Chapter 17 Peptides and Proteins
INTRODUCTION
In dermatology, there are often many ways to accomplish any particular goal. One method to reverse cutaneous signs of aging is through the use of prescription retinoids, such as tretinoin or tazarotene. However, these substances are irritating to the skin, resulting in the peeling and stinging characteristic of barrier damage. In recent years, the trend in cosmeceuticals has been for products that improve the appearance of aging skin without the irritation of topical retinoids. This has resulted in the popularity of peptides, which have demonstrated cosmeceutical effects both in vitro and in vivo that could result in clinical aging skin improvement. This chapter discusses the current use of cosmeceutical peptides to aid the practicing dermatologist in understanding the science behind these new ingredients and how to utilize them for cutaneous antiaging treatment.
TERMINOLOGY AND DEFINITIONS
Peptides are short chains of amino acid sequences that make up larger proteins. There are three main categories of peptides currently being used in cosmeceutical products (Box 17.1). This increase in peptide technology has arisen because of the technology to synthesize fragments that mimic peptide sequences in collagen and elastin with the ability to stimulate production of new collagen and elastin. Other peptides are currently available that function primarily as carriers of cofactors for important enzymatic steps in collagen production. Peptide fragments also exist that are able to modulate neurotransmitter release. Since some wrinkling of the skin is caused by collagen breakdown, while other wrinkles are caused by hyperkinetic facial muscle movement, peptides for sale that have actions to inhibit or reverse these actions could have clinical antiaging cosmeceutical benefits..
INDICATIONS AND BIOLOGIC ACTIVITY
Clinically, photoaged skin has wrinkles that are coarse, while the wrinkles of naturally aged skin are much finer. Both of these wrinkles are due, in part, to the loss of collagen in the skin. Chronologically aged skin shows decreased production of new collagen, as well as increased proteolytic activity resulting in increased collagen degradation. Aging fibroblasts show decreased synthesis of mRNA for type I collagen, which is the major collagen in the skin. Also, in skin cell cultures, aging fibroblasts proliferate at a slower rate than fetal fibroblasts. Therefore, natural aging is at least partly the result of the limited replicative capacity of dermal fibroblasts, as well as the overexpression of proteolytic activity of matrix metalloproteinase-1 (MMP-1, interstitial collagenase).
Additionally, both photoaged and naturally aged human skin have lower procollagen type I mRNA and protein compared to younger skin. The balance between collagen synthesis and collagen breakdown appears to be different in the same individual from photoaged to naturally aged skin. While both show greater MMP activity, it is even higher in the skin aged from ultraviolet (UV) radiation exposure. The precocious elastosis seen in actinically damaged skin is also at least partially responsible for the difference in the appearance of the sun-induced versus age-induced wrinkling. The elastotic degeneration of sun-damaged skin may be due to chronic injury to fibroblasts that results in thicker elastotic fibers with accentuated microfibril dense masses. In the photoaged skin, both mRNA and protein production are reduced when compared to sun-protected skin of the same individual. UV radiation is well known to stimulate MMP-1, which in turn damages and degrades collagen. It is likely that the degraded and damaged collagen has further deleterious effects on dermal fibroblast function after repeated injury. Thus, there is a need for cosmeceuticals capable of either increasing collagen regeneration or preventing its further demise. Peptides and proteins for antiaging purposes were developed with this end in mind.
• Signal peptides
It is well recognized that the ability to stimulate proteins of the extracellular matrix, including collagen and elastin, or to diminish the activity of collagenase, or both, could result in clinical improvement of the wrinkles and lines that are seen in both photoaged and naturally aged skin. Thus, direct stimulation of collagen-producing human fibroblasts and/or downregulation of fibroblast collagenase production are the mechanisms by which a cosmeceutical may clinically improve lines and wrinkles. Bioactive peptides were originally developed as part of wound-healing research on the growth and stimulation of human skin fibroblasts. These same peptides are now being studied for their ability to act as growth factors via activation of protein kinase C, a key enzyme for cell growth and migration. Wound studies of human keratinocyte cultures show a stimulatory effect from topical application of neuropeptides, such as gastrin-releasing peptide. Certain amino acid chains in specific lengths and sequences have been found to stimulate human skin dermal fibroblast growth in vitro. One study of elastin-derived peptides showed that valine-glycine-valine-alanine-proline-glycine (VGVAPG) significantly stimulated human skin fibroblast production, probably mediated through a binding of the peptide to a plasmalemmal receptor of human skin fibroblasts. This same hexapeptide sequence has been found to stimulate human dermal skin fibroblasts, downregulate elastin expression, and to be chemotactic for fibroblasts. Other studies have shown the peptide sequence tyrosine-tyrosine-arginine-alanine-aspartame-aspartame-alanine (YYRADDA) to inhibit procollagen C-proteinase, which cleaves C propeptide from type I procollagen. This could result in decreased collagen breakdown. A specific amino acid sequence, lysine-threonine-threonine-lysine-serine (KTTKS), found on type I procollagen, has been found to stimulate feedback regulation of new collagen synthesis, resulting in an increased production of extracellular matrix proteins. A number of these signal peptides have made the transition from research to practical application.
