CHAPTER 286 Pediatric Spondylolisthesis
Spondylolisthesis was first described more than 200 years ago when a Belgian obstetrician combined the Greek roots for vertebra (spondylos) and slippage (listhesis) to explain an osseous protuberance found to compromise the birth canal during delivery.1 After almost a century, the term came to characterize forward slippage of L5 on S1. Initial theories of its cause were based on the presumed effects of axial body weight on the lumbar-sacral-pelvic junction with resultant subluxation of the facet joint.2 Similar mechanistic hypotheses prevailed over the following 100 years, such as injury or trauma as inciting events in the setting of underlying lumbosacral defects or excessive body weight as in Blake’s description of a 26-year-old multiparous woman who experienced a weight gain of 100 lb before slippage was detected.3,4 The current understanding of spondylolisthesis is based on the etiology, severity, and likelihood for progression and stems from the classification scheme provided by Newman in the 1960s.5
Epidemiology
Spondylolisthesis is a relatively common problem, with more than 30% of lumbar fusions being performed for this diagnosis.6 Each type of spondylolisthesis has differences in demographics, natural history, severity, levels involved, and treatment considerations. The pediatric population is most affected by the dysplastic and isthmic types. Degenerative spondylolisthesis is more commonly seen in women and at the L4-5 level, whereas isthmic spondylolisthesis most commonly affects L5-S1 and is seen predominantly in men. Hereditary factors appear to play some role in the development of congenital and isthmic spondylolisthesis.7–11 Certain types of isthmic spondylolisthesis contain a dysplastic elongated pars, which has been found to have a strong familial component.12 Most agree, however, that environmental factors play a major role in the development and progression of spondylolisthesis. Slip progression has been linked with the adolescent growth spurt, a greater degree of slippage on initial evaluation, and lumbosacral kyphosis. Factors associated with a higher risk for progressive slippage include younger age (by demonstration of decreased skeletal maturity), slippage of greater than 50%, slip angle greater than 40 to 50 degrees (with 0 to 10 degrees being normal), female gender, a dome-shaped sacrum, and a dysplastic lumbosacral junction. Diagnosis after the onset of adulthood portends a lower rate of significant progression.
Classification
Newman classified spondylolisthesis into five groups in 1963 (Table 286-1).5 This was later published by Wiltse, and colleagues in 1976.13
| DYSPLASTIC |
Modified from Wiltse LL, Newman PH, Macnab I. Classification of spondylolysis and spondylolisthesis. Clin Orthop Relat Res. 1976;117:23-29.
Dysplastic
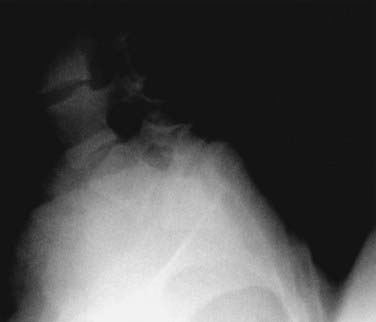
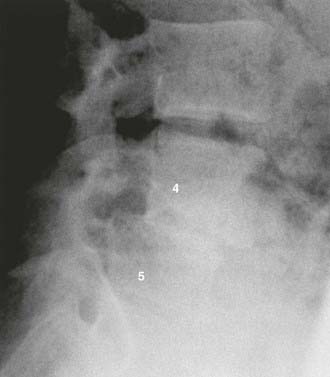
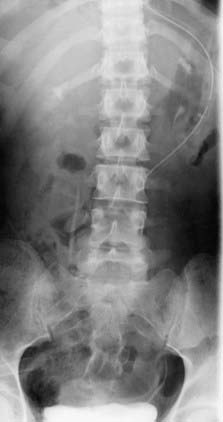
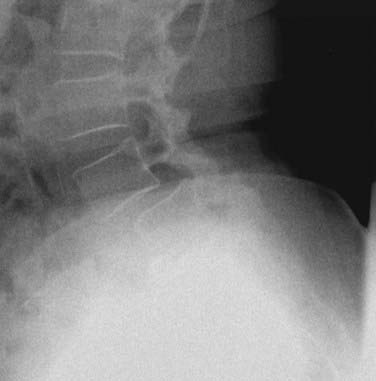
Dysplastic (congenital) spondylolisthesis results from a congenital defect at the L5-S1 articulation. Facet defects such as hypoplasia or malorientation or sacral deficiency results in vertebral subluxation. Although some degree of compromised integrity exists, the pars interarticularis is intact in children with congenital spondylolisthesis. Most patients have an abnormal orientation of the facets and a dome-shaped sacral promontory with lack of the normal buttress effect provided by the posterior elements. In contrast to other types of spondylolisthesis, progression is common because the dysplastic facets permit further slippage.14 This anterior translation may result in neuroforaminal stenosis and compression of the exiting nerve root and become symptomatic with radiculopathy. If the slippage progresses to spondyloptosis, severe neurological conditions such as cauda equina syndrome can occur as the nerve roots become entrapped by the lamina, intact pars interarticularis, and the sacral dome (Fig. 286-1). Dysplastic spondylolisthesis occurs approximately twice as frequently in females as males and was found to account for 14% to 21% of cases in two large series.15–17 It can be subdivided into subtypes A to C (Table 286-2, Figs. 286-2 to 286-7).
| SUBTYPE A |
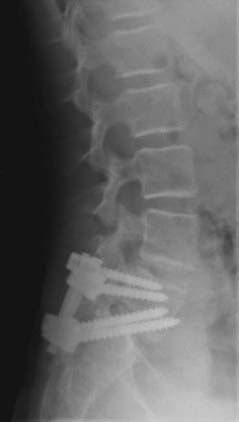
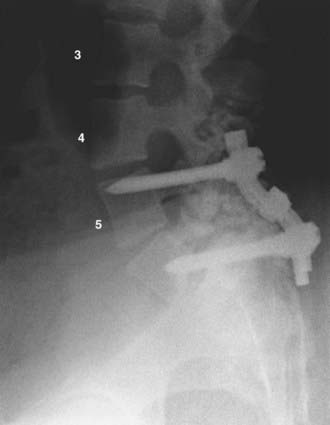
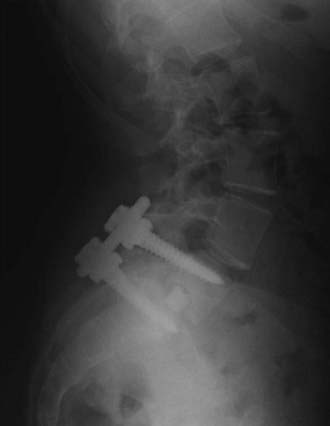
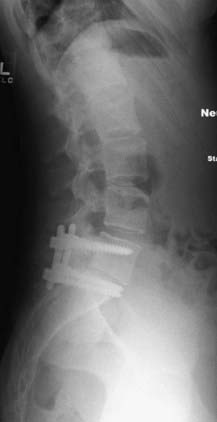
FIGURE 286-3 Lateral plain radiograph of the patient in Figure 286-2 after L5-S1 transforaminal lumbar interbody fusion with reduction and decompression. The S1 screws traverse the L5 inferior end plate.
