158 Pediatric Overdoses
• Children less than 6 years old suffer the majority of toxic exposures seen annually in the United States.
• Many over-the-counter medications, household substances, and prescription medications can cause significant toxicity in young children when these substances are ingested in amounts as small as 1 to 2 pills or 1 to 2 teaspoons.
• As with adults, supportive care is the mainstay of treatment for pediatric overdoses. Specific antidotes and treatments are the same for pediatric patients as for adults.
• Salicylate overdose in the pediatric patient manifests similarly to overdose in adults, but the classic finding of a mixed metabolic acidosis and respiratory alkalosis may not be present. Methylsalicylate toxicity from oil of wintergreen ingestion can progress much more rapidly than toxicity from other salicylates, and treatment should not be delayed.
• When evaluating a child for potential caustic substance ingestion, the absence of burns in the mouth and oropharynx does not preclude burns in the esophagus and stomach.
• Sulfonylurea exposure in the pediatric patient warrants prolonged observation because of the risk of delayed hypoglycemia.
Epidemiology
Pediatric exposures to toxic substances represent the majority of cases reported to poison centers in the United States. Children less than 5 years old accounted for 52%, or 1.29 million, of total reported exposures in 2009. In contrast, exposure-related fatalities in the same age group represented only 1.8%, or 21, of total fatalities in 2009, and 18 of these fatalities were classified as unintentional exposures.1 This finding reflects the fact that toxic exposure in the child younger than 5 years old is generally the result of an inquisitive toddler exploring his or her environment.
The “One Pill Can Kill” list refers to a group of agents that are known to cause serious toxicity or death when they are ingested in very small quantities by a small child (Box 158.1). The emergency physician must be familiar with these agents to manage these patients appropriately and to anticipate the potential for poor outcomes. Many of these agents are covered in more detail in other chapters in this section.
Pathophysiology
Over-the-Counter Agents
Camphor
Camphor is an aromatic terpene ketone, originally distilled from the bark of the camphor tree and now synthesized from turpentine oil. It is a common ingredient in some topical and vaporized medications intended to treat musculoskeletal pain or symptoms of common flu-like illnesses (Table 158.1). Camphor is marketed as an analgesic, an antipruritic, and an antitussive, and it is also found in older formulations of mothballs.
| PRODUCT | CAMPHOR CONTENT (%) |
|---|---|
| Camphorated oil | 20.0 |
| Campho-Phenique | 10.8 |
| Camphor spirits | 10.0 |
| Vicks VapoRub | 4.8 |
| Heet | 3.60 |
| Tiger Balm | 11% |
The exact mechanism by which camphor produces toxicity is unknown, although the cyclic ketone of its hydroaromatic terpene group is hypothesized to be a neurotoxin. Camphor is highly lipophilic, resulting in rapid movement across cell membranes and a large volume of distribution. Its metabolites are stored in fat deposits and are cleared over a prolonged period of time, which may be responsible for the delayed onset of seizures associated with camphor toxicity.2 Camphor may also cause gastrointestinal toxicity from its direct effect on mucosal surfaces.
Doses between 750 and 1500 mg, and doses as low as 500 mg in some case reports, are associated with seizures and death.2 For this reason, the U.S. Food and Drug Administration ruled in 1982 that products could not contain more than 11% camphor. However, some commercially available formulations contain 500 mg in 1 teaspoonful of product. In addition, a case series of pediatric seizures attributed to camphor toxicity highlights the role that camphor still plays in some ethnic and cultural practices. Illegally sold, high-concentration camphor products pose a risk in these populations.3
Salicylates
Salicylates are present in numerous over-the-counter products and are marketed as analgesics, antipyretics, and antiinflammatory agents (Table 158.2). Several Asian herbal remedies sold as topical treatments for musculoskeletal pain also contain salicylates.
