Chapter 594 Pediatric Stroke Syndromes
594.1 Arterial Ischemic Stroke (AIS)
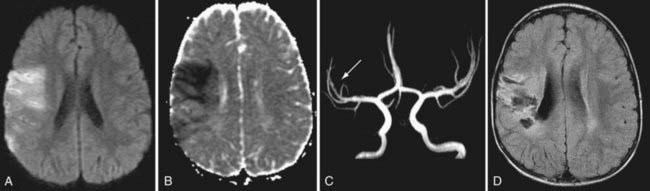
In children the diagnosis of stroke is frequently delayed or missed. This is due to subtle and nonspecific clinical presentations, a complicated differential diagnosis (Chapter 594.4) and a lack of awareness by primary care pediatric physicians. The acute onset of a focal neurologic deficit in a child is stroke until proven otherwise. The most common focal presentation is hemiparesis but acute visual, speech, sensory, or balance deficits also occur. Children with these presentations require urgent neuroimaging and consultation with a child neurologist as emergency interventions may be indicated. AIS is a clinical and radiographic diagnosis. CT imaging can demonstrate larger mature AIS and exclude hemorrhage, however MRI identifies early and small infarcts and is therefore required to exclude ischemic stroke. Diffusion weighted MRI (DWI) can demonstrate AIS within minutes of onset, and MR angiography can confirm vascular occlusion and suggest arteriopathy as the underlying cause (Fig. 594-1).
Most etiologies for AIS are well-established; some represent only potential associations (Tables 594-1 and 594-2). AIS often remains idiopathic although most children have identifiable, frequently multiple risk factors. Three main categories of etiology should be considered: arteriopathic, cardiac, and hematological; full investigation often reveals multiple risk factors in a given individual.
Table 594-1 COMMON RISK FACTORS FOR ARTERIAL ISCHEMIC STROKE IN CHILDREN
| MAJOR CATEGORY | EXAMPLES |
|---|---|
| Arteriopathic |
Table 594-2 MISCELLANEOUS AND GENETIC RISK FACTORS FOR STROKE
From Roach ES, Golomb MR, Adams R, et al: Management of stroke in infants and children, Stroke 39:2644–2691, 2008, Table 2, p 6.
HERNS, hereditary endotheliopathy with retinopathy, nephropathy, and stroke; MELAS, mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes; MERRF, myoclonic epilepsy with ragged red fibers.
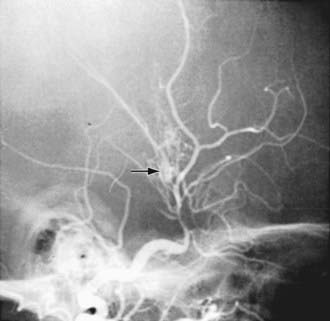
Arteriopathy refers to disorders of the cerebral arteries and has emerged as the leading cause of childhood AIS, present in >50% of children. Idiopathic arterial stenosis has been termed focal cerebral arteriopathy (FCA), and may be more specfically identified as transient cerebral arteriopathy (TCA) or postvaricella angiopathy (PVA). These diseases likely represent focal, localized, unilateral vasculitis. More diffuse or bilateral vasculitis may be primary or associated with systemic inflammatory conditions (Table 594-3). Arterial dissection can be spontaneous or post-traumatic and can affect extracranial internal carotid or vertebral arteries, or intracranial arteries. Moyamoya disease may be idiopathic or associated with other conditions (NF-1, trisomy 21, sickle cell anemia, radiation therapy) and demonstrates progressive occlusion of the distal internal carotid arteries (Table 594-4, Fig. 594-2). Congenital malformations of the craniocervical arteries, including PHACES syndrome, may predispose to AIS.
From Roach ES, Golomb MR, Adams R, et al: Management of stroke in infants and children, Stroke 39:2644–2691, 2008, Table 5, p 8.
