Chapter 21 Penetrating Brain Injury
• In the presence of penetrating brain injury, the first step is to initiate advanced trauma life support resuscitation with early transportation to definitive care.
• The major principles of management of penetrating brain injuries include early decompression with conservative débridement of the brain. Surgeons should avoid disrupting functional eloquent cortex when chasing deep-seated fragments. In addition, it is important to identify and remove superficial expanding hematoma and foreign material, reconstruct the anterior skull base, and attempt a watertight dural closure.
• One should attempt to avoid secondary injury (i.e., meningitis, seizures, delayed vasospasm, and thromboembolic stroke) through protocol-driven neurocritical care, which includes monitoring intracranial pressure and drainage of cerebrospinal fluid.
• It is essential to maintain a high suspicion for acute and delayed neurovascular injuries (i.e., traumatic aneurysms) with subsequent endovascular evaluation and treatment when indicated.
• Delayed or immediate restoration of normal anatomy can be accomplished through craniofacial reconstruction techniques.
Historical Background
No discussion of the neurosurgical management of penetrating brain injury is complete without first acknowledging Dr. Harvey Cushing’s contributions during World War I. His landmark 1918 British Journal of Surgery article consisted of a retrospective review and classification of 219 cranial injuries and associated rates of mortality.1 In this paper he advocated aggressive débridement of necrotic tissue, removal of all in-driven debris, and meticulous scalp and dural closure. With this strategy he was able to decrease the incidence of brain abscesses and thereby lower the operative mortality rate from 55% to 29% within a 3-month period (Table 21.1).
This approach continued throughout World War II and was refined by Dr. Donald Matson in his classic monograph published in 1958.2 Matson described the principal tenets of performing initial lifesaving interventions, preventing infection, preserving neurological function, and restoring normal anatomy. These strategies hold as true today as then and have developed into modern advanced trauma life support (ATLS) guidelines,3 aseptic surgical techniques, prevention of secondary injury through neurocritical care, and craniofacial reconstruction.
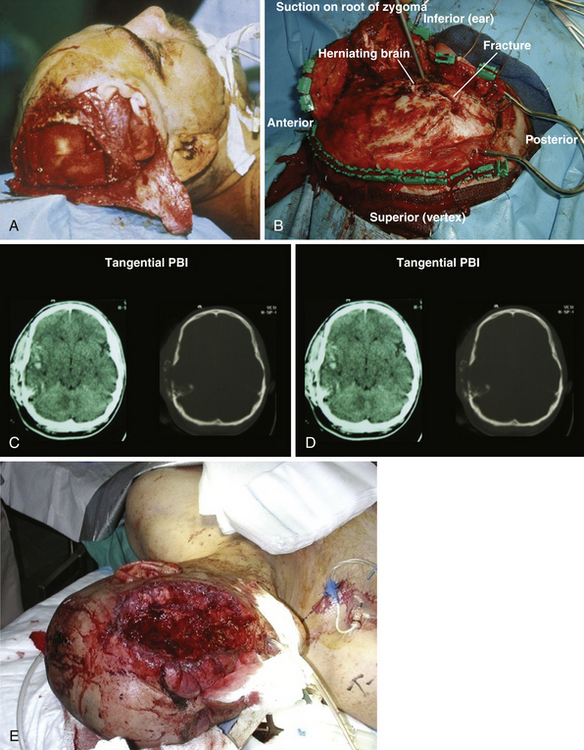
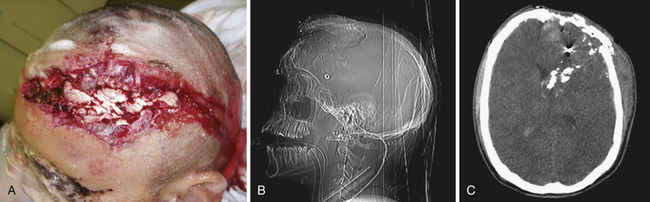
Cushing’s strategy of débridement continued to be applied in both the Korean War and the Vietnam conflict where early experiences with less aggressive approaches were met with higher infection rates and increased mortality rates.4–6 It was not until the Israel/Lebanon conflict of 1982, when computed tomography (CT) first became routine in the assessment of head injury, that a more conservative approach was advocated in favor of brain preservation. This approach was validated by 6 years of follow-up in the studies by Brandvolt and associates and Levi and colleagues and by 8 years of follow-up in a similar group of patients from the Iran-Iraq war of 1986 by Amirjamshidi and co-workers revealing no statistically significant increase in delayed infection or seizure disorders.7–9 A report of early experience with conservative débridement in Operation Desert Storm continued to find similar results10 (Fig. 21.1).
In 1995, the Brain Trauma Foundation developed the first traumatic brain injury (TBI) guidelines with the assistance of international experts in the field to address the need for protocol-driven health care based upon the most current scientific literature. In 2001, guidelines for the care of PBI were created toward the same end with the support of the Brain Injury Association, the International Brain Injury Association, the American Association of Neurological Surgeons (AANS), and members of the Congress of Neurological Surgeons (CNS). These guidelines stand as the most comprehensive evidence-based evaluation of interventions specific to the care of PBI and are summarized in Table 21.2.11 It is worth mentioning, however, that despite the exhaustive literature search used to develop the guidelines, no recommendation achieves a level beyond that of option and this underscores the need for further research. No level I or II data exist on the subject.
TABLE 21.2 2001 Guidelines for the Management of Penetrating Brain Injury
| Topic | Conclusion | Recommendation Level |
|---|---|---|
| I. Antibiotic prophylaxis | The use of prophylactic broad-spectrum antibiotics is recommended for patients with PBI. | Option |
| II. Seizure prophylaxis | The use of prophylactic antiseizure medications in the first week after PBI is recommended to prevent early post-traumatic seizures. | Option |
| III. ICP monitoring | Early ICP monitoring is recommended when the clinician is unable to assess the neurological examination accurately or if CT scans suggest elevated intracranial pressure. The need to evacuate a mass lesion is unclear. | Option |
| IV. Management of CSF leaks | Surgical correction is recommended for CSF leaks that do not close spontaneously, or are refractory to temporary CSF diversion. During the primary surgery, every effort should be made to close the dura and prevent CSF leaks. | Option |
| V. Neuroimaging | CT scanning of the head is strongly recommended. Angiography is recommended in PBI when a vascular injury is suspected. Routine MRI is not recommended for use in the acute management of missile-induced PBI. | Option |
| VII. Surgical management | Evacuation of hematomas with mass effect, conservative débridement of missile tract without retrieval of remote fragments, débridement of nonviable scalp, bone, or dura before primary closure, and watertight repair of open-air sinus injuries is recommended. | Option |
| VIII. Vascular complications | CT angiography and/or conventional angiography should be considered when vascular injury is suspected. If traumatic intracranial aneurysm (TICA) or arteriovenous fistula (AVF) is identified, surgical or endovascular management is recommended. | Option |
CSF, cerebrospinal fluid; CT, computed tomography; ICP, intracranial pressure; MRI, magnetic resonance imaging; PBI, penetrating brain injury.
