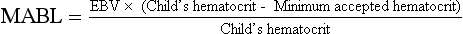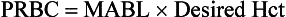Pediatrics
A Anatomy and physiology
a) During fetal development, oxygenation and carbon dioxide (CO2) elimination are accomplished through the placenta. Oxygenated blood to the fetus travels from the placenta through the umbilical vein through the ductus venosus near the liver to the inferior vena cava. The foramen ovale, the opening between the right and left atria, allows the oxygenated blood direct access to the left heart circulation. From the left atrium, the blood is transferred to the left ventricle and then to the body. The blood returns to the placenta through two umbilical arteries. Deoxygenated blood from the superior vena cava flows into the right atrium. It is then ejected into the pulmonary artery. Because of high pulmonary vasculature pressure, the blood bypasses the lungs and is instead transferred through the ductus arteriosus to the aorta. The blood travels to the placenta through the umbilical arteries.
b) Clamping of the umbilical cord increases systemic vascular resistance, increasing aortic and left-sided heart pressures and allowing the foramen ovale to close and the lungs to assume their role in oxygenation. Pulmonary vascular resistance decreases, and the ductus arteriosus closes as arterial oxygen pressure (Po2) levels increase.
c) Hypoxia, hypercarbia, and acidosis lead to persistent pulmonary hypertension and continued maintenance of fetal circulation. The diagnosis is made when right radial (preductal) and umbilical line (postductal) samples reveal a Po2 difference of 20 mmHg. Shunting continues across a patent ductus arteriosus, resulting in hypoxemia and reversal of acidosis.
d) Treatment of persistent pulmonary circulation includes hyperventilation, maintenance of adequate oxygenation, and alkalosis.
e) Neonatal cardiac output is heart rate dependent because of a noncompliant left ventricle and fixed stroke volume.
f) The pediatric basal heart rate is higher than that of adults, although parasympathetic stimulation, hypoxia, or deep anesthesia can cause profound bradycardia and decreased cardiac output.
g) Sympathetic nervous system and baroreceptor reflexes are immature. Infants have low catecholamine stores and decreased responsiveness to exogenous catecholamines. Infants cannot respond to hypovolemia with vasoconstriction. Therefore, hypovolemia is suspected when there is hypotension in the absence of an increased heart rate.
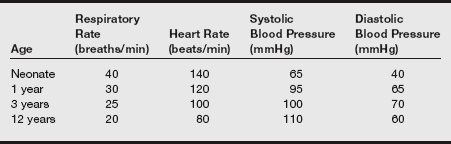
h) Normal parameters are given in the table on pg. 485.
i) Physiologic anemia of the newborn: Hematocrit at birth is 50%, 80% of which is fetal hemoglobin. Fetal hemoglobin binds more strongly to O2 than adult hemoglobin. This facilitates O2 uptake in utero. After birth, the presence of fetal hemoglobin causes a shift in the oxyhemoglobin curve to the left and a decrease in O2 delivery to the tissues. At age 1 to 3 months, hemoglobin levels decrease, and levels of 2,3-diphosphoglycerate increase. This causes a shift of the oxyhemoglobin curve to the right and increased O2 delivery to tissues.
a) Metabolic rate, CO2 production, and O2 consumption are increased.
b) Functional residual capacity and O2 reserves are decreased.
c) Infants have a paradoxical response to hypoxia—initial hyperpnea followed by respiratory depression and depressed response to hypercarbia.
d) The larynx is at C2 to C4 in children and at C3 to C6 in adults. This results in increased difficulty in alignment of the pharyngeal and laryngeal axes. A straight blade is useful for laryngoscopy in children.
e) Children have a stiff, omega-shaped epiglottis. The vocal cords slant up and back.
f) The narrowest part of the pediatric airway is the cricoid cartilage, as opposed to the adult glottis. The cricoid cartilage can form a seal around the endotracheal tube (ETT), eliminating the need for a cuffed tube. The cartilage is funnel shaped. Do not force fit the ETT. Properly fitted tubes allow a leak at 15 to 25 cm H2O.
g) Children have large occiputs that flex the head onto the chest, large tongues, and small chins. Tonsils and adenoids grow rapidly from ages 4 to 7 and may obstruct breathing.
h) Infants are obligatory nasal breathers. The position of the epiglottis in relation to the soft palate allows simultaneous breathing and sucking or drinking.
i) The neonatal trachea is 4 cm. Flexion of the head onto the chest forces the ETT to extend deeper into the right mainstem. Extension of the head may dislodge the tube.
j) The number of alveoli increases until age 6 years. Mature levels of surfactant are reached at 35 weeks of gestation. Decreased amounts of alveoli and surfactant in the neonatal period increase the risk of infant respiratory distress syndrome.
k) Increased work of breathing in the infant results from a decreased amount of type I muscle fibers in the diaphragm; this causes a predisposition to fatigue. Poor chest wall mechanics, lack of rib cage rigidity, horizontal orientation of the ribs, weak intercostal muscles, and increased fatigue result in paradoxical chest movements in the newborn.
a) Cranial sutures are not fused in infants; the cranium is pliable. Fluid status is indicated by fullness of the fontanels.
b) Myelination of the nervous system continues until age 3 years. The spinal cord ends at L1 in adults and at L3 in pediatric patients. This is important to consider when using regional anesthesia techniques in the pediatric population.
c) Preterm and low birthweight infants are at risk for intracranial hemorrhage resulting from fragile cerebral vessels. Intracranial bleeding may result from hypoxia, hypercarbia, hyperglycemia or hypoglycemia, hypernatremia, or wide variations in blood pressure.
a) The total body water in proportion to body weight is higher in neonates than in adults. Whereas the kidneys function in utero to eliminate urine into the amniotic fluid, the placenta eliminates waste.
b) Neonates have the complete number of nephrons at birth. Nephrons are immature in function until age 6 to 12 months.
c) The glomerular filtration rate (GFR) is decreased by renal vasoconstriction, low plasma flow in the renal system, and low blood pressure. GFR increases until age 1 year.
d) Infants are obligate sodium excretors because of their inability to conserve sodium. Renal tubules are not responsive to the renin–angiotensin–aldosterone system. Infants’ kidneys cannot concentrate urine, leading to an increased risk of dehydration. The ability to reabsorb glucose is also impaired. If excessive glucose is given intravenously, the result is osmotic diuresis.
e) Pediatric patients have a tendency to develop acidosis because the metabolic rate and CO2 production are double those of adults. There is a decreased ability to conserve bicarbonate and to excrete acids.
a) Near birth, the fetal liver increases glycogen stores. Preterm infants are at increased risk for hypoglycemia because of a lack of glycogen stores.
b) Hepatic metabolism of drugs is decreased in the early weeks of life. The liver functions at the adult level by age 2 years.
a) Infants are at risk for hypothermia from the following:
(1) Increased ratio of surface area to body weight
(2) Ineffective shivering mechanism
(3) Decreased amounts of subcutaneous fat present in preterm infants
b) Heat loss results from the following:
(1) Radiation: This is the transfer of heat between two objects of different temperatures not in direct contact. Reduce radiant loss by decreasing the temperature gradient (raise the room temperature closer to patient temperature). This factor is the major way that patients lose heat.
(2) Convection: This is the transfer of heat to moving molecules such as air or liquid. Cover exposed skin.
(3) Evaporation: This occurs through the skin and respiratory systems, including sweat, insensible water loss through skin, wounds, respiratory tract, and evaporation of liquids applied to the skin.
(4) Conduction: This is the transfer of heat from a warm infant to a cool object in direct contact.
c) Patients assume room temperature under anesthesia, a condition termed poikilothermia.
d) Nonshivering thermogenesis: Infants have impaired shivering capabilities. Autonomic nervous system activation during periods of cold results in metabolism of brown fat stores. Brown fat is located around the neck, kidneys, axilla, and adrenals in addition to spaces between shoulders, under the sternum, and along the spine. Fatty acids in the brown fat stores are oxidated in an exothermic reaction to produce heat. Nonshivering thermogenesis can occur. The consequence of hypothermia that initiates nonshivering thermogenesis is acidosis until age 1 to 2 years.
e) Hypothermia in neonates results in the release of norepinephrine, peripheral and pulmonary vasoconstriction, increasing acidosis, increased pulmonary pressures and right-to-left shunting, and eventually hypoxia, further perpetuating the cycle.
f) Avoid hypothermia by instituting the following: Increase room temperature, cover the patient’s head and exposed extremities, and use overhead warming light. Beware of burns. Use recommended distances for safe use. Heat and humidify delivered gases.
B Pediatric pharmacologic considerations
a) Immature organ systems are responsible for existing pharmacologic differences between infants and children.
b) Physiologic characteristics that modify the pharmacokinetic (what the body does to the drug) and pharmacodynamic (what the drug does to the body) activity include differences in total body water (TBW) composition, immaturity of metabolic degradation pathways, reduced protein binding, immaturity of the blood-brain barrier, greater proportion of blood flow to the vessel-rich organs (brain, heart, liver, and lungs), reductions in glomerular filtration, a smaller functional residual capacity, and increased minute ventilation.
c) TBW, expressed in liters, is determined as a percentage of total body weight (1 L of water weighs 1 kg). The changes in TBW, intracellular fluid (ICF), and extracellular fluid (ECF) during maturation are listed in the following table.
2. Volume of drug distribution
a) Infants have a larger extracellular fluid compartment and greater TBW content.
b) There is a greater adipose content and a higher ratio of water to lipid. Fat content is approximately 12% at birth, doubling by 6 months of age and reaching 30% at 12 months of age.
c) These factors lower plasma drug concentrations when water-soluble drugs are administered according to weight.
d) A larger drug loading dose is required to achieve the desired plasma concentration. The effect of immaturity on the volume of distribution is not as evident for lipophilic drugs that are transported across cell membranes.
a) Total plasma protein is decreased in infants, reaching equivalent adult concentrations by childhood.
b) Both albumin and alpha 1-acid glycoprotein (AAG) concentrations are diminished at birth but reach the adult equivalency by infancy (age 4 weeks).
a) Phase II reactions, which are immature at birth, consist of conjugation or synthesis. Conjugation couples the drug with an endogenous substrate (glucuronidation, methylation, acetylation, and sulfation) to facilitate excretion.
b) Newborns lack the capacity to efficiently conjugate bilirubin (decreased glucuronyl transferase activity), and metabolize acetaminophen, chloramphenicol, and sulfonamides.
c) Although the necessary enzyme systems are present at birth, enzyme activity is reduced, increasing drug elimination half-lives.
