55 Pediatric Bone and Soft Tissue Tumors
Osteosarcoma and Ewing sarcoma are first and second in incidence of childhood bone tumors and compose 2.6% and 2.1% of pediatric solid tumors (approximately 260 and 200 cases annually), respectively.1 Most primary osteosarcoma lesions are managed by amputation or limb-sparing procedures and intensive chemotherapy. Pulmonary metastases are managed by surgical excision. For primary lesions in an unresectable site, control with conventional photon radiotherapy is limited. With proton-beam therapy, 60% local control was achieved for patients with axial skeletal tumors.2 Hypofractionated, accelerated radiotherapy afforded durable response in 14 patients requiring palliation of primary or metastatic lesions.3 For 17 patients with spine osteosarcoma, radiotherapy after intralesional surgery improved survival.4 A dose escalation trial of 153-samarium-ethylenediaminetetramethylphosphonic acid followed by peripheral stem cell rescue demonstrated minimal toxicity and prolonged response in patients with recurrent or metastatic osteosarcoma.5
Other rare bone tumors may present in childhood. Non-Hodgkin lymphoma of bone responds well to multiagent chemotherapy, and radiotherapy is required only for nonresponders.6 Malignant fibrous histiocytoma of bone can be controlled with radiotherapy.7 Hemangioendothelial sarcoma of bone has a variable presentation and natural history and may be multifocal in one limb or bone.8 Radiotherapy to 40 to 60 Gy affords good local control.8–11 Benign giant cell tumor of bone presents in the pediatric age group in locations not amenable to surgery.12 Radiation to greater than 40 Gy resulted in a local control rate of 75% to 85% in two series.13,14 Proton-beam therapy has also been used with similar control rates.2 Local control of aneurysmal bone cyst in three adolescents was achieved with 40 Gy.15
Ewing Sarcoma of Bone
Epidemiology and Cytogenetics
A case-control study of environmental and familial factors did not identify risk factors for Ewing sarcoma.16 A reciprocal translocation t(11;22)(q24;q12) characterizes Ewing sarcoma of bone and is shared with extraosseous Ewing sarcoma and peripheral primitive neuroectodermal tumor (of bone or soft tissue), and helps define the Ewing sarcoma family of tumors.17,18 Mapping of the translocation break point indicates fusion of the FLI-1 gene on 11q24 with the EWS gene of 22q12.19 One function of the resulting fusion protein is repression of a tumor suppressor gene that encodes for the type II receptor of transforming growth factor beta.20 Patients harboring the less frequent EWS-ERG fusion protein resulting from a t(21;22)(q22; q12) translocation were compared with patients with the EWS-FLI1 transcript. Differences in clinical phenotype and outcome were not observed.21
As transcriptional repression by NKX2.2 is mediated through histone deacetylases (HDACs), these findings identify a novel therapeutic approach for Ewing sarcoma. Specifically, HDAC inhibitors may prove clinically effective by reversing NKX2.2-mediated transcriptional repression. This line of investigation exemplified how scientific investigations can indicate promising novel approaches to cancer treatment.22
Clinical Presentation and Routes of Spread
Ewing sarcoma of bone may present as late as the fourth and fifth decades of life, but the mean and median age (15 to 16 years) reflect the predominance of presentation between ages 10 and 20 years (approximately 65% of cases).23–25 Age at diagnosis younger than 5 years is rare, as is diagnosis in blacks. The male/female ratio is 1.5 : 1. Pain is the most common presenting symptom (>90%), and two-thirds of patients complain of a mass lesion. When a long bone is the primary site, the tumor is typically in the metaphysis or diaphysis. Three-fourths of primary sites are extremities or pelvic bones, with the femur as the single bone most frequently involved (Fig. 55-1).26 Other features at presentation include pathologic fracture (16%), systemic symptoms including fever (10% to 30%), and metastases (10% to 25%).23 Of 122 patients in the Intergroup Ewing Sarcoma Studies (IESS-I and IESS-II) with metastases at diagnosis, 53% had lung metastases with or without other metastases and 43% had bone metastases with or without other metastases.27
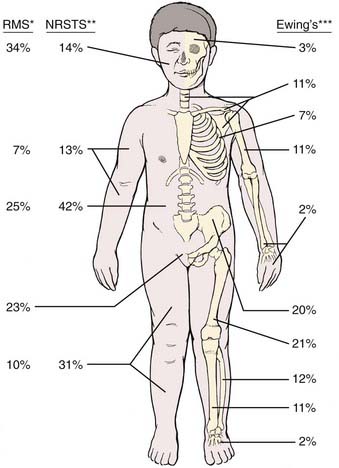
FIGURE 55-1 • Distribution of primary sites for rhabdomyosarcoma (RMS),80 nonrhabdomyosarcoma soft tissue sarcoma (NRSTS),165–167 and Ewing sarcoma.265 *For RMS, the head and neck site may be subdivided as 8% orbit, 8% other head, and 18% parameningeal.The pelvic sites may be subdivided as 11% bladder and prostate, and 12% female genital or paratestis. **For NRSTS, the trunk data include pelvic sites. ***For Ewing sarcoma, certain sites by subdivision are 5% vertebral column; 4% scapula; 12% ilium; and 8% sacrum, coccyx, and pubis.
Pathologic Conditions
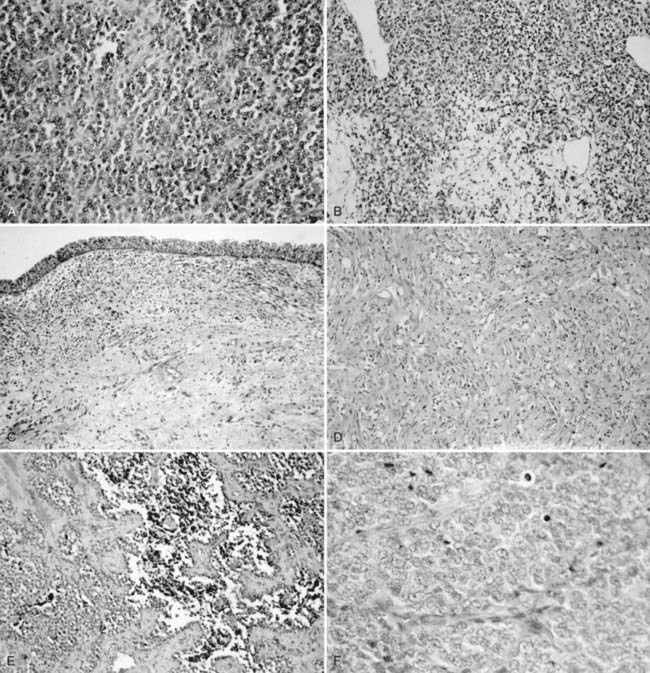
The histogenesis of Ewing sarcoma is uncertain. The classic or “typical” variant is shown in Fig. 55-2A. A variant featuring greater range of cell size and more frequent mitoses has been termed atypical and may also have a lobular, alveolar, or organoid organizational pattern. The primitive round-cell sarcoma of bone is less common and on light microscopy may have cells of greater size and shape, more pink cytoplasm, and a more prominent matrix. Ultrastructural features are distinct from Ewing sarcoma of bone and include the presence of cytoplasmic organelles, intracytoplasmic attachments, and developed intercellular attachments. Neurosecretory granules are not apparent in contrast to the peripheral primitive neuroectodermal tumor.28 There is at present insufficient clinical experience to determine if the subtypes differ by age at diagnosis, site of presentation, probability of metastases, and response to therapy.
Diagnostic and Staging Studies
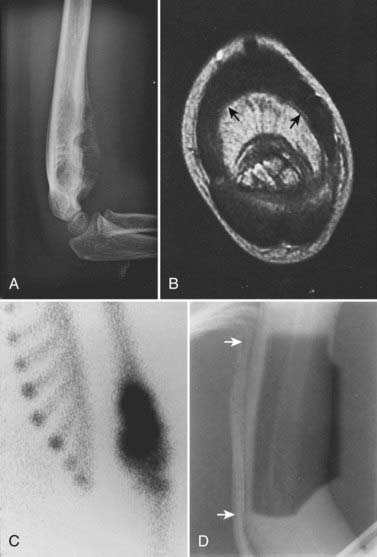
Laboratory studies at presentation, in addition to routine hematology and chemistry, should include serum lactase dehydrogenase level and histologic assessment of bone marrow. Chest computed tomography (CT) and bone scan are required. Imaging studies of the primary lesion include plain radiographs that typically show a central destructive lesion with variable sclerosis and osteolysis. Periosteal elevation by the tumor mass stimulates calcium deposition that may form layers with an “onion skin” appearance and that may, at the periphery of the elevation, demonstrate the Codman triangle sign (Fig. 55-3). A soft tissue component may show radiating calcium spicules. Bone scan will also show the extent of the primary lesion.29 Although CT assessment of the primary site is superior to magnetic resonance imaging (MRI) for identification of tumor matrix calcification and periosteal reaction, MRI has several advantages. The assessment of bone cortex is best seen in the axial plane. Sagittal and coronal images show (on T1 sequences) the extent of marrow infiltration. Major blood vessels are well seen. Soft tissue tumor mass and adjacent normal muscle are distinguished on T2-weighted images.30 The coronal and sagittal images are best suited for determining the gross tumor volume (GTV), but correlation with the bone scan is mandatory.
After treatment, an elevated serum lactase dehydrogenase level may herald relapse.31 Plain radiographs, CT, and MRI of the primary lesion will show primary site recurrence.30,32 The bone scan should return to normal within a median of 12 months (range 6 to 18 months) after radiotherapy. Recurrence of uptake at a median of 10 months after radiation (range 6 to 12 months) or persistent uptake signals primary site tumor regrowth or, occasionally, pathologic fracture or ectopic bone formation.29
Staging System
No staging system is in common use for Ewing sarcoma of bone. Pretreatment tumor characteristics that portend a poor prognosis include presence of metastases,23 proximal or central sites,33 volume greater than either 100 or 500 ml,33,34 and gross soft tissue extension.35
Standard Treatment
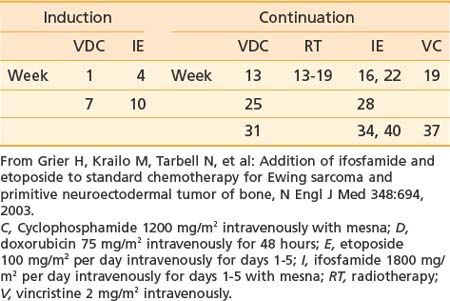
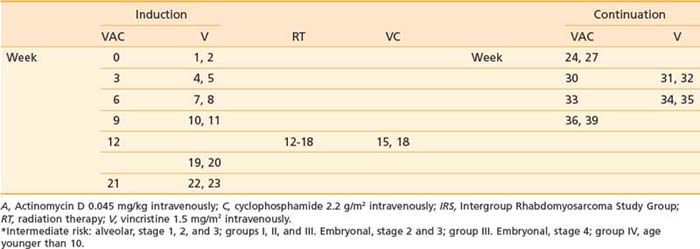
Treatment of Ewing sarcoma requires prompt initiation of chemotherapy at completion of diagnostic evaluation. Treatment of the primary site with radiotherapy, surgery, or both follows induction chemotherapy. Intergroup Study 0091 demonstrated the superiority of a six-drug regimen.36 A modified regimen for patients receiving definitive radiotherapy on the subsequent Children’s Oncology Group (COG) Study AEWS 0031 is shown in Table 55-1 and represents a contemporary standard.
The propensity for Ewing sarcoma to extend within the medullary cavity or into adjacent soft tissues has limited the application of limb-sparing procedures successfully used for osteosarcoma patients. Historically, surgical resection was restricted to “expendable” bones (e.g., rib, clavicle, proximal fibula, scapula). The apparent benefit for the surgical approach in retrospective studies (in comparison with radiotherapy) resulted, in part, from selection bias, for which there was no reliable control.37 The proportion of nonmetastatic patients having surgery alone in Intergroup Study 0091 was 38%, with 23% having surgery and radiotherapy and 39% having radiotherapy alone. In the first two European Cooperative Ewing Sarcoma Studies (CESS 81 and 86), the proportion of patients having surgery with or without postoperative radiotherapy was 66%, and the number having preoperative radiotherapy was 5%. In the subsequent European Intergroup Cooperative Ewing Sarcoma Study (EICESS 92), the proportions were 37% and 42%, respectively.38
Role of Radiotherapy
Radiotherapy is used pre- or postoperatively or definitively for the primary tumor and for treatment of pulmonary and skeletal metastases. Management of the primary tumor follows induction chemotherapy. The indications for surgery versus radiotherapy as initial local therapy are still evolving. For the very young child who would have significant leg-length discrepancy after definitive radiotherapy, amputation and prosthetic replacement may be preferred. Smaller lesions in expendable bones should also be considered for primary surgery, as should very large lesions (e.g., in the iliac wing). Sites with pathologic fracture can have definitive radiotherapy if healing occurs during induction chemotherapy; otherwise, surgery is required. After primary surgery, adjuvant radiotherapy is indicated for intralesional resection or positive or close margins. It must be emphasized that the surgical intent should be to completely resect the tumor with negative margins because “debulking” is not an appropriate approach. Although recommended margins of resection are 2 to 5 cm, COG studies define minimal acceptable margins as at least 1 cm for bony margin, at least 5 mm for fat or muscle planes, and at least 2 mm for facial planes. If such minimal resection margins are achieved, postoperative radiotherapy is not indicated. In the three CESS/EICESS trials, local failure occurred in 20% of patients with intralesional resection who had postoperative radiotherapy. The same study showed a less than 5% local failure risk for patients who had good histologic response to initial chemotherapy followed by wide resection (with or without postoperative radiotherapy). The patients given preoperative radiotherapy had a local failure risk of 5%.38
Controversy persists regarding whether surgery or radiation affords greatest likelihood of local control. This debate is particularly pertinent for primary sites such as the pelvis for which surgery often entails extirpative resections. Although no randomized trial has compared local control modalities and no such study is likely to transpire, retrospective studies suggest that local recurrence rates after surgery are lower than those following definitive radiation. However, selection factors that portend worse outcome clearly bias such studies in favor of surgery because larger tumors with poor response to therapy involving sites less amenable to surgery are referred more often for definite radiation. Selection bias notwithstanding, data presented in abstract form analyzes local control modalities in the patients with nonmetastatic Ewing sarcoma of the bone treated on Intergroup Study-0091.36 A total of 329 patients were included, with 122 receiving surgery, 142 definitive radiation, and 65 both local control modalities. At 5 years, cumulative incidences of disease progression were 22.1% for surgery, 36.9% for radiation, and 48.1% for combined surgery and radiation. The investigators used definitive radiation as the reference group and reported that the hazard ratio for local failure was 0.38 (95% CI 0.15-0.98; P = 0.04) for surgery and 0.77 (95% CI 0.34-1.75) for surgery plus radiation and the hazard ratios for death for these two comparisons were 0.77 (95% CI 0.44-1.34) and 1.46 (95% CI 0.88-2.42), respectively. Although confounding factors introduce clear bias in favor of surgery and results therefore must be interpreted with circumspection, rates of disease progression, distant failure, and death did not differ between the surgery and radiation groups. However, patients undergoing surgery had lower local failure rates than those receiving definitive radiation.