Modified from Aarabi B, Alden TD, Chestnut RM, et al. Management and prognosis of penetrating brain injury. J Trauma 2001;51:3-43.
Craniofacial injuries sustained during OEF and OIF have resulted in further modifications to the management of patients with severe PBI.12–18 Although aggressive débridement of the brain is no longer used, early and aggressive cranial decompression with subsequent watertight dural closure is the current accepted practice (see Fig. 21.1).18 This strategy affords the patient protection from significant elevations in intracranial pressure (ICP) during the long flights, unaccompanied by neurosurgeons, from operative theaters back to the United States. This initial stabilization is then followed by protocol-driven neurocritical care aimed at preventing secondary neurological decline and maximizing neurological recovery. Though the data are still under review, preliminary analysis appears favorable15,19 (Fig. 21.2).
Prognosis
Civilian PBI has high mortality rates that remain refractory to medical and neurosurgical advances based on the most recent case series.20,21 The mortality rate in most retrospective cohorts is greater than 70% owing in large part to the higher percentage of gunshot wounds (see Fig. 21.2), suicides, and the ubiquitous absence of cranial protection (helmets) compared to military injuries.22–26 Other variables that differentiate these two groups are presented in Table 21.3. The reported civilian mortality rates from gunshot wounds to the head (GSWH) in the literature range from 23% to 92% with those series on the lower end including a number of cases without dural violation.27–29 Death from PBI occurs shortly after injury with 70% of patients succumbing after the first 24 hours23,25 (Table 21.4).
TABLE 21.3 Comparison of Civilian and Military Penetrating Brain Injury
| Factor | Civilian | Military |
|---|---|---|
| Population | Heterogeneous | Homogeneous (younger overall age) |
| Mechanism | Gunshot/suicide | Blast/shrapnel |
| Environment | Modern sanitation | Possibly contaminated |
| Prehospital care | Variable | Immediate |
| Tertiary care | Hours | Variable |
| Injury severity score (ISS)∗ | Low | High |
TABLE 21.4 Prognostic Variables
| Topic | Conclusion | Data Class |
|---|---|---|
| I. Age | Age > 50 years is associated with higher rate of mortality, but significance is unclear. | III |
| II. Cause of injury (suicide) | Self-inflicted GSWH carry a higher rate of mortality than those for other causes. | II |
| III. Mode of injury | Perforating wounds have a higher mortality rate than tangential or penetrating PBIs. | III |
| IV. Caliber of weapon | No demonstrated effect. | |
| V. Hypotension | Hypotension is associated with increased mortality rate. | III |
| VII. Coagulopathy | Coagulopathy is associated with increased mortality rate. | III |
| VIII. Respiratory distress | Respiratory distress is associated with increased mortality rate. | III |
| IX. GCS | In civilian injuries, low GCS score correlates with higher mortality rate and unfavorable outcome. In military injuries, low GCS score correlates with unfavorable outcome. | I (civilian) III (military) |
| X. Pupil size and response | The presence of bilateral fixed and dilated pupils is highly predictive of fatal outcome. | III |
| XI. ICP | High ICP is predictive of higher mortality rate. | II |
GCS, Glasgow Coma Scale; GSWH, gunshot wounds to the head; ICP, intracranial pressure; PBI, penetrating brain injury.
Modified from Aarabi B, Alden TD, Chestnut RM, et al. Management and prognosis of penetrating brain injury. J Trauma 2001;51:44-86.
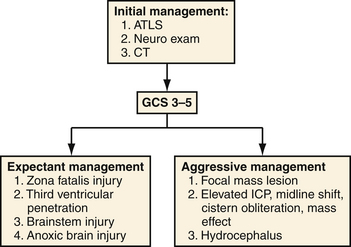
Prognostic indicators were classified as a separate section in the 2001 PBI guidelines and are summarized in Table 21.4.11 Of these factors and consistent with data in other forms of TBI, a postresuscitation Glasgow Coma Scale (GCS) score stands as the most reliable predictor of death and other unfavorable outcomes.30 This measure is a consistent predictor for both civilian and military populations but appears to have the greatest correlation with civilian mortality. In series segregating out patients with poor presenting neurological function or upon subgroup analysis of those with GCS scores of 3 to 5, mortality rate rises to 87% to 100%.25–31 A recommended triage for both military and civilian patients is located in Figure 21.3.
Because of the many dissimilar characteristics between civilian and military populations it has been difficult to correlate the weight of certain prognostic factors and to compare treatment measures across groups; however, a recent study by Dubose and associates compared the epidemiology and outcomes of military and civilian severe TBI.19 The Joint Trauma Theater Registry (JTTR) database and the American College of Surgeons’ National Trauma Data Bank (NTDB), both the largest patient databases of their kind, were queried for severe TBI and PBI. Subgroup analysis then selected for isolated PBI from gunshot wounds and statistically matched counterparts across the same period of 2003 to 2006. The results showed a threefold increase toward neurosurgical intervention in the JTTR group compared with civilian, particularly in the way of ICP monitoring (13.8% vs. 1.7%). Outcomes showed a tenfold decrease in mortality rate in the JTTR over their NTDB counterparts (5.6% vs. 47.9%). Although a dramatic difference in mortality rates between these populations is not new, this study excludes the confounding differences in explosive fragment PBI and GSWH injuries and allows for a more direct comparison of pertinent factors.