Table 594-4 RISK FACTORS FOR MOYAMOYA DISEASE
| PATIENTS (n) | |
|---|---|
| No associated conditions (idiopathic) | 66 |
| Neurofibromatosis type 1 | 16 |
| Asian heritage | 16 |
| Cranial therapeutic radiation | 15 |
| Hypothalamic-optic system glioma | 8 |
| Craniopharyngioma | 4 |
| Medulloblastoma, with Gorlin syndrome | 1 |
| Acute lymphocytic leukemia, intrathecal chemotherapy | 2 |
| Down syndrome | 10 |
| Congenital cardiac anomaly, previously operated | 7 |
| Renal artery stenosis | 4 |
| Hemoglobinopathy (2 sickle cell anemia, 1 “Bryn Mawr”) | 3 |
| Other (hematologic: 1 spherocytosis, 1 idiopathic thrombocytopenic purpura) | 2 |
| Giant cervicofacial hemangiomas | 3 |
| Shunted hydrocephalus | 3 |
| Idiopathic hypertension requiring medication | 3 |
| Hyperthyroidism (1 with Graves syndrome) | 2 |
Other syndromes, 1 patient each: Reye (remote), Williams, Alagille, cloacal exstrophy, renal artery fibromuscular dysplasia, and congenital cytomegalic inclusion virus infection (remote). Two patients had unclassified syndromic presentations. There were 4 blacks, 2 of whom had sickle cell disease.
From Roach ES, Golomb MR, Adams R, et al: Management of stroke in infants and children, Stroke 39:2644–2691, 2008, Table 6, p 11.
Amlie-Lefond C, Sebire G, Fullerton HJ. Recent developments in childhood arterial ischaemic stroke. Lancet Neurol. 2008;7:425-435.
Benseler SM, Silverman E, Aviv RI, et al. Primary central nervous system vasculitis in children. Arthritis Rheum. 2006;54:1291-1297.
Bernard TJ, Goldenberg NA, Armstrong-Wells J, et al. Treatment of childhood arterial ischemic stroke. Ann Neurol. 2008;63:679-696.
Birnbaum J, Hellmann DB. Primary angiitis of the central nervous system. Arch Neurol. 2009;66:704-709.
Chollet F, Tardy J, Albucher JF, et al. Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomized placebo-controlled trial. Lancet Neurol. 2011;10:123-130.
Danchaivijitr Cox TC, Saunders DE, Ganesan V. Evolution of cerebral arteriopathies in childhood arterial ischemic stroke. Ann Neurol. 2006;59:620-626.
De Tiege X, Van Bogaert P, Aeby A, et al. Primary angitis of the central nervous system: neurologic deterioration despite treatment. Pediatrics. 2011;127:el086-el090.
deVeber G, Kirkham F. Guidelines for the treatment and prevention of stroke in children. Lancet Neurol. 2008 Nov;7:983-985.
Donnan GA, Fisher M, Macleod M, et al. Stroke. Lancet. 2008;371:1612-1622.
Elbers J, Benseler SM. Central nervous system vasculitis in children. Curr Opin Rheumatol. 2008;20:47-54.
Farrar MA, Lin CSY, Krishnan AV, et al. Acute, reversible axonal energy failure during stroke-like episodes in MELAS. Pediatrics. 2010;126(3):e734-e739.
Goldenberg NA, Bernard TJ, Fullerton HJ, et al. Antithrombotic treatments, outcomes, and prognostic factors in acute childhood-onset arterial ischaemic stroke: a multicentre, observational, cohort study. Lancet Neurol. 2009;8:1120-1127.
Herak DC, Antolic MR, Krleza JL, et al. Inherited prothrombotic risk factors in children with stroke, transient ischemic attack, or migraine. Pediatrics. 2009;123:e653-e660.
Ikram MA, Seshadri S, Bis JC, et al. Genomewide association studies of stroke. N Engl J Med. 2009;360:1718-1728.
Jea A, Smith ER, Robertson R, et al. Moyamoya syndrome associated with down syndrome: outcome after surgical revascularization. Pediatrics. 2005;116:e694-e701.
Kirton A, deVeber G. Advances in perinatal ischemic stroke. Pediatr Neurol. 2009;40:205-214.
Lin HC, Chien CW, Ho JD. Herpes zoster ophthalmicus and the risk of stroke. Neurology. 2010;74:792-797.
Mallick AA, Ganesan V, O’Callaghan FJK. Mortality from childhood stroke in England and Wales, 1921-2000. Arch Dis Child. 2010;95:12-19.
Mitre N, Mack K, Babovic-Vuksanovic D, et al. Ischemic stroke as the presenting symptom of primary hyperparathyroidism due to multiple endocrine neoplasia type 1. J Pediatr. 2008;153:582-585.
Monagle P, Chalmers E, Chan A, et al. Antithrombotic therapy in neonates and children: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:887S-968S.