The contribution of confounding factors to these conclusions is highlighted by a separate analysis of the same Intergroup Study. In this published report, local control modalities were compared for patients with pelvic Ewing sarcoma enrolled on INT-0091. Seventy-five patients were randomized to receive doxorubicin, vincristine, cyclophosphamide, and dactinomycin (VACA) or VACA alternating with ifosfamide and etoposide (VACA-IE). Neither tumor size nor local control modality affected event-free survival (EFS) or risk of local failure. Cumulative incidence of local failure was 25% for the 12 patients who had surgery, 25% for the 44 who had definitive radiation, and 10.5% for the 19 who had both (P = 0.46).39 In contrast, systemic therapy did influence the risk of local failure. The 5-year cumulative incidence of local failure was 30% for those receiving VACA and only 11% for the patients treated with VACA-IE (P = 0.06), a difference that was evident regardless of local control modality.40
Simulation
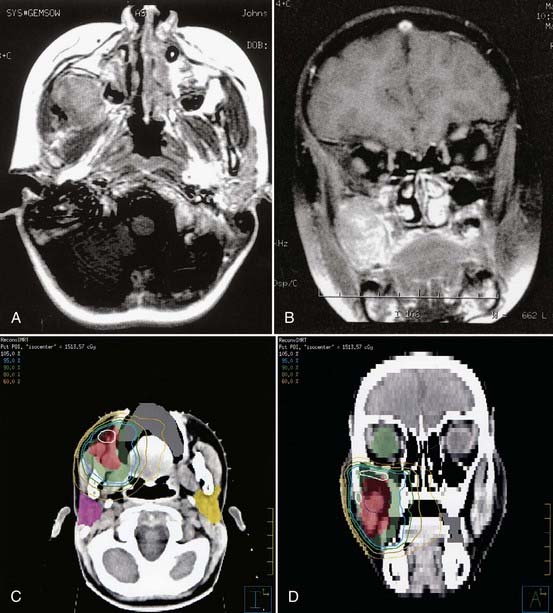
Ewing sarcoma can occur in virtually any bone, and therefore the principles of simulation and treatment planning are site-dependent. The initial decision for treatment planning is determination of optimal patient position. For an extremity lesion, a typical plan employs parallel opposed portals and the limb must be positioned so that the lesion, its adjacent soft tissue extension, and the biopsy or surgical scar are all encompassed while leaving a strip of soft tissue outside the port. The avoidance of circumferential irradiation facilitates lymphatic drainage and prevents the late appearance of distal edema. A combination of limb rotation and beam angulation (gantry rotation) usually permits the necessary soft tissue sparing for lesions that have mostly anterior or posterior extension. For the patient with an iliac or sacral primary lesion, the prone position, with or without the use of the “belly board” (which allows bowel to fall forward) will help reduce bowel dose. Bladder distention helps keep the intestine away from the treated volume in patients with a pubic or ischial primary tumor. The patient with a scapular primary tumor may be positioned prone with the involved limb extending off the treatment couch and moved cephalad to place the scapula in a plane that accommodates opposed tangential ports and that maximally spares underlying lung. Immobilization of the treated limb should be done using plaster of Paris or commercially available thermal-reaction polymers. Figure 55-3 illustrates the imaging studies of a child with a primary tumor of the distal left humerus that had anterior soft tissue extension.
Portal margins are based on prechemotherapy imaging studies. Particular attention must be paid to soft tissue extension in axial planes and intramedullary extension of long bones. The contribution of both MRI and bone scan imaging is essential. The typical presentation of the primary lesion in a long bone usually requires inclusion of the adjacent epiphysis in the port. Historically, the volume extended to include the opposite epiphysis, but this is no longer required.41 For the COG study AEWS 0031, the margin requirements were clinical target volume (CTV) equivalent to GTV plus 1.5 cm and planning target volume (PTV) equivalent to CTV plus 0.5 to 1 cm. A reduced portal (CTV = 1 cm) is based on the postinduction chemotherapy, preradiotherapy imaging if there has been regression of soft tissue extension. The reduced portal maintains the same margin around the tumor extent in bone, but is planned to reflect the regression of the soft tissue extension. This is done after a tumor dose of 45 Gy. Exceptions may be made to the rule that the CTV includes soft tissue extension as it existed at the time of diagnosis. Examples include margins where the medial surface of primary lesions in the ileum or rib have displaced bowel or lung (respectively), which then returned to a more anatomic location with regression. The same concept applies for low pelvic lesions that displace the bladder at the time of diagnosis, but do so to a lesser extent after induction chemotherapy. After resection of a primary lesion with microscopic residual, the margins are the original GTV plus 2 to 2.5 cm to 45 Gy; thereafter, a reduced port covers the area of residual tumor (CTV plus PTV = 1.5 to 2 cm) to an additional 5.4 Gy (three fractions).
Radiation Treatment Plan
If radiotherapy is given after resection of the primary lesion, the total dose may be limited to 50.4 Gy for the patient with positive margins. Gross residual tumor requires full dose (55.8 Gy). Single institution reports, however, suggest that the postoperative total dose may be reduced to 30 Gy.42 For the patient with a rib primary tumor who has risk factors for pleural involvement, treatment should encompass the entire, ipsilateral hemithorax (1.5 Gy/day to 15 to 18 Gy) before reducing the port to a CTV appropriate to the rib primary tumor (Fig. 55-4).43
Critical Normal Tissues
Ewing sarcoma can occur adjacent to virtually every important normal tissue. Fig. 55-5 illustrates the treatment plan for a primary lesion in the third cervical vertebra using proton-beam therapy to minimize dose to the spinal cord. When treating an extremity, the plan should spare a strip of normal tissue. The epiphyseal growth plate opposite the primary tumor can usually be excluded from treatment. Inclusion of only half the growth plate may lead to an exaggerated angle of the affected limb to its adjacent bone. Radiation-induced limb shortening is a function of age at diagnosis and radiotherapy dose. In a study from the orthovoltage era, growth loss for children receiving approximately 50 Gy was about 25% of the remaining (incremental) growth in the unaffected limb.44 Leg dysfunction in patients treated at the National Cancer Institute (NCI) was scored as minor or moderate in 18 of 22 patients. One patient required amputation.45 Late functional results from a study of patients with primary tumors of an extremity who received hyperfractionated radiotherapy (1.2 Gy twice daily; total of 50.4 to 60 Gy) were very good and were subjectively improved over standard fractionation. No pathologic fractures occurred.46 Five-year survivors of lower extremity osteosarcoma and Ewing sarcoma were assessed for limb function and quality of life. Children treated after age 12 and females fared less well. No difference was observed between those having amputation or a limb-sparing procedure.47
Reports of radiation-induced osteosarcomas usually describe patients whose tumor doses were 60 Gy or more. A multi-institutional report demonstrated no secondary sarcomas in patients receiving 48 Gy or less.48 The NCI Surveillance, Epidemiology, and End Results Program included 595 patients with Ewing sarcoma. One osteosarcoma and three soft tissue sarcomas developed in radiotherapy ports.49 Secondary sarcomas developed in 3 of 674 patients enrolled in the CESS trials.50
After radiotherapy, widely distributed lytic changes of radiation osteitis will be seen on the plain radiograph in the initial 2 to 3 years. A localized area of lysis may herald recurrence of Ewing sarcoma or, if at least 5 years have elapsed, a treatment-related bone sarcoma.51
Outcome
Several American institutions have reported treatment results for large patient numbers with 5- and 10-year follow-up. The Mayo Clinic report includes 105 patients with nonmetastatic disease treated from 1969 to 1982. Five-year survival was 42%, and local control was maintained in 73% of patients.23 The NCI report included 80 patients with nonmetastatic disease treated between 1968 and 1980. The 5- and 10-year survival rates were 51% and 39%, respectively. Local control was 80%.25 The IESS-I trial enrolled 342 patients with nonmetastatic disease from 1973 to 1978 in a randomized trial of three treatment regimens. The 5- and 10-year survival rates for the best arm of the study were 65% and 60%, respectively. Local control was 85% and did not differ significantly by treatment assignment.56 The failure pattern for nonmetastatic patients on IESS 0091 was 5% for local only (experimental arm), 20% for systemic (standard or experimental arm), and 2% to 5% for local and systemic or unknown (both arms).36 The EICESS 92 trial enrolled 479 nonmetastatic patients. The 5-year relapse-free survival rate was 59%.53
Several patient and tumor characteristics affect outcome. Three that are interrelated are site, size, and extraosseous extension. Many studies indicate that pelvic, sacral, and other central sites have a worse prognosis. These lesions are typically larger than those at other sites and are more likely to have extraosseous extension. In IESS-I, patients with nonpelvic primary tumors had an overall 5-year survival rate of 57% and, on the superior treatment regimen, 72%. The 5-year survival for patients with a pelvic primary lesion was 34% (no difference by treatment arm), and relapses continued after 5 years. The survival rate was statistically significantly higher in IESS-II for patients with pelvic primary tumors, 63% at 5 years, and the local failure declined from 28% to 12%. The improvement was attributed to more intensive chemotherapy, more aggressive surgery, and the effect of CT on radiation treatment planning.54 For patients with nonpelvic primary lesions, the IESS-II study (1978-1982) compared a regimen similar to the best arm of IESS-I (control arm) with a more intensive schedule of the same drugs. Five-year survival in the intensive arm was 77% and in the control arm was 63% (P = 0.05).55 (The comparable figure for the best arm of IESS-I was 72%, as noted earlier.52,55) The local failure for IESS-II patients with nonpelvic disease was 9%. No site group (proximal, distal, rib, or other) was prognostic for an effect on survival.55
In another report, the presence of soft tissue extension in patients with localized disease was more predictive of failure than large tumor size.35 Significant prognostic factors (endpoint: 10-year EFS) in CESS 86 included tumor size greater than 200 ml (EFS 36%) and less than 200 ml (EFS 63%), as well as poor histologic response (poor response EFS 38% versus good response EFS 64%).56 To assess the relationship of these two variables, patients from the CESS and French EW88 trials were combined and the analysis restricted to 385 nonmetastatic patients who had initial chemotherapy followed by surgery (with or without postoperative radiotherapy). Histologic response (which was independent of tumor size) became the only significant prognostic factor. Based on the percentage of viable cells in the surgical specimen, three prognostic groups were determined (endpoint: 5-year EFS): low risk (<10% cells), 73% EFS; intermediate risk (10% to 49% cells), 56% EFS; high risk (>50% cells), 37% EFS.57 Patients younger than age 3 constituted only 2.6% of the patients enrolled on five IESS protocols. Their 5-year survival rate was identical to that of older patients.58
Outcome of therapy at various uncommon sites has been published: rib, survival rate 50% to 60%43,59; vertebral column, 5-year EFS 36% to 44%60,61; small bones of the hands and feet, 6 of 7 alive; calcaneus, 1 of 5 alive62; and bones of the head and neck, survival rate 80% (see Fig. 55-5).63
Patients with metastases at diagnosis do not have a uniformly fatal outcome. The CESS trials enrolled 114 patients with metastases confined to the lung and pleura. The 10-year EFS for bilateral metastases was 24%; unilateral was 53%. Survival was better for patients who received whole-lung irradiation.64 When patients with skeletal metastases were included, the 4-year EFS was 27%.65 Bone marrow ablative therapy (including total-body irradiation) for patients with bone and marrow metastases did not improve outcome compared with conventional therapy in a report from the Memorial Sloan-Kettering Cancer Center.66
Soft Tissue Sarcoma
Epidemiology
Soft tissue sarcomas in children have an annual incidence of about 650 cases, or 6% of the approximate total of 10,500 new cancer cases per year in U.S. children younger than age 15.1 They therefore rank among pediatric solid tumors after central nervous system and neuroblastoma, before bone tumors and retinoblastoma, and at about the same incidence as Wilms tumor. Slightly more than half of patients have rhabdomyosarcoma (RMS). The remainder, non-RMS soft tissue sarcomas (NRSTSs), are a conglomerate of many types of heterogeneous origin that are individually rare. For both types, the male/female ratio is about 1.2 : 1 and the incidence in both blacks and whites is 8 to 9 per million.67
Causal Factors and Genetics of Rhabdomyosarcoma
An environmental factors study of 322 children enrolled in Intergroup RMS Study (IRS)-III found that parental use of marijuana and cocaine in the year before a child’s birth was highly correlated with the subsequent diagnosis of RMS. The risk was greater (fivefold) for maternal use of cocaine.68
The normal development from uncommitted mesenchymal cell to myoblast (myogenesis) and the cell’s aberrant transformation to malignancy are being elucidated. Myogenic transcription factors are a group of DNA-binding proteins that bind to promoter regions for muscle-specific genes and promote their expression. Several of these factors (MyoD, Myf5, myogenin) are variably expressed in RMS and therefore help to identify a small, blue, round cell tumor without characteristic phenotypic features as RMS and not as another sarcoma such as extraosseous Ewing sarcoma. Defects in the normal pathway are associated with the two major histologic subtypes of RMS: embryonal and alveolar. The embryonal type correlates with loss of heterozygosity at chromosome 11p15.5, with presumed loss of a tumor suppressor locus.69,70 The alveolar subtype features a translocation t(2;13) (q35;q14) that results in the fusion of normally unassociated transcription factors (PAX 3) and the activation domain of FKHR. A variant translocation that produces the PAX 7-FKHR fusion transcript is associated with a better outcome.71 A study of 45 tissue samples of RMS using comparative genomic hybridization and fluorescence in situ hybridization methods demonstrated that genomic gains and losses were similar in alveolar and embryonal histologies, and that they did not differ between the two alveolar fusion transcript subtypes. Both histologies had a 1 : 4 rate of genomic amplification, which, for the embryonal subtype, was most common in those with anaplastic features. One locus of amplification included the gene for insulin-like growth factor type 1 receptor, which has been implicated in the induction of RMS.72
The cancer family syndrome of Li-Fraumeni includes childhood RMS and NRSTS and is characterized by p53 suppressor gene germline mutations.73
Pathologic Conditions
An international committee of pathologists expert in RMS reviewed the histologies of 800 randomly chosen cases from IRS-II.74 The resulting classification retains the basic division between the embryonal and alveolar subtypes, omits the formerly used pleomorphic subtype, and further refines the embryonal category as follows: favorable prognosis, botryoid embryonal and spindle cell (SC) embryonal; intermediate prognosis, all other embryonal; and unfavorable prognosis, all alveolar subtypes (includes cases with any alveolar component) and undifferentiated sarcoma. Examples are shown in Figs. 55-2B through F.
