Pediatric Respiratory Emergencies
Lower Airway Obstruction
Asthma
Introduction and Epidemiology
Asthma, the most prevalent chronic disease of childhood, affects almost 7 million children in the United States.1 In the past 25 years, childhood asthma prevalence rates have more than doubled.1 The public health burden posed by this disease, as assessed by emergency department (ED) visits, hospitalizations, and deaths, remains at a historically high level. About 3% of all ED visits among children are for asthma, which accounts for 750,000 such visits annually.1 Similarly, about 3% of all hospitalizations for children are due to asthma, totaling about 200,000 per year.1 In addition, there are astonishing racial disparities among children with this condition. Compared with white children, black children have a 60% higher prevalence rate, a 260% higher ED visit rate, a 250% higher hospitalization rate, and a 500% higher death rate due to asthma.1
Clinical Features
History
A more comprehensive history should include questions about asthma triggers, such as URIs, cigarette smoke, allergies, or exercise. Inquiries about fever and hydration status should be part of a complete review of systems. A past medical history of frequent asthma exacerbations, ED visits for asthma, or hospitalizations to either the general ward or the intensive care unit would raise the concern for poorly controlled asthma. The impact that asthma has on the child’s life may be gauged by the monthly frequency of daytime or nighttime symptoms, such as cough, wheezing, shortness of breath, or chest tightness, as well as missed days of school or restricted activity. Persistent asthma has been defined as having at least 3 days per week of symptoms or use of SABA or awakening at night with asthma symptoms several nights per month.2 Conversely, some will consider any child treated for an asthma exacerbation in the ED to have persistent asthma. A child who meets criteria for persistent asthma should be receiving daily anti-inflammatory therapy, and those older than 5 years should be monitoring symptoms with a peak flow meter.2 If the child is wheezing for the first time, inquiries about other possible causes of wheezing (see Differential Considerations) should be made. Family and social histories should focus on asthma, cystic fibrosis, or atopic disease and the adequacy of support systems at home.
Physical Examination
In the initial focused physical examination of the wheezing child, vital signs should be obtained and the level of consciousness assessed. A child who is anxious, restless, or lethargic may be hypoxemic. No single asthma score has been universally adopted to assess degree of illness or treatment responses.3,4 Most asthma scores include key clinical factors, such as respiratory rate, degree of wheezing, inspiratory to expiratory ratio, use of accessory muscles, and oxygen saturation in room air.4 Such scores can assist in the assessment of the pretreatment degree of illness at ED triage and in tracking of the child’s response to therapy.
Diagnostic Strategies
Pulse Oximetry and Arterial Blood Gas Analysis
Adjunctive studies, such as arterial oxygen saturation measured by pulse oximetry, may assist in determining the initial degree of illness.5 Pulse oximetry is noninvasive and inexpensive, and it provides objective data about the degree of illness of a wheezing child. The oxygen saturation of any child with respiratory distress should be determined soon after ED arrival, and supplemental oxygen should be provided if the oxygen saturation is 92% or less.
Peak Expiratory Flow Rate
Measurement of the peak expiratory flow rate (PEFR) is a means of obtaining an objective assessment of exacerbation severity, but it has limited utility in the evaluation of acutely ill children. Young children, in particular, may be unable to properly comply with this testing, and in one study just two thirds of children older than 5 years were able to complete PEFR testing during an asthma exacerbation.3 Ideally, the PEFR should be determined with the child standing and the best of three attempts recorded. Therefore, moderately to severely ill or younger children may not be able to cooperate with this assessment.
Chest Radiographs
URIs marked by low-grade fever and coughing are common triggers of asthma exacerbations. These signs overlap with those found among children with pneumonia, making it difficult to determine the necessity of obtaining a chest radiograph in the evaluation of an acutely wheezing child. No set of predictors has been found that can accurately identify children likely to have abnormalities on chest radiography.6 Hyperinflation, interstitial markings, and atelectasis are common radiographic findings that may be seen in a wheezing child, but these should not result in initiation of antibiotic therapy or other changes in management. More serious conditions associated with asthma, such as pneumonia, pneumomediastinum, and pneumothoraces, are much less common. Rarely is an unsuspected diagnosis made on the basis of a chest radiograph in an acutely wheezing child, even if the child has never wheezed before.7
It should not be a routine practice to obtain chest radiographs for all wheezing children, even those who are wheezing for the first time or those who are being hospitalized.7 Chest radiographs should be considered for those with focal chest findings, fever, extreme distress, subcutaneous emphysema, or a history of choking. Reassessment after treatment to evaluate for the resolution of focal findings may further decrease the need to obtain a chest radiograph. This selective approach will be more cost-effective and lessen unnecessary radiation exposure and overuse of antibiotics. On the other hand, clinicians may have a lower threshold to obtain chest radiographs for infants with first-time wheezing because of a slightly greater likelihood of uncovering an anatomic abnormality.
Differential Considerations
Although most children with wheezing have asthma, other conditions should be considered. A differential diagnosis for childhood asthma is listed in Table 169-1. Of these conditions, bronchiolitis, laryngotracheobronchitis (croup), pneumonia, and gastroesophageal reflux are those that clinicians will encounter most often. Bronchiolitis is the one disease that is most commonly confused with asthma. Although the viruses associated with bronchiolitis infect children of all ages, clinical bronchiolitis marked by wheezing is seen almost exclusively in those younger than 12 months. Patients with bronchiolitis typically present between November and March. There is much clinical overlap between asthma and bronchiolitis, and the two cannot be distinguished by physical examination findings alone. A complete discussion of bronchiolitis is included later in this chapter.
Table 169-1
Differential Diagnosis of Asthma
| CONDITION | DISTINGUISHING CHARACTERISTICS |
| Infections | |
| Bronchiolitis | Infant, preceding upper respiratory infection, seasonal, no history of atopy, no family history of asthma |
| Laryngotracheobronchitis (croup) | Inspiratory stridor, barky cough, fever, response to humidified air |
| Pneumonia | Focal wheezing, rhonchi, rales, grunting, fever |
| Tuberculosis | Diffuse adenopathy, weight loss, prolonged fever |
| Bronchiolitis obliterans | Prolonged cough or chest pain, inhalational exposure to toxin |
| Anatomic or Congenital | |
| Gastroesophageal reflux | Frequent emesis, weight loss, aspiration |
| Cystic fibrosis | Diarrhea, weight loss, chronic cough, salty sweat |
| Congestive heart failure | Rales, murmur, gallop, hepatosplenomegaly, cardiomegaly or pulmonary vascular congestion on chest radiograph |
| Tracheoesophageal fistula | Choking, coughing, cyanosis with feeds |
| Mediastinal mass | Chest pain, mediastinal density on chest radiograph |
| Vascular ring | Stridor, cyanosis, apnea, high-pitched brassy cough, dysphagia |
| Acquired | |
| Foreign body aspiration | History of choking, toddler, asymmetrical pulmonary examination, unilateral hyperinflation on chest radiograph |
| Anaphylaxis | Abrupt onset, urticarial rash, angioedema, history of allergies |
Management
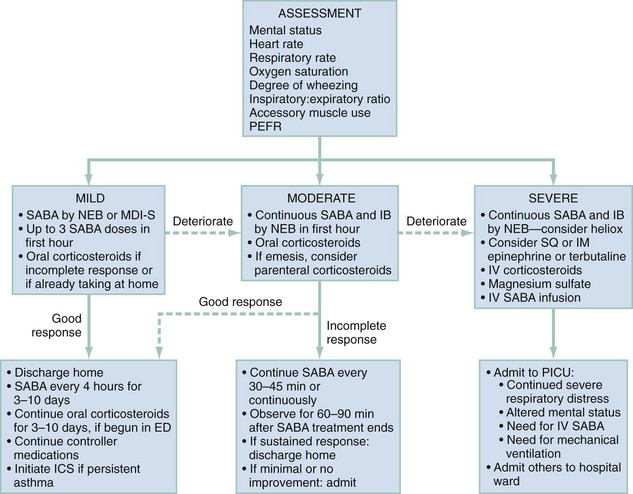
For purposes of patient management, it is best to stratify children by degree of illness on the basis of the initial clinical assessment (Fig. 169-1). This will help ensure the timely initiation of an appropriately aggressive approach for sicker children while minimizing adverse effects from unnecessary therapy among those with milder exacerbations. Of course, during the ED stay, the illness severity may change, making frequent examinations to assess response to therapy essential.
Mild Exacerbation
A mild exacerbation is characterized by alertness, slight tachypnea, expiratory wheezing only, mildly prolonged expiratory phase, minimal accessory muscle use, and oxygen saturation of greater than 95%. Children who are able to provide a PEFR should have a value greater than 70% of personal best. Patients with a mild exacerbation, especially those who were not receiving any asthma therapy before the ED visit, will usually require SABA therapy only. The Expert Panel of the National Heart, Lung, and Blood Institute (NHLBI) recommends that patients receive therapy every 20 minutes in the first hour of care.2 Children with mild exacerbations often improve promptly with just one or two SABA treatments. Many of these patients are managed without systemic corticosteroids. However, systemic corticosteroids may be given to those who are already undergoing a course of treatment with them before ED arrival or to those who do not respond promptly to SABA therapy (see later section on moderate exacerbation).
