Chapter 100 Pediatric Spinal Deformities and Deformity Correction
Pediatric spinal deformity can result from congenital anomalies, neuromuscular disorders, genetic conditions, connective tissue disorders, skeletal dysplasia, and developmental (idiopathic) causes.1,2 Each category of spinal deformity has a typical behavior dictated by the pathophysiology of the underlying condition.
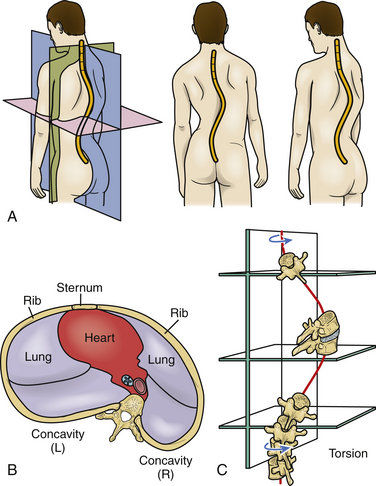
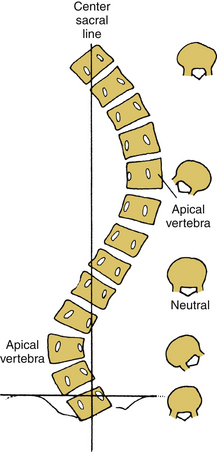
Scoliosis, kyphosis, and lordosis refer to deviations from normal spinal alignment. In the coronal plane, the spine is normally straight. In the sagittal plane, the thoracic region is kyphotic (range, 20–40 degrees), the lumbar region is lordotic, and the transition over the thoracolumbar region is relatively straight (Fig. 100-1).3 Scoliosis, curvature in the coronal plane, is also associated with transverse rotation, as well as with pathologic lordosis or kyphosis (Figs. 100-2 and 100-3).3–5 Therefore, the terms lordoscoliosis and kyphoscoliosis are frequently used to characterize the three-dimensional nature of a deformity. When more than one pathologic curvature exists along the length of the spine, the primary (or major) curve is designated on the basis of its size and rigidity. The secondary (or minor) curve(s), even if compensatory, may be rigid or have a “structural” component. Surgical planning should address the magnitude and flexibility of all the curves in all three planes.
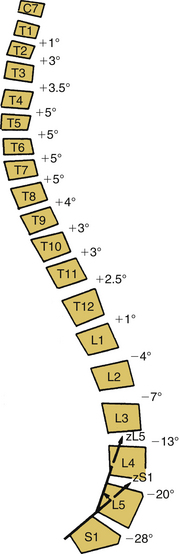
FIGURE 100-1 Spinal sagittal alignment is shown with the segmental angulation between vertebrae.
(From Bernhardt M, Bridwell KH: Segmental analysis of the sagittal plane alignment of the normal thoracic and lumbar spines and thoracolumbar junction. Spine [Phila Pa 1976] 14:717–721, 1989, with permission.)
Growth of the Spine
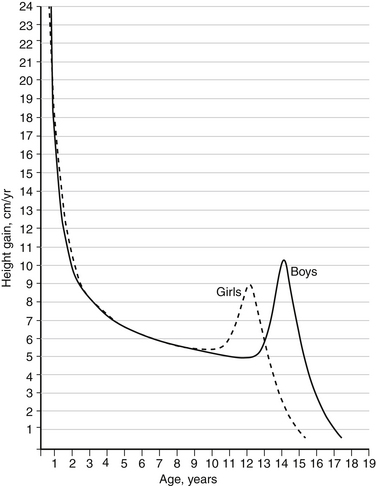
Pediatric spinal deformities are usually not clinically evident at birth. However, they progress in proportion to spinal growth. Therefore, anticipating and modifying the growth potential of the vertebral elements composing the deformity is essential. Two periods of rapid growth occur in children: the first between birth and 3 years and the second during the adolescent years. The timing and duration of the adolescent growth spurt can be determined by monitoring the growth velocity (Fig. 100-4). The spine grows heterogeneously; that is, during the adolescent growth spurt, the thoracic spine grows 1.2 cm per year and, in contrast, the lumbar spine grows 0.6 cm per year. Thus, the effect of spinal fusion on future growth can be estimated, although it should be remembered that a spine with scoliosis grows with progressive deformity. Apical vertebral growth exacerbates the deformity with further rotation, displacement, and tilting of the vertebrae.
Several methods for this assessment of skeletal maturity have been reported, including Risser stage, presence of triradiate cartilage, and hand films.6–10 The Risser stage is a method based on the degree of iliac ossification, with stages 1 through 4 corresponding to sequential ossification of each quarter of the iliac crest from ventral to dorsal11 (Fig. 100-5). Stage 4 reflects completion of spinal growth, and stage 5 is defined as fusion to the ilium. Alternatively, hand films can be obtained for the assessment of skeletal maturity, without the need to expose the pelvis to radiation, as is required for both the Risser stage and assessment of the triradiate cartilage.8,9
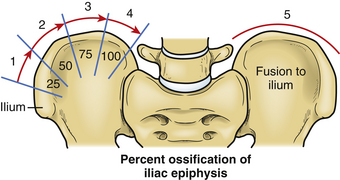
FIGURE 100-5 The Risser stage of iliac ossification can be used to estimate remaining maturity and growth.
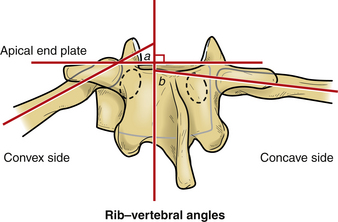
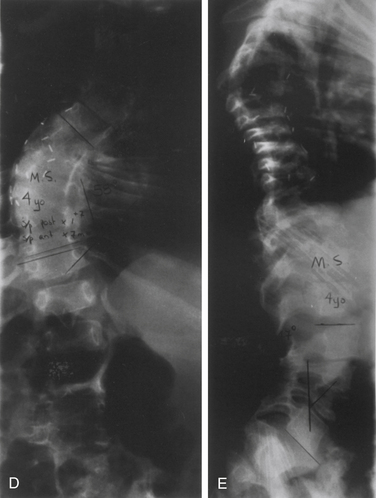
The rib–vertebral angle difference (RVAD) is another measure that can guide decision making in pediatric spinal deformity. This measure is an important prognostic indicator for infantile scoliosis.12 The RVAD is the difference between the angles formed by a line along the rib head and perpendicular to the base of the apical vertebra on the right and left sides of the spine. Spontaneous resolution of the scoliosis is expected in 85% to 90% of the cases if the RVAD is less than 20 degrees, but progression is expected with an RVAD greater than 20 degrees.12
Evaluation
Clinical Evaluation
Initial evaluation should begin with a detailed history, including the prenatal, birth, and cognitive and motor developmental history.9 Details of the suspected spinal disorder should be documented, including symptoms, deficits, onset, and progression, as well as disability and the quality of life. Past medical history can be a significant contributor, especially with congenital spinal disorders, which can be associated with other anomalies.13
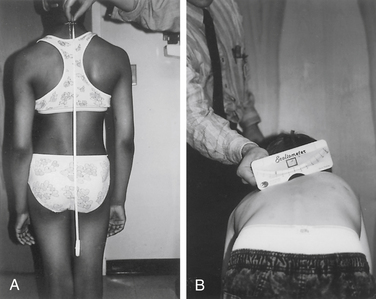
The scoliometer or inclinometer is used to quantify the rib prominence and paralumbar prominence. A scoliometer reading greater than 5 degrees is associated with a scoliosis of at least 10 degrees (Fig. 100-6).