| PRODUCT | ACTIVE COMPONENT | CONTENT |
|---|---|---|
| Alka-Seltzer Plus | Acetylsalicylic acid | 325 mg/tablet |
| Ben Gay Arthritis Formula | Methylsalicylate | 30% |
| Clearasil Ultra Acne Scrub | Salicylic acid | 2% |
| Heet | Methylsalicylate | 18% |
| Oil of wintergreen | Methylsalicylate | 98% |
| Pepto-Bismol | Bismuth subsalicylate | 262 mg/15 mL |
| Sebulex Dandruff Shampoo | Salicylic acid | 2% |
Oil of wintergreen represents a specific concern in the pediatric population because of its extremely high concentration. One teaspoon of 98% oil of wintergreen contains 7000 mg of methylsalicylate, equivalent to 86 baby aspirin, a potentially lethal dose for children weighing less than 23 kg. This product has a pleasing aroma, thus rendering it particularly vulnerable to accidental ingestion. A review of pediatric salicylate poisonings found that all published cases of life-threatening toxicity or death resulted from oil of wintergreen or Asian herbal oil ingestions.4
Topical Anesthetics
Topical anesthetics are found in various pain-relieving products ranging from teething gels to hemorrhoid creams (Table 158.3). Amide anesthetics, which include lidocaine and dibucaine, work by blocking voltage-gated sodium channels and preventing action potential propagation. In toxic doses, these agents can cause CNS hyperstimulation secondary to central blocking of inhibitory pathways that can progress to seizures, respiratory depression, and coma. Amides can also cause cardiac toxicity because of their antiarrhythmic properties, but this is most frequently seen in intravenous, rather than oral, exposures.5
| PRODUCT | CONTENT |
|---|---|
| Anbesol Maximum Strength | Benzocaine, 20.0% |
| Baby Orajel | Benzocaine, 7.5% |
| Baby Anbesol Gel | Benzocaine, 7.5% |
| EMLA Cream | 25 g each of lidocaine and prilocaine/1 g |
| Vagisil Cream | Benzocaine, 5% |
Benzocaine is an ester anesthetic whose metabolites can cause methemoglobinemia in toxic doses. Methemoglobin is formed by oxidation of iron from the ferrous (Fe2+) to the ferric (Fe3+) state within the hemoglobin molecule. This process causes a leftward shift in the hemoglobin-oxygen dissociation curve and decreases hemoglobin’s oxygen carrying capacity. Patients less than 6 months old have a relative deficiency of methemoglobin reductase and may be more susceptible to toxicity.5
Prilocaine is an amide compound that has been shown to cause methemoglobinemia as its primary toxicity in overdose.5 Both prilocaine and lidocaine are components in EMLA cream, so this particular cream can cause either CNS toxicity or methemoglobinemia in overdose.
A literature review found published cases of seizures resulting after single ingestions of 5 to 25 mL of viscous lidocaine by children 2 years of age or younger.5 Although dibucaine is less commonly prescribed than lidocaine, it is 10 times more potent, and ingestion of 2 to 3 teaspoons has caused death secondary to cardiopulmonary arrest.6 Published reports of benzocaine-induced toxicity vary; cyanosis secondary to methemoglobinemia may result from oral doses in the range of 15 to 40 mg/kg, although the development of methemoglobinemia may be idiosyncratic, rather than dose related.
Caustics
Many household products are caustic agents and can cause significant toxicity with small exposures. Caustic agents are classified as alkaline or acid corrosives, depending on their pH (Table 158.4). Passed in 1970, the Federal Hazardous Substances Act and the Poison Prevention Packaging Act stated that caustic agents with a concentration higher than 10% must be placed in child-resistant containers. By 1973, the household product concentration limit had been lowered to 2%.