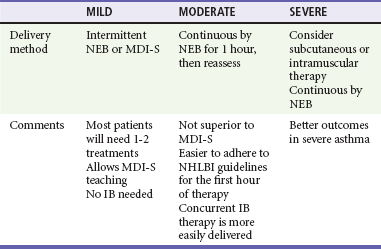
Nebulizers versus Metered-Dose Inhalers with Spacers.: There is considerable debate about the optimal method for delivery of SABAs to children with acute asthma. About three fourths of pediatric emergency medicine physicians report using NEBs to administer SABAs, regardless of illness severity.8 NEBs provide a passive means of receiving aerosolized medication. Precise coordination between respiration and aerosol delivery is not needed, and medications such as anticholinergics as well as humidified oxygen may be delivered concurrently with the SABA. However, delivery is inefficient, with only about 10% of the drug in the reservoir delivered to the small airways.9,10 In addition, administration takes about 10 minutes, increasing respiratory therapy time and costs, and an external power source is needed, limiting portability.
On the other hand, spacers used with MDIs provide a reservoir of medication that is available to be inhaled. Therefore, precise coordination between actuation and inhalation is not needed, and there is no need for breath-holding. Drug deposition in the oropharynx and systemic absorption are reduced with the use of a spacer.11 The decreased administration time associated with MDI-S use may result in reduced costs.12–14 A recent cost analysis determined that the use of MDIs with spacers in place of NEBs to treat children with mild to moderate asthma exacerbations in the ED could yield significant cost savings.15 The portability of the MDI-S allows older children to use it during the school day. Face mask–equipped spacers are available for children too young to use the spacer’s mouthpiece, although mouthpieces are preferable for older children to decrease nasal filtering of drug, which may reduce lung deposition. After each actuation, children should take five to eight breaths to completely empty the spacer.
These NEB disadvantages along with the development and widespread use of spacers have led investigators to assess the role of MDI-S for delivery of SABAs in the ED. Numerous clinical trials and meta-analyses have consistently demonstrated that delivery by MDI-S is at least as effective as delivery by NEBs.11,16–23 Among children 1 to 4 years old, MDI-S use was associated with a greater reduction in wheezing and a lower hospitalization rate.14 One systematic review evaluated trials in which a total of 2066 children with acute asthma were randomized to receive SABAs by one of these two methods.17 Those children treated with MDI-S had shorter ED length of stay and a reduced hospitalization rate that was not statistically significant. The American College of Chest Physicians and American College of Asthma, Allergy, and Immunology concluded that both the NEB and MDI-S are appropriate for the delivery of SABA in the ED.24 Thus, compared with NEBs, MDI-S administration has been shown to be equally effective for children of all ages,14,16 with a wide range of illness severity and by multiple outcome measures.
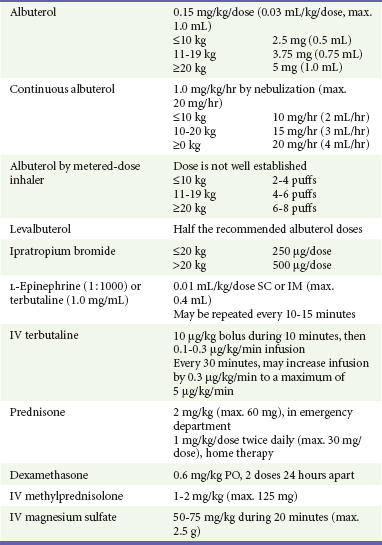
Typically, when racemic albuterol is administered by intermittent nebulization, a dose of 0.15 mg/kg is placed in the reservoir of a NEB. This dose is well established as one that is both effective and safe.25,26 Optimal dosing for albuterol administered by MDI-S is not as well defined. A review assessed 10 randomized controlled trials comparing MDI-S with NEBs for delivery of SABAs to children with acute asthma.11 In some studies, up to seven times more drug was placed in the NEB reservoir compared with that released by MDI-S, yet outcomes were similar. This reflects the inefficiency inherent with NEB delivery, with much drug being lost to the environment. Multiple puffs of SABAs delivered by MDI-S seem to be well tolerated, even by young children.14,16 In one trial, children 1 to 4 years old treated with six puffs of albuterol by MDI-S had less tachycardia than did those treated with 2.5 mg of albuterol by NEB.14 The 2007 NHLBI guidelines state that “equivalent bronchodilation can be achieved either by high doses (4-12 puffs) of a SABA by MDI with a valved holding chamber … or by nebulizer”; they suggest a dose of four to eight puffs.2 Table 169-2 provides recommended SABA doses stratified by the patient’s weight.
Racemic Albuterol versus Levalbuterol.: Another consideration in the use of SABAs is the potential role of levalbuterol. Racemic albuterol is an equal mix of the active R-albuterol and the inactive S-albuterol. R-Albuterol produces bronchodilation as well as side effects such as tachycardia and tremors. S-Albuterol was long thought to be inert. However, there is some evidence that S-albuterol may increase reactivity to histamine, have proinflammatory effects, and exhibit “characteristics of a typical contractile agent.”27–33 Further, there seems to be preferential retention of S-albuterol in the lungs of healthy volunteers34–36; this may account for diminished effectiveness with frequent dosing. Levalbuterol is pure R-albuterol without the S component. In theory, levalbuterol should be more effective than racemic albuterol at half the dose because there are no competing harmful effects from the S-isomer.
Studies assessing the use of levalbuterol for treatment of children with acute asthma have not consistently demonstrated this theoretic advantage. In the first of these clinical trials, levalbuterol (1.25 mg) was compared with racemic albuterol (2.5 mg) in the ED treatment of more than 500 children with acute asthma.37 The use of levalbuterol was associated with a decreased need for hospitalization. However, the baseline hospitalization rate in this study was high even though patients with all degrees of illness severity were enrolled. Subsequently, three other randomized trials comparing the ED use of the two drugs have failed to find a levalbuterol benefit.38–40 These three studies analyzed children with a wide range of illness severities and baseline hospitalization rates and used various outcome measures, such as asthma scores, pulmonary function test results, and hospitalization rates. Racemic albuterol was demonstrated to be as effective as levalbuterol under each of these circumstances.
Similarly, a clinical trial failed to demonstrate a benefit with the use of continuously nebulized levalbuterol.41 Children who failed to respond to ED treatment and required hospitalization were randomized to receive either continuously nebulized levalbuterol or albuterol. There were no differences in the two groups with respect to time that continuous therapy was needed or time to discharge home. The acquisition cost of levalbuterol is more than 10 times greater than that of racemic albuterol.40 Until there are more compelling data to demonstrate conclusively that the additional costs of levalbuterol are offset by the need for fewer nebulizations, decreased length of ED or hospital stay, or decreased need for hospitalization, racemic albuterol should remain the drug of choice for children with acute asthma exacerbations.
Disposition.: Most children with a mild exacerbation will be able to be discharged home. Those sustaining clinical improvement 60 minutes after the most recent SABA treatment may be discharged. SABAs should be continued for the next 3 to 10 days. If systemic corticosteroid therapy was administered in the ED, it should also be continued for 3 to 10 days; 2 mg/kg/day of oral prednisone is recommended. Children should continue all other asthma controller medications, including inhaled corticosteroids (ICS).
For those who are not already receiving ICS, it is unclear if prescribing them at ED discharge leads to improved short-term outcomes. Among adult asthmatics discharged from the ED after acute asthma exacerbations, the addition of inhaled flunisolide did not lead to improved outcomes.42 Of note, though, compliance with the inhaled medication was low, and many patients were lost to follow-up. On the other hand, adults randomized to inhaled budesonide after ED discharge had a marked decrease in relapse rates, frequency of SABA use, and asthma symptoms.43 A review concluded that there is “insufficient evidence that ICS therapy provides additional benefit” when it is added to systemic corticosteroids at ED discharge.44 Pediatric emergency medicine physicians rarely prescribe ICS at ED discharge, even to children who have persistent asthma.45
Rather than prescribing ICS to prevent ED relapse, emergency physicians should consider longer term goals for those with persistent disease. National guidelines state that ICS are the medications of choice when long-term controller therapy is initiated for children with persistent asthma.2 These drugs are safe and well tolerated at recommended doses. Longitudinal studies show that daily use of ICS may decrease growth velocity, but these changes are small and reversible.46,47 Therefore, emergency physicians should identify children who, during the preceding month, have had frequent asthma symptoms, nighttime awakenings, and the need for frequent use of SABAs for asthma control. As stated by national asthma guidelines, initiation of ICS therapy at ED discharge “should be considered” for these patients.2 Those already taking low doses of daily ICS may benefit from an increase in dosing. Initiation or increase of ICS would be in addition to the 3- to 10-day course of systemic corticosteroids after ED discharge.
Summary.: For children with mild asthma exacerbations, racemic albuterol should be administered every 20 minutes, as needed (see Fig. 169-1). Most children will respond promptly to therapy and be well enough to be discharged home after one or two treatments. Systemic corticosteroids may be considered for those who exhibit a suboptimal response to SABAs (see later section on moderate exacerbation). NEBs and MDI-S are each reasonable options for delivery of SABAs intermittently. Table 169-2 lists recommended doses for SABAs and other asthma medications in the ED, and Table 169-3 provides a recommended strategy for SABA administration. Rather than base the method of delivery on the issue of efficacy, clinicians should assess other factors. Determination of the number of treatments a child is likely to need, the anticipated cooperation with a given delivery method, the need to deliver concurrent medications, and costs will help guide decision-making.
Moderate Exacerbation
Anticholinergics.: Stimulation of airway cholinergic receptors results in reflex bronchoconstriction, which may be blocked with the use of anticholinergic agents such as IB. This medication is available as an MDI and as a solution for nebulization that may be mixed directly with racemic albuterol. The MDI formulation should not be given to patients with allergy to peanut or soy because it contains soya lecithin; this is not a concern with the solution for nebulization.