Imaging Evaluation
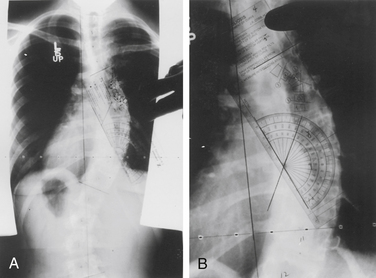
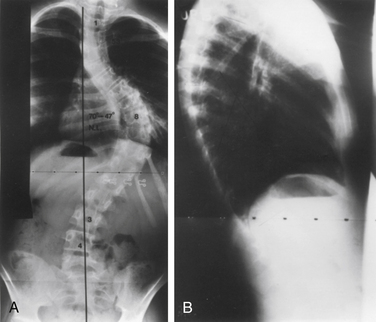
Initial evaluation of suspected spinal deformity often includes full-length (36-inch) posteroanterior (PA) and lateral spinal radiographs to assess global and regional spinal alignment. PA images evaluate for scoliosis, with measures including Cobb angle and coronal balance. Coronal balance is typically measured as the distance between a vertical line from the center of the C7 vertebral body (C7 plumb line) and the central sacral vertical line (CSVL) (Fig. 100-7). Lateral full-length spinal radiographs can be used to assess for regional kyphosis and lordosis and for global sagittal balance. Sagittal balance is typically measured as the distance between a vertical line from the center of the C7 plumb line and the dorsal rostral corner of the S1 vertebral body. If the C7 plumb line is ventral to the dorsal rostral corner of the S1 vertebral body, the sagittal balance measure is reflected as a positive value, and if dorsal, it is reflected as a negative value.
The flexibility of scoliotic curves can be assessed with side-bending PA films to the left and to the right. Alternatively, the same information may be obtained with the patient placed over a bolster or with the use of traction. The flexibility of kyphotic deformities can be assessed with a bolster placed under the apex of the kyphosis, and the flexibility of lordotic deformities may be assessed with the spine and pelvis placed in flexion.
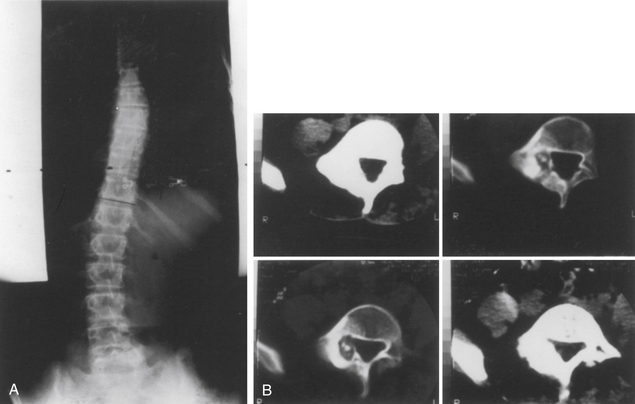
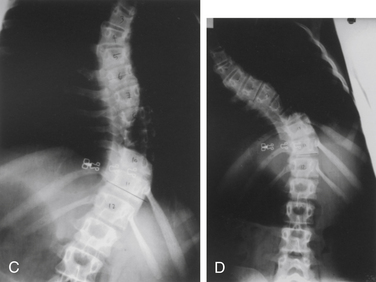
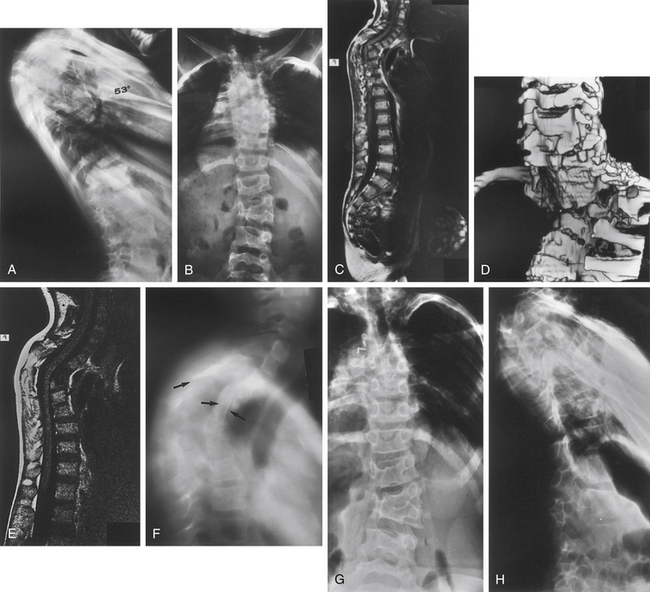
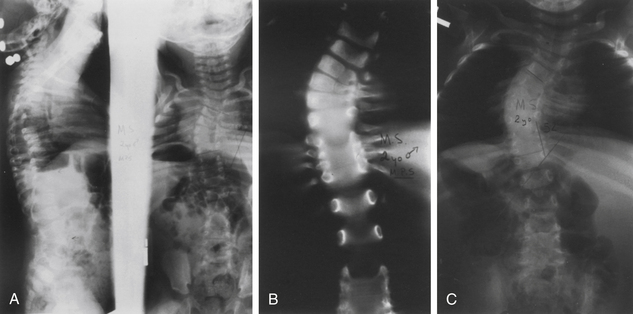
CT imaging provides greater detail of the bony anatomy and a three-dimensional view of complex deformities; such information may facilitate planning of surgical treatment.14,15 CT clearly defines congenital deformities with underlying anomalies, such as hemivertebra or unsegmented bars that may be occult on plain-film radiographs (Fig. 100-8).
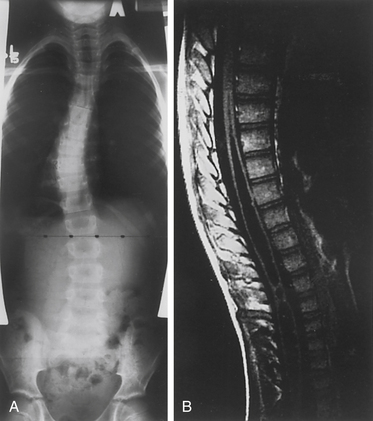
In the setting of spinal deformity, MRI can be used to evaluate for central canal and foraminal stenosis, as well as for underlying abnormalities, which may warrant treatment or alter surgical planning. Associated abnormalities, such as tethered cord, syringomyelia, and tumors, occur in up to 15% to 38% of congenital spinal deformity patients16–21 (Fig. 100-9). Although MRI is often used as an adjunct in the imaging evaluation of pediatric spinal deformity, several specific indications necessitate this evaluation, including severe pain; neurologic findings, including motor weakness, muscle atrophy, and upper motor neuron signs; early-onset scoliosis with a Cobb angle greater than 20 degrees; atypical scoliosis curve patterns (e.g., left thoracic curves, sharp angular curves, congenital deformities, and curves that are >70 degrees); scoliosis curves with a rapid progression (>1 degree per month); neurofibromatosis; deformity associated with myelomeningocele; and lack of apical lordosis in idiopathic scoliosis9,22 (Fig. 100-10).