5. Rectal and oral drug administration
a) Drugs are usually formulated as liquids for oral administration in children.
b) Midazolam may be administered orally for premedication, and the rectal route may be selected for the administration of acetaminophen, opioids, barbiturates, and benzodiazepines.
c) Both routes rely on passive diffusion for drug absorption. The resulting plasma drug concentration depends on the molecular weight, degree of drug ionization, and lipid solubility.
d) Orally administered drugs are generally reserved for older children because gastric pH is elevated in neonates at birth (pH 6 to 8), and although decreased to a pH level of 1 to 3 within 24 hours, adult gastric pH values are not consistent until age 2 years.
e) Gastric absorption is reduced after oral administration of acidic drugs in infants. Gastric emptying time reaches adult values by 6 months of age. Although gastric emptying time does not affect drug absorption, it may alter peak drug concentration.
f) Acetaminophen, a metabolite of phenacetin, is a popular and safe analgesic and antipyretic commonly administered to children during the perioperative period.
g) The analgesic and antipyretic effects of acetaminophen are equivalent to those of aspirin when the drugs are administered in equipotent dosages.
h) Suppositories should not be divided in an attempt to provide the exact calculated dose because the suspended acetaminophen is distributed unevenly within the suppository. Recommended acetaminophen doses have been based on the age of the child, weight, body surface area calculations, and fractions of adult dosages.
i) Currently recommended oral and rectal doses of acetaminophen range from 10 to 15 mg/kg every 4 hours. Because of the variable absorption of acetaminophen suppositories, some practitioners have advocated the administration of larger initial rectal dosages. It should be emphasized that subsequent rectal doses should be decreased (20 mg/kg), and the dosing interval should be extended to every 6 to 8 hours.
j) After acetaminophen administered during the perioperative period, the parents should be informed as to the time of administration and be advised of appropriate acetaminophen dosages (60-65 mg/kg/day).
k) The daily acetaminophen dosage administered either rectally or orally should be limited to 100 mg/kg/day for children and 75 mg/kg/day for infants.
l) Sedation with nasally administered midazolam (0.2 mg/kg) may be achieved in as little as 10 to 20 minutes and is explained in part through drug absorption via the olfactory mucosa. Nasal administration is unpleasant because midazolam produces a burning of the nasal mucosa.
m) Oral fentanyl, although effective in producing significant sedation, has been plagued by significant side effects, including facial pruritus (up to 80%) and postoperative nausea and vomiting, seven times greater than when a child receives an oral meperidine, midazolam, or atropine premedicant.
n) Water-soluble drugs (atropine, fentanyl, lidocaine, morphine) may be administered via inhalation; however, only 5% to 10% of the administered dose will reach the systemic circulation.
Inhalation agents
a) Although tidal volume is similar between children and adults (5-7 mL/kg), children have greater minute ventilation and a higher ratio of tidal volume to functional residual capacity (5:1) compared with adults (1.5:1).
b) The greater minute ventilation and higher cardiac output in infants and children are responsible for rapid inhalation anesthetic uptake and rapidly increasing alveolar anesthetic concentration. In addition, their decreased distribution of adipose tissue and decreased muscle mass affect the rate of equilibration among the alveoli, blood, and brain.
c) The percentage of blood flow to the vessel-rich organs is greater than in adults, and the blood-gas partition coefficients are lower in infants and children.
d) Anesthetic requirements are known to change with age. Neonates have a somewhat lower minimum alveolar concentration (MAC) than infants, which peaks at around 30 days of age.
e) MAC is higher in infants from age 1 to 6 months of age; thereafter, MAC values are known to decrease with increasing age.
f) Myocardial depression may be exaggerated when inhalation anesthetics are administered to pediatric patients. A more rapid rise FA/FI ratio, the greater percentage of blood flow to the vessel-rich organs, and higher administered anesthetic concentrations are central to the cause of myocardial depression.
g) Inhalation induction is more rapid in pediatric patients and is accompanied by a higher incidence of myocardial depression than in adults.
a) The MAC of isoflurane in oxygen is 1.6% in infants and children.
b) Inhalation induction with isoflurane produces more adverse respiratory events (breath-holding, coughing, and laryngospasm with copious secretions) than sevoflurane.
c) Administration of isoflurane to adults produces dose-dependent decreases in peripheral vascular resistance, but increases in heart rate maintain blood pressure. This touted advantage (e.g., increase in heart rate to maintain blood pressure) does not occur in infants.
d) Anesthetic induction in infants with isoflurane produces significant decreases in heart rate, blood pressure, and mean arterial pressure that are not corrected with prior atropine administration.
a) The MAC of desflurane in oxygen is 9% for infants and 6% to 10% for children.
b) Desflurane has the lowest blood-gas partition coefficient of all the inhalation anesthetics (0.42), which facilitates a rapid induction, rapid alterations in anesthetic depth, and emergence.
c) Similar to isoflurane, desflurane is pungent and is associated with more adverse respiratory events during inhalation induction, including breath-holding, laryngospasm, coughing, and increased secretions with accompanying hypoxia.
d) After inhalation induction with sevoflurane, desflurane is appropriate for the maintenance of general anesthesia with face mask, ETT, or laryngeal mask airway (LMA).
e) As in adults, dramatic increases in desflurane concentrations may induce sympathetic stimulation evidenced by tachycardia and hypertension.
a) The MAC of sevoflurane in oxygen is 3% for infants up to 6 months of age, decreasing to 2.5% to 2.8% up to 1 year of age. The MAC of sevoflurane in oxygen is 2% to 3%.
b) Sevoflurane produces a more rapid induction and emergence than halothane because of its low blood-gas partition coefficient.
c) Sevoflurane is readily accepted for mask induction, and its safe cardiovascular profile (compared with halothane) is responsible for the increasing popularity of sevoflurane in pediatric anesthesia.
d) Minute ventilation is significantly lower, and respiratory rate increases until apnea occurs.
e) Sevoflurane metabolism may produce concentration-dependent elevations in serum fluoride levels that decline when sevoflurane is discontinued.
f) Some clinicians, when performing longer procedures, use sevoflurane for anesthetic induction and subsequently introduce either desflurane or isoflurane for anesthetic maintenance. This clinical decision reduces patient cost and limits sevoflurane exposure.
g) Sevoflurane does not sensitize the myocardium to the effects of endogenous and exogenous catecholamines, but concentration-dependent myocardial depression may occur.
a) A variety of terms are used interchangeably when referring to postoperative agitation. These include emergence delirium, emergence agitation, and postanesthetic excitement. These terms describe altered behavior in the immediate postoperative period manifesting as nonpurposeful restlessness, crying, moaning, incoherence, and disorientation (known here as emergency delirium [ED]).
b) Case reports also suggest that ED occurs more frequently in preschool-aged children (younger than age 6 years).
c) The reported incidence of ED is between 25% and 80%, although the incidence has been difficult to pinpoint because previous studies are confounded by the previously mentioned varying definitions.
d) The Pediatric Anesthesia Emergence Delirium (PAED) scale for the assessment of ED is listed in the following box.
e) Fortunately, ED is self-limiting but may manifest for as long as 45 minutes.
f) In a search for the causation of ED, several emerging themes have been examined. Proposed etiologies include rapid emergence in a strange environment, pain upon awakening, and preoperative behavior.
g) Several strategies have been advocated for the prevention of ED, although a scientific, clinically tested strategy for prevention has yet to be advanced. After inhalation induction with sevoflurane, propofol infusion for maintenance has been demonstrated to reduce ED.
h) Some anesthesia providers advocate the substitution of sevoflurane with isoflurane, yet no studies have detailed the effectiveness of this strategy.
i) The phenomenon of ED is clearly increased after the administration of sevoflurane (and likely desflurane).
Intravenous anesthetics
a) Infants and children have a higher proportion of cardiac output delivered to vascular-rich tissues (i.e., heart, brain, kidneys, and liver).
b) Intravenously administered drugs are readily taken up by these tissues and are subsequently redistributed to muscle and fat—tissues that are less well perfused.
c) Intravenously administered drugs may have a prolonged duration of action in infants and children because of decreased percentages of muscle and fat.
d) The central nervous system (CNS) effects of opioids and barbiturates may also be prolonged because of the immaturity of the blood-brain barrier.
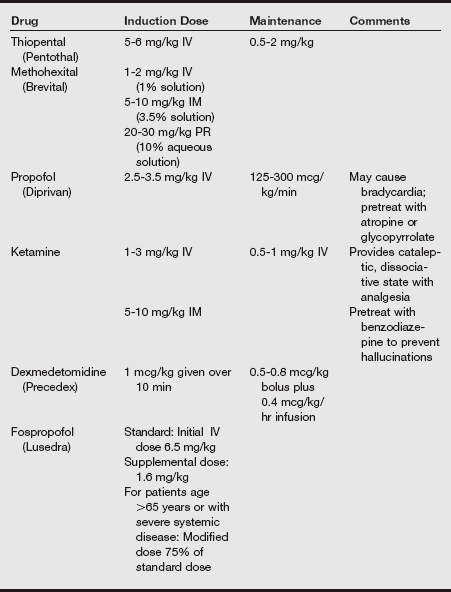
e) Although this evidence suggests that intravenously administered anesthetic doses should be reduced, one must also recall the effect of increased body water. Increased doses of thiopental, propofol, and ketamine are required, presumably because of a greater volume of distribution.
a) Propofl has a rapid onset and a short duration of action and has been established as a sole agent for induction and maintenance of general anesthesia or may be combined with an opioid and nitrous oxide to provide total intravenous (IV) anesthesia.
b) Propofol may be delivered as a continuous infusion for short diagnostic and radiologic procedures and is used as a primary sedative in chronically ventilated intensive care patients.
c) Its antiemetic properties may reduce the incidence of postoperative nausea and vomiting in children undergoing strabismus correction.
d) Infants require larger induction doses (2.5-3 mg/kg) than children (2-2.5 mg/kg). These induction doses produce moderate decreases in systolic blood pressure.
e) The pain that accompanies IV administration may be reduced with the addition of as little as 0.2 mg/kg of lidocaine.