| PRODUCT | CAUSTIC INGREDIENT(S) |
|---|---|
| Alkaline Corrosives | |
| Drain cleaners | Sodium hydroxide (lye) |
| Oven cleaners | Sodium hydroxide |
| Hair relaxers | Sodium hydroxide |
| Automatic dishwasher detergents | Sodium tripolyphosphate |
| Sodium metasilicate | |
| Household ammonia cleaning solutions (glass cleaners, antirust products, floor strippers, toilet bowl cleaners, wax removers) | Ammonium hydroxide |
| Acidic Corrosives | |
| Drain cleaners | Sulfuric acid |
| Rust removers | Hydrofluoric acid |
| Oxalic acid | |
| Toilet bowl cleaners | Hydrochloric acid |
| Sulfuric acid | |
| Phosphoric acid | |
| Tire cleaning agent | Ammonium bifluoride |
Hydrofluoric Acid
Hydrofluoric acid and related compounds, such as ammonium bifluoride, ammonium fluoride, potassium bifluoride, and sodium bifluoride, are found in rust removers, automobile wheel cleaners, toilet bowl cleaners, air conditioner coil cleaners, dentifrices, and insecticides. The bifluorides can form hydrofluoric acid in the presence of body water and can thus cause delayed presentation of symptoms.7 The fluoride ion is directly toxic to numerous cellular enzymes and also forms complexes with calcium and magnesium. These complexes precipitate in tissues and cause significant pain and tissue destruction. At high enough levels of fluoride, these complexes can lead to systemic depletion of calcium and magnesium that can precipitate life-threatening arrhythmias.
Calcium Channel Blockers
Calcium channel blocker overdose causes severe hypotension and bradycardia, although reflex tachycardia may also be seen in overdose of the vascular tone–predominant dihydropyridines. Calcium channel blockade is responsible for dysrhythmias ranging from heart block to idioventricular arrhythmias. Impaired insulin release and systemic insulin resistance lead to the classic finding of hyperglycemia. Pulmonary edema may occur; the mechanism is unknown, but the edema may be caused by selective precapillary vasodilation or aggressive fluid resuscitation.8
Data on the minimum dose required to produce significant toxicity in children are mixed, but published cases have reported that one to two pills caused significant morbidity or death in children less than 6 years of age.9
Calcium channel blocker overdose and beta-blocker overdose are commonly discussed together in the adult literature because of their similarity of presentation and the therapeutic coadministration of these medications. A review of the pediatric literature found no published cases of death in young children as a result of accidental ingestion of beta-blockers, so these agents do not appear on the “One Pill Can Kill” list.10
Clonidine and Topical Imidazolines
The exact mechanism of clonidine toxicity is not fully understood. Because of functional overlap between α2 and µ receptors, symptoms of clonidine toxicity can mimic those of opioid toxicity. Peripheral α1-receptor stimulation may cause a transient period of hypertension before centrally mediated bradycardia and hypotension predominate. In contrast to clonidine, the topical imidazolines are specifically designed to induce local α2-mediated peripheral vasoconstriction for their desired clinical effect. Toxicity from these agents may manifest as agitation, tachycardia, and hypertension, although CNS and respiratory depression similar to that seen with clonidine toxicity have also been reported with these agents.11,12
A minimum toxic dose of imidazoline has not been established. A clonidine level as low as 0.01 mg/kg has been shown in case reports to cause altered mental status in children, which correlates with ingestion of a single 0.1-mg tablet by a 10-kg child. Larger doses have been associated with more severe symptoms.12
Diphenoxylate-Atropine (Lomotil)
Lomotil has classically been included on most “One Pill Can Kill” lists, but one review of the literature revealed a paucity of data demonstrating that significant morbidity is caused by small doses.13 Most deaths are attributed to ingestion of multiple pills or repetitive, incorrect dosing over time. No established minimum toxic dose exists, but the review found no reports of significant toxicity with ingestions of fewer than six to eight tablets in small children.
Sulfonylureas
Death is rare with sulfonylurea exposure. Only one case was found in a literature review.14 However, many children less than 6 years old become symptomatic from sulfonylurea ingestion, and numerous case reports have noted symptoms in children who ingested of one to two pills.