Idiopathic Scoliosis: Early Onset and Adolescent Onset
Idiopathic scoliosis has a familial tendency and a bimodal frequency distribution. With the early-onset type the majority of cases occur in infancy and a second major peak arises during adolescence. Idiopathic scoliosis is divided into two groups: early onset (<5 years) and late onset (5 years to skeletal maturity).23,24 The most common type is adolescent idiopathic scoliosis (AIS).
The diagnosis of AIS is one of exclusion. In idiopathic scoliosis, deformity is the most common reason for referral. Occasionally, low-grade, activity-related back pain can result from lumbar curves or curves greater than 40 degrees. Atypical cases demand further evaluation to establish a diagnosis.9,22
Adolescent Idiopathic Scoliosis
Clinical Features
The natural history of AIS was studied thoroughly by Weinstein, who followed a cohort of patients for over 40 years, and by Collis and Ponseti.25–28 Factors that correlate with progression of idiopathic curves include physiologic younger age, female gender, curve magnitude, and double curves. During the adolescent years, curves typically progress an average of 1 degree per month and curves in excess of 50 degrees have a high risk of progression even after skeletal maturity. Finally, there is a significant inverse relationship between the pulmonary vital capacity and magnitude of thoracic scoliosis. Thoracic curves greater than 70 degrees are associated with a vital capacity less than predicted for size.
Classification
Lenke et al. developed the currently most commonly applied classification for AIS.29,30 Based on both coronal and sagittal radiographs, it was developed to aid in determination of the appropriate vertebral levels to be included in an arthrodesis. The classification system includes six curve types (numbered 1 through 6), a lumbar spine modifier (A, B, or C) that is based on deviation of the apical lumbar vertebra, and a sagittal plane modifier (−, N, or +).
Treatment
Bracing may be indicated in patients with AIS for curves greater than 20 degrees that have progressed more than 5 degrees. Adolescents with significant remaining growth potential who have curves between 25 degrees and 40 degrees may also warrant bracing, despite no documented progression.31 Bracing is typically stopped in adolescents if the curve progresses to surgical dimensions (45–50 degrees) or when skeletal maturity is reached.
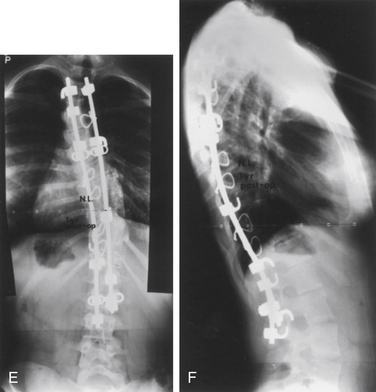
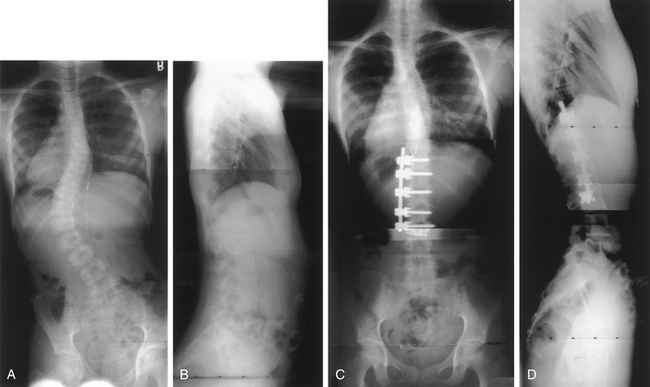
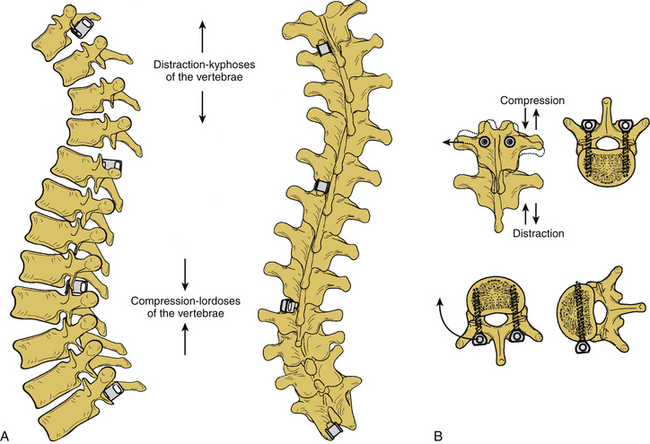
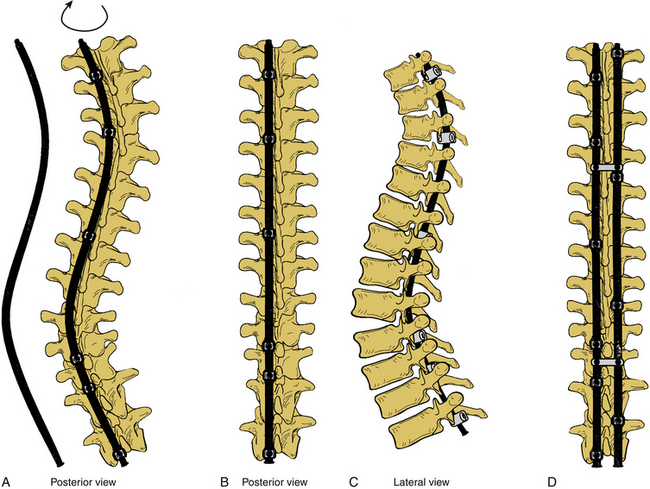
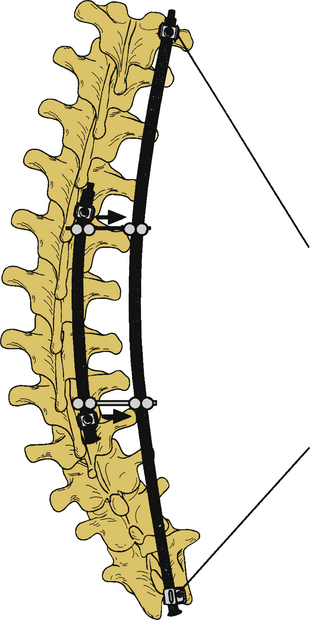
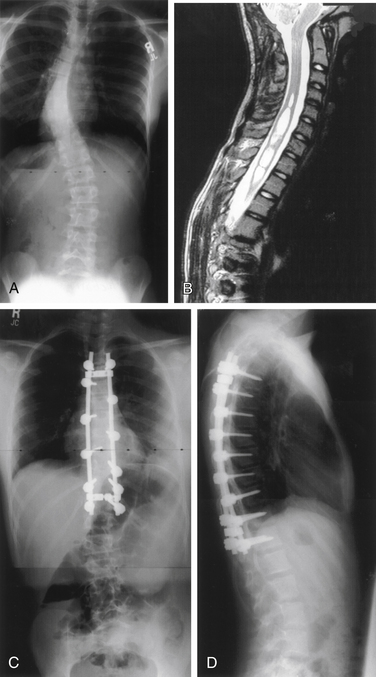
Surgery for AIS is often recommended in growing children once curves reach 45 degrees. Surgery is also recommended for skeletally mature teenagers for curves greater than 50 degrees, because curves of this severity have a high risk of progression in adult life.25,26,32 The goals of surgery include stopping curve progression, restoring alignment, balancing the spine in all anatomic planes, and minimizing the number of vertebral levels that are fused (Figs. 100-11 to 100-13). Current dorsal instrumentation systems include hooks, wires, and/or pedicle screws that are typically connected with dual rods (Figs. 100-14 to 100-16). Pedicle screw instrumentation of pediatric patients in experienced hands has been shown to be safe.33–37 Regardless of the instrumented system selected, the ultimate success of the procedure depends on achieving solid bony fusion.