There are several anatomic site associations for the histologic types of RMS. The botryoid tumor occurs in the bladder and vagina, usually in infants and younger children, and 31% of SC variant cases occur in the paratesticular site. Other sites are head and neck, orbit, and extremity.75 About half of patients have an embryonal type that has no particular propensity for specific anatomic sites. Alveolar RMS predominates in extremity and truncal sites. A rare, unfavorable variant containing anaplastic cells is found most often in lower extremity, retroperitoneum and pelvis, and paratesticular sites.76 Whether histologic features are an independent prognostic factor is uncertain. The IRS staging system uses primary site in preference to histologic findings; however, all nonmetastatic patients with alveolar histologic findings were assigned to the intermediate risk trial of IRS-V. The distribution of histologic types on IRS-IV for nonmetastatic patients was 70% embryonal, 20% alveolar, 4% undifferentiated sarcoma, and 6% other.77
Clinical Presentation
Males with RMS predominate in the ratio of 3 : 2, and in IRS-I the race distribution was 80% white, 12% black, and 8% other.78 Median age at presentation is 5 years; and the distribution on IRS-III was 6% less than 1 year and 66% less than 10 years.79 The proportion of all patients presenting with dissemination is 17%.80
Symptoms at presentation relate to anatomic site of the primary tumor. Primary site distribution is shown in Fig. 55-1. The parameningeal site is composed of four sites: nasopharynx and nasal cavity, paranasal sinuses, middle ear and mastoid, and infratemporal space and pterygopalatine fossa. Symptoms from a parameningeal primary tumor are protean and include cranial nerve palsy, facial pain and swelling, meningeal irritation, nasal voice, mouth breathing, trismus, and painless adenopathy.81 Parameningeal primary tumors may erode skull base bone, transgress the dura, and invade brain; however, subarachnoid space dissemination is an uncommon event at diagnosis. Orbital tumors present with unilateral proptosis, chemosis, impaired mobility, ptosis, and lid or conjunctival mass.82 The other head and neck lesions present as a superficial mass that may cause seventh nerve palsy if arising in the parotid region or a mass lesion that interferes with speech or swallowing.
The second most frequent primary site is genitourinary (23%) divided between bladder and prostate (11%) and other genitourinary sites (female genital tract and paratesticular) (12%).78 The typical presentation is of bladder distention or outlet obstruction. Sarcoma botryoids may protrude from the vagina. Paratesticular presentation is as a painless mass. Next in frequency are extremity lesions (17%), which present with an enlarging, usually painless mass.
Routes of Spread
RMS is usually locally invasive and therefore frequently unresectable. In IRS-II patients with nonmetastatic tumor at presentation, 64% had only a biopsy or subtotal resection, approximately 20% had microscopic residual disease, and 16% had complete resection.80 A retrospective review of IRS-I and IRS-II patients in clinical groups I and II provided data on lymphatic metastases in 592 cases. Nodal spread was not influenced by gender, age, or histologic features. It was influenced by site as follows: 41% prostate, 26% paratesticular, 12% extremity, 3% trunk, 7% head and neck, 0% orbit, and 14% all sites.83 The inclusion of group I patients in the denominator minimizes the estimate of nodal involvement.
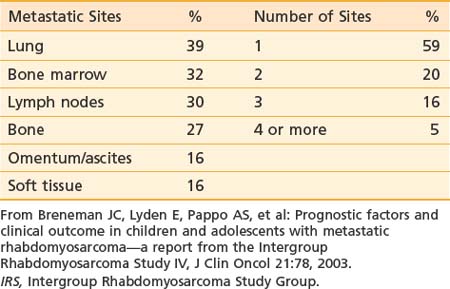
At presentation, 17% of IRS-II patients had hematogenous metastases (most common) or, less frequently, involvement of distant lymph node groups or malignant pleural or peritoneal effusion. Hematogenous metastases occur most frequently in lung. Both bone and bone marrow metastases are common.84 Unusual sites (e.g., heart, breast, and subcutaneous tissues) can occur.85,86 Brain metastases at diagnosis are rare.87 The pattern and frequency of metastatic sites for IRS-IV patients are shown in Table 55-2.
Diagnostic and Staging Studies
Patients with RMS require diagnostic studies that consist of both standard and site-specific assessments. In addition to the routine history, detailed family history of cancer should be obtained. In addition to the routine physical examination, an examination under anesthesia may be required for genitourinary presentations, and direct nasopharyngoscopy and laryngoscopy may be needed for upper airway primary tumors. Routine blood cell count and a standard chemistry assay are done. Renal function is assessed. Detailed assessment of the primary tumor with MRI or CT is required to determine location, invasiveness, and size. For orbit and parameningeal primary sites, the MRI should include the brain to characterize intracranial extension. Sagittal and coronal views are useful for radiotherapy planning.88 For genitourinary primary tumors, the entire urinary tract should be imaged with MRI or CT. Studies to assess dissemination include abdominal and chest CT and bone scan.89 A comparison of imaging with positron emission tomography to conventional imaging demonstrated that pet scanning was superior in detecting node and bone metastases.90 Bone marrow aspirate and biopsy are required, as is lumbar puncture for cerebrospinal fluid examination (chemistry and cytologic studies) for parameningeal sites.
Staging System
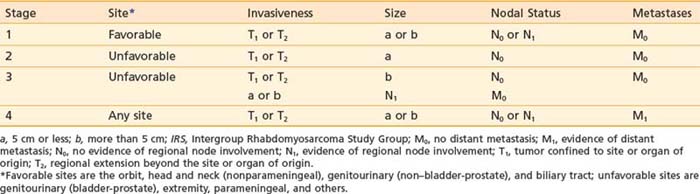
In the United States, 80% to 85% of patients with RMS are entered on the IRS trials that have been ongoing since 1972. The IRS grouping system for classifying patients in prognostic categories and for determining therapy is thus a de facto national standard. The IRS groups are described in Table 55-3. The system, which is surgical and pathologic, assesses extent of resection for patients with localized disease. It is clearly prognostic for treatment outcome but does not account for the variation in surgical aggressiveness as a first therapeutic event. Accordingly, the IRS committee designed a pretreatment staging system that separately categorizes the primary tumor (T), the regional nodal status (N), the presence of metastases (M), and the primary anatomic site. The system (Table 55-4) was retrospectively assessed and found to be of prognostic significance and to be reproducible.91,92 The TNM system was evaluated prospectively in IRS-III and was used to assign treatment in IRS-IV. The pretreatment staging system uses five features to determine stage: (1) primary site, (2) primary tumor invasiveness, (3) size, (4) nodal status, and (5) metastases. Primary sites are divided into favorable—orbit, nonparameningeal head and neck, biliary, and genitourinary (but not bladder or prostate); and unfavorable—all others, including bladder and prostate, extremity, and parameningeal. Invasiveness categories are T1, confined to anatomic site of origin; and T2, extension or fixation to surrounding tissues. T stage data are obtained, but they do not alter stage assignment. Size, rather than T stage, was believed to be a more reliable parameter and therefore affects stage assignment. Size (maximum tumor diameter) is divided into two categories: a, less than or equal to 5 cm; and b, greater than 5 cm. Nodal status is determined clinically: N0 indicates regional nodes clinically uninvolved, and N1 indicates regional nodes clinically involved. M staging assignment is M0, no distant metastases; and M1, indicating that metastases are present, often hematogenous, and including distant lymph nodes, pleural or peritoneal dissemination (or malignant effusion), and cytologically positive cerebrospinal fluid.
Table 55-3 Intergroup Rhabdomyosarcoma Study Clinical Group Classification
Standard Therapy
Surgery
Treatment of RMS is multidisciplinary. The role of surgery in the therapeutic strategy is, in part, driven by primary site location. Many primary tumors are technically unresectable by virtue of site, size, and invasiveness. Resection of others would entail organ sacrifice, limb amputation, or cosmetic disfigurement. Among localized patients on IRS-IV, 62% of patients were in this category (group III). The use of surgery as a first therapeutic maneuver yielded margins as follows: group I (clear margins), 23%; and group II (microscopically positive margins), 15%.77 Control of the primary site requires adjuvant radiotherapy for group II patients and definitive radiotherapy for the largest subset, group III. A subset of patients with incomplete resection (node-negative, group II) may be amenable to elective re-excision of the primary site before starting other treatment to achieve negative margins and thus restrict management to a group I approach. When this strategy was followed for patients with trunk and extremity primary tumors in IRS-I and IRS-II, there was an apparent survival benefit.93 In addition to the initial diagnostic and therapeutic role for surgery, the regression that occurs with induction chemotherapy (± radiotherapy) may render some group III primary tumors resectable. This opportunity was studied in IRS-III. A group of 83 patients (primary sites: extremity, trunk, retroperitoneum) had a second-look surgical procedure after induction chemotherapy and radiotherapy; 20% had complete resection of their initially unresected primary tumor. In addition, approximately 60% of partial responders and nonresponders had specimens from their second-look procedure consistent with pathologic complete response.93a
Chemotherapy
The most effective chemotherapeutic agents for RMS are vincristine, actinomycin D, and cyclophosphamide (VAC). The schema and drug doses for VAC as used in the intermediate-risk IRS-V trial are shown in Table 55-5. That study compares VAC with a regimen wherein topotecan (a topoisomerase I inhibitor) replaces actinomycin. Irinotecan (CPT-11), also a topoisomerase I inhibitor, is being evaluated in a phase II, “upfront window” study of stage IV patients enrolled in IRS-V.
Radiotherapy
Radiotherapy is not required for the patient with embryonal RMS who at diagnosis is node negative and has resection with negative surgical margins (group I).78 Postoperative adjuvant radiotherapy is given to group I patients with alveolar histology and all group II patients.94 Definitive radiotherapy is required for group III patients enrolled on COG protocols. The practice of restricting the use of radiotherapy has been studied by the International Society of Pediatric Oncology.95 In a comparison with COG-sponsored trials, 5-year survival and EFS were generally improved in the COG protocols.95 Radiotherapy is also given with curative intent to the primary site in group IV patients along with treatment, when feasible, to metastatic sites (except bone marrow).
Simulation
RMS occurs in every anatomic site, and the details of simulation and planning are site-dependent. For all patients, whether or not sedation is required, immobilization in a reproducible, comfortable position is essential. Plaster of Paris, thermoplastic polymer, or vacuum-evacuated polystyrene foam bead bags are useful. Patient laser-beam setup points should be translated in the appropriate plane to the immobilization device to ensure reproducible positioning. Most patients can be positioned supine. For treatment of head and neck sites, a younger child should be elevated (below the neck) over the simulator table 2 to 3 inches to allow the neck to be extended. This will move structures of the oral cavity farther away from the thyroid gland and the pharynx. Principles for positioning and immobilizing a patient with a primary tumor of an extremity are the same as for Ewing sarcoma. CT-based simulation is essential for three-dimensional treatment planning.96 Sedation or anesthesia is required for many children younger than age 3.97 A reward-based training and desensitization program by an experienced nurse will enable children in the 3- to 9-year age group to cooperate.98
Radiotherapy Plan
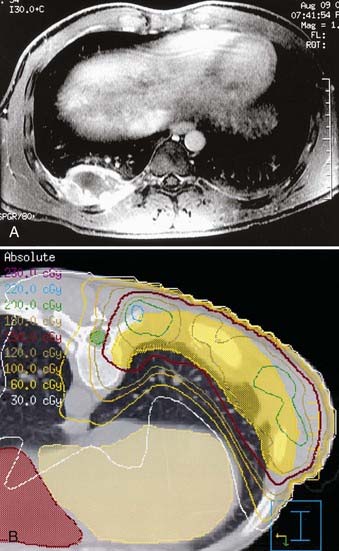
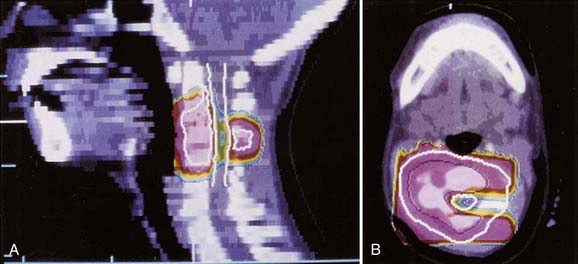
Patients with parameningeal primary tumors may have intracranial extension. This usually represents displacement of dura rather than brain parenchymal invasion. A 1.5-cm margin (CTV) above this extension is appropriate. Whole-cranial radiotherapy is not needed unless there is evidence of hematogenous metastases, diffusely involved meninges, or cytologically positive cerebrospinal fluid (in which case, spinal subarachnoid space radiotherapy is required). Figure 55-6 shows the MRI and intensity-modulated radiotherapy isodose plan for a child with parameningeal RMS arising in the pterygoid space.
Planning should always attempt to exclude cornea, lenses, and pituitary for orbit lesions. A proton-beam plan elegantly excludes extraorbital normal structures.99 For a primary tumor of an extremity, inclusion of muscle from origin to insertion is not necessary. Figure 55-7 illustrates the combination of postoperative brachytherapy and external-beam radiotherapy for a 2-year-old boy with embryonal RMS of the right soleus muscle. Pleural or peritoneal involvement or malignant effusion requires that the initial port cover the hemithorax or entire peritoneal surface, respectively. For primary pelvic tumors, careful attention must be paid to the caudal (perineal) margin because it may not always be clearly seen on axial imaging studies.