Studies have shown that use of SABAs with IB is more effective than SABAs alone.47,48 In a randomized, double-blind clinical trial, three doses of IB administered concurrently with the first three SABA treatments were shown to be superior to just one dose of IB.49 In another study, more than 400 children were randomized to receive racemic albuterol and prednisone alone or that therapy plus IB.48 Those judged to be moderately ill did not experience an IB benefit. However, among those with an initial PEFR less than 50% predicted, the use of IB resulted in a significantly lower hospitalization rate. A systematic review and meta-analysis compared the use of SABAs plus anticholinergics with SABAs alone among children older than 18 months.50 In the 16 trials assessed, combination therapy was associated with significantly lower hospitalization rates and improvements in asthma scores and pulmonary function test results. These investigators concluded that multiple doses of IB added to SABAs should be standard treatment of children with moderate to severe asthma exacerbations.
Clinical benefits after IB use may be delayed for up to 60 minutes.49 However, it is inexpensive, and because less than 1% is absorbed systemically, it is virtually free of adverse effects.50 IB should be administered to children with moderate to severe exacerbations. Three doses may be mixed with racemic albuterol and delivered concurrently and continuously by NEB in the first hour of care (see Table 169-3). This means of administration, although not superior to delivery by MDI-S, will help ensure compliance with the goal of the equivalent of three albuterol treatments in the first hour of care.2 Alternatively, four to eight puffs of IB may be given every 20 minutes in the first hour of care,2 but these children will also need to receive a substantial number of puffs of SABAs, and as clinicians care for other patients, there may be delays in receiving appropriately aggressive therapy. In summary, sicker patients require three albuterol treatments soon after ED arrival. Continuous delivery by NEB during 1 hour may be preferred to intermittent MDI-S, not for superior efficacy but to help ensure timely medication delivery.
Systemic Corticosteroids.: There are compelling data to show that the prompt use of corticosteroids can decrease the need for hospitalization and that they should be used routinely for patients with moderate disease.4,51–56 Clinicians must decide the optimal agent and route of administration.
Oral versus Parenteral.: Two early clinical trials established the efficacy of parenterally administered corticosteroids in the ED. Compared with those treated with placebo, adults treated with intravenous methylprednisolone had a lower hospitalization rate,51 as did children treated with intramuscular methylprednisolone.52 Scarfone and colleagues were the first to demonstrate the efficacy of orally administered corticosteroids in this setting.4 Children treated with frequent SABAs and oral prednisone had a reduced need for hospitalization compared with those treated with SABA therapy alone. Further, a meta-analysis determined that compared with placebo, oral corticosteroids were effective in reducing the need for hospitalization among children with acute asthma exacerbations.55
There have been few clinical trials directly comparing oral and parenteral therapy. In one small study, there were no differences in any outcome measures for children in the ED with moderate to severe asthma who were treated with equal doses of either intravenous or oral methylprednisolone.57 The most recent NHLBI guidelines recommend oral administration of corticosteroids because this is less invasive and the benefits seem to be equivalent to those of parenteral therapy.58,59 Further, oral corticosteroid therapy is inexpensive, the drugs are rapidly and completely absorbed, and this mode of administration provides the potential for out-of-hospital administration either at home or in a physician’s office.
Prednisone versus Dexamethasone.: As with SABAs, clinicians have a choice in the specific corticosteroid to be used. Oral prednisone has been the drug of choice in this setting. Significant clinical benefits begin 2 hours after administration, are most pronounced among the sickest children, and result in a decreased need for hospitalization.4 Dexamethasone phosphate may be given orally or parenterally and has a substantially longer (36-72 hours) half-life than prednisone (18-36 hours).60 Investigators treated children in the ED with either 0.6 mg/kg of dexamethasone or 2 mg/kg of prednisone in a randomized fashion.60 Those in the dexamethasone group were provided one additional dose to take the following day, whereas those in the prednisone group were given a prescription for an additional 4 days of prednisone. There were no differences in hospitalization or relapse rates or symptom persistence. Significantly fewer dexamethasone patients vomited the study drug in the ED and reported noncompliance with it after ED discharge. Of note is that Orapred, a more palatable form of oral prednisone, was not used in this study.
These data suggest that either dexamethasone or prednisone may be used in the treatment of moderately ill children with acute asthma. Given that clinical benefits from corticosteroids are delayed and that all moderately ill children will require corticosteroids whether or not they require hospitalization, they should be administered as soon as possible after ED arrival in an attempt to hasten clinical improvement and perhaps to prevent the need for hospitalization.53,56 Because SABAs are administered by inhalation and corticosteroids may be given orally, most children with a moderate asthma exacerbation can be managed without the insertion of an intravenous line. This avoids unnecessary pain and anxiety as well as the delays in drug administration associated with intravenous line insertion. Intramuscular therapy is a reasonable option for children who vomit orally administered corticosteroids yet do not require an intravenous line for other reasons.
Inhaled Corticosteroids.: The use of ICS for the ED treatment of acute asthma is an area of ongoing research. In three clinical trials, ICS were compared with oral prednisone in the ED setting.61–63 Scarfone and colleagues treated children with either nebulized dexamethasone or oral prednisone.61 The two groups had similar rates of hospitalization, although there was a trend toward greater rate of improvement among the dexamethasone-treated children. A potential limitation to the widespread use of nebulized dexamethasone is that it contains sodium bisulfite, a preservative that may induce wheezing among allergic individuals. Budesonide, an ICS that has a high topical activity and low systemic absorption, is effective in the treatment of children with croup.64 Investigators from India found three nebulized doses of budesonide to be superior to one dose of prednisone in the treatment of acute asthma.62 In another trial, fluticasone-treated children with asthma were more likely to be hospitalized and experienced a significantly smaller degree of improvement compared with those treated with prednisone.63 Finally, investigators determined that children with asthma treated in the ED with inhaled triamcinolone had lower hospitalization rates and relapse rates compared with those treated with either prednisone or intravenous corticosteroids.65
Rather than replacing systemic corticosteroids, other investigators have assessed whether the addition of ICS to systemic therapy results in clinical benefits; however, few such studies have been performed in children, and few have assessed the need for hospitalization.66–68 Thus the niche for ICS in the ED treatment of acute asthma is still being defined. Additional areas for further investigation include determination of the optimal agent, the proper dose, and the appropriate mode of delivery and defining of the population of patients most likely to benefit from this therapy. At this time, there is no proven role for the routine use of ICS in the ED setting. Because of its greater bioavailability and proven benefits, moderately ill children should receive systemic corticosteroid therapy.
Intermittent versus Continuous Therapy.: Children requiring very frequent intermittently nebulized albuterol may benefit from receiving albuterol continuously instead. In one clinical trial among asthmatic children, patients were randomized to receive the same total dose of albuterol nebulized either intermittently or continuously during 2 hours.69 Those in the continuous group had a greater mean improvement in their asthma scores and significantly less respiratory therapy time, although there were no differences in mean PEFR or admission rates. A systematic review found that those treated with continuously nebulized SABAs had lower rates of hospitalization, greater improvements in pulmonary function test results, and similar rates of adverse events compared with those treated intermittently.70
Perhaps the greatest advantage of continuous over intermittent therapy is one of a practical nature: it allows greater compliance with the goal of delivering the equivalent of three intermittent albuterol treatments in the first hour of care.2 In addition, this method will result in less respiratory therapy time and costs; it has been shown to be safe,71,72 and it may benefit the sickest patients the most.73,74 On the other hand, young children may not tolerate a face mask for prolonged periods.
Many clinicians find it helpful to determine the total of racemic albuterol that would be delivered if three treatments were to be given intermittently during an hour, place that total dose in the NEB reservoir, and administer it continuously during an hour. Alternatively, a dose range may be used on the basis of the child’s weight (see Table 169-2).
Summary.: A suggested approach to the management of children with moderately acute asthma is as follows. Supplemental oxygen should be provided if the initial oxygen saturation is 92% or less in room air. Albuterol and IB should be administered continuously by nebulization for 1 hour (see Fig. 169-1). This will ensure that an appropriate amount of each is delivered in the first hour of ED care. As soon as possible after ED arrival, a single dose of either oral prednisone or dexamethasone should be given. Intramuscular dexamethasone is an option for children who vomit the initial oral corticosteroid dose within 15 minutes of its administration or who vomit repeated doses.
After 1 hour of therapy, a clinical reassessment should be made; evaluation at this time is better than the initial assessment at predicting the need for hospitalization.75 At this point, patients can generally be grouped into one of three categories: markedly improved, not improved or worse, or slightly improved. Children who are markedly improved may be observed without SABAs to ensure that there is no clinical deterioration. It is wise to delay a disposition decision until at least 60 minutes after the most recent SABA treatment so that a clinical relapse may be noted. In making the disposition decision, the clinician should determine the patient’s physical examination findings and also consider the frequency of prior hospitalizations and ED visits and issues such as compliance and support systems. The medications and education to be provided at ED discharge are the same as outlined for those with a mild exacerbation.
Children who remain moderately ill after the first hour should continue to be treated aggressively with SABAs, either continuously or with frequent administration of intermittent therapy. If, after 2 hours, subjective and objective measures reveal that the degree of respiratory distress is unchanged or worse, hospitalization is warranted. On the other hand, there will be a subset of patients who demonstrate some degree of clinical improvement at the 2-hour assessment but are not yet well enough to be sent home. One study showed that among prednisone-treated children who would have been hospitalized after 2 hours of ED therapy, less than half were actually hospitalized when aggressive SABA therapy was continued for an additional 2 hours, and none returned to the ED within 48 hours of discharge.4 Presumably, the onset of prednisone’s effects allowed these children to avoid hospitalization. Therefore, the continuing treatment of such children with SABAs for a total of 3 to 4 hours, perhaps in an ED observation area, would be expected to avoid the need for many hospitalizations.