Early-Onset Scoliosis
Clinical Features
Idiopathic scoliosis typically develops during late childhood (juvenile type) and can create serious cosmetic and functional problems. Pulmonary compromise is an additional concern in the rare cases of scoliosis developing before 5 years of age. According to the study of Nilsonne and Lundgren, severe curves (>100 degrees) resulted in death from cardiac or pulmonary causes in 60% of cases.38 This significant morbidity and mortality underscores the importance of identifying these patients early. Because idiopathic infantile scoliosis is so rare, meticulous examination of these patients and radiography are mandatory to exclude congenital or neurologic causes of the scoliosis. Routine brainstem and spinal cord MRI is reasonable for excluding CNS abnormalities in patients younger than 8 years presenting with a spinal deformity of greater than 20 degrees.
In comparison with adolescent and juvenile idiopathic scoliosis, the curves in infantile scoliosis are commonly (in 50–75% of patients) left thoracic curves, with boys more commonly affected than are girls. Increased risk of curve progression is associated with double curves, large curves, and significant rotational deformity. Mehta found that risk of progression was related to the RVAD.12 This angle is formed by a line along the rib head perpendicular with the base of the apical vertebra (see Fig. 100-12). If the difference between the angles on the concave and convex sides exceed 20 degrees, then progression is probable.
Treatment
Spinal instrumentation without fusion is the preferred technique for patients younger than 9 years of age.39–49 Serial distraction is carried out every 6 to 9 months with the ratcheted distraction rod. The patient is protected in a brace at all times, and once adolescence is reached, a formal instrumentation and fusion is carried out. Complications with this technique are common and include hook dislodgement, rod breakage, skin breakdown, and early fusion without bone graft. Despite the frequent complications, subfascial instrumentation for early-onset or juvenile scoliosis is a reasonable alternative to the inevitable cardiopulmonary problems associated with curve progression. Some surgeons perform ventral discectomy over the apical segments as the initial procedure, followed by dorsal subcutaneous rod insertions. This makes sense in the more severe curves and in the presence of thoracic lordosis.
Congenital Disorders
A broad range of congenital spinal disorders occur.50 Although many of these may not be evident at birth, each is thought to result from errors during development.51 Congenital spine anomalies most commonly occur sporadically and have an incidence of approximately 1 per 1000 to 2000.52–54 However, not all congenital spinal disorders are sporadic.
Congenital spinal malformations are not infrequently accompanied by associated anomalies, most frequently the organ systems affected develop at a similar phase of embryogenesis. Genitourinary anomalies are the most common, occurring in up to 25%.55 The cardiovascular system also develops anomalies, including ventricular septal defects, atrial septal defects, dextrocardia, and tetralogy of Fallot, in approximately 10% of cases.16
Congenital Scoliosis
Scoliosis is an abnormal curvature of the spine in the coronal plane. Congenital scoliosis is distinguished by the presence of anomalous vertebrae at birth. Although the vertebral abnormality is present at birth, typically no evidence of deformity presents until the growth phases of childhood or adolescence. Balanced spine anomalies may go undiagnosed until adulthood or are discovered incidentally. Infantile idiopathic scoliosis and juvenile idiopathic scoliosis also present with scoliosis in childhood, but these are distinguished from congenital scoliosis by their lack of vertebral anomalies.56
The presentation of congenital scoliosis varies and depends on the type and level of the spine anomaly, their number, and the degree of deformity. At the extremes, anomalies can result in rapidly progressive scoliosis with significant morbidity in early childhood or can result in minimal or no deformity throughout life. Approximately one quarter of patients with congenital scoliosis can be expected to experience no progression; one half progress slowly; and one quarter progress rapidly.50 With advancements in CT and MRI have come concomitant advances with classification and improvements in the surgical management.
