12 Peptic ulcer disease
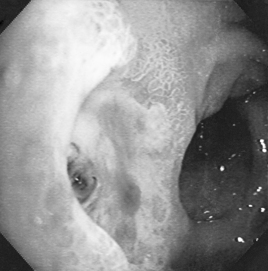
The term ‘peptic ulcer’ describes a condition in which there is a discontinuity in the entire thickness of the gastric or duodenal mucosa that persists as a result of acid and pepsin in the gastric juice (Fig. 12.1). Oesophageal ulceration due to acid reflux is generally classified under GORD. This definition excludes carcinoma and lymphoma, which may also cause gastric ulceration, and also excludes other rare causes of gastric and duodenal ulceration such as Crohn’s disease, viral infections and amyloidosis. About 10% of the population in developed countries is likely to be affected at some time by peptic ulcer, with the prevalence for active ulcer disease being about 1% at any particular point in time.
Epidemiology
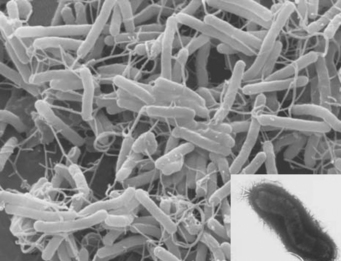
Infection by H. pylori, a spiral bacterium of the stomach, remains an important epidemiological factor in causing peptic ulcer (Fig. 12.2). Most H. pylori infections are acquired by oral–oral and oral–faecal transmission. The most important risk factors for H. pylori infection are low social class, overcrowding and home environment during childhood, for example, bed sharing. Transmission may occur within a family, a fact demonstrated by the finding that family members, especially spouses, may have the same strain of H. pylori. H. pylori seropositivity increases with age as colonisation persists for the lifetime of the host. Subjects who become infected with H. pylori when young are more likely to develop chronic or atrophic gastritis with reduced acid secretion that may protect them from developing duodenal ulcer. However, it may promote development of gastric ulcer as well as gastric cancer. Duodenal ulcer seems to develop in those who are infected with H. pylori at the end of childhood or later. Historically, developing countries have a higher ratio of duodenal ulcer to gastric ulcer but as rates of H. pylori infection decline with improvements in hygiene and rates of gastric ulcer increase with the use of ulcerogenic drugs, this ratio of duodenal to gastric ulcer is declining. The prevalence of H. pylori still tends to be higher in the Asian adult population in whom a lower parietal cell mass has been found. These factors together with slower metabolism may explain the greater efficacy of PPIs in Asian populations. There may be other genetic, environmental or cultural factors influencing peptic ulcer disease.
Pathogenesis
Helicobacter pylori
This organism is a Gram-negative microaerophilic bacterium found primarily in the gastric antrum of the human stomach (see Fig. 12.2). Ninety-five percent or more of duodenal ulcers and 80–85% of gastric ulcers are associated with H. pylori. The bacterium is located in the antrum and the acid-secreting microenvironment of the corpus of the stomach is less hospitable to the bacterium. In the developed world, reinfection rates are low, about 0.3–1.0% per year, whereas in the developing world reinfection rates are higher, approximately 20–30%. Ulcerogenic strains of H. pylori, ulcer-prone hosts, age of infection and interaction with other ulcerogenic factors such as NSAIDs determine peptic ulcer development following H. pylori infection. The contribution of H. pylori infection to the risk of ulcers in NSAID users is not clear but there appears to be an additive effect. The risk of peptic ulcer in long-term NSAID users is greater in those who test positive for H. pylori and eradication of H. pylori in these patients prior to commencing NSAID treatment has been shown to reduce the risk of H. pylori NSAID-associated peptic ulcer.
Non-steroidal anti-inflammatory drugs
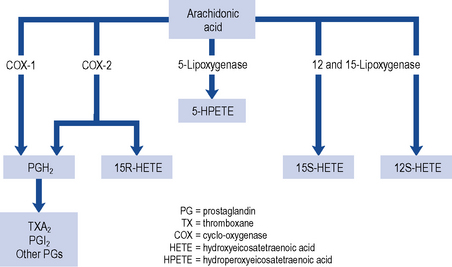
Three patterns of mucosal damage are caused by NSAIDs. These include superficial erosions and haemorrhages, silent ulcers detected at endoscopy and ulcers causing clinical symptoms and complications. Weak acid NSAIDs, such as acetylsalicylic acid, are concentrated from the acidic gastric juice into mucosal cells, and may produce acute superficial erosions via inhibition of COX and by mediating the adherence of leucocytes to mucosal endothelial cells. Enteric coating may prevent this superficial damage but does not demonstrate any clinical benefit in terms of reduction of gastro-intestinal bleeding or ulceration (Bhatt et al., 2008). The major systemic action of NSAIDs that contributes to the formation of ulcers is the reduction of mucosal prostaglandin production. All NSAIDs share the ability to inhibit COX (Fig. 12.3). The presence of NSAID-induced ulcers does not correlate with abdominal pain and NSAIDs themselves often mask ulcer pain. Approximately 20% of patients taking NSAIDs experience symptoms of dyspepsia but symptoms correlate poorly with the presence of mucosal damage. Ulcers and ulcer complications occur in approximately 4% of NSAID users every year. Patients taking NSAIDs have a four-fold increase in risk of ulcer complications compared with non-users. The risk of ulcer bleeding in low-dose aspirin users is two- to three-fold and there may be differences in risk factors. For example, the risk with aspirin is less influenced by age than the risk associated with NSAIDs (McQuaid and Laine, 2006) and H. pylori may have greater influence on the risk of bleeding with low-dose aspirin than with NSAIDs (Lanza et al., 2009).
Each year, in the UK population over the age of 60 years, there are ∼︀3500 hospitalisations and over 400 deaths associated with NSAIDs. The risk of ulcer complications (Box 12.1) is progressive depending upon the number of risk factors present (Lanza et al., 2009). The most important risk factors are a history of ulcer complications and advancing age, particularly over 75 years. Ulcers have been found to be more common in patients who have taken NSAIDs for less than 3 months, with the highest risk observed during the first month of treatment. The risk increases with higher doses of NSAID but mucosal damage occurs with even very low doses of NSAIDs, particularly aspirin. Corticosteroids alone are an insignificant ulcer risk, but potentiate the ulcer risk when added to NSAIDs, particularly in daily doses of at least 10 mg prednisolone (Lanza et al., 2009).