Severe Exacerbation
Figure 169-1 provides an outline for the approach to management of severely ill children. They should be attached to a cardiorespiratory monitor and blood pressure cuff with continuous monitoring of oxygen saturation by pulse oximeter. As with the moderately ill child, supplemental oxygen and continuously nebulized albuterol and IB should be provided soon after arrival. To achieve an oxygen saturation of 92% or greater, it may be necessary to use a non-rebreathing face mask. Severely ill children may be too sick to tolerate oral medications and will likely need an intravenous catheter for other indications. Therefore, an intravenous line should be established as soon as possible and a dose of methylprednisolone given.
Subcutaneous or Intramuscular Therapy.: For children with very poor inspiratory flow, nebulized SABAs may not be effectively delivered to the smallest airways. Short inspiratory time, prolonged expiration, and low inspiratory pressures will impair delivery of inhaled medications. Here, subcutaneous or intramuscular terbutaline or epinephrine should be used, especially if an intravenous line is yet to be established. Terbutaline may be preferable because it is a more selective agent with fewer side effects, such as tremors, vomiting, or palpitations. Very ill anxious young children who are uncooperative with the inhalation treatments may also benefit from this therapy. There are no data to suggest that one mode of administration is superior to the other, although intramuscular therapy is recommended for children with bronchospasm due to anaphylaxis.76 Subcutaneous or intramuscular therapy may be repeated every 10 to 15 minutes, as needed.
Magnesium Sulfate.: There is accumulating evidence that magnesium sulfate may benefit adults and children with severe asthma. Meta-analyses determined that use of magnesium resulted in improved outcomes for both adults77 and children.78 In two separate trials, children with a suboptimal response to initial SABA therapy who were randomized to receive magnesium had significantly greater improvements in pulmonary function compared with those treated with placebo.79,80 In contrast, Scarfone and colleagues conducted a randomized, controlled trial assessing the use of 75 mg/kg of magnesium in asthmatic children.81 They sought to determine if magnesium was efficacious as a component of initial therapy for children with moderate to severe exacerbation, without waiting to judge response to early albuterol therapy. No magnesium benefits were found for this population.
Magnesium is inexpensive and has minimal adverse effects.82 The most common adverse effect is hypotension; this may be avoided by infusion of the dose during 20 minutes. The most recent NHLBI guidelines recommend the consideration of magnesium for select patients; this represents a key difference from prior reports. At this point, existing literature indicates that magnesium should be considered for moderately ill patients who have a suboptimal response to SABAs, IB, and corticosteroids as well as for all severely ill children.
Intravenous Short-Acting Beta2-Agonists.: The NHLBI guidelines conclude that there are insufficient data to make recommendations for the use of intravenous SABAs.2 Similarly, a systematic review of randomized, controlled trials failed to support this practice.83 However, of the 15 studies included, just three were performed in children and just three assessed the combination of intravenous and inhaled SABAs compared with inhaled SABAs alone.84 Rather than definitively demonstrating lack of efficacy, these data highlight the need for more and larger clinical trials.
Heliox.: Heliox is a low-density mixture (often in a 70 : 30 ratio) of helium and oxygen that results in less turbulent flow through narrowed airways. Theoretically, its use is associated with decreased work of breathing, less respiratory muscle fatigue, and lower likelihood of ventilatory failure. In one trial, children with acute severe asthma treated with heliox had a significantly greater decrease in pulsus paradoxus and dyspnea index, and increase in PEFR, compared with others. A more recent study compared the use of heliox versus oxygen alone to deliver continuously nebulized SABAs.85 At 240 minutes, children in the heliox group had greater improvement as assessed by decreased asthma scores and need for hospitalization. However, after reviewing 10 clinical trials assessing the use of heliox, investigators concluded that there is insufficient evidence to support the use of heliox for all patients with asthma,86 although it may be considered for severely ill children who are not responding to more conventional therapy.2
Mechanical Ventilation.: When making decisions about the need for mechanical ventilation of the severely ill patient, one should assess the entire clinical picture, including duration of wheezing, illness severity, response to therapy, and ABG results. Making this decision on the basis of the ABG results alone should be discouraged. For example, the child with a pH of 7.10 and a PaCO2 of 55 who shows marked improvement with intravenous SABA therapy may not require ventilatory assistance, yet the child with a pH of 7.18 and a PaCO2 of 50 who appears fatigued and is not responding to therapy probably does. Ketamine is a bronchodilator and is the drug of choice for sedation and analgesia of the asthmatic child who requires intubation.
There are many potential therapies that are not recommended for the treatment of acutely ill asthmatic children. These include methylxanthines such as aminophylline, routine use of antibiotics in the absence of known bacterial infection, aggressive hydration, chest physiotherapy, mucolytics, sedation, and noninvasive ventilation.2
Bronchiolitis
Epidemiology
Bronchiolitis is a seasonal disease, with most cases occurring between November and April in temperate climates. It accounts for approximately 3% of all ED visits in the United States.87 Overall, approximately 19 to 27% of children presenting to an ED with bronchiolitis are admitted for inpatient management.87,88 This disease accounts for more than 20% of acute care hospitalizations for children younger than 1 year, and the total cost for all bronchiolitis-related hospitalizations is more than $500 million per year.89 Hospitalization rates vary according to many factors, and these rates have increased dramatically during the past two decades. In the United States, Hispanic children and those of Alaskan or American Indian descent are more likely to be admitted to the hospital after presenting with bronchiolitis.88,90 Boys and younger infants are also more likely to need admission.91 Other factors that seem to be associated with hospitalization are poverty, household crowding, exposure to environmental tobacco smoke, and daycare attendance.92 Clearly, underlying chronic medical conditions, such as congenital heart disease and chronic lung disease, lead to a more severe course in patients with this disease.
Bronchiolitis is rarely fatal, with an average mortality rate of 2.0 per 100,000 live births in the United States. Low birth weight (<2500 g), low 5-minute Apgar score, high birth order, and young maternal age are associated with an increased risk of death.93,94 Breast-feeding, on the other hand, appears to be associated with a less severe clinical course.95
Distinguishing Principles of Disease
Many viruses are implicated as the underlying cause of bronchiolitis. Respiratory syncytial virus (RSV), the most common agent identified in children diagnosed with this disease, is estimated to cause up to 70% of cases in previously healthy children.96 Other viruses commonly isolated are parainfluenza, human metapneumovirus, influenza, adenovirus, bocavirus, and rhinovirus.96–101
Pathophysiology
Most respiratory viruses that cause bronchiolitis in children are transmitted from one host to another by fomites spread from hand to nose or by droplets produced by sneezing or coughing of respiratory secretions. Shedding of the virus often begins before the onset of significant clinical symptoms and can continue for 2 to 3 weeks in an immunocompetent infant. The typical incubation period is 2 to 8 days from the time of initial contact.102
In an infected patient, viral replication often begins in the epithelial cells of the upper airway before spreading to the mucosal surfaces of the lower respiratory tract. The infected epithelial cells are generally destroyed by lysis or apoptosis, which results in the desquamation of these cells and release of host inflammatory mediators.103 Affected lungs demonstrate epithelial cell necrosis, monocytic inflammation and edema of the peribronchial tissues, and mucus and fibrin plugging of the distal airways on histologic examination. These findings translate into the clinical findings of wheezing and lower airway obstruction in an infant with bronchiolitis. Younger infants, whose distal airways are of smaller caliber and whose immune systems lack active immunity to most respiratory viruses, are prone to more severe clinical symptoms.92 Severe lower airway obstruction leads to air trapping and atelectasis, resulting in mismatched ventilation and perfusion and hypoxemia. In addition, younger infants are at increased risk for fatigue, leading to hypercarbia and respiratory failure.
Clinical Features
Typically, infants with bronchiolitis are younger than 12 months and present during the winter months. The first symptoms are generally those of a URI, such as nasal congestion and copious rhinorrhea. This is followed within a few days by development of a tight cough, often associated with difficulty in feeding. Some parents will report audible wheezing as well. A history of fever is common but not universal; one study reported fever in approximately one third of patients admitted with bronchiolitis.104 Very young infants may present with a history of apnea, and this may precede the onset of typical symptoms of respiratory infection. It is essential to ascertain information about the infant’s hydration status, including the amount and frequency of oral intake, urine output, vomiting, and diarrhea.
Physical Examination
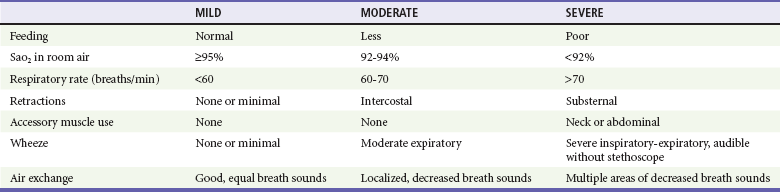
As in any pediatric patient, an assessment of vital signs and general appearance is crucial to the evaluation of an infant with bronchiolitis. Common vital sign abnormalities include fever, tachycardia, tachypnea, and hypoxia. Pulse oximetry is noninvasive and inexpensive, and it provides objective data about the degree of illness of a wheezing child. The oxygen saturation (SaO2) of any moderately or severely ill infant should be obtained soon after ED arrival as an adjunct to the physical examination. With the use of pulse oximetry, an ABG analysis is generally unnecessary to assess a patient’s oxygenation. Thus the acquisition of an ABG analysis should be reserved for those with severe disease and impending respiratory failure to measure the extent of hypercarbia and respiratory acidosis. Irritability or lethargy may be present, especially in those infants with more severe disease. Nasal flaring and retractions are visible signs of respiratory distress that may be present. Lung auscultation often reveals decreased air movement, rales, rhonchi, wheezing, and increased ratio of expiratory to inspiratory times. Using these physical examination findings, the clinician may stratify patients into mild, moderate, and severe categories of disease (Table 169-4).