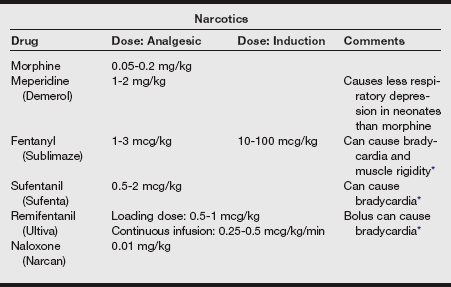
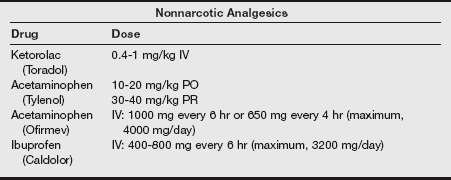
f) Additional strategies suggested for decreasing the pain of injection include a slower injection of propofol into a rapid-running IV line or the injection into larger IV catheters placed in the antecubital space. Induction agents and analgesics are listed in the following tables.
Neuromuscular relaxants
a) Increases in ECF volume and the ongoing maturation of neonatal skeletal muscle and acetylcholine receptors affect the pharmacokinetics and pharmacodynamics of neuromuscular relaxants.
b) The effective doses of clinical neuromuscular blocking drugs in various age groups are listed in the following table.
Effective Doses (ED95) of Clinical Neuromuscular Blocking Drugs (mcg/kg)

*Should be used for emergency airway stabilization in children younger than 12 years. Not for routine intubation.
c) The neuromuscular junction is incompletely developed at birth, maturing after 2 months of age. Skeletal muscle, acetylcholine receptors, and the accompanying biochemical processes essential in neuromuscular transmission mature during infancy into childhood.
d) The presynaptic release of acetylcholine is slowed compared with in adults, which explains the decreased margin of safety for neuromuscular transmission in neonates. The acetylcholine receptors of newborns are anatomically different from adult receptors, which may explain the sensitivity of neonates to the nondepolarizing class of neuromuscular relaxants.
e) This neuromuscular immaturity may be demonstrated with the appearance of fade after tetanic stimulation in the absence of neuromuscular blocking drugs.
a) Because succinylcholine contains acetylcholine moieties, its IV administration will reproduce the effects of acetylcholine when it interacts with nicotinic and muscarinic receptors, provoking both sympathetic and parasympathetic cardiovascular responses.
b) Stimulation of the parasympathetic ganglia or direct stimulation of cardiac muscarinic receptors produces sinus bradycardia, junctional rhythms, unifocal premature ventricular contractions, and ventricular fibrillation.
c) The prior administration of atropine 0.02 mg/kg will block cardiac muscarinic receptors and minimize the decreases in heart rate.
d) Dysrhythmia is more common in children, particularly after repeated doses in the presence of hypoxia or a concurrent electrolyte imbalance.
e) Myoglobinemia may occur in up to 20% of children who receive IV succinylcholine.
f) The prior administration of a small dose of a nondepolarizing neuromuscular blocking drug will modify the degree of myoglobinuria.
g) Myalgia is common after succinylcholine administration.
h) Succinylcholine is a known triggering agent for the development of malignant hyperthermia.
i) Neonates are more resistant to the effects of succinylcholine than children and adults. This sensitivity is illustrated by the effective dose in 95% of the population (ED95) for neonates (620 mcg/kg), infants (729 mcg/kg), children (423 mcg/kg), and adults (290 mcg/kg).
j) The increase in dose requirement is in part a result of the increased volume of distribution within the large extracellular compartment.
k) Plasma cholinesterase activity is reduced in neonates; however, the duration of action after a single dose is of expected duration (6-10 minutes). A longer duration of action after a single bolus dose suggests the presence of an inherited deficiency of plasma cholinesterase activity.
l) IM succinylcholine may facilitate endotracheal intubation in children without suitable IV access. Because of the increased volume of distribution, a larger dose is required to achieve satisfactory relaxation. Although a dose of 3 mg/kg will produce satisfactory relaxation in 85% of patients, an IM dose of 4 mg/kg in the deltoid muscle will provide skeletal muscle relaxation in all, with a duration of action of up to 21 minutes.
m) To attenuate the effects of succinylcholine at both the nicotinic and muscarinic receptors, atropine at a dose of 0.02 mg/kg may be combined in the same syringe with the calculated dose of succinylcholine or in an additional syringe, which is administered in a selected muscle group before succinylcholine administration.
n) Unexpected cardiac arrest has been reported after the routine administration of succinylcholine, with fewer than 40% of patients successfully resuscitated.
o) Succinylcholine should not be routinely used for airway management in children younger than 8 years of age.
Nondepolarizing neuromuscular blocking agents
a) Infants and children are more sensitive than adults to the effects of nondepolarizing neuromuscular blocking drugs.
b) A lower plasma concentration of the selected neuromuscular relaxant is required to achieve the desired clinical level of neuromuscular blockade.
c) This does not imply that the selected dosage should be decreased because infants have a greater volume of distribution.
d) The larger volume of distribution and slower drug clearance result in longer half-life elimination, decreasing the need for repeated drug dosing (longer dosing intervals).
e) Neuromuscular function monitoring must be used to guide repeated administration of these drugs in all pediatric patients.
f) The selection of a nondepolarizing neuromuscular relaxant should take into consideration the desired degree and duration of skeletal muscle paralysis, the immaturity of organ systems, and the associated side effects of the selected relaxant.
a) An intermediate-acting neuromuscular relaxant that is metabolized by nonspecific esterases and spontaneous breakdown of the parent compound by Hofmann elimination.
b) Cisatracurium also uses Hofmann elimination and nonspecific ester hydrolysis for the metabolism of the parent compound.
c) The duration of action of atracurium is relatively the same as in adults.
d) The volume of distribution is greater in infants, yet the clearance is more rapid. Accordingly, an intubating dose (0.5 mg/kg) may be administered in infants and children with the same expected duration of action.
e) Atracurium (intubating dose, 0.5 mg/kg; maintenance dose, 0.2-0.3 mg/kg) and cisatracurium (intubating dose, 0.1 mg/kg; maintenance, dose 0.08-0.1 mg/kg) may be the drugs of choice for the infant because these drugs are independent of mature organ function for elimination.
a) Vecuronium produces minimal alterations in cardiovascular function and stimulates the release of histamine.
b) Infants are more sensitive to the effects of vecuronium than children (ED95 0.047 vs. 0.081 mg/kg).
c) Vecuronium may be administered as a continuous infusion at a rate of 0.8 to 1 mcg/kg/min.
a) An intermediate-acting neuromuscular blocker with a rapid to intermediate onset of 60 to 90 seconds after an intubating dose of 0.6 mg/kg.
b) The potency of rocuronium is greater in infants than children; however, its onset is faster in children.
c) Unlike vecuronium, rocuronium in intubating doses may produce transient increases in heart rate.
d) Skeletal muscle relaxation can be maintained with repeat doses of 0.075 to 0.125 mg/kg.
e) In clinical situations in which IV access is not available, rocuronium may be administered intramuscularly. Acceptable intubating conditions in lightly anesthetized infants occurs 2.5 to 3 minutes after a deltoid IM dose of 1000 mcg/kg and within 3 minutes after 1800 mcg/kg in children.
f) The onset of action approximates the onset of succinylcholine after IM injection.
g) Rocuronium injection into the deltoid provides a faster onset of twitch and ventilatory depression than does injection into the quadriceps muscle group.
h) A disadvantage of this route of administration is the accompanying prolonged duration of relaxation—in excess of 60 minutes.
i) Rocuronium may also be administered by continuous infusion at doses of 0.004 to 0.016 mg/kg/min.
5. Antagonism of neuromuscular blockade
a) Residual neuromuscular blockade places infants and children at risk of hypoventilation and the inability to independently and continuously maintain a patent airway.
b) Because of increased basal oxygen consumption, impaired respiratory function will lead to arterial oxygen desaturation and CO2 retention. The resulting acidosis will potentiate residual neuromuscular blockade.
c) Accordingly, infants and children must have neuromuscular function restored at the conclusion of the surgical procedure. The detection of residual neuromuscular blockade requires the integration of clinical criteria and the assessment of neuromuscular blockade via a peripheral nerve stimulator.
d) Conventional doses of the anticholinesterase inhibitors (50-60 mcg/kg of neostigmine or 500-1000 mcg/kg of edrophonium) combined with appropriate doses of atropine or glycopyrrolate are acceptable for antagonism of nondepolarizing neuromuscular blockade.
e) Useful clinical signs of successful antagonism of neuromuscular blockade include the ability to flex the arms, lift of the legs, and flex the thighs upon the abdomen, providing evidence of the return of abdominal muscle tone, in addition to the return of a normal train-of-four response as assessed by the peripheral nerve stimulator.
f) Neonates are capable of generating a negative inspiratory force of −70 cm H2O with the first few breaths after birth.
g) An negative inspiratory force of at least −32 cm H2O has been found to correspond with leg lift, which is indicative of the adequacy of ventilatory reserve required before tracheal extubation.
h) Clinical investigation is ongoing in examining a novel antagonist of neuromuscular blockade. Sugammadex, a water-soluble, modified γ-cyclodextrin, is being investigated as a reversal of steroidal neuromuscular blocking agents.
i) The drug does not affect acetylcholinesterase, eliminating the need for the co-administration of an anticholinergic.
j) The application of Sugammadex for antagonism of neuromuscular blockade in the pediatric population is being studied.
k) Muscle relaxant reversal: See the following table.
| Drug | Dose |
| Neostigmine | 0.03-0.07 mg/kg |
| Pyridostigmine (Regonol) | 0.2 mg/kg |
| Edrophonium | 0.7-1.4 mg/kg |
| Atropine | 0.01-0.02 mg/kg |
| Glycopyrrolate (Robinul) | 0.01 mg/kg |
C Pediatric anesthesia equipment
The child’s age, weight, and proposed surgical procedure guide the selection of essential pediatric anesthesia equipment. The anesthesia workroom should be appropriately stocked with a variety of sizes of masks, airways, LMAs, laryngoscope blades, ETTs, ETT stylets, blood pressure cuffs, pulse oximeter probes, calibrated pediatric fluid sets, syringe pumps for the delivery of both fluids and drugs, an assortment of IV catheters, tape, and arm boards.
a) The pediatric face mask is designed to fit the smaller facial features of children and eliminate mechanical dead space.
b) Contemporary masks are manufactured from transparent plastics and have a soft, inflatable cuff that sits on the face.
c) The transparent feature allows continuous observation of skin color, the presence of condensation from ventilation exhalation, and the appearance of gastric contents in case vomiting occurs.
a) Appropriately sized oral airways must be readily available. Because of infants’ relatively large tongues, the pediatric airway is predisposed to airway obstruction after the induction of general anesthesia.
b) Oral airways that are too large may produce airway obstruction, inhibit venous and lymphatic drainage, and subsequently produce macroglossia, creating further airway compromise.
c) The oral airway should be inserted with the aid of a tongue blade, displacing the tongue toward the floor of the mouth to allow smooth insertion of the airway.
d) Insertion and rotation of an oral airway in children 5 to 10 years old should be performed with caution because the insertion or rotation may dislodge loose deciduous teeth.
e) Nasal airways are infrequently used in children younger than 1 to 2 years of age. The internal diameter of the nasal airway may unnecessarily increase the work of breathing. Adenoid hypertrophy may make nasal airway placement difficult and produce severe epistaxis.
a) The goal of ETT selection is the placement of an appropriately sized tube that allows controlled ventilation but minimizes laryngeal or tracheal injury.