Radiotherapy for group III patients on IRS-IV was a randomized comparison between an experimental arm (hyperfractionated radiotherapy) and a standard fractionation control arm.100 No survival or local control advantage was evident in the twice-a-day group and it was concluded that once-a-day fractionation remains the standard of care: Group III patients are treated once per day, all fields each session, 5 days per week, 1.8 Gy per fraction, to a total of 50.4 Gy. Large volumes may be treated with a reduced daily dose of 1.5 Gy. The IRS-V protocol addresses the role of “second-look” surgery after 12 weeks of chemotherapy for most group III patients. Those patients with sufficient response to induction chemotherapy may have surgery if it is anticipated that form and function will be maintained and that surgical margins will be clear. The postoperative radiotherapy dose is reduced as determined by the margin status: 36-Gy negative margins and 41.4-Gy microscopically involved margins. For gross residual tumor, the total dose remains 50.4 Gy. Patients not qualifying for second-look surgery can also have dose reduction to 41.4 Gy if they have clinical and biopsy evidence of complete response; otherwise, they receive 50.4 Gy.
In IRS-V, radiotherapy is not given to group I patients with embryonal histologic findings but is dictated for group I patients with alveolar histologic findings (41.4 Gy). Group II patients also receive 41.4 Gy if node positive and 36 Gy if node negative. Patients in group IV receive a daily and total dose to the primary tumor that would be used in the absence of dissemination (if a “local” group I, II, or III). Total dose to metastatic sites is 50.4 Gy, but should also account for the constraints of normal tissue tolerance (Table 55-6).
Table 55-6 Normal Tissue Tolerance With Conventional Fractionation
| Organ | Total Dose, Gy |
|---|---|
| Kidney | 14.4 |
| Whole liver | 23.4 |
| Bilateral lungs | 14.4 |
| Whole brain | |
| >3 yr | 30.6 |
| <3 yr | 23.4 |
| Optic nerve and chiasm | 46.8 |
| Spinal cord | 45 |
| Gastrointestinal tract | 45 |
| Whole abdomen and pelvis | 30 at 1.50/day |
| Heart | 30.6 |
| Lens | 14.4 |
| Lacrimal gland/cornea | 41.4 |
Brachytherapy
Use of brachytherapy is restricted by the invasive character of localized RMS and the issues of confinement and nursing care for a radioactive child. The foremost proponent of brachytherapy has been the group at Institut Gustave-Roussy in Villejuif, France. Their early experience with small, subcutaneous sites (e.g., lip, nasolabial fold, perineum) led them to treat vagina and other gynecologic sites and head and neck sites. The technique was usually after loading 192-iridium (192Ir) at approximately 0.50 Gy per hour to an average total of 60.8 Gy (pelvic sites) and 64 Gy (head and neck, perineum, extremities). Three-fourths of patients had RMS. The survival rate of the entire group was 78%.101 In an update that excluded pelvic primary sites, the 5-year local failure-free survival (FFS) rate was 66% in 72 children. There was a need for cosmetic rehabilitation in 24%, mainly for head and neck sites.102 The same group achieved bladder preservation (mean dose 52 Gy) in 8 of 12 patients with bladder or prostate RMS. They recommend their approach in patients with tumor size of 4 cm or less.103 Although late sequelae requiring surgical correction in vulvar and vaginal RMS occur, the French group reports successful childbearing in 2 of 17 patients.104
The University of Amsterdam group reports an intraoperative mold technique for head and neck sites. The mold is placed operatively in the surgical defect, and thereafter brachytherapy is accomplished using 192Ir over 50 to 70 hours (dose of 40-50 Gy). Surgical removal and plastic closure follows. Good healing and encouraging early cosmetic outcome were reported; however, 5 of 15 patients failed locally.105
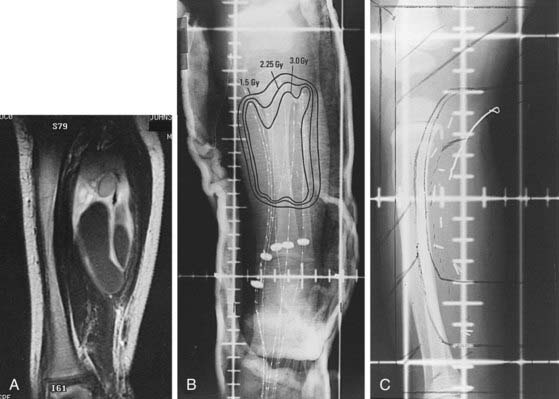
The development of the remote afterloading high-dose-rate brachytherapy device preserves the normal tissue-sparing advantage and eliminates the problem of nursing care for the radioactive child. It also permits the use of fractionated brachytherapy. Investigators at Ohio State University have used a regimen of 3 Gy twice a day to a total dose of 36 Gy delivered to the postinduction chemotherapy volume. Patients were treated for primary tumors of the tongue, buccal mucosa, chest wall, clitoris, and vagina.106 The indications for brachytherapy are evolving.107 Conservative indications include group III patients with good response to induction chemotherapy and small residual tumor volume (see Fig. 55-7), and group II patients with an initial GTV less than 5 cm with primary tumors in sites such as extremity; superficial trunk; scalp and face; oral cavity and oropharynx; uterus, vagina, or vulva; and bladder or prostate. It may also be used after less than full-dose external-beam radiotherapy as a volume-restricted tumor bed boost.
Critical Normal Tissues
The growing normal tissues of a pediatric RMS patient should be shielded whenever possible, consistent with sufficient margin about the GTV. Examples of common problems include protection of contralateral lens (always), ipsilateral lens (if possible), and pituitary in patients with orbital and other head and neck tumors; unerupted molars in patients with head and neck tumors; larynx and thyroid gland when treating neck adenopathy; female breast buds when thorax lesions are present; epiphyseal growth plates and joints in extremity primary tumor sites; kidneys in lower paravertebral sites; capital femoral epiphyses and rectum in pelvic sites; and the small intestine in abdominal sites. When a high dose of radiation to the abdomen is required, temporary surgically implanted tissue expanders to displace bowel should be considered. Controlled iatrogenic pneumothorax to avoid radiation pneumonitis has been successfully used for primary chest wall tumors.108
Tolerance doses as recommended in IRS-V using daily fractions of 1.8 Gy are listed in Table 55-6. These general guidelines may require modification for very young patients or those with a large CTV. For example, 45 Gy to a large volume of small intestine, particularly in a postlaparotomy patient, may lead to radiation enteritis or small bowel obstruction.
Outcome
Five-year survival of all patients in the IRS studies has shown improvement: 56% in IRS-I, 63% in IRS-II, and 72% in IRS-III. The 3-year FFS rate for nonmetastatic patients on IRS-IV was 77% (76% for IRS-III). The pattern of failure (3-year cumulative incidence) was 10% local, 4% regional, and 7% distant. Prognostic factors for 3-year FFS included 83% for clinical group I, 86% for group II, and 73% for group III. Also, the pattern of failure for age group was 55% for age younger than 1 year, 83% for age 1 to 9 years, and 68% for age 10 years and older. Outcomes by histologic findings and risk group are shown in Table 55-7.77
Table 55-7 Three-Year Failure-Free Survival Rate for IRS-IV Patients (Without Metastases) by Risk Group and Histologic Findings
| Risk Group* | ||
|---|---|---|
| Histologic Findings | Low | Intermediate |
| Embryonal | 93% | 76% |
| Alveolar/undifferentiated | 72% | 55% |
IRS, Intergroup Rhabdomyosarcoma Study Group.
* Low risk: stage 1, clinical groups I, II, III; and stage 2 and 3, clinical groups I and II. Intermediate risk: stage 2 and 3, clinical group III.
From Crist WM, Anderson JR, Meza JL, et al: Intergroup Rhabdomyosarcoma Study-IV: results for patients with nonmetastatic disease, J Clin Oncol 19:3091, 2001.
The failure pattern for group II patients enrolled on IRS-I to IV was assessed as 8% local failure, 4% regional failure, and 14% distant failure.109 Local control in group III patients varies with site. The 5-year estimate of cumulative local failure risk on IRS-IV was 5% orbit, 12% head and neck, 16% parameningeal, 19% bladder and prostate, 7% extremity, and 14% other sites.100 IRS-IV patients with metastatic disease (group IV) had 3-year FFS of 25%; however, a favorable subset with embryonal histologic findings, age younger than 10 years, and one or two metastatic sites had 3-year FFS of 45%.110 A pooled analysis of American and European trials that assessed risk factors for outcome showed a 50% EFS for children between ages 1 and 10 who had a favorable primary site, no bone-marrow involvement, and only one or two metastatic sites.111
The special problems in management of infants younger than age 1 have been reviewed and the effect of age on outcome has been variable. Data to support omission of radiotherapy are lacking.112–115 In general, there is a greater reliance on second-look surgery, and brachytherapy is encouraged.
The late sequelae of radiotherapy are site-related and have been reviewed by Heyn.116 Specific, site-related reports are noted in the following section. The risk for second malignant neoplasms in 1026 survivors enrolled on IRS-I and IRS-II was 1.7% at 10 years. Treatment with both an alkylating agent plus radiotherapy, compared with only one modality, carried increased risk, and a contributing factor was a family history of neurofibromatosis or Li-Fraumeni syndrome.117 The 20-year cumulative risk of second malignant neoplasms in 4367 patients on IRS-I-IV was 3.5%. Secondary leukemia or lymphoma and sarcoma were equally likely.118
Outcome by Site
Head and Neck
The orbit is the most common head and neck site for RMS. The history of improved outcome of these patients has been reviewed by Sagerman.119 Patients with orbital tumors enrolled in IRS-I and II had 3-year local control of 94%.120 For 186 patients enrolled on IRS studies through 1992, the 10-year EFS was 86%.121 An early review of IRS-IV suggested improved 3-year EFS compared with IRS-III.122 Five-year survival for patients with alveolar histologic findings was 74%.123 The recommended total dose is 45 Gy. A European cooperative group trial omitted radiotherapy for patients achieving complete remission with chemotherapy and 38% avoided radiotherapy; however, one-third of patients failed locally with a 4-year EFS of 62%.124 Late effects have been tabulated for patients on IRS-I: 90% had decreased vision, usually secondary to cataract formation; half of patients had facial asymmetry; and 61% had decreased stature resulting from radiotherapy.125 Growth impairment was 24% for IRS-III patients, possibly reflecting improved treatment planning.126
Tumors of the parameningeal sites constitute half of all head and neck primaries. An early IRS report noted that the pattern of failure in these patients included meningeal extension in 35% and that outcome thereafter was fatal in 90%.127 Subsequent single-institution reports found a lesser risk.128,129 The IRS strategy was altered in 1977 to include whole-cranial prophylactic radiotherapy and intrathecal chemotherapy for patients who had cranial nerve palsy, skull-base erosion, or intracranial extension. In addition, radiotherapy began concurrently with chemotherapy for patients with these high-risk features. Three-year survival improved from 41% to 68% and by site was 73% for middle ear and mastoid, 69% for nasopharynx and nasal cavity, 68% for infratemporal fossa, and 60% for paranasal sinuses.130 The subsequent IRS trials omitted prophylactic intrathecal methotrexate and cranial and spinal radiotherapy without a decline in outcome. Five-year EFS was influenced by histologic factors and impingement of the primary tumor on adjacent meninges: alveolar and impingement was present in 48%, and embryonal and impingement on adjacent meninges was not present in 81%.131 A trial was conducted to eliminate radiotherapy in patients younger than age 3. With intensified chemotherapy, only 11% avoided radiotherapy or radical surgery.132 The remaining 25% of patients with head and neck RMS have involvement of the scalp, pinnae, face, parotid region, masseter muscle, cheek, buccal mucosa, oral cavity, oropharynx, hypopharynx, and larynx. Eighty-nine patients without metastases in IRS-I and IRS-II had a 3-year survival rate of 83%. Local control for the patients who received at least 40 Gy was 93%.133
An analysis of late effects of therapy in 213 patients with parameningeal and other nonorbital head and neck sites revealed growth retardation in 48%, with 19% receiving growth hormone. Facial and mandibular bone hypoplasia developed in 35%, of whom one-fifth had reconstructive surgery. Other sequelae included poor dentition, decreased vision and hearing, and poor school performance.134
Genitourinary Sites
The treatment approach for patients with bladder and prostate tumors on IRS-I relied on radical surgery. In IRS-II, induction chemotherapy was used to delay radiotherapy or reduce radiotherapy dose. In each study, the proportion of patients alive and with a functional bladder at 3 years was approximately 23%.135 For IRS-III, standard dose radiotherapy (45 Gy) was given at week 6. The proportion of patients alive with a functional bladder at 3 years was 60%.136 The presence of postradiation maturing rhabdomyoblasts led to possibly unnecessary cystectomy in some IRS-III patients.137 Approximately 20% of bladder primary patients may have tumor confined to the bladder dome.138 Forty such patients in IRS-I and IRS-II had partial cystectomy and 31 survived (24 bladder symptom–free). The benefit of radiotherapy was uncertain.139
Late sequelae in patients with bladder or prostate RMS were assessed in 54 patients with bladder preservation of 109 survivors enrolled on IRS-I and IRS-II. Bladder function was satisfactory in 73% of patients. Renal dysfunction and hypertension were uncommon. There was statural growth retardation in 10%.140
Paratesticular RMS is characterized by relatively early diagnosis, a predominance of embryonal histology (93%), and a 27% proportion of the more favorable SC variant histologic features. The patients with SC variant were more likely to be group I or II compared with the non-SC types (98% versus 80%) and less likely to involve lymph nodes (16% versus 36%). The overall rate of nodal involvement (determined pathologically) was 31%.75 Retroperitoneal lymph node dissection was required for IRS-III, but not IRS-IV, which resulted in an increase in the proportion of IRS-IV patients in clinical group I. These patients therefore did not receive nodal radiotherapy and experienced a decrease in 3-year FFS, which was worse in boys age 10 and older: FFS was 78% in IRS-III and 68% in IRS-IV. The recommendation for retroperitoneal lymph node dissection was reinstituted for boys age 10 and older for IRS-V.141 Group IV patients can have a protracted natural history, and cure is possible. The 3-year survival rate is 64%.142,143 Radiotherapy should therefore be given to nodal and other metastatic sites as recommended for group IV patients.
