified with fluoroscopy, using a K-wire. The point of skin entry will be at the medial border of the sternocleidomastoid muscle. Firm pressure is applied digitally at this level between the sternocleidomastoid muscle and the trachea, and pointed toward the cervical spine. The larynx and esophagus are displaced medially, and the carotid artery is displaced laterally (Fig. 49-10). The esophagus could be made more prominent with the insertion of a nasogastric tube, and the carotid pulse is augmented with sympathomimetics. After palpation of the anterior cervical spine, an 18-gauge spinal needle is placed in the disc space of concern under fluoroscopic guidance. A guidewire is passed through the spine needle and then removed. A 3- to 5-mm skin incision is made, depending on the procedure and instruments to be used. To assist the placement of the endoscope or the working cannula, 3- to 5-mm dilators are passed through the K-wire. Specific instruments are then used to perform the discectomy, with papain (chemonucleolysis), loop-shaped electrode (automated PCD), laser, microcurette, and microforceps, as well as graft for fixation (Figs. 49-11 to 49-14).
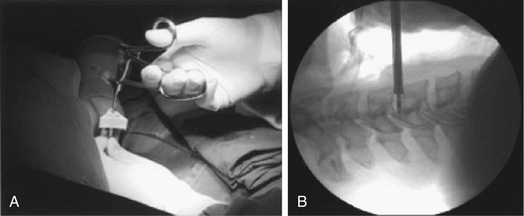
FIGURE 49-12 Intraoperative view (A) and C-arm view (B) of manual discectomy using microforceps.
(Modified from Lee SH, Ahn Y, Choi WC, et al: Immediate pain improvement is a useful predictor of long-term favorable outcome after percutaneous laser disc decompression for cervical disc herniation. Photomed Laser Surg 24:508–513, 2006.)
Outcomes, Results, and Complications
Good-to-excellent clinical outcomes were reported in the literature, ranging from 80% to 85% for automated PCD,78–81 75% to 94.5% for laser PCD,86,87,89,90 80.2% to 94.5% for PCD with manual resection and laser,91–94 and 86.36% for chemonucleolysis.76 Predictors of good outcome were found to be related to radiating arm pain, lateral disc location herniation, and immediate postoperative pain relief.91,93 Radiographically, Ahn et al. showed a significant decrease in the disc height by 11.2%, with maintenance of overall and focal sagittal alignments.92 There was no segmental instability or spontaneous fusion noted. Interestingly, complication rates were less than 1%, without any catastrophic ones.76,80,81,86,87,89–91,94 Most serious complications were infection81 and postoperative hematoma due to rupture of the inferior thyroid artery,80 not of major vessels. Good knowledge of the cervical anatomy, understanding the safety zone of work for each level,96 and frequent fluoroscopic checking will help to avoid complications in these procedures.
Li et al. compared retrospectively the percutaneous cervical disc nucleoplasty (42 patients) with the percutaneous cervical discectomy (38 patients).97 The average follow-up was 12 ± 4 to 5 months. There was no significant difference of the clinical outcomes for the two groups, but operative time was significantly lower for the nucleoplasty group.
Ruetten et al. conducted a prospective, randomized, controlled study to compare the results of full-endoscopic anterior cervical discectomy (FACD) (60 patients) with those of ACDF (60 patients) in mediolateral soft disc herniations.98 Patients were followed for a period of 2 years. Operative time and blood loss were less in the FACD group. There was no significant difference in the clinical outcomes, the progression of preexisting adjacent disc degeneration, or the increase of the postoperative kyphotic angle at the operated segment between the two groups. Revision rates were 6.1% for the ACDF group and 7.4% for the FACD group.
Jho H.D. Microsurgical anterior cervical foraminotomy for radiculopathy: a new approach to cervical disc herniation. J Neurosurg. 1996;84:55-56.
Khoo L.T., Perez-Cruet M.J., Laich D.T., Fessler R.G. Posterior cervical microendoscopic foraminotomy. In: Perez-Cruet M.J., Fessler R.G., editors. Outpatient spinal surgery. St Louis: Quality Medical Publishing; 2006:71-93.