Selective cyclo-oxygenase-2 inhibitors
The gastro-intestinal side effects of conventional NSAIDs are mediated through the inhibition of COX-1 (see Fig. 12.3). COX-1 stimulates synthesis of homeostatic prostaglandins while COX-2 is predominantly induced in response to inflammation. Selective COX-2 inhibitors tend not to reduce the mucosal production of protective prostaglandins to the same extent as NSAIDs. COX-2 inhibitors are, therefore, considered to be safer than non-selective NSAIDs in patients at high risk of developing gastro-intestinal mucosal damage. Although studies have confirmed the reduction of endoscopic and symptomatic ulcers (Hooper et al., 2004), an increase in cardiovascular risk, including heart attack and stroke, has resulted in the withdrawal of some COX-2 inhibitors from the market. Additional contraindications are now in place for those COX-2 inhibitors that remain on the market. Amongst the new contraindications is the recommendation that they should not be taken by patients with established heart or cerebrovascular disease, or taken in combination with low-dose aspirin as this negates any beneficial gastro-intestinal protective effects. The need for and choice of anti-inflammatory agent should therefore take into account gastro-intestinal, cardiovascular and other risks such as potential cardio-renal effects. For all agents, the lowest effective dose should be used for the shortest duration.
Nitric oxide-releasing NSAIDs
Nitric oxide (NO)-releasing NSAIDs are being investigated to see if the gastric mucosa protection associated with nitric oxide prevents ulceration when prostaglandins are inhibited by NSAIDs (Fiorucci et al., 2007). Nitric oxide is coupled to the NSAID via an ester, resulting in prolonged release of nitric oxide. Nitric oxide itself has anti-inflammatory effects adding to the potency of the NSAID. Animal studies suggest NO-releasing agents, such as naproxcinod, have minimum cardiovascular and gastro-intestinal toxicity.
Patient assessment
Presenting symptoms of dyspepsia require careful assessment to judge the risk of serious disease or to provide appropriate symptomatic treatment. Symptom subgroups such as ulcer, reflux and dysmotility type may be useful in identifying the predominant symptom subgroup to which a patient belongs. Many patients have symptoms which fit more than one subgroup (Box 12.2). Many patients seek reassurance, lifestyle advice and symptomatic treatment with a single consultation, others have chronic symptoms. In some cases, medications may be the cause of dyspepsia and should be reviewed (Box 12.3).
Patients at any age who present with alarm features (Box 12.4) should be referred for endoscopic investigation. These groups of patients are at a higher risk of underlying serious disease such as cancer, ulcers or severe oesophagitis. Referral is also recommended for patients over the age of 55 if symptoms are unexplained or persistent despite initial management (NICE, 2004; SIGN, 2003). Malignant disease is rare in young people and in those without alarm features.
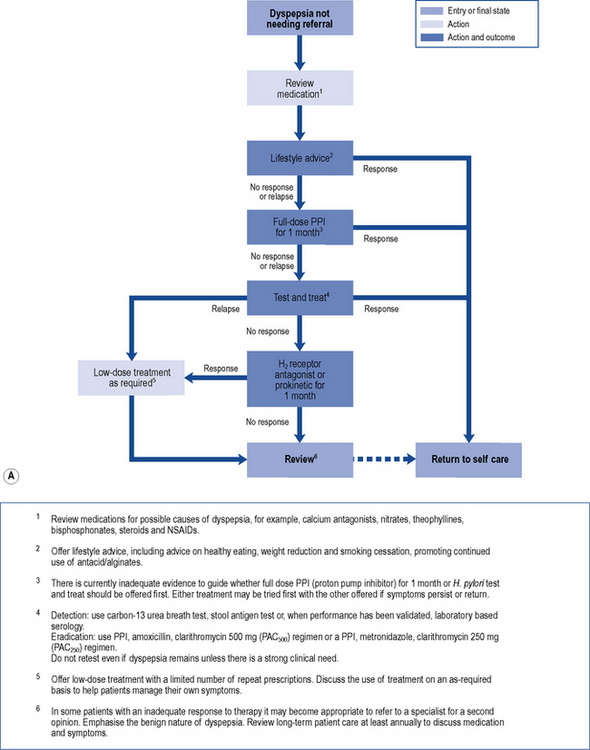
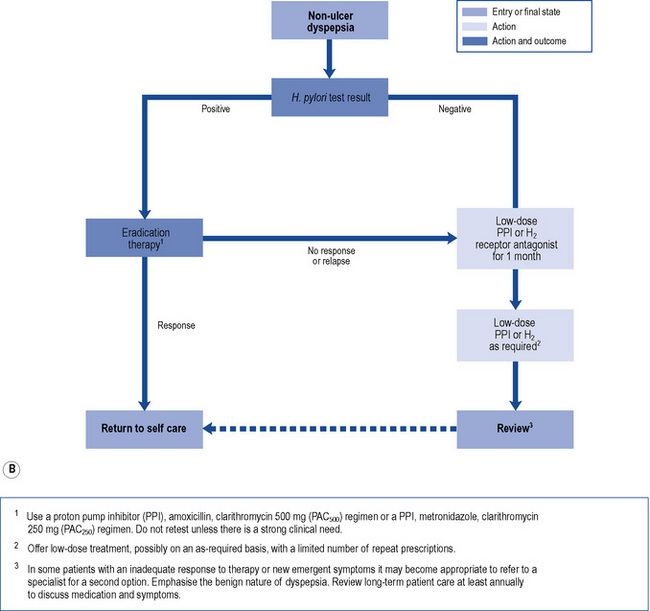
In those patients who do not have reflux-like dyspepsia, testing for the presence of H. pylori is recommended. Eradication treatment should be prescribed for those who test positive and empirical acid suppression for those who test negative. The small proportion of patients with symptoms due to ulcers should be cured. Overall, in functional dyspepsia, symptom control is poor but a small and significant benefit of eradication treatment has been shown. Acid suppression is only of benefit in a small proportion of patients with functional dyspepsia. There is no evidence to support other pharmacological therapies and non-pharmacological strategies may have a future role in functional dyspepsia. Patients should be reassured that the condition is common and not serious. National guidelines (NICE, 2004) provide algorithms to guide practitioners through the management of patients presenting with dyspepsia (Fig. 12.4A and B).
Investigations
Endoscopy
Endoscopy is generally the investigation of choice for diagnosing peptic ulcer, and the procedure is sensitive, specific and safe. However, it is also invasive and expensive. Routine endoscopy in patients presenting with dyspepsia without alarm features (see Box 12.4) is not necessary. Endoscopic investigation should be undertaken in patients with alarm features and in those patients over 55 years who present with unexplained or persistent symptoms of dyspepsia. Biopsies may be taken to exclude malignancy and uncommon lesions such as Crohn’s disease.
