Chapter 31 Pediatric and Adult Scoliosis
• The clinician must be alert for any evidence of underlying neurological disease, including asymmetrical abdominal reflexes, abnormalities on neurological examination, or a cavovarus foot deformity. On radiographs, hyperkyphosis over the apex of the curvature, an unusual curve pattern, marked trunk shift, a left thoracic curve, or lack of curve rotation may indicate an underlying neurological condition. These patients should undergo magnetic resonance imaging (MRI).
• Patients presenting with significant pain, those who have rapid progression, and children younger than 10 years of age should also have an MRI screening of the entire spine prior to initiation of treatment.
• Shoulder asymmetry, unilateral Sprengel’s deformity, spasm from acute pain, or a leg-length discrepancy can give the false clinical appearance of scoliosis. Standing full-length scoliosis films are the gold standard for diagnosis.
• Bracing and surgery are the mainstays of treatment for scoliosis. Treatment must be tailored to the patient and family. Bracing is typically indicated in growing children with progressive curves measuring 20 to 45 degrees. In attempts to prevent curve progression, bracing is most effective in skeletally immature patients (Risser 0, 1, or 2) and in females who are less than 12 months past menarche. Casting is well tolerated in the young child and may be a preferable technique over bracing because of compliance. It has been shown to be curative in some cases of infantile scoliosis.
• When the curve magnitude exceeds 50 degrees in adolescent idiopathic scoliosis, surgical treatment is offered, typically with posterior spinal fusion.
• Adult scoliosis has a wide range of causes; most cases appear to relate to the degenerative process of aging or adolescent scoliosis in an adult. Other causes of deformity include osteoporosis, trauma, infection, and iatrogenic factors.
• Treatment for adult scoliosis is driven by the patient who wishes to regain function without pain. Correction of sagittal plane balance is essential to obtaining satisfactory results in this treatment.
Part I. Scoliosis in the Pediatric Patient
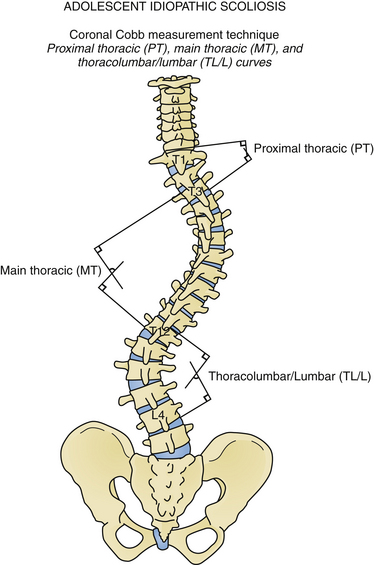
Scoliosis represents a complex three-dimensional deformity of the spine and thorax.1,2 Scoliosis is defined as a coronal deformity of the spine with a Cobb angle measuring greater than 10 degrees (Fig. 31.1).3 Small-magnitude curves may progress during periods of skeletal growth.4 Larger curves may continue to increase, even in adulthood.5,6 Thus, a pediatric patient with scoliosis should be followed until skeletal maturity, and young adults with large untreated curves should be monitored periodically for curve progression.
Congenital, neuromuscular, and idiopathic curves are commonly diagnosed in childhood. Congenital scoliosis results from malformation of the vertebral elements, either due to failure of formation or failure of segmentation. A hemivertebra with a contralateral bar results in the greatest risk of curve progression.7 Particularly in small children, it is difficult to predict which congenital curves will be progressive and will subsequently require surgical intervention. Because of a high associated risk, all children with congenital scoliosis require evaluation for concomitant renal, cardiac, and intrathecal abnormalities. Owing to the structural abnormalities, bracing in congenital scoliosis is typically not indicated. Neuromuscular curves are those associated with an underlying neurological difference, either structural (Chiari malformation, syrinx, tethered cord, diastematomyelia) or systemic (e.g., cerebral palsy, Charcot-Marie-Tooth disease, muscular dystrophy, neurofibromatosis). As idiopathic scoliosis is a diagnosis of exclusion, neurological causes for the deformity must be ruled out by medical history, clinical examination, radiographs, and in selected cases, axial imaging.