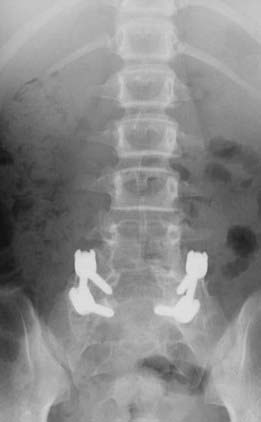
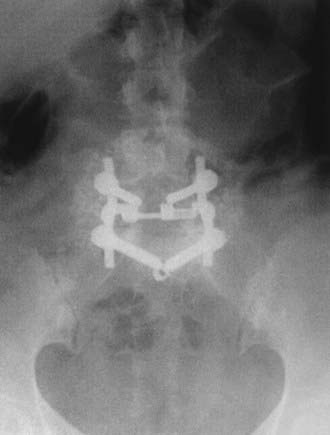
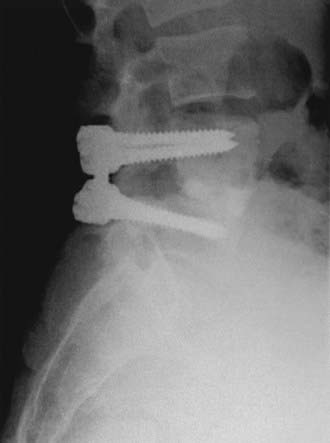
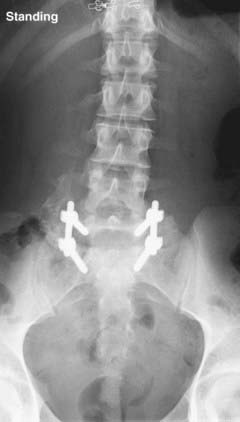
FIGURE 286-4 Anteroposterior radiograph of the patient in Figure 286-2 treated by L5-S1 transforaminal lumbar interbody fusion with reduction and decompression.
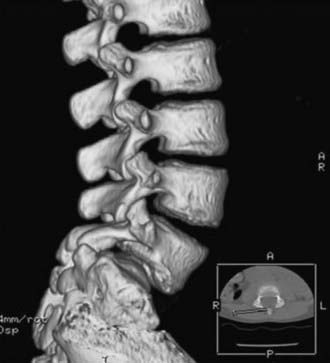
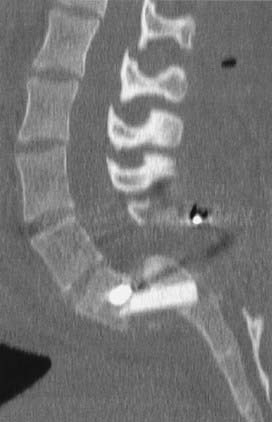
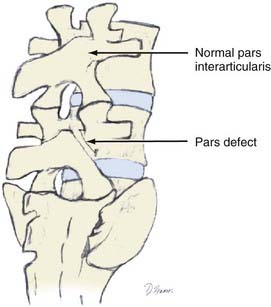
Isthmic
Isthmic spondylolisthesis refers to vertebral subluxation caused by a defect in the pars interarticularis of the posterior spine (spondylolysis) (Figs. 286-8 and 286-9). The pars interarticularis plays a strategic role in the configuration of the spine in that it supports the anterior and posterior columns through the connection of the pedicle with the lamina and facet joints. Because it provides such functional integrity to the lumbar spine, if compromised it may allow subluxation.7,18–21
There are three subclassifications of isthmic spondylolisthesis (Table 286-3). The first is thought to result from stress fractures within the pars interarticularis (subtype A). The second type (subtype B) is an elongation rather than an actual break in the pars. Subtype C, the most uncommon type, refers to an acute fracture of the pars.
TABLE 286-3 The Three Subclassifications of Isthmic Spondylolisthesis
| SUBTYPE A |
Approximately 90% of cases result from an L5 pars defect and involve the L5-S1 joint. The second most common defect arises from compromise of the L4 pars, which leads to L4-5 anterior subluxation.7,18–24
These defects typically occur in adolescents and represent the most common type of spondylolisthesis in this age group. Males are more commonly affected than females; however, in those with high-grade isthmic spondylolisthesis, females are affected four times more commonly than males, with the highest rates reported in Alaskan female Eskimos. Hereditary factors appear to play a role in the isthmic types in that an increased incidence in first-degree relatives ranging from 19% to 69% has been cited in different series. When compared with the dysplastic types with abnormal vertebral architecture, isthmic spondylolisthesis is less likely to progress because dysplastic features are not a characteristic of this type (Figs. 286-10 and 286-11).
Degenerative
Although Newman is credited with the term degenerative spondylolisthesis, Junghanns first described lumbar spondylolisthesis without a pars defect as “pseudospondylolisthesis” in the 1930s.25,26 As would be expected, this condition primarily affects the older population. Rosenberg and associates’ studies of 20 skeletons and 200 patients with degenerative spondylolisthesis found this condition to occur up to six times more frequently at the L4-5 level than at adjoining levels.27,28 Lower extremity symptoms were described in 153 patients, 85 of whom had neurological findings on objective evaluation. Of these patients, signs and symptoms requiring hospitalization developed in 39, and 29 eventually required operative treatment after conservative measures failed.27 In addition, a fourfold increase in listhesis was found in patients with sacralization of L5.