Lee S.H., Lee J.H., Choi W.C., et al. Anterior minimally invasive approaches for the cervical spine. Orthop Clin North Am. 2007;38:327-337.
Perez-Cruet M.J., Samartzis D., Fessler R.G. Microendoscopic cervical laminectomy. In: Perez-Cruet M.J., Khoo L.T., Fessler R.G., editors. An anatomic approach to minimally invasive spine surgery. St Louis: Quality Medical Publishing; 2006:349-366.
Santiago P., Fessler R.G. Minimally invasive surgery for the management of cervical spondylosis. Neurosurgery. 2007;60(Supp1 1):S160-S165.
Saringer W., Nöbauer I., Reddy M., et al. Microsurgical anterior cervical foraminotomy (uncoforaminotomy) for unilateral radiculopathy: clinical results of a new technique. Acta Neurochir (Wien). 2002;144:685-694.
1. Aldrich F. Posterolateral microdisectomy for cervical monoradiculopathy caused by posterolateral soft cervical disc sequestration. J Neurosurg. 1990;72(3):370-377.
2. Crandall P.H., Batzdorf U. Cervical spondylotic myelopathy. J Neurosurg. 1966;25(1):57-66.
3. Henderson C.M., Hennessy R.G., Shuey H.M.Jr., Shackelford E.G. Posterior-lateral foraminotomy as an exclusive operative technique for cervical radiculopathy: a review of 846 consecutively operated cases. Neurosurgery. 1983;13(5):504-512.
4. Ratliff J.K., Cooper P.R. Cervical laminoplasty: a critical review. J Neurosurg. 2003;98(Suppl 3):230-238.
5. Khoo L.T., Perez-Cruet M.J., Laich D.T., Fessler R.G. Posterior cervical microendoscopic foraminotomy. In: Perez-Cruet M.J., Fessler R.G., editors. Outpatient spinal surgery. St. Louis: Quality Medical Publishing, Inc; 2006:71-93.
6. Kumar V.G., Rea G.L., Mervis L.J., McGregor J.M. Cervical spondylotic myelopathy: functional and radiographic long-term outcome after laminectomy and posterior fusion. Neurosurgery. 1999;44(4):771-777. discussion 777–778
7. Wang M.Y., Green B.A. Laminoplasty for the treatment of failed anterior cervical spine surgery. Neurosurg Focus. 2003;15(3):E7.
8. Wang M.Y., Shah S., Green B.A. Clinical outcomes following cervical laminoplasty for 204 patients with cervical spondylotic myelopathy. Surg Neurol. 2004;62(6):487-492. discussion 492–493
9. Fessler R.G., Khoo L.T. Minimally invasive cervical microendoscopic foraminotomy: an initial clinical experience. Neurosurgery. 2002;51(Suppl 5):S37-S45.
10. Hilibrand A.S., Robbins M. Adjacent segment degeneration and adjacent segment disease: the consequences of spinal fusion? Spine J. 2004;4(Suppl 6):190S-194S.
11. Ishihara H., Kanamori M., Kawaguchi Y., et al. Adjacent segment disease after anterior cervical interbody fusion. Spine J. 2004;4(6):624-628.
12. Hosono N., Yonenobu K., Ono K. Neck and shoulder pain after laminoplasty. A noticeable complication. Spine (Phila Pa 1976). 1996;21(17):1969-1973.
13. Siddiqui A., Yonemura K.S. Posterior cervical mircoendoscopic dis-kectomy and laminoforaminotomy. In: Kim D.H., Fessler R.G., Regan J.J., editors. Endoscopic spine surgery and instrumentation: percutaneous procedures. New York: Thieme; 2005:66-73.
14. Albert T.J., Vacarro A. Postlaminectomy kyphosis. Spine (Phila Pa 1976). 1998;23(24):2738-2745.
15. Kaptain G.J., Simmons N.E., Replogle R.E., Pobereskin L. Incidence and outcome of kyphotic deformity following laminectomy for cervical spondylotic myelopathy. J Neurosurg. 2000;93(Suppl 2):199-204.
