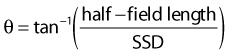53 Pediatric Central Nervous System Tumors
Epidemiology and Etiology
Pediatric central nervous system (CNS) tumors account for nearly 20% of all childhood neoplasms, exceeded in incidence only by leukemia, and are the leading cause of cancer death in childhood. Incidence is about 1 per 30,000 children each year.1 Although roughly two thirds of all patients are alive 5 years after diagnosis, survivors suffer from neurologic, cognitive, psychologic, and endocrinologic sequelae of both the disease and its treatment. Boys and girls are equally affected, except for medulloblastoma, germ cell tumors, and ependymoma, in which more boys are affected.2–4 Tumors can rarely appear in association with genetic syndromes (Table 53-1), but more often appear to arise spontaneously. The tumors are sometimes found to harbor somatic genetic changes, such as mutations in single genes in atypical teratoid rhabdoid tumor (ATRT; INI1) or high-grade astrocytoma (p53), aberrant gene expression pathways in medulloblastoma (SHH, Wnt, and Notch), and chromosomal losses or gains in high-grade astrocytoma (10), medulloblastoma (17p, 8q24), ependymoma (22), and germ cell tumors (12p).5 Some tumors, do not have a consistent genetic or chromosomal abnormality. Environmental exposures, aside from radiation, have been difficult to associate consistently with development of any CNS tumor. High-dose ionizing radiation clearly increases the risk of developing a meningioma or malignant glioma.6
Table 53-1 Syndromes Associated With Pediatric Central Nervous System Tumors
| Syndrome | Associated CNS Tumors |
|---|---|
| Basal cell nevus (Gorlin) | Medulloblastoma |
| Cowden | Dysplastic gangliocytoma of the cerebellum (Lhermitte-Duclos) |
| Li-Fraumeni | Astrocytomas, medulloblastoma, choroid plexus tumors |
| Neurofibromatosis 1 | Pilocytic astrocytoma, malignant peripheral nerve sheath tumor |
| Neurofibromatosis 2 | Schwannoma, meningioma, ependymoma |
| Rhabdoid tumor predisposition | Atypical teratoid rhabdoid tumor |
| Tuberous sclerosis | Subependymal giant cell astrocytoma |
| Turcot | Medulloblastoma, glioblastoma |
| von Hippel-Lindau | Hemangioblastoma |
CNS, Central nervous system.
Anatomy
In contrast to adults, the majority of pediatric CNS tumors are infratentorial (i.e., cerebellum, brain stem, and fourth ventricle) and primary in origin. The location of the tumor may be related to cause; for example, germ cell tumors are likely to develop in the midline periventricular region after abnormal migration of stem cells, and medulloblastoma may arise in the cerebellar external granule cell layer. Tumor location also dictates presenting symptoms (see Clinical Presentations, in the following text). For example, intrasellar or parasellar lesions may produce hormonal and visual abnormalities. Anatomy likewise plays a key role in determination of treatment and prognosis. For instance, pilocytic astrocytomas of the cerebrum or cerebellum are often completely resected and fully cured, whereas those in the diencephalon or brainstem can less easily be resected and require radiotherapy or chemotherapy. Patients with ependymoma, or embryonal and germ cell tumors involving the ventricles, may require more extensive postoperative irradiation as the circulation of the cerebrospinal fluid (CSF) is an important route for tumor dissemination.
Pathologic Conditions
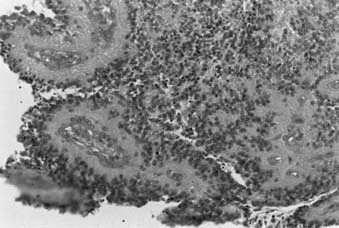
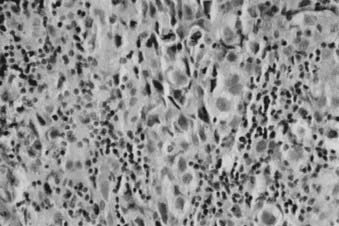
Whereas most adult primary brain tumors are high-grade astrocytomas, the heterogeneity of histologic diagnoses in children is striking. Photomicrographs of the most common tumors, pilocytic astrocytoma, medulloblastoma, ependymoma, and germinoma, are shown in Fig. 53-1 through Fig. 53-4. Historically, brain tumors have been identified by correlative normal cells of putative origin: astrocytes, neurons, and oligodendroglia. However, biologic proof of such derivation is wanting.7 There is only minimal correlation between the incidence of particular histologic types of cancer in the brain and the absolute number of potential precursor cells in that region.8
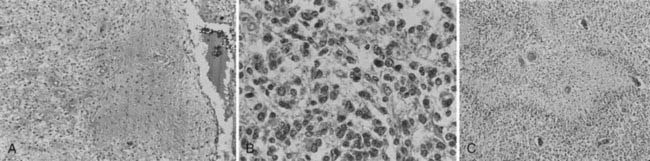
FIGURE 53-1 • Astrocytoma. A, Grade I; B, grade II; C, grade III.
(Courtesy Dr. Richard Davis, University of California–San Francisco.)
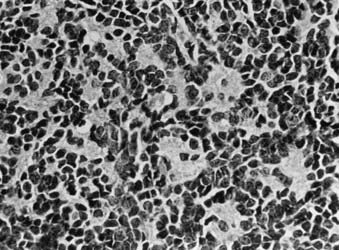
FIGURE 53-3 • Medulloblastoma: Homer-Wright rosettes.
(Courtesy Dr. Richard Davis, University of California–San Francisco.)
At present, the most recent classification by the World Health Organization (WHO) sorts CNS tumors as follows: tumors of neuroepithelial tissue, tumors of cranial and paraspinal nerves, tumors of the meninges, tumors of the sellar region, lymphomas and hematopoietic neoplasms, germ cell tumors, or metastatic tumors.5 Neuroepithelial tumors can be subtyped further into astrocytic, oligodendroglial, oligoastrocytic, ependymal, choroid plexus, neuronal, pineal, or embryonal tumors. Although the majority of pediatric brain tumors are reported to be glial in origin (e.g., pilocytic and diffuse astrocytomas), children also display a diversity of tumors seldom or never seen in adults: medulloblastoma, choroid plexus papilloma and carcinoma, ganglioglioma, astroblastoma, pleomorphic xanthoastrocytoma (PXA), and dysembryoplastic neuroepithelial tumor (DNT). Previously, medulloblastoma and other embryonal neoplasms were all lumped together as primitive neuroectodermal tumors (PNETs), but now are known to be biologically distinct as medulloblastoma, atypical teratoid rhabdoid tumor, pineoblastoma, ependymoblastoma, and other supratentorial PNETs.9 Certain adult tumors, such as oligodendroglioma, oligoastrocytoma, and well-differentiated astrocytoma, are relatively uncommon in children, and such diagnoses should be questioned for accuracy. For example, DNT is commonly mistaken as oligodendroglioma and PXA sometimes misinterpreted as glioblastoma.10 Calcification and cyst formation are representative of a chronic course and typically indicative of more indolent tumors, such as craniopharyngioma, epidermoid tumor, ganglioglioma, and pilocytic astrocytoma. For the pediatric patient with a CNS tumor, consultation with a neuropathologist skilled in the range of childhood brain tumor diagnoses is highly warranted, especially because diagnosis determines treatment planning.