Ovbiagele B, Nath A. Increasing incidence of ischemic stroke in patients with HIV infection. Neurology. 2011;76:444-450.
Pappachan J, Kirkham FJ. Cerebrovascular disease and stroke. Arch Dis Child. 2008;93:890-898.
Perry JJ, Stiell IG, Sivilotti MLA, et al. High risk clinical characteristics for subarachnoid haemorrhage in patients with acute headache: prospective cohort study. BMJ. 2010;341:c5204.
Rea D, Brandsema JF, Arnstrong D, et al. Cerebral arteriopathy in children with neurofibromatosis type 1. Pediatrics. 2009;124:e476-e483.
Roach ES, Golomb MR, Adams R, et al. Management of stroke in infants and children. Stroke. 2008;39:2644-2691.
Scott RM, Smith ER. Moyamoya disease and moyamoya syndrome. N Engl J Med. 2009;360:1226-1237.
Sebire G. Transient cerebral arteriopathy in childhood. Lancet. 2006;368:8-10.
Veeravagu A, Guzman R, Patil CG, et al. Moyamoya disease in pediatric patients: outcomes of neurosurgical interventions. Neurosurg Focus. 2008;24:1-9.
594.2 Cerebral Sinovenous Thrombosis (CSVT)
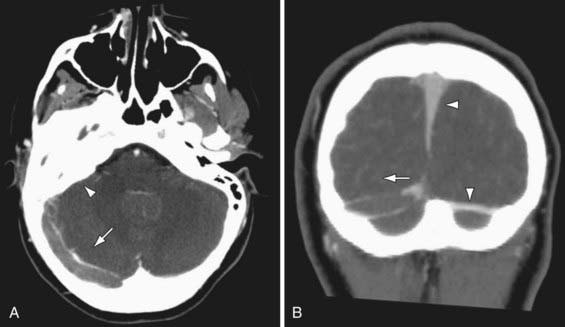
Clinical presentations are often more gradual, variable, and nonspecific compared to AIS. Neonates typically present with diffuse neurologic signs and seizures. Children may present with progressive headache, papilledema, diplopia secondary to 6th nerve palsies (frequently misdiagnosed as idiopathic intracranial hypertension), or acute focal deficits. Seizures, lethargy, and confusion are common. Diagnosis requires a high clinical suspicion and purposeful imaging of the cerebral venous system. Nonenhanced CT is very insensitive for CSVT, and contrast CT venography (CTV) is usually necessary to demonstrate filling defects in the cerebral venous system (Fig. 594-3). However MRI includes diffusion imaging of the brain parenchyma and modern MR venography (MRV) can be comparable to CTV in accuracy. Intraventricular hemorrhage in term infants suggests CSVT.
The Virchow triad is helpful in understanding the risk factors for CSVT (Table 594-5). Hypercoagulable states are frequently associated with childhood venous thrombosis including CSVT. Prothrombotic states frequently detected in childhood CSVT include inherited (e.g., prothrombin gene 20210A mutation) and acquired (e.g., antiphospholipid antibodies) conditions, prothrombotic medications (asparaginase, oral contraceptives), and common childhood illnesses, including iron deficiency anemia and severe dehydration. Systemic diseases associated with increased CSVT risk include leukemia, inflammatory bowel disease, and nephrotic syndrome.
Table 594-5 COMMON RISK FACTORS FOR CEREBRAL SINOVENOUS THROMBOSIS IN CHILDREN
| MAJOR CATEGORIES (VIRCHOW TRIAD) | EXAMPLES |
|---|---|
| Blood coagulation |
deVeber G, Andrew M, Canadian Pediatric Ischemic Stroke Study Group. Cerebral sinovenous thrombosis in children. N Engl J Med. 2001;345:417-423.
Kenet G, Kirkham F, Niederstadt T, et al. Risk factors for recurrent venous thromboembolism in the European collaborative paediatric database on cerebral venous thrombosis: a multicentre cohort study. Lancet Neurol. 2007;6:595-603.
Monagle P, Chalmers E, Chan A, et al. Antithrombotic therapy in neonates and children: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:887S-968S.
Moharir MD, Shroff M, Stephens D, et al. Anticoagulants in pediatric cerebral sinovenous thrombosis: a safety and outcome study. Ann Neurol. 2010;67:590-599.
Sebire G, Tabarki B, Saunders DE, et al. Cerebral venous sinus thrombosis in children: risk factors, presentation, diagnosis and outcome. Brain. 2005;128:477-489.