Complications
Clinicians caring for children with bronchiolitis need to understand the typical course of the disease, both for their ability to accurately diagnose and manage these patients and to advise parents about the recovery phase. The worst phase of the illness that may necessitate hospitalization generally occurs in the first few days, and median length of hospital stay has been reported to be between 2 and 3 days.105,106 However, the entire course of illness can last much longer, with a median duration of 12 days.107 Coughing and noisy breathing, in particular, can last for more than 4 weeks.
Bacterial acute otitis media is the most common condition associated with bronchiolitis, with a prevalence of up to 60%. The bacterial pathogens are similar to those recovered in other children with acute otitis media; thus it should be treated according to standard recommendations. Other concurrent bacterial infections are rare. In one study of more than 2000 children hospitalized with RSV bronchiolitis, approximately 1% also had a urinary tract infection (UTI).108 Pathologic bacteremia and meningitis were not found in any of these patients. Similar rates of UTI without bacteremia are found in febrile children with clinical bronchiolitis, with or without documented RSV infection.109 Infants younger than 8 weeks with fever and bronchiolitis present a unique dilemma for emergency physicians. The rate of serious bacterial infections (SBIs), defined as UTI, bacteremia, bacterial meningitis, or bacterial enteritis, among all febrile infants younger than 8 weeks is reported to be up to 12%. However, in infants with documented RSV infection or clinical bronchiolitis at the time of ED presentation, the rate of SBI is substantially lower.110,111 In a large, prospective, multicenter study, Levine and colleagues reported that 7% of febrile infants younger than 61 days who were RSV positive had a concurrent SBI, compared with 12.5% of those who were RSV negative. Of the patients with SBIs, most (82%) had a UTI. Bacteremia was rare and occurred only in infants younger than 1 month. None of the RSV-positive infants had bacterial meningitis. As a result, most would advocate performance of a workup for UTI in febrile infants between 1 and 2 months of age who are known to be RSV positive or have clinical bronchiolitis. Additional testing to obtain culture specimens of cerebrospinal fluid and blood may be done selectively. Similarly, these infants may not require empirical antibiotic therapy for presumed SBIs. On the other hand, in the first month of life, all febrile infants should undergo testing for evaluation for SBIs and be empirically treated with antibiotics regardless of RSV status.
Apnea is commonly reported in young infants with bronchiolitis, especially those who are admitted for inpatient management. Eight percent of admitted patients have a history of apnea, and nearly 3% will have apnea during the hospital stay.112,113 Risk factors for development of in-hospital apnea include age younger than 1 month in full-term infants, postconceptional age younger than 48 weeks in preterm infants, and history of apnea before admission. The absence of all of these risk factors has a high negative predictive value for the development of in-hospital apnea.113
The long-term outcomes after bronchiolitis have also been examined extensively. It is clear that those who experience the disease in infancy have a higher prevalence of lower respiratory diseases, including asthma, in adolescence and adulthood.114–117 Whether there is a causal relationship remains undetermined.
Differential Considerations
Asthma is the condition that has the most clinical overlap with bronchiolitis. Physical examination findings alone cannot distinguish the two. Younger age, presentation during winter months, antecedent URI symptoms, and absence of prior or family history of atopic disease and wheezing suggest bronchiolitis as the cause of wheezing in an individual patient. Some infants will have clinical features consistent with both conditions. For example, a 12-month-old may present in July with a URI and wheezing for the first time. For this child, a clinician may choose to initiate therapy for acute asthma. A complete discussion of other conditions that should be differentiated from bronchiolitis is included earlier in this chapter and in Table 169-1.
Diagnostic Strategies
There is tremendous variability in the use of diagnostic imaging; in one series, a chest radiograph was obtained for more than 70% of infants hospitalized with bronchiolitis.118 In children with clinical findings that are typical for bronchiolitis, however, radiographic imaging is rarely necessary. Hyperinflation, atelectasis, and peribronchial cuffing are the findings most commonly associated with this disease. In ambulatory patients with acute lower respiratory infections, obtaining of a chest radiograph does not affect clinical outcome,119 and this practice has been associated with increased use of unnecessary antibiotics.118 Further, the likelihood of a chest radiograph’s revealing findings inconsistent with the clinical diagnosis of bronchiolitis is less than 1%.120 Diagnostic imaging may be helpful in patients with severe distress, significant hypoxia, or atypical presentation or clinical course. In summary, as recommended by the American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis, “clinicians should diagnose bronchiolitis and assess disease severity on the basis of history and physical examination. Clinicians should not routinely order laboratory and radiologic studies for diagnosis.”121
Management
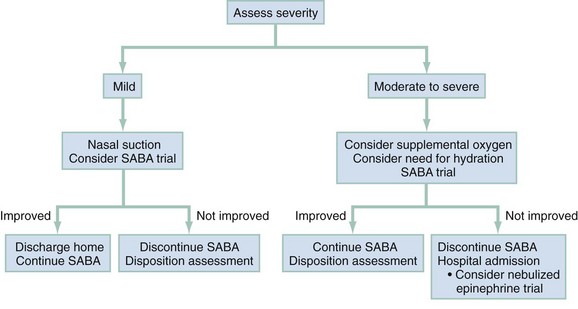
Whereas the diagnosis of bronchiolitis is fairly straightforward, the management of children with the disease often presents clinicians with confusing and controversial dilemmas. The literature is often contradictory, making it difficult to reach a consensus. As a result, there exists wide practice variation in the management of bronchiolitis.122–124 However, it is clear that a consistent, evidence-based approach to this disease can lead to more efficient and effective care.113,125–128 Supportive care, such as providing hydration and supplemental oxygen, is the cornerstone of therapy for affected children.121 A management strategy, stratified by the patient’s initial degree of illness, is outlined in Figure 169-2.
SABAs are the treatment of choice for children with wheezing due to asthma. However, the evidence supporting their use in wheezing caused by bronchiolitis is less favorable than for asthma. In a meta-analysis of 22 clinical trials, a small short-term benefit in clinical score was observed for children with bronchiolitis treated with SABAs. This treatment had no significant effect on rates or duration of hospitalization. Although rare, adverse effects such as tachycardia, decreased oxygen saturation, flushing, and hyperactivity occurred more frequently in children treated with SABAs.129 Thus the American Academy of Pediatrics does not recommend the routine use of SABAs for bronchiolitis; instead, clinicians should consider a trial of such medications to determine if a patient has a positive clinical response.121
Similar controversy exists with respect to the utility of nebulized epinephrine in treatment of bronchiolitis. A meta-analysis of 14 studies concluded that there is not enough evidence to support the use of epinephrine for inpatients, but it does provide some clinical benefit over other bronchodilators and placebo for outpatients.130 Treatment does not decrease the rate of hospitalization or the length of hospital stay for admitted patients.131 One difficulty with the use of epinephrine in the ED is that it is not a treatment that can be continued at home. Thus nebulized epinephrine should be considered for children with moderate to severe distress in whom beta-agonist therapy was not effective and who will likely require hospitalization. As with SABAs, nebulized epinephrine should be continued only for those patients who demonstrate a clinical benefit.