Idiopathic scoliosis is the most common type of scoliosis in children. Based on age at diagnosis, idiopathic scoliosis is traditionally classified into infantile (age less than 3 years), juvenile (age 3 to 10 years), and adolescent (age greater than 10 years). More recently, the term early-onset scoliosis has been used for spinal deformities in children under the age of 5 years, including both idiopathic, congenital, and neuromuscular curves, in order to acknowledge the special challenges of treating very young children with scoliosis.8 Infantile neuromuscular scoliosis is frequently progressive, but if the curve is flexible it may be effectively managed with bracing and casting (Fig. 31.2).9 Idiopathic infantile scoliosis is more common in males than females, and patients may present with a left-sided curve. Factors associated with progressive infantile idiopathic scoliosis include age at treatment, rib vertebral angle greater than 20 degrees, a phase II rib, or a curve greater than 60 degrees.9–11
Although the cause of idiopathic scoliosis is unknown, family members of affected patients have an increased risk of developing scoliosis, reflecting a genetic component to the disease.12–14 Several genes have been associated with familial scoliosis.15,16 The incidence of adolescent idiopathic scoliosis (AIS) with a curvature greater than 30 degrees ranges from 1 to 3 in 1000 children.17–23 Small curves are common in both males and females, although progression is more common in female children.
Natural history studies of scoliosis reveal the greatest morbidity from early-onset scoliosis, with higher mortality rates and decreased pulmonary function for thoracic curves.24 One series of patients with untreated juvenile and adolescent scoliosis revealed only one death from cor pulmonale, and overall mortality rates similar to those for the average population.6 Back pain could not be correlated with the severity of the deformity, although patients with lumbar curves had more back pain.6 Patients with thoracic curves have been noted to have poor pulmonary function because of decreased lung volumes from the coronal plane deformity.6,25 Thoracic curves measuring 70 degrees may result in noticeable changes on pulmonary function testing, and curves greater than 100 degrees may result in clinical symptoms.24
Curves are at risk for progression during periods of rapid skeletal growth, specifically the first 2 years of life and the adolescent growth spurt.4 There is increased risk of curve progression for large curves and for skeletally immature patients (Table 31.1).4 Bone age, peak height velocity, Risser sign, and elbow and hand maturity indices may aid in determining risk of curve progression.26–30 Active investigation is under way to identify genetic markers that would assist in predicting curve progression.31 For adolescent idiopathic scoliosis, thoracic and double curve patterns are at higher risk of progression than thoracolumbar curves.4 Curves less than 30 degrees at skeletal maturity are thought to be mostly stable and unlikely to progress over time.5,32 Curves greater than 50 degrees at skeletal maturity commonly progress at a rate of up to 1 degree per year for thoracic curves.5,32 Curves between 35 and 50 degrees at skeletal maturity present a diagnostic dilemma due to insufficient clinical data and should be followed radiographically into adulthood.
TABLE 31.1 Adolescent Idiopathic Scoliosis Treatment Algorithm
| Growing Child |
| Open triradiate cartilage, Risser 0, 1, peak height velocity of 8-10 cm/year growth42 |
| Curve Size∗ | Treatment | Follow-up Interval |
|---|---|---|
| ≤25 degrees | Observation | 4-6 months |
| 25-45 degrees | Bracing (may consider in Risser 0 at 20-25 degrees with documented progression) | 4 months |
| >45 degrees | Surgery—consider anterior release with open triradiate cartilage |
| Adolescent Nearing Maturity |
| Closed triradiate, Risser 3-4, postmenarchal females |
| Curve Size∗ | Treatment | Follow-up Interval |
|---|---|---|
| ≤30 degrees | Observation | 12 months to document no progression |
| 35-45 degrees | Consider bracing, efficacy not determined | 4 months if braced 6-12 months otherwise |
| >50 degrees | Surgery |
| Skeletally Mature Child |
| Risser 5, menarche + 2 years in females, cessation of growth5,6 |
| Curve Size∗ | Treatment | Follow-up Interval |
|---|---|---|
| ≤50 degrees | Observation | 1-2 years |
| >50 degrees | Surgery |
Note: Superscript numbers refer to references for this chapter, listed online.