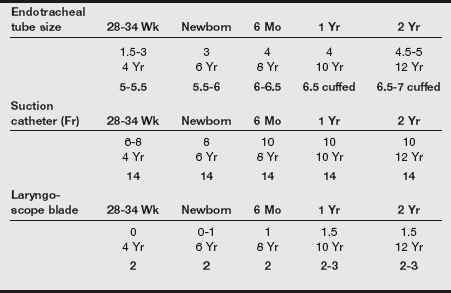
b) Because of patient variability, many formulas exist for the determination of the correct ETT size and for the depth of insertion. Despite countless practitioner recommendations, there is no agreed upon standard formula.
c) In addition, many practitioners fail to appreciate the differences in the internal diameter of small ETTs. ETTs for neonates are sized by the internal diameter, yet the external diameters may differ by as much as 0.9 mm among manufacturers in tubes with identical internal diameters.
d) The approximate size of ETT for children 2 years of age and older may be determined with the following formula: 16 + Age ÷ 4.
e) To accommodate the variability in patient airway size, ETTs one-half size larger and one-half size smaller should be immediately available.
f) The depth of ETT insertion from the dental alveoli may be estimated using the “1, 2, 3, 4/7, 8, 9, 10” rule. For example, the ETT is inserted to a depth of 7 cm in a neonate weighing 1 kg and to a depth of 8 cm in a 2-kg neonate.
g) Another approximate method is to insert the ETT to a depth in centimeters three times the internal diameter of the ETT in millimeters. For example, a 3-mm ETT should be inserted to a depth of 9 cm.
h) Uncuffed ETTs are marked distally with a double black line that provides a visual indication of the depth of the ETT.
i) During intubation, the ETT is passed until the double black line has reached the level of the vocal cords.
j) Regardless of formula or technique used for assessing the depth of an ETT, the method for confirming placement is auscultation of bilateral breath sounds.
k) The table below provides approximate sizes of ETTs, suction catheters, and laryngoscope blades for preterm infants through 12-year-old children.
l) If resistance is encountered with laryngeal advancement of the ETT, the ETT should be withdrawn and an ETT a half size smaller in internal diameter should be selected.
m) After proper placement is confirmed, the anesthesia provider should listen over the child’s mouth (preferably with the aid of a stethoscope) while simultaneously squeezing the reservoir bag and noting the pressure at which an air leak is appreciated.
n) Positive-pressure ventilation may be ineffective when an air leak is detected at 8 to 10 cm H2O.
o) A large or tight-fitting ETT that does not permit a detectable air leak until 25 to 30 cm H2O may be too tight at the level of the cricoid cartilage and may result in postintubation laryngeal edema (“croup”).
p) The selected uncuffed or cuffed ETT should have a demonstrable air leak detected at 20 to 25 cm H2O.
q) Specialized uncuffed and cuffed oral and nasal ETTs may be chosen for otolaryngologic, ophthalmologic, and dental procedures.
r) The uncuffed ETT is a common choice in infants and neonates provided there is an accepted leak.
s) When an unacceptable leak occurs, for instance in a child about to undergo laparoscopy, a cuffed tube may be selected (a half size smaller than the previously selected tube) to minimize repeat laryngoscopies and the cuff inflated, allowing for a leak up to but no greater than to 25 cm H2O.
t) Newer research has demonstrated that the use of cuffed ETTs (a half size smaller than a correctly calculated tube) has not resulted in an increased incidence in laryngotracheal edema or postextubation croup.
a) The LMA is used for short surgical procedures that do not require endotracheal intubation (herniorrhaphy, peripheral extremity surgical procedures) or resuscitation situations.
b) The LMA is available in sizes specific for neonates, infants, children, and adolescents, as shown in the following table.
| Laryngeal Mask Airway Size | Suggested Inflation Volume | Patient Weight Guidelines (kg) |
| 1 | 4 mL | Neonates: Up to 5 kg |
| 1½ | 7 mL | Infants: 5-10 |
| 2 | 10 mL | Infants and children: 10-20 |
| 2½ | 14 mL | Children: 20-30 |
| 3 | 20 mL | 30-50 |
| 4 | 30 mL | 50-70 |
| 5 | 40 mL | 70-100 |
From Brain AIJ, Denman WT, Goudsouzian NG. LMA-Classic and LMA-Flexible Instruction Manual. San Diego: LMA North America; 2011.
c) The inflation of the pharyngeal cuff can produce undue pressure on pharyngeal structures. Similar to the case in an adult ETT cuff, the LMA cuff may be expanded during the course of the anesthetic procedure with the administration of nitrous oxide.
d) The initial volume of air injected into the laryngeal cuff may be regulated by identifying the amount of air and airway pressure that produces an audible leak. This pressure is generally between 15 and 25 cm H2O.
e) Removal of the LMA in pediatric patients can be associated with biting, pulmonary edema, severe laryngospasm, and separation of the tube from the pharyngeal mask.
6. Pediatric breathing circuits
a) The ideal pediatric breathing circuit should be lightweight, minimize dead space, consist of low resistance and a low compressible volume, be adaptable for both spontaneous and controlled ventilation, be capable of providing humidification and warming of inspired gases, and permit the collection and scavenging of exhaled anesthetic gases.
b) For children with decreased minute ventilation (recent opioid administration or high concentrations of potent inhalation agent), fresh gas flow rates may need to be increased or ventilation controlled.
c) The circle breathing system is the most common anesthetic gas delivery system, and technological advancements in anesthesia machine design have decreased the resistance imparted by the absorbent canisters and the one-way inspiratory and expiratory valves.
d) The breathing tubing for the pediatric circle systems is a smaller diameter than the adult tubing and has a lower compression volume, allowing accurate delivery of desired tidal volumes.
e) The circle breathing system is characterized by the presence of CO2 absorbent canisters and a total of three valves (a one-way inspiratory valve, a one-way expiratory valve, and a pop-off or pressure-limiting [APL] valve) that directs exhaled gas to the scavenging system.
f) Advantages of the circle system include the conservation of potent inhalation agents, the ability to retain both heat and humidity, and the ease of collecting and scavenging waste gases.
g) The reservoir bag contains the anesthesia machine–delivered anesthetic mixture inspired by the patient and serves as a visual and tactile monitor of ventilation.
h) Reservoir bags range in size from 0.5 to 6 L. The selected reservoir bag must be appropriate for the patient’s size (i.e., capable of containing a volume in excess of the child’s inspiratory capacity).
i) The use of an inappropriately small reservoir bag may restrict respiratory efforts, and the use of a large reservoir bag inhibits the ability to use the reservoir bag as a monitor of ventilation.
D Perioperative care
Pediatric preoperative preparation
a) Appropriate anesthetic evaluation and management depend on a thorough understanding of the surgical and anesthetic requirements for the proposed procedure. All possible sources of medical information, including the patient chart, physical examination of the child, and the parental or guardian interview are essential.
b) The review of the chart should focus on the medical history (beginning with the gestational history), previous hospitalizations, previous medical or surgical experiences, the presence of chronic illness or infectious disease, and any family history of anesthetic complications (e.g., family history of atypical pseudocholinesterase).
c) The child should also be evaluated for proper growth and development as determined by a review of norms and percentages for age and gender. Developmental delay may suggest a prenatal pathologic condition, the presence of a chronic illness, or the presence of a concurrent neurologic or neuromuscular disease.
d) The examination of any previous anesthetic records is invaluable in gleaning information regarding previous anesthetic encounters.
e) The parent or guardian verifies information obtained during the chart review during the face-to-face interview and physical examination of the child.
f) The physical examination allows the anesthesia provider to evaluate the child’s general health. If not previously evaluated, the child’s ears and nose should be examined.
g) Children between the ages of 5 and 9 years should be examined for the presence of loose teeth, and these should be noted on the evaluation. A loose deciduous tooth that is in danger of being dislodged during airway management should be removed after anesthetic induction with the consent of a parent.
3. Preoperative laboratory testing
a) Preoperative laboratory tests should be ordered based on abnormal findings from the medical history and physical examination.
b) Preoperative hemoglobin determination has characteristically been obtained to provide an assessment of “anesthetic fitness.” An “adequate” hemoglobin concentration is essential for oxygen delivery and has been arbitrarily defined as a hemoglobin of 10 g/dL or a hematocrit of 30%. The determination of an acceptable value requires an understanding of the child’s current medical history, the proposed surgical procedure, and an understanding of global oxygen transport and use. The value of a “routine” hemoglobin determination has been questioned for some time and rarely has been found to affect the anesthetic management of children.
c) Children who benefit from preoperative hemoglobin determinations include premature infants less than 60 weeks of postconceptual age, children with concurrent cardiopulmonary disease, children with known hematologic dysfunction (sickle cell disease), and children in whom major blood loss is anticipated during the surgical procedure.
d) The time constraints of preoperative evaluation hinder the child’s psychological preparation, which has ostensibly become the responsibility of the parent or guardian and the surgeon. Children’s exhibited behavior is age dependent and shaped by fears of parental separation, postoperative pain, the potential for disfigurement, and loss of control. Children during the first 6 months of age readily accept strangers and can be separated from their parents, but children from 6 months to 5 years of age become distressed when separated from their parents.
e) Parental preparation is important. One of the most important tasks is allaying the fear of the parent and family members. The anesthesia provider will foster trust and confidence through a courteous and understandable explanation of the anesthetic experience. Parental anxiety may be driven by personal past anesthetic experiences, such as painful IV catheter placement; coerced mask induction; and postoperative pain, nausea, and vomiting.
f) Parents offer invaluable information regarding their child’s past anesthetic experiences. When the child appears for a repeat surgical procedure, the parents may have important information relative to “what works” and may be helpful in detailing a successful approach.
g) Parental presence during anesthetic induction in preschool-aged and young children may allay the fears of separation for both the parent and child.
a) Guidelines for preoperative fasting are listed in the following table.