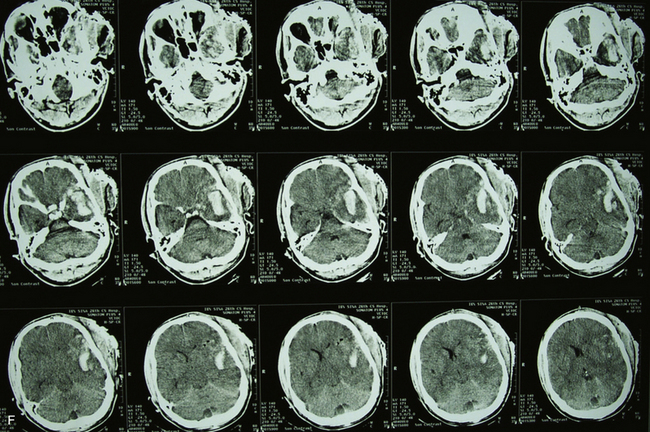
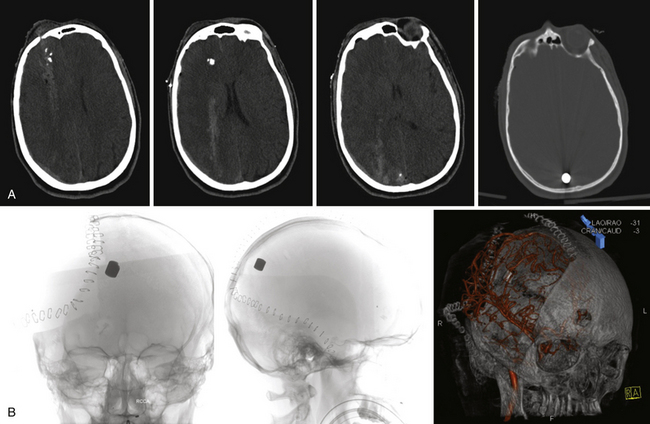
To make these populations truly comparable, however, the high rate of suicides and point-blank injuries in the civilian population needs to be accounted for in the analysis. In the most recent case series of civilian GSWH, Hofbauer and colleagues analyzed the impact of shooting distance on mortality rate in contact, near-contact, intermediate range, and at distance.21 Those at intermediate and long distances had a 0% mortality rate of 19 patients treated. In contrast, the contact group showed mortality rates of 91% with self-inflicted wounds (n = 46) and 100% with non-self-inflicted wounds (n = 4). Until variables of mechanism and modes of injury can be more appropriately matched, treatment effects across civilian and military PBI will be difficult to discern (Figs. 21.4 and 21.5).
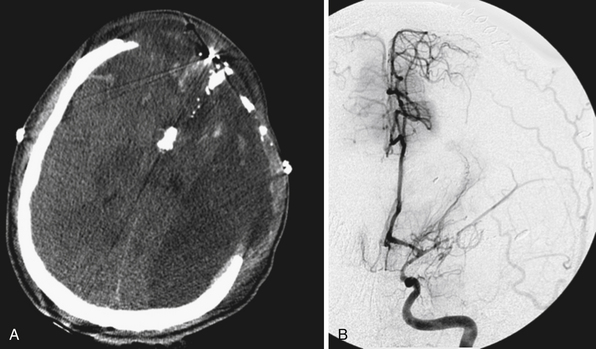
FIGURE 21.5 A and B, Computed tomography (CT) scan of the pilot in Figure 21.10 reveals the degree of bone fragmentation resulting in a secondary projectile phenomenon. Bony fragments drove into the depths of the sylvian fissure, resulting in stenosis and occlusion of the middle cerebral artery major divisions and a large hemispheric infarct.
Pathophysiology
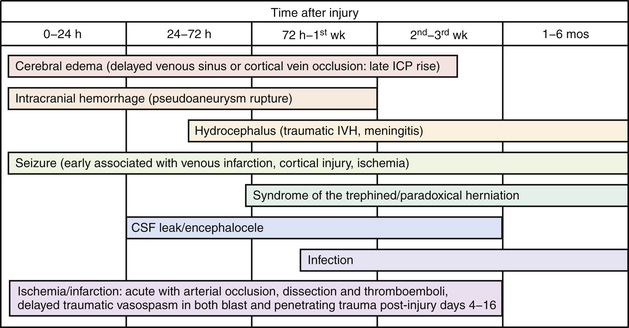
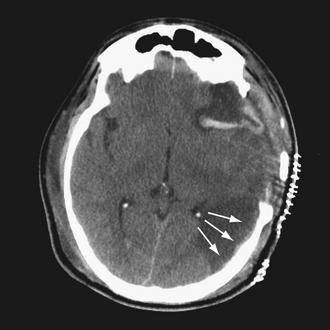
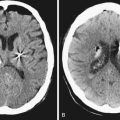
The pathophysiology of penetrating head injury is in many ways similar to all forms of severe brain injury. Injury can be classified as either primary (directly resulting from the penetrating force) or secondary (injury sustained from complications of the initial penetrating injury). Initial neurological cell death occurs immediately adjacent to the trajectory of the penetrating object (see Fig. 21.4). Subsequent cell death can occur as a result of increased ICP, mass effect from space-occupying lesions, stroke from initial vessel injury (see Fig. 21.5) or delayed ischemic neurological deficits resulting from traumatic vasospasm (Fig. 21.6), complications from infection, uncontrolled seizure activity, and delayed hydrocephalus.32–38 Surgical and critical care management strategies must therefore be directed at mitigating complications from the secondary neurological injury described here37,38 (Fig. 21.7).