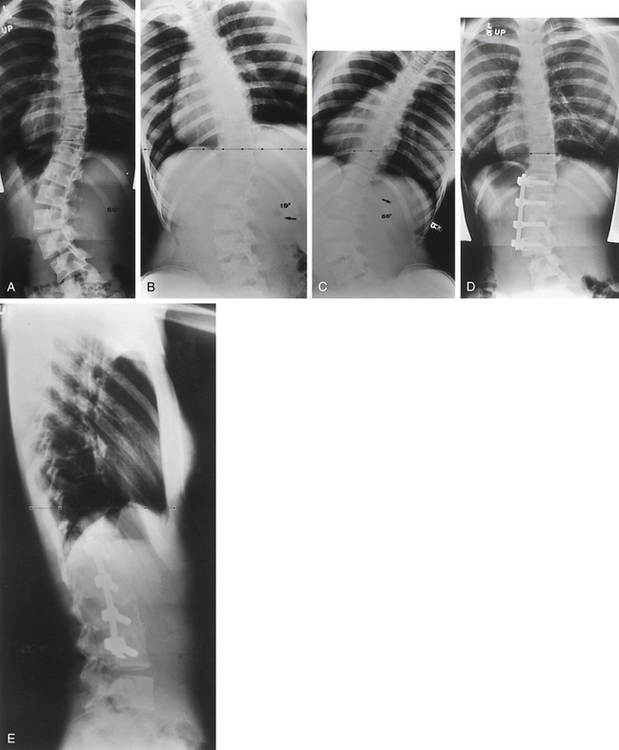
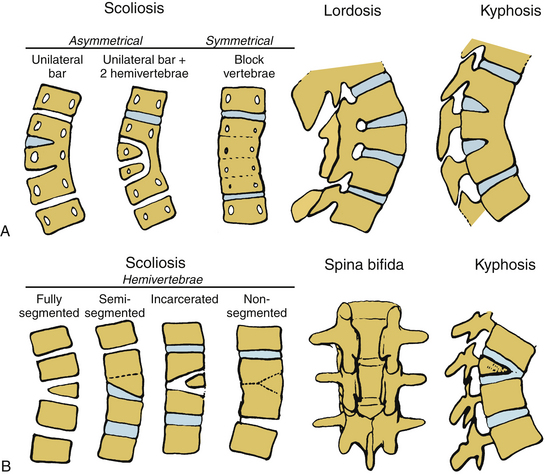
Congenital abnormalities of the spine are classified based on embryologic development of the spine, with categories including failures of formation, failures of segmentation, and mixed anomalies9,57 (Fig. 100-17). Failures of formation can range from mild wedging to complete absence of a vertebral body. The most common cause of congenital scoliosis is a hemivertebra, which typically consists of a wedged vertebral body with a single pedicle and hemilamina (Figs. 100-18 to 100-20). Failures of segmentation result in unilateral or bilateral bony fusion between vertebrae.58,59 The most common is the unilateral unsegmented bar, consisting of a bony block including the disc space and facet joints (Fig. 100-21). Among the various complex combinations of vertebral anomalies that can coexist is the unsegmented bar with contralateral hemivertebra, which can result in significant progressive scoliosis.60
An understanding of the natural history of congenital spine anomalies is important in order to define optimal treatment strategies. Of 202 patients with congenital scoliosis, 11% were nonprogressive, 14% had limited progression, and 75% progressed significantly.56 The type of anomaly is an important factor in the likelihood of progression. The most severe congenital scolioses may result from a unilateral unsegmented bar with a contralateral hemivertebra at the same level. The significant potential for this combination of anomalies to produce deformity argues for treating the patient immediately, without allowing for a period of observation.56,61 Patient age is also an important factor in determining capacity for deformity progression, with the greatest risk typically arising during the preadolescent growth spurt. Cases in which deformity presents in the first years of life are often associated with significant growth imbalance and are at high risk of significant deformity.
In cases of severe or progressive congenital scoliosis, surgery is often the most effective treatment. Surgical options include hemivertebra excision, convex hemiepiphysiodesis, fusion in situ, spinal instrumentation, and thoracoplasty with vertical expansion prosthetic titanium rib (VEPTR).50 Hemivertebra excision offers the ability to directly address the biomechanical decompensation and can provide immediate and often significant deformity correction.62–64 Convex hemiepiphysiodesis involves excision of the disc and fusion on the convex side of the curve and relies on remaining growth potential on the side of the concavity.65 Although in situ fusion with casting or bracing has been the traditional treatment, the use of dorsal instrumented arthrodesis has become more popular in cases requiring fusion. Patients with progressive curves and congenital rib fusions may benefit from thoracoplasty and VEPTR.44,46 It is important to recognize the surgical morbidity that can accompany complex reconstructions for congenital scoliosis. Reames et al. reported a modern series of more than 2000 patients from the Scoliosis Research Society treated for congenital scoliosis, in which the morbidity rate was more than 10% and the mortality rate was 0.3%.66
Dysplastic Spondylolisthesis
Spondylolisthesis is the malalignment of one vertebral body relative to an adjacent level. The most widely accepted classification of spondylolisthesis describes five types, including dysplastic (type I), isthmic (type II), degenerative (type III), traumatic (type IV), and pathologic (type V).67 Dysplastic spondylolisthesis is a congenital disorder that results from a defect in the facet complex, typically L5-S1, that permits pathologic movement. The development of spondylolisthesis is extremely rare in infancy and usually requires ambulation.
Initial treatment of dysplastic spondylolisthesis should be nonoperative, unless there has been documented progression in a young patient or a slippage more severe than 50% of the initial presentation. Surgical treatment includes decompression with fusion in situ or decompression, fixation with pedicle screws, and fusion. Instrumentation facilitates partial or complete reduction but is associated with an increased rate of new neurologic deficit.68
Congenital Lordosis
Congenital lordosis results from dorsal defects in segmentation and is much rarer than other congenital spinal deformities.69 Congenital lordosis is usually accompanied by a concomitant coronal plane deformity (lordoscoliosis) due to a dorsolateral unsegmented bar. The incidence of neurologic deficits with congenital lordosis is much lower than with congenital kyphosis.
Congenital Kyphosis
Congenital kyphosis is a sagittal plane deformity resulting from vertebral anomalies, including failures of formation and segmentation.9 Congenital kyphosis often results in neurologic deficit without treatment. There are three distinct types: failure of formation of the vertebral body (type I), failure of segmentation of the vertebral body with ventral unsegmented bar (type II), and mixed failure of formation and segmentation (type III).70 Type I is the most common and the one that most frequently results in severe deformity and neurologic deficits. Type II produces less deformity and is considerably less likely to result in neurologic deficit. The severity of kyphosis produced with type II depends on the differential growth between ventral vertebral and dorsal structures. Type III is very rare and is thought to behave in a fashion similar to type I.
In general, nonoperative treatment is not recommended for congenital kyphosis because of the significant risk of neurologic deficits with progression. Dorsal fusion alone is adequate in patients younger than 5 years with kyphosis less than 55 degrees. Combined ventral and dorsal procedures are often required in older patients or those with kyphosis greater than 55 degrees. Surgical correction of congenital kyphosis can be associated with significant risk of morbidity and mortality. Adverse events are associated with kyphosis greater than 60 degrees and spinal cord compression evident on preoperative imaging.71
Neuromuscular Disorders
Cerebral Palsy
Among nonambulatory cerebral palsy patients, approximately 70% develop scoliosis by the age of 15 years.72 Although these curves can progress into adulthood, the greatest progression occurs intuitively during the times of peak growth (~2–4 degrees per month).73,74 These deformities are typically long sweeping curves that include pelvic obliquity and may also include significant kyphosis or hyperlordosis. Although braces may be used to temporarily treat these deformities, many patients ultimately require surgical intervention.75 Surgical correction often includes long segmental instrumentation including pelvic fixation. Significant pelvic and hip contractures should be treated prior to spinal fusion, since pelvic fixation could exacerbate these conditions.
Neuromuscular Dystrophies and Myopathies
Both scoliosis and sagittal plane deformities are common among patients with primary disorders of the nervous system or muscular system, or both. The Scoliosis Research Society has classified neuromuscular scoliosis including categories of upper motor neuron pathology (e.g., cerebral palsy, Rett syndrome), lower motor neuron and mixed pathology (e.g., Charcot-Marie-Tooth atrophy, spinal muscular atrophy, spinal cord injury, myelomeningocele), and primary myopathies (e.g., Duchenne muscular dystrophy, myopathies).76
The risk of deformity depends on the severity of the underlying disorder. For example, almost all patients with Duchenne muscular dystrophy develop collapsing deformity and many require surgical treatment after becoming nonambulatory. Up to 70% of patients with severe cerebral palsy develop scoliosis by the age of 7.72,77 Among patients with myelomeningocele at L3 or above, at least 70% develop significant deformity. By the time these deformities develop, many of these patients are nonambulatory and may suffer from significant comorbidities, including osteopenia, pulmonary compromise, and malnutrition.