Tricyclic Antidepressants
Several case reports published since the 1970s demonstrated that one or two pills can kill a small child. Significant morbidity or mortality tends to occur at doses greater than 15 to 30 mg/kg; ingestion of one or two 150-mg pills by a 10-kg toddler can easily reach this level.15
Presenting Signs and Symptoms
Camphor
Camphor toxicity most often develops 5 to 90 minutes from the time of ingestion, but some case reports have noted presentations as late as 9 hours after ingestion.16 Clinical toxicity may first manifest with gastrointestinal complaints, from oral burning to nausea and vomiting. Initial CNS effects of hyperactivity such as irritability, hyperreflexia, and myoclonic jerking may progress to seizure, delirium, and coma. Seizures are common and may persist for up to 24 hours, although this does not seem to have prognostic implications.2 When death occurs, it is usually the result of status epilepticus or respiratory failure.
Salicylates
Symptoms of oil of wintergreen ingestion may progress much faster than symptoms caused by other agents because of the high concentrations of salicylate. One review found that patients who progressed to life-threatening toxicity from oil of wintergreen ingestion almost uniformly experienced emesis within minutes to a few hours of ingestion, followed by signs of CNS toxicity.4
Topical Anesthetics
Lidocaine or dibucaine overdose manifests with signs and symptoms of CNS toxicity that progress from agitation, confusion, or ataxia to generalized seizures and coma. Cardiac toxicity, such as heart block or arrhythmia, is infrequently seen with ingestion other than dibucaine and usually occurs only after CNS toxicity has manifested. The onset of symptoms has been reported to occur any time from minutes to hours following exposure, with significantly faster onset if the exposure involves aspiration or the respiratory tract.5
Caustics
The severity and rapidity of symptom development are related to the type of agent, concentration, volume, viscosity, duration of contact, and pH. The emergency physician must keep in mind that an absence of oropharyngeal burns does not exclude esophageal injury.17
Hydrofluoric Acid
Local apparent injury may be minimal because of a paucity of free hydrogen ions, which are responsible for the caustic tissue injuries caused by other acids. A hallmark sign of dermal exposure to hydrofluoric acid is pain out of proportion to physical findings. Evidence of ingestion commonly manifests with gastrointestinal symptoms of nausea, vomiting, and abdominal pain. Respiratory symptoms ranging from dyspnea to acute respiratory distress syndrome can indicate direct inhalational injury, increased secretions caused by fluoride’s effect on acetylcholinesterase, or muscle fatigue.7
Calcium Channel Blockers
Bradycardia and hypotension are the hallmarks of calcium channel blocker overdose. Patients may exhibit sequelae of hypoperfusion and shock, including mental status changes, lactic acidosis, renal or hepatic failure, and intestinal ischemia. Patients may have cardiac arrhythmias including second- and third-degree heart block. Seizures rarely occur in the absence of cardiovascular collapse because these agents, with the exception of nimodipine, do not cross the blood-brain barrier. CNS manifestations out of proportion to the cardiovascular effects should raise the suspicion of coingestions.11 Hyperglycemia is a hallmark of calcium channel blocker toxicity.
Clonidine and Topical Imidazolines
Clonidine toxicity often resembles opioid intoxication, with symptoms consisting of miosis, bradycardia, hypotension, and, in severe cases, respiratory depression and hypothermia. CNS depression is a common symptom. Unlike with opioid intoxication, patients are often rousable by stimulation, but they may quickly return to a depressed mental state.12,18 Toxicity from topical imidazolines also manifests most commonly with altered mental status and respiratory depression.12 Both topical imidazolines and clonidine may cause tachycardia or bradycardia and hypertension or hypotension.