∗ Measurement variability for Cobb angle is reported at ±5-6 degrees.75,76
Indications
Bracing and surgery are the mainstays of treatment for scoliosis. Treatment must be tailored to the patient and family. Bracing is typically indicated in growing children with progressive curves measuring 20 to 45 degrees.33 In attempts to prevent curve progression, bracing is most effective in skeletally immature patients (Risser 0 or 1, 2) and in females who are less than 12 months past menarche.33 Numerous types of braces have been described,34–38 but a TLSO (thoracic lumbar spine orthosis) is commonly used.33 Brace wear has been shown to be efficacious if the brace is worn.39,40 The brace is discontinued at the completion of growth. Casting is well tolerated in the young child and may be a preferable technique over bracing owing to derotational molding of the deformity and improved compliance.9,10 It has been shown to be curative in some cases of infantile scoliosis, although in some instances these curves are self-resolving.9
When the curve magnitude exceeds 50 degrees in AIS, surgical treatment is offered, typically with posterior spinal fusion. Fusion of smaller curves can be considered in skeletally immature patients who have been documented to have significant progression or in patients who find the appearance and trunk shift unacceptable. Pain is not typically an indication for surgery. Patients with severe deformity or who are skeletally immature (open triradiate cartilages) may also be treated with anterior release or posterior fusion to improve correction and prevent subsequent rotational and coronal or “crankshaft” deformity.41–44 In the era of pedicle screw fixation, the necessity of anterior procedures is debated in the literature and may be a matter of surgeon preference.45 Anterior release, fusion, and instrumentation in the thoracic spine may be performed through a thoracotomy or through a thoracoscopic approach.46,47 Anterior procedures, particularly open surgery, may result in decreased pulmonary function.46–48 Large curves and complex deformities can also be addressed by osteotomies through a posterior approach to achieve satisfactory correction. These procedures may include, in order of increased correction and neurological risk, Ponte/Smith-Peterson osteotomies, pedicle subtraction osteotomies, and vertebral column resections.49–51
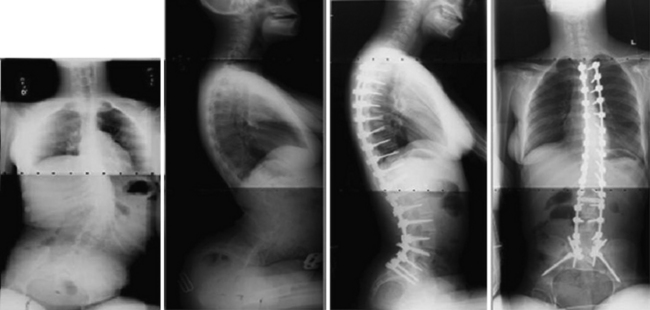
Neuromuscular scoliosis is guided by similar treatment principles. Progressive imbalance from muscle weakness or spasticity may result in a sweeping thoracolumbar or C-type curvature and subsequent pelvic obliquity, truncal shift, and difficulty with wheelchair seating and caregiving.52,53 The role of bracing in the neuromuscular population is not well defined, but may be a helpful delay tactic. Custom wheelchair seating systems can also serve as an external brace in many instances. Surgical management is indicated when bracing or wheelchair positioning can no longer accommodate the patient’s deformity and the curve progresses to greater than 50 degrees (Fig. 31.3).52 Smaller curves may continue to progress in adult patients with neuromuscular scoliosis, although curves greater than 50 degrees increase at a faster rate.54 Patients with underlying cardiac or pulmonary deficits as classically seen in Duchenne muscular dystrophy may benefit from early surgery performed for smaller magnitude curves rather than waiting for the curve to progress while the patient’s medical status deteriorates.55 New corticosteroid treatment protocols for Duchenne muscular dystrophy may redefine these indications.56 Surgical treatment of nonambulatory patients typically involves fusion from the upper thoracic spine to the pelvis, although recent literature has shown improved mobility in paraplegic children fused to L5. Long-term results from this technique are not established. In ambulatory patients with an underlying neuromuscular condition, longer fusions are typically indicated to avoid subsequent decompensation.