16. Perez-Cruet M.J., Samartzis D., Fessler R.G. Microendoscopic cervical laminectomy. In: Perez-Cruet M.J., Khoo L.T., Fessler R.G., editors. An anatomic approach to minimally invasive spine surgery. St. Louis: Quality Medical Publishing, Inc; 2006:349-366.
17. Yonenobu K., Okada K., Fuji T., et al. Causes of neurologic deterioration following surgical treatment of cervical myelopathy. Spine (Phila Pa 1976). 1986;11(8):818-823.
18. Khoo L.T., Bresnahan L., Fessler R.G. Cervical endoscopic foraminotomy. Fessler R.G., Sekhar L., editors. Atlas of neurosurgical techniques: spine and peripheral nerves. New York: Thieme. 2006;vol 1:785-792.
19. Coric D., Adamson T. Minimally invasive cervical microendoscopic laminoforaminotomy. Neurosurg Focus. 2008;25(2):E2.
20. Ruetten S., Komp M., Merk H., Godolias G. Full-endoscopic cervical posterior foraminotomy for the operation of lateral disc herniations using 5.9-mm endoscopes: a prospective, randomized, controlled study. Spine (Phila Pa 1976). 2008;33(9):940-948.
21. Hilton D.L.Jr. Minimally invasive tubular access for posterior cervical foraminotomy with three-dimensional microscopic visualization and localization with anterior/posterior imaging. Spine J. 2007;7(2):154-158.
22. Gala V.C., O’Toole J.E., Voyadzis J.M., Fessler R.G. Posterior minimally invasive approaches for the cervical spine (abstract v). Orthop Clin North Am. 2007;38(3):339-349.
23. Santiago P., Fessler R.G. Minimally invasive surgery for the management of cervical spondylosis. Neurosurgery. 2007;60(Supp1 1):S160-S165.
24. Holly L.T., Moftakhar P., Khoo L.T., et al. Minimally invasive 2-level posterior cervical foraminotomy: preliminary clinical results. J Spinal Disord Tech. 2007;20(1):20-24.
25. Cağlar Y.S., Bozkurt M., Kahilogullari G., et al. Keyhole approach for posterior cervical discectomy: experience on 84 patients. Minim Invasive Neurosurg. 2007;50(1):7-11.
26. Ruetten S., Komp M., Merk H., Godolias G. A new full-endoscopic technique for cervical posterior foraminotomy in the treatment of lateral disc herniations using 6.9-mm endoscopes: prospective 2-year results of 87 patients. Minim Invasive Neurosurg. 2007;50(4):219-226.
27. Song J.K., Christie S.D. Minimally invasive cervical stenosis decompression. Review. Neurosurg Clin North Am. 2006;17(4):423-428.
28. Yabuki S., Kikuchi S. Endoscopic partial laminectomy for cervical myelopathy. J Neurosurg Spine. 2005;2(2):170-174.
29. Hong G.L., Yang X.Y., Yang S.Y. [Minimally-invasive surgical treatment of cervical radiculomyelopathy]. Zhonghua Wai Ke Za Zhi. 2004;42(6):340-342.
30. Boehm H., Greiner-Perth R., El-Saghir H., Allam Y. A new minimally invasive posterior approach for the treatment of cervical radiculopathy and myelopathy: surgical technique and preliminary results. Eur Spine J. 2003;12(3):268-273.
31. Yuguchi T., Nishio M., Akiyama C., et al. Posterior microendoscopic surgical approach for the degenerative cervical spine. Neurol Res. 2003;25(1):17-21.
32. Witzmann A., Hejazi N., Krasznai L. Posterior cervical foraminotomy. A follow-up study of 67 surgically treated patients with compressive radiculopathy. Neurosurg Rev. 2000;23(4):213-217.
33. Burke T.G., Caputy A. Microendoscopic posterior cervical foraminotomy: a cadaveric model and clinical application for cervical radiculopathy. J Neurosurg. 2000;93(Suppl 1):126-129.