Tumor grade less often determines treatment and outcome, compared with adults. Although the WHO employs a traditional I to IV scale of increasing anaplasia, some tumors have no variability in grade, or, for some, tumor grade may not distinguish outcome or be mistakenly reassuring of outcome. All medulloblastomas are recorded as grade IV, although there is now a movement to subclassify these neoplasms based on anaplasia, desmoplasia, and nodularity.10 Ependymomas are graded only as well-differentiated (WHO grade II) or anaplastic (WHO grade III), although numerous studies have failed to show a difference in outcome.11 All pilocytic astrocytomas are graded as I. Nevertheless, suprasellar and other midline pilocytic tumors often require chemotherapy or irradiation and result sometimes in death, unlike the grade I cerebral and cerebellar gangliogliomas and pilocytic astrocytomas cured simply by surgical removal. The majority of astrocytomas in children are pilocytic astrocytomas (WHO grade I) and do not undergo anaplastic transformation, in distinction to diffuse astrocytomas (well-differentiated astrocytoma [WHO II], anaplastic astrocytoma [WHO III], and glioblastoma multiforme [WHO IV]), more commonly seen in adults. Recently the diagnosis of pilomyxoid astrocytoma has been adopted by the WHO as a grade II, more aggressive variant of pilocytic astrocytoma.5
Clinical Presentations
The presenting symptoms and signs of intracranial lesions in the developing child vary, depending on tumor location, grade or growth rate, and age at presentation (Table 53-2). These factors also influence the time to diagnosis. A long prodrome over months to years often indicates a more favorable histology and grade, whereas a short prodrome of just weeks or months may indicate an aggressive neoplasm. Symptoms and signs may be generalized (e.g., developmental delay, irritability, growth failure), or secondary to an increase in intracranial pressure (e.g., headache, vomiting, papilledema). If the sutures have not yet fused, toddlers often manifest increased intracranial pressure chronically by macrocephaly with an increased head circumference, and sometimes split cranial sutures or tense, open anterior fontanelle.
Table 53-2 Frequent Symptoms and Signs of Pediatric Central Nervous System Tumors
| Infants | Children |
|---|---|
| Failure to thrive | Headaches |
| Bulging fontanelles | Nausea and vomiting |
| Arrested development | Lethargy |
| Head nodding | Ataxia |
| Nystagmus | Cranial nerve palsies |
| Seizures | |
| Visual field abnormalities |
Routes of Spread
Childhood astrocytomas, other gliomas, and a number of other brain tumors generally do not relapse or recur far from the primary lesion. Unfortunately, as vital structures are compressed within the confines of the skull, it is destruction of the primary organ, rather than metastasis, that results in great morbidity and mortality. Malignancies such as medulloblastomas, ATRT, pineoblastoma, supratentorial PNET, germ cell tumors, and sometimes ependymoma can spread through the ventricular system and metastasize along the meninges or spine. It is particularly common for medulloblastoma to metastasize beyond the posterior fossa to involve the meninges or parenchyma of the spine or cerebrum. Although brain tumors can metastasize outside the CNS, this is currently thought to be a rare occurrence, and the documentation of such events in earlier reports may have been the result of tumor cells that spread through unfiltered shunts during an era when chemotherapy was seldom used for brain tumors.12 Hematogenous and lymphatic tumor spread are not relevant in brain tumors.
Diagnostic and Staging Studies
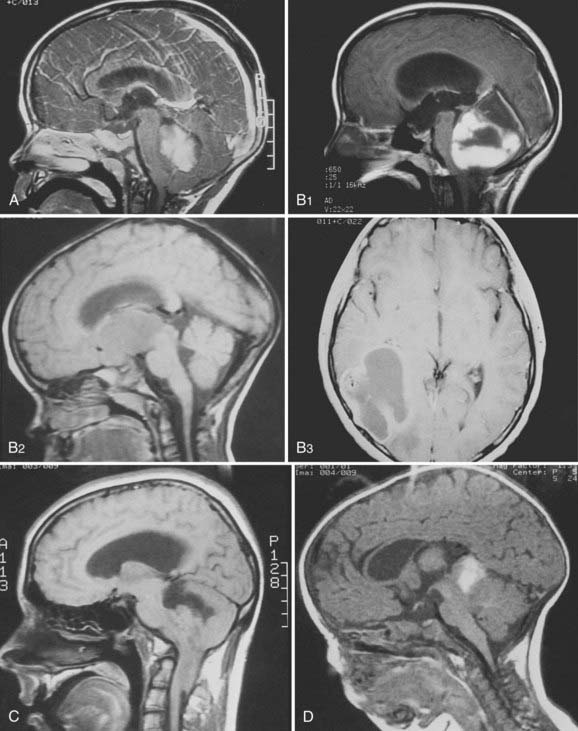
Dramatic improvement in neuroimaging during the past 2 decades have resulted in major advances in diagnosis and targeting of therapy for lesions of the CNS. Young children often require sedation or general anesthesia for any study, but magnetic resonance imaging (MRI) yields the best definition of parenchymal lesions, as well as clear views of the posterior and middle cranial fossa. MRI has replaced myelography for evaluation of the spine, and is therefore the modality of choice for evaluation of the craniospinal axis. Preoperative MRI with gadolinium contrast should include images of the brain, and when there is a posterior fossa tumor or a supratentorial tumor suspected to be embryonal or germ cell, the entire neuraxis should be imaged. Postoperative MRI scan with gadolinium contrast needs to be performed within 48 hours of surgery to evaluate the extent of resection, prior to the onset of postoperative edema and potentially artifactual enhancement. All these MRI data are of critical importance for planning further treatment and for defining the gross tumor volume (GTV) and the clinical treatment volume. Following radiotherapy, the results of treatment are followed by routine interval MRI scans, and in cases of ambiguous findings, MRI techniques such as spectroscopy and diffusion-weighted imaging, or positron emission tomography (PET), may be used to distinguish between recurrent tumor and treatment effects, such as radiation necrosis. Figure 53-5 demonstrates the radiographic appearance of the common tumors by MRI.
Staging System
In contrast to staging systems for other types of cancer, CNS tumors are staged mostly by extent of metastasis using the M scoring of the Chang staging system (Table 53-3), with some consideration of extent of resection.13 Tumor extent at diagnosis by the Chang T score is no longer used. Indeed, T3B brainstem invasion in medulloblastoma was once considered to place a patient at higher risk of recurrence, but has now been shown not to be predictive.14 At present, medulloblastoma in children ages 3 and older is risk-stratified as average- and high-risk. Less than total resection (>1.5 cm2 residual), any metastasis (M+), or severe anaplasia on the histologic specimen place a child into high-risk status. Only complete resection, no metastasis, and absence of diffuse anaplasia are consistent with average risk. For other pediatric CNS tumors, histologic type and M-stage largely indicate risk and dictate dosage and volume of radiotherapy, whereas extent of removal merits therapy based only on the histologic type.
| T1 | Tumor <3 cm |
| T2 | Tumor >3 cm |
| T3A | Extension into the aqueduct of foramen |
| T3B | Invasion of brain stem |
| T4 | Midbrain, third ventricle, or upper cord involved |
| M0 | No metastasis |
| M1 | Microscopic cerebrospinal fluid involvement |
| M2 | Gross seeding of third ventricle |
| M3 | Gross seeding of cord |
| M4 | Extracentral nervous system metastasis |
Standard Therapeutic Approaches
The benefit of performing maximal debulking of the tumor mass safely should be sought, because it improves prognosis and limits adjuvant therapy.15 Only for germ cell tumors has extent of removal been shown not to influence outcome.16 In some instances, surgical resection or biopsy is not performed, namely some optic pathway gliomas (i.e., pilocytic astrocytomas), diffuse intrinsic pontine gliomas, and some germ cell tumors diagnosed by tumor markers. Diffuse intrinsic pontine gliomas are often diagnosed simply by radiographic appearance on MRI of a ventral pontine tumor engulfing the basilar artery in a child with strabismus, ataxia, and weakness over just weeks or several months. Elevated serum or CSF alpha-fetoprotein in the setting of a sellar or pineal tumor is considered diagnostic of an NGGCT, and some individuals consider an elevated serum or CSF beta-human chorionic gonadotropin of less than 50 IU/L without elevation of alpha-fetoprotein diagnostic of germinoma. Except in very young children, incomplete surgical resection of most high-grade malignancies requires postoperative radiotherapy.
Radiotherapy is a key component of treatment for most pediatric CNS tumors. Although planning techniques and the ability to localize tumor volumes have improved significantly in recent years, it is difficult to correlate this progress with improvements in survival. Likewise, it has been difficult to prove any benefit from altered fractionation schemes that have come from radiobiologic studies. In contrast, recent studies have suggested that sophisticated treatment planning and sparing of normal tissues have perhaps mitigated neurocognitive sequelae associated with radiation therapy to the young brain (see Toxicity of Radiation Therapy, in the following text).