Diphenoxylate-Atropine (Lomotil)
The classic teaching regarding Lomotil toxicity is that it exhibits a biphasic course: an early, anticholinergic-predominant phase lasting 2 to 3 hours followed by a prolonged, opioid-predominant phase. This notion was challenged by a review in 1991 that suggested that most patients exhibited a prolonged opioid toxidrome, and only 11% presented with a biphasic course.19 In serious intoxications, patients may exhibit CNS excitation, coma, or profound respiratory depression.
Sulfonylureas
The clinical presentation of sulfonylurea toxicity is that of hypoglycemia: lethargy, confusion, headache, seizures, tachycardia, and diaphoresis. If untreated, hypoglycemia can result in permanent neurologic sequelae or death. The onset of symptoms is usually seen within 8 hours of ingestion, but it can be delayed with ingestion of extended-release formulations. Chlorpropamide, glyburide, and the long-acting form of glipizide tend to have a longer clinical effect. Delayed presentation also seems to correlate with patients in whom a prophylactic dextrose drip is started; this practice may mask emerging hypoglycemia and create a false sense of security.14
Tricyclic Antidepressants
The TCA-poisoned pediatric patient presents similarly to the adult patient, with an anticholinergic syndrome of delirium, mydriasis, flushing, tachycardia, dry mucous membranes, or hyperthermia. Hypotension and a widened QRS complex are concerning findings. Pediatric patients exhibit electrocardiographic (ECG) changes similar to those seen adult patients, but no correlation between ECG findings and prognosis has been established.15,18 As with adults, seizure is an ominous sign of potential impending circulatory collapse. When death occurs, it is usually the result of cardiac arrest.
Treatment
Prehospital
The administration of syrup of ipecac at home is not recommended. A 2004 position paper from the American Academy of Clinical Toxicology (AACT) and the European Association of Poisons Centres and Clinical Toxicologists (EAPCCT) stated that data were insufficient to support or refute the use of syrup of ipecac in the prehospital setting.20 In highly toxic ingestions by patients in remote locations (i.e., when hospital evaluation will be delayed by hours), ipecac may have a limited role in the prehospital setting if it is administered very early.21
Hospital
Gastric decontamination in toxic ingestions has historically been a controversial topic and poses unique problems in the pediatric patient. The AACT and EAPCCT’s joint position on the routine use of gastric lavage states that the weight of evidence does not demonstrate a beneficial clinical effect and that the risk of adverse outcomes such as aspiration, laryngospasm, or perforation outweighs the questionable benefits.22 An additional issue concerning lavage in the pediatric patient is that smaller children may not easily accommodate the 36 to 40 French catheter often needed to remove particulate material effectively.
Single-dose activated charcoal is similarly not recommended for routine use by the AACT and EAPCCT but may be considered in specific cases.23 Risks associated with charcoal administration include vomiting and aspiration, decreased effectiveness of oral antidotes, and an obscured view of the oropharynx should intubation or endoscopy become necessary.21 Data from volunteers implied that the reduction of toxin absorption may not be clinically significant if activated charcoal is administered more than 1 hour following ingestion, but a potential benefit after 1 hour cannot be excluded.23 Charcoal use is contraindicated in a patient without an intact or protected airway. Convincing a toddler to drink a gritty slurry may prove extremely difficult, and the distress caused by forcibly administering this therapy must be weighed against the benefits on a case-by-case basis.
Whole-bowel irrigation is not routinely recommended but should be considered in a patients who has ingested sustained-release or enteric-coated tablets, particularly if the patient presents more than 2 hours following ingestion.24 This procedure is contraindicated in cases of ileus, bowel obstruction or perforation, hemodynamic instability, or compromised airway.