Women are affected five times more frequently than men and black women three times more frequently than white women. The superior facet of the affected level often undergoes hypertrophic changes that cause stenosis of the lateral recess, thereby compounding the already present compromise in central canal diameter. Although the percentage of slippage was not reliably correlated with clinical symptoms, Rosenberg found that at the L3 level, even a small amount of listhesis may produce pronounced symptoms. Spondylolisthesis was associated with a higher lumbosacral angle, and the most common complaint was pain in the back, buttock, or thigh. The symptoms of spondylolisthesis may not remain constant and often occur in discrete periods. The sitting position was noted to improve symptoms in many cases. According to Rosenberg, the L5 nerve root provided the most common source of objective physical findings when present.27
Hormonal factors resulting in ligamentous laxity may contribute to the higher incidence of degenerative spondylolisthesis in women.29 This was concluded in part by Imada and associates, who demonstrated in their review of women who had undergone oophorectomy a three times higher incidence of degenerative spondylolisthesis.30 Nadaud and colleagues, however, failed to find evidence of estrogen receptors on facet joint capsular ligaments in their review of 14 patients who underwent spinal fusion surgery.31 Grobler and coworkers demonstrated the role of anatomic morphology when they compared computed tomography (CT) scans of facet joints affected by degenerative spondylolisthesis with scans of normal lumbar spines.32 They found a more sagittally oriented L4-5 facet joint in patients with degenerative spondylolisthesis than in normal individuals, as well as a greater transverse articular dimension and joint surface depth. Osteophyte formation within the joint may be primarily responsible for these findings.
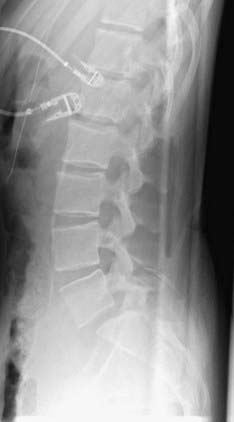
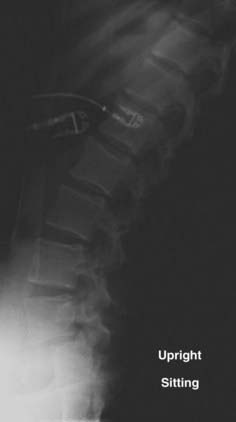
In a prospective study of 40 patients with a minimum 5 years of follow-up, Matsunaga and coauthors reviewed the natural history of degenerative spondylolisthesis.33 They determined that women with a mean age of 55 were most commonly affected and that L4 was most commonly involved (87.5% versus 10% at L5). In this study, 30% of the patients experienced progression of slippage by 5% or greater, with a mean initial slippage rate of 12.4% per 5 years in the progression group and 14.5% per 5 years in the group without progression. Although there was no correlation between gender and progression of slippage, a positive correlation was found among activity, particularly repetitive anterior flexion, and progression of slippage. Within the group experiencing progression, 75% were involved in activities requiring repetitive flexion, whereas only 10% of the group without progression performed similar activities. They were unable to demonstrate a significant difference in lumbosacral angle, lamina angle, or facet inclination angle between the groups. Factors not found to be associated with progression of slippage included spur formation of the involved vertebral body, sclerosis of the end plates, and ossification of the ligaments. Using Carter’s criteria, more patients with degenerative spondylolisthesis were found to have joint laxity than normal individuals. Progression of slippage did not correlate with clinical deterioration. The most common symptoms were low back pain and gluteal pain, which were present in 98% of patients. Lower extremity pain and numbness were also present in 48% of patients, whereas intermittent claudication was seen in 13% and one patient had bowel and bladder dysfunction. In a follow-up review of nonsurgically treated patients over a 10-year period by the same authors, progression of spondylolisthesis occurred in 34% of patients.34 They again were unable to correlate this progression with clinical symptoms. All patients who were noted to be neurologically intact at initial evaluation remained so at the 10-year follow-up. However, 83% of the patients with initial neurological symptoms experienced further neurological deterioration, thus providing evidence for recommending early treatment in symptomatic individuals (Figs. 286-12 and 286-13).
Traumatic
Although bracing alone may suffice for the treatment of many fractures of the posterior element, open reduction with segmental fixation and fusion is most often required in patients with progressive displacement of the involved level (Figs. 286-14 to 286-18).
Pathologic
Many local and systemic processes may compromise the integrity of the intervertebral articulation and result in subluxation of the vertebral body. Focal processes (subtype B), which compromise bones or ligaments, include infection, neoplasia, iatrogenic causes, or other processes, whereas systemic causes (subtype A) include generalized bone or connective tissue disorders (Table 286-4). Systemic disorders such as osteoporosis, osteogenesis imperfecta, syphilitic disease, Ehlers-Danlos disease, arthrogryposis, Paget’s disease, and Marfan’s syndrome are a few of the more commonly cited afflictions resulting in this type of spondylolisthesis.
| SUBTYPE A |
| Systemic causes+ |
| SUBTYPE B |
| Focal processes |
Iatrogenic
Iatrogenic spondylolisthesis has been suggested as a potential sixth type and is usually described as occurring after decompressive surgery involving laminectomies or facetectomies. Iatrogenic disruption of the posterior elements may result in anterolisthesis or retrolisthesis by the same basic principles as described earlier (Fig. 286-19).
Measurement
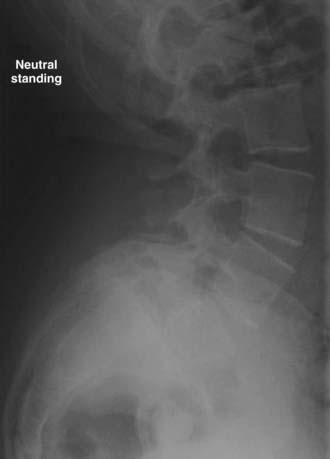
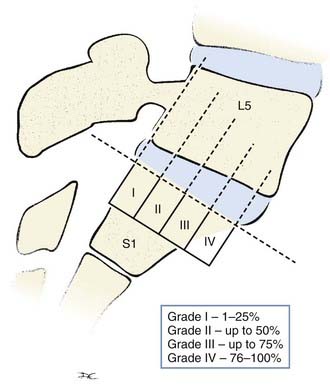
The Meyerding classification is the most widely used system for grading spondylolisthesis4 (Fig. 286-20). It involves the percentage of anterior translation relative to the adjacent level. On a lateral view through the center of the vertebral body, the vertebral column is divided into quarters. The subsequent grade is determined simply by the degree of anterior slippage. With each quarter of vertebral body slippage on the adjacent level, a grade is added. For example, grade I refers to less than 25% slippage, whereas grade IV corresponds to 75% to 100% translation. If there is greater than 100% translation, it is referred to as spondyloptosis and is often associated with deformity and neurological deficits.
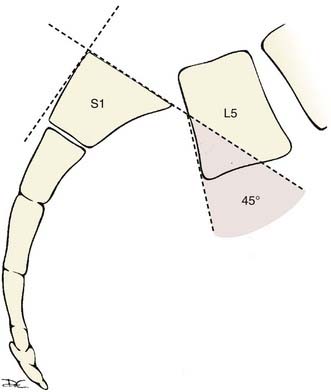
The slip angle is another important descriptor of spondylolisthesis and refers to the kyphotic angle resulting from the slippage. A line drawn parallel to the L5 superior end plate intersects a line drawn perpendicular to the posterior cortex of the S1-2 vertebral bodies and creates this angle (Fig. 286-21). As the L5 vertebral body slides forward over the sacrum, it tilts into kyphosis. The resultant kyphosis is quantified by measuring the slip angle as just described. Spondylolisthesis with slip angles greater than 50% are likely to progress.