Preoperative Fasting Recommendations*
| Ingested Materials | Minimum Fasting Period† (hr) |
| Clear liquids‡ | 2 |
| Breast milk | 4 |
| Infant formulaठ| 6 |
| Nonhuman milk§ | 6 |
| Light meal¶ | 6 |
*These recommendations apply to healthy patients who are undergoing elective procedures. They are not intended for women in labor. Following the guidelines does not guarantee complete gastric emptying.
†The fasting periods noted apply to all ages.
‡Examples of clear liquids include water, fruit juices without pulp, carbonated beverages, clear tea, and black coffee.
§Because nonhuman milk is similar to solids in gastric emptying time, the amount ingested must be considered when determining an appropriate fasting period.
¶A light meal typically consists of toast and clear liquids. Meals that include fried or fatty foods or meat may prolong gastric emptying time. Both the amount and type of foods ingested must be considered when determining an appropriate fasting period.
From American Society of Anesthesiologists Committee. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: an updated report by the ASAC on Standards and Practice Parameters. Anesthesiology. 2011; 114(3):495-511.
b) Prolonged fasting may produce irritability as a result of thirst and hunger. Prolonged fasting may also alter fluid balance, producing preinduction hypovolemia and hypoglycemia.
c) Hypoglycemia is especially problematic in premature infants. Preoperative access to clear fluids (e.g., apple juice, water) 2 hours before anesthetic induction has been shown to have a minimal impact on the resultant gastric volume and pH.
a) Upper respiratory infection
(1) Upper respiratory infections (URIs) are common in the pediatric age group; are seasonal in occurrence; and may be accompanied by cough, pharyngitis, tonsillitis, and croup.
(2) Children with an active or resolving URI have increased airway reactivity, a propensity for the development of atelectasis and mucous plugging of the airways, and the potential to experience postoperative arterial hypoxemia.
(3) Bronchial reactivity may persist for 6 to 8 weeks after a viral lower respiratory tract infection, although this fact has been challenged by experts.
(4) The presence of chronic respiratory disease (asthma or bronchopulmonary dysplasia) requires a thorough assessment to ensure that the disease is well controlled and the child is not currently experiencing an exacerbation.
(5) The anesthesia provider must understand the child’s routine pharmacologic management. A history of steroid use necessitates consideration of steroid supplementation throughout the perioperative period.
(6) Healthy children who are scheduled for the placement of tympanostomy tubes frequently have rhinitis. In deciding whether to proceed with anesthesia, additional patient history must be obtained to differentiate between a chronic allergic or an acute infectious presentation and to determine whether there is lower airway involvement.
(7) The assessment of the color and the duration of nasal drainage will assist in deciding whether rhinorrhea is chronic or acute. Purulent nasal discharge associated with pharyngitis, cough, or fever may indicate a bacterial or viral URI.
(8) Additional information may be obtained by questioning the parents regarding their assessment of the child’s current health. Helpful questions include: Does your child appear sick? Is your child eating, sleeping, and playing normally? Is there anyone in the family (including siblings) who is currently ill? Children with chronic allergic rhinorrhea who exhibited a clear nasal drainage without accompanying signs of illness (no cough, pharyngitis, wheezing, or associated fever) are probably in satisfactory condition for elective general anesthesia with no imposed increased risk.
(9) Lower respiratory tract dysfunction typically accompanies viral or bacterial URI. This combination may be associated with a greater frequency of laryngospasm (fivefold greater incidence) and bronchospasm (10-fold greater incidence) during anesthetic management, particularly when endotracheal intubation is performed.
(10) Although mild URI may be inconsequential during the intraoperative period, significant problems may develop in the immediate postoperative period. Studies have noted an increase in the incidence of postintubation croup, hypoxemia, and bronchospasm in patients with URIs compared with asymptomatic children.
(11) Multiple factors must be considered when one is deciding whether to cancel an elective procedure. Children with signs and symptoms of acute airway dysfunction should have further medical evaluation by a pediatrician. A white blood cell count of 12,000 to 15,000/mm3 suggests the presence of infection, and the surgery should be canceled. Clearly, elective surgery should be postponed for children who have a cough and pharyngitis accompanied by fever and wheezing.
(12) Current recommendations for patients with URI infections are listed in the box on pg. 505.
(1) The parent may relay a previous history of a heart murmur, or a murmur may be discovered during the physical examination. A heart murmur may be detected in up to 50% of pediatric patients.
(2) It is important to properly classify the murmur as either innocent or pathologic before anesthetic intervention. The murmur may not impose a functional limitation; however, this may change with the physiologic stress associated with an anesthetic.
(3) Children with “functional” murmurs are generally asymptomatic without the presence of cyanosis and are growing appropriately. An example of a functional murmur is the Still vibratory systolic murmur, which is common in children between the ages of 2 and 6 years.
(4) If the heart murmur has been previously detected and the child has undergone an evaluation, the parent may be able to provide the information as to the relevance of the murmur. Previous records may also contain information as to the significance and whether additional testing was performed to assess the physiologic significance.
(5) It is important that a pediatrician or cardiologist evaluate previously undiagnosed murmurs before the induction of anesthesia.
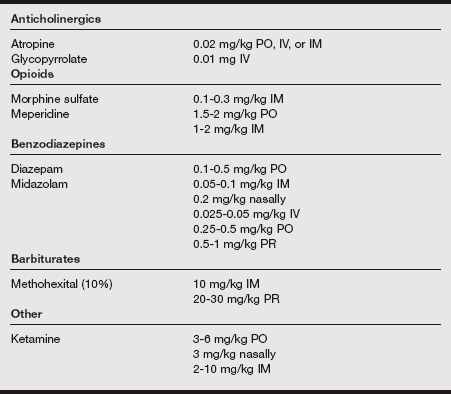
a) The selection and administration of premedication for pediatric patients require an understanding of the desired goals, the planned surgical procedure (inpatient or outpatient procedure), the familiarity and previous experiences with the particular drug, and the availability of nursing staff to monitor the child after the drug’s administration.
b) The ideal premedicant should be dependable with a rapid and reliable onset and offset and should be devoid of undesirable effects.
c) The commonly prescribed pediatric premedicants are listed in the following table.
a) Patient blood pressure and heart rate are monitored for the assessment of the cardiovascular system.
b) Pulse oximetry and capnography are used for the assessment of the adequacy of oxygenation and ventilation, a temperature probe for intermittent or continuous assessment of core body temperature, and a neuromuscular function monitor for the evaluation of the child’s response to the administration of neuromuscular blocking drugs.
c) A precordial or esophageal stethoscope should be used for the continuous assessment of heart rate during anesthetic induction and throughout the perioperative period.
d) Some circumstances require the application of arterial and central venous pressure monitoring. Small multilumen catheters are available for pediatric patients. These catheters are advantageous when large blood losses are expected (e.g., during burn débridement and skin grafting). However, these catheters have long, thin lumens that may severely limit the rate at which IV fluid or blood may be administered.
e) When rapid flow is required, a peripheral IV line may be used for maintenance and deficit fluid replacement, and a single-lumen venous catheter placed within the femoral vein may be reserved for colloid and blood administration.
a) Mask induction is the most popular and is easily accomplished in infants younger than 8 months of age, as well as in children.
b) The essential monitoring modalities for inhalation induction include a precordial stethoscope and a pulse oximeter.
c) Anesthetic induction is begun with a 70:30 mixture of nitrous oxide and oxygen via mask or a “cupped hand” that is placed on the child’s chin with the anesthetic mixture directed toward the mouth and nose.
d) A pacifier may quiet the infant during the induction, or the infant may suck on the end of the anesthesia provider’s gloved finger. Sevoflurane is added to the nitrous oxide–oxygen mixture beginning with a 2% concentration, with a rapid increase to 8%.
e) The mask may then be introduced as the inspired concentration is increased. The anesthesia provider should await the return of respiration and avoid the temptation to administer a breath because this may produce coughing and laryngospasm.
f) Unconsciousness is produced with inspired sevoflurane concentrations 6% to 8%. After the loss of consciousness, nitrous oxide is discontinued, and sevoflurane is administered in 100% oxygen. Because of the low blood gas solubility coefficient and rapid uptake, the choice to use nitrous oxide is provider specific.
g) At this time, the anesthesia provider should begin to assist respiration and promptly decrease the inspired anesthetic concentration of sevoflurane to 2% to 2.5%. Controlled ventilation with high-inspired concentrations of inhalation agent aggravates myocardial depression, precipitating the development of sudden cardiac arrest.
h) During assisted ventilation, IV access should be established. The age of the child, proposed surgical procedure, and ease of airway management during induction are the determining factors for whether to proceed with IV access.
i) For elective surgical procedures, neonates may be managed with a 24-gauge catheter, infants with a 22-gauge catheter, and children with a 20-gauge catheter.
j) Surgical procedures with expected large third-space fluid loss or blood loss require an additional IV catheter. Preferred sites for IV access include the nondominant upper extremity (dorsum of the hand, antecubital fossa) and the lower extremity (dorsum of the foot, saphenous vein).
k) After the establishment of IV access, preparations are made for endotracheal intubation. Intubation may be accomplished using the inhaled anesthetic agent without muscle relaxation or subsequent to the administration of a nondepolarizing neuromuscular relaxant.
l) The administration of a neuromuscular relaxant decreases the potential for the cardiovascular depression that accompanies the administration of high concentrations of inhalation agents that may be required to facilitate laryngoscopy and intubation.
m) Whatever method is selected, the inhalation agent should be discontinued immediately before laryngoscopy. This practice minimizes the contamination of the operating room with free-flowing inhalation agent from the patient breathing circuit, and, more importantly, the delivery of high inspired anesthetic concentrations is avoided immediately after intubation during the confirmation of ETT placement. After confirmation, the ETT is secured and the position of the tube at the alveolar ridge or lip is noted on the anesthetic record.