Interstitial brachytherapy allows for the delivery of high doses of radiation to a tumor region. One retrospective study reports the largest series of pediatric brain tumors treated with iodine-125 (I-125) brachytherapy.17 Twenty-eight children were treated with temporary, high-activity 125I brachytherapy for recurrent or persistent supratentorial tumors. The study is most useful in its documentation of acute and late toxicities. No grade 3 or grade 4 acute or late toxicities occurred. However, 22 patients (79% of 28 total patients) required at least one repeat operation following brachytherapy, and 17 of these 22 patients had evidence of necrosis in the resected specimen. Furthermore, little improvement was found compared with conventional methods.
To date, outcome data with use of proton beam radiotherapy for CNS tumors is limited, and advocated mostly on theoretical grounds. Merchant and colleagues compared models of photon and proton dose characteristics and their predicted relationship to cognitive function in children treated for brain tumors.18 They found that small critical normal structures such as the cochlea and hypothalamus, which were anatomically separated from the PTV, received substantially less radiation using protons compared with photons. Proton radiotherapy would therefore be expected to reduce the risks of endocrine deficits and hearing loss. In addition, protons decreased the low (0-20 Gy) and intermediate (20-40 Gy) doses to the cerebrum in patients receiving focal radiation. Using longitudinal models of radiation dose-cognitive effects, their data indicate that proton radiotherapy would mitigate intellectual decline from radiation treatment.
The benefit of proton beam radiotherapy in children may be best exemplified by its use for craniospinal irradiation (CSI). Comparisons of proton beam, conventional 3-D radiation, and IMRT for treatment of the posterior fossa and spinal column suggest superior sparing of normal structures by protons. In particular, protons are likely to mitigate long-term toxicities related to hearing, endocrine, and cardiac functions. For example, 90% of the cochlea received 101.2% of posterior fossa boost dose with conventional radiation techniques, 33.4% with IMRT, and only 2.4% with protons. Similarly, 50% of the heart received 72.2%, 29.5%, and 0.5% of the posterior fossa boost dose for conventional x-ray, IMRT, and protons, respectively.19
Toxicity of Radiation Therapy
A wide range of potential toxicities complicates the implementation of radiation therapy in the treatment of tumors of the craniospinal axis. These toxicities can be severe and debilitating, particularly in pediatric patients. Care should be taken to minimize these effects. Treatment of intracranial tumors can result in damage to the eye, ear structures, brain, and hypothalamic-pituitary axis, as well as growth abnormalities. Treatment of the spine can result in growth deficits and damage to the spinal cord. Specific potential acute side effects of radiation to the CNS include epilation, skin reactions, otitis, hematopoietic depression, and somnolence. Specific late toxicities of such radiation include radionecrosis, myelopathy, leukoencephalopathy, vascular injury, neuropsychologic sequelae, endocrine dysfunction, bone and tooth abnormalities, ocular complications, ototoxicity, and induction of second primary tumors. Table 53-4 delineates the radiation doses associated with late toxicities that may result from radiation therapy to the CNS.
Table 53-4 Late Toxicities of Central Nervous System Irradiation
| Structure | Late Effect | Threshold Dose |
|---|---|---|
| Spinal cord | Chronic progressive myelitis | 45 Gy |
| Brain | Radiation necrosis Intellectual deficits |
54 Gy 12-18 Gy |
| Lens of eye | Cataract formation | 8 Gy |
| Retina | Radiation retinopathy | 45 Gy |
| Optic nerve | Optic neuritis | 50 Gy |
| Inner ear | Sensorineural hearing loss | 40-50 Gy |
Spinal Cord
Radiation therapy can result in severe damage to the spinal cord, with functional transection of the cord at the affected level representing the most dire of such toxicities. Radiation damage takes the form of chronic progressive myelitis. The 5% incidence of radiation myelopathy probably lies between 57 Gy and 61 Gy in the absence of chemotherapy, using standard fractionation. At doses less than 45 Gy the risk of myelitis is less than 0.2%. At 73 Gy the risk of myelitis approaches 50%.20,21 A number of reports have indicated that tolerance of the cervical spinal cord to radiation toxicity is somewhat higher than 45 Gy in adults. However, it is unclear what the cervical spinal cord tolerance is in pediatric patients.
Brain
Acute reactions during radiation therapy, thought to result from disruption of the blood-brain barrier, are uncommon. However, there are reports of edema following single conventional fractions.22 Clinically, apparent acute changes are more common with hypofractionated doses, such as are used in radiosurgery.23 Subacute reactions are more common and are thought to be due to transient demyelination.24 These effects typically occur within the first few months following radiation and usually resolve within 6 to 9 months. Cranial nerve palsies have also been reported.25 Severe subacute effects such as rapidly progressive ataxia are rarer and are generally associated with fractions larger than 2.0 Gy and total doses larger than 50 Gy.26
The late effects of radiation are primarily due to radiation necrosis. Symptoms are related to the neuroanatomic location of necrosis (sensory, motor, speech/receptive deficits, seizures) and may, in addition, be due to increased intracranial pressure. Focal necrosis is uncommon with doses less than 60 Gy given with conventional fractionation.27
Large-volume radiation therapy can result in diffuse white matter changes. Clinically, these can result in lassitude, personality change, or neurocognitive deficits. Multiple studies have examined the effect of whole-brain radiation therapy on intellect in patients treated for leukemia. Radiation-associated depression in intelligence quotient (IQ) has been noted by a number of authors.28,29 Halberg and colleagues30 compared three groups of patients. The first group received 18 Gy (1.8 Gy per daily fraction) cranial irradiation, and the second received 24 Gy cranial irradiation. The third group consisted of other oncology patients who did not receive cranial irradiation. Lower IQ scores were noted in the group receiving 24 Gy. High-dose methotrexate and female sex appear to increase risk of intellectual deficits.31,32 The effects of cranial irradiation are also more severe the younger the child. Intellectual deficits resulting from radiation most commonly result in difficulty acquiring new knowledge, decreased processing speed, and memory deficits (most frequently short-term).33
Pediatric CNS tumors require a multimodality approach, including surgery, chemotherapy, and radiation. Attribution of toxicities such as neurocognitive decline to a single modality, most often radiotherapy, is not supported by the literature. Kiehna and colleagues34 conducted a prospective trial of 120 children and young adults to evaluate neurocognitive aptitudes before, during, and after CRT. In this comprehensive study from St. Jude Children’s Research Hospital, investigators used the Conners continuous performance test to measure selective attention, sustained attention, impulsivity, and reaction time. During a 6- to 7-week course of CRT, children exhibited problems with cognitive processing, attention, and reaction times. Overall attention indices worsened during CRT, with significant risk factors including progression of symptoms prior to CRT, young age, and diagnoses of craniopharyngioma or low-grade astrocytoma.34
The group at St. Jude Children’s Research Hospital has also evaluated academic achievement and verbal memory performance in children after CRT. Factors including female gender, number of surgeries, and hydrocephalus, predicted worse verbal memory performance at baseline. Of key importance, however, verbal memory stabilized and improved over time, rather than declined.35 In a similar study from the same group, serial assessments of academic achievement revealed that reading was the aptitude most susceptible to decline after CRT, whereas math and spelling skills remained stable.36
Ear
Radiation-induced sensorineural hearing loss is dose-dependent and is more severe in younger patients. Hearing loss can result from doses greater than 40 to 50 Gy, usually developing within 6 to 12 months of treatment.37 High-frequency hearing loss is seen in 25% to 50% of patients who received greater than 50 to 60 Gy to inner ear structures.38–40 Hearing loss is typically attributed to radiation changes induced in the cochlea and vasculature. Ototoxicity related to cisplatinum chemotherapy is well documented.41,42 Cranial irradiation before or concurrent with cisplatinum chemotherapy enhances ototoxicity.41,43 Chronic otitis can also develop following radiation therapy because of obstruction of the eustachian canal.