• Carrier peptides
Another function of peptides in cosmeceuticals is to stabilize and deliver metals such as copper, an important trace element necessary for wound healing and enzymatic processes. There are several ways in which copper, if delivered into the skin, may have anti-skin-aging benefits. Free radicals have recently been implicated in both chronologic and actinic aging because they cause damage to collagen. Superoxide dismutase acts as an important antioxidant and requires copper as a cofactor. In addition, lysyl oxidase, an important enzyme in collagen and elastin production, is also dependent on copper.
It has been postulated that the tripeptide glycyl-L-histidyl-L-lysine (GHK) facilitates copper uptake by cells. Copper spontaneously complexes with this tripeptide, which is found on the alpha II chain of human collagen. Its release from the collagen helix during wounding or inflammation may result in a feedback stimulation of new collagen. The GHK sequence has been found to stimulate collagen synthesis by fibroblasts as well as increasing levels of both MMP-2 and MMP-2 mRNA. It also increases the level of tissue inhibitors of metalloproteinase-1 (TIMP-1) and TIMP-2. As such, it is believed to aid in dermal tissue remodeling. Therefore, the tripeptide alone may be beneficial as a signal peptide. The major effect of this peptide, however, is felt to be the delivery of copper into the skin rather than the GHK peptide as the active ingredient. Laboratory studies on both experimental rat and cultured human fibroblasts have demonstrated stimulation of both type I collagen and glycosaminoglycan synthesis by the copper-peptide complex. In addition, chondroitin sulfate and dermatan sulfate were also enhanced. This type of data has resulted in incorporation of the copper tripeptide complex in facial cosmeceutical creams.
• Neurotransmitter-modulating peptides
The neurotransmitter-modulating peptides were developed as topical simulators of the botulinum neurotoxins. One such peptide—acetyl-glutamyl-glutamyl-methoxyl-glutaminyl-arginyl-arginylamide—has been synthesized and shown to have antiwrinkle activity. This peptide, currently marketed as Argireline®, inhibits neurotransmitter release by interference with the formation or stabilization of the protein complex required to drive calcium-dependent exocytosis when studied with in vitro models. These in vitro studies have resulted in incorporation of this peptide into certain cosmeceutical products. The clinical results of this peptide’s inhibitory effect on neurotransmitter release may raise the threshold for minimal muscle activity, requiring more signal to achieve movement and thus reducing subconscious muscle movement over time. If delivered to targeted facial muscles, there could be a decrease in dynamic facial lines and wrinkles. Several other peptides have been formulated which act on different parts of the neuromuscular junction or affect certain neurotransmitters.
• Peptide mechanisms of action
The recurring theme in peptide studies is that protein subfragments of collagen and elastin, which are liberated during cellular processes, can act as feedback stimulators of their own neosynthesis. They can, in essence, signal or at least mimic the signals that result in synthesis of new proteins of the extracellular matrix. Carrier proteins, by contrast, have the primary function in cosmeceuticals of delivering ionized elements such as copper in the skin where the copper then may have benefits for enhanced collagen production. Lastly, the mechanism of action of neurotransmitter-modulating peptides is to act through various mechanisms in order to inhibit the effect of neurotransmitters at the neuromuscular junction. However, for any type of peptide to be biologically active in vivo, it must be first stabilized in the product and adequately delivered into the viable dermis or musculature where these processes occur. This is especially important for neurotransmitter-modulating peptides, which must penetrate to the level of the neuromuscular junction to have an effect. One way in which this is achieved is through linkage of the amino acid sequence to a lipophilic fatty acid, such as palmitic acid, in order to enhance skin stability and improve skin delivery. Penetration enhancers are very important in cosmeceutical peptide technology, since penetration of these large molecular weight molecules is difficult and efficacy cannot be achieved without entry into the skin.
COSMECEUTICAL PEPTIDE USE
Small chain peptides in specific sequences may be able to imitate some biologic processes to stimulate repair, while other oligopeptides may inhibit processes that accelerate the signs of aging in the skin (Fig. 17.1). The major benefit of signal peptides and carrier peptides is to enhance collagen production without the irritation that is seen with prescription retinoids (Figs 17.2 and 17.3). Cosmeceutical peptides have the advantage of not increasing transepidermal water loss, thus preserving the barrier function that is often compromised with the use of retinoids. The optimal way to incorporate peptides into a skin care protocol is to use them after applying topical retinoids to reduce dehydration and irritation (Fig. 17.4). The peptides, such as signal and carrier peptide products, may be used with retinoids daily to improve tolerance of the retinoid and result in better patient compliance.