Clinical Findings
Low back pain is the primary complaint in most symptomatic patients. It is described as worse with activity and improved with rest, thus alluding to its mechanical nature. The pain should localize to the area of listhesis and is frequently worse on the side with spondylosis if unilateral. Isthmic spondylolisthesis often causes a hyperlordotic posture and tight hamstrings. Reduced lumbar mobility and paraspinal muscle spasms may also be complaints. On questioning or examination, many patients report worsening of symptoms with maneuvers involving hyperextension and rotation, such as standing on one leg while leaning backward. Factors associated with a higher incidence of pain include listhesis greater than 25%, degeneration at the level of slippage, and spondylolisthesis of L3 or L4.27,35 Symptoms attributable to secondary or additional processes such as neurogenic claudication may also be seen. Differentiation of vascular claudication from neurogenic claudication is essential in the evaluation of back pain with associated radicular symptoms. Reduction of pain with forward flexion (“shopping cart sign”) is a hallmark of neurogenic causes and should not be present in patients with vascular claudication. In addition, those with neurogenic claudication frequently complain of worsening of pain on extension. Patients with vascular claudication may complain of pain worsened with bicycle riding, often more severe than the pain in patients with neurogenic claudication. Higher grade spondylolisthesis may cause increased pain with flexion because the listhesis may be accentuated with flexion and possibly decreased with extension, which may provide evidence supporting slippage rather than central stenosis as the primary causative factor generating the patient’s symptoms.
Other ailments such as osteoarthritis of the hip may confound the findings on physical examination. It is prudent to assess for pain with provocative hip motion, and it should not be present in patients with pure spondylolisthesis. However, 11% to 17% of patients with degenerative spondylolisthesis have associated osteoarthritis of the spine, which may be responsible for a variable degree of the symptoms.36 Normal strength and sensation on objective lower extremity testing are common, and reflexes are frequently normal to decreased.
High-grade spondylolisthesis results in lumbosacral kyphosis at the level of the spondylolisthesis. To compensate for this change in sagittal balance, patients assume an exaggerated lumbar lordotic stance with adjustments in pelvic tilt. The tight hamstring syndrome is often seen in patients with isthmic spondylolisthesis; such patients appear stooped over, maintain excessive flexion of the hip and knee, and ambulate with a waddling gait.37 This hip and knee flexion also serves to reduce tension on the stretched nerve root in those with concomitant radiculopathy.
Diagnostic Studies
Although many patients are referred with magnetic resonance imaging (MRI) and CT scans, basic plain films (anteroposterior, lateral, oblique, flexion-extension, and occasionally standing scoliosis views) are the standard for diagnosis of spondylolisthesis. Plain oblique films may demonstrate the so-called Scotty dog collar, which describes a fracture of the pars interarticularis. The neck of the Scotty dog corresponds to the pars, which will demonstrate a radiolucency representing a fracture or defect. The eyes are represented by the pedicle, the hind legs are represented by the spinous processes, the ear is the superior articular facet, the forelegs are the inferior facets, and the nose is the transverse process. Amato and colleagues found a spot lateral view of the lumbosacral junction to have high sensitivity (84%) in diagnosing a defect of the pars.38 Although not commonly performed, a bone scan may provide clues with new lesions; however, its sensitivity diminishes with long-term lesions. Single-photon emission computed tomography has diagnostic sensitivity in finding defects, but its specificity is poor. The utility of MRI in evaluating soft tissue structures, including the neural elements, disk herniation or degeneration, annular disruption, and the presence of neoplastic or infectious processes, provides an excellent means of ruling in or out other associated causes in the evaluation of spondylolisthesis. MRI, however, has a relatively poor ability to accurately characterize bone lesions, which renders it less useful for describing pars defects. Despite certain inherent limitations, such as lower sensitivity for smaller defects in the axial plane, CT scanning provides the highest sensitivity and specificity for diagnosing spondylolisthesis. Although introducing a needle into the thecal sac is not without risk, CT myelography provides excellent visualization of the bony elements, as well as compression of critical neural structures.
Natural History
Counseling patients on the progression of their spondylolisthesis is an important component of the management of this condition. Unfortunately, there are conflicting opinions among experts regarding factors contributing to progression. Less skeletal maturation, listhesis greater than 50%, slip angle (lumbosacral kyphosis) greater than 40% to 50% (normal, 0% to 10%), female gender, a dome-shaped sacrum, and a dysplastic lumbosacral junction are a few of the factors commonly associated with progression of slippage.39,40 Conversely, slippage of less than 30% is not linked to further progression unless associated with spina bifida occulta or puberty.41 The overall risk for progression has been reported to be around 4% to 5%.35,42 After their review of CT imaging of isthmic spondylolysis and normal lumbar spines, Grobler and colleagues found a smaller transverse articular dimension in the isthmic group to be the only morphologic finding with a significant difference.32 It is widely accepted that the adolescent growth spurt contributes to the progression of various forms of spinal deformity, including progression of slippage. Disk degeneration has also been attributed to the progression of adolescent listhesis, and some authors believe that this should be evaluated because its presence may cause the surgeon to be more inclined to consider fusion surgery.43,44 Other authors, however, have failed to demonstrate significant progression of slippage during adolescence.39 Sacralization of the lumbar vertebra with an isthmic defect at L4 has been associated with a greater likelihood of progression than an L4 defect without a transitional vertebra.45 Interestingly, in this same study, sacralization with an L5 isthmic defect showed less slippage than the same condition without a transitional vertebra.45 In a long-term follow-up study (minimum of 20 years) of 255 patients, mean slip progression was found to be 4 mm. Of the patients with slippage greater than 10 mm, 11% were adolescents and 5% were adults.35 In their report of 86 pediatric athletes (6 to 20 years of age) with spondylolysis and a mean initial slippage of 10.1%, Muschik and associates noted progression in 44%, stability of the slippage in 47%, and actual improvement in 9%. Over a 4.8-year period, 12% of the patients demonstrated greater than 10-mm progression of their slippage, whereas a single patient experienced greater than 20% slippage. In this study, spina bifida occulta and continued activity were not found to be associated with increased risk for slippage.46 The sacral morphology of pediatric patients with developmental L5-S1 spondylolisthesis was recently demonstrated to have certain unique characteristics that are thought to predispose to progression of slippage. Wang and colleagues found a lower sacral table angle and higher sacral kyphosis to more frequently be associated with spondylolisthesis. They also correlated a lower sacral table angle with increasing grade of slippage.47 The phenomenon of “autofusion” refers to the end stage of disk space collapse together with end plate and facet osteophyte formation and results in segmental fusion of the involved listhesis and, at times, diminution of symptoms in a subset of patients. During their review of the natural history of degenerative spondylolisthesis, Matsunaga and associates found that patients experiencing significant disk space collapse, end plate sclerosis, and spurring rarely progressed to higher grades of listhesis.33
Treatment
Conservative Treatment
As described earlier, the symptoms of degenerative spondylolisthesis are often intermittent in nature. Nonsteroidal anti-inflammatory medications, bed rest, bracing, heat, exercise, and traction were found by Rosenberg to be unpredictable in their ability to treat a patient’s symptoms.27 Although often used for their diagnostic and therapeutic utility, epidural steroid injections have not been shown to provide lasting results in most cases. In a study by Rosen and coworkers, less than 25% of patients had any long-term relief of symptoms after epidural steroid injections.48 Overall, 10% to 15% of patients will fail conservative therapy and need to undergo surgical treatment.27,48
Surgical Treatment
Initially popularized by Gill and colleagues,49 resection of the lamina and pars as a means of decompression without fusion has largely been abandoned as primary treatment of spondylolisthesis because it is associated with increased slippage and worsened back pain.