Endocrine
Whole-cranial irradiation or more focal radiation that includes the hypothalamic-pituitary axis can result in neuroendocrine abnormalities. The neuroendocrine complications that can result from radiation therapy are summarized in Table 53-5. Growth hormone (GH) production appears to be the most prone to disruption by radiation therapy. GH deficiency worsens over time and may follow radiation doses as low as 12 Gy.44 This appears to be a result of decreased GH-releasing hormone (GH-RH) in the hypothalamus, as GH deficiency is seen in patients undergoing hypothalamic radiation with pituitary sparing. Clinically, GH deficiency can result in short stature, bone loss, and metabolic abnormalities. Treatment with synthetic GH can allow children to maintain their expected growth percentile despite irradiation.
| Hormone Abnormality | Threshold Dose to Hypothalamic Axis |
|---|---|
| Growth hormone deficit | 18–25 Gy |
| ACTH deficit | 40 Gy |
| TRH/TSH deficit | 40 Gy |
| Precocious puberty | 20 Gy |
| LH/FSH deficit | 40 Gy |
| Hyperprolactinemia | 40 Gy |
ACTH, Corticotropin; LH/FSH, luteinizing hormone/follicle-stimulating hormone; TRH/TSH, thyrotropin-releasing hormone/thyrotropin.
Results in Selected Specific Central Nervous System Tumors
Medulloblastoma
Medulloblastoma comprises approximately 20% of pediatric CNS tumors, although one third of medulloblastomas occur in adults.45 Up to one third of medulloblastomas in children are associated with dissemination beyond the primary site at the time of diagnosis. Age younger than 3 years, inability to perform a gross total surgical removal of tumor, metastases, and diffuse anaplasia in the histologic specimen are associated with poor survival. Historically, standard treatment delivered 36 Gy to the brain and spine with a boost to the posterior fossa totalling 54 Gy. Significant abnormalities in intellectual development, growth, and hormonal function commonly occurred after CSI.46,47
Today, CSI followed by chemotherapy is the standard of care for both average- and high-risk children ages 3 and older. In average-risk disease (defined as gross totally resected tumor without severe anaplasia, and no metastases), 23.4 Gy to the craniospinal axis, plus a boost to 54 Gy to the posterior fossa, followed by chemotherapy, is standard and has resulted in 5-year survival of 80% or better.48 Areas of active investigation in average-risk disease are lowering of the craniospinal dosage to 18 Gy, conformal posterior fossa radiotherapy to the tumor volume, and intensification of chemotherapy with autologous peripheral blood stem cell support.49 In high-risk disease (defined as subtotally resected tumor, severely anaplastic histology, or presence of metastases), 36 Gy craniospinal plus a boost at the posterior fossa to 54 Gy, followed by chemotherapy is standard, and results in 5-year survival rates of 50% to 70%. Incorporation of chemotherapy during irradiation and intensification of chemotherapy following radiation therapy are subjects of current trials. Among infants with medulloblastoma, many approaches are competing in trials, particularly chemotherapy with limited posterior fossa radiation, high-dose methotrexate with or without later CSI,50 or very-high-dose chemotherapy without radiation therapy. Chemotherapeutics typically used in medulloblastoma include cisplatin, vincristine, cyclophosphamide, sometimes lomustine, and etoposide, or occasionally topotecan or thiotepa.
Simulation
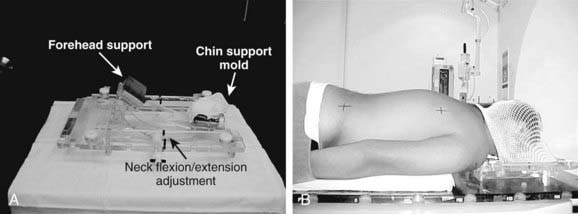
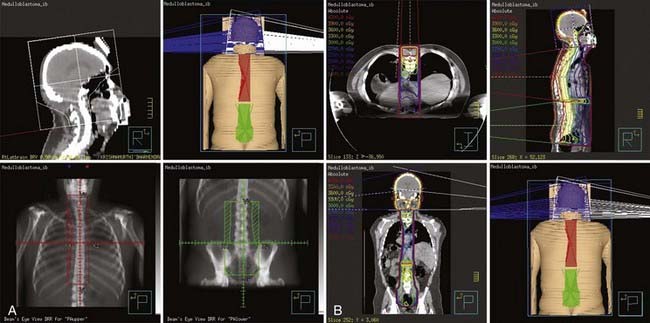
The vast majority of patients receiving CSI are children. Many have neurologic deficits, making it difficult for them to be motionless and cooperative for any length of time. More than any other patients, they require proper stabilization during treatment setup, and often assistive anesthesia. The field arrangement usually consists of opposed lateral brain portals and a posterior spinal axis field, all of which are matched in the region of the cervical spine. This set-up usually requires that patients be placed in the prone position. For most patients, the prone position is less comfortable than the supine, and it is therefore less likely to be consistently maintained throughout the treatment course unless the patient is positioned in a thermoplastic mold or a similar device (e.g., an Alpha Cradle). This is less problematic for cooperative adult patients than with children. Small children requiring anesthesia present a problem because the anesthesiologist requires access to the airway. These patients are usually treated in either a full-body plaster cast with an open facial area or using a customizable immobilization device that supports the forehead and the mandible (Fig. 53-6), with access for anesthesia and airway suction devices.
Craniospinal Irradiation Considerations
When treating the craniospinal axis, it is essential to be aware of potential technique limitations. Different techniques to deliver CSI have been described and variously address some of these known limitations.51–53
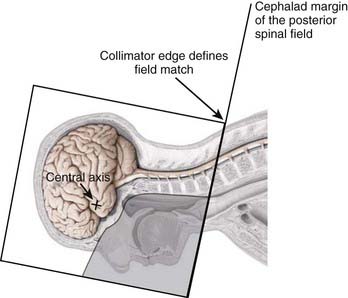
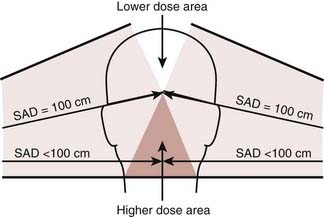
Craniospinal treatment is complicated by the fact that several adjacent fields must be used. Typically, the brain is treated through lateral opposed fields, whereas the spinal axis is treated with one or two posterior spinal fields (depending on the length of the spine). Field matching between the two posterior fields is usually accomplished by calculating the separation between the two fields on the skin surface, the so-called “skin-gap” calculation. It is important to match the adjacent radiation treatment fields at the anterior edge of the spinal cord, or alternatively at the posterior edge of the vertebral column. This approach prevents relative overdose of the spinal cord, but will result in a relative cold spot. Matching the inferior border of the lateral cranial fields with the superior border of the most cephalad spinal field is more complicated, because the cephalad aspect of the posterior spine field is diverging cephalad and the caudal aspect of the brain fields is diverging caudad and posteriorly. The first step is to rotate the collimator of the lateral fields so that its inferior border is parallel to the divergence of the superior aspect of the spinal field (Fig. 53-7). This prevents the superior aspect of the spinal field from exiting into the anterior-superior aspect of the brain fields. In effect, it moves the anterior portion of the lateral brain fields cephalad and out of the path of the spine field. Second, to avoid overlap resulting from inferior divergence of the caudal border of the lateral fields into the cephalad aspect of the posterior spinal field, the foot of the couch is rotated toward the collimator so that the caudal field margins of the two fields become parallel and cross the patient’s neck in a straight line, that is, perpendicular to the patient’s sagittal axis (Fig. 53-8).
Field junctions are usually moved two or three times during a course of treatment to spread out the dose inhomogeneities at the junction.54 The field junctions are often shifted by adjusting either cranial or caudal portions of the abutting fields using asymmetric jaws.