Nonoperative treatments for neuromuscular deformities include bracing and wheelchair seating systems that provide support for the head, trunk, pelvis, and extremities. Although several studies suggest that bracing does not correct the deformity,75,78–82 it can support young children and flexible deformities (e.g., hypotonic myopathies, some types of cerebral palsy, spina bifida, and spinal muscular atrophy) (Fig. 100-22).
Neuromuscular curves are often sweeping and involve significant portions of the spine. Indications for surgical intervention are not precisely defined, but typically curves greater than 50 degrees are considered for surgical treatment. Bracing can be used in an attempt to delay surgical treatment if the curve is flexible. Occasionally fusion may be required for curves less than 50 degrees. Ambulatory status and often significant comorbidities of this patient population result in significant morbidity and mortality associated with surgical correction. A recent series by the membership of the Scoliosis Research Society of 4657 patients with neuromuscular scoliosis who underwent surgical correction demonstrated overall morbidity and mortality rates of 18% and 0.3%, respectively.66
Several muscular dystrophies and myopathies are associated with developmental anomalies of spinal alignment. Surgical treatment typically includes fusion with segmental instrumentation and incorporating the pelvis for pelvic obliquity greater than15 degrees. Patients with these disorders have a higher incidence of malignant hyperthermia when exposed to anesthetic agents. Depolarizing agents should not be used if neuromuscular blockade is necessary for surgery, and nondepolarizing agents should be used. It is essential to perform a thorough cardiac and pulmonary evaluation for these patients due to the incidence of associated anomalies. Pulmonary compromise can result from spinal deformity and decreased muscle function. Despite an increase in complication rates when vital capacity falls to less than 40% of predicted, it is possible to perform successful surgery, with the caveat that the patient may require a tracheostomy for long-term ventilation.83–86 Deformity correction is unlikely to improve pulmonary function but may slow its progression.87 Preoperative assessment of cardiac function is important in patients with neuromuscular deformity, particularly Duchenne muscular dystrophy and myotonic dystrophy. For Duchenne muscular dystrophy, steroid treatment offers the potential to prolong ambulation and delay the onset of scoliosis.88
Archer I.A. Surgical treatment of late-onset idiopathic thoracic scoliosis. The Leeds procedure. J Bone Joint Surg [Br]. 1987;69(5):709-714.
Dickson R.A. Conservative treatment for idiopathic scoliosis. J Bone Joint Surg [Br]. 1985;67(2):176-181.
Dickson R.A., Ferguson R.L. Medical and congenital comorbidities associated with spinal deformities in the immature spine. J Bone Joint Surg [Am]. 2007;89(Suppl 1):34-41.
Hedequist D.J., Hall J.E., Emans J.B. The safety and efficacy of spinal instrumentation in children with congenital spine deformities. Spine (Phila Pa 1976). 2004;29(18):2081-2086. discussion 2087
Lenke L.G., Betz R.R., Harms J., et al. Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg [Am]. 2001;83(8):1169-1181.
Sanders J.O., Browne R.H., McConnell S.J., et al. Maturity assessment and curve progression in girls with idiopathic scoliosis. J Bone Joint Surg [Am]. 2007;89(1):64-73.
Smith J.S., Abel M.F., Shaffrey C.I., et al. Decision making in pediatric spinal deformity. Neurosurgery. 2008;63(Suppl 3):54-68.
Weinstein S.L., Zavala D.C., Ponseti I.V. Idiopathic scoliosis: long-term follow-up and prognosis in untreated patients. J Bone Joint Surg [Am]. 1981;63(5):702-712.
1. Bradford D.S., Lonstein J.E., Moe J.H., et al, editors. Moe’s textbook of scoliosis and other spinal deformities. Philadephia: WB Saunders, 1987.
2. Weinstein S.L., editor. The pediatric spine: principles and practice. Philadelphia: Raven Press, 1994.
3. Bernhardt M., Bridwell K.H. Segmental analysis of the sagittal plane alignment of the normal thoracic and lumbar spines and thoracolumbar junction. Spine (Phila Pa 1976). 1989;14(7):717-721.
4. Deacon P., Archer I.A., Dickson R.A. The anatomy of spinal deformity: a biomechanical analysis. Orthopedics. 1987;10(6):897-903.
5. Deacon P., Dickson R.A. Vertebral shape in the median sagittal plane in idiopathic thoracic scoliosis. A study of true lateral radiographs in 150 patients. Orthopedics. 1987;10(6):893-895.
6. Ryan P.M., Puttler E.G., Stotler W.M., et al. Role of the triradiate cartilage in predicting curve progression in adolescent idiopathic scoliosis. J Pediatr Orthop. 2007;27(6):671-676.
7. Sanders J.O., Browne R.H., Cooney T.E., et al. Correlates of the peak height velocity in girls with idiopathic scoliosis. Spine(Phila Pa 1976). 2006;31(20):2289-2295.
8. Sanders J.O., Browne R.H., McConnell S.J., et al. Maturity assessment and curve progression in girls with idiopathic scoliosis. J Bone Joint Surg [Am]. 2007;89(1):64-73.
9. Smith J.S., Abel M.F., Shaffrey C.I., et al. Decision making in pediatric spinal deformity. Neurosurgery. 2008;63(Suppl 3):54-68.
10. Song K.M., Little D.G. Peak height velocity as a maturity indicator for males with idiopathic scoliosis. J Pediatr Orthop. 2000;20(3):286-288.
11. Risser J.C., Norquist D.M., Cockrell B.R.Jr., et al. The effect of posterior spine fusion on the growing spine. Clin Orthop Relat Res. 1966;46:127-139.
12. Mehta M.H. The rib-vertebra angle in the early diagnosis between resolving and progressive infantile scoliosis. J Bone Joint Surg [Br]. 1972;54(2):230-243.
13. Ferguson R.L. Medical and congenital comorbidities associated with spinal deformities in the immature spine. J Bone Joint Surg [Am]. 2007;89(Suppl 1):34-41.
14. Bush C.H., Kalen V. Three-dimensional computed tomography in the assessment of congenital scoliosis. Skeletal Radiol. 1999;28(11):632-637.
15. Nakajima A., Kawakami N., Imagama S., et al. Three-dimensional analysis of formation failure in congenital scoliosis. Spine (Phila Pa 1976). 2007;32(5):562-567.
16. Basu P.S., Elsebaie H., Noordeen M.H. Congenital spinal deformity: a comprehensive assessment at presentation. Spine (Phila Pa 1976). 2002;27(20):2255-2259.
17. Belmont P.J.Jr., Kuklo T.R., Taylor K.F., et al. Intraspinal anomalies associated with isolated congenital hemivertebra: the role of routine magnetic resonance imaging. J Bone Joint Surg [Am]. 2004;86(8):1704-1710.
18. Bradford D.S., Heithoff K.B., Cohen M. Intraspinal abnormalities and congenital spine deformities: a radiographic and MRI study. J Pediatr Orthop. 1991;11(1):36-41.