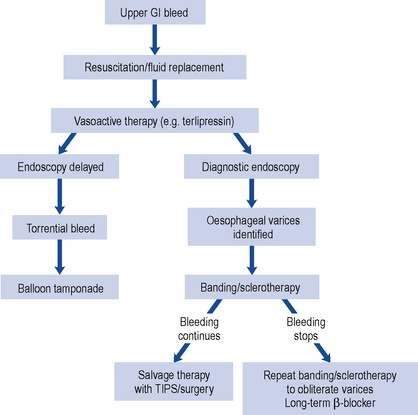
Patients with upper gastro-intestinal bleeding have traditionally undergone endoscopy whether as an emergency or on the next available list. Most patients do not require endoscopy and in those at low risk, endoscopy and admission to hospital can be avoided by application of a scoring system. The most widely used is the Rockall risk scoring system which includes endoscopic findings to predict poor outcome. Pre-endoscopic scoring systems are available (Stanley et al., 2009) such as the abbreviated Rockall score or the Glasgow–Blatchford score (GBS) which identify low-risk patients who can be managed safely without endoscopy or admission to hospital.
H. pylori detection
There are several methods of detecting H. pylori infection. They include non-invasive tests such as serological tests to detect antibodies, [13C] urea breath tests and stool antigen tests. Urea breath tests have a sensitivity and specificity over 90% and are accurate for both initial diagnosis and confirmation of eradication. The breath test is based on the principle that urease activity in the stomach of infected individuals hydrolyses urea to form ammonia and carbon dioxide. The test contains carbon-labelled urea which, when hydrolysed, results in production of labelled carbon dioxide which appears in the patient’s breath. The stool antigen test uses an enzyme immunoassay to detect H. pylori antigen in stool. This test also has a sensitivity and specificity over 90% and can be used in the initial diagnosis and also to confirm eradication. However, the breath test is preferable and more convenient. Serological tests are based on the detection of anti-H. pylori IgG antibodies but are not able to distinguish between active or previous exposure to infection. Near patient serology tests are not recommended (Malfertheiner et al., 2007) as they are inaccurate.
Treatment
Complications of peptic ulcer disease
Bleeding peptic ulcer
A number of pharmacological agents have been used for endoscopic injection therapy such as 1:10,000 adrenaline (epinephrine), human thrombin and fibrin glue. Mechanical endoscopic treatment options include thermocoagulation using a heater probe or endoscopic clipping. Combination therapies are superior to monotherapy and a combination of adrenaline 1:10,000 with either thermal or mechanical treatment is recommended (SIGN, 2008; Barkun et al., 2010). Need for surgery, re-bleeding rates and mortality are reduced but bleeding recurs in about 10% of patients and can cause death. Patients with uncontrolled bleeding should receive repeat endoscopic treatment, arterial embolisation or surgery. The risk of recurrent bleeding following endoscopic therapy is reduced by increasing intragastric pH during the first 3 days after the initial bleed and eradication of H. pylori. Biopsies taken at the time of endoscopy are used to detect H. pylori, or the urea breath test can be used once oral intake is established and H. pylori eradication therapy is indicated in those who test positive. Successful eradication of H. pylori reduces the rate of re-bleeding to a greater extent than antisecretory non-eradicating therapy (Gisbert et al., 2004). Following successful H. pylori eradication and healing of the ulcer, there is no need to continue maintenance antisecretory therapy beyond 4 weeks unless required for prophylaxis of ulcer complications in those continuing to take aspirin or NSAIDs (SIGN, 2008).
Acid suppression reduces the re-bleeding rate and should be given to those patients at high risk of re-bleeding following endoscopic haemostatic therapy. The rationale for this is based on the fact that gastric acid inhibits clot formation and if intragastric pH is maintained above 6 during the first 3 days after the initial bleed, there is opportunity for clot stabilisation and haemostasis. Meta-analysis suggests PPIs significantly reduce re-bleeding rates compared with H2-receptor antagonists and are the preferred choice of treatment (Leontiadis et al., 2006). In similar dosage regimens, there is no data to suggest any PPI is more efficacious than another. The optimal dose and route of PPI is unknown in this indication, although reduction in mortality is observed in high-risk patients when high dose PPI therapy is given (e.g. 80 mg bolus omeprazole, pantoprazole or esomeprazole followed by 8 mg/h for 72 h) following endoscopic haemostasis (Leontiadis et al., 2007; SIGN, 2008; Barkun et al., 2010).
The use of intravenous PPI therapy before endoscopy in patients with upper gastro-intestinal bleeding does not affect clinical outcome such as re-bleeding, need for surgery or mortality (Dorward et al., 2006). However, this may reduce the need for endoscopic therapy (Lau et al., 2007) as demonstrated in Asian patients in whom PPIs are more effective. Its benefits are not clear and it is not possible to identify patients with a greater likelihood of being at high risk. Therefore, the use of PPIs is not recommended prior to diagnosis by endoscopy (SIGN, 2008) but may be beneficial if early endoscopy is delayed (Barkun et al., 2010).
In those patients at low risk for re-bleeding and in whom endoscopic therapy is not indicated, usual therapeutic doses of oral PPI are given for 4 weeks to heal the ulcer. Aspirin or NSAIDs should be avoided but if strongly indicated, these patients are given concomitant PPI therapy following successful eradication of H. pylori. The effect of H. pylori eradication on the risk of recurrent ulcer bleeding is greater in patients taking low-dose aspirin than in those taking NSAIDs (Chan et al., 2001). A possible explanation (Lanza et al., 2009) for this might be that aspirin provokes bleeding in H. pylori ulcers and after healing, aspirin is less likely to cause ulceration.
In patients for whom there is a clear indication to continue aspirin therapy, addition of a PPI is of benefit in the prevention of recurrent bleeding in aspirin users (Lai et al., 2002). Clopidogrel alone is not a safer alternative than this combination in terms of prevention of recurrent ulcer bleeding. Cardiovascular and gastro-intestinal risks must be taken into consideration when deciding how long aspirin should be discontinued after a gastro-intestinal bleed. In some cases, low-dose aspirin can be restarted with concurrent PPI treatment within 7 days (Barkun et al., 2010). When the combination of aspirin and clopidogrel is indicated, concomitant PPI therapy is recommended in patients at high risk of gastro-intestinal complications despite the potential drug interaction between PPIs and clopidogrel. An increased risk of myocardial infarction has been observed with the combination of clopidogrel and PPIs (MHRA, 2009). Causality is unclear but it is suggested that through competitive enzyme inhibition, PPIs metabolised by CYP2C19 reduce the conversion of clopidogrel to its active metabolite. Concomitant use is discouraged but if necessary, separation of dosage timing is recommended (Laine and Hennekens, 2010).