34. Benglis D.M., Guest J.D., Wang M.Y. Clinical feasibility of minimally invasive cervical laminoplasty. Neurosurg Focus. 2008;25(2):E3. Review
35. Wang M.Y., Green B.A., Coscarella E., et al. Minimally invasive cervical expansile laminoplasty: an initial cadaveric study. Neurosurgery. 2003;52(2):370-373. discussion 373
36. Holly L.T., Foley K.T. Percutaneous placement of posterior cervical screws using three-dimensional fluoroscopy. Spine (Phila Pa 1976). 2006;31(5):536-540. discussion 541
37. Wang M.Y., Levi A.D. Minimally invasive lateral mass screw fixation in the cervical spine: initial clinical experience with long-term follow-up. Neurosurgery. 2006;58(5):907-912. discussion 907–912
38. Sehati N., Khoo L.T. Minimally invasive posterior cervical arthrodesis and fixation. Neurosurg Clin North Am. 2006;17(4):429-440. Review
39. Fong S., Duplessis S. Minimally invasive lateral mass plating in the treatment of posterior cervical trauma: surgical technique. J Spinal Disord Tech. 2005;18(3):224-228.
40. Wang M.Y., Prusmack C.J., Green B.A., et al. Minimally invasive lateral mass screws in the treatment of cervical facet dislocations: technical note. Neurosurgery. 2003;52(2):444-447. discussion 447–448
41. Scoville WB. Rupture of the lateral cervical disk and its operative technique. Proceedings of the Harvey Cushing meeting, Boston, 1946.
42. Spurling R., Scoville W.B. Lateral rupture of the cervical intervertebral discs: a common cause of shoulder and arm pain. Surg Gynaecol Obstet. 1944;78:350-358.
43. Frykholm R. Deformities of dural pouches and strictures of dural sheaths in the cervical region producing nerve root compression. J Neurosurg. 1947;4:403-413.
44. Williams R.W. Microcervical foraminotomy: a surgical alternative for intractable radicular pain. Spine (Phila Pa 1976). 1983;8:708-716.
45. Krupp W., Schattke H., Müke R. Clinical results of the foraminotomy as described by Frykholm for the treatment of lateral cervical disc herniation. Acta Neurochir (Wien). 1990;107(1-2):22-29.
46. Herkowitz H.N., Kurz L.T., Overholt D.P. Surgical management of cervical soft disc herniation. A comparison between the anterior and posterior approach. Spine (Phila Pa 1976). 1990;15(10):1026-1030.
47. Jödicke A., Daentzer D., Kästner S., et al. Risk factors for outcome and complications of dorsal foraminotomy in cervical disc herniation. Surg Neurol. 2003;60(2):124-129. discussion 129–130
48. Adamson T.E. Microendoscopic posterior cervical laminoforaminotomy for unilateral radiculopathy: results of a new technique in 100 cases. J Neurosurg. 2001;95(Suppl 1):51-57.
49. Robinson R.A., Smith G.W. Anterolateral cervical disc removal and interbody fusion for cervical disc syndrome. Bull Johns Hopkins Hosp. 1955;96:223-224.
50. Cloward R. The anterior approach for removal of ruptured cervical disks. J Neurosurg. 1958;15:602-617.
51. Cloward R. Treatment of acute fractures and fracture dislocation of cervical spine by vertebral body fusion. A report of 11 cases. J Neurosurg. 1961;18:205-209.
52. Mayer H.M. Minimally invasive spine surgery, ed 2. New York: Springer; 2006. p 55
53. Hilibrand A.S., Carlson G.D., Palumbo M.A., et al. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg [Am]. 1999;81(4):519-528.
54. Verbiest H. A lateral approach to the cervical spine: technique and indications. J Neurosurg. 1968;28(3):191-203.
55. Hakuba A. Trans-unco-discal approach. A combined anterior and lateral approach to cervical discs. J Neurosurg. 1976;45(3):284-291.
56. Snyder G.M., Bernhardt M. Anterior cervical fractional interspace decompression for treatment of cervical radiculopathy. A review of the first 66 cases. Clin Orthop Relat Res. 1989;246:92-99.
57. Jho H.D. Microsurgical anterior cervical foraminotomy for radiculopathy: a new approach to cervical disc herniation. J Neurosurg. 1996;84(2):155-160.