Mechanisms of Penetrating Brain Injury
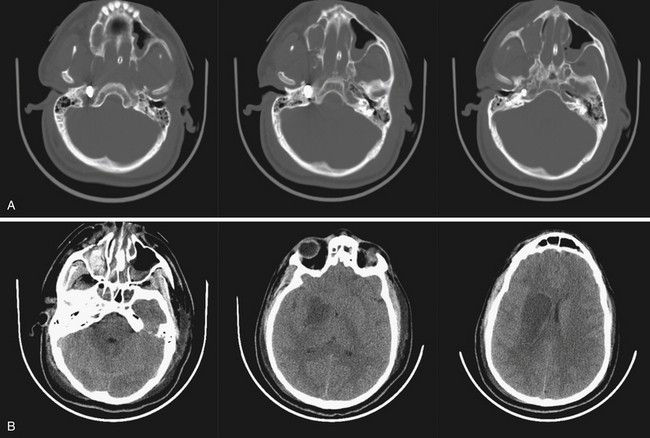
Open head injury mechanisms have been described in various systems of classification from high and low velocity to blunt, explosive, penetrating, and perforating. For the purpose of this discussion, anything related to gunshot wounds to the head will be explicitly defined, and penetrating fragment injuries will be referred to as explosive or blast fragmentation injuries. Blunt injuries will include open, comminuted, and depressed skull fractures with in-driven bone fragments as the result of a nonpenetrating external force (i.e., “under the body armor,” Fig. 21.8). Discussion concerning nonprojectile penetrating injuries (i.e., stab wound from knives or sticks) will be excluded given the relative infrequency in the referenced studies. As previously mentioned, the most common mechanism shared between civilian and military populations are GSWH. The lethality of GSWH is greater than the vast majority of penetrating blast fragment injuries and has been assumed to be partially responsible for the disparity of favorable outcomes in the civilian population. The ratio of fragment to GSWH, approximately 7:2, remains relatively consistent among wartime conflicts.1,2,4,5,7,8,15,31
Ballistics, or the study of the dynamic properties of a projectile, is useful to understand when reconstructing what occurred at time zero of the impact.39 Terminal or wounding ballistics is perhaps even more relevant to the neurosurgeon as it describes how the projectile behaves after impacting tissue. Kinetic energy (KE), also known as wounding energy, is the quantity of energy that a projectile transfers to the brain and creates damage. Kinetic energy is calculated by the equation KE = (Mass) (Velocity)2, revealing the relative weight that velocity conveys to a missile’s wounding potential.
In low-velocity wounds, the injury is made primarily by the projectile itself, crushing tissue within its immediate trajectory in creating the permanent cavity. High-velocity wounds, in contrast, create a more complex pattern of injury based on the whether the projectile penetrates or perforates the cranial vault and the degree of the KE imparted to the tissue. The KE of a perforating wound can be calculated as equal to (Mass) (Impact velocity − Exit velocity)2. The release of KE into tissue causes a phenomenon of cavitation: a brief, compressive force that expands tangentially from the tract of primary injury, generating a destructive wake. After the KE is depleted, and the cavity has expanded to its maximal size, the area begins to collapse under negative pressure and may suck in surrounding debris. The cavity may then subsequently undergo further expansions and contractions in decreasing amplitudes depending on the viscoelastic properties of the tissue. In inelastic tissue, such as brain, the permanent cavity can become tenfold larger than the diameter or profile of the offending projectile.
Other characteristics of a projectile include caliber, shape, yaw, precession and nutation, and ballistic coefficient. A round’s caliber is defined by the internal diameter of the weapon’s barrel, therefore representing the widest diameter of the bullet. This is can be measured in both inches (.45) and millimeters (9-mm) and essentially approximates the bullet’s mass. The shape of a projectile may be sharp or round nosed, or may be completely spherical as is the case in some explosive munitions. The shape will be the main determinant of the other following characteristics. Yaw is the rotation of a bullet around its long axis. As the yaw of a projectile increases to 90 degrees, the size of the permanent cavity, and therefore tissue destruction, is maximized. The potentially large discrepancy between in-flight yaw and tissue-travel yaw explains why an exit wound may be many times larger than the entry wound. The precession and nutation are the circular motions of a projectile in flight and contribute more to in-flight stability than impact damage. The ballistic coefficient, or drag, is the force that resists a bullet’s forward velocity. This, along with initial velocity, will determine the effective range of the weapon. Other factors not discussed are fragmentation and explosive potential, ricochet (Fig. 21.9), jacketed versus unjacketed rounds, and hollow-nosed or soft-pointed cartridges.
Management Recommendations in the Setting of Penetrating Brain Injuries
Evaluation, Diagnostics, and Medical Management
A penetrating brain injury can be an overwhelming “shock to the senses,” blinding the examiner from identifying other additional life-threatening issues. As a general management pearl, focusing solely on the head injury to the exclusion of a more meticulous systematic evaluation is to be avoided. A thorough review of the entire patient as recommended by the primary and secondary survey of ATLS will prevent this trap. Thinking of the examination and injury analysis as a complete “crime scene investigation” will allow the examiner to consider the details of the mechanism involved and record them for later consideration in the management of the patient.
In the setting of multitrauma, concomitant thoracic, abdominal, and cranial trauma may require simultaneous intervention. The recent combination of abdominal ultrasonic FAST (focused assessment with sonography for trauma) examinations in conjunction with head CT scans in the trauma bay can avoid significant treatment delays in the radiology suite. Alternatively, imaging done in the operating room with the use of a mobile CT scanner can facilitate diagnoses in patients too hemodynamically unstable to be transported to radiology. Individuals with multiple missile injuries should be triaged with a low threshold for immediate surgery if major visceral injury is suspected. If a neurological examination is not obtainable, the placement of a ventriculostomy or ICP monitor is advised to better manage the effects of intracranial hemorrhage or cerebral edema.40
Imaging modalities such as three-dimensional CT volumetric reconstruction is a luxury in some environments but is helpful for fragment path delineation and re-creation of the zone of injury. Standard CT stands as the most common and useful tool when evaluated penetrating brain injury. It allows for the analysis, with rapid precision, of the trajectory path, resultant intracranial hemorrhages, concomitant orbital and skull base fractures, and associated disrupted structures.41 The addition of CT angiography, when available, helps provide a reasonable initial assessment of vascular injuries. It may direct the clinician to proceed to angiography if further concern exists (Figs. 21.9 and 21.10). The role for immediate angiography is presented in Box 21.1. When evaluating the CT, questions specifically to be answered include the following:
1. Does the injury include the vascular structures, skull base, ventricles, orbits, or cranial nerves?
2. Does it cross the zona fatalis or brainstem?
3. Is there evidence of anoxic brain injury or diffuse axonal injury?
Remember that fragments can also be dynamic particles and positional movements may be missed between static CT images. When trying to determine if a projectile is still mobile, flexion and extension plain skull radiographs may be useful. Because of the mechanism of injury and the high likelihood of retained fragments with ferromagnetic properties, magnetic resonance imaging (MRI) is not recommended as an initial imaging modality in PBI (see Box 21.3).