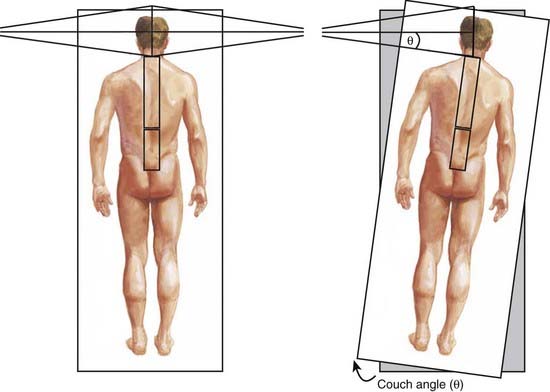
Although the practice of turning the couch for the lateral cranial fields solves the field-matching problem, depending on the location of the cranial field central axes (or anterior-superior position of the isocenter), it may cause the most inferior aspect of the opposite temporal fossa to be underdosed or missed (Fig. 53-9). This problem is generally avoided in practice by placing the isocenter for the brain fields in the central portion of the brain in the midsagittal plane.
Another note of caution should be added here, because when the foot of the couch is rotated toward the collimator for treatment of each of the lateral brain fields, the treatment distance (SSD) in the caudal half of the lateral brain field is effectively decreased whereas the cephalad half is increased (Fig. 53-10). The change in SSD becomes larger as the distance from the isocenter increases. The SSD is usually shorter by 1 to 2 cm in the cervical spine, depending on the couch angle and the location of the isocenter of the lateral brain fields. The length by which the distance is decreased, which must be measured at the appropriate distance from the isocenter, can be found by measuring the distance between the sagittal laser line when the couch is set to 0 degrees versus where it is when the couch is rotated to the treatment position. It is important to recognize that this change in SSD is in the same direction for both of the lateral brain fields.
It is difficult to treat the entire brain without having divergence into the eye on the opposite side. The problem is analogous to that described in Fig. 53-9, in which the contralateral temporal lobe may be underdosed. One solution is to set the isocenter, and thereby the central axis of the beam, at the lateral canthus of the eye, thereby eliminating the beam divergence. Although this approach may reduce eye irradiation, this technique generally cannot be used in the setting of CSI. In the setting of CSI, when the couch is rotated to eliminate the caudal beam divergence of the lateral cranial fields, the opposite eye is projected more cephalad and cannot be adequately shielded without also blocking brain tissue. A gantry angle here will not solve the problem because that motion causes both eyes to move posteriorly with respect to the beam. Although the anterior-posterior motion can be changed only by a gantry angle, the cephalad-caudad motion can be changed only by a couch rotation.
In general, an attempt should be made to keep the patient’s posterior surface horizontal, an isodose distribution should be calculated on a sagittal contour, and the dose gradient within the spinal canal should be considered when the dose prescription is formulated. All fields must be carefully matched to prevent relative overdose or underdose at junctional sites (Fig. 53-11).54,55
Ependymomas
Ependymomas occur most frequently in the posterior fossa and are treated with 54 Gy with a local field, achieving relatively good 5-year survival rates.56 Less-than-complete surgical resection leads to a very high rate of recurrence, in spite of adjuvant radiotherapy. Most centers treat with local therapy only. There is no proven benefit from chemotherapy in children with ependymoma, although novel agents are now bing investigated at relapse as well as radiosurgery.
Radiation Therapy Guidelines
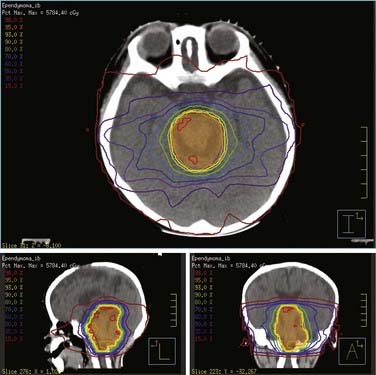
The dose should be prescribed to the highest isodose surface that encompasses both PTV and CTV with the least inhomogeneity (Fig. 53-12). Ideally, 95% of the PTV should be covered by 95% of the prescription dose; PTV dose should be within +10% and −5% of the prescription. These guidelines aim to minimize target dose heterogeneity and consequently possible associated irreversible neurologic sequelae. Without compromising the target coverage, the treatment plan should aim to minimize dose to the spinal cord, brainstem, optic chiasm, optic nerves, auditory system (cochleae), hypothalamic-pituitary complex, and temporal lobes. In situations in which the critical structure tolerance will be exceeded, a reduction in the target volume (staged cone-down) will be required after the tolerance dose has been reached. This may require that portions of the PTV, CTV, or GTV in or near the region of the critical structure will be underdosed. For example, when the cumulative spinal cord dose has reached 50 Gy, the spinal cord should be excluded from the treatment (or excluded after 54 Gy if disease extends into foramen magnum).
Brainstem Gliomas
Diffuse brainstem gliomas are nearly universally fatal within 3 years of diagnosis.57,58 Children present abruptly, often with a month or less of weakness, ataxia, and cranial neuropathies, and typically an abducens paresis producing horizontal strabismus. These poor-prognosis, diffuse pontine gliomas are usually treated with radiation only. Although radiotherapy is often performed without biopsy, the influence of surgical intervention or biopsy on this disease (excluding dorsally exophytic lesions) is uncertain. Hyperfractionated radiotherapy of 1.17 Gy twice a day to a total dose of 70.2 Gy has been shown to produce similar survival to that of other treatment schemes, with an acceptable rate of morbidity.59 Current clinical trials are testing novel molecular therapeutics, antiangiogenesis agents, and radiosensitizers in this tumor.
Radiation Therapy Guidelines
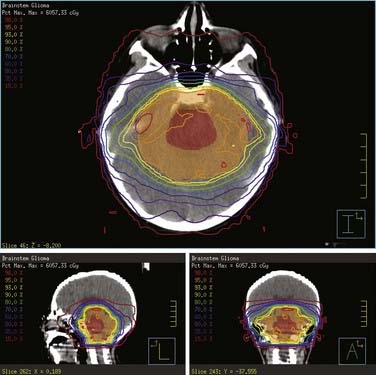
Three-dimensional conformal treatment techniques, including IMRT, should be used in the treatment of brainstem glioma patients (Fig. 53-13). Two-dimensional techniques, with a midline prescription point, are discouraged, given their inherent limitations to spare uninvolved surrounding anatomic structures and tendency to deposit higher doses within the temporal lobes. More than 95% of the PTV should be covered by the 95% isodose surface (CTV and GTV must be within the prescription volume); the PTV dose should be within +10% and −5% of the prescription to minimize dose heterogeneity within the brainstem. Doses to the spinal cord, brainstem, optic chiasm, optic nerves, auditory system (cochleae), hypothalamic-pituitary complex, and temporal lobes should be monitored, recorded, and maintained within the accepted tolerance limits.
Cerebral and Cerebellar Astrocytomas
The prognosis for astrocytomas is much better for children than for adults, particularly because many of the tumors are low-grade pilocytic (WHO grade I) or diffuse astrocytomas (WHO grade II). Even the rate of diffuse WHO grade II astrocytomas that undergo anaplastic transformation is much less than that in adults.60 Thus, the role of radiotherapy following surgery for low-grade astrocytomas is controversial, and, in practice today, is deferred following gross total resection and frequently in subtotally resected low-grade gliomas until there is further evidence of growth.61 The majority of cerebellar astrocytomas carry a good prognosis when treated with surgery alone and are often of the pilocytic type. Even with recurrent or subtotally resected cerebellar tumors, irradiation is withheld and repeat surgery usually pursued in lieu of radiotherapy.
For diencephalic or chiasmatic astrocytomas at the hypothalamus or chiasm, often minimally amenable to resection, carboplatin and vincristine chemotherapy are used in children presenting before age 10 to stabilize or reduce tumor burden and delay radiotherapy until the child is older and can better tolerate acute and long-term effects of radiation.62
Germ Cell Tumors
Intracranial germ-cell tumors (GCTs) represent 3% to 11% of pediatric and 1% of adult brain tumors.63 Intracranial GCTs compose two distinct histologic groupings: germinomas are the most common histologic subtype, whereas NGGCT represent less than one third of intracranial GCTs and consist of embryonal carcinoma, endodermal sinus (yolk sac) tumor, choriocarcinoma, mature and immature teratoma, and GCT of mixed cellular origin.5 Historically, radiation alone, frequently encompassing a large treatment volume, constituted the gold standard of treatment for intracranial GCTs.64–66 However, the morbidity of radiation therapy in children,67–72 particularly CSI, prompted many investigators to explore approaches that reduce the volume and dose of radiotherapy while preserving high cure rates.