58. Saringer W., Nöbauer I., Reddy M., et al. Microsurgical anterior cervical foraminotomy (uncoforaminotomy) for unilateral radiculopathy: clinical results of a new technique. Acta Neurochir (Wien). 2002;144(7):685-694.
59. Saringer W.F., Reddy B., Nöbauer-Huhmann I., et al. Endoscopic anterior cervical foraminotomy for unilateral radiculopathy: anatomical morphometric analysis and preliminary clinical experience. J Neurosurg. 2003;98(Suppl 2):171-180.
60. Jho H.D., Kim W.K., Kim M.H. Anterior microforaminotomy for treatment of cervical radiculopathy: part 1—disc-preserving “functional cervical disc surgery.”. Neurosurgery. 2002;51(Suppl 5):S46-S53.
61. Choi G., Lee S.H., Bhanot A., et al. Modified transcorporeal anterior cervical microforaminotomy for cervical radiculopathy: a technical note and early results. Eur Spine J. 2007;16(9):1387-1393.
62. Johnson J.P., Filler A.G., McBride D.Q., Batzdorf U. Anterior cervical foraminotomy for unilateral radicular disease. Spine (Phila Pa 1976). 2000;25(8):905-909.
63. Taşçioğlu A.O., Attar A., Taşçioğlu B. Microsurgical anterior cervical foraminotomy (uncinatectomy) for cervical disc herniation. Report of three cases. J Neurosurg. 2001;94(Suppl 1):121-125.
64. Lee J.Y., Löhr M., Impekoven P., et al. Small keyhole transuncal foraminotomy for unilateral cervical radiculopathy. Acta Neurochir (Wien). 2006;148(9):951-958.
65. Balasubramanian C., Price R., Brydon H. Anterior cervical microforaminotomy for cervical radiculopathy—results and review. Minim Invasive Neurosurg. 2008;51(5):258-262.
66. Hacker R.J., Miller C.G. Failed anterior cervical foraminotomy. J Neurosurg. 2003;98(Suppl 2):126-130.
67. Hong W.J., Kim W.K., Park C.W., et al. Comparison between transuncal approach and upper vertebral transcorporeal approach for unilateral cervical radiculopathy—a preliminary report. Minim Invasive Neurosurg. 2006;49(5):296-301.
68. Smith L., Garvin P.J., Gesler R.M., Jennings R.B. Enzyme dissolution of the nucleus pulposus. Nature. 1963;198:1311-1312.
69. Smith L. Enzyme dissolution of the nucleus pulposus in humans. JAMA. 1964;187:137-140.
70. Hijikata S. Percutaneous nucleotomy. A new concept technique and 12 years’ experience. Clin Orthop Relat Res. 1989;238:9-23.
71. Ascher P.W. Application of the laser in neurosurgery. Lasers Surg Med. 1986;2:91-97.
72. Smith G.W., Nichols P.Jr. The technic of cervical discography. Radiology. 1957;68(5):718-720.
73. Grubb S.A., Kelly C.K. Cervical discography: clinical implications from 12 years of experience. Spine (Phila Pa 1976). 2000;25(11):1382-1389.
74. Zeidman S.M., Thompson K., Ducker T.B. Complications of cervical discography: analysis of 4400 diagnostic disc injections. Neurosurgery. 1995;37(3):414-417.
75. Guyer R.D., Ohnmeiss D.D., Mason S.L., Shelokov A.P. Complications of cervical discography: findings in a large series. J Spinal Disord. 1997;10(2):95-101.
76. Hoogland T., Scheckenbach C. Low-dose chemonucleolysis combined with percutaneous nucleotomy in herniated cervical disks. J Spinal Disord. 1995;8(3):228-232.
77. Courtheoux F., Theron J. Automated percutaneous nucleotomy in the treatment of cervicobrachial neuralgia due to disc herniation. J Neuroradiol. 1992;19(3):211-216.
78. Li J., Yan D.L., Zhang Z.H. Percutaneous cervical nucleoplasty in the treatment of cervical disc herniation. Eur Spine J. 2008;17(12):1664-1669.