Early-onset scoliosis has unique considerations. Thoracic fusion in a small child is unacceptable and may later lead to thoracic insufficiency syndrome and significant shortening of the trunk. Delay tactics include casting, bracing, and halo gravity traction.9 When curves do not respond to these measures, surgical intervention may be undertaken to prevent curve progression and allow for spinal growth.57,58 Several growing spine systems are in use, including growing spine-based and rib-based devices.57,59–62 There is limited consensus as to the indications and applications of these devices. These are seen as a last resort because the child is then committed to twice yearly lengthening procedures and a high rate of complications, including infection, wound problems, early unintended fusion, implant failure, and rarely neurological complications.57 A planned definitive fusion is performed at the completion of the lengthening procedures. For growing rods, a dual-rod construct has been shown to have fewer complications and improved correction, although a single rod can be used in conjunction with a brace.60,63 The implants are frequently quite prominent. Prior to initiating surgical treatment, nutritional and medical status must be optimized. Large curves result in increased work of breathing and energy expenditure. Thus, children with significant deformity are often malnourished and require dietary supplementation and in many instances gastrostomy tube placement to improve soft tissue coverage and reduce wound complications.
Diagnosis
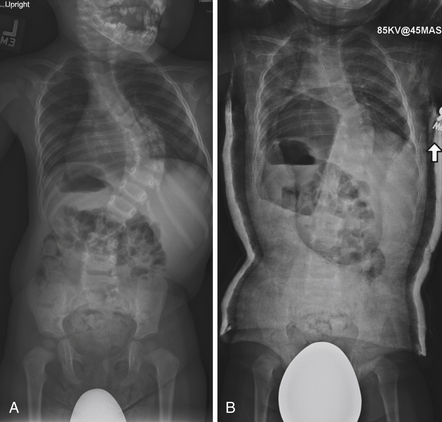
All patients with presumed idiopathic scoliosis should be evaluated with a medical history, complete neurological examination, and radiographs. A family history should be taken for scoliosis or a history of underlying neurological abnormalities. Females should be asked about age at menarche, because this landmark is an excellent predictor of skeletal maturity. On physical examination, the patient’s back should be carefully examined for asymmetry, trunk shift, and sagittal plane alignment. AIS is balanced in the coronal plane and typically hypokyphotic, so the presence of kyphosis or excessive truncal shift is an indication for axial imaging64 (Fig. 31.4). Asymmetrical abdominal reflexes may be associated with a neural axis abnormality.65 The Adams’ forward bend test may reveal a thoracic or lumbar rib prominence resulting from rotational deformity. The pelvis should be assessed for an occult leg-length discrepancy, which may give the appearance of scoliosis. The skin should be examined for masses, café au lait patches, and axillary or inguinal freckling indicative of neurofibromatosis. A hairy patch, dimple, or pigmentation over the lumbosacral region suggests spinal dysraphism. The patient should be examined for excess ligamentous laxity or pectus deformity, raising the possibility of an underlying syndrome.