BOX 21.1 Surgical Recommendations for Penetrating Brain Injury
1. Avoid use of the entry/exit wound when planning scalp incision.
2. Identify global/hemispheric injuries and role for large decompressive craniectomy.
3. Separate cranial-facial-orbital compartments with autologous bone or titanium mesh for support and watertight repair with dural substitutes.
4. Initiate early repair and fixation of the orbital bandeau to support future cranial vault reconstruction.
5. Perform conservative débridement of the trajectory path and imbedded fragments.
6. Consider intraoperative cerebral angiography in patients with combined penetrating neck and brain injury.
BOX 21.3 Criteria for Intracranial Angiography Following Penetrating Brain Injury
1. Penetrating injury through pterion, orbit, or posterior fossa
2. Penetrating fragment with intracranial hematoma
3. Known cerebral artery sacrifice or pseudoaneurysm at the time of initial exploration
4. Blast-induced penetrating injury with Glasgow Coma Scale score <8
5. Transcranial Doppler or computed tomography angiographic evidence of severe vasospasm
Outside the need for immediate operative intervention, patients with penetrating brain injury should initially be managed by applying fundamental critical care principles.37,38,40 After establishing basic life support and applying initial resuscitation via ATLS guidelines, obtaining an accurate initial neurological examination is most essential next step. In the ensuing time course, avoidance of hypoxia and hypotension is critical, as is fluid resuscitation with efforts taken to avoid hypotonic fluid administration. Efforts to monitor and mitigate the effects of elevated ICP should proceed rapidly following admission depending on the patient’s physical examination.
Surgical Management
The tenets of surgical treatment should be directed at preventing secondary insults due to raised ICP, delayed infection, and ischemia. Common neurosurgical management principles include brainstem decompression, reducing ICP, restoring anatomical continuity, and obtaining hemostasis. For injuries in which a focal insult has occurred, such as an open depressed skull fracture, débridement of the entry wound with fracture elevation may be all that is necessary. In patients with hemispheric or global injury, evacuation of mass lesions (hematoma) should be performed in conjunction with a large decompressive craniectomy to ameliorate secondary cerebral edema and ICP elevations. Early obliteration of basal cisterns, loss of sulcal and gyral pattern, midline shift not explained by associated mass lesion, or hemispheric edema are indicative of a diffuse insult with a less clear role for intervention. Surgical recommendations specific to PBI are summarized in Box 21.1.
The role for removal of deep embedded fragments has been discussed at length and attempts should be tempered by the potential for associated injury caused by deep tract dissection.7–10 Only superficial fragments that readily present themselves at the cortical surface or that can be dislodged with gentle irrigation should undergo primary débridement. Delayed fragment removal (metal, bone, or environmental elements) is indicated in certain situations and categorized in Box 21.2.
Preoperative Planning
Avoidance of iatrogenic injury is always of paramount importance to the neurological surgeon. Violation of major venous sinuses and cortical veins are complications that can arise when performing large decompressions. Awareness of alterations in normal anatomy and an evaluation of the fragment or missile pathway should always precede intervention. A plan should provide for a rim of bone around the major venous sinuses to allow for dural tuck-up sutures if a tear of the transverse or sigmoid sinus is suspected. If a depressed bone fragment compromises the venous sinus, this bone should be carefully elevated with a bone flap that encompasses the surrounding bone and allows direct repair if necessary. The use of muscle, fascia, dura, vein, and synthetic allograft has been described for sinus repairs and all are effective in many circumstances.42 Preserving normal venous outflow is essential in these cases and should be confirmed by CT angiography or cerebral angiography. Delayed venous occlusions may result in a poor outcome with subsequent venous infarction, swelling, bleeding, neurological deterioration, and death. The role for anticoagulation in PBI-related sinus occlusion has not been well studied.
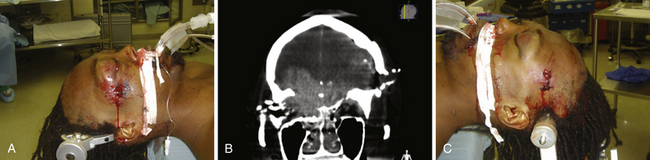
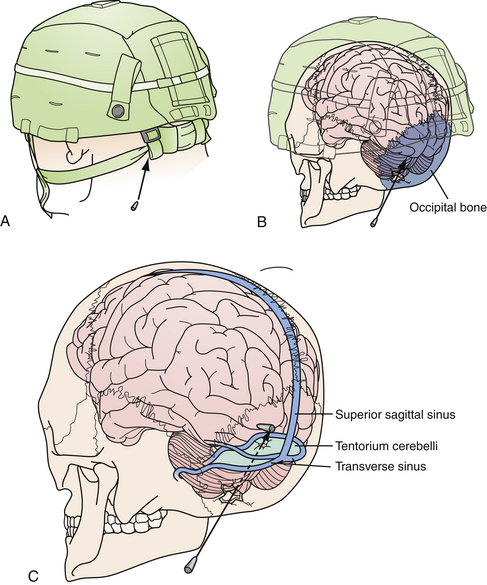
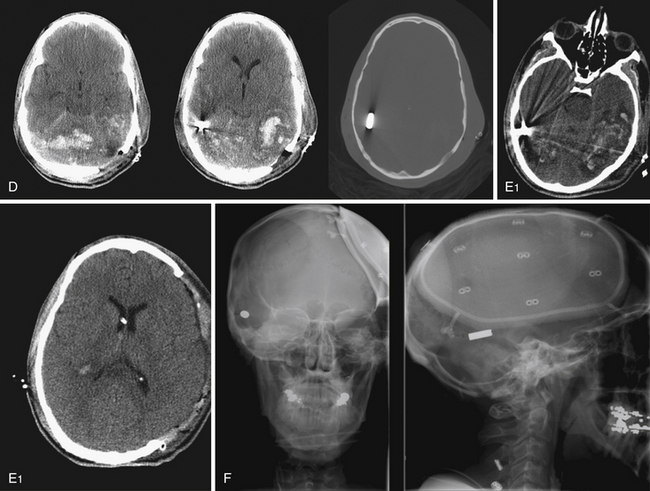
Those penetrating wounds without exit may have resulted from significant ricochet on the inner aspect of the calvarium. This has been reported in up to 30% in autopsy series.43 These rounds may then travel in an opposite direction and penetrate another intracranial compartment or stop at a dural margin. Special consideration should be given to injuries that cross two dural compartments, in particular those that penetrate from the posterior fossa to the supratentorial space. These rounds may require staged decompressive procedures to access separate mass lesions or mitigate ICP elevations in different compartments. In rare situations, a delayed venous infarction may result in supratentorial ICP elevations in a trajectory that crosses the tentorium from a posterior fossa entry (see Fig. 21.8). Typically the posterior fossa should be decompressed first, given the proximity to the brainstem and the potential for immediate death, followed by supratentorial decompression if necessary (see Fig. 21.8).