79. Nardi P.V., Cabezas D., Cesaroni A. Percutaneous cervical nucleoplasty using coblation technology. Clinical results in fifty consecutive cases. Acta Neurochir Suppl. 2005;92:73-78.
80. Bonaldi G., Minonzio G., Belloni G., et al. Percutaneous cervical diskectomy: preliminary experience. Neuroradiology. 1994;36(6):483-486.
81. Bonaldi G., Baruzzi F., Facchinetti A., et al. Plasma radio-frequency-based diskectomy for treatment of cervical herniated nucleus pulposus: feasibility, safety, and preliminary clinical results. AJNR Am J Neuroradiol. 2006;27(10):2104-2111.
82. Birnbaum K. Percutaneous cervical disc decompression. Surg Radiol Anat. 2009;31(5):379-387.
83. Lee S.H., Lee J.H., Choi W.C., et al. Anterior minimally invasive approaches for the cervical spine. Orthop Clin North Am. 2007;38(3):327-337.
84. Li K., Qin H., Chen J. [Clinical application of percutaneous laser disc decompression in the treatment of cervical disc herniation]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2007;21(5):465-467.
85. Pedachenko E.G., Chebotareva L.L., Khizhniak M.V., et al. [Percutaneous laser diskectomy in cervical osteochondrosis]. Zh Vopr Neirokhir Im N N Burdenko. 2001;1:3-5.
86. Chiu J.C., Clifford T.J., Greenspan M., et al. Percutaneous microdecompressive endoscopic cervical discectomy with laser thermodiskoplasty. Mt Sinai J Med. 2000;67(4):278-282.
87. Choy D.S. Percutaneous laser disc decompression (PLDD): twelve years’ experience with 752 procedures in 518 patients. J Clin Laser Med Surg. 1998;16(6):325-331.
88. Hellinger J. Technical aspects of the percutaneous cervical and lumbar laser-disc-decompression and -nucleotomy. Neurol Res. 1999;21(1):99-102.
89. Choy D.S. Clinical experience and results with 389 PLDD procedures with the Nd:YAG laser, 1986 to 1995. J Clin Laser Med Surg. 1995;13(3):209-213.
90. Siebert W. Percutaneous laser discectomy of cervical discs: preliminary clinical results. J Clin Laser Med Surg. 1995;13(3):205-207.
91. Lee S.H., Ahn Y., Choi W.C., et al. Immediate pain improvement is a useful predictor of long-term favorable outcome after percutaneous laser disc decompression for cervical disc herniation. Photomed Laser Surg. 2006;24(4):508-513.
92. Ahn Y., Lee S.H., Shin S.W. Percutaneous endoscopic cervical discectomy: clinical outcome and radiographic changes. Photomed Laser Surg. 2005;23(4):362-368.
93. Ahn Y., Lee S.H., Lee S.C., et al. Factors predicting excellent outcome of percutaneous cervical discectomy: analysis of 111 consecutive cases. Neuroradiology. 2004;46(5):378-384.
94. Chiu J.C., Clifford T.J., Greenspan M., et al. Percutaneous microdecompressive endoscopic cervical discectomy with laser thermodiskoplasty. Mt Sinai J Med. 2000;67(4):278-282.
95. Chiu J.C. Endoscopic assisted microdecompression of cervical disc and foramen. Surg Technol Int. 2008;17:269-279.
96. Lee S.H., Kim K.T., Jeong B.O., et al. The safety zone of percutaneous cervical approach: a dynamic computed tomographic study. Spine (Phila Pa 1976). 32(20), 2007 Sep 15. E569–E574, 2007
97. Li J., Yan D.L., Gao L.B., et al. [Comparison of percutaneous cervical disc nucleoplasty and cervical discectomy for the treatment of cervical disc herniation]. Zhonghua Wai Ke Za Zhi. 2006;44(12):822-825.
98. Ruetten S., Komp M., Merk H., Godolias G. Full-endoscopic anterior decompression versus conventional anterior decompression and fusion in cervical disc herniations. Int Orthop. 2009;33(6):1677-1682.