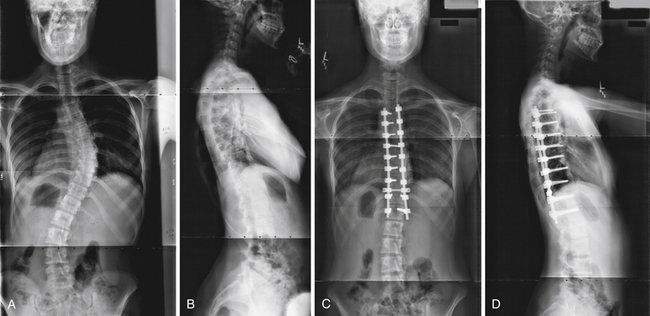
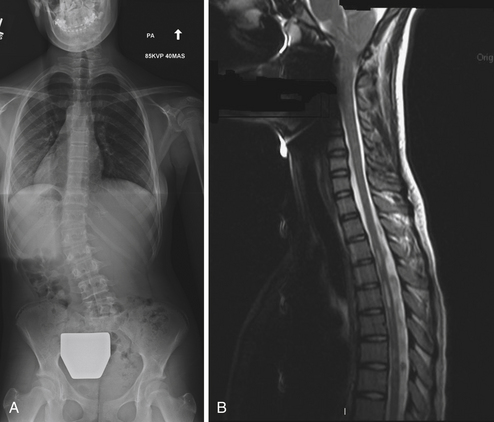
Neural axis abnormalities are found in 13% to 26% of patients with juvenile scoliosis and 2.6% to 14% of patients with adolescent-onset scoliosis.65–67 Up to 50% of patients with a left thoracic rather than right thoracic curve may have an underlying neural axis abnormality.67–69 As many as 20% to 50% of children with infantile scoliosis may have an underlying neural axis abnormality.67,70 No difference in treatment outcome has been reported based on the presence of a neurological abnormality, although a large syrinx, tethered cord, or Chiari malformation may require surgical intervention (Fig. 31.5).69 In some instances, scoliosis may improve after treatment of the neural axis abnormality, but this is not predictable.
The idiopathic scoliosis curve types have been classified by King and associates71 and more recently by Lenke and colleagues (Fig. 31.6).72 These classifications assist in choosing fusion levels and help identify idiopathic curve types for research purposes. With the Lenke classification, premeasured radiographs have high inter- and intrarater reliability,72,73 although there is only poor to good interobserver reliability on unmeasured radiographs.73,74 Cobb angle measurement error among different observers is 5 to 6 degrees; thus, more than 5 degrees increase in curve size is necessary in order to document curve progression.75,76 Digital imaging systems may improve measurement accuracy.75,76 Children who may be candidates for treatment should be followed with radiographs several times annually during periods of rapid growth.
Evidence-Based Medicine
Treatment for scoliosis includes observation, bracing or casting, and surgery. There are no studies that support the use of activity modification, manipulation, exercises, physical therapy, or electrical stimulation for the correction of scoliosis.77–80 Casting has been shown to be efficacious for infantile curves at high risk of progression. Braces, if worn consistently, may prevent curve progression.39,40
Surgical treatment is undertaken to prevent further curve progression, but has the added benefit of deformity correction. The importance of specific correction objectives are debated among surgeons and include coronal plane correction, restoration of thoracic kyphosis and lumbar lordosis, sagittal balance, and vertebral derotation.81 Beginning with Harrington rods, a variety of implants have been used including hooks, wires, and screws.82 Pedicle screws are now the gold standard due to no violation of the canal, superior rotational and curve correction, resulting improvements in pulmonary function tests (PFTs).82–85 Pedicle screws can be placed using anatomical, fluoroscopic, or computed tomography (CT)-guided navigation.86 The role of intraoperative CT is under development, but may be of great assistance in complex deformity and congenital cases when the anatomical landmarks are unreliable. Posterior spinal fusion for scoliosis results in improved patient perception of appearance and improved functional outcome scores. Long fusions limit spine motion and have the theoretical risk of increased stress and degenerative changes at adjacent motion segments.87
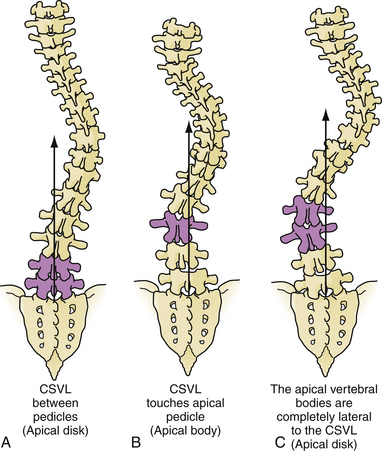
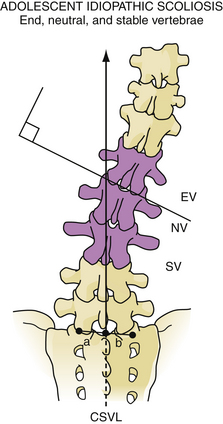
Selection of fusion level and density of implants is subject to significant variability in practice and is beyond the scope of this text.71,88,89 Definitions of the end, neutral, and stable vertebrae may aid in selection of fusion levels (Fig. 31.7). Occasionally, levels can be preserved in thoracolumbar curves by using an anterior versus posterior approach. Bracing or casting is not routinely recommended postoperatively because new fixation techniques are quite sturdy and reliable.