Studies on the use of nebulized anticholinergic agents (e.g., ipratropium bromide) have not been conclusive. Whereas one study in the ED setting reported a decreased need for additional treatment of patients receiving an anticholinergic medication in addition to SABAs,132 a similarly designed ED study found no benefit.133 There is currently not sufficient evidence to recommend the use of anticholinergic agents for young children with wheezing and suspected bronchiolitis.134
Many of the symptoms of bronchiolitis are a result of increased and thickened respiratory secretions. A great deal of literature supports the use of nebulized hypertonic saline in the treatment of cystic fibrosis, in which clearance of thickened secretions is vital.135 Although there is not yet enough literature to definitively recommend its use for bronchiolitis, one study suggested that nebulized hypertonic saline is a safe medication that reduces the length of stay for hospitalized children.136 Thus far, studies have not demonstrated any clinically significant benefits associated with its use in ED patients.137 Chest physiotherapy has also been examined as a means to clear respiratory secretions. A meta-analysis of three randomized, controlled trials revealed no improvement in clinical score, length of stay, or oxygen requirement after chest physiotherapy.138
Systemic corticosteroids are well established as being effective treatment of wheezing due to acute asthma. Despite reports that more than half of infants may be prescribed corticosteroids when they are diagnosed with bronchiolitis,124 well-designed controlled trials have demonstrated no benefit for their use in terms of rate of admission, clinical score, or any other outcome.121,139,140 Specifically, Corneli and colleagues conducted a double-blind, randomized trial comparing oral dexamethasone with placebo in 600 children with acute moderate to severe bronchiolitis. The investigators concluded that oral dexamethasone had no significant effect on the rate of hospitalization, respiratory status after 4 hours of observation, or later outcomes, such as length of inpatient stay, repeated medical visits, and adverse events. Another multicenter trial did, however, demonstrate a reduction in hospitalization rates in patients treated with the combination of oral dexamethasone and nebulized epinephrine.141 Further research is needed to clarify the role of systemic corticosteroids in the treatment of bronchiolitis. ICS also provide no positive effect on clinical course.142
Whereas infants with severe bronchiolitis requiring intensive care and mechanical ventilation frequently have concurrent or secondary bacterial infections,143 this is an uncommon complication for most children. Despite some reports that clarithromycin may hasten recovery in RSV bronchiolitis,144 there is no evidence for the routine use of antibiotics for bronchiolitis, and they should be reserved for patients with true bacterial infections.139,145
Ribavirin is a specific antiviral agent directed toward treatment of RSV infections. Several smaller studies suggested a small benefit with respect to duration of mechanical ventilation and length of stay in patients with severe disease.146 Its high cost and potential risks to caregivers, however, limit its role in routine management. Ribavirin may be indicated in selected cases of documented RSV bronchiolitis with severe respiratory compromise.121
Prophylaxis
Although emergency physicians generally do not have a role in the administration of preventive medications, they should be aware of the options available to their patients. Palivizumab (Synagis) consists of monoclonal antibodies against RSV. Whereas RSV-specific immune globulin is not effective in treatment of the acute disease process,147 palivizumab is effective in reducing hospitalization rates for RSV in certain high-risk populations.148–151 It is recommended for most children younger than 24 months with chronic lung disease, congenital heart disease, or prematurity and is administered as a monthly intramuscular injection during the high-prevalence months.152
Disposition
An essential component in the evaluation and management of bronchiolitis in the ED is the ability to predict the severity of its clinical course. Because it is a dynamic disease, evaluations at a single point in time may not be sufficient to fully estimate its severity; thus serial examinations are necessary. A number of demographic and clinical features have been associated with a severe clinical course. These factors, which may mandate hospitalization, include age younger than 3 months, gestational age less than 34 weeks, ill appearance, hypoxemia (SaO2 < 95%), tachypnea (>70 breaths/min), and significant atelectasis on the chest radiograph (if it is obtained). Most of the literature, however, focuses on inpatients. In addition to younger age and prematurity, a history of hemodynamically significant congenital heart disease, chronic lung disease, and immunocompromised state have been associated with higher morbidity and mortality among inpatients.121,153
References
1. Akinbami, L. The state of childhood asthma, United States, 1980-2005. Adv Data. 2006;381:1.
2. National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma—Summary Report 2007. J Allergy Clin Immunol. 2007;120(Suppl 1):94.
3. Gorelick, MH, Stevens, MW, Schultz, TR, Scribano, PV. Performance of a novel clinical score, the Pediatric Asthma Severity Score (PASS), in the evaluation of acute asthma. Acad Emerg Med. 2004;11:10.
4. Scarfone, RJ, Fuchs, SM, Nager, AL, Shane, SA. Controlled trial of oral prednisone in the emergency department treatment of children with acute asthma. Pediatrics. 1993;92:513.
5. Geelhoed, GC, Landau, LI, Le Souef, PN. Evaluation of SaO2 as a predictor of outcome in 280 children presenting with acute asthma. Ann Emerg Med. 1994;23:1236.
6. Walsh-Kelly, CM, Kim, MK, Hennes, HM. Chest radiography in the initial episode of bronchospasm in children: Can clinical variables predict pathologic findings? Ann Emerg Med. 1996;28:391.
7. Gershel, JC, et al. The usefulness of chest radiographs in first asthma attacks. N Engl J Med. 1983;309:336.
8. Tien, I, Dorfman, D, Kastner, B, Bauchner, H. Metered-dose inhaler: The emergency department orphan. Arch Pediatr Adolesc Med. 2001;155:1335.
9. Kacmarek, RM. The interface between patient and aerosol generator. Respir Care. 1991;36:952.
10. Rubilar, L, Castro-Rodriguez, JA, Girardi, G. Randomized trial of salbutamol via metered-dose inhaler with spacer versus nebulizer for acute wheezing in children less than 2 years of age. Pediatr Pulmonol. 2000;29:264.
11. Amirav, I, Newhouse, MT. Metered-dose inhaler accessory devices in acute asthma: Efficacy and comparison with nebulizers: A literature review. Arch Pediatr Adolesc Med. 1997;151:876.
12. Camargo, C, Kennedy, P. Assessing costs of aerosol therapy. Respir Care. 2000;45:756.
13. Dewar, AL, Stewart, A, Cogswell, JJ, Connett, GJ. A randomised controlled trial to assess the relative benefits of large volume spacers and nebulisers to treat acute asthma in hospital. Arch Dis Child. 1999;80:421.
14. Leversha, AM, Campanella, SG, Aickin, RP, Asher, MI. Costs and effectiveness of spacer versus nebulizer in young children with moderate and severe acute asthma. J Pediatr. 2000;136:497.
15. Doan, Q, Shefrin, A, Johnson, D. Cost-effectiveness of metered-dose inhalers for asthma exacerbations in the pediatric emergency department. Pediatrics. 2011;127:e1105.
16. Castro-Rodriguez, JA, Rodrigo, GJ. Beta-agonists through metered-dose inhaler with valved holding chamber versus nebulizer for acute exacerbation of wheezing or asthma in children under 5 years of age: A systematic review with meta-analysis. J Pediatr. 2004;145:172.
17. Cates, CJ, Crilly, JA, Rowe, BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. (2):2006.
18. Freelander, M, Van Asperen, PP. Nebuhaler versus nebuliser in children with acute asthma. BMJ (Clin Res Ed). 1984;288:1873.
19. Lee, N, et al. Comparison of efficacy and safety of albuterol administered by power-driven nebulizer (PDN) versus metered-dose inhaler (MDI) with Aerochamber and mask in young children with asthma. J Allergy Clin Immunol. 1991;87:307.
20. Parkin, PC, et al. Randomised trial spacer vs nebuliser for acute asthma. Arch Dis Child. 1995;72:239.
21. Williams, JR, Bothner, JP, Swanton, RD. Delivery of albuterol in a pediatric emergency department. Pediatr Emerg Care. 1996;12:263.
22. Ploin, D, et al. High-dose albuterol by metered-dose inhaler plus a spacer device versus nebulization in preschool children with recurrent wheezing: A double-blind, randomized equivalence trial. Pediatrics. 2000;106(Pt 1):311.
23. Wildhaber, JH, et al. Inhalation therapy in asthma: Nebulizer or pressurized metered-dose inhaler with holding chamber? In vivo comparison of lung deposition in children. J Pediatr. 1999;135:28.
24. Dolovich, MB, et al. Device selection and outcomes of aerosol therapy: Evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and Immunology. Chest. 2005;127:335.
25. Schuh, S, et al. High-versus low-dose, frequently administered, nebulized albuterol in children with severe, acute asthma. Pediatrics. 1989;83:513.
26. Schuh, S, et al. Nebulized albuterol in acute childhood asthma: Comparison of two doses. Pediatrics. 1990;86:509.
27. Mazzoni, L, Naef, R, Chapman, ID, Morley, J. Hyperresponsiveness of the airways following exposure of guinea pigs to racemic mixtures and distomers of beta2-selective sympathomimetics. Pulm Pharmacol. 1994;7:367.
28. Johansson, F, Rydberg, I, Aberg, G, Andersson, RG. Effects of albuterol enantiomers on in vitro bronchial reactivity. Clin Rev Allergy Immunol. 1996;14:57.
29. Templeton, AG, et al. Effects of S-salbutamol on human isolated bronchus. Pulm Pharmacol Ther. 1998;11:1.
30. Volcheck, GW, Gleich, GJ, Kita, H. Pro- and anti-inflammatory effects of beta adrenergic agonists on eosinophil response to IL-5. J Allergy Clin Immunol. 1998;101(Suppl):35.
31. Leff, AR, et al. Effect of enantiomeric forms of albuterol on stimulated secretion of granular protein from human eosinophils. Pulm Pharmacol Ther. 1997;10:97.
32. Yamaguchi, H, McCullough, JR. S-Albuterol exacerbates calcium responses to carbachol in airway smooth muscle cells. Clin Rev Allergy Immunol. 1996;14:47.
33. Mitra, S, et al. S)-Albuterol increases intracellular free calcium by muscarinic receptor activation and a phospholipase C–dependent mechanism in airway smooth muscle. Mol Pharmacol. 1998;53:347.
34. Dhand, R, et al. Preferential pulmonary retention of (S)-albuterol after inhalation of racemic albuterol. Am J Respir Crit Care Med. 1999;160:1136.
35. Lipworth, BJ, Clark, DJ, Koch, P, Arbeeny, C. Pharmacokinetics and extrapulmonary β2 adrenoceptor activity of nebulised racemic salbutamol and its R and S isomers in healthy volunteers. Thorax. 1997;52:849.
36. Cockcroft, DW, Swystun, VA. Effect of single doses of S-salbutamol, R-salbutamol, racemic salbutamol, and placebo on the airway response to methacholine. Thorax. 1997;52:845.
37. Carl, JC, Myers, TR, Kirchner, HL, Kercsmar, CM. Comparison of racemic albuterol and levalbuterol for treatment of acute asthma. J Pediatr. 2003;143:731.
38. Qureshi, F, et al. Clinical efficacy of racemic albuterol versus levalbuterol for the treatment of acute pediatric asthma. Ann Emerg Med. 2005;46:29.
39. Hardasmalani, MD, DeBari, V, Bithoney, WG, Gold, N. Levalbuterol versus racemic albuterol in the treatment of acute exacerbation of asthma in children. Pediatr Emerg Care. 2005;21:415.