19. McMaster M.J. Occult intraspinal anomalies and congenital scoliosis. J Bone Joint Surg [Am]. 1984;66(4):588-601.
20. Mohanty S., Kumar N. Patterns of presentation of congenital scoliosis. J Orthop Surg [Hong Kong]. 2000;8(2):33-37.
21. Ozerdemoglu R.A., Denis F., Transfeldt E.E. Scoliosis associated with syringomyelia: clinical and radiologic correlation. Spine (Phila Pa 1976). 2003;28(13):1410-1417.
22. Ouellet J.A., LaPlaza J., Erickson M.A., et al. Sagittal plane deformity in the thoracic spine: a clue to the presence of syringomyelia as a cause of scoliosis. Spine (Phila Pa 1976). 2003;28(18):2147-2151.
23. Dickson R.A. Conservative treatment for idiopathic scoliosis. J Bone Joint Surg [Br]. 1985;67(2):176-181.
24. Dickson R.A., Archer I.A. Surgical treatment of late-onset idiopathic thoracic scoliosis. The Leeds procedure. J Bone Joint Surg [Br]. 1987;69(5):709-714.
25. Weinstein S.L., Ponseti I.V. Curve progression in idiopathic scoliosis. J Bone Joint Surg [Am]. 1983;65(4):447-455.
26. Weinstein S.L., Zavala D.C., Ponseti I.V. Idiopathic scoliosis: long-term follow-up and prognosis in untreated patients. J Bone Joint Surg [Am]. 1981;63(5):702-712.
27. Weinstein S.L., et al. Idiopathic scoliosis. Natural history. Spine (Phila Pa 1976). 1986;11:780-783.
28. Collis D.K., Ponseti I.V., et al. Long-term follow-up of patients with idiopathic scoliosis not treated surgically. J Bone Joint Surg [Am]. 1969;51:425-445.
29. Lenke L.G., Betz R.R., Harms J., et al. Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg [Am]. 2001;83(8):1169-1181.
30. Smith J.S., Shaffrey C.I., Kuntz C.T., et al. Classification systems for adolescent and adult scoliosis. Neurosurgery. 2008;63(Suppl 3):16-24.
31. Lonstein J.E., Winter R.B. The Milwaukee brace for the treatment of adolescent idiopathic scoliosis. A review of one thousand and twenty patients. J Bone Joint Surg [Am]. 1994;76(8):1207-1221.
32. Ascani E., Bartolozzi P., Logroscino C.A., et al. Natural history of untreated idiopathic scoliosis after skeletal maturity. Spine. 1986;11(8):784-789.
33. Barr S.J., Schuette A.M., Emans J.B. Lumbar pedicle screws versus hooks. Results in double major curves in adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 1997;22(12):1369-1379.
34. Brown C.A., Lenke L.G., Bridwell K.H., et al. Complications of pediatric thoracolumbar and lumbar pedicle screws. Spine (Phila Pa 1976). 1998;23(14):1566-1571.
35. Hedequist D.J., Hall J.E., Emans J.B. The safety and efficacy of spinal instrumentation in children with congenital spine deformities. Spine (Phila Pa 1976). 2004;29(18):2081-2086. discussion 2087
36. O’Brien M.F., Lenke L.G., Mardjetko S., et al. Pedicle morphology in thoracic adolescent idiopathic scoliosis: is pedicle fixation an anatomically viable technique? Spine (Phila Pa 1976). 2000;25(18):2285-2293.
37. Suk S.I., Kim W.J., Lee S.M., et al. Thoracic pedicle screw fixation in spinal deformities: are they really safe? Spine (Phila Pa 1976). 2001;26(18):2049-2057.
38. Nilsonne U., Lundgren K.D. Long-term prognosis in idiopathic scoliosis. Acta Orthop Scand. 1968;39:456-465.
39. Thompson G.H., Akbarnia B.A., Campbell R.M.Jr. Growing rod techniques in early-onset scoliosis. J Pediatr Orthop. 2007;27(3):354-361.
40. Blakemore L.C., Scoles P.V., Poe-Kochert C., et al. Submuscular Isola rod with or without limited apical fusion in the management of severe spinal deformities in young children: preliminary report. Spine (Phila Pa 1976). 2001;26(18):2044-2048.
41. Thompson G.H., Akbarnia B.A., Kostial P., et al. Comparison of single and dual growing rod techniques followed through definitive surgery: a preliminary study. Spine (Phila Pa 1976). 2005;30(18):2039-2044.
42. Akbarnia B.A., Marks D.S., Boachie-Adjei O., et al. Dual growing rod technique for the treatment of progressive early-onset scoliosis: a multicenter study. Spine (Phila Pa 1976). 2005;30(Suppl 17):S46-S57.
43. Campbell R.M.Jr., Hell-Vocke A.K. Growth of the thoracic spine in congenital scoliosis after expansion thoracoplasty. J Bone Joint Surg [Am]. 2003;85(3):409-420.
44. Campbell R.M.Jr., Smith M.D., Hell-Vocke A.K. Expansion thoracoplasty: the surgical technique of opening-wedge thoracostomy. Surgical technique. J Bone Joint Surg [Am]. 2004;86(Suppl 1):51-64.
45. Campbell R.M.Jr., Smith M.D., Mayes T.C., et al. The characteristics of thoracic insufficiency syndrome associated with fused ribs and congenital scoliosis. J Bone Joint Surg [Am]. 2003;85(3):399-408.
46. Campbell R.M.Jr., Smith M.D., Mayes T.C., et al. The effect of opening wedge thoracostomy on thoracic insufficiency syndrome associated with fused ribs and congenital scoliosis. J Bone Joint Surg [Am]. 2004;86(8):1659-1674.
47. Campbell R.M.Jr. Operative strategies for thoracic insufficiency syndrome by vertical expandable prosthetic titanium rib expansion thoracoplasty. Oper Tech Orthop. 2005;15:315-325.
48. Gollogly S., Smith J.T., Campbell R.M. Determining lung volume with three-dimensional reconstructions of CT scan data: a pilot study to evaluate the effects of expansion thoracoplasty on children with severe spinal deformities. J Pediatr Orthop. 2004;24(3):323-328.
49. Hell A.K., Campbell R.M., Hefti F. The vertical expandable prosthetic titanium rib implant for the treatment of thoracic insufficiency syndrome associated with congenital and neuromuscular scoliosis in young children. J Pediatr Orthop B. 2005;14(4):287-293.
50. Oskouian R.J.Jr., Sansur C.A., Shaffrey C.I. Congenital abnormalities of the thoracic and lumbar spine. Neurosurg Clin North Am. 2007;18(3):479-498.
51. Giampietro P.F., Dunwoodie S.L., Kusumi K., et al. Progress in the understanding of the genetic etiology of vertebral segmentation disorders in humans. Ann N Y Acad Sci. 2009;1151:38-67.
52. Giampietro P.F., Blank R.D., Raggio C.L., et al. Congenital and idiopathic scoliosis: clinical and genetic aspects. Clin Med Res. 2003;1(2):125-136.