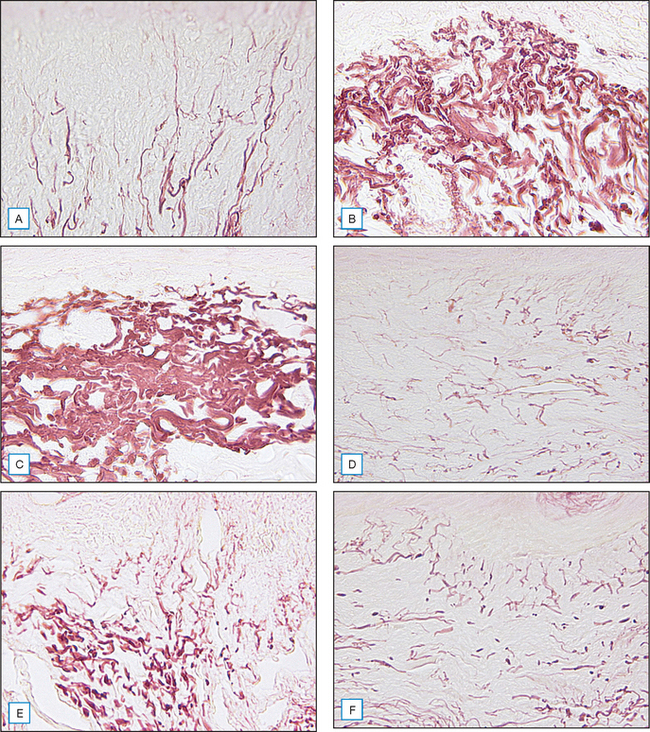
Fig. 17.1 (A–F) Dermal elastin may increase following the use of pentapeptides.
(Courtesy of Karl Lintner)
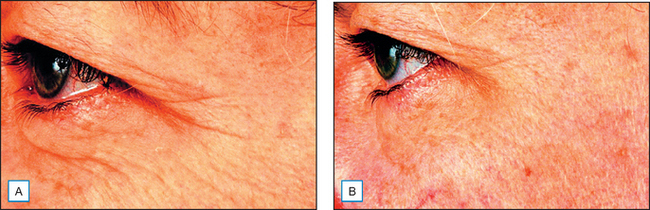
Fig. 17.2 The appearance of periorbital fine lines and wrinkles following 4 months’ use of a pentapeptide: example 1. (A) Before. (B) After.
(Courtesy of Karl Lintner)
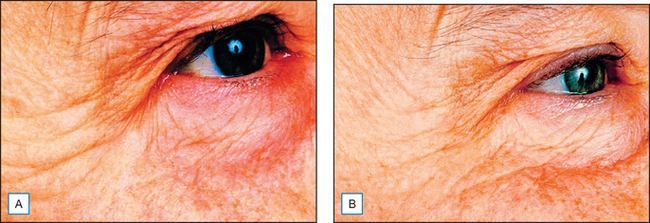
Fig. 17.3 The appearance of periorbital fine lines and wrinkles following 4 months’ use of a pentapeptide: example 2. (A) Before. (B) After.
(Courtesy of Karl Lintner)
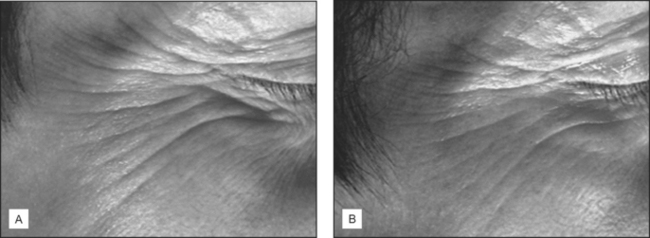
Fig. 17.4 Moisturizers containing pentapeptides may soften lines of facial expression. (A) Before. (B) Four months after.
(Courtesy of Karl Lintner)
Another growing use of peptides is in the reduction of the appearance of cellulite and striae distensae. There are a number of cosmeceutical products currently on the market that have incorporated some of the same peptides marketed for wrinkle reduction. These include the GHK-copper peptide as well as the GQMQRR, VGVAPG, and KTTKS sequences to name a few.
ADVERSE EFFECTS OF PEPTIDES
As with most cosmeceutical preparations, the major concern is one of allergic contact dermatitis. Proteins and peptides are some of the most common allergens. The daily casual use of these products will determine whether or not an allergy is present. Manifestations of allergic contact dermatitis would be an erythematous papular rash with itching. With copper delivery into the skin, there are theoretical concerns of free ions triggering reactions like the Fenton reaction, which results in free radical generation. In general, however, these peptide cosmeceuticals are safe in the currently marketed formulations.