Stress ulcers
Severe physiological stress such as head injury, spinal cord injury, burns, multiple trauma or sepsis may induce superficial mucosal erosions or gastroduodenal ulcerations. These may lead to haemorrhage or perforation. Mechanical ventilation and the presence of coagulopathies place patients at particular risk of stress-related mucosal bleeding and may warrant prophylactic treatment (Quenot et al., 2009). Diminished blood flow to the gastric mucosa, decreased cell renewal, diminished prostaglandin production and, occasionally, acid hypersecretion are involved in causing stress ulceration. Intravenous acid-suppression therapy, histamine H2-receptor antagonists and PPIs, and nasogastric tube administration of sucralfate (4–6 g daily in divided doses) have been used to prevent stress ulceration in the intensive care unit until the patient tolerates enteral feeding. The most commonly used regimen is intravenous ranitidine 50 mg every 8 h reducing to 25 mg in severe renal impairment. Current evidence does not support routine use of prophylaxis and reports of complications associated with bacterial overgrowth in the gastro-intestinal tract in patients receiving acid suppressants should limit use only to those at high risk. Complications include association with hospital acquired Clostridium difficile diarrhoea (Dial et al., 2004) and pneumonia (Herzig et al., 2009).
Uncomplicated peptic ulcer disease
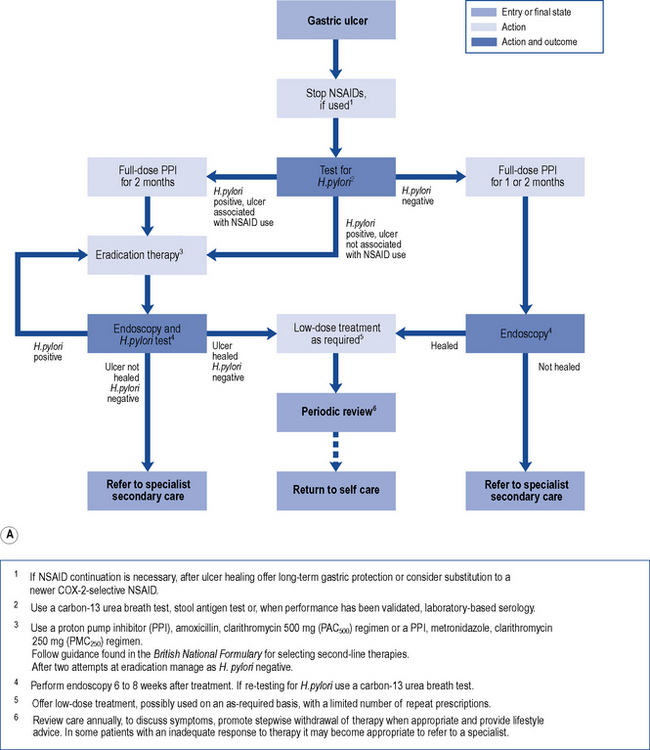
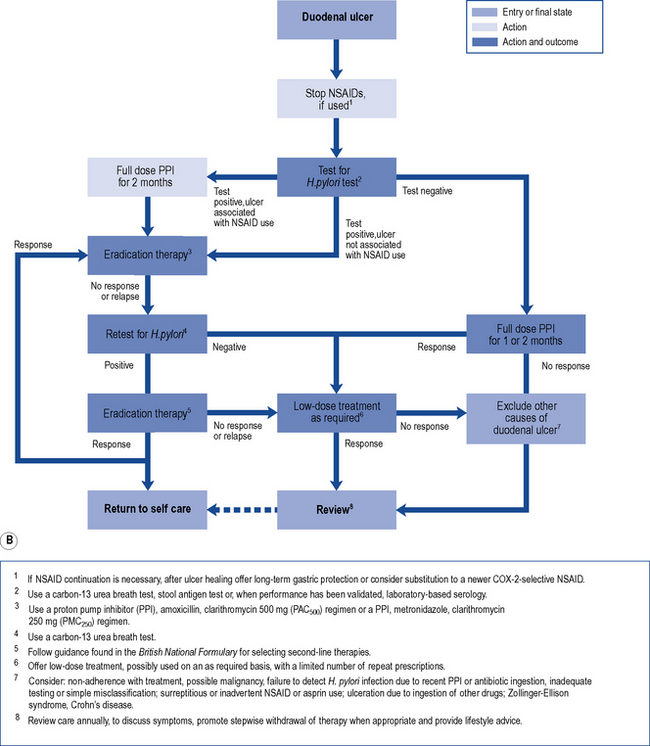
Treatment of endoscopically proven uncomplicated peptic ulcer disease has changed dramatically in recent years (Fig. 12.5A and B). Curing of H. pylori infection and discontinuation of NSAIDs are key elements for the successful management of peptic ulcer disease.
H. pylori eradication
It is known that H. pylori infection is associated with over 90% of duodenal ulcers and 80% of gastric ulcers. Cure of this infection with antibiotic therapy and simultaneous treatment with conventional ulcer-healing drugs facilitates symptom relief and healing of the ulcer and reduces the ulcer relapse rate. Antibiotics alone, or acid-suppressing agents alone, do not eradicate H. pylori. Both therapies act synergistically as growth of the organism occurs at elevated pH and antibiotic efficacy is enhanced during growth. Additionally, increasing intragastric pH may enhance antibiotic absorption. Recent studies limited to one country (Italy) suggest that sequential antibiotic treatment may be advantageous in overcoming emerging antibiotic resistance but the complexity of sequential regimens has the potential to affect adherence (Jafri et al., 2008)
High eradication rates are achieved by a short course of triple therapy consisting of a PPI, clarithromycin and amoxicillin or metronidazole in a twice-daily simultaneous regimen. European guidelines recommend 1 week of therapy, whereas the US guidelines recommend 10–14 days of therapy and achieve 7–9% better eradication rates (Malfertheiner et al., 2007).
A lower dose of clarithromycin (250 mg twice daily) is effective and recommended when combined with metronidazole (NICE, 2004). However, some prescribers prefer to recommend 500 mg twice daily to achieve consistency and avoid prescribing errors.
If eradication is successful, uncomplicated active peptic ulcers heal without the need to continue ulcer-healing drugs beyond the duration of eradication therapy (Gisbert et al., 2004). Patients with persistent symptoms after eradication therapy should have their H. pylori status rechecked. This should be carried out no sooner than 4 weeks after discontinuation of therapy to avoid false-negative results due to suppression rather than eradication of the organism. If the patient is H. pylori positive, an alternative eradication regimen should be given. If eradication was successful but symptoms persist, gastro-oesophageal reflux or other causes of dyspepsia should be considered.