Late sequelae attributable to radiotherapy in 86 IRS-I and IRS-II survivors included testicular dysfunction when testis shielding was not used, bone and soft tissue hypoplasia, and four instances of chronic diarrhea in 37 patients. Bladder function was unaffected by radiotherapy.144 Another report confirmed the risk of gastrointestinal sequelae and impaired statural growth.145
The IRS protocols I through IV included 151 girls with genital tract primary tumors. Fifty-four percent occurred in the vagina, 64% were group III, and 87% had embryonal or botryoid histologic features. Five-year survival was 87% for nonmetastatic patients, but was better (98%) for girls 1 to 9 years old. Radiation therapy was used in less than one-third of patients.146 Surgical resection for vaginal primaries has declined from IRS-I (100%) to IRS-IV (13%).147 One-third of girls enrolled in trials conducted by International Society of Pediatric Oncology were cured with chemotherapy alone. The authors noted the efficacy of brachytherapy when local therapy was required.148 Late effects were assessed in 12 girls who had attained pubertal age. Normal puberty occurred in 11, and 2 had three children. There were vaginal or other sequelae as follows: none in five, minimal in three who required surgery to allow intercourse, and serious in four (colorectal, vaginal, urethral, and ureteral stenosis).104 The late effects of radiotherapy in girls treated for pelvic RMS may be severe and require surgical intervention for correction.95
Extremity Sites
Among 102 patients with an extremity primary tumor in IRS-I, 44% had alveolar histologic features (25 of 48 in groups III and IV).149 The 5-year survival in IRS-III was 74%.79 In IRS-IV, 76 of 139 patients had surgical evaluation of regional lymph nodes and 38 (50%) were positive. The 5-year FFS for node-positive patients was 35% compared with 70% for node-negative patients.150 The efficacy of radiation therapy in the control of node metastases from extremity primaries was demonstrated in IRS-II.151 The local failure rate in group III patients on IRS-IV was 7%.106
Other Sites
Thirty-six patients with primary tumors in the perineum were treated in IRS-I and IRS-II. For groups II and III, the number failing locally and 3-year survival rates were 2 of 11 and 46% in group II, and 5 of 15 and 37% in group III.152 RMS of the chest and abdominal wall was reported in 20 patients on IRS-I. Disease-free survival was attained in 6 of 11 chest wall and 2 of 9 abdominal wall patients.153 Four teenage girls have been enrolled on IRS protocols to 1992 with primary, locoregional breast RMS. All survive.154 RMS in the paraspinal soft tissue was greater than 5 cm in diameter in half of patients and, in 55% of cases in IRS-I and IRS-II, exhibited either undifferentiated histologic findings or extraosseous Ewing sarcoma. Fifty-six percent of patients required laminectomy for spinal cord compression at the time of diagnosis. Local or regional failure was a component of failure in 12 of 27 relapse patients.155 Intrathoracic primary tumors (mediastinum, lung, pleura) occurred in 17 IRS-I and IRS-II patients. The benefit of radiotherapy was inconclusive.156 Intra-abdominal RMS occurs in various sites. Embryonal or botryoid histologic features were present in 24 of 25 patients with a biliary tract RMS, and 17 were disease-free. Aggressive surgery was not necessary and these patients are considered a “favorable” site in the IRS staging system.157 Gastric RMS may present as an unknown primary lesion.158 Radiotherapy contributed to survival in a report of hepatic RMS.159 There were 101 patients enrolled on IRS-I and IRS-II with a retroperitoneal primary tumor. The median tumor size was 10 cm, and there was local or regional failure in 60% of evaluable patients. Five-year survival was 30%.160
Nonrhabdomyosarcoma Soft Tissue Sarcoma
Single-institution retrospective reviews of NRSTS report 5-year survival rates from 50% to 89%. Most confirm the substantial survival rate discrepancy between group I (IRS) patients (approximately 80%) and group III patients (12% to 30%).161–168 In one report, 5-year relapse-free survival increased from 21% to 70% with postoperative radiotherapy (dose > 45 Gy).161 Another report showed the 5-year survival rate to vary by grade: 92% for low-grade and 59% for high-grade.162 One series (n = 154) had the following site distribution: 45% extremity, 35% trunk, and 15% head and neck. The primary site distribution compiled from five large series is depicted in Fig. 55-1. The most common histologic finding was synovial sarcoma (23%), followed by malignant fibrous histiocytoma and neurogenic sarcoma (16% each). Metastases were typically of trunk and lower extremity sites, whereas upper-extremity tumors were usually in groups I or II (IRS). There was a strong correlation between invasiveness, histologic grade, and size greater than 5 cm, with, for example, a 12% disease-free survival rate in the 42% of patients with T2 lesions greater than 5 cm (all grades).168 All of these studies confirm the lack of prognostic significance of primary site in contrast to RMS. A prospective trial conducted by the Pediatric Oncology Group concluded in 1994. There was no benefit of adjuvant chemotherapy for patients with NRSTS, group I or II (IRS), grade 1 or 2. Also, postoperative radiotherapy was not needed in group I patients, grade 1 or 2.169,170
The general principle for the use of postoperative radiotherapy in pediatric NRSTS is that it is indicated for the patient equivalent to IRS group II or III. For group I patients with clear but close margins, or uncertain margins, the adverse prognostic factors of higher T stage, larger size, and higher grade should influence the decision to treat. A report of 88 group I patients assessed outcome based on margin and grade. With margins less than 1 cm, high grade, and postoperative radiotherapy, none of seven recurred. Without radiotherapy, three of seven recurred. For 20 high-grade patients with margins of 1 cm or more, none received radiotherapy and four recurred.171
In a series of 121 group I and II children, factors predicting local failure were positive surgical margins, intra-abdominal site, and omission of radiation therapy.172 A report of local failure for group III patients showed improvement from 9 of 12 to 3 of 15 children when radiotherapy dose (median) was increased from 39.6 Gy to 59.4 Gy.173
For synovial sarcoma, histologic subtype (monophasic or biphasic) does not affect prognosis, but higher grade and size greater than 5 cm does. A multivariate analysis of 39 patients with localized synovial sarcoma demonstrated the significance of fusion transcript type on 5-year survival: SYT-SSX2 (48%) versus SYT-SSX1 (24%).174 The 5-year survival for IRS group III and IV patients was 17% in one series that spanned 30 years but was 58% in a contemporary German cooperative group trial. In that trial, the median radiotherapy dose was 50 Gy and four of five patients not getting radiotherapy experienced local relapse.175–177 Another report showed a reduced local failure rate with postoperative radiotherapy, but an unstated proportion were IRS group I.178 The largest retrospective study of pediatric patients noted improved overall survival in radiotherapy patients.179 A high risk of local recurrence is associated with synovial sarcoma of pleuropulmonary primary site.180
Fibrosarcoma is usually low-grade. In a series of 66 children, local recurrence was noted in one-third, most of which were seen in the first 2 years (range of 1 month to 6 years). All patients with local recurrence had a positive margin, as did 46% of those with no recurrence.181 A review of 110 children noted a metastasis rate of 7% in patients younger than age 5 despite a local recurrence rate of 43%. The metastatic rate in patients older than age 10 years was 50%.182
Malignant fibrous histiocytoma occurred in the head and neck area in 6 of 16 children in two series. Postoperative radiotherapy (30 to 55 Gy) and chemotherapy were effective in three of five children.183,184 A higher dose in a mainly adult series (60 Gy) resulted in a 68% (actuarial) rate of local control at 5 years.185 The angiomatoid malignant fibrous histiocytoma is a variant seen in the age range of 5 to 25 years (median 13 years). Its clinical presentation is usually as a painless, nodular, subcutaneous lesion thought to be a hematoma or hemangioma. Local recurrence was common and usually appeared within 1 year of diagnosis. Metastasis was uncommon.186 In a series of 28 pediatric patients with malignant peripheral nerve sheath tumor, 39% had neurofibromatosis syndrome, type 1, which had no affect on outcome. Resectability was the prognostic factor most influencing 5-year survival: 65% for IRS groups I and II and 0% for groups III and IV. Radiotherapy was recommended for group II patients.187 Liposarcoma is usually low-grade and the benefit of chemotherapy is not apparent. Cure is accomplished by complete resection. Radiotherapy is effective against microscopic residual disease (42 to 60 Gy, median dose of 57 Gy), but its role in pediatric patients with gross residual disease has not been adequately tested.188,189
The Askin tumor (malignant small cell tumor of the thoracopulmonary region during childhood) is recognized (along with extraosseous Ewing sarcoma and peripheral primitive neuroectodermal tumor) to share the characteristic t(11 : 22) chromosomal translocation.190–192 For patients with known or suspected pleural involvement, intrapleural colloidal 32-phosphate (32P) should be considered in addition to external-beam radiotherapy.193 In the IRS studies to 1991, 130 patients with extraosseous Ewing sarcoma were registered. Primary site distribution was 32% trunk, 26% extremity, 18% head and neck, 20% retroperitoneal and genitourinary, and 5% other. Forty-six percent were in group III and their 10-year FFS rate was 55%.194
The alveolar soft part sarcoma occurs in the age range of 10 to 30. It arises primarily in the extremities (mainly the thigh) and has a predilection for the right side. The natural history is for the gradual evolution to distant metastases (lung, bone, brain) over a protracted time, ranging to 15 years.195,196 In a pediatric series, 1 patient of 11 died of disease (6 of 11 received radiotherapy).197 A review of epithelioid sarcoma noted a median age of 23 years with the lower age range of 4 years. The lesions usually arise in the hand, forearm, or pretibial region.198 Lymph node involvement occurs. Four of five patients treated postoperatively to a median dose of 68 Gy were locally controlled and disease free.199 Postoperative therapy may be useful for the intra-abdominal desmoplastic small round cell tumor that occurs mainly in adolescents. In one report, 2 of 17 patients survived.200 The malignant melanoma of soft parts (clear cell sarcoma) may occur in older children. It usually involves tendons in the foot, knee, and other limb sites, and nodal metastases may occur. Radiotherapy may prevent local recurrence, but distant metastasis is common.201 Other rare extremity sarcomas of childhood include the extraskeletal myxoid chondrosarcoma,202 the malignant rhabdoid tumor,203 and hemangiopericytoma.204 Mesothelioma may occur in children.205
Children develop various primary organ sarcomas for which radiotherapy may be required; however, the experience with radiotherapy is limited or anecdotal. Examples include the pleuropulmonary blastoma,206 malignant rhabdoid tumor of the kidney,207 clear cell sarcoma of the kidney,208 and undifferentiated (embryonal) sarcoma of the liver.209,210 Leiomyosarcoma of bowel has been reported in two girls with acquired immunodeficiency syndrome.211
Aggressive Fibromatosis
Aggressive fibromatosis (desmoid tumor) is occasionally seen in children. Definitive radiotherapy may be required for lesions of the shoulder girdle or head.212–215 A dose of 50 to 55 Gy in standard fractions with generous margins is appropriate; however, alternative treatments including chemotherapy, hormonal therapy, and nonsteroidal anti-inflammatory drugs should be considered.216–218
Aggressive fibromatosis is a slow-growing fibroblastic tumor arising from musculoaponeurotic stromal elements and thus can occur in virtually any anatomic site. In the United States, 2.4 to 4.3 new cases of desmoid are diagnosed per million, with incidence peaking at four distinct age ranges, including the pediatric population.219 Although data pertaining to pediatric desmoids are sparse, published reports suggest that pediatric desmoids may be particularly aggressive and difficult to control.220,221 This, combined with long-term morbidity associated with radiation therapy in children220 have led to a schism in which, on the one hand, more aggressive local control measures are recommended to improve outcome for pediatric desmoids patients, whereas on the other hand external-beam radiation therapy approaches are delayed or eliminated in this patient population. Recent and ongoing investigations are evaluating protocols that rely on systemic agents and omit external-beam radiation therapy; however, early data suggest the need for more intensified local therapy for optimal disease control.222,223
In treating children, many practitioners are inclined to avoid or delay adjuvant radiation if a gross-total resection has been achieved, even if surgical margins are positive. Although understandable given the potential long-term effects of radiation in children, this approach must be weighed against the high likelihood of tumor recurrence that then entails complex, prolonged, and aggressive salvage therapy. In fact, studies show that pediatric desmoids are more likely to recur locally than adult desmoids. Published studies suggest that desmoids in children may be biologically less responsive to radiation therapy than their counterparts in adults.220,221,224,225
In the largest study assessing the role of radiation therapy in pediatric desmoids, Merchant et al.220 reported the experience of St. Jude Children’s Research Hospital in which 13 pediatric desmoids were treated with radiotherapy in either the primary or salvage setting to a median dose of 50 Gy. With all but one patient progressing after external-beam radiation therapy, the authors concluded that pediatric desmoids are likely to recur after external-beam radiation therapy and are less responsive to radiation than desmoids occurring in adults.
1 Gurney JG, Severson RK, Davis S, et al. Incidence of cancer in children in the United States. Cancer. 1995;75:2186.
2 Hug EB, Fitzek MM, Munzenrider JE, et al. Locally challenging osteo- and chondrogenic tumors of the axial skeleton: results of combined proton and photon radiation therapy using three-dimensional treatment planning. Int J Radiat Oncol Biol Phys. 1995;31:467.
3 Lombardi F, Gandola L, Fossati-Bellani F, et al. Hypofractionated accelerated radiotherapy in osteogenic sarcoma. Int J Radiat Oncol Biol Phys. 1992;24:761.
4 Ozaki T, Flege S, Liljenqvist U, et al. Osteosarcoma of the spine. Cancer. 2002;94:1069.
5 Anderson PM, Arndt CAS, Rodriquez V, et al. High-Dosel53 SM-EDTMP (Samarium Ethylenediaminetetramethylphosphonate) therapy in locally recurrent or metastatic osteosarcoma. Med Pediatr Oncol. 2001;37:183. [abstract O81]
6 Wollner N, Lane JM, Marcove RC, et al. Primary skeletal non-Hodgkin’s lymphoma in the pediatric age group. Med Pediatr Oncol. 1992;20:506.
7 Nishida J, Sim FH, Wenger DE, et al. Malignant fibrous histiocytoma of bone. A clinicopathologic study of 81 patients. Cancer. 1997;79:482.
8 Campanacci M, Boriani S, Giunti A. Hemangioendothelioma of bone: a study of 29 cases. Cancer. 1980;46:804.
9 Wold LE, Unni KK, Beabout JW, et al. Hemangioendothelial sarcoma of bone. Am J Surg Pathol. 1982;6:59.
10 Finsterbush A, Husseini N, Rousso M. Multifocal hemangioendothelioma of bones in the hand: a case report. J Hand Surg. 1981;6:353.
11 Welles L, Dorfman H, Valentine ES, et al. Low grade malignant hemangioendothelioma of bone: a disease potentially curable with radiotherapy. Med Pediatr Oncol. 1994;23:144.