Operative Techniques
The hazards of poor surgical management in these injuries include improperly fashioning an incision using the traumatic scalp laceration, poor hemostasis, inadequate initial repair of skull base defect, and generally inadequate cranial decompression (Fig. 21.11). Basing an incision behind the zone of injury allows placement of a large craniotomy or craniectomy incision in normal tissue whose healing properties are not compromised. This avoids delayed wound breakdown, CSF leakage, and related infections.
In the operating room, the patient should be positioned in such a manner that allows for proximal carotid exposure if a skull base arterial injury is suspected. This should be done pre emptively if a fracture or fragment penetration includes the petrous pyramid, sphenoid bone, anterior clinoid, or optic strut (Fig. 21.12). Preparation for a potential donor bypass graft would include having the contralateral leg prepped with saphenous vein marked or arm preparation for possible radial artery graft. Additional foresights include having a Doppler readily available to delineate the superficial temporal artery before skin incision.
Injuries that include the frontal sinus or an anterior skull base defect may require autologous fascia or fat to close the defect. This may involve prepping the lower leg for harvesting of the fascia lata, an excellent autologous dural substitute, and the abdomen for a fat graft. Other considerations for incorporating the abdomen into the surgical field are for possible implantation of a postcraniectomy bone flap. It is important that the right anterior abdominal wall be used in lieu of the left to avoid the potential compromise of a percutaneous gastrostomy site. Implanting the bone flap prevents the autologous bone from being separated from the patient, allowing it to remain sterile, viable, and available. Alternatively, the autologous bone can be split and used for immediate reconstruction of the cranial base or orbital bandeau. If possible, this measure allows a more solid foundation for future cranial vault reconstruction and helps to prevent a possible CSF leak or encephalocele.
Endovascular Considerations
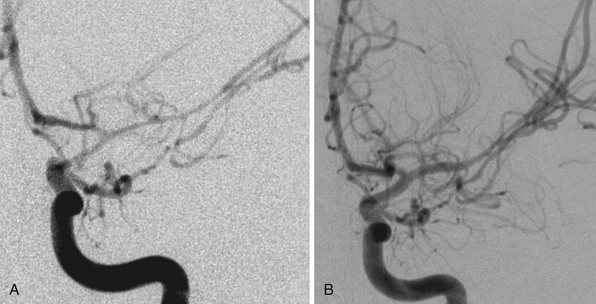
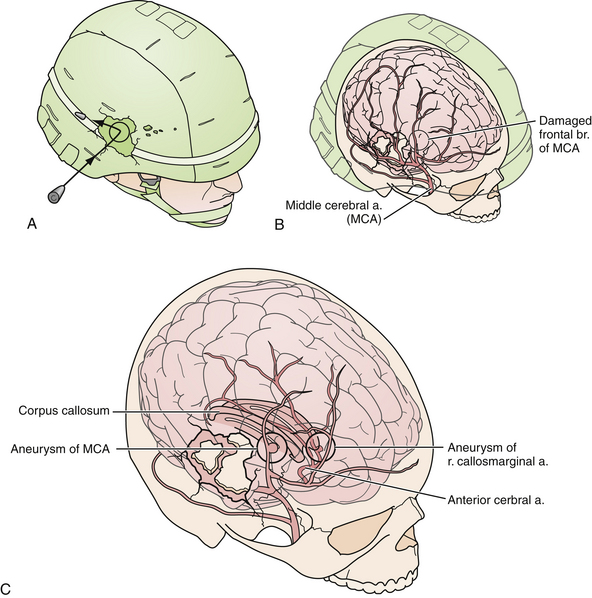
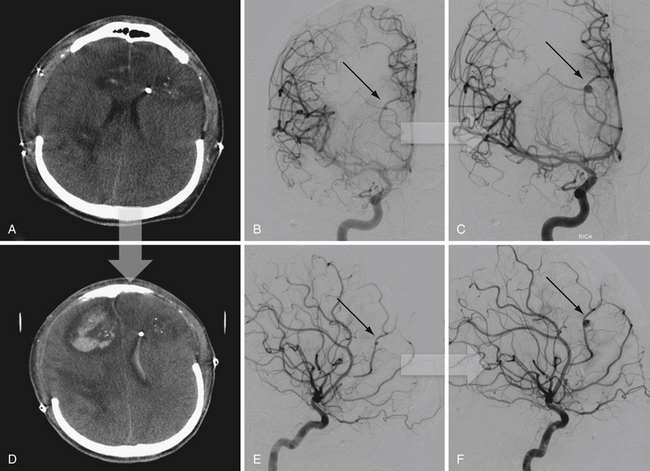
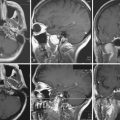
In addition to decompression and reconstruction of the cranial vault, adjunctive endovascular procedures may be required to prevent delayed ischemic or hemorrhagic deterioration. In some cases CT angiography may not demonstrate peripherally located small aneurysms or those hidden in venous contamination, bone, or metal scatter artifact. Modern digital subtraction cerebral angiography will demonstrate these smaller aneurysms more effectively and may allow for immediate treatment if necessary. The indications for immediate angiography are discussed in Box 21.312–14,16,17,44,45 (Figs. 21.13 and 21.14).