Published reports corroborate poorer prognosis for patients with NGGCT compared with those with germinomas.65,73 Radiation alone produces 5-year survival rates as low as 30% to 40%.74 More recent studies that demonstrate excellent response rates to chemotherapy have shifted the standard treatment of NGGCT to combined-modality therapy consisting of chemotherapy and radiation, and these approaches are now being explored in North America.75 Many or most investigators still consider craniospinal rather than local-field radiotherapy standard in this disease.
Of the various histologic subtypes of intracranial GCTs, germinomas represent the most common and prognostically favorable subgroup. Historically, intracranial germinoma has been treated with radiation alone, producing excellent cure rates.63,64,66 The significant long-term toxicity of radiation, particularly in children,67–72 has prompted investigation to explore alternative treatment that minimizes the dose and volume of irradiation. However, these alternative approaches must preserve the high cure rates established with radiation alone.
Several series have reported excellent clinical outcome with preirradiation chemotherapy followed by focal irradiation.76–81 The appeal of this approach lies in lower radiation dose and smaller radiation fields that presumably translate into reduced long-term toxicity. Radiation alone continues to be the gold standard for the treatment of intracranial germinoma. Promising approaches such as combined chemotherapy and focal, reduced-dose irradiation are being compared with radiation alone in a prospective, randomized trial.
In addition to the role of chemotherapy, a point of controversy in the treatment of localized germinoma has been the appropriate field of radiation.82 Current evidence substantiates omitting CSI from the treatment of localized germinoma. Spinal failure rates of less than 10% in the absence of CSI, reported in most contemporary series, do not justify routine incorporation of CSI into the treatment strategy for localized germinoma.73,83–87 Nevertheless, the precise irradiation field appropriate for localized germinoma remains unclear. Several studies report higher recurrence rates associated with radiation targeting the tumor volume only.73,83,88,89 We recommend whole-ventricular irradiation, followed by a boost to the primary tumor for localized germinoma when using radiation alone.90 The literature supports a dose of greater than or equal to 45 Gy to the primary tumor for germinomas treated with radiation alone. In the context of combined chemotherapy and radiation, many investigators have tailored the dose of radiation to the tumor response observed following chemotherapy. Doses of radiation following chemotherapy range from 24 to 40 Gy.
Radiation Therapy Guidelines
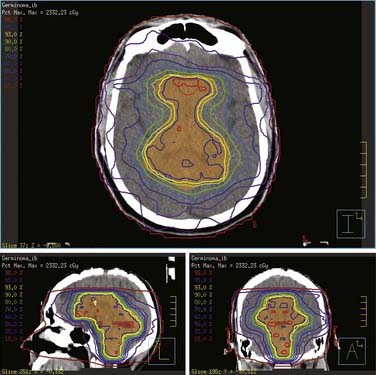
Patients with intracranial, localized germinoma are generally simulated in a supine position with maximal comfortable neck flexion and head-only thermoplastic mask for immobilization. It is critical to ensure that the chin, forehead, and nasal bridge fit snugly inside the mask to minimize patient motion. Whenever possible, WV irradiation should be delivered with photon energies of 6 mV or more and either a four-field plan, consisting of an opposed lateral pair and anterior-to-posterior beams (angled to avoid the orbits), or IMRT (Fig. 53-14). The use of an opposed lateral pair should be avoided because of a relative increase in the temporal lobe dose compared with more conformal multibeam techniques.91 Similar principles should be followed for the involved-field boost.
Craniopharyngiomas
These lesions are not malignant but, because of their location in the suprasellar region, are often associated with high morbidity. The judgment and skill of the operating surgeon is therefore critical if significant endocrine and visual problems are to be avoided. There is a suggestion that subtotal resection followed by local irradiation to 54 Gy offers progression-free survival similar to that of gross total resection with a much lower rate of morbidity, but contradictory studies are published as well.92
Radiation Therapy Guidelines
Because these are relatively rare tumors, with fewer than 100 cases annually, radiation guidelines have not been developed.93 After decompressive surgery, patients may be observed even in the presence of residual disease and treated at the time of progression. Radiosurgery can be used to treat small solid residual tumors and intracystic isotopes (e.g., 32p colloid) are useful for treating recurrent or persistent cystic components 2 to 3 cm in diameter or larger. Mixed solid-cystic masses are best addressed by decompressive surgery followed by adjuvant radiation. The treatment volume should include both solid and cystic tumor (GTV) as designated on an MRI, a conservative CTV expansion, usually 1.0 cm or smaller, and an appropriate PTV margin (0.3-0.5 cm). A dose of 54 Gy is delivered to the target volume using conventional fractionation (1.8 Gy/fraction). Highly-conformal techniques, such as IMRT or 3D-CRT, should be employed in the treatment of these patients to minimize radiation dose outside of the tumor volume. Because these tumors generally arise in the sellar and suprasellar region, it is important to carefully monitor the dose to the optic apparatus (optic nerves, chiasm, and optic radiations) as well as to the hypothalamus and bilateral temporal lobes during treatment planning. Target dose heterogeneity should be carefully manipulated to avoid placing areas of relative high dose (“hot spots”) in the vicinity of critical structures. Because cyst formation in response to radiation therapy is frequently observed, patients should ideally receive at least biweekly MRI scans to ensure that the tumor target remains within the radiation field. Radiation-related morbidity is quite common and includes a variety of endocrinologic, neurologic, and cognitive sequelae.94,95
1 Central Brain Tumor Registry of the US. Statistical Report: Primary Brain Tumors in the United States, 2000–2004. Hinsdale, IL: Central Brain Tumor Registry of the United States; 2008.
2 Gurney JG, Smith MA, Bunin GR. CNS and miscellaneous intracranial and intraspinal neoplasms. In: Ries LAG, Smith MA, Gurney JG, et al, editors. Cancer incidence and survival among children and adolescents: United States SEER Program 1975–1995. NIH Pub no. 99-4649. Bethesda, MD: National Cancer Institute, SEER Program; 1999:51-63.
3 McGuire CS, Sainani KL, Fisher PG. Incidence patterns for ependymoma: a SEER study. J Neurosurg. 2009;110:725-729.
4 Goodwin TL, Sainani KL, Fisher PG. Incidence patterns of central nervous system germ cell tumors: a SEER study. J Pediatr Hematol Oncol, Aug. 2009;31(8):541-544.
5 Louis DN, Ohgaki H, Wiestler OD, et al, editors. WHO classification of tumors of the central nervous system. Lyon, France: International Agency for Research on Cancer, 2007.
6 Neglia JP, Robison LL, Stovall M, et al. New primary neoplasms of the central nervous system in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2006;98:1528-1537.
7 Packer RJ. Brain tumors in children. Curr Opin Pediatr. 1995;7:64-72.
8 Conway PD, Oechler HW, Kun LE, et al. Importance of histologic condition and treatment of pediatric cerebellar astrocytoma. Cancer. 1991;67:2772-2775.
9 Pomeroy SL, Tamayo P, Gaasenbeek M, et al. Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature. 2002;415:436-442.
10 Burger PC, Scheithauer BW. Tumors of the central nervous system. In: AFIP Atlas of Tumor Pathology, fourth series, fascicle 7. Washington DC: American Registry of Pathology; 2007.
11 Bouffet E, Perilongo G, Canete A, et al. Intracranial ependymomas in children: a critical review of prognostic factors and a plea for cooperation. Med Pediatr Oncol. 1998;40:51-57.
12 Rickert CH. Extraneural metastases of paediatric brain tumours. Acta Neuropathol. 2003;105:309-327.
13 Chang CH, Housepian EM, Herbert CJr. An operative staging system and a megavoltage radiotherapeutic technic for cerebellar medulloblastomas. Radiolog. 1969;93:1351-1359.