12 Dahlin DC. Giant-cell tumor of vertebrae above the sacrum: a review of 31 cases. Cancer. 1977;39:1350.
13 Schwartz LH, Okunieff PG, Rosenberg A, et al. Radiation therapy in the treatment of difficult giant cell tumors. Int J Radiat Oncol Biol Phys. 1989;17:1085.
14 Bennett CJ, Marcus RB, Million RR, et al. Radiation therapy for giant cell tumor of bone. Int J Radiat Oncol Biol Phys. 1993;26:299.
15 Marks RD, Scruggs HJ, Wallace KM, et al. Megavoltage therapy in patients with aneurysmal bone cysts. Radiology. 1976;118:421.
16 Buckley JD, Pendergrass TW, Buckley CM, et al. Epidemiology of osteosarcoma and Ewing’s sarcoma in childhood. A study of 305 cases by the children’s cancer group. Cancer. 1998;83:1440.
17 Turc-Carel C, Philip I, Berger MP, et al. Chromosomal translocations in Ewing’s sarcoma. N Engl J Med. 1983;309:497.
18 Whang-Peng J, Triche TJ, Knutsen T, et al. Chromosome translocation in peripheral neuroepithelioma. N Engl J Med. 1984;311:584.
19 Delattre O, Zucman J, Melot T, et al. The Ewing family of tumors-a subgroup of small-round-cell tumors defined by specific chimeric transcripts. N Engl J Med. 1994;331:294.
20 Hahm K, Cho K, Lee C, et al. Repression of the gene encoding the TGF-b type II receptor is a major target of the EWS-FLI1 oncoprotein. Nature Genetics. 1999;23:222.
21 Ginsberg JP, de Alava E, Ladanyi M. EWS-FLI1and EWS-ERG gene fusions are associated with similar clinical phenotypes in Ewing’s sarcoma. J Clin Oncol. 1999;17:1809.
22 Owen LA, Kowalewski AA, Lessnick SL. EWS/FLI mediates transcriptional repression via NKX2.2 during oncogenic transformation in Ewing’s sarcoma. PLoS ONE. 2008;3(4):e1965.
23 Wilkins RM, Pritchard DJ, Burgert EO, et al. Ewing’s sarcoma of bone: Experience with 140 patients. Cancer. 1986;58:2551.
24 Mameghan H, Fisher RJ, O’Gorman-Hughes D, et al. Ewing’s sarcoma: Long-term follow-up in 49 patients treated from 1967 to 1989. Int J Radiat Oncol Biol Phys. 1993;25:431.
25 Kinsella TJ, Miser JS, Waller B, et al. Long-term follow-up of Ewing’s sarcoma of bone treated with combined modality therapy. Int J Radiat Oncol Biol Phys. 1991;20:389.
26 Kissane JM, Askin FB, Foulkes M, et al. Ewing’s sarcoma of bone: clinicopathologic aspects of 303 cases from the Intergroup Ewing’s Sarcoma Study. Hum Pathol. 1983;14:773.
27 Cangir A, Vietti TJ, Gehan EA, et al. Ewing’s sarcoma metastatic at diagnosis: results and comparison of two Intergroup Ewing’s Sarcoma studies. Cancer. 1990;66:887.
28 Horowitz ME, Malawer MM. Ewing’s sarcoma family of tumors: Ewing’s sarcoma of bone and soft tissue and the peripheral primitive neuroectodermal tumors. In: Pizzo PA, Poplack DG, editors. Principles and practice of pediatric oncology. ed 2. Philadelphia: JB Lippincott; 1993:795.
29 Hugenholtz EAL, Piers DA, Kamps WA, et al. Bone scintigraphy in nonsurgically treated Ewing’s sarcoma at diagnosis and follow-up: prognostic information of the primary tumor site. Med Pediatr Oncol. 1994;22:236.
30 Boyko OB, Cory DA, Cohen MD, et al. MR imaging of osteogenic and Ewing’s sarcoma. AJR Am J Roentgenol. 1987;148:317.
31 Farley FA, Healey JH, Caparros-Sison B, et al. Lactase dehydrogenase as a tumor marker for recurrent disease in Ewing’s sarcoma. Cancer. 1987;59:1245.
32 Reinus WR, Gilula LA, Donaldson S, et al. Prognostic features of Ewing sarcoma on plain radiograph and computed tomography scan after initial treatment: a Pediatric Oncology Group Study (8346). Cancer. 1993;72:2503.
33 Jürgens H, Exner U, Gadner H, et al. Multidisciplinary treatment of primary Ewing’s sarcoma of bone. A 6-year experience of a European Cooperative Trial. Cancer. 1988;61:23.
34 Sailer SL, Harmon DC, Mankin HJ, et al. Ewing’s sarcoma: surgical resection as a prognostic factor. Int J Radiat Oncol Biol Phys. 1988;15:43.
35 Mendenhall CM, Marcus RB, Enneking WF, et al. The prognostic significance of soft tissue extension in Ewing’s sarcoma. Cancer. 1983;51:913.
36 Grier H, Krailo M, Tarbell N, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing’s sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348:694.
37 Barbieri E, Emiliani E, Zini G, et al. Combined therapy of localized Ewing’s sarcoma of bone: analysis of results in 100 patients. Int J Radiat Oncol Biol Phys. 1990;19:1165.
38 Shuck A, Ahrens S, Paulussen M, et al. Local therapy in localized Ewing tumors: results of 1058 patients treated in the CESS 81, CESS 86 and EICESS 92 trials. Int J Radiat Oncol Biol Phys. 2003;55:168.
39 Yock TI, Krailo M, Fryer CJ, et al. Local control in pelvic Ewing sarcoma: analysis from INT-0091—a report from the Children’s Oncology Group. J Clin Oncol. 2006;24(24):3838.
40 DuBois S, Krailo M, Cook EF, et al: Evaluation of local control in patients with non-metastatic Ewing sarcoma of the bone: a report from the Children’s.
41 Donaldson SS, Torrey M, Link MP, et al. A multidisciplinary study investigating radiotherapy in Ewing’s sarcoma: end results of POG #8346. Int J Radiat Oncol Biol Phys. 1998;42:125.
42 Merchant TE, Kushner BH, Sheldon JM, et al. Effect of low-dose radiation therapy when combined with surgical resection for Ewing sarcoma. Med Pediatr Oncol. 1999;33:65.
43 Schuck A, Dunst J, Ahrens S, et al. Hemithorax irradiation in Ewing tumors of the chest wall. Med Pediatr Oncol. 2001;37:177. [abstract O58]
44 Gonzalez D, Breur K. Clinical data from irradiated growing long bones in children. Int J Radiat Oncol Biol Phys. 1983;9:841.
45 Jentzsch K, Binder H, Cramer H, et al. Leg function after radiotherapy for Ewing’s sarcoma. Cancer. 1981;47:1267.
46 Marcus R, Cantor A, Heare TC, et al. Local control and function after twice a day radiotherapy for Ewing’s sarcoma of bone. Int J Radiat Oncol Biol Phys. 1991;21:1509.
47 Nagarajan R, Clohisy DR, Greenberg M, et al. Function and quality of life (QOL) of survivors of pediatric lower extremity bone tumors: a report from the Childhood Cancer Survivor Study (CCSS). Proc Am Soc Clin Oncol. 2002;21:395a. [abstract 1577]
48 Kuttesch JF, Wexler LH, Marcus RB, et al. Second malignancies after Ewing’s sarcoma: radiation dose-dependency of secondary sarcomas. J Clin Oncol. 1996;14:2818.
49 Travis LB, Curtis RE, Hankey BF, et al. [Letter to the editor]: Second cancers in patients with Ewing’s sarcoma. Med Pediatr Oncol. 1994;22:296.
50 Dunst J, Ahrens S, Paulussen MP, et al. Second malignancies after treatment for Ewing’s sarcoma: a report of the CESS-studies. Int J Radiat Oncol Biol Phys. 1998;42:379.
51 Ehara S, Kattapuram SV, Egglin TK. Ewing’s sarcoma: radiographic pattern of healing and bony complications in patients with long-term survival. Cancer. 1991;68:1531.
52 Nesbit ME, Gehan EA, Burgert EO, et al. Multimodal therapy for the management of primary, nonmetastatic Ewing’s sarcoma of bone: a long-term follow-up of the First Intergroup Study. J Clin Oncol. 1990;8:1664.
53 Ahrens S, Paulussen M, Craft AW, et al. Ewing tumor of bone—updated report of the European Intergroup Cooperative Ewing’s Sarcoma Study EICESS 92. Proc Am Soc Clin Oncol. 2002;21:393a. [abstract 1568]
54 Evans RG, Nesbit ME, Gehan EA, et al. Multimodal therapy for the management of localized Ewing’s sarcoma of pelvic and sacral bones: a report from the Second Intergroup Study. J Clin Oncol. 1991;9:1173.
55 Burgert EO, Nesbit ME, Garnsey LA, et al. Multimodal therapy for the management of nonpelvic, localized Ewing’s sarcoma of bone: Intergroup Study IESS-II. J Clin Oncol. 1990;8:1514.
56 Paulussen M, Ahrens S, Dunst J, et al. Localized Ewing tumor of bone: final results of the Cooperative Ewing’s Sarcoma Study CESS 86. J Clin Oncol. 2001;19:1818.
57 Le Deley MC, Ahrens S, Paulussen M, et al. Histological response is the main prognostic factor of survival in localized Ewing tumour (ET) treated with chemotherapy alone before surgery. Med Pediatr Oncol. 2001;37:178. [abstract O60]
58 Maygarden SJ, Askin FB, Siegal GP, et al. Ewing sarcoma of bone in infants and toddlers: a clinicopathologic report from the Intergroup Ewing’s Study. Cancer. 1993;71:2109.
59 Thomas PRM, Foulkes MA, Gilula LA, et al. Primary Ewing’s sarcoma of the ribs: a report from the Intergroup Ewing’s Sarcoma Study. Cancer. 1983;51:1021.
60 Venkateswaran L, Rodriquez-Galindo C, Merchant TE, et al. Primary Ewing tumor of the vertebrae: clinical characteristics, prognostic factors, and outcome. Med Pediatr Oncol. 2001;37:30.
61 Helfre S, Habrand JJ, Zucker JM, et al. Primary Ewing’s sarcoma of the spine: report of the French Society of Pediatric Oncology. Med Pediatr Oncol. 2001;37:178. [abstract O59]
62 Shirley SK, Askin FB, Gilula LA, et al. Ewing’s sarcoma in bones of the hands and feet: a clinicopathologic study and review of the literature. J Clin Oncol. 1985;3:686.
63 Siegal GP, Oliver WR, Reinus WR, et al. Primary Ewing’s sarcoma involving the bones of the head and neck. Cancer. 1987;60:2829.
64 Paulussen M, Ahrens S, Craft AW, et al. Ewing’s tumors with primary lung metastases: survival analysis of 114 (European Intergroup) cooperative Ewing’s sarcoma studies patients. J Clin Oncol. 1998;16:3044.
65 Paulussen M, Ahrens S, Burdach, et al. Primary metastatic (stage IV) Ewing tumor: survival analysis of 171 patients from the EICESS studies. Ann Oncol. 1998;9:275.
66 Meyers PA, Krailo MD, Ladanyi M, et al. High-dose melphalan, etoposide, total-body irradiation, and autologous stem-cell reconstitution as consolidation therapy for high-risk Ewing’s sarcoma does not improve prognosis. J Clin Oncol. 2001;19:2812.
67 Robison L. General principles of the epidemiology of childhood cancer. In: Pizzo PA, Poplack DG, editors. Principles and practice of pediatric oncology. ed 3. Philadelphia: JB Lippincott; 1997:1.
68 Grufferman S, Schwartz AG, Ruymann FB, et al. Parents’ use of cocaine and marijuana and increased risk of rhabdomyosarcoma in their children. Cancer Causes Control. 1993;4:217.
69 Scrable HJ, Witte DP, Lampkin BC, et al. Chromosomal localization of the human rhabdomyosarcoma locus by mitotic recombination mapping. Nature. 1987;329:645.
70 Scrable HJ, Cavenee W, Ghavimi F, et al. A model for embryonal rhabdomyosarcoma tumorigenesis that involves genome imprinting. Proc Natl Acad Sci USA. 1989;86:7480.
71 Sorenson PH, Lynch JC, Qualman SJ, et al. PAX3-FKHR and PAX7-FKHR gene fusions are prognostic indicators in alveolar rhabdomyosarcoma: a report from the Children’s Oncology Group. J Clin Oncol. 2002;20:2672.
72 Bridge JA, Liu J, Qualman SJ, et al. Genomic gains and losses are similar in genetic and histologic subsets of rhabdomyosarcoma, whereas amplification predominates in embryonal with anaplasia and alveolar subtypes. Genes Chromosomes Cancer. 2002;33:310.
73 Malkin D, Li FP, Strong LC, et al. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990;250:1233.
74 Asmar L, Gehan EA, Newton WA, et al. Agreement among and within groups of pathologists in the classification of rhabdomyosarcoma and related childhood sarcomas: report of an international study of four pathology classifications. Cancer. 1994;74:2579.
75 Leuschner I, Newton WA, Schmidt D, et al. Spindle cell variants of embryonal rhabdomyosarcoma in the paratesticular region: a report of the Intergroup Rhabdomyosarcoma Study. Am J Surg Pathol. 1993;17:221.
76 Kodet R, Newton WA, Hamoudi AB, et al. Childhood rhabdomyosarcoma with anaplastic (pleomorphic) features: a report of the Intergroup Rhabdomyosarcoma Study. Am J Surg Pathol. 1993;17:443.
77 Crist WM, Anderson JR, Meza JL, et al. Intergroup Rhabdomyosarcoma Study-IV: results for patients with nonmetastatic disease. J Clin Oncol. 2001;19:3091.
78 Maurer HM, Beltangady M, Gehan EA, et al. The Intergroup Rhabdomyosarcoma Study-I: a final report. Cancer. 1988;61:209.
79 Crist W, Gehan EA, Ragab AH, et al. The Third Intergroup Rhabdomyosarcoma Study. J Clin Oncol. 1995;13:610.
80 Maurer HM, Gehan EA, Beltangady M, et al. The Intergroup Rhabdomyosarcoma Study-II. Cancer. 1993;71:1904.
81 Wharam MD. Rhabdomyosarcoma of parameningeal sites. Semin Radiat Oncol. 1997;7:212.
82 Wharam MD, Maurer HM. Management of orbital rhabdomyosarcoma. In: Jacobs C, editor. Cancers of the head and neck. Boston: Martinus Nijhoff; 1987:223.