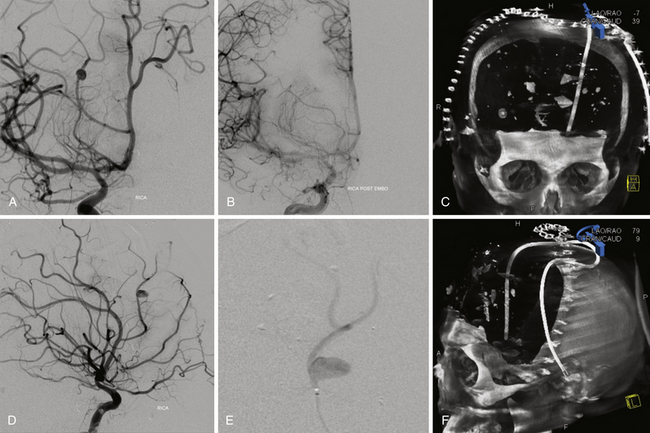
FIGURE 21.14 Same patient from Figure 21.8, preembolization (A and B) and after PAO and embolization with Onyx (C and D). The black arrows indicate the enlarged pseudoaneurysm, the empty arrows the subtracted Onyx cast, and the white arrows differentiate the cast from in-driven bone fragments on the dyna computed tomography (CT) scan (E and F).
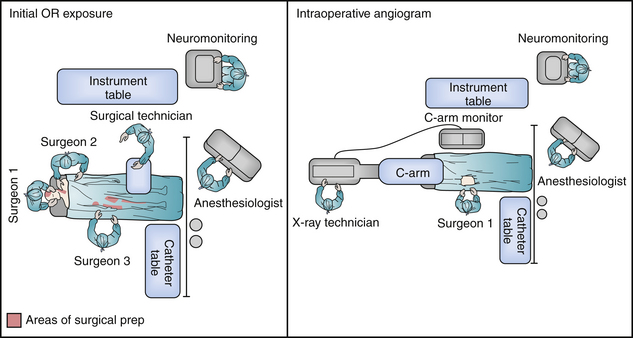
In some circumstances, an intraoperative cerebral angiogram can be coordinated to facilitate a simultaneous cranial decompression and cerebrovascular intervention. This proves essential in scenarios that include associated penetrating head and neck injuries, allowing for identification of the cervical vasculature injury in parallel with a timely cerebral decompression. The details to be considered in a well-orchestrated operation include (1) employing radiology technicians facile with the use of fluoroscopy, (2) use of a radiolucent headholder before pinning, (3) appropriate prep and draping for femoral arterial sheath placement, and (4) positioning the patient in a 180-degree turn from anesthesia. In this manner the surgical/endovascular team has access to the groin, neck, and cranial vault. The C-arm is then brought in at the head, directly in line with the patient, to allow the simulation of biplane positions (Fig. 21.15).
Delayed cerebral angiograms should also be considered in those patients who demonstrate a neurological deterioration that may be related to development of cerebral vasospasm, venous sinus occlusion, or de novo pseudoaneurysm formation in previously occluded branches. In the intensive care unit (ICU), morning sedation holidays with diligent neurological examinations and daily monitoring of transcranial Doppler (TCD) prove to be our least invasive and most reliable measures toward detection of such events. In the future, noninvasive near-infrared cerebral oximetry, cerebral blood flow probes, xenon CT, CT perfusion, continuous electroencephalography (EEG), and brain tissue oxygen probes may allow earlier and more sensitive identification of these problems.
Conclusion
Penetrating brain injury is a high-mortality emergency for the neurological surgeon. Advances that have improved the outcomes in the military population include better prehospital care in the field, a more standardized approach to the treatment of PBI, and early neurosurgical intervention to include ICP monitoring, early decompression, reconstruction of the anterior skull base, and watertight dural closure. The use of endovascular techniques has increased our ability to manage vascular complications as a result of PBI. In time, measures learned from the treatment of PBI in the military population may be conferred to civilian patients provided they survive their initial injury and ensuing field and emergency room resuscitations.
Aarabi B., Alden T.D., Chestnut R.M., et al. Management and prognosis of penetrating brain injury. J Trauma. 2001;51:3-86.
Bell R.S., Vo A.H., Neal C.J., et al. Military traumatic brain and spinal column injury: a 5-year study of the impact of blast and other military grade weaponry on the central nervous system. J Trauma. 2009;66(Suppl 4):S104-S111.
Bullock M.R., Povlishock J.T. Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24(1):11-106.
Cushing H. A series of wounds involving the brain and its enveloping structures. Br J Surg. 1918;5:558-684.
Matson D. The Management of Acute Craniocerebral Injuries Due to Missiles. Washington, DC: Office of the Surgeon General, Department of the Army; 1958.
Please go to expertconsult.com to view the complete list of references.
1. Cushing H. A series of wounds involving the brain and its enveloping structures. Br J Surg. 1918;5:558-684.
2. Matson D. The Management of Acute Craniocerebral Injuries Due to Missiles. Washington, DC: Office of the Surgeon General, Department of the Army; 1958.
3. Kortbeek J.B., Ali J., Antoine J.A., et al. Advanced trauma life support, 8th edition, the evidence for change. J Trauma. 2008;64(6):1638-1650.
4. Hammon W.M. Analysis of 2187 consecutive penetrating wounds of the brain from Vietnam. J Neurosurg. 1971;34:127-131.
5. Meirowsky A.M. The retention of bone fragments in brain wounds. Milit Med. 1968;133:887-890.
6. Wannamaker G.T., Pulaski E.J. Pyogenic neurosurgical infections in Korean battle casualties. J Neurosurg. 1958;15:512-518.
7. Brandvold B., Levi L., Feinsod M., George E.D. Penetrating craniocerebral injuries in the Israeli involvement in the Lebanese conflict, 1982-1985. Analysis of a less aggressive surgical approach. J Neurosurg. 1990;72:15-21.
8. Levi L., Borovich B., Guilburd J.N., et al. Wartime neurosurgical experience in Lebanon, 1982-1985. II. Closed craniocerebral injuries. Israel J Med Sci. 1990;26:555-558.
9. Amirjamshidi A., Abbassioun K., Rahmat H. Minimal debridement or simple wound closure as the only surgical treatment in war victims with low-velocity penetrating head injuries. Indications and management protocol based upon more than 8 years follow-up of 99 cases from Iran-Iraq conflict. Surg Neurol. 2003;60(2):105-110. discussion 110-111
10. Chaudhri K.A., Choudhury A.R., Moutaery K.R., Cybulski G.R. Penetrating craniocerebral shrapnel injuries during “Operation Desert Storm”: early results of a conservative surgical treatment. Acta Neurochir. 1994;126:120-123.
11. Aarabi B., Alden T.D., Chestnut R.M., et al. Management and prognosis of penetrating brain injury. J Trauma. 2001;51:3-86.