53. Shands A.R.Jr., Eisberg H.B. The incidence of scoliosis in the state of Delaware: a study of 50,000 minifilms of the chest made during a survey for tuberculosis. J Bone Joint Surg [Am]. 1955;37(6):1243-1249.
54. Wynne-Davies R. Congenital vertebral anomalies: aetiology and relationship to spina bifida cystica. J Med Genet. 1975;12(3):280-288.
55. Byrd S.E., Darling C.F., McLone D.G., et al. MR imaging of the pediatric spine. Magn Reson Imaging Clin North Am. 1996;4(4):797-833.
56. McMaster M.J., Ohtsuka K. The natural history of congenital scoliosis. A study of two hundred and fifty-one patients. J Bone Joint Surg [Am]. 1982;64(8):1128-1147.
57. Winter R.B., Moe J.H., Eilers V.E. Congenital scoliosis: a study of 234 patients treated and untreated. Part 2. Treatment. J Bone Joint Surg [Br]. 1968;50:1-15.
58. Christ B., Schmidt C., Huang R., et al. Segmentation of the vertebrate body. Anat Embryol (Berl). 1998;197(1):1-8.
59. Keynes R.J., Stern C.D. Mechanisms of vertebrate segmentation. Development. 1988;103(3):413-429.
60. McMaster M.J. Congenital scoliosis caused by a unilateral failure of vertebral segmentation with contralateral hemivertebrae. Spine (Phila Pa 1976). 1998;23(9):998-1005.
61. McMaster M.J. Spinal growth and congenital deformity of the spine. Spine (Phila Pa 1976). 2006;31(20):2284-2287.
62. Nakamura H., Matsuda H., Konishi S., et al. Single-stage excision of hemivertebrae via the posterior approach alone for congenital spine deformity: follow-up period longer than ten years. Spine (Phila Pa 1976). 2002;27(1):110-115.
63. Ruf M., Harms J. Hemivertebra resection by a posterior approach: innovative operative technique and first results. Spine (Phila Pa 1976). 2002;27(10):1116-1123.
64. Ruf M., Jensen R., Harms J. Hemivertebra resection in the cervical spine. Spine (Phila Pa 1976). 2005;30(4):380-385.
65. Hedequist D., Emans J. Congenital scoliosis: a review and update. J Pediatr Orthop. 2007;27(1):106-116.
66. Reames D.L., Smith J.S., Fu K.G., et al. Complications in the surgical treatment of 19,360 cases of pediatric scoliosis: a review of the Scoliosis Research Society Morbidity and Mortality Database. Spine (Phila Pa 1976). 2011;36(18):1484-1494.
67. Wiltse L.L., Newman P.H., Macnab I. Classification of spondylolisis and spondylolisthesis. Clin Orthop Relat Res. 1976;117:23-29.
68. Fu K.G., Smith J.S., Polly D.W.Jr., et al. Morbidity and mortality in the surgical treatment of 605 pediatric patients with isthmic or dysplastic spondylolisthesis. Spine (Phila Pa 1976). 2011;36(4):308-312.
69. Lonstein J.E. Congenital spine deformities: scoliosis, kyphosis, and lordosis. Orthop Clin North Am. 1999;30(3):387-405, viii.
70. Winter R.B., Moe J.H., Wang J.F. Congenital kyphosis. Its natural history and treatment as observed in a study of one hundred and thirty patients. J Bone Joint Surg [Am]. 1973;55(2):223-256.
71. Kim Y.J., Otsuka N.Y., Flynn J.M., et al. Surgical treatment of congenital kyphosis. Spine (Phila Pa 1976). 2001;26(20):2251-2257.
72. Saito N., Ebara S., Ohotsuka K., et al. Natural history of scoliosis in spastic cerebral palsy. Lancet. 1998;351(9117):1687-1692.
73. Thometz J.G., Simon S.R. Progression of scoliosis after skeletal maturity in institutionalized adults who have cerebral palsy. J Bone Joint Surg [Am]. 1988;70(9):1290-1296.
74. Majd M.E., Muldowny D.S., Holt R.T. Natural history of scoliosis in the institutionalized adult cerebral palsy population. Spine (Phila Pa 1976). 1997;22(13):1461-1466.
75. Terjesen T., Lange J.E., Steen H. Treatment of scoliosis with spinal bracing in quadriplegic cerebral palsy. Dev Med Child Neurol. 2000;42(7):448-454.
76. Berven S., Bradford D.S. Neuromuscular scoliosis: causes of deformity and principles for evaluation and management. Semin Neurol. 2002;22(2):167-178.
77. Abel M.F., Blanco J.S., Pavlovich L., et al. Asymmetric hip deformity and subluxation in cerebral palsy: an analysis of surgical treatment. J Pediatr Orthop. 1999;19(4):479-485.
78. Holmes K.J., Michael S.M., Thorpe S.L., et al. Management of scoliosis with special seating for the non-ambulant spastic cerebral palsy population—a biomechanical study. Clin Biomech (Bristol, Avon). 2003;18(6):480-487.
79. Miller A., Temple T., Miller F. Impact of orthoses on the rate of scoliosis progression in children with cerebral palsy. J Pediatr Orthop. 1996;16(3):332-335.
80. Muller E.B., Nordwall A. Brace treatment of scoliosis in children with myelomeningocele. Spine (Phila Pa 1976). 1994;19(2):151-155.
81. Noble-Jamieson C.M., Heckmatt J.Z., Dubowitz V., et al. Effects of posture and spinal bracing on respiratory function in neuromuscular disease. Arch Dis Child. 1986;61(2):178-181.
82. Olafsson Y., Saraste H., Al-Dabbagh Z. Brace treatment in neuromuscular spine deformity. J Pediatr Orthop. 1999;19(3):376-379.
83. Bach J.R., Sabharwal S. High pulmonary risk scoliosis surgery: role of noninvasive ventilation and related techniques. J Spinal Disord Tech. 2005;18(6):527-530.
84. Kurz L.T., Mubarak S.J., Schultz P., et al. Correlation of scoliosis and pulmonary function in Duchenne muscular dystrophy. J Pediatr Orthop. 1983;3(3):347-353.
85. Wazeka A.N., DiMaio M.F., Boachie-Adjei O. Outcome of pediatric patients with severe restrictive lung disease following reconstructive spine surgery. Spine (Phila Pa 1976). 2004;29(5):528-534. discussion 535
86. Yuan N., Skaggs D.L., Dorey F., et al. Preoperative predictors of prolonged postoperative mechanical ventilation in children following scoliosis repair. Pediatr Pulmonol. 2005;40(5):414-419.
87. Yuan N., Fraire J.A., Margetis M.M., et al. The effect of scoliosis surgery on lung function in the immediate postoperative period. Spine (Phila Pa 1976). 2005;30(19):2182-2185.
88. Alman B.A., Raza S.N., Biggar W.D. Steroid treatment and the development of scoliosis in males with Duchenne muscular dystrophy. J Bone Joint Surg [Am]. 2004;86(3):519-524.