Treatment of NSAID-associated ulcers
NSAID-associated ulcers may be H. pylori positive. Although the presence of H. pylori may enhance the efficacy of acid suppression, eradication is generally recommended in infected patients with NSAID-associated ulcers as it is difficult to differentiate between H. Pylori or NSAID as the cause of the ulcer (Malfertheiner et al., 2009). If NSAIDs are discontinued, most uncomplicated ulcers heal using standard doses of a PPI, H2-receptor antagonist, misoprostol or sucralfate. Healing is impaired if NSAID use is continued. Studies have demonstrated conflicting results, in terms of the comparative healing rates between PPIs and H2-receptor antagonists, in this situation (Yeomans et al., 2006; Goldstein et al., 2007). PPIs demonstrate higher healing rates at 4 weeks but similar healing rates to H2-receptor antagonists at 8 weeks. There is no evidence that high-dose PPI is better than treatment with the standard dose. Although effective, misoprostol use is limited by treatment-related adverse events.
Prophylaxis of NSAID ulceration
NSAIDs should be avoided in patients who are at risk of gastro-intestinal toxicity (see Box 12.1). However, some patients with chronic rheumatological conditions may require long-term NSAID treatment, in which case the lowest effective dose should be used. Dyspepsia is not a risk factor for ulcer complications but in those patients at high risk of ulcer complication, the NSAID should be stopped and investigation undertaken if dyspepsia develops.
Data suggests that H. pylori increases, has no effect on or decreases ulcer risk in NSAID users (Malfertheiner et al., 2007, 2009). The value of eradication of H. pylori in chronic NSAID users is, therefore, unclear but there may be some benefit in screening and eradicating H. pylori in patients about to start NSAIDs. The benefit is less apparent in those patients at low risk of peptic ulcer. In patients with a history of bleeding or non-bleeding ulcer, guidelines recommend screening for and eradicating H. pylori before starting low-dose aspirin (Bhatt et al., 2008). When using NSAIDs in patients with a previous bleeding ulcer, PPI maintenance therapy is more effective secondary prophylaxis than H. pylori eradication alone, but a combination of both treatments is additive.
Treatment options for ulcer prophylaxis in patients at risk of peptic ulcer but who require NSAIDs, include co-therapy with acid-suppressing agents or a synthetic prostaglandin analogue, or substitution of a selective COX-2 inhibitor for a non-selective NSAID (Hooper et al., 2004). Comparison of study outcomes requires interpretation of whether ulcers are detected symptomatically or by endoscopy. The prostaglandin analogue, misoprostol at a dose of 800 µcg daily is effective at reducing NSAID-associated ulcer complications and symptomatic ulcers. Adverse effects, primarily diarrhoea, abdominal pain and nausea, limit its use as lower doses are less effective. PPIs are effective at reducing endoscopically diagnosed ulcers and dyspepsia symptoms but the effect on symptomatic ulcers is unclear. Studies have not demonstrated any advantage in using higher than standard doses of PPIs to reduce risk of ulcers. Standard doses of H2-receptor antagonists are effective at reducing the risk of endoscopic duodenal ulcers. However, reduction in the risk of gastric ulcers requires double this dose. Gastroprotective agents licensed for prophylaxis of NSAID ulceration are listed in Table 12.1.
Table 12.1 Drugs for prophylaxis for NSAID-induced ulceration
| Drug | Licensed indication | Prophylaxis dose |
|---|---|---|
| Omeprazole | Prophylaxis of further DU or GU | 20 mg every day |
| Esomeprazole | Prophylaxis of DU or GU | 20 mg every day |
| Lansoprazole | Prophylaxis of DU or GU | 15–30 mg every day |
| Pantoprazole | Prophylaxis of DU or GU | 20 mg daily |
| Misoprostol | Prophylaxis of DU or GU | 200 µcg 2–4 times a day |
| Ranitidine | Prophylaxis of DU | 150 mg twice a day |
| Ranitidine | Prophylaxis of DU (unlicensed) | 300 mg twice day |
DU, duodenal ulcer; GU, gastric ulcer.
In low-dose aspirin users, standard-dose PPIs are more effective than high dose H2-receptor antagonists in preventing recurrent ulcer bleeding following ulcer healing and eradication of H. pylori (Ng et al., 2010). However, in those patients who do not have a history of peptic ulcer bleeding, high-dose H2-receptor antagonists might be an alternative to PPIs (Taha et al., 2009).
Given the contraindications to selective COX-2 inhibitors, their use is limited and further studies are required to clarify their place in minimising risk of ulcers in both primary and secondary prophylaxis. In patients with no history of peptic ulcer bleeding but with risk factors, a combination of COX-2 inhibitor with a PPI was similar in efficacy to a combination of non-selective NSAID with a PPI (Scheiman et al., 2006). In patients with a history of ulcer bleeding, a combination of selective COX-2 inhibitor with a PPI reduced recurrent ulcer bleeding compared to COX-2 inhibitor alone (Chan et al., 2007). This was not compared to a combination of a non-selective NSAID and a PPI. Although an earlier study suggested COX-2 inhibitors alone offered similar protection to that offered by a combination of non-selective NSAID with PPI (Lai et al., 2005), results suggest that in high-risk patients with a history of gastro-intestinal bleeding in whom an NSAID is indicated where alternative analgesic therapies have failed and in whom there are no contraindications to selective COX-2 inhibitors, a combination of PPI with a selective COX-2 inhibitor may be the safest strategy.
Gastro-oesophageal reflux disease
GORD is the term used to describe any symptomatic clinical condition or histopathological alteration resulting from episodes of reflux of acid, pepsin and, occasionally, bile into the oesophagus from the stomach (Moayyedi and Talley, 2006). Heartburn is the characteristic symptom, and the patient may also complain of acid regurgitation and dysphagia. Complications include oesophageal stricture, oesophageal ulceration and formation of specialised columnar-lined oesophagus at the gastro-oesophageal junction known as Barrett’s oesophagus (Shaheen and Richter, 2009). The mechanism of acid reflux is multifactorial and involves transient lower oesophageal sphincter relaxations, reduced tone of the lower oesophageal sphincter, hiatus hernia and abnormal oesophageal acid clearance. The severity of inflammation of the oesophageal mucosa is described as categories of oesophagitis (Los Angeles A–D). However, approximately two-thirds of patients with GORD have normal mucosa on endoscopy. Hypersensitivity to normal acid exposure may be the cause of symptoms in this group of patients. Progression from non-erosive reflux disease to erosive oesophagitis and Barrett’s oesophagus is rare.