9. Parental presence during anesthetic induction
a) Children older than 1 year of age may have difficulty with parental separation and may require premedication to ease their anxiety.
b) In addition to preoperative medication, a new strategy some anesthetic departments have adopted is to have one parent present for anesthetic induction. Parents prefer to stay with their children during diagnostic procedures such as bone marrow biopsy, immunization, dental rehabilitation, and the induction of anesthesia.
c) Anesthesia departments may have age limitations, not allowing parental presence for children less than 12 to 18 months of age. Clearly, the parent should not be invited to participate in the induction of a child with a “full stomach” or compromised airway.
a) IV induction is generally reserved for children with an existing IV line. An IV induction may be clinically indicated when a child has a full stomach or a history of gastroesophageal reflux.
b) IV induction is quicker and more dependable, facilitating the rapid securing of the airway with endotracheal intubation.
c) Venipuncture can be a frightening experience for needle-phobic child. Oral premedication with midazolam before the child enters the operating room may be beneficial to decrease the child’s anxiety and gain his or her cooperation.
d) The pain associated with preoperative IV access may be eased with the subcutaneous injection of local anesthetic via a 30-gauge needle. Additional analgesia may be provided by the administration of a 50:50 mixture of nitrous oxide and oxygen during venipuncture.
e) The administration of concentrations in excess of 50% may produce significant disorientation and muscle rigidity, impeding the ability to obtain IV access.
f) The timely application of a eutectic mixture of local anesthetic also minimizes the pain of venipuncture. Suitable IV sites are identified preoperatively and marked with an ink pen. The eutectic mixture of local anesthetic is applied well in advance (30-60 minutes) to ensure effectiveness and covered with a Tegaderm dressing.
a) On rare occasions, an intramuscularly administered drug may be required in uncooperative children or in children who refuse alternative routes (oral, nasal, or rectal) for premedication.
b) Ketamine produces dose-dependent unconsciousness and analgesia, and the child may appear to be in a catatonic state.
c) Parents who witness the administration of ketamine should be warned that their child might exhibit spontaneous involuntary movements and nystagmus.
d) Ketamine is a cardiac stimulant that produces an increase in systemic blood pressure and heart rate. Additional undesirable effects include bronchodilation, increases in intraocular and intracranial pressure, disorientation, unpleasant dreaming, and hallucinations.
e) The psychogenic effects may be decreased with the concomitant administration of a benzodiazepine. IM ketamine in a dose of 2 to 3 mg/kg facilitates inhalation induction in children who are reluctant to be subjected to inhalation induction or venipuncture.
f) Ketamine doses between 5 and 10 mg/kg are associated with a lengthy recovery period and the inability to accept oral fluids.
g) Ketamine may be injected in a small volume because a variety of formulated concentrations exist. Ketamine is particularly advantageous in children with cardiovascular instability because the cardiovascular system is stimulated via the CNS.
h) Intramuscular (IM) midazolam may be used to induce sleep. IM midazolam (0.1-0.15 mg/kg) may also be used as a premedicant. IM induction is less reliable and more uncomfortable for the child.
E Pediatric airway management
1. The normal pediatric airway
Differences Between the Adult and Pediatric Airway
| Pediatric | Adult | |
| Laryngeal location | C2-C4 | C4-C6 |
| Narrowest location of airway | Cricoid | Glottis |
| Shape of epiglottis | Omega shaped | V-shaped |
| Right mainstem bronchus | Less vertical | More vertical |
| Tongue | Relatively larger | Relatively smaller |
| Cricoid | Conical shape | Cylindrical shape |
| Head | Pronounced occiput | Flatter occiput |
a) These factors interact to maintain pharyngeal airway patency.
b) The infant larynx is located in a more cephalad position (C3-C4 interspace) achieving the adult position (C5) by 6 years of age. This position allows infants to swallow and breathe simultaneously.
c) The epiglottis is described as omega or U shaped, is short and stiff, and projects posteriorly at a 45-degree angle, which increases the difficulty of vocal cord visualization.
d) Because of these anatomic differences, the use of a straight laryngoscope blade placed into the vallecula improves visualization of the glottic opening.
e) The infant tongue is significantly larger in relation to the oral cavity, increasing the size and volume of soft tissue within the oral cavity.
f) There is a smaller submental space for displacement of the tongue during laryngoscopy.
g) Unlike neonates and infants, children have an increase in oropharyngeal tissue with the appearance and hypertrophy of the tonsil and adenoids between the ages of 2 and 7 years.
h) The size of the tongue and the position of the epiglottis increase the difficulty of mask ventilation.
i) Because of the more cephalad laryngeal position, the tongue lies closer to the palate and easily occludes it, producing upper airway obstruction. This explains why neonates and small infants are obligate nose breathers.
j) Attempted mask ventilation of an infant may be unsuccessful until the mouth is opened and the tongue is swept away from the palate.
k) These anatomic differences also make the airway appear more anterior during laryngoscopy. Various pediatric oral airways sizes should be immediately available before the administration of sedatives or the induction of general anesthesia.
l) The infant head and occiput are large relative to the shoulders and upper body. With slight head extension and a small towel placed under the shoulders, the alignment of the pharyngeal and laryngeal axis can be improved, and the infant will assume a proper intubating position.
m) The maintenance of a neutral head position is more helpful than an exaggerated head tilt.
n) Because of the higher position of the infant larynx and the larger occiput, the traditionally applied “sniffing” position may actually hinder glottic visualization.
o) A thorough dental evaluation should be conducted. Loose teeth should be suspected between 5 and 12 years of age.
a) Etiology: The incidence of laryngospasm may be greater in the pediatric population because of specific practices in the anesthetic management of infants and children. Several factors generally associated with the development of laryngospasm are listed in the following box.
The risk of laryngospasm is increased on induction when airway instrumentation is attempted before an adequate depth of anesthesia has been achieved, without the benefit of neuromuscular blocking drugs, and in infants and children with residual effects of previous URIs.
b) Prevention of laryngospasm: The prevention of laryngospasm requires an understanding of the risk factors. Measures that may be undertaken for the prevention of laryngospasm are listed in the following box.
(1) Incomplete airway obstruction may be evident as “grunting” or audible inspiratory and expiratory sounds as heard through a precordial stethoscope accompanied by tracheal tug and thoracoabdominal asynchrony. A decrease in arterial oxygen saturation may also occur.
(2) Management of laryngospasm on induction of anesthesia consists of three essential processes. First, the responsible noxious stimuli should be discontinued (surgical stimulation, attempted airway instrumentation during “light” anesthesia, removal of pharyngeal secretions with gentle suctioning). Next, anesthetic depth should be increased by the delivery of increased concentration of inhalation agent or the IV administration of a small dose of propofol. Third, gentle positive-pressure ventilation using 100% oxygen should be attempted using a properly applied face mask with concurrent airway opening maneuvers (slight head extension, chin lift and jaw thrust). On occasion, this may require two individuals, one to firmly apply the face mask and open the airway and one individual to attempt positive-pressure ventilation.
(3) The transition to complete airway obstruction becomes evident with the absence of inspiratory and expiratory sounds, as well as the inability to deliver positive-pressure ventilation. Further deterioration of arterial oxygen saturation with accompanying bradycardia may occur despite the continued application of positive-pressure ventilation, which may not be effective. The administration of succinylcholine is then required to break the laryngospasm.
(4) If complete airway obstruction continues unabated, the IV or IM administration of atropine and succinylcholine should be administered without delay.
(5) In the absence of IV access, IM succinylcholine (4 mg/kg) and atropine (0.02 mg/kg) are administered in the deltoid or gluteus maximus muscle. After IM administration, the vocal cords will begin to relax within 60 seconds, permitting positive-pressure ventilation and relaxation to facilitate endotracheal intubation.
(6) With continued deterioration in arterial oxygen saturation, intubation may be required before the onset of skeletal muscle relaxation. In an extreme situation, the application of lidocaine to the vocal cords may produce sufficient relaxation, allowing endotracheal intubation.
(7) The direct application of pressure to the laryngospasm notch, the space located immediately behind the ear, bounded anteriorly by the mandibular rami, posteriorly by the mastoid process, and cephalad by the base of the skull is advocated by some practitioners. Bilateral firm and direct application of pressure toward the skull base produces an anterior displacement of the mandible. In addition to producing a jaw thrust, the intense stimulation with postcondylar pressure in a lightly anesthetized patient often produces a ventilatory sigh. This maneuver may be successful for the treatment of laryngospasm.
3. The difficult pediatric airway
a) A difficult airway may be defined as difficulty in accomplishing mask ventilation or endotracheal intubation. The identification of a difficult pediatric airway begins with a thorough history followed by physical examination of the mouth, head, and neck.
b) A history of snoring, difficulty breathing with feeding, current or recent URI, and history of croup should be obtained.
c) Previous anesthetic records are an invaluable resource in determining the history of difficult airway management. However, a prior uneventful anesthetic does not preclude the possibility of difficulty of airway management with succeeding anesthetics.
d) The physical examination should focus on the assessment of craniofacial skeletal features, specifically the size and shape of the mandible and maxilla, the size of the tongue in relation to the oral cavity, the absence of dentition, the presence of loose dentition, and the range of motion of the neck.
e) The pathologic conditions that affect pediatric airway management are listed in the box on pg. 512.
a) Category I. These individuals have a normal-appearing airway and minimal or no sternal retractions with an age-appropriate respiratory rate and normal arterial oxygen saturation.
b) Category II. These individuals presents with moderate airway distress or significant airway disease (e.g., laryngeal papillomatosis). The anesthetic history demonstrates previous successful airway management.
c) Category III. The delineating characteristic of this group is an abnormal airway identified by physical examination (micrognathia, macroglossia, palate deformities, or prominent dentition). Individuals with maxillary or mandibular pathology (e.g., Treacher Collins syndrome, Down syndrome) are grouped here. Included in this group are individuals with mediastinal mass.
d) Category IV. Individuals in this category have significant respiratory distress exemplified by marked sternal retractions, significantly decreased arterial oxygen saturations, and marked increases in respiratory rate that may ultimately lead to respiratory fatigue. An example is the pediatric patient presenting with an inhaled foreign body.
a) This is often best accomplished in a controlled environment (operating suite) with general anesthesia and the presence of surgical expertise to obtain a surgical airway should there be an inability to provide mask ventilation.
b) For individuals who are category I and II, an inhalation induction with sevoflurane, with the application of continuous positive airway pressure (5-10 cm H2O) minimizes soft tissue obstruction and pharyngeal collapse with increasing depths of anesthesia. After confirmation of the ease of positive-pressure ventilation and acceptable arterial oxygen concentration—then and only then—should a neuromuscular relaxant be administered to facilitate endotracheal intubation.
c) Individuals who are category III and IV require advanced preparation for airway management and require the immediate availability of surgical expertise during airway management.
d) Airway adjuncts, including size-appropriate LMAs, oral airways, various laryngoscope blades, video laryngoscopes, flexible fiberoptic bronchoscopes, or any other difficult airway adjunct requested, should be immediately available. A flexible fiberoptic bronchoscope with an external diameter of 2.2 to 4.0 mm will traverse a 2.5- to 4.5-mm internal diameter ETT. These individuals are best managed with spontaneous ventilation.
e) A slow and deliberate inhalation induction is accomplished with sevoflurane. The administration of a neuromuscular relaxant should be avoided because total airway obstruction may follow with the loss of pharyngeal and laryngeal tone.