83 Lawrence W, Hays DM, Heyn R, et al. Lymphatic metastases with childhood rhabdomyosarcoma: a report from the Intergroup Rhabdomyosarcoma Study. Cancer. 1987;60:910.
84 Raney RB, Tefft M, Maurer HM, et al. Disease patterns and survival rate in children with metastatic soft-tissue sarcoma: a report from the Intergroup Rhabdomyosarcoma Study (IRS)-I. Cancer. 1988;62:1257.
85 Pratt CB, Dugger DL, Johnson WW, et al. Metastatic involvement of the heart in childhood rhabdomyosarcoma. Cancer. 1973;31:1492.
86 Howarth CB, Caces JN, Pratt CB. Breast metastases in children with rhabdomyosarcoma. Cancer. 1980;46:2520.
87 Spunt SL, Anderson JR, Teot LA, et al. Routine brain imaging is unwarranted in asymptomatic patients with rhabdomyosarcoma arising outside the head and neck region that is metastatic at diagnosis. A report from the Intergroup Rhabdomyosarcoma Study Group. Cancer. 2001;92:121.
88 Yousem DM, Lexa FJ, Bilaniuk LT, et al. Rhabdomyosarcoma in the head and neck: MR imaging evaluation. Radiology. 1990;177:683.
89 Quddus FF, Espinola D, Kramer SS, et al. Comparison between x-ray and bone scan detection of bone metastases in patients with rhabdomyosarcoma. Med Pediatr Oncol. 1983;11:125.
91 Lawrence W, Gehan EA, Hays DM, et al. Prognostic significance of staging factors of the UICC staging system in childhood rhabdomyosarcoma: a report from the Intergroup Rhabdomyosarcoma Study (IRS-II). J Clin Oncol. 1987;5:46.
92 Rodary C, Flamant F, Donaldson SS. An attempt to use a common staging system in rhabdomyosarcoma: a report of an international workshop initiated by the International Society of Pediatric Oncology (ISPO). Med Pediatr Oncol. 1989;17:210.
93 Hays DM, Lawrence W, Wharam MD, et al. Primary reexcision for patients with “microscopic residual” tumor following initial excision of sarcomas of trunk and extremity sites. J Pediatr Surg. 1989;24:5.
93a Wiener E: Personal communication.
94 Wolden SL, Anderson JR, Crist WM, et al. Indications for radiotherapy and chemotherapy after complete resection in rhabdomyosarcoma: a report from the Intergroup Rhabdomyosarcoma Studies I to III. J Clin Oncol. 1999;17:3468.
96 Michalski JM, Sur RK, Harms WB, et al. Three dimensional conformal radiation therapy in pediatric parameningeal rhabdomyosarcomas. Int J Radiat Oncol Biol Phys. 1995;33:985.
97 Bucholtz JD. Issues concerning the sedation of children for radiation therapy. Oncol Nurs Forum. 1992;19:649.
98 Slifer KJ, Bucholtz JD, et al. Behavioral training of motion control in young children undergoing radiation treatment without sedation. J Pediatr Oncol Nursing. 1994;11:55.
99 Hug EB, Adams J, Fitzek M. Fractionated, three-dimensional, planning-assisted proton-radiation therapy for orbital rhabdomyosarcoma: a novel technique. Int J Radiat Oncol Biol Phys. 2000;47:979.
100 Donaldson SS, Meza J, Breneman JC, et al. Results from the IRS-IV randomized trial of hyperfractionated radiotherapy in children with rhabdomyosarcoma—a report from the IRSG. Int J Radiat Oncol Biol Phys. 2001;51:718.
101 Flamant F, Chassagne D, Cosset JM, et al. Embryonal rhabdomyosarcoma of the vagina in children: conservative treatment with curietherapy and chemotherapy. Eur J Cancer. 1979;15:527.
102 Habrand JL, Ganry O, Gerbaulet A, et al. Interstitial brachytherapy (IB) in the management of soft tissue sarcomas (STS) in children. Med Pediatr Oncol. 1994;23:188. [abstract 0–75]
103 Haie-Meder C, Flamant F, Revillon Y, et al. Brachytherapy in the multidisciplinary therapy of prostate and/or bladder rhabdomyosarcoma in children. Med Pediatr Oncol. 1994;23:188. [abstract 0–74]
104 Flamant F, Gerbaulet A, Nihoul-Fekete C, et al. Long-term sequelae of conservative treatment by surgery, brachytherapy, and chemotherapy for vulval and vaginal rhabdomyosarcoma in children. J Clin Oncol. 1990;8:1847.
105 Schouwenburg PF, Kupperman D, Bakker FP, et al. New combined treatment of surgery, radiotherapy, and reconstruction in head and neck rhabdomyosarcoma in children: the AMORE protocol. Head and Neck. 1998;20:283.
106 Nag S, Grecula J, Ruymann FB. Aggressive chemotherapy, organ-preserving surgery, and high-dose-rate remote brachytherapy in the treatment of rhabdomyosarcoma in infants and young children. Cancer. 1993;72:2769.
107 Nag S, Olson T, Ruymann R, et al. High-dose-rate brachytherapy in childhood sarcomas: a local control strategy preserving bone growth and function. Med Pediatr Oncol. 1995;25:463.
108 Ortega JA, Stowe SM, Shore NA, et al. Prevention of radiation pneumonitis by controlled pneumothorax in childhood rhabdomyosarcoma of the chest wall. Int J Radiat Oncol Biol Phys. 1985;11:2033. [letter]
109 Smith LM, Anderson JR, Qualman SJ, et al. Which patients with microscopic disease and rhabdomyosarcoma experience relapse after therapy? A report of the Soft Tissue Sarcoma Committee of the Children’s Oncology Group. J Clin Oncol. 2001;19:4058.
110 Breneman JC, Lyden E, Pappo AS, et al. Prognostic factors and clinical outcome in children and adolescents with metastatic rhabdomyosarcoma—a report from the Intergroup Rhabdomyosarcoma Study IV. J Clin Oncol. 2003;21:78.
112 Ragab AH, Heyn R, Tefft M, et al. Infants younger than 1 year of age with rhabdomyosarcoma. Cancer. 1986;58:2606.
113 Koscielniak E, Harms D, Schmidt D, et al. Soft tissue sarcomas in infants younger than 1 year of age: a report of the German Soft Tissue Sarcoma Study Group (CWS-81). Med Pediatr Oncol. 1989;17:105.
114 Salloum E, Flamant F, Rey A, et al. Rhabdomyosarcoma in infants under 1 year of age: experience of the Institut Gustave-Roussy. Med Pediatr Oncol. 1989;17:424.
115 Lobe TE, Wiener ES, Hays DM, et al. Neonatal rhabdomyosarcoma: the IRS experience. J Pediatr Surg. 1994;29:1167.
116 Heyn R. Late effects of therapy in rhabdomyosarcoma. Clin Oncol. 1985;4:287.
117 Heyn R, Haeberlen V, Newton WA, et al. Second malignant neoplasms in children treated for rhabdomyosarcoma. J Clin Oncol. 1993;11:262.
118 Spunt SL, Meza JL, Anderson JR, et al. Second malignant neoplasms (SMN) in children treated for rhabdomyosarcoma: a report from the Intergroup Rhabdomyosarcoma Studies (IRS) I–IV. Proc Am Soc Clin Oncol. 2001;20:369a. [abstract 1473a]
119 Sagerman RH. Orbital rhabdomyosarcoma: a paradigm for irradiation. Radiology. 1993;187:605.
120 Wharam M, Beltangady M, Hays D, et al. Localized orbital rhabdomyosarcoma: an interim report of the Intergroup Rhabdomyosarcoma Study Committee. Ophthalmology. 1987;94:251.
121 Oberlin O, Rey A, Anderson J, et al. Treatment of orbital rhabdomyosarcoma: survival and late effects of treatment—results of an international workshop. J Clin Oncol. 2001;19:197.
122 Wharam MD, Anderson JR, Laurie F, et al. Failure-free survival for orbit rhabdomyosarcoma patients on Intergroup Rhabdo myosarcoma Study IV (IRS-IV) is improved compared to IRS-III. Proc Am Soc Clin Oncol. 1997;16:578a. [abstract 1864]
123 Kodet RI, Newton WA, Hamoudi AB, et al. Orbital rhabdomyosarcoma and related tumors in childhood: relationship of morphology to prognosis—an Intergroup Rhabdomyosarcoma Study. Med Pediatr Oncol. 1997;29:51.
124 Rosseau P, Flamant F, Quintana E, et al. Primary chemotherapy in rhabdomyosarcoma and other malignant mesenchymal tumors of the orbit: results of the International Society of Pediatric Oncology MMT 84 Study. J Clin Oncol. 1994;12:516.
125 Heyn R, Ragab A, Raney B, et al. Late effects of therapy in orbital rhabdomyosarcoma in children: a report from the Intergroup Rhabdomyosarcoma Study. Cancer. 1986;57:1738.
126 Raney RB, Anderson JR, Kollath J, et al. Late effects of therapy in 94 patients with localized rhabdomyosarcoma of the orbit: report from the Intergroup Rhabdomyosarcoma Study (IRS)-III, 1984–1991. Med Pediatr Oncol. 2000;34:413.
127 Tefft M, Fernandez C, Donaldson M, et al. Incidence of meningeal involvement by rhabdomyosarcoma of the head and neck in children: a report of the Intergroup Rhabdomyosarcoma Study (IRS). Cancer. 1978;42:253.
128 Chan RC, Sutow WW, Lindberg RD. Parameningeal rhabdomyosarcoma. Radiology. 1979;13:211.
129 Berry MP, Jenkins RDT. Parameningeal rhabdomyosarcoma in the young. Cancer. 1981;48:281.
130 Raney RB, Tefft M, Newton WA, et al. Improved prognosis with intensive treatment of children with cranial soft tissue sarcomas arising in nonorbital parameningeal sites: a report from the Intergroup Rhabdomyosarcoma Study. Cancer. 1987;59:147.
131 Raney RB, Meza J, Anderson JR, et al. Treatment of children and adolescents with localized parameningeal sarcoma: experience of the Intergroup Rhabdomyosarcoma Study Group protocols IRS-II through-IV, 1978–1997. Med Pediatr Oncol. 2002;38:22.
132 Spooner D, Gaze M, Habrand JL, et al. Treatment of young children (age <3 years) with parameningeal (PM) rhabdomyosarcoma (RMS): experience of the SIOP MMT 89 study. Med Pediatr Oncol. 2001;37:182.
133 Wharam MD, Beltangady MS, Heyn RM, et al. Pediatric orofacial and laryngopharyngeal rhabdomyosarcoma: an Intergroup Rhabdomyosarcoma Study report. Arch Otolaryngol Head Neck Surg. 1987;113:1225.
134 Raney RB, Asmar L, Vassilopoulou-Sellin R, et al. Late complications of therapy in 213 children with localized, nonorbital soft-tissue sarcoma of the head and neck: a descriptive report from the Intergroup Rhabdomyosarcoma Studies (IRS)-II and -III. Med Pediatr Oncol. 1999;33:362.
135 Raney RB, Gehan EA, Hays DM, et al. Primary chemotherapy with or without radiation therapy and/or surgery for children with localized sarcoma of the bladder, prostate, vagina, uterus, and cervix. Cancer. 1990;66:2072.
136 Hays D, Raney RB, Crist W, et al. Improved survival and bladder preservation among patients with bladder/prostate primary tumors in Intergroup Rhabdomyosarcoma Study (IRS) III. Proc Am Soc Clin Oncol. 1991;10:318. [abstract 1119]
137 Heyn R, Newton WA, Raney RB, et al. Preservation of the bladder in patients with rhabdomyosarcoma. J Clin Oncol. 1997;15:69.
138 Hays DM, Lawrence W, Crist WM, et al. Partial cystectomy in the management of rhabdomyosarcoma of the bladder: a report from the Intergroup Rhabdomyosarcoma Study. J Pediatr Surg. 1990;25:719.
139 Hays DM, Raney RB, Wharam MD, et al. Children with vesical rhabdomyosarcoma (RMS) treated by partial cystectomy with neoadjuvant or adjuvant chemotherapy, with or without radiotherapy: a report of the Intergroup Rhabdomyosarcoma Study (IRS) Committee. Am J Pediatr Hematol Oncol. 1995;17:46.
140 Raney B, Heyn R, Hays DM, et al. Sequelae of treatment in 109 patients followed for 5 to 15 years after diagnosis of sarcoma of the bladder and prostate: a report from the Intergroup Rhabdomyosarcoma Study Committee. Cancer. 1993;71:2387.
141 Wiener ES, Anderson JR, Ojimba JI, et al. Controversies in the management of paratesticular rhabdomyosarcoma: is staging retroperitoneal lymph node dissection necessary for adolescents with resected paratesticular rhabdomyosarcoma? Semin Pediatr Surg. 2001;10:146.
142 Curnes JT, Pratt CB, Hustu HO. Five-year survival after disseminated paratesticular rhabdomyosarcoma. J Urol. 1977;118:662.
143 Raney RB, Tefft M, Lawrence W, et al. Paratesticular sarcoma in childhood and adolescence: a report from the Intergroup Rhabdomyosarcoma Studies I and II, 1973-1983. Cancer. 1987;60:2337.
144 Heyn R, Raney RB, Hays DM, et al. Late effects of therapy in patients with paratesticular rhabdomyosarcoma. J Clin Oncol. 1992;10:614.
145 Hughes LL, Baruzzi MJ, Ribeiro RC, et al. Paratesticular rhabdomyosarcoma: delayed effects of multimodality therapy and implications for current management. Cancer. 1994;73:476.
146 Arndt C, Donaldson S, Andrassy R, et al. What constitutes optimal therapy for rhabdomyosarcoma (RMS) of the female genitourinary tract? Med Pediatr Oncol. 2000;35:278. [abstract O–37]
147 Andrassy RJ, Wiener ES, Raney RB, et al. Progress in the surgical management of vaginal rhabdomyosarcoma: a 25-year review from the Intergroup Rhabdomyosarcoma Study Group. J Pediatr Surg. 1999;34:731.
148 Martelli H, Oberlin O, Rey A, et al. Conservative treatment for girls with nonmetastatic rhabdomyosarcoma of the genital tract: a report from the Study Committee of the International Society of Pediatric Oncology. J Clin Oncol. 1999;17:2117.