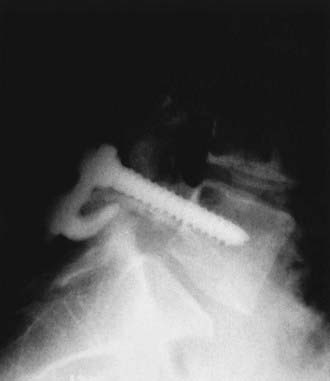
Although generally reserved for low-grade spondylolisthesis in patients who have failed conservative treatment, direct repair of the pars defect through various constructs with the use of wires, screws, or other techniques is an option for some pediatric patients (Fig. 286-22). This technique has been compared with intertransverse fusion with or without segmental instrumented fusion for the treatment of pediatric spondylolisthesis.17
The literature is replete with data regarding outcomes after surgery consisting of decompression only.50–57 The degree of decompression performed by any surgeon for any individual patient is dictated by many variables, and the odds of decompression alone resulting in instability is difficult to predict. However, should progressive instability develop, worsening symptoms may necessitate repeated surgery.
In situ fusion is preferred by many spine surgeons for spondylolysis and spondylolisthesis in adolescents because it has been found to have a lower number of complications than reduction procedures while maintaining comparable outcomes.15 Higher grade spondylolisthesis has been shown to have a lower fusion rate, however, especially when combined with an increased slip angle. Although more radical decompression is often necessary to adequately relieve neural compression, some studies have determined that limited resection of the articular process with or without in situ intertransverse fusion has significantly better results than aggressive facet joint resection without fusion and that decompression without fusion has good outcomes with only limited progression of slippage, provided that pars and facet joint integrity is preserved.56,57
A randomized controlled trial of 50 patients with degenerative lumbar spondylolisthesis in which decompression alone was compared with decompression and intertransverse process fusion demonstrated significantly improved outcomes when decompression with arthrodesis of the intertransverse process was performed, despite the fact that pseudoarthrosis developed in nine patients in the fusion arm.58
Although the addition of instrumented fusion has been shown to improve fusion rates over bony fusion techniques, improvement in clinical outcome has previously been more difficult to demonstrate.59,60 A meta-analysis performed in 1994 determined that fusion surgery, regardless of whether instrumentation was used, was superior to decompression alone in achieving good clinical outcomes in patients with degenerative spondylolisthesis.61 Instrumented fusion techniques have also been found to be very expensive in cost-effectiveness studies62; however, the current wide variations in outcomes studies for spondylolisthesis make these types of studies difficult to interpret by practicing spine surgeons.
Kornblum and coworkers associated pseudoarthrosis with worse clinical outcomes in a long-term outcomes study of patients treated with decompression, decompression and bone fusion, and decompression and instrumented fusion. Based on their findings, a recommendation may be made for instrumented fusion for degenerative spondylolisthesis.63 In addition, although posterolateral fusion is an effective option for most patients, it often does not improve the slip angle in those with higher grade spondylolisthesis. Consequently, there is heightened concern regarding slip progression and pseudoarthrosis in patients with slip angles greater than 25 degrees, grade III or IV spondylolisthesis, presence of a dome-shaped sacrum or trapezoidal L5 segment, or greater than 50% lordosis of the lumbar spine. A 6% rate of cauda equina syndrome was reported by Schoenecker and coauthors in patients treated with in situ arthrodesis for grade III to IV spondylolisthesis.64
Posterior segmental instrumented fusion is an integral part of the current treatment algorithm for spondylolisthesis. It provides a means of correcting the slip angle and can be performed with or without reduction. Improved body posture and biomechanics are thereby possible. Improved fusion rates for higher grade spondylolisthesis65 and more aggressive decompression can be achieved through instrumentation. Instrumentation is not without risk, however, and one prospective randomized study of 77 patients with grades I to III isthmic spondylolisthesis found that the risk-to-benefit ratio for instrumentation may be different for low versus higher grade spondylolisthesis.66 Interestingly, the authors found a higher radiographic fusion rate in the noninstrumented patients (78% versus 65%) than in the instrumented group. Although both groups demonstrated significant improvement, blood loss and longer operative times were noted in the instrumented group. Nerve root injury after the placement of pedicle screws occurred in 5.4%.
Other studies have demonstrated realignment alone without decompression to be adequate for achieving good outcomes (satisfactory relief of back pain in 89% and improvement in radicular symptoms in 93%) in patients with unstable slippage.67 In a retrospective review of degenerative spondylolisthesis treated with or without instrumented fusion, recovery was positively correlated with lumbar lordosis of the fused segments and pedicle screw fixation. Better restoration of sagittal balance and reduction of slippage were proposed as factors in the improvement in back pain with instrumentation.68 Lenke and coworkers were able to demonstrate 80% improvement in symptoms in 56 patients undergoing posterolateral fusion without decompression for isthmic spondylolisthesis. Interestingly, however, definite fusion was seen in only 50% at follow-up.69
Age at treatment influences outcome. Despite a high rate of fusion in both groups, in their study of more than 40 patients undergoing posterolateral fusion without decompression for isthmic spondylolisthesis, Haraldsson and Willner found significant relief of symptoms in 95% of adolescents, whereas only 57% of adults reported the same degree of improvement.70 An aged spine with resultant degenerative disease was proposed as a factor affecting these results.