Management of GORD focuses on symptom control rather than endoscopic findings, and therefore careful symptom evaluation is required (Box 12.5). Patients with alarm features or those who fail to respond to medical treatment should be referred for endoscopic investigation. H. pylori eradication is not recommended in the management of GORD (Malfertheiner et al., 2007).
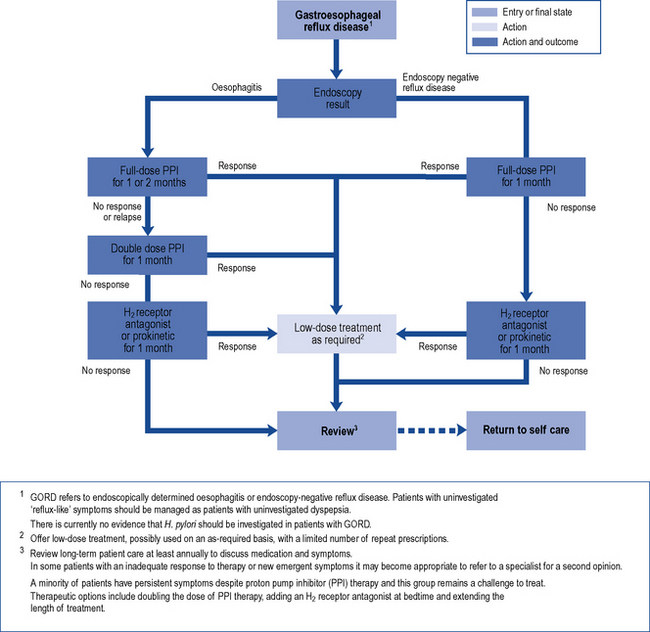
Strategies for initial treatment include lifestyle measures such as weight loss and smoking cessation in combination with antacids. These measures may be effective in patients with no significant impairment of quality of life. When quality of life is impaired, acid-suppression therapy is the basis of effective treatment. A course of standard-dose PPI therapy is most effective for symptom relief, healing of oesophagitis and maintenance of remission in patients with GORD (Fig. 12.6). Compared with erosive oesophagitis, there is a diminished response to acid suppression in non-erosive reflux disease but PPIs remain the most effective agents. Most patients respond after 4 weeks’ treatment and after initial control of symptoms, therapy can be withdrawn. If symptoms return, intermittent courses can be given or, alternatively, on-demand single doses can be taken immediately as symptoms occur. Patients who relapse frequently may require continuous maintenance therapy using the lowest dose of acid suppression which provides effective symptom relief.
Ulcer-healing drugs
Proton pump inhibitors
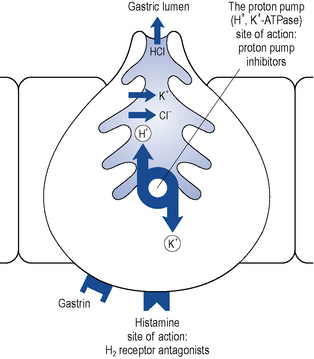
The PPIs are all benzimidazole derivatives that control gastric acid secretion by inhibition of gastric H+, K+-ATPase, the enzyme responsible for the final step in gastric acid secretion from the parietal cell (Fig. 12.7).
The PPIs are inactive prodrugs that are carried in the bloodstream to the parietal cells in the gastric mucosa. The prodrugs readily cross the parietal cell membrane into the cytosol. These drugs are weak bases and therefore have a high affinity for acidic environments. They diffuse across the secretory membrane of the parietal cell into the extracellular secretory canaliculus, the site of the active proton pump (see Fig. 12.7). Under these acidic conditions the prodrugs are converted to their active form, which irreversibly binds the proton pump, inhibiting acid secretion. Since the ‘active principle’ forms at a low pH it concentrates selectively in the acidic environment of the proton pump and results in extremely effective inhibition of acid secretion. The different PPIs (omeprazole, esomeprazole, lansoprazole, pantoprazole and rabeprazole) bind to different sites on the proton pump, which may explain their differences in potency on a milligram per milligram basis.
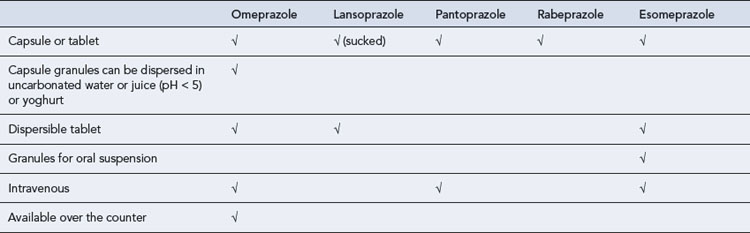
PPIs require an enteric coating to protect them from degradation in the acidic environment of the stomach. This delays absorption and a maximum plasma concentration is reached after 2–3 h. Different formulations have been developed for patients with swallowing difficulties but these still rely upon some form of enteric coating (Table 12.2). New immediate-release formulations are under development which may overcome problems with enteric-coated granules blocking enteral feeding tubes.
Adverse drug reactions
Experience suggests that PPIs are a remarkably safe group of drugs. The most commonly reported side effects are diarrhoea, headaches, abdominal pain, nausea, fatigue and dizziness which resolve on drug discontinuation (Table 12.3). Possible mechanisms for diarrhoea include bacterial overgrowth, changes in intestinal pH and bile salt abnormalities. Diarrhoea is most commonly associated with lansoprazole, particularly in the elderly. Some cases of persistent chronic watery diarrhoea associated with lansoprazole have been diagnosed as microscopic colitis. This may be explained by the unique ability of lansoprazole to inhibit colonic proton pumps which may have an effect on colonic secretion and pH. Apart from the association between lansoprazole and diarrhoea, the incidence of adverse reactions is similar among the drugs in this group.
| Proton pump inhibitors | H2-receptor antagonists | Sucralfate |
|---|---|---|
| Diarrhoea | Diarrhoea | Constipation |
| Headache | Headache | |
| Abdominal pain | Abdominal pain | |
| Nausea | Confusion | |
| Fatigue | ||
| Dizziness |
Drug interactions
H2-receptor antagonists
Adverse drug reactions
H2-receptor antagonists are a remarkably safe group of drugs with a lower risk of side effects than PPIs. The risk of any adverse reaction is below 3% and serious adverse reactions account for less than 1%. Diarrhoea and headache are the most common and occasionally mental confusion and rashes have been reported (see Table 12.3). Hepatotoxicity is a rare adverse effect. Cimetidine, due to its antiandrogenic effects, has been associated with gynaecomastia and impotence when used in high doses.