12. Armonda R.A., Bell R.S., Vo A.H., et al. Wartime traumatic cerebral vasospasm: recent review of combat casualties. Neurosurgery. 2006;59:1215-1225. discussion 1225
13. Bell R., Vo A., Porter C., et al. Wartime neurovascular injuries: review of the effectiveness of early, aggressive, endovascular management in the setting of blast-related cerebral vasospasm. Neurosurgery. 2006;59(2):455-456.
14. Bell R.S., Vo A.H., Roberts R., et al. Wartime traumatic aneurysms: acute presentation, diagnosis, and multimodal treatment of 64 craniocervical arterial injuries. Neurosurgery. 2010;66:66-79.
15. Bell R.S., Vo A.H., Neal C.J., et al. Military traumatic brain and spinal column injury: a 5-year study of the impact of blast and other military grade weaponry on the central nervous system. J Trauma. 2009;66(Suppl 4):S104-S111.
16. Bell R.S., Vo A.H., Roberts R., et al. Wartime traumatic aneurysms: acute presentation, diagnosis, and multimodal treatment of 64 craniocervical arterial injuries. Neurosurgery. 2010;66(1):66-79. discussion 79
17. Bell R.S., Ecker R.D., Severson M.A.3rd, et al. The evolution of the treatment of traumatic cerebrovascular injury during wartime. Neurosurg Focus. 2010;28(5):E5.
18. Bell R.S., Mossop C.M., Dirks M.S., et al. Early decompressive craniectomy for severe penetrating and closed head injury during wartime. Neurosurg Focus. 2010;28(5):E1.
19. Dubose J.J., Barmparas G., Inaba K., et al. Isolated severe traumatic brain injuries sustained during combat operations: demographics, mortality outcomes and lessons to be learned from contrasts to civilian counterparts. J Trauma. 2011;70(1):11-16. discussion 16-18
20. Murano T., Mohr A.M., Lavery R.F., et al. Civilian craniocerebral gunshot wounds: an update in predicting outcomes. Am Surg. 2005;71(12):1009-1014.
21. Hofbauer M., Kdolsky R., Figl M., et al. Predictive factors influencing the outcome after gunshot injuries to the head: a retrospective cohort study. J Trauma. 2010;69(4):770-775.
22. Ruff R.M., Marshall L.F., Crouch J., et al. Predictors of outcome following severe head trauma: follow-up data from the Traumatic Coma Data Bank. Brain Injury. 1993;7:101-111.
23. Aldrich E.F., Eisenberg H.M., Saydjari C., et al. Predictors of mortality in severely head-injured patients with civilian gunshot wounds: a report from the NIH Traumatic Coma Data Bank. Surg Neurol. 1992;38:418-423.
24. Hernesniemi J. Penetrating craniocerebral gunshot wounds in civilians. Acta Neurochir (Wien). 1979;49:199-205.
25. Kaufman H.H., Levy M.L., Stone J.L., et al. Patients with Glasgow Coma Scale scores 3, 4, 5 after gunshot wounds to the brain. Neurosurg Clin North Am. 1995;6:701-714.
26. Siccardi D., Cavaliere R., Pau A., et al. Penetrating craniocerebral missile injuries in civilians: a retrospective analysis of 314 cases. Surg Neurol. 1991;35:455-460.
27. Grahm T.W., Williams F.C., Harrington T., Spetzler R.F. Civilian gunshot wounds to the head: a prospective study. Neurosurgery. 1990;27:696-700.
28. Martins R.S., Siqueira M.G., Santos M.T.S., et al. Prognostic factors and treatment of penetrating gunshot wounds to the head. Surg Neurol. 2003;60:98-104.
29. Zafonte R.D., Wood D.L., Harrison-Felix C.L., et al. Penetrating head injury: a prospective study of outcomes. Neurol Res. 2001;23:219-226.
30. Guidelines for the management of patients with penetrating brain injury; neurological measures: level of consciousness and Glasgow Coma Scale. J Trauma. 2001;51:64-70.
31. Aarabi B. Surgical outcome in 435 patients who sustained missile head wounds during the Iran-Iraq War. Neurosurgery. 1990;27:692-695.
32. Aarabi B., Taghipour M., Alibaii E., Kamgarpour A. Central nervous system infections after military missile head wounds. Neurosurgery. 1998;42:500-507. discussion 507-509
33. Hagan R.E. Early complications following penetrating wounds of the brain. J Neurosurg. 1971;34:132-141.
34. Hamer J., Krastel A. Cerebral vasospasm after brain injury. Neurochirurgia. 1976;19:185-189.
35. Marshall L.F., Bruce D.A., Bruno L., Langfitt T.W. Vertebrobasilar spasm: a significant cause of neurological deficit in head injury. J Neurosurg. 1978;48:560-564.
36. Martin N.A., Doberstein C., Alexander M., et al. Posttraumatic cerebral arterial spasm. J Neurotrauma. 1995;12:897-901.
37. Chestnut R.M., Marshall L.F., Klauber M.R., et al. The role of secondary brain injury in determining outcome from severe head injury. J Trauma. 1993;34:216-222.
38. Wald S.L., Shackford S.R., Fenwick J. The effect of secondary insults on mortality and long-term disability after severe head injury in a rural region without a trauma system. J Trauma. 1993;34:377-381. discussion 381-382
39. Swan K.G., Swan R.C. Wound ballistics for the civilian surgeon. Surg Ann. 1985;17:163-187.
40. Bullock M.R., Povlishock J.T. Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24(1):11-106.
41. Neuroimaging in the management of penetrating brain injury. J Trauma. 2001;51:7-11.
42. Meirowsky A.M. Wounds of the dural sinuses. J Neurosurg. 1953;10:496-514.
43. Kirkpatrick J.D., DiMaio V. Civilian gunshot wounds of the brain. J Neurosurg. 1978;49:185-198.
44. Aarabi B. Traumatic aneurysms of brain due to high velocity missile head wounds. Neurosurgery. 1988;22:1056-1063.
45. Amirjamshidi A., Rahmat H., Abbassioun K. Traumatic aneurysms and arteriovenous fistulas of intracranial vessels associated with penetrating head injuries occurring during war: principles and pitfalls in diagnosis and management. A survey of 31 cases and review of the literature. J Neurosurg. 1996;84:769-780.