The use of interbody grafts for the treatment of spondylolisthesis is a common strategy. Together with posterior instrumentation, interbody grafts have demonstrated superior stability in cadaver studies when compared with standalone anterior cages or posterior-only instrumentation for degenerative spondylolisthesis.71 Combined interbody and instrumented posterior fusion techniques have also resulted in improved clinical outcomes when compared with instrumented posterolateral fusion techniques alone for spondylolytic spondylolisthesis (45% versus 75% excellent outcome for back pain).72 The same authors later determined anterior lumbar interbody fusion to be superior to posterolateral fusion in terms of fusion rates and maintenance of reduction. Both anterior lumbar interbody fusion and posterolateral fusion were combined with pedicle screw fixation to augment the fusion in this study.73 Various techniques for achieving anterior interbody fusion from a posterior approach have been developed.74
In addition to its role as a salvage procedure for failed posterior fusion, anterior surgery allows the posterior fusion to heal under compression. However, there are inherent complications that must be considered when proposing an anterior approach, and the results are varied in comparison to posterior approaches.75 Retrograde ejaculation, damage to the great vessels and urogenital system, intraperitoneal injury, ileus, and other complications unique to this approach have been described.76–79 Many authors agree that the degree of correction achieved is often greater when an anterior approach is combined with posterior fusion, with or without decompression, and there are benefits to combining posterior stabilization with anterior fusion. Although a statistically significant difference in outcome could not be established, in Muschik and Zippel’s retrospective comparison of 70 children treated by combined instrumented posterior fusion and in situ ventral fusion versus ventral fusion alone for L5 spondylolisthesis, lower rates of pseudoarthrosis and lumbosacral kyphosis, a lesser degree of slippage, and a shorter time to achieve fusion were demonstrated in the combined anterior/posterior group.80
Blackburne JS, Velikas EP. Spondylolisthesis in children and adolescents. J Bone Joint Surg Br. 1977;59:490-494.
Frennered AK, Danielson BI, Nachemson AL. Natural history of symptomatic isthmic low-grade spondylolisthesis in children and adolescents: a seven-year follow-up study. J Pediatr Orthop. 1991;11:209-213.
Gill GG, Manning JG, White HL. Surgical treatment of spondylolisthesis without spine fusion; excision of the loose lamina with decompression of the nerve roots. J Bone Joint Surg Am. 1955;37:493-520.
Hresko MT, Labelle H, Roussouly P, et al. Classification of high-grade spondylolistheses based on pelvic version and spine balance: possible rationale for reduction. Spine. 2007;32:2208-2213.
Kawakami M, Tamaki T, Ando M, et al. Lumbar sagittal balance influences the clinical outcome after decompression and posterolateral spinal fusion for degenerative lumbar spondylolisthesis. Spine. 2002;27:59-64.
Kinoshita T, Ohki I, Roth KR, et al. Results of degenerative spondylolisthesis treated with posterior decompression alone via a new surgical approach. J Neurosurg. 2001;95:11-16.
Laurent LE, Einola S. Spondylolisthesis in children and adolescents. Acta Orthop Scand. 1961;31:45-64.
Lenke LG, Bridwell KH, Bullis D, et al. Results of in situ fusion for isthmic spondylolisthesis. J Spinal Disord. 1992;5:433-442.
Lonstein JE. Spondylolisthesis in children: cause, natural history, and management. Spine. 1999;24:2640-2648.
Lundin DA, Wiseman D, Ellenbogen RG, et al. Direct repair of the pars interarticularis for spondylolysis and spondylolisthesis. Pediatr Neurosurg. 2003;39:195-200.
Mac-Thiong J-M, Labelle H. A proposal for a surgical classification of pediatric lumbosacral spondylolisthesis based on current literature. Eur Spine J. 2006;15:1425-1435.
Matsunaga S, Ijiri K, Hayashi K. Nonsurgically managed patients with degenerative spondylolisthesis: a 10- to 18-year follow-up study. J Neurosurg. 2000;93:194-198.
Molinari RW, Bridwell KH, Lenke LG, et al. Complications in the surgical treatment of pediatric high-grade, isthmic dysplastic spondylolisthesis. A comparison of three surgical approaches. Spine. 1999;24:1701-1711.
Muschik M, Zippel H. Spondylolisthesis in children and adolescents: surgical treatment with and without dorsal transpedicular instrumentation. J Bone Joint Surg Br. 1997;79:326-327.
Newman PH. The etiology of spondylolisthesis. J Bone Joint Surgery Br. 1963;45:39-59.
Poussa M, Schlenzka D, Seitsalo S, et al. Surgical treatment of severe isthmic spondylolisthesis in adolescents: reduction or fusion in situ. Spine. 1993;18:894-901.
Saraste H. Long-term clinical and radiological follow-up of spondylolysis and spondylolisthesis. J Pediatr Orthop. 1987;7:631-638.
Turner RH, Bianco AJJr. Spondylolysis and spondylolisthesis in children and teen-agers. J Bone Joint Surg Am. 1971;53:1298-1306.
Wiltse LL, Winter RB. Terminology and measurement of spondylolisthesis. J Bone Joint Surg Am. 1983;65:768-772.
Wiltse LL, Newman PH, Macnab I. Classification of spondylolysis and spondylolisthesis. Clin Orthop Relat Res. 1976;117:23-29.
1 Herbiniaux G. Traite sur Divers Accouchements Laborieux et sur les Polypes de la Matrice. Brussels: De Boubers, 1782.
2 Kilian HF. Schilderungen neuer Beckenformen and ihres Verhaltens in Leben. Mannheim, Germany: Verlag von Bassermann and Mathey; 1854.
3 Neugebauer FL. A new contribution to the history and etiology of spondylolisthesis. New Sydenham Society Select Monogr. 1888;121B:1-64.
4 Meyerding HW. Spondylolisthesis. Surg Gynecol Obstet. 1932;54:371-377.
5 Newman PH. The etiology of spondylolisthesis. J Bone Joint Surgery Br. 1963;45:39-59.
6 Davis H. Increasing rates of cervical and lumbar spine surgery in the United States 1979-1990. Spine. 1994;19:1117-1123.
7 Wynne-Davies R, Scott JH. Inheritance and spondylolisthesis: a radiographic family survey. J Bone Joint Surg Br. 1979;61:301-305.