Smith R, Hourihan MD. Investigating suspected cerebral venous thrombosis. BMJ. 2007;334:794-795.
594.3 Hemorrhagic Stroke (HS)
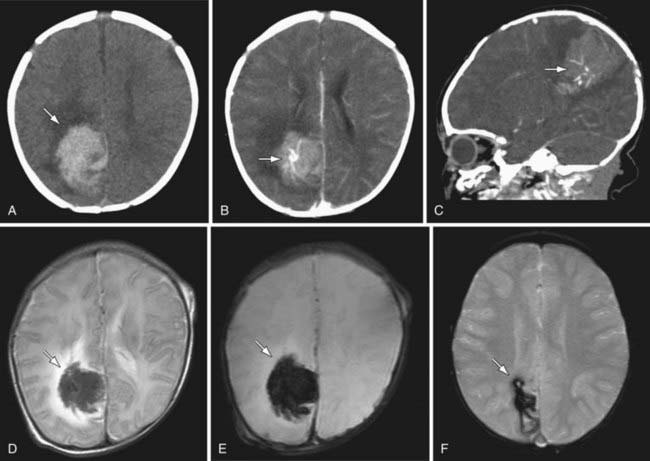
Clinical presentations vary according to location, cause, and rate of bleeding. Acute hemorrhages may feature instantaneous or thunderclap headache, loss of consciousness, and nuchal rigidity in addition to focal neurologic deficits and seizures. HS can be rapidly fatal. In bleeds associated with vascular malformations, pulsatile tinnitus, cranial bruit, macrocephaly, and high-output heart failure may be present. Diagnosis relies on imaging, and CT is highly sensitive to acute HS. However lumbar puncture may be required to exclude subarachnoid hemorrhage. Modern MRI is highly sensitive to even small amounts of acute hemorrhage and it images residual blood products long-term following most parenchymal bleeds (Fig. 594-4). Angiography by CT, MR, or conventional means is often required to exclude underlying vascular abnormalities (e.g., vascular malformations, aneurysms).
Causes of HS include vascular malformations and systemic disorders (Table 594-6). Arteriovenous malformations (AVM) are the most common cause of childhood subarachnoid and intraparenchymal HS and may occur anywhere in the brain. Risk of AVM bleeding is approximately 2-4% per year throughout life. Other vascular malformations leading to HS include cavernous angiomas (cavernomas), dural arteriovenous fistulas, and vein of Galen malformations. Cerebral aneurysms are an uncommon cause of subarachnoid hemorrhage in children and may suggest an underlying disorder (e.g., polycystic kidney disease, infective endocarditis). A common cause for HS is bleeding from a pre-existing brain tumor. Arterial diseases that usually cause ischemic stroke can also predispose to HS including the central nervous system (CNS) vasculitides and moyamoya disease. Additional causes of parenchymal HS include hypertensive hemorrhage and hematologic disorders such as thrombocytopenic purpura, hemophilia, acquired coagulopathies (e.g., disseminated intravascular coagulation, liver failure), anticoagulant therapy (e.g., warfarin), or illicit drug use. Ischemic infarcts may undergo hemorrhagic transformation, particularly in CSVT, and can be difficult to differentiate from primary HS.
Table 594-6 POTENTIAL RISK FACTORS FOR HEMORRHAGIC STROKE IN CHILDREN
| MAJOR CATEGORIES | EXAMPLES |
|---|---|
| Vascular disorder |
Neonatal hemorrhagic strokes are different. Cranial ultrasound can detect most significant neonatal bleeds. In the preterm infant, germinal matrix bleeding and intraventricular hemorrhage are common (Chapter 93.3). Subarachnoid and subdural blood may be imaged in up to 25% of normal term newborns. Term HS is poorly studied and includes the etiologies listed earlier, but may be idiopathic in >50% of cases. Term intraventricular bleeding is often secondary to either deep CSVT or choroid plexus angiomas.
Al-Shahi Salman R, Labovitz DL, Stapf C. Spontaneous intracerebral haemorrhage. BMJ. 2009;339:284-289.
Gomis P, Graftieau P, Sercombe R, et al. Randomized, double-blind, placebo-controlled, pilot trial of high-dose methylprednisolone in aneurysmal subarachnoid hemorrhage. J Neurosurg. 2010;112:681-688.