14 Zeltzer PM, Boyett JM, Finlay JL, et al. Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: conclusions from the Children’s Cancer Group 921 randomized phase III study. J Clin Oncol. 1999;17:832-845.
15 Partap S, Fisher PG. Update on new treatments and developments in childhood brain tumors. Curr Opin Pediatr. 2007;19:670-674.
16 Kaur H, Singh D, Peereboom DM. Primary central nervous system germ cell tumors. Curr Treat Options Oncol. 2003;4:491-498.
17 Chuba PJ, Zamarano L, Hamre M, et al. Permanent I-125 brain stem implants in children. Childs Nerv Syst. 1998;14:570-577.
18 Merchant TE, Hua CH, Shukla H, et al. Proton versus photon radiotherapy for common pediatric brain tumors: Comparison of models of dose characteristics and their relationship to cognitive function. Pediatr Blood Cancer. 2008;51:110-117.
19 St Clair WH, Adams JA, Bues M, et al. Advantage of protons compared to conventional X-ray or IMRT in the treatment of a pediatric patient with medulloblastoma. Int J Radiat Oncol Biol Phys. 2004;58:727-734.
20 Schultheisis TE, Kun LE, Ang KK, et al. Radiation response of the central nervous system. Int J Radiat Oncol Biol Phys. 1995;31:1093-1112.
21 Wong CS, Van Dyke J, Milosevic M, et al. Radiation myelopathy following single courses of radiotherapy and retreatment. Int J Radiat Oncol Biol Phys. 1994;30:575-581.
22 Kramer S, Lee KF. Complications of radiation therapy: the central nervous system. Semin Roentgenol. 1974;9:75-83.
23 Loeffler JS, Siddon RL, Wen PY, et al. Stereotactic radiosurgery of the brain using a standard linear accelerator: a study of early and late effects. Radiother Oncol. 1990;17:311-321.
24 Boldrey E, Sheline G. Delayed transitory clinical manifestations after radiation treatment of intracranial tumors. Acta Radiol Ther Phys Biol. 1966;3:5-10.
25 Littman P, Rosenstock J, Gale G, et al. The somnolence syndrome in leukemic children following reduced daily dose fractions of cranial radiation. Int J Radiat Oncol Biol Phys. 1984;10:1851-1853.
26 Lampert H, Alegria A. Treatment of post-poliomyelitis paralysis of long duration. Acta Neurol Latinoam. 1964;10:160-171.
27 Halperin EC. Pediatric brain stem tumors: patterns of treatment failure and their implications for radiotherapy. Int J Radiat Oncol Biol Phys. 1985;11:1293-1298.
28 Rowland JH, Glidewell OJ, Sibley RF, et al. Effects of different forms of central nervous system prophylaxis on neuropsychologic function in childhood leukemia. J Clin Oncol. 1984;2:1327-1335.
29 Copeland DR, Fletcher JM, Pfefferbaum-Levine B, et al. Neuropsychological sequelae of childhood cancer in long-term survivors. Pediatrics. 1985;75:745-753.
30 Halberg FE, Kramer JH, Moore IM, et al. Prophylactic cranial irradiation dose effects on late cognitive function in children treated for acute lymphoblastic leukemia. Int J Radiat Oncol Biol Phy. 1992;22:13-16.
31 Waber DP, Tarbell NJ, Kahn LM, et al. The relationship of sex and treatment modality to neuropsychologic outcome in childhood acute lymphoblastic leukemia. J Clin Oncol. 1992;10:810-817.
32 Hardenbergh PH, Golden J, Billet A, et al. Intracranial germinoma: the case for lower dose radiation therapy. Int J Radiat Oncol Biol Phys. 1997;39:419-426.
33 Mulhern RK, Ochs J, Fairclough D. Deterioration of intellect among children surviving leukemia: IQ test changes modify estimates of treatment toxicity. J Consult Clin Psychol. 1992;60:477-480.
34 Kiehna EN, Mulhern RK, Li C, et al. Changes in attentional performance of children and young adults with localized primary brain tumors after conformal radiation therapy. J Clin Oncol. 2008;24:5283-5290.
35 Di Pinto M, Conklin H, Li C, et al: Predicting verbal memory performance after conformal radiation therapy for children with craniopharyngioma, ependymoma, and low grade astrocytoma. Poster presentation presented at the Annual Meeting for the International Neuropsychological Society (INS), February, 2008, Waikoloa, Hawaii.
36 Conklin HM, Li C, Xiong X, et al: Predicting change in academic achievement after conformal radiation therapy for localized ependymoma. Paper presented at the biannual meeting of the International Neuropsychological Society, February 7–10, 2007, Portland, Oregon.
37 Grau C, Overgaard J. Postirradiation sensorineural hearing loss: a common but ignored late radiation complication. Int J Radiat Oncol Biol Phys. 1996;36:515-517.
38 Anteunis LJ, Engel JA, Hendriks JJ, et al. A prospective longitudinal study on radiation-induced hearing loss. Am J Surg. 1994;168:408-411.
39 Kwong DL, Wei WI, Sham JS, et al. Sensorineural hearing loss in patients treated for nasopharyngeal carcinoma: a prospective study of the effect of radiation and cisplatin treatment. Int J Radiat Oncol Biol Phys. 1996;36:281-289.
40 Low WK, Fong KW. Long-term hearing status after radio-therapy for nasopharyngeal carcinoma. Auris Nasus Larynx. 1998;25:21-24.
41 Schell MJ, McHaney VA, Green AA, et al. Hearing loss in children and young adults receiving cisplatin with or without prior cranial irradiation. J Clin Oncol. 1989;7:754-757.
42 McHaney VA, Thibadoux G, Hayes FA, et al. Hearing loss in children receiving cisplatin chemotherapy. J Pediatr. 1983;102:314-317.
43 Walker DA, Pillow J, Waters KD, et al. Enhanced cis-platinum ototoxicity in children with brain tumors who have received simultaneous or prior cranial irradiation. Med Pediatr Oncol. 1989;17:48-52.
44 Merchant TE, Goloubeva O, Pritchard DL, et al. Radiation dose-volume effects on growth hormone secretion. Int J Radiat Oncol Biol Phys. 2002;52:1264-1270.
45 Curran EK, Le GM, Propp JM, et al. Gender affects survival from medulloblastoma only in older children and adults: a study from the Surveillance Epidemiology End Results Registry. Pediatr Blood Cancer. 2009;52:60-64.
46 Silber JH, Radcliffe J, Peckham V, et al. Whole-brain irradiation and decline in intelligence: The influence of dose and age on IQ score. J Clin Oncol. 1992;10:1390-1396.
47 Duffner PK. Long-term effects of radiation therapy on cognitive and endocrine function in children with leukemia and brain tumors. Neurologist. 2004;10:293-310.
48 Packer RJ, Gajjar A, Vezina G, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24:4202-4208.
49 Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7:813-820.
50 Rutkowski S, Bode U, Deinlein F. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med. 2005;352:978-986.
51 Narayana A, Jeswani S, Paulino AC. The cranial-spinal junction in medulloblastoma: does it matter? Int J Radiat Oncol Biol Phys. 1999;44:81-84.
52 Mah K, Danjoux CE, Manship S, et al. Computed tomographic simulation of craniospinal fields in pediatric patients: improved treatment accuracy and patient comfort. Int J Radiat Oncol Biol Phys. 1998;41:997-1003.
53 Miralbell R, Bleher A, Huguenin P, et al. Pediatric medulloblastoma: radiation treatment technique and patterns of failure. Int J Radiat Oncol Biol Phys. 1997;37:523-529.
54 Kiltie AE, Povall JM, Taylor RE. The need for the moving junction in craniospinal irradiation. Br J Radiol. 2000;73:650-654.
55 Tatcher M, Glicksman AS. Field matching considerations in craniospinal irradiation. Int J Radiat Oncol Biol Phys. 1989;17:865-869.
56 Merchant TE, Mulhern RK, Krasin MJ, et al. Preliminary results from a phase II trial of conformal radiation therapy and evaluation of radiation-related CNS effects for pediatric patients with localized ependymoma. J Clin Oncol. 2004;22:3156-3162.