8 Albanese M, Pizzutillo PD. Family study of spondylolysis and spondylolisthesis. J Pediatr Orthop. 1982;2:496-499.
9 Friberg S. Studies on spondylolisthesis. Acta Chir Scand. 1939;82(suppl 55):1-140.
10 Laurent LE, Einola S. Spondylolisthesis in children and adolescents. Acta Orthop Scand. 1961;31:45-64.
11 Turner RH, Bianco AJJr. Spondylolysis and spondylolisthesis in children and teen-agers. J Bone Joint Surg Am. 1971;53:1298-1306.
12 Lonstein JE. Spondylolisthesis in children: cause, natural history, and management. Spine. 1999;24:2640-2648.
13 Wiltse LL, Newman PH, Macnab I. Classification of spondylolysis and spondylolisthesis. Clin Orthop Relat Res. 1976;117:23-29.
14 Ward CV, Latimer B. Human evolution and the development of spondylolysis. Spine. 2005;30:1808-1814.
15 Poussa M, Schlenzka D, Seitsalo S, et al. Surgical treatment of severe isthmic spondylolisthesis in adolescents: reduction or fusion in situ. Spine. 1993;18:894-901.
16 Zamani MH, MacEwen GD. Herniation of the lumbar disc in children and adolescents. J Pediatr Orthop. 1982;2:528-533.
17 Lundin DA, Wiseman D, Ellenbogen RG, et al. Direct repair of the pars interarticularis for spondylolysis and spondylolisthesis. Pediatr Neurosurg. 2003;39:195-200.
18 Grobler LJ, Wiltse LL. Classification, non-operative, and operative treatment of spondylolisthesis. Frymoyer JW, Ducker TB, Hadler NM, et al, editors. The Adult Spine: Principles and Practice, vol 2. 1991:1655-1704.
19 Hutton WC, Cyron BM. Spondylolysis. The role of the posterior elements in resisting the intervertebral compressive force. Acta Orthop Scand. 1978;49:604-609.
20 Jackson DW, Wiltse LL, Cirincione RJ. Spondylolysis in the female gymnast. Clin Orthop Relat Res. 1976;117:68-73.
21 Wiltse LL, Winter RB. Terminology and measurement of spondylolisthesis. J Bone Joint Surg Am. 1983;65:768-772.
22 Ganju A. Isthmic spondylolisthesis. Neurosurg Focus. 2002;13:E1.
23 Hresko MT, Labelle H, Roussouly P, et al. Classification of high-grade spondylolistheses based on pelvic version and spine balance: possible rationale for reduction. Spine. 2007;32:2208-2213.
24 Mac-Thiong J-M, Labelle H. A proposal for a surgical classification of pediatric lumbosacral spondylolisthesis based on current literature. Eur Spine J. 2006;15:1425-1435.
25 Junghanns H. Spondylolisthesen ohne Spalt in Zwischengelenkstuck. Arch Orthop Unfallchir. 1930;29:118-127.
26 Newman PH. Spondylolisthesis, its cause and effect. Ann R Coll Surg Engl. 1955;16:305-323.
27 Rosenberg NJ. Degenerative spondylolisthesis. Predisposing factors. J Bone Joint Surg Am. 1975;57:467-474.
28 Rosenberg NJ, Bargar WL, Friedman B. The incidence of spondylolysis and spondylolisthesis in nonambulatory patients. Spine. 1981;6:35-38.
29 Bassewitz H, Herkowitz H. Lumbar stenosis with spondylolisthesis: current concepts of surgical treatment. Clin Orthop Relat Res. 2001;384:54-60.
30 Imada K, Matsui H, Tsuji H. Oophorectomy predisposes to degenerative spondylolisthesis. J Bone Joint Surg Br. 1995;77:126-130.
31 Nadaud MC, McClure S, Weiner BK. Do facet joint capsular ligaments contain estrogen receptors? Application to pathogenesis of degenerative spondylolisthesis. Am J Orthop. 2001;30:753-754.
32 Grobler LJ, Robertson PA, Novotny JE, et al. Etiology of spondylolisthesis. Assessment of the role played by lumbar facet joint morphology. Spine. 1993;18:80-91.
33 Matsunaga S, Sakou T, Morizono Y, et al. Natural history of degenerative spondylolisthesis. Pathogenesis and natural course of the slippage. Spine. 1990;15:1204-1210.
34 Matsunaga S, Ijiri K, Hayashi K. Nonsurgically managed patients with degenerative spondylolisthesis: a 10- to 18-year follow-up study. J Neurosurg. 2000;93:194-198.
35 Saraste H. Long-term clinical and radiological follow-up of spondylolysis and spondylolisthesis. J Pediatr Orthop. 1987;7:631-638.
36 Fitzgerald JA, Newman PH. Degenerative spondylolisthesis. J Bone Joint Surg Br. 1976;58:184-192.
37 Newman PH. A clinical syndrome associated with severe lumbo-sacral subluxation. J Bone Joint Surg Br. 1965;47:472-481.
38 Amato M, Totty WG, Gilula LA. Spondylolysis of the lumbar spine demonstration of defects and laminal fragmentation. Radiology. 1984;153:627-629.
39 Fredrickson BE, Baker D, McHolick WJ, et al. The natural history of spondylolysis and spondylolisthesis. J Bone Joint Surg Am. 1984;66:699-707.
40 Speck GR, McCall IW, O’Brien JP. Spondylolisthesis: the angle of kyphosis. Spine. 1984;9:659-660.
41 Blackburne JS, Velikas EP. Spondylolisthesis in children and adolescents. J Bone Joint Surg Br. 1977;59:490-494.
42 Frennered AK, Danielson BI, Nachemson AL. Natural history of symptomatic isthmic low-grade spondylolisthesis in children and adolescents: a seven-year follow-up study. J Pediatr Orthop. 1991;11:209-213.
43 Schlenzka D, Poussa M, Seitsalo S, et al. Intervertebral disc changes in adolescents with isthmic spondylolisthesis. J Spinal Disord. 1991;4:344-352.
44 Szypryt EP, Twining P, Mulholland RC, et al. The prevalence of disc degeneration associated with neural arch defects of the lumbar spine assessed by magnetic resonance imaging. Spine. 1989;14:977-981.
45 Kim NH, Suk KS. The role of transitional vertebrae in spondylolysis and spondylolytic spondylolisthesis. Bull Hosp Jt Dis. 1997;56:161-166.
46 Muschik M, Haehnel H, Robinson PN, et al. Competitive sports and the progression of spondylolisthesis. J Pediatr Orthop. 1996;16:364-369.
47 Wang Z, Parent S, Mac-Thiong J-M, et al. Influence of sacral morphology in developmental spondylolisthesis. Spine. 2008;33:2185-2191.