40. Ralston, ME, et al. Comparison of levalbuterol and racemic albuterol combined with ipratropium bromide in acute pediatric asthma: A randomized controlled trial. J Emerg Med. 2005;29:29.
41. Andrews, T, et al. High-dose continuous nebulized levalbuterol for pediatric status asthmaticus: A randomized trial. J Pediatr. 2009;155:205.
42. Brenner, BE, Chavda, KK, Camargo, CA, Jr. Randomized trial of inhaled flunisolide versus placebo among asthmatic patients discharged from the emergency department. Ann Emerg Med. 2000;36:417.
43. Rowe, BH, et al. Inhaled budesonide in addition to oral corticosteroids to prevent asthma relapse following discharge from the emergency department: A randomized controlled trial. JAMA. 1999;281:2119.
44. Edmonds, ML, et al. Inhaled steroids in acute asthma following emergency department discharge. Cochrane Database Syst Rev. (3):2000.
45. Scarfone, RJ, Zorc, JJ, Angsuco, CJ. Emergency physicians’ prescribing of asthma controller medications. Pediatrics. 2006;117:821.
46. Guilbert, TW, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354:1985.
47. Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med. 2000;343:1054.
48. Qureshi, F, Pestian, J, Davis, P, Zaritsky, A. Effect of nebulized ipratropium on the hospitalization rates of children with asthma. N Engl J Med. 1998;339:1030.
49. Schuh, S, et al. Efficacy of frequent nebulized ipratropium bromide added to frequent high-dose albuterol therapy in severe childhood asthma. J Pediatr. 1995;126:639.
50. Rodrigo, GJ, Castro-Rodriguez, JA. Anticholinergics in the treatment of children and adults with acute asthma: A systematic review with meta-analysis. Thorax. 2005;60:740.
51. Littenberg, B, Gluck, EH. A controlled trial of methylprednisolone in the emergency treatment of acute asthma. N Engl J Med. 1986;314:150.
52. Tal, A, Levy, N, Bearman, JE. Methylprednisolone therapy for acute asthma in infants and toddlers: A controlled clinical trial. Pediatrics. 1990;86:350.
53. Rachelefsky, G. Treating exacerbations of asthma in children: The role of systemic corticosteroids. Pediatrics. 2003;112:382.
54. Rowe, BH, et al. Corticosteroid therapy for acute asthma. Respir Med. 2004;98:275.
55. Rowe, BH, et al. Early emergency department treatment of acute asthma with systemic corticosteroids. Cochrane Database Syst Rev. (1):2001.
56. Scarfone, RJ, Friedlaender, E. Corticosteroids in acute asthma: Past, present, and future. Pediatr Emerg Care. 2003;19:355.
57. Barnett, PL, Caputo, GL, Baskin, M, Kuppermann, N. Intravenous versus oral corticosteroids in the management of acute asthma in children. Ann Emerg Med. 1997;29:212.
58. Harrison, BD, et al. Need for intravenous hydrocortisone in addition to oral prednisolone in patients admitted to hospital with severe asthma without ventilatory failure. Lancet. 1986;1:181.
59. Ratto, D, et al. Are intravenous corticosteroids required in status asthmaticus? JAMA. 1988;260:527.
60. Qureshi, F, Zaritsky, A, Poirier, MP. Comparative efficacy of oral dexamethasone versus oral prednisone in acute pediatric asthma. J Pediatr. 2001;139:20.
61. Scarfone, RJ, et al. Nebulized dexamethasone versus oral prednisone in the emergency treatment of asthmatic children. Ann Emerg Med. 1995;26:480.
62. Devidayal, SS, Singhi, S, Kumar, L, Jayshree, M. Efficacy of nebulized budesonide compared to oral prednisolone in acute bronchial asthma. Acta Paediatr. 1999;88:835.
63. Schuh, S, et al. A comparison of inhaled fluticasone and oral prednisone for children with severe acute asthma. N Engl J Med. 2000;343:689.
64. Klassen, TP, et al. The efficacy of nebulized budesonide in dexamethasone-treated outpatients with croup. Pediatrics. 1996;97:463.
65. Macias, CG, Felner, EI, Gan, V. Inhaled corticosteroids may be superior to systemic corticosteroids in children with moderate-to-severe acute asthma. Pediatr Asthma Allergy Immunol. 2003;16:121.
66. Edmonds, ML, Camargo, CA, Jr., Pollack, CV, Jr., Rowe, BH. The effectiveness of inhaled corticosteroids in the emergency department treatment of acute asthma: A meta-analysis. Ann Emerg Med. 2002;40:145.
67. Edmonds, ML, Camargo, CA, Jr., Pollack, CV, Jr., Rowe, BH. Early use of inhaled corticosteroids in the emergency department treatment of acute asthma. Cochrane Database Syst Rev. (3):2003.
68. Rodrigo, GJ. Rapid effects of inhaled corticosteroids in acute asthma: An evidence-based evaluation. Chest. 2006;130:1301.
69. Khine, H, Fuchs, SM, Saville, AL. Continuous vs intermittent nebulized albuterol for emergency management of asthma. Acad Emerg Med. 1996;3:1019.
70. Camargo, CA, Jr., Spooner, CH, Rowe, BH. Continuous versus intermittent beta-agonists in the treatment of acute asthma. Cochrane Database Syst Rev. (4):2003.
71. Papo, MC, Frank, J, Thompson, AE. A prospective, randomized study of continuous versus intermittent nebulized albuterol for severe status asthmaticus in children. Crit Care Med. 1993;21:1479.
72. Katz, RW, et al. Safety of continuous nebulized albuterol for bronchospasm in infants and children. Pediatrics. 1993;92:666.
73. Rudnitsky, GS, et al. Comparison of intermittent and continuously nebulized albuterol for treatment of asthma in an urban emergency department. Ann Emerg Med. 1993;22:1842.
74. Lin, RY, et al. Continuous versus intermittent albuterol nebulization in the treatment of acute asthma. Ann Emerg Med. 1993;22:1847.
75. Kelly, AM, Kerr, D, Powell, C. Is severity assessment after one hour of treatment better for predicting the need for admission in acute asthma? Respir Med. 2004;98:777.
76. Stevenson, MD, Ruddy, RM. Asthma and allergic emergencies. In: Fleisher GR, Ludwig S, eds. Textbook of Pediatric Emergency Medicine. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:1067.
77. Rowe, BH, et al. Intravenous magnesium sulfate treatment for acute asthma in the emergency department: A systematic review of the literature. Ann Emerg Med. 2000;36:181.
78. Cheuk, DK, Chau, TC, Lee, SL. A meta-analysis on intravenous magnesium sulphate for treating acute asthma. Arch Dis Child. 2005;90:74.
79. Ciarallo, L, Sauer, AH, Shannon, MW. Intravenous magnesium therapy for moderate to severe pediatric asthma: Results of a randomized, placebo-controlled trial. J Pediatr. 1996;129:809.
80. Ciarallo, L, Brousseau, D, Reinert, S. Higher-dose intravenous magnesium therapy for children with moderate to severe acute asthma. Arch Pediatr Adolesc Med. 2000;154:979.
81. Scarfone, RJ, et al. A randomized trial of magnesium in the emergency department treatment of children with asthma. Ann Emerg Med. 2000;36:572.
82. Scarfone, RJ. Use of magnesium sulfate in the treatment of children with acute asthma. Clin Pediatr Emerg Med. 1999;1:6.
83. Travers, A, et al. Intravenous beta2-agonists for acute asthma in the emergency department. Cochrane Database Syst Rev. (2):2001.
84. Browne, GJ, Penna, AS, Phung, X, Soo, M. Randomised trial of intravenous salbutamol in early management of acute severe asthma in children. Lancet. 1997;349:301.
85. Kim, IK, et al. Helium/oxygen-driven albuterol nebulization in the treatment of children with moderate to severe asthma exacerbations: A randomized, controlled trial. Pediatrics. 2005;116:1127.
86. Rodrigo, G, Pollack, C, Rodrigo, C, Rowe, BH. Heliox for nonintubated acute asthma patients. Cochrane Database Syst Rev. (4):2006.
87. Mansbach, JM, Emond, JA, Camargo, CAJr. Bronchiolitis in US emergency departments 1992 to 2000: Epidemiology and practice variation. Pediatr Emerg Care. 2005;21:242.
88. Johnson, DW, et al. Differences in admission rates of children with bronchiolitis by pediatric and general emergency departments. Pediatrics. 2002;110:e49.
89. Pelletier, AJ, Mansbach, JM, Camargo, CAJr. Direct medical costs of bronchiolitis hospitalizations in the United States. Pediatrics. 2006;118:2418.
90. Liu, LL, et al. Asthma and bronchiolitis hospitalizations among American Indian children. Arch Pediatr Adolesc Med. 2000;154:991.
91. Shay, DK, et al. Bronchiolitis-associated hospitalizations among US children, 1980-1996. JAMA. 1999;282:1440.
92. Coffin, SE. Bronchiolitis: In-patient focus. Pediatr Clin North Am. 2005;52:1047.
93. Shay, DK, et al. Bronchiolitis-associated mortality and estimates of respiratory syncytial virus–associated deaths among US children, 1979-1997. J Infect Dis. 2001;183:16.
94. Holman, RC, et al. Risk factors for bronchiolitis-associated deaths among infants in the United States. Pediatr Infect Dis J. 2003;22:483.
95. Chatzimichael, A, et al. The role of breastfeeding and passive smoking on the development of severe bronchiolitis in infants. Minerva Pediatr. 2007;59:199.