57 Fisher PG, Breiter SN, Carson BS, et al. A clinicopathologic reappraisal of brainstem tumor classification: identification of pilocytic astrocytoma and fibrillary astrocytoma as distinct entities. Cancer. 2000;89:1569-1576.
58 Donaldson SS, Laningham F, Fisher PG. Advances toward an understanding of brain stem gliomas. J Clin Oncol. 2006;24:1266-1272.
59 Mandell LR, Kadota R, Freeman C, et al. There is no role for hyperfractionated radiotherapy in the management of children with newly diagnosed diffuse intrinsic brainstem tumors: results of a Pediatric Oncology Group phase III trial comparing conventional vs. hyperfractionated radiotherapy. Int J Radiat Oncol Biol Phys. 1999;43:959-964.
60 Broniscer A, Baker SJ, West AN, et al. Clinical and molecular characteristics of malignant transformation of low-grade glioma in children. J Clin Oncol. 2007;25:682-689.
61 Fisher PG, Tihan T, Goldthwaite PT, et al. Outcome analysis of childhood low-grade astrocytomas. Pediatr Blood Cancer. 2008;51:245-250.
62 Packer RJ, Ater J, Allen J, et al. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg. 1997;86:747-754.
63 Hoffman HJ, Otsubo H, Hendrick EB, et al. Intracranial germ-cell tumors in children. J Neurosurg. 1991;74:545-551.
64 Wolden SL, Wara W, Larson DA, et al. Radiation therapy for primary intracranial germ-cell tumors. Int J Radiat Oncol Biol Phys. 1995;32:943.
65 Kretschmar CS. Germ cell tumors of the brain in children: A review of current literature and new advances in therapy. Cancer Invest. 1997;15:187-198.
66 Bamberg M, Kortmann RD, Caliminus G, et al. Radiation therapy for intracranial germinoma: results of the German cooperative prospective trials MAKEI 83/86/89. J Clin Oncol. 1999;17:2585-2592.
67 Chapman CA, Waber DP, Bernstein JH, et al. Neurobehavioral and neurologic outcome in long-term survivors of posterior fossa brain tumors: role of age and perioperative factors. J Child Neurol. 1995;10:209-212.
68 Ellenberg L, McComb JG, Siegel SE, et al. Factors affecting intellectual outcome in pediatric brain tumor patients. Neurosurgery. 1987;21:638-644.
69 Moore BD, Ater JL, Copeland DR. Improved neuropsychological outcome in children with brain tumors diagnosed during infancy and treated without cranial irradiation. J Child Neurol. 1992;7:281-290.
70 Packer RJ, Sutton LN, Atkins TE, et al. A prospective study of cognitive function in children receiving whole-brain radiotherapy and chemotherapy: 2-year results. J Neurosurg. 1989;70:707-713.
71 Radcliffe J, Packer RJ, Atkins TE, et al. Three- and four-year cognitive outcome in children with noncortical brain tumors treated with whole-brain radiotherapy. Ann Neurol. 1992;32:551-554.
72 Waber DP, Tarbell NJ, Fairclough D, et al. Cognitive sequelae of treatment in childhood acute lymphoblastic leukemia: Cranial radiation requires an accomplice. J Clin Oncol. 1995;13:2490-2496.
73 Matsutani M, Sano K, Takakura K, et al. Primary intracranial germ cell tumors: a clinical analysis of 153 histologically verified cases. J Neurosurg. 1997;86:446-455.
74 Fuller BG, Kapp DS, Cox R. Radiation therapy of pineal region tumors: 25 new cases and a review of 208 previously reported cases. Int J Radiat Oncol Biol Phys. 1994;28:229-245.
75 Bouffet E, Baranzelli MC, Patte C, et al. Combined treatment modality for intracranial germinomas: Results of a multicentre SFOP experience. Société Française d’Oncologie Pédiatrique. Br J Cancer. 1999;79:1199-1204.
76 Baranzelli MC, Patte C, Bouffet E, et al. Nonmetastatic intracranial germinoma: the experience of the French Society of Pediatric Oncology. Cancer. 1997;80:1792-1797.
77 Buckner JC, Peethambaram PP, Smithson WA, et al. Phase II trial of primary chemotherapy followed by reduced-dose radiation for CNS germ cell tumors. J Clin Oncol. 1999;17:933-940.
78 Fouladi M, Grant R, Baruchel S, et al. Comparison of survival outcomes in patients with intracranial germinomas treated with radiation alone versus reduced-dose radiation and chemotherapy. Childs Nerv Syst. 1998;14:596-601.
79 Kitamura K, Shirato H, Sawamura Y, et al. Preirradiation evaluation and technical assessment of involved-field radiotherapy using computed tomographic (CT) simulation and neoadjuvant chemotherapy for intracranial germinoma. Int J Radiat Oncol Biol Phys. 1999;43:783-788.
80 Sawamura Y, Shirato H, Ikeda J, et al. Induction chemotherapy followed by reduced-volume radiation therapy for newly diagnosed central nervous system germinoma. J Neurosurg. 1998;88:66-72.
81 Kretschmar C, Kleinberg L, Greenberg M, et al. Pre-radiation chemotherapy with response-based radiation therapy in children with central nervous system germ cell tumors: A report from the Children’s Oncology Group. Pediatr Blood Cancer. 2007;48:285-291.
82 Paulino AC, Wen BC, Mohideen MN. Controversies in the management of intracranial germinomas. Oncology. 1999;13:513-521.
83 Aoyama H, Shirato H, Yoshida H, et al. Retrospective multi-institutional study of radiotherapy for intracranial non-germinomatous germ cell tumors. Radiother Oncol. 1998;49:55-59.
84 Dattoli MJ, Newall J. Radiation therapy for intracranial germinoma: the case for limited volume treatment. Int J Radiat Oncol Biol Phys. 1990;19:429-433.
85 Haddock MG, Schild SE, Scheithaver BW, et al. Radiation therapy for histologically confirmed primary central nervous system germinoma. Int J Radiat Oncol Biol Phys. 1997;38:915-923.
86 Linstadt D, Wara WM, Edwards MS, et al. Radiotherapy of primary intracranial germinomas: the case against routine craniospinal irradiation. Int J Radiat Oncol Biol Phys. 1988;15:291-297.
87 Sugiyama K, Uozumi T, Arita K, et al. Clinical evaluation of 33 patients with histologically verified germinoma. Surg Neurol. 1994;42:200-210.
88 Shibamoto Y, Abe M, Yamashita J, et al. Treatment results of intracranial germinoma as a function of the irradiated volume. Int J Radiat Oncol Biol Phys. 1988;15:285-290.
89 Shirato H, Nishio M, Sawamura Y, et al. Analysis of long-term treatment of intracranial germinoma. Int J Radiat Oncol Biol Phys. 1997;37:511-515.
90 Haas-Kogan DA, Missett BT, Wara WM, et al. Radiation therapy for intracranial germ cell tumors. Int J Radiat Oncol Biol Phys. 2003;56:511-518.
91 Roberge D, Kun LE, Freeman CR. Intracranial germinoma: on whole-ventricular irradiation. Pediatr Blood Cancer. 2005;44:358-362.
92 Hetelekidis S, Barnes PD, Tao ML, et al. 20-year experience in childhood craniopharyngioma. Int J Radiat Oncol Biol Phys. 1993;27:189-195.
93 Suh JH, Gupta N. Role of radiation therapy and radiosurgery in the management of craniopharyngiomas. Neurosurg Clin N Am. 2006;17:143-148. vi-vii
94 Merchant TE. Craniopharyngioma radiotherapy: endocrine and cognitive effects. J Pediatr Endocrinol Metab. 2006;19(Suppl 1):439-446.
95 Scarzello G, Buzzaccarini MS, Perilongo G, et al. Acute and late morbidity after limited resection and focal radiation therapy in craniopharyngiomas. J Pediatr Endocrinol Metab. 2006;19(Suppl 1):399-405.