F Pediatric intravenous fluid and blood therapy
a) Intravascular fluid balance is influenced by a number of preoperative and perioperative circumstances. Preoperative IV fluid administration minimizes the degree of dehydration that accompanies the NPO (nothing by mouth) period. Unless there exists a compelling reason to place an IV catheter preoperatively, IV therapy is generally avoided in pediatric patients until general anesthesia has been induced via inhalation.
b) Perioperative fluid homeostasis is altered by a number of factors, including inhalation agent administration, surgical trauma, and preoperative fasting.
c) Potent inhalation agents produce peripheral vasodilation and varying degrees of myocardial depression, decreasing systemic blood pressure and end-organ perfusion.
d) Dehydration after prolonged preoperative oral fluid abstinence aggravates these decreases in systemic blood pressure.
e) The delivery of cold, dry anesthetic gases via an ETT bypasses normal anatomic humidification, increasing the loss of fluid from the respiratory tract. These insensible respiratory fluid losses can be minimized with the use of active or passive humidification systems during the intraoperative period.
f) General anesthesia modifies the neuroendocrine control of fluid balance. Surgical stress increases plasma glucose levels. Hyperglycemia induces an osmotic-induced renal loss of free water.
g) Anesthetic agents modify neuroendocrine regulation of fluids and electrolytes.
h) Surgical trauma modifies fluid balance, the degree of which depends on the invasiveness of the surgical procedure and potential blood loss.
i) IV fluids are used to replace intraoperative blood loss and fluid loss resulting from fluid shifts that develop from evaporative and third-space fluid losses. Physiologic parameters, such as heart rate, blood pressure, capillary refill time, urine output, and ongoing blood loss, are continually assessed. In addition, it is essential to monitor and accurate estimated blood loss because pediatric patients do not tolerate excessive loss well.
j) The rate of intraoperative fluid administration is continuously modified to maintain circulatory homeostasis. Peripheral surgical procedures (extremity procedures) have minimal evaporative or third-space fluid losses. However, intracavitary procedures (intraabdominal or intrathoracic procedures) are associated with greater blood loss, third-space fluid loss, and substantial evaporative fluid losses that approach 10 mL/kg of body weight per hour.
k) When administering fluids to pediatric patients younger than 2 years of age, one should consider use of a Buretrol and or IV fluid pump to avoid excess fluid administration.
2. Pediatric fluid compartments
a) The growth of newborns is accompanied by a decrease in the relative fluid compartment volumes of TBW and ECF volumes during the first year of life followed by additional decreases in ECF later in childhood.
b) Whereas the TBW of premature infants is as high as 80% of total body weight, the TBW of term infants is approximately 70% to 75% of total body weight. The adult value of TBW (55%-60%) is reached between 6 months and 1 year of age.
3. Maintenance fluid calculation
a) The most direct and widely accepted method for determining IV fluid requirements is based on body weight.
b) The hourly maintenance fluid level is determined by the “4-2-1” formula and is calculated as shown in the following tables.
Hourly Fluid Requirements: the “4-2-1” Formula
| Weight (kg) | Fluid |
| 0-10 | 4 mL/kg/hr for each kilogram of body weight |
| 10-20 | 40 mL + 2 mL/kg/hr for each kilogram >10 kg |
| >20 | 60 mL + 1 mL/kg/hr for each kilogram >20 kg |
| Sample Calculated Fluid Requirements | Maintenance Fluid per Hour |
| 4 kg | 16 mL |
| 9 kg | 36 mL |
| 15 kg | 50 mL |
| 30 kg | 70 mL |
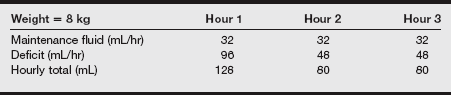
c) Preoperative fluid deficits develop during the period of time in which the child has not received oral or IV maintenance fluids.
d) The preoperative fluid deficit is calculated by determining the hourly maintenance fluid rate and multiplying this rate by the number of hours the child has been without IV or oral intake.
e) The calculated fluid deficit is replaced following the guidelines wherein half of the fluid deficit is replaced during the first hour with the remainder divided in half and replaced over the course of the subsequent 2 hours.
f) In addition to the calculated maintenance and deficit fluids necessary to replace insensible fluid losses, additional IV fluid is required to replace third-space fluid losses that occur with surgical trauma. Lactated Ringer’s solution, 0.9% normal saline, and Plasmalyte are acceptable for the replacement of insensible and third-space fluid losses at the rate of 1 to 2 mL/kg/hr.
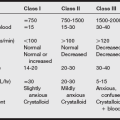
g) Expected third-space fluid losses can be categorized as minimal surgical trauma (an additional 3-4 mL/kg/hr), moderate surgical trauma (5-6 mL/kg/hr), and major surgical trauma (7-10 mL/kg/hr).
4. Glucose-containing solutions
a) Most anesthesia providers administer a glucose-free IV solution (lactated Ringer’s solution) for maintenance fluid administration in the replacement of third-space and intraoperative blood loss.
b) If the child has had an extended NPO period, a plasma glucose level may be determined at the time of IV catheter insertion after inhalation induction.
c) Hypoglycemia is likely to develop in a variety of clinical circumstances. Examples include infants who are premature, infants of mothers with diabetes, children with diabetes who have received a portion of daily insulin preoperatively, and children who receive glucose-based parenteral nutrition.
d) A glucose-containing IV solution is administered to these patients as a controlled piggyback infusion with frequent plasma glucose determinations performed to avoid hyperglycemia.
e) Infants born of mothers with diabetes and infants of mothers who receive glucose-containing solutions during labor may require a continuation of these solutions for the prevention of rebound hypoglycemia.
f) Premature infants who have had less time to store glycogen in the liver than term infants are more susceptible to hypoglycemia. For this reason, premature infants may receive an infusion of 5% dextrose in 0.2% normal saline.
5. Crystalloid intravenous fluids
a) Crystalloid IV solutions are advantageous for perioperative administration because they are the least expensive of the available IV solutions and are acceptable for the replacement of preoperative, intraoperative, and postoperative isotonic fluid deficits. Unlike colloid solutions, crystalloid solutions do not produce allergic reactions.
b) Crystalloid IV solutions can be further subdivided by their tonicity in relation to plasma (hypotonic, isotonic, or hypertonic).
a) The intravascular volume may be estimated by multiplying the child’s weight by the estimated blood volume.
b) The estimated blood volumes are as follows: premature infants, 90 to 100 mL/kg; full-term newborns, 80 to 90 mL/kg; infants age 3 months to 3 years, 75 to 80 mL/kg; and children older than 6 years, 65 to 70 mL/kg. For example, the estimated blood volume of a 6-month-old infant who weighs 7 kg is 525 mL (7 kg × 75 mL/kg = 525 mL).
c) Ongoing surgical blood loss requires frequent reassessment of the child’s physiologic responses. Moderate to severe decreases in intravascular volume produce tachycardia, hypotension, narrowed pulse pressure, low urine output, decreased central venous pressure, pallor, and slow capillary refill.
d) A sudden decrease in blood pressure in neonates and infants with rate-dependent cardiac output is indicative of significant intravascular volume depletion.
a) Allowable blood loss must be defined individually for each patient based on current medical condition, surgical procedure, and cardiovascular and respiratory function.
b) Children with normal cardiovascular function may tolerate a lower hematocrit and may compensate with an increased cardiac output if a higher inspired oxygen concentration is provided to improve oxygen delivery. An exception is premature infants.
c) The incidence of apnea is higher in neonates and premature infants with hematocrit levels below 30%. The anesthesia provider, surgeon, and neonatologist should agree on a target hematocrit level, and this discussion should be documented in the medical record.
d) The allowable blood loss may be calculated by means of the following formula:
e) Blood loss may be replaced with suitable crystalloid solutions (0.9% normal saline, lactated Ringer’s solution) by administering 3 mL for each milliliter of blood loss.
f) Blood loss that is less than the calculated permissible blood loss may be replaced with colloid (1 mL for every milliliter of blood loss).
g) When the blood loss equals or exceeds the calculated allowable loss, transfusion should be considered. Before transfusion is performed, a current hemoglobin and hematocrit should be obtained. The surgeon should be included in the decision process. These discussions and the resultant hemoglobin and hematocrit are recorded in the anesthetic record.
h) The volume of packed red blood cells (PRBCs) to be infused may be determined by the following formula:
i) Blood loss in excess of one blood volume can lead to coagulopathies and a reduction in clotting factors. Platelets, fresh-frozen plasma, and cryoprecipitate should be considered.
a) Before blood component therapy is initiated, the proper equipment (filters, infusion devices, blood warming devices) should be obtained and tested.
b) Standard blood transfusion sets contain a 170- to 200-μm filter. Microaggregate filters (20-40 μm) may be placed between the blood-dispensing bag and the filtered infusion set, although no studies prove these filters to be of benefit over the standard 170- to 200-μm filter.
c) Infusion pumps (syringe or piston driven) selected for the infusion of blood and blood products should be licensed for this function by the manufacturer. An excessive infusion rate can produce RBC lysis.
d) Blood should be warmed before infusion and used with an appropriate blood-warming device such as a Hotline fluid warmer. The selected blood component containers may be placed under the forced-air warming blanket or the measured aliquot of blood drawn into a syringe may be warmed with the hand. Syringes should not be placed into water baths because bacterial contamination may occur.
e) The blood bank can dispense small aliquots of blood into a calibrated syringe or provide 50- to 100-mL bags of the selected blood product transferred from an assigned donor unit.
f) Blood used for neonatal transfusion is preferably less than 1 week old to preserve 2,3-diphosphoglycerate levels and irradiated to prevent graft-versus-host disease. When PRBCs are transfused, the blood should not be diluted before transfusion because this may contribute to hypervolemia.