Sucralfate
Adverse drug reactions
Constipation appears to be the most common problem with sucralfate, and this is thought to be related to the aluminium content (see Table 12.3). About 3–5% of a dose is absorbed, and therefore there is a risk of aluminium toxicity with long-term treatment. This risk is correspondingly greater in patients with renal impairment. Caution is required to avoid oesophageal bezoar formation around a nasogastric tube in patients managed in the intensive care unit.
Patient care
Patient monitoring
Some common therapeutic problems in the treatment of peptic ulcer disease are summarised in Table 12.4.
Table 12.4 Common therapeutic problems in peptic ulcer disease
| Comments | |
|---|---|
| Ulcer-like symptoms of dyspepsia are not specific for peptic ulcer disease and are often present in functional (non-ulcer) dyspepsia | Predominant heartburn differentiates GORD from dyspepsia. Patients with GORD are likely to respond to antisecretory therapy |
| In uncomplicated patients with ulcer-like symptoms of dyspepsia, there is controversy about whether a 1-month course of acid suppression or test and for H. pylori should be carried out initially | Identification and eradication of H. pylori will benefit those with ulcers and a small proportion of H. pylori-positive patients with functional dyspepsia. A course of acid suppression will have similar outcomes but ulcers may relapse. Eradication of H. pylori plays no role in management of GORD |
| A test and treat policy is cost-effective compared with initial endoscopy in patients with uncomplicated dyspepsia | There is no evidence to support widespread eradication of H. pylori in primary care |
| Patients on proton pump inhibitors (PPIs) can have false-negative results for H. pylori | PPIs should be withdrawn at least 2 weeks before urea breath test or biopsy urease testing (endoscopy) |
| Following a 7-day eradication therapy regimen, antisecretory therapy can normally be stopped | Longer courses of acid-suppressive therapy should be reserved for patients with active ulcers complicated by bleeding and/or NSAID use |
| First-line eradication therapy comprises twice-daily PPI, amoxicillin 1 g and clarithromycin 500 mg | Metronidazole can be substituted in those patients allergic to amoxicillin and for second-line therapy. When combined with metronidazole, the dose of clarithromycin can be reduced to 250 mg |
| Use of high dose intravenous PPIs | Intravenous PPI use is not recommended before endoscopic diagnosis in gastro-intestinal bleeding. High dose intravenous PPI is recommended for 72 h after endoscopic haemostatic therapy in patients at high risk of re-bleeding from peptic ulcer |
| In patients with NSAID-associated active peptic ulcer disease who test positive for H. pylori, the cause of the ulcer may not be confirmed | The ulcer should be healed with a PPI for 4 weeks and H. pylori eradicated. Eradication of H. pylori reduces the risk of recurrent bleeding |
| The treatment of NSAID-associated ulcers may differ depending upon whether or not the NSAID must be continued | If NSAIDs are withdrawn, healing rates at 4 weeks are best with PPIs and are similar between H2-receptor antagonists and PPIs after 8 weeks. PPIs are continued only if NSAIDs are continued |
| Patients in whom low-dose aspirin is indicated but have risk factors for peptic ulcer disease | Use of aspirin should be considered carefully, especially for primary prophylaxis of cardiovascular disease. Enteric coating does not reduce this risk. Clopidogrel is not a safer alternative than aspirin in combination with a PPI |
| There is an increased risk of bleeding with dual antiplatelet therapy | PPIs are thought to decrease conversion of clopidogrel to active metabolite, thus decreasing efficacy of clopidogrel. If dual antiplatelet therapy is indicated after PCI, use H2 receptor antagonist unless high risk (previous gastro-intestinal bleed), when PPI indicated |
| NSAID use in elderly patients | Patients over 65 years of age are at increased risk of peptic ulcer disease associated with NSAIDs and often present with ‘silent ulcers’. NSAIDs (prescription and non-prescription) should be avoided in the elderly |
| Patients who are candidates for COX-2 inhibitors | COX-2 inhibitors are associated with less gastro-intestinal toxicity than non-selective NSAIDs. COX-2 inhibitors are contraindicated in cardiovascular disease. The reduction in gastro-intestinal risk associated with COX-2 inhibitors is similar to reduction in risk associated with non-selective NSAIDs in combination with gastroprotective agents |
| Criteria and regimens to administer for stress ulcer prophylaxis are unclear | Intravenous H2-receptor antagonist therapy is given to patients at risk until enteral feeding is tolerated. Definite risk factors include mechanical ventilation, presence of coagulopathy and spinal cord injury. Controversial risks include head injury, sepsis, burns, multiple trauma, steroid therapy |
| Patients who require long-term PPI therapy | Those in whom long-term NSAIDs or low-dose aspirin are indicated and who have risk factors for associated upper gastro-intestinal complications. Patients with endoscopically diagnosed GORD who either have severe erosive oesophagitis or severe symptoms which can only be controlled with maintenance therapy |
PCI, percutaneous coronary intervention.
Questions
Answers
Answers
Case 12.3
| Reference range | ||
|---|---|---|
| Sodium | 138 mmol/L | 135–145 mmol/L |
| Potassium | 3.9 mmol/L | 3.4–5.0 mmol/L |
| Creatinine | 110 µmol/L | 75–155 µmol/L |
| Blood glucose | 6.8 mmol/L | <11.1 mmol/L |
| Total cholesterol | 4.5 mmol/L | <4.0 mmol/L |
| Haemoglobin | 12.0 g/dL | 11.5–16.5 g/dL |
Answers
Barkun A.N., Bardou M., Kuipers E.J., et al. International consesus recommendations on the management of patients with nonvariceal upper gastro-intestinal bleeding. Ann. Intern. Med.. 2010;152:101-113.
Bhatt D.L., Scheiman J., Abraham N.S., et al. Expert consensus document on reducing the gastro-intestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J. Am. Coll. Cardiol.. 2008;52:1502-1517.
Chan F.K., Chung S.C., Suen B.Y., et al. Preventing recurrent upper gastro-intestinal bleeding in patients with Helicobacter pylori infection who are taking low dose aspirin or naproxen. N. Engl. J. Med.. 2001;344:967-973.
Chan F.K.L., Wong V.W.S., Suen B.Y., et al. Combination of a cyclo-oxygenase-2 inhibitor and a proton-pump inhibitor for prevention of recurrent ulcer bleeding in patients at very high risk: a double-blind, randomised trial. Lancet. 2007;369:1621-1626.
Dial S., Alrasadi K., Manoukian C., et al. Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case–control studies. Can. Med. Assoc. J.. 2004;171:33-38.