96. Henrickson, KJ, Hoover, S, Kehl, KS, Hua, W. National disease burden of respiratory viruses detected in children by polymerase chain reaction. Pediatr Infect Dis J. 2004;23(Suppl):11.
97. Williams, JV, et al. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med. 2004;350:443.
98. Smyth, RL, Openshaw, PJ. Bronchiolitis. Lancet. 2006;368:312.
99. Korppi, M, et al. Rhinovirus-associated wheezing in infancy: Comparison with respiratory syncytial virus bronchiolitis. Pediatr Infect Dis J. 2004;23:995.
100. van den Hoogen, BG, et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719.
101. van den Hoogen, BG, et al. Prevalence and clinical symptoms of human metapneumovirus infection in hospitalized patients. J Infect Dis. 2003;188:1571.
102. Black, CP. Systematic review of the biology and medical management of respiratory syncytial virus infection. Respir Care. 2003;48:209.
103. Dakhama, A, Lee, YM, Gelfand, EW. Virus-induced airway dysfunction: Pathogenesis and biomechanisms. Pediatr Infect Dis J. 2005;24(Suppl):159.
104. El-Radhi, AS, Barry, W, Patel, S. Association of fever and severe clinical course in bronchiolitis. Arch Dis Child. 1999;81:231.
105. Klassen, TP, et al. Dexamethasone in salbutamol-treated inpatients with acute bronchiolitis: A randomized, controlled trial. J Pediatr. 1997;130:191.
106. Richter, H, Seddon, P. Early nebulized budesonide in the treatment of bronchiolitis and the prevention of postbronchiolitic wheezing. J Pediatr. 1998;132:849.
107. Swingler, GH, Hussey, GD, Zwarenstein, M. Duration of illness in ambulatory children diagnosed with bronchiolitis. Arch Pediatr Adolesc Med. 2000;154:997.
108. Purcell, K, Fergie, J. Concurrent serious bacterial infections in 2396 infants and children hospitalized with respiratory syncytial virus lower respiratory tract infections. Arch Pediatr Adolesc Med. 2002;156:322.
109. Kuppermann, N, et al. Risks for bacteremia and urinary tract infections in young febrile children with bronchiolitis. Arch Pediatr Adolesc Med. 1997;151:1207.
110. Levine, DA, et al. Risk of serious bacterial infection in young febrile infants with respiratory syncytial virus infections. Pediatrics. 2004;113:1728.
111. Titus, MO, Wright, SW. Prevalence of serious bacterial infections in febrile infants with respiratory syncytial virus infection. Pediatrics. 2003;112:282.
112. Willwerth, BM, Harper, MB, Greenes, DS. Identifying hospitalized infants who have bronchiolitis and are at high risk for apnea. Ann Emerg Med. 2006;48:441.
113. Vogel, AM, et al. Variations in bronchiolitis management between five New Zealand hospitals: Can we do better? J Paediatr Child Health. 2003;39:40.
114. Gomez, R, Colas, C, Sebastian, A, Arribas, J. Respiratory repercussions in adults with a history of infantile bronchiolitis. Ann Allergy Asthma Immunol. 2004;93:447.
115. Hyvarinen, M, Piippo-Savolainen, E, Korhonen, K, Korppi, M. Teenage asthma after severe infantile bronchiolitis or pneumonia. Acta Paediatr. 2005;94:1378.
116. Sznajder, M, et al. Respiratory development of 5- to 6-year-old children experiencing a first bronchiolitis episode before age one. Eur Ann Allergy Clin Immunol. 2005;37:392.
117. Piippo-Savolainen, E, Korppi, M, Korhonen, K, Remes, S. Adult asthma after non–respiratory syncytial virus bronchiolitis in infancy: Subgroup analysis of the 20-year prospective follow-up study. Pediatr Int. 2007;49:190.
118. Christakis, DA, et al. Variation in inpatient diagnostic testing and management of bronchiolitis. Pediatrics. 2005;115:878.
119. Swingler, GH, Hussey, GD, Zwarenstein, M. Randomised controlled trial of clinical outcome after chest radiograph in ambulatory acute lower-respiratory infection in children. Lancet. 1998;351:404.
120. Schuh, S, et al. Evaluation of the utility of radiography in acute bronchiolitis. J. Pediatr. 2007;150:429.
121. American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118:1774.
122. Plint, AC, et al. Practice variation among pediatric emergency departments in the treatment of bronchiolitis. Acad Emerg Med. 2004;11:353.
123. Brand, PL, Vaessen-Verberne, AA. Differences in management of bronchiolitis between hospitals in The Netherlands. Dutch Paediatric Respiratory Society. Eur J Pediatr. 2000;159:343.
124. Willson, DF, et al. Effect of practice variation on resource utilization in infants hospitalized for viral lower respiratory illness. Pediatrics. 2001;108:851.
125. Perlstein, PH, et al. Evaluation of an evidence-based guideline for bronchiolitis. Pediatrics. 1999;104:1334.
126. Perlstein, PH, et al. Sustaining the implementation of an evidence-based guideline for bronchiolitis. Arch Pediatr Adolesc Med. 2000;154:1001.
127. Kotagal, UR, et al. Impact of a bronchiolitis guideline: A multisite demonstration project. Chest. 2002;121:1789.
128. Todd, J, Bertoch, D, Dolan, S. Use of a large national database for comparative evaluation of the effect of a bronchiolitis/viral pneumonia clinical care guideline on patient outcome and resource utilization. Arch Pediatr Adolesc Med. 2002;156:1086.
129. Gadomski, AM, Bhasale, AL. Bronchodilators for bronchiolitis. Cochrane Database Syst Rev. (3):2006.
130. Hartling, L, et al. Epinephrine for bronchiolitis. Cochrane Database Syst Rev. (1):2004.
131. Wainwright, C, et al. A multicenter, randomized, double-blind, controlled trial of nebulized epinephrine in infants with acute bronchiolitis. N Engl J Med. 2003;349:27.
132. Naspitz, CK, Sole, D. Treatment of acute wheezing and dyspnea attacks in children under 2 years old: Inhalation of fenoterol plus ipratropium bromide versus fenoterol. J Asthma. 1992;29:253.
133. Schuh, S, et al. Efficacy of adding nebulized ipratropium bromide to nebulized albuterol therapy in acute bronchiolitis. Pediatrics. 1992;90:920.
134. Everard, ML, et al. Anticholinergic drugs for wheeze in children under the age of two years. Cochrane Database Syst Rev. (3):2005.
135. Elkins, MR, et al. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med. 2006;354:229.
136. Kuzik, BA, et al. Nebulized hypertonic saline in the treatment of viral bronchiolitis in infants. J Pediatr. 2007;151:266.
137. Grewal, S, et al. A randomized trial of nebulized 3% hypertonic saline with epinephrine in the treatment of acute bronchiolitis in the emergency department. Arch Pediatr Adolesc Med. 2009;163:1007.
138. Perrotta, C, Ortiz, Z, Roque, M. Chest physiotherapy for acute bronchiolitis in paediatric patients between 0 and 24 months old. Cochrane Database Syst Rev. (1):2007.
139. Corneli, HM, et al. A multicenter, randomized, controlled trial of dexamethasone for bronchiolitis. N Engl J Med. 2007;357:331.
140. Patel, H, Platt, R, Lozano, JM, Wang, EE. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database Syst Rev. (3):2004.
141. Plint, AC, et al. Epinephrine and dexamethasone in children with bronchiolitis. N Engl J Med. 2009;360:2079.
142. Blom, D, et al. Inhaled corticosteroids during acute bronchiolitis in the prevention of post-bronchiolitic wheezing. Cochrane Database Syst Rev. (1):2007.
143. Thorburn, K, et al. High incidence of pulmonary bacterial co-infection in children with severe respiratory syncytial virus (RSV) bronchiolitis. Thorax. 2006;61:611.
144. Tahan, F, Ozcan, A, Koc, N. Clarithromycin in the treatment of RSV bronchiolitis: A double-blind, randomised, placebo-controlled trial. Eur Respir J. 2007;29:91.
145. Spurling, GK, Fonseka, K, Doust, J, Del Mar, C. Antibiotics for bronchiolitis in children. Cochrane Database Syst Rev. (1):2007.
146. Ventre, K, Randolph, AG. Ribavirin for respiratory syncytial virus infection of the lower respiratory tract in infants and young children. Cochrane Database Syst Rev. (1):2007.
147. Fuller, H, Del Mar, C. Immunoglobulin treatment for respiratory syncytial virus infection. Cochrane Database Syst Rev. (4):2006.
148. Feltes, TF, et al. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr. 2003;143:532.
149. IMpact–RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102(Pt 1):531.
150. Parnes, C, et al. Palivizumab prophylaxis of respiratory syncytial virus disease in 2000-2001: Results from the Palivizumab Outcomes Registry. Pediatr Pulmonol. 2003;35:484.
151. Romero, JR. Palivizumab prophylaxis of respiratory syncytial virus disease from 1998 to 2002: Results from four years of palivizumab usage. Pediatr Infect Dis J. 2003;22(Suppl):46.
152. American Academy of Pediatrics. Respiratory syncytial virus. In: Pickering LK, Baker CJ, Long SS, McMillan JA, eds. Red Book: 2006 Report of the Committee on Infectious Diseases. 27th ed. Elk Grove, Ill: American Academy of Pediatrics; 2006:560–566.
153. Purcell, K, Fergie, J. Driscoll Children’s Hospital respiratory syncytial virus database: Risk factors, treatment and hospital course in 3308 infants and young children, 1991 to 2002. Pediatr Infect Dis J. 2004;23:418.