Complications can be limited by diligent preoperative planning and clear communication with the medical and anesthesiology teams. The complication rates for posterior spinal fusion is reported at 5% to 15% for idiopathic scoliosis patients90–92 and 28% to 33% for neuromuscular scoliosis patients.93,94 Higher complication rates are associated with prolonged surgical and anesthetic time, increased blood loss, hybrid constructs, and renal disease.90,92 Growing spine patients face a 48% complication rate.60 All patients should have blood products available. Tranexamic acid may help reduce blood loss.95 Thoracic surgery should be performed with intraoperative somatosensory evoked potentials (SSEPs) and motor evoked potentials (MEPs) in collaboration with a neuromonitoring team and neurologist.96 These tests require special anesthetic techniques such as total intravenous anesthesia. The Stagnara wakeup test can be used in instances when signals cannot be obtained in the patient and serves as a secondary method to assess neurological function intraoperatively. From an anesthesia standpoint, significant lead time may be required. The airway should be carefully protected, and the wound should be filled with saline to prevent pulmonary air embolus from deep inspiration at the time of the wakeup test. Forceful injection of thrombin products into the pedicle tract has been reported to cause neurological injury and should be avoided.97 Neurological deficit can present in a delayed fashion postoperatively, likely due to vascular effects on the spinal cord.98 Cases with osteotomies, high blood loss, and preoperative kyphosis or myelopathy are at greater risk of neurological complication. Thus, for high-risk procedures, careful postoperative monitoring, maintenance of blood pressure with fluid or in some instances with pressors, and adequate maintenance of hemoglobin should be ensured.
Part II. Adult Spinal Deformity: Considerations in Evaluation and Treatment
Spinal deformity in the adult is increasingly recognized as a significant health care issue. With an aging population there are also increasing functional expectations of an older population in Western societies. The costs of caring for the elderly who lose autonomy due to spine deformity are substantial. Pain and disability are closely associated with spinal deformity in the adult population. One prevalence study99 reports a 60% rate of spine deformity in a population over 60 years of age.
The treatment of adult spinal deformity (ASD) has received rather sparse attention compared to deformity in the pediatric population. Despite the large number of adults affected by spine deformities and the high potential for marked loss of function,100 adult deformity remains poorly understood. Progress in research is hampered by the diversity of disorders associated with spinal deformity in the adult, the lack of a coherent system for classifying patients, and until recently, poorly understood correlations between deformity parameters and patient-reported disability.
Key Radiographic Parameters and Health-Related Quality of Life
Although ASD has a wide range of causes, most cases appear to relate to the degenerative process of aging (de novo degenerative spinal deformity, or DDS) or adolescent scoliosis in an adult (ASA).101 Other causes of deformity include osteoporosis, trauma, infection, and iatrogenic factors. For DDS and ASA, the two largest categories, a common pathway appears to lead to pain and loss of function.
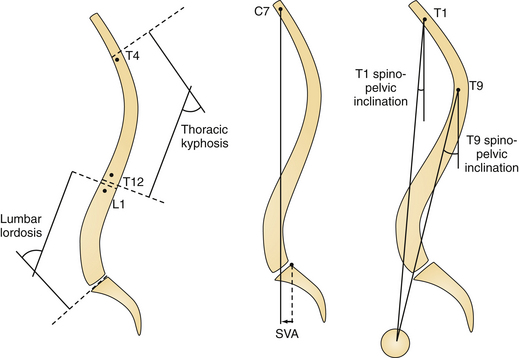