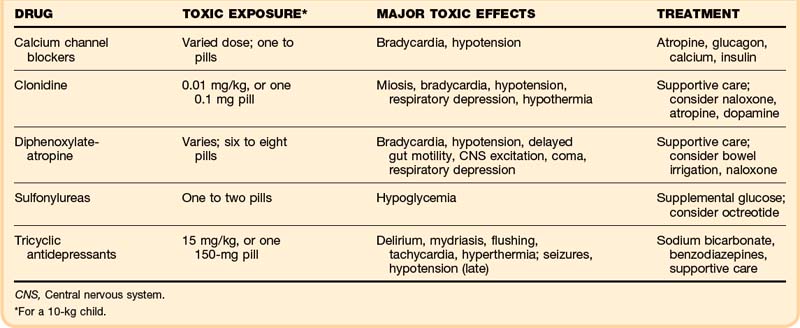
Agents with specific treatment antidotes or guidelines are discussed next (Table 158.5).
Salicylates
Depending on the clinical symptoms, blood salicylate concentration, and evidence of organ toxicity, urine and serum alkalinization or hemodialysis should be initiated following the same guidelines as for adult patients (see Chapter 144). Salicylate ingestion is a situation for which activated charcoal administration should be considered, given the high potential for significant morbidity.
Caustics
The administration of corticosteroids in the acute management of caustic ingestion is controversial, and the current evidence does not support use of these agents in preventing stricture formation.25
Calcium Channel Blockers
Calcium channel blocker overdoses in the pediatric patient should be managed aggressively following the same algorithm as for adult ingestions, with the use of atropine, calcium, glucagon, vasopressors, or hyperinsulinemia-euglycemic therapy, as warranted (see Chapter 148). Because of the danger of delayed and prolonged presentation, activated charcoal administration or whole-bowel irrigation should be considered in these patients if ingestion of extended-release formulations is suspected.
Clonidine
Naloxone has been shown in some case reports to reverse both cardiovascular and respiratory depression caused by clonidine ingestion, but this is not well studied, and the use of naloxone is recommended only in severe cases or when intubation is imminent.18 Atropine may be used to treat refractory bradycardia, and refractory hypotension may be treated with vasopressors; dopamine is the agent most commonly studied.
Tricyclic Antidepressants
Pediatric TCA overdoses should be managed aggressively according to the same algorithm that is applied to adults: alkalinization with sodium bicarbonate, benzodiazepines for seizures, and supportive care (see Chapter 147). Given the lethality of a large enough dose and the concern for delayed gastric emptying secondary to anticholinergic effects, activated charcoal should be considered in the first 1 to 2 hours of a known exposure.
Follow-Up and Next Steps in Care
Observation and Admission Versus Discharge
Children with suspected salicylate exposure who are found to have a positive blood level require hospital admission with levels rechecked every 2 hours until they are clinically improving, have a decreasing and nontoxic salicylate level, and have an alkalemic blood pH.11
![]() Documentation
Documentation
Patient Instructions
• Document a discussion with the patient or parent regarding the diagnosis, warning signs, what to do, follow-up, and when to return.
• With pediatric accidental ingestions, document poison prevention counseling.
• Families should be given the phone number for the poison control center to address further concerns (1-800-222-1222).
• Whenever concern exists about the situation surrounding the ingestion or when an ingestion has occurred in an infant younger than 12 months, social services should be contacted.
Greene S, Harris C, Singer J. Gastrointestinal decontamination of the poisoned patient. Pediatr Emerg Care. 2008;24:176–186.
Henry K, Harris CR. Deadly ingestions. Pediatr Clin North Am. 2006;53:293–315.
Michael JB, Sztajnkrycer MD. Deadly pediatric poisons: nine common agents that kill at low doses. Emerg Med Clin North Am. 2004;22:1019–1050.
1 Bronstein AC, Spyker DA, Cantilena LR, Jr., et al. 2009 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 27th annual report. Clin Toxicol (Phila). 2010;48:979–1178.
2 Love JN, Sammon M, Smereck J. Are one or two dangerous? Camphor exposure in toddlers. J Emerg Med. 2004;27:49–54.
3 Khine H, Weiss D, Graber N, et al. A cluster of children with seizures caused by camphor poisoning. Pediatrics. 2009;123:1269–1272.