48 Rosen CD, Kahanovitz N, Bernstein R, et al. A retrospective analysis of the efficacy of epidural steroid injections. Clin Orthop Relat Res. 1988;228:270-272.
49 Gill GG, Manning JG, White HL. Surgical treatment of spondylolisthesis without spine fusion; excision of the loose lamina with decompression of the nerve roots. J Bone Joint Surg Am. 1955;37:493-520.
50 Epstein JA, Epstein BS, Lavine L. Nerve rot compression associated with narrowing of the lumbar spinal canal. J Neurol Neurosurg Psychiatry. 1962;25:165-176.
51 Epstein NE, Epstein JA, Carras R, et al. Degenerative spondylolisthesis with an intact neural arch: a review of 60 cases with an analysis of clinical findings and the development of surgical management. Neurosurgery. 1983;13:555-561.
52 Epstein NE. Decompression in the surgical management of degenerative spondylolisthesis: advantages of a conservative approach in 290 patients. J Spinal Disord. 1998;11:116-122.
53 Silvers HR, Lewis PJ, Asch HL. Decompressive lumbar laminectomy for spinal stenosis. J Neurosurg. 1993;78:695-701.
54 Kinoshita T, Ohki I, Roth KR, et al. Results of degenerative spondylolisthesis treated with posterior decompression alone via a new surgical approach. J Neurosurg. 2001;95:11-16.
55 Kristof RA, Aliashkevich AF, Schuster M, et al. Degenerative lumbar spondylolisthesis-induced radicular compression: nonfusion-related decompression in selected patients without hypermobility on flexion-extension radiographs. J Neurosurg. 2002;97:281-286.
56 Herron LD, Trippi AC. L4-5 degenerative spondylolisthesis. The results of treatment by decompressive laminectomy without fusion. Spine. 1989;14:534-538.
57 Lombardi JS, Wiltse LL, Reynolds J, et al. Treatment of degenerative spondylolisthesis. Spine. 1985;10:821-827.
58 Herkowitz HN, Kurz LT. Degenerative lumbar spondylolisthesis with spinal stenosis. A prospective study comparing decompression with decompression and intertransverse process arthrodesis. J Bone Joint Surg Am. 1991;73:802-808.
59 Zdeblick TA. A prospective, randomized study of lumbar fusion. Preliminary results. Spine. 1993;18:983-991.
60 Fischgrund JS, Mackay M, Herkowitz HN, et al. 1997 Volvo Award winner in clinical studies. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective, randomized study comparing decompressive laminectomy and arthrodesis with and without spinal instrumentation. Spine. 1997;22:2807-2812.
61 Mardjetko SM, Connolly PJ, Shott S. Degenerative lumbar spondylolisthesis. A meta-analysis of literature 1970-1993. Spine. 1994;19:2256S-2265S.
62 Kuntz KM, Snider RK, Weinstein JN, et al. Cost-effectiveness of fusion with and without instrumentation for patients with degenerative spondylolisthesis and spinal stenosis. Spine. 2000;25:1132-1139.
63 Kornblum MB, Fischgrund JS, Herkowitz HN, et al. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective long-term study comparing fusion and pseudarthrosis. Spine. 2004;29:726-733.
64 Schoenecker PL, Cole HO, Herring JA, et al. Cauda equina syndrome after in situ arthrodesis for severe spondylolisthesis at the lumbosacral junction. J Bone Joint Surg Am. 1990;72:369-377.
65 Molinari RW, Bridwell KH, Lenke LG, et al. Complications in the surgical treatment of pediatric high-grade, isthmic dysplastic spondylolisthesis. A comparison of three surgical approaches. Spine. 1999;24:1701-1711.
66 Moller H, Hedlund R. Instrumented and noninstrumented posterolateral fusion in adult spondylolisthesis—a prospective randomized study: part 2. Spine. 2000;25:1716-1721.
67 Lee TC. Reduction and stabilization without laminectomy for unstable degenerative spondylolisthesis: a preliminary report. Neurosurgery. 1994;35:1072-1076.
68 Kawakami M, Tamaki T, Ando M, et al. Lumbar sagittal balance influences the clinical outcome after decompression and posterolateral spinal fusion for degenerative lumbar spondylolisthesis. Spine. 2002;27:59-64.
69 Lenke LG, Bridwell KH, Bullis D, et al. Results of in situ fusion for isthmic spondylolisthesis. J Spinal Disord. 1992;5:433-442.
70 Haraldsson S, Willner S. A comparative study of spondylolisthesis in operations on adolescents and adults. Arch Orthop Trauma Surg. 1983;101:101-105.
71 Cagli S, Crawford NR, Sonntag VK, et al. Biomechanics of grade I degenerative lumbar spondylolisthesis. Part 2: treatment with threaded interbody cages/dowels and pedicle screws. J Neurosurg. 2001;94:51-60.
72 Suk SI, Lee CK, Kim WJ, et al. Adding posterior lumbar interbody fusion to pedicle screw fixation and posterolateral fusion after decompression in spondylolytic spondylolisthesis. Spine. 1997;22:210-219.
73 Suk KS, Jeon CH, Park MS, et al. Comparison between posterolateral fusion with pedicle screw fixation and anterior interbody fusion with pedicle screw fixation in adult spondylolytic spondylolisthesis. Yonsei Med J. 2001;42:316-323.
74 Bohlman HH, Cook SS. One-stage decompression and posterolateral and interbody fusion for lumbosacral spondyloptosis through a posterior approach. Report of two cases. J Bone Joint Surg Am. 1982;64:415-418.
75 Gaines RW, Nichols WK. Treatment of spondyloptosis by two stage L5 vertebrectomy and reduction of L4 onto S1. Spine. 1985;10:680-686.
76 Samudrala S, Khoo LT, Rhim SC, et al. Complications during anterior surgery of the lumbar spine: an anatomically based study and review. Neurosurg Focus. 1999;7(6):E9.
77 Staehli LM, Zehnder T, Schwarzenbach O, et al. Venous injury in lumbar anterior spine surgery. Swiss Med Wkly. 2006;136:670-671.
78 Rajaraman V, Vingan R, Roth P, et al. Visceral and vascular complications resulting from anterior lumbar interbody fusion. J Neurosurg. 1999;91(suppl 1):60-64.
79 Raskas DS, Delamarter RB. Occlusion of the left iliac artery after retroperitoneal exposure of the spine. Clin Orthop Relat Res. 1997;338:86-89.
80 Muschik M, Zippel H. Spondylolisthesis in children and adolescents: surgical treatment with and without dorsal transpedicular instrumentation. J Bone Joint Surg Br. 1997;79:326-327.