Gupta SN, Kechli AM, Kanamalla US. Intracranial hemorrhage in term newborns: management and outcomes. Pediatr Neurol. 2009;40:1-12.
Lo WD, Lee J, Rusin J, et al. Intracranial hemorrhage in children: an evolving spectrum. Arch Neurol. 2008;65:1629-1633.
Meyer-Heim AD, Boltshauser E. Spontaneous intracranial haemorrhage in children: aetiology, presentation and outcome. Brain Dev. 2003;25:416-421.
Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373:1632-1642.
Runchey S, McGee S. Does this patient have a hemorrhagic stroke? JAMA. 2010;313:2280-2286.
van Gijn J, Kerr RS, Rinkel GJE. Subarachnoid haemorrhage. Lancet. 2007;369:306-318.
594.4 Differential Diagnosis of Strokelike Events
Adam Kirton and Gabrielle deVeber
The diagnosis of stroke in childhood requires a high index of suspicion, balanced with awareness of the differential diagnosis. Acute onset of a focal neurologic deficit or in neonates, seizures, should be considered stroke until proven otherwise and assessed with neuroimaging. Pediatric stroke must be differentiated from other strokelike disorders (Table 594-7) that may require their own urgent specific treatment.
Table 594-7 DIFFERENTIAL DIAGNOSIS OF STROKELIKE EPISODES IN CHILDREN
| DISORDER | CLINICAL DISTINCTION FROM STROKE | IMAGING DISTINCTION FROM STROKE |
|---|---|---|
| Migraine | Evolving or “marching” symptoms, short duration, complete resolution, headache, personal or family history of migraine | Typically normal Migrainous infarction is rare |
| Seizure | Positive symptoms, Todd paralysis is postseizure and limited | Normal or may identify source of seizures (e.g., malformation, old injury) |
| Infection | Fever, encephalopathy, gradual onset, meningismus | Normal or signs of encephalitis/cerebritis, which are typically diffuse and bilateral AIS and CSVT can occur in bacterial meningitis |
| Demyelination | Gradual onset, multifocal symptoms, encephalopathy Accompanying optic neuritis or transverse myelitis | Multifocal lesions, typical appearance (e.g., patchy in ADEM, ovoid in MS), typical locations (e.g., pericallosal in MS), less likely to show restricted diffusion |
| Hypoglycemia | Risk factor (e.g., insulin therapy), related to meals, additional systemic symptoms | Bilateral, symmetric May see restricted diffusion Posterior dominant pattern |
| Watershed infarction due to global HIE | Risk factor (e.g., hypotension, sepsis, heart disease), bilateral deficits | Bilateral, symmetric restricted diffusion in border zones between major arteries (watersheds) |
| Hypertensive encephalopathy (PRES) | Documented hypertension, bilateral visual symptoms, encephalopathy | Posterior dominant, bilateral, patchy lesions involving gray and white matter; usually no restricted diffusion |
| Inborn errors of metabolism | Pre-existing delays/regression, multisystem disease, abnormal biochemical profiles | May have restricted diffusion lesions but bilateral, symmetrical, not within vascular territories MR spectroscopy changes (e.g., high lactate in MELAS) |
| Vestibulopathy | Symptoms limited to vertigo, imbalance (i.e., no weakness) Gradual onset | Normal |
| Acute cerebellar ataxia | Sudden onset bilaterally symmetric ataxia postviral | Normal |
| Channelopathy | Syndromic cluster of symptoms not localizing to single lesion Gradual onset, progressive evolution |
Normal |
| Alternating hemiplegia | History contralateral events Choreoathetosis/dystonia |
Normal |
ADEM, acute disseminated encephalomyelitis; AIS, arterial ischemic stroke; CSVT, cerebral sinovenous thrombosis; HIE, hypoxic-ischemic encephalopathy; MELAS, mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes; MR, magnetic resonance; MS, multiple sclerosis; PRES, posterior reversible leukoencephalopathy syndrome.
Inborn Errors of Metabolism
Mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes (MELAS; Chapter 591.2) is the classic example, though other mitochondrial disease can mimic stroke. Features favoring MELAS would include a history of developmental regression, posterior (and often bilateral) lesions not respecting vascular territories on MRI, and elevated serum or cerebrospinal fluid lactate (MR spectroscopy). In contrast to these types of “metabolic infarction,” children with Fabry disease and homocystinuria are at risk of true ischemic stroke.