4 Davis JE. Are one or two dangerous? Methyl salicylate exposure in toddlers. J Emerg Med. 2007;32:63–69.
5 Curtis LA, Dolan TS, Seibert HE. Are one or two dangerous? Lidocaine and topical anesthetic exposures in children. J Emerg Med. 2009;37:32–39.
6 Dayan PS, Litovitz TL, Crouch BI, et al. Fatal accidental dibucaine poisoning in children. Ann Emerg Med. 1996;28:442–445.
7 Perry HE. Pediatric poisonings from household products: hydrofluoric acid and methacrylic acid. Curr Opin Pediatr. 2001;13:157–161.
8 Arroyo AM, Kao LW. Calcium channel blocker toxicity. Pediatr Emerg Care. 2009;25:532–538.
9 Ranniger C, Roche C. Are one or two dangerous? Calcium channel blocker exposure in toddlers. J Emerg Med. 2007;33:145–154.
10 Love JN, Sikka N. Are 1–2 tablets dangerous? Beta-blocker exposure in toddlers. J Emerg Med. 2004;26:309–314.
11 Michael JB, Sztajnkrycer MD. Deadly pediatric poisons: nine common agents that kill at low doses. Emerg Med Clin North Am. 2004;22:1019–1050.
12 Eddy O, Howell JM. Are one or two dangerous? Clonidine and topical imidazolines exposure in toddlers. J Emerg Med. 2003;25:297–302.
13 Thomas TJ, Pauze D, Love JN. Are one or two dangerous? Diphenoxylate-atropine exposure in toddlers. J Emerg Med. 2008;34:71–75.
14 Little GL, Boniface KS. Are one or two dangerous? Sulfonylurea exposure in toddlers. J Emerg Med. 2005;28:305–310.
15 Rosenbaum TG, Kou M. Are one or two dangerous? Tricyclic antidepressant exposure in toddlers. J Emerg Med. 2005;28:169–174.
16 Ruha AM, Graeme KA, Field A. Late seizure following ingestion of Vicks VapoRub. Acad Emerg Med. 2003;10:691.
17 Riffat F, Cheng A. Pediatric caustic ingestion: 50 consecutive cases and a review of the literature. Dis Esophagus. 2009;22:89–94.
18 Henry K, Harris CR. Deadly ingestions. Pediatr Clin North Am. 2006;53:293–315.
19 McCarron MM, Challoner KR, Thompson GA. Diphenoxylate-atropine (Lomotil) overdose in children: an update (report of eight cases and review of the literature). Pediatrics. 1991;87:694–700.
20 American Academy of Clinical Toxicology, European Association of Poisons Centres and Clinical Toxicologists. Position paper: ipecac syrup. J Toxicol Clin Toxicol. 2004;42:133–143.
21 Greene S, Harris C, Singer J. Gastrointestinal decontamination of the poisoned patient. Pediatr Emerg Care. 2008;24:176–186.
22 Vale JA, Kulig K, American Academy of Clinical ToxicologyEuropean Association of Poisons Centres and Clinical Toxicologists. Position paper: gastric lavage. J Toxicol Clin Toxicol. 2004;42:933–943.
23 Chyka PA, Seger D, Krenzelok EP, Vale JA. American Academy of Clinical Toxicology and European Association of Poisons Centres and Clinical Toxicologists. Position paper: single-dose activated charcoal. Clin Toxicol (Phila). 2005;43:61–87.
24 American Academy of Clinical Toxicology, European Association of Poisons Centres and Clinical Toxicologists. Position paper: whole bowel irrigation. J Toxicol Clin Toxicol. 2004;42:843–854.
25 Peclova D, Navratil T. Do corticosteroids prevent oesophageal stricture after corrosive ingestion? Toxicol Rev. 2005;24:125–129.