ON THE FOREFRONT
The most recent development of peptide use is in the realm of antimicrobial treatment. Peptides are currently being formulated to simulate the effects of the human β-defensins and cathelicidins. These naturally produced antimicrobial peptides are found in human and animal epithelial keratinocytes and neutrophils, and decreased levels have been noted in patients with atopic dermatitis and susceptibility to skin infection. Their expression is upregulated in response to inflammation and skin injury, and they have been demonstrated to have antibacterial, antiviral, and antifungal activity. Currently Helix BioMedix Inc. is utilizing this new technology to market one such peptide, Oligopeptide-10, in a new acne treatment system. Further research into the effects of topical canthelicidin-simulating peptides needs to be performed, however, since excessive levels of these peptides have also been indicated to have a pathogenic role in the development of rosacea. A recent study by Yamasaki et al found that patients with rosacea have elevated levels of stratum corneum tryptic enzyme and cathelicidins, and this ultimately leads to increased abnormal inflammatory peptides in the skin and the symptoms associated with the disease.
SUMMARY
Cosmeceutical peptides are currently in the marketplace and their success is reflected in commercial sales. Unfortunately there are no double-blinded, placebo-controlled studies to assess their proposed effects on the skin. Limited, mostly company-sponsored studies, both in vitro and in vivo, are often used to determine the benefit of ingredients in cosmeceutical products. An additional limitation is the frequent omission of the concentration of the cosmeceutical peptide that is incorporated into the final cosmetic product. Lack of peptide concentration details on cosmetic products, which have incorporated the peptide into their commercial formulas, severely reduces one’s ability to draw a conclusion regarding the product’s likely usefulness. Peptides have a growing body of knowledge regarding their utility as cell regulators, but further research is required to determine their long-term effects as cosmeceuticals in the antiaging arena. Table 17.1 lists some of the most commonly encountered cosmeceutical peptides and their proposed effects in the skin.
Blanes-Mira C, Clemente J, Jodas G, et al. A synthetic hexapeptide (argireline) with anti-wrinkle activity. International Journal of Cosmetic Science. 2002;24:303–310.
Blanes-Mira C, Merino JM, Valera E, et al. Small peptides patterned after the N-terminus domain of SNAP-25 inhibit SNARE complex assembly and regulated exocytosis. Journal of Neurochemistry. 2004;88:124–135.
Braff M, Zaiou M, Fierer J, et al. Keratinocyte production of canthelicidin provides direct activity against bacterial skin pathogens. Infection and Immunity. 2005;73:6771–6781.
Chung JH, Seo JY, Choi HR, et al. Modulation of skin collagen metabolism in aged and photoaged human skin in vivo. Journal of Investigative Dermatology. 2001;117:1218–1224.
Katayama K, Armendariz-Borunda J, Raghow R, et al. A pentapeptide from type I procollagen promotes extracellular matrix production. Journal of Biological Chemistry. 1993;268:9941–9944.
Khorramizadeh MR, Tredget EE, Telasky C, et al. Aging differentially modulates the expression of collagen and collagenase in dermal fibroblasts. Molecular and Cellular Biochemistry. 1999;194:99-108.
Lintner K. Promoting production in the extracellular matrix without compromising barrier. Cutis. 2002;70(6 suppl):13–16.
López-García B, Lee P, Gallo R. Expression and potential function of canthelicidin antimicrobial peptides in dermatophytosis and tinea versicolor. Journal of Antimicrobial Chemotherapy. 2006;57:877–882.
Robinson LR, Fitzgerald NC, Doughty DG, et al. Topical palmitoyl pentapeptide provides improvement in photoaged human facial skin. International Journal of Cosmetic Science. 2005;27:155.
Simeon A, Emonard H, Hornebeck W, et al. The tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+ stimulates matrix metalloproteinase-2 expression by fibroblast cultures. Life Sciences. 2000;67:2257–2265.
Simeon A, Wegrowski Y, Bontemps J, et al. Expression of glycosaminoglycan and small proteoglycans in wounds: modulation by the tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+. Journal of Investigative Dermatology. 2000;115:962–968.
Varoni J, Spearman D, Perone P, et al. Inhibition of type I procollagen synthesis by damaged collagen in photoaged skin and by collagenase-degraded collagen in vitro. American Journal of Pathology. 2001;158:931–942.
Yamasaki K, Di Nardo A, Bardan A, et al. Increased serine protease activity and canthelicidin promotes skin inflammation in rosacea. Nature Medicine. 2007;13:975–980.