G Pediatric regional anesthesia
a) Regional anesthesia provides perioperative analgesia (minimizing the risk of respiratory depression), modifies the metabolic responses to anesthesia and surgery, and improves patient outcome.
b) Pediatric patients generally receive a peripheral or centrally administered regional anesthetic after the induction of general anesthesia. The inherent fear of needles and pain, the fear of neurologic injury in a combative child, and the difficulty in providing adequate sedation to ensure patient mobility during the introduction of the block often necessitates the safe execution of the regional anesthetic during general anesthesia.
c) Regional anesthetic procedures in children should be performed only by anesthesia providers with previous training, demonstrated skill in adult regional anesthetic procedures, and knowledge of the appropriate applications of each technique in the pediatric population.
d) Caudal anesthesia is a frequently used residual anesthetic technique that provides analgesia for lower abdominal, genital, urinary and lower extremity procedures.
e) The detection of intravascular injection in the child during the concurrent administration of inhalation agents has been the subject of several studies. Infants may have a greater risk of amide local anesthetic toxicity because of their decreased levels of AAG.
f) The routine administration of epinephrine-containing test doses of local anesthetics may be counterproductive during the concurrent administration of myocardial depressant drugs.
H Genitourinary procedures
a) A cystoscopy is usually a brief procedure, allowing for administration of general anesthesia by a mask or LMA.
b) Hypospadias repair, chordee release, and repair of undescended testicles are usually performed with the patient under general anesthesia with an ETT secured. If the testicle cannot be located directly, an intraabdominal approach with muscle relaxation may be required.
2. Preoperative assessment and patient preparation
a) History and physical examination
(1) Review systems to determine the presence of comorbidities.
(2) Laboratory tests: As indicated by the history and physical examination
(3) Diagnostic tests: As indicated by the history and physical examination
b) Patient preparation: Administer midazolam, 0.5 mg/kg orally 30 minutes before the procedure, if desired.
a) Monitoring equipment is standard.
b) Additional equipment includes warming devices for prolonged procedures.
c) Position: Supine for orchiopexy, chordee release, and hypospadias repair; lithotomy position for cystoscopy. Pad all pressure points and maintain proper body alignment to prevent nerve and soft tissue injuries.
d) A standard pediatric tabletop is used.
e) IV fluids: Depending on the patient’s age and size, a 22- or 24-gauge catheter should be sufficient. Infuse 0.9% normal saline or lactated Ringer’s solution at a rate of 2 to 4 mL/kg/hr. Additional fluid may be needed to replace fluid deficit and blood loss.
4. Perioperative management and anesthetic technique
a) Mask induction with oxygen, nitrous oxide, and sevoflurane. If the patient has a preexisting IV line established, an IV induction is indicated. Muscle relaxation may be used if desired for intubation purposes, but it is not required for the procedure.
b) Obtain a protected airway with an appropriately sized ETT. Ensure proper placement and secure with tape.
c) Maintenance: Oxygen, nitrous oxide, and sevoflurane, halothane or isoflurane with an IV fluid rate as calculated. Maintain normothermia. Manipulation of the testicles or peritoneum may produce a profound vagal response. Pretreat with atropine or glycopyrrolate may be warranted. Caudal anesthesia prevents this response. If bradycardia occurs and is refractory to atropine, treat with a fluid bolus (10-20 mL) or epinephrine (10-20 mcg) intravenously.
I Hernia repair
2. Preoperative assessment and patient preparation
a) History and physical examination
(1) Cardiac: Routine assessment
(2) Respiratory: Patients with prolonged ventilation or immature lungs are more susceptible to tracheomalacia, subglottic stenosis, and bronchopulmonary dysplasia.
(3) Neurologic: Premature infants may display transient apneic and bradycardic episodes in response to hypoxemia. Premature infants are more prone to seizure disorders. Complications from general anesthesia may occur from effects on the immature CNS.
(5) Gastrointestinal: The patient may have abdominal compression if the umbilical hernia is large. Consider rapid-sequence induction for incarcerated hernia and bowel obstructions.
(6) Endocrine: Premature infants are more prone to hypoglycemia. Check frequently blood glucose level.
a) Monitoring equipment is standard.
b) Additional equipment: Use a pediatric circle or Bain circuit. Warm and humidify gases. A warming pad may be used on the table.
c) Position: The patient is supine.
d) Fluids: 0.9% normal saline or lactated Ringer’s solution. Dextrose solutions may be used for infants younger than 1 month old.
4. Perioperative management and anesthetic technique
a) General anesthesia with an ETT is required if laparoscopic hernia repair is to be accomplished.
b) General anesthesia, mask, or ETT. Caudal block may be performed after induction for postoperative pain and provides approximately 6 to 12 hours of pain relief postoperatively.
c) Induction: Mask or endotracheal intubation with halothane or sevoflurane, oxygen, or nitrous oxide. Obtain an IV line after induction. Administer atropine intravenously (0.02 mg/kg) before laryngoscopy, vecuronium (0.1 mg/kg), or cisatracurium (0.1 to 0.2 mg/kg). If applicable, perform a caudal block with bupivacaine (0.25%) 1 mL/kg after induction (onset of 15 minutes).
d) Maintenance: Caudal block will decrease the amount of volatile agent. Increase the MAC before incision to avoid laryngospasm.
e) Stretching or traction of the inguinal structures can cause mild, moderate, or severe bradycardia
f) Emergence: Extubate when the patient is fully awake. IV reversal is with neostigmine (0.07 mg/kg) and glycopyrrolate (0.01 mg/kg) is required.
J Myringotomy
a) History and physical examination
(1) A careful family history should be obtained preoperatively, including any history of family problems with anesthesia.
(2) Respiratory: Many pediatric patients presenting for myringotomy have a history of frequent URIs. Each patient must be evaluated on an individual basis. Because the removal of middle ear fluid usually resolves the frequent URIs, surgery should not be delayed because of symptoms of URI. Instead, it is recommended to place the patient on supplemental oxygen in the postoperative period. It is controversial whether these patients have an increased increased risk of hyperactive airways and laryngospasm.
(3) Dental: Inspection of the airway and questioning of the parents should identify any loose teeth.
a) Monitoring equipment is standard.
b) Additional equipment: None is needed.
c) Standard emergency drugs are used (including atropine, lidocaine, epinephrine, and succinylcholine).
d) Standard pediatric airway equipment.
e) IV fluids: Depending on the age of the patient, expected length of procedure, and history and physical findings, an IV catheter may be available for emergency use but is not started routinely. If necessary, use a 20- or 22-gauge peripheral catheter and normal saline or lactated Ringer’s solution at 2 to 4 mL/kg/hr.
4. Perioperative management and anesthetic technique
a) Mask general anesthesia is usually adequate for uncomplicated myringotomy of otherwise healthy patients.
b) Induction: Mask inhalation induction with an inhalation anesthetic such as or sevoflurane, nitrous oxide, and oxygen. If an existing IV catheter is present, routine IV induction is appropriate. Routine IV induction is preferred in older children and adults.
c) Maintenance: Standard maintenance with halothane, sevoflurane or isoflurane, nitrous oxide, and oxygen. Muscle relaxation and opiates are not routinely used.
d) Emergence: The airway is maintained until the patient is fully awake. The patient should be placed in the lateral position while being transported to the recovery room. In older children and adults, antiemetics should be considered.
K Tonsillectomy and adenoidectomy
2. Preoperative assessment and patient preparation
a) History and physical examination
(1) Cardiac: Echocardiography may be useful to determine the presence of right-sided heart failure from chronic airway obstruction. These patients are at increased risk for negative-pressure pulmonary edema and volume overload.
(2) Respiratory: Evaluate for potentially difficult airway management. If the child exhibits signs and symptoms of URI, there may be an increased risk of laryngospasm intraoperatively and postoperatively. The choice to cancel or proceed with surgery must be evaluated on an individual basis.
(1) Judicious use of preoperative medication in children with obstructive apnea
(2) Midazolam, 0.5 mg/kg orally in children without obstruction
(3) Antisialagogue is strongly recommended
(4) Laboratory tests: As indicated by the history and physical examination
(5) Diagnostic tests: As indicated by the history and physical examination
a) Monitoring equipment: Standard
c) Standard emergency drugs: Atropine, lidocaine, epinephrine, and succinylcholine
d) Standard pediatric tabletop: ETTs (RAE or straight), laryngoscope blade and handle, oral airways, lubricant, gauze, and emergency drugs
e) IV fluids: Depending on the age and size of the patient, a 22- or 24-gauge catheter is probably sufficient; infusion of normal saline or lactated Ringer’s solution at 2 to 4 mL/kg/hr.
4. Perioperative management and anesthetic technique
a) Mask induction is performed with oxygen, nitrous oxide, and sevoflurane.
b) Obtain IV access; administer narcotic; give atropine as indicated; and administer muscle relaxant if desired (not necessary).
c) Intubate with an appropriate-size ETT. Ensure correct placement by auscultation of breath sounds, movement of chest, and note the end-tidal CO2 wave. Tape in the midline.
d) An acetaminophen (Tylenol) suppository, 25 to 30 mg/kg, may be given at this point for postoperative pain control.
e) A mouth gag will be inserted by the surgeon after the airway is secured. It is essential that a deep plane of anesthesia is achieved before insertion. Tube placement should be reconfirmed.
f) Maintenance: Standard with a volatile agent, oxygen, and nitrous oxide. Muscle relaxation is not required. Opiates are given at induction.
g) At the end of the case, reverse the remaining muscle relaxant if used. Carefully suction the stomach and oropharynx. Extubate when the patient is fully awake because of the increased risk of laryngospasm with the presence of blood in the airway. An alternative method is true deep extubation after patient initiates the first breath. Transfer the patient to the recovery unit in lateral-head down position so secretions do not pool in back of airway, thus increasing risk of laryngospasm.
a) Nausea and vomiting can be treated with the following:
(1) Metoclopramide, 0.15 mg/kg/dose; maximum, 10 mg.
(2) Ondansetron, 0.01 to 0.5 mg/kg/dose; maximum, 4 mg, with or without dexamethasone.
(1) The severity of pain depends on the history of chronic infections and the surgical technique.
(2) The site may be infiltrated with local anesthetic.
(3) Opioids may increase nausea and vomiting.
(4) Nonsteroidal anti-inflammatory agents are not recommended because of the increased risk of bleeding.
(5) Pain usually resolves in 7 to 10 days with sloughing of tissue.
(6) Admission to hospital may be required if pain prevents adequate oral intake.
(1) This occurs in first 6 hours postoperatively. Bleeding can occur immediately after surgery or can occur up to the sixth postoperative day.
(2) A bleeding tonsil that forces the patient to return to the operating room has several implications.
(a) The patient is hypovolemic: Establish IV access and infuse a balanced salt solution or lactated Ringer’s solution.
(b) The airway may be compromised by the presence of blood and edema. Have an alternative plan for establishing a protected airway. The use of an ETT that is 0.5 mm smaller than the original tube is recommended because of the presence of edema.
(c) Plan rapid-sequence induction. The patient is considered to have a full stomach because of the presence of blood.