Dorward S., Sreedharan A., Leontiadis G.I., et al. Proton pump inhibitor treatment initiated prior to endoscopic diagnosis in upper gastro-intestinal bleeding. Cochrane Database Syst. Rev.. (4, No):2006. CD005415
Fiorucci S., Santucci L., Distrutti E. NSAIDs, Coxibs CINOD and H2S-releasing NSAIDs, what lies beyond the horizon? Dig. Liver Dis.. 2007;39:1043-1051.
Gisbert J.P., Khorrami S., Carballo F., et al. H. pylori eradication therapy vs. antisecretory non-eradication therapy (with or without long-term maintenance antisecretory therapy) for the prevention of recurrent bleeding from peptic ulcer. Cochrane Database Syst. Rev.. (2, No):2004. CD004062
Goldstein J.L., Johanson J.F., Hawkey C.J., et al. Clinical trial: healing of NSAID-associated gastric ulcers in patients continuing NSAID therapy – a randomised study comparing rantidine with esomeprazole. Aliment. Pharmacol. Ther.. 2007;26:1101-1111.
Herzig S.J., Howell M.D., Ngo L.H., et al. Acid-suppressive medication use and the risk for hospital-acquired pneumonia. J. Am. Med. Assoc.. 2009;301:2120-2128.
Hooper L., Brown T.J., Elliott R.A., et al. The effectiveness of five strategies for the prevention of gastro-intestinal toxicity induced by non-steroidal anti-inflammatory drugs: systematic review. Br. Med. J.. 2004;329:948-952.
Jafri N.S., Hornung C.A., Howden C.W. Meta-analysis: sequential therapy appears superior to standard therapy for Helicobacter pylori infection in patients naïve to treatment. Ann. Intern. Med.. 2008;148:923-931.
Lai K.C., Lam S.K., Chu K.M., et al. Lansoprazole for the prevention of recurrences of ulcer complications from long-term low dose aspirin use. N. Engl. J. Med.. 2002;346:2033-2038.
Lai K.C., Chu K.M., Hui W.M., et al. Celecoxib compared with lansoprazole and naproxen to prevent gastro-intestinal ulcer complications. Am. J. Med.. 2005;118:1271-1278.
Laine L., Hennekens C. Proton pump inhibitor and clopidogrel interaction: fact or fiction? Am. J. Gastroenterol.. 2010;105:34-41.
Lanza F.L., Chan F.K., Quigley E.M., Practice Parameters Committeee of the American College of Gastroenterology. Guidelines for prevention of NSAID-related ulcer complications. Am. J. Gastroenterol.. 2009;104:728-738.
Lau J.Y., Leung W.K., Wu J.C., et al. Omeprazole before endoscopy in patients with gastro-intestinal bleeding. N. Engl. J. Med.. 2007;356:1631-1640.
Leontiadis G.I., Sharma V.K., Howden C.W. Proton pump inhibitor treatment for acute peptic ulcer bleeding. Cochrane Database Syst. Rev.. (1, No):2006. CD002094
Leontiadis G.I., Sharma V.K., Howden C.W. Proton pump inhibitor therapy for peptic ulcer bleeding: cochrane collaboration meta-analysis of randomised controlled trials. Mayo Clin. Proc.. 2007;82:286-296.
Malfertheiner P., Megraud F., O’Morain C., et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772-781.
Malfertheiner P., Chan F.K.L., McColl E.L. Peptic ulcer disease. Lancet. 2009;374:1449-1461.
McQuaid K.R., Laine L. Systematic review and meta-analysis of adverse events of low-dose aspirin and clopidogrel in randomised controlled trials. Am. J. Med.. 2006;119:624-638.
MHRA. Clopidogrel and proton pump inhibitors: interaction. Drug Safety Update. 2009;2:2-3.
Moayyedi P., Talley N.J. Gastro-oesophageal reflux disease. Lancet. 2006;367:2086-2100.
National Institute for Health and Clinical Excellence, North of England Dyspepsia Guideline Development Group. Dyspepsia – management of dyspepsia in adults in primary care. In Clinical Guideline 17. London: NICE; 2004.
Ng F.H., Wong S.Y., Lam K.F., et al. Famotidine is inferior to pantoprazole in preventing recurrence of aspirin-related peptic ulcers or erosions. Gastroenterology. 2010;138:82-88.
Quenot J.P., Theiry N., Barbar S. When should stress ulcer prophylaxis be used in the ICU? Curr. Opin. Crit. Care. 2009;15:139-143.
Scheiman J.M., Yeomans N.D., Talley N.J., et al. Prevention of ulcers by esomeprazole in at-risk patients using non-selective NSAIDs and COX-2 inhibitors. Am. J. Gastroenterol.. 2006;101:701-710.
Scottish Intercollegiate Guidelines Network. Dyspepsia. Guideline No. 68. Edinburgh: Scottish Intercollegiate Guidelines Network; 2003.
Scottish Intercollegiate Guidelines Network. Management of Acute Upper and Lower Gastro-intestinal Bleeding Guideline 105. Edinburgh: Scottish Intercollegiate Guideline Network; 2008.
Shaheen N.J., Richter J.E. Barrett’s oesophagus. Lancet. 2009;373:850-861.
Stanley A.J., Ashley D., Dalton H.R., et al. Outpatient management of patients with low risk upper gastro-intestinal haemorrhage: multicentre validation and prospective evaluation. Lancet. 2009;373:42-47.
Taha A.S., McCloskey C., Prasad R., et al. Famotidine for the prevention of peptic ulcers and oesophagitis in patients taking low dose aspirin (FAMOUS): a phase III, randomised, double-blind, placebo-controlled trial. Lancet. 2009;374:119-125.
Yeomans N.D., Svedberg L.E., Naesdal J. Is ranitidine therapy sufficient for healing peptic ulcers associated with non-steroidal anti-inflammatory drug use? Int. J. Clin. Pract.. 2006;60:1401-1407.
Boparai V., Rajagopalan J., Triadafilopoulos G. Guide to the use of proton pump inhibitors in adult patients. Drugs. 2008;68:925-947.
Latimer N., Lord J., Grant R.L., et al. Cost effectiveness of COX-2 selective inhibitors and traditional NSAIDs alone or in combination with a proton pump inhibitor for people with arthritis. Br. Med. J.. 2009;339:b2538.
Lazzaroni M., Bianchi Porro G. Management of NSAID-induced gastro-intestinal toxicity. Drugs. 2009;69:51-69.
Van Pinxteren B., Numans M.E., Bonis P.A., et al. Short-term treatment with proton pump inhibitors, H2 receptor antagonists and prokinetics for gastro-oesophageal reflux disease-like symptoms and endoscopy negative reflux disease. Cochrane Database Syst. Rev.. (3, No):2004. CD002095