149 Hays DM, Soule EH, Lawrence W, et al. Extremity lesions in the Intergroup Rhabdomyosarcoma Study (IRS-I): a preliminary report. Cancer. 1982;48:1.
150 Neville HL, Andrassy RJ, Lobe TE, et al. Preoperative staging, prognostic factors, and outcome for extremity rhabdomyosarcoma: a preliminary report from the Intergroup Rhabdomyosarcoma Study IV (1991–1997). J Pediatr Surg. 2000;35:317.
151 Heyn R, Beltangady M, Hays D, et al. Results of intensive therapy in children with localized alveolar extremity rhabdomyosarcoma: a report from the Intergroup Rhabdomyosarcoma Study. J Clin Oncol. 1989;7:200.
152 Raney RB, Crist W, Hays D, et al. Soft tissue sarcoma of the perineal region in childhood. Cancer. 1990;65:2787.
153 Raney RB, Ragab AH, Ruymann FB, et al. Soft-tissue sarcoma of the trunk in childhood: results of the Intergroup Rhabdomyosarcoma Study. Cancer. 1982;49:2612.
154 Hays DM, Donaldson SS, Shimada H, et al. Primary and metastatic rhabdomyosarcoma in the breast: neoplasms of adolescent females, a report from the Intergroup Rhabdomyosarcoma Study. Med Pediatr Oncol. 1997;29:181.
155 Ortega JA, Wharam M, Gehan EA, et al. Clinical features and results of therapy for children with paraspinal soft tissue sarcoma: a report of the Intergroup Rhabdomyosarcoma Study. J Clin Oncol. 1991;9:796.
156 Crist WM, Raney RB, Newton W, et al. Intrathoracic soft tissue sarcomas in children. Cancer. 1982;50:598.
157 Spunt SL, Lobe TE, Pappo AS, et al. Aggressive surgery is unwarranted for biliary tract rhabdomyosarcoma. J Pediatr Surg. 2000;35:309.
158 Fox KR, Moussa SM, Mitre RJ, et al. Clinical and pathologic features of primary gastric rhabdomyosarcoma. Cancer. 1990;66:772.
159 Horowitz ME, Etcubanas E, Webber BL, et al. Hepatic undifferentiated (embryonal) sarcoma and rhabdomyosarcoma in children: results of therapy. Cancer. 1987;59:396.
160 Crist WM, Raney RB, Tefft M, et al. Soft tissue sarcomas arising in the retroperitoneal space in children: a report from the Intergroup Rhabdomyosarcoma Study (IRS) Committee. Cancer. 1985;56:2125.
161 Jenkin D, Sonley M. Soft-tissue sarcomas in the young: medical treatment advances in perspective. Cancer. 1980;46:621.
162 Sollaccio RJ, Conrad C, Mendenhall NP, et al. Soft tissue sarcoma in children: analysis of prognostic factors. Int J Radiat Oncol Biol Phys. 1986;12:133. [abstract 82]
163 Horowitz ME, Pratt CB, Webber BL, et al. Therapy for childhood soft-tissue sarcomas other than rhabdomyosarcoma: a review of 62 cases treated at a single institution. J Clin Oncol. 1986;4:559.
164 Salloum E, Flamant F, Caillaud JM, et al. Diagnostic and therapeutic problems of soft tissue tumors other than rhabdomyosarcoma in infants under 1 year of age: a clinicopathological study of 34 cases treated at the Institut Gustave-Roussy. Med Pediatr Oncol. 1990;18:37.
165 Hayani A, Mahoney DH, Hawkins HK, et al. Soft-tissue sarcomas other than rhabdomyosarcoma in children. Med Pediatr Oncol. 1992;20:114.
166 Skene AI, Barr L, Robinson M, et al. Adult type (nonembryonal) soft tissue sarcomas in childhood. Med Pediatr Oncol. 1993;21:645.
167 Marcus KC, Grier HE, Shamberger RC, et al. Childhood soft tissue sarcoma: a 20-year experience. J Pediatr. 1997;131:603.
168 Rao BN. Nonrhabdomyosarcoma in children: prognostic factors influencing survival. Semin Surg Oncol. 1993;9:524.
169 Dillon P, Maurer H, Jenkins J, et al. A prospective study of nonrhabdomyosarcoma soft tissue sarcomas in the pediatric age group. J Pediatr Surg. 1992;27:241.
170 Maurer HM, Cantor A, Salzberg A, et al. Adjuvant chemotherapy vs observation for localized nonrhabdomyosarcoma soft tissue sarcoma (NRSTS) in children. Proc Am Soc Clin Oncol. 1992;11:363. [abstract 1248]
171 Blakely ML, Spurbeck WW, Pappo AS, et al. The impact of margin of resection on outcome in pediatric nonrhabdomyosarcoma soft tissue sarcoma. J Pediatr Surg. 1999;34:672.
172 Spunt SL, Poquette CA, Hurt YS, et al. Prognostic factors for children and adolescents with surgically resected nonrhabdomyosarcoma soft tissue sarcoma: an analysis of 121 patients treated at St. Jude Children’s Research Hospital. J Clin Oncol. 1999;17:3697.
173 Spunt SL, Hill DA, Motosue AM, et al. Clinical features and outcome of initially unresected nonmetastatic pediatric nonrhabdomyosarcoma soft tissue sarcoma. J Clin Oncol. 2002;20:3225.
174 Kawai A, Woodruff J, Healey JH, et al. SYT-SSX gene fusion as a determinant of morphology and prognosis in synovial sarcoma. N Engl J Med. 1998;338:153.
175 Schmidt D, Thum P, Harms D, et al. Synovial sarcoma in children and adolescents: a report from the Kiel Pediatric Tumor Registry. Cancer. 1991;67:1667.
176 Ladenstein R, Treuner J, Koscielniak E, et al. Synovial sarcoma of childhood and adolescence: report of the German CWS-81 Study. Cancer. 1993;71:3647.
177 Pappo AS, Fontanesi J, Luo X, et al. Synovial sarcoma in children and adolescents: the St. Jude Children’s Research Hospital Experience. J Clin Oncol. 1994;12:2360.
178 Okcu MF, Despa S, Choroszy M, et al. Synovial sarcoma in children and adolescents: thirty-three years of experience with multimodal therapy. Med Pediatr Oncol. 2001;37:90.
179 Okcu MF, Treuner A, Mattke A, et al. Synovial sarcoma of childhood and adolescence: multicenter multivariate analysis of outcome of 220 patients,. Proc American Soc Clin Oncol. 2002;21:391a. [abstract 1560]
180 Essary LR, Vargas SO, Fletcher CD. Primary pleuropulmonary synovial sarcoma. Reappraisal of a recently described anatomic subset. Cancer. 2002;94:459.
181 Dehner LP, Askin FB. Tumors of fibrous tissue origin in childhood: a clinicopathologic study of cutaneous and soft tissue neoplasms in 66 children. Cancer. 1976;38:888.
182 Soule EH, Pritchard DJ. Fibrosarcoma in infants and children: a review of 110 cases. Cancer. 1977;40:1711.
183 Raney RB, Allen A, O’Neill J, et al. Malignant fibrous histiocytoma of soft tissue in childhood. Cancer. 1986;57:2198.
184 Cole CH, Magee JF, Gianoulis M, et al. Malignant fibrous histiocytoma in childhood. Cancer. 1993;71:4077.
185 Fagundes HM, Lai PP, Dehner LP, et al. Postoperative radiotherapy for malignant fibrous histiocytoma. Int J Radiat Oncol Biol Phys. 1992;23:615.
186 Enzinger FM. Angiomatoid malignant fibrous histiocytoma: a distinct fibrohistiocytic tumor of children and young adults simulating a vascular neoplasm. Cancer. 1979;44:2147.
187 DeCou JM, Rao BN, Parham DM, et al. Malignant peripheral nerve sheath tumors: the St. Jude Children’s Research Hospital experience. Ann Surg Oncol. 1995;2:524.
188 Shmookler BM, Enzinger FM. Liposarcoma occurring in children: an analysis of 17 cases and review of the literature. Cancer. 1983;52:567.
189 La Quaglia MP, Spiro SA, Ghavimi F, et al. Liposarcoma in patients younger than or equal to 22 years of age. Cancer. 1993;72:3114.
190 Askin FB, Rosai J, Sibley RK, et al. Malignant small cell tumor of the thoracopulmonary region in childhood: a distinctive clinicopathologic entity of uncertain histogenesis. Cancer. 1979;43:2438.
191 Kinsella TJ, Triche TJ, Dickman PS, et al. Extraskeletal Ewing’s sarcoma: results of combined modality treatment. J Clin Oncol. 1993;1:489.
192 Jurgens H, Bier V, Harms D, et al. Malignant peripheral neuroectodermal tumors: a retrospective analysis of 42 patients. Cancer. 1988;61:349.
193 Shamberger RC, Grier HE, Weinstein HJ, et al. Chest wall tumors in infancy and childhoods. Cancer. 1989;63:774.
194 Raney RB, Asmar L, Newton WA, et al. Ewing’s sarcoma of soft tissues in childhood: a report from the Intergroup Rhabdomyosarcoma Study, 1972 to 1991. J Clin Oncol. 1997;15:574.
195 Lieberman PH, Foote FW, Stewart FW, et al. Alveolar soft-part sarcoma. JAMA. 1966;198:121.
196 Heller DS, Frydman CP, Gordon RE, et al. An unusual organoid tumor: alveolar soft part sarcoma or paraganglioma? Cancer. 1991;67:1894.
197 Pappo AS, Parham DM, Cain A, et al. Alveolar soft part sarcoma in children and adolescents: clinical features and outcome of 11 patients. Med Pediatr Oncol. 1996;26:81.
198 Enzinger FM. Epithelioid sarcoma: a sarcoma simulating a granuloma or a carcinoma. Cancer. 1970;26:1029.
199 Shimm DS, Suit HD. Radiation therapy of epithelioid sarcoma. Cancer. 1983;52:1022.
200 Gerald WL, Miller HK, Battifora H, et al. Intra-abdominal desmoplastic small round-cell tumor: report of 19 cases of a distinctive type of high-grade polyphenotypic malignancy affecting young individuals. Am J Surg Pathol. 1991;15:499.
201 Sara AS, Evans HL, Benjamin RS. Malignant melanoma of soft parts (clear cell sarcoma): a study of 117 cases, with emphasis on prognostic factors. Cancer. 1990;65:367.
202 Hachitanda Y, Tsuneyoshi M, Daimaru Y, et al. Extraskeletal myxoid chondrosarcoma in young children. Cancer. 1988;61:2521.
203 Kent AL, Mahoney DH, Gresik MV, et al. Malignant rhabdoid tumor of the extremity. Cancer. 1987;60:1056.
204 Ferrari A, Casanova M, Bisogno G, et al. Hemangiopericytoma in pediatric ages. Cancer. 2001;92:2692.
205 Kauffman SL, Stout AP. Mesothelioma in children. Cancer. 1964;17:539.
206 Manivel JC, Priest JR, Watterson J, et al. Pleuropulmonary blastoma. The so-called pulmonary blastoma of childhood. Cancer. 1988;62:1516.
207 Weeks DA, Beckwith JB, Mierau GW, et al. Rhabdoid tumor of kidney: a report of 111 cases from the National Wilms’ Tumor Study Pathology Center. Am J Surg Pathol. 1989;13:439.
208 Sotelo-Avila C, Gonzalez-Crussi F, Sadowinski S, et al. Clear cell sarcoma of the kidney: a clinicopathologic study of 21 patients with long-term follow-up evaluation. Hum Pathol. 1986;16:1219.
209 Miettinen M, Kahlos T. Undifferentiated (embryonal) sarcoma of the liver: epithelial features as shown by immunohistochemical analysis and electron microscopic examination. Cancer. 1989;64:2096.
210 Leuschner I, Schmidt D, Harms D. Undifferentiated sarcoma of the liver in childhood: morphology, flow cytometry, and literature review. Hum Pathol. 1990;21:68.
211 Chadwick EG, Connor EJ, Hanson CG, et al. Tumors of smooth-muscle origin in HIV-infected children. JAMA. 1990;263:3182.
212 Leibel SA, Wara WM, Hill DR, et al. Desmoid tumors: local control and patterns of relapse following radiation therapy. Int J Radiat Oncol Biol Phys. 1983;9:1167.
213 Sherman NE, Romsdahl M, Evans H, et al. Desmoid tumors: a 20-year radiotherapy experience. Int J Radiat Oncol Biol Phys. 1990;19:37.
214 McCollough WM, Parson JT, Van Der Griend R, et al. Radiation therapy for aggressive fibromatosis: the experience at the University of Florida. J Bone Joint Surg Am. 1991;73:717.
215 Acker JC, Bossen EH, Halperin EC. The management of desmoid tumors. Int J Radiat Oncol Biol Phys. 1993;26:851.
216 Goepfert H, Cangir A, Ayala AG, et al. Chemotherapy of locally aggressive head and neck tumors in the Pediatric Age Group. Am J Surg. 1982;144:437.
217 Sportiello DJ, Hoogerland DL. A recurrent pelvic desmoid tumor successfully treated with tamoxifen. Cancer. 1991;67:1443.
218 Waddell WR. Treatment of intra-abdominal and abdominal wall desmoid tumors with drugs that affect the metabolism of cyclic 3′,5′-adenosine monophosphate. Ann Surg. 1975;181:299. 218
219 Reitamo JJ, Scheinin TM, Hayry P. The desmoid syndrome: new aspects in the cause, pathogenesis and treatment of the desmoid tumor. Am J Surg. 1986;151(2):230.
220 Merchant TE, et al. Long-term results with radiation therapy for pediatric desmoid tumors. Int J Radiat Oncol Biol Phys. 2000;47(5):1267.
221 Faulkner LB, et al. Pediatric desmoid tumor: retrospective analysis of 63 cases. J Clin Oncol. 1995;13(11):2813.
222 Skapek SX, et al. Vinblastine and methotrexate for desmoid fibromatosis in children: results of a pediatric oncology group phase II trial. J Clin Oncol. 2007;25(5):501.
223 Heinrich MC, et al. Clinical and molecular studies of the effect of imatinib on advanced aggressive fibromatosis (desmoid tumor). J Clin Oncol. 2006;24(7):1195.
224 Buitendijk S, et al. Pediatric aggressive fibromatosis: a retrospective analysis of 13 patients and review of literature. Cancer. 2005;104(5):1090.
225 Spear MA, et al. Individualizing management of aggressive fibromatoses. Int J Radiat Oncol Biol Phys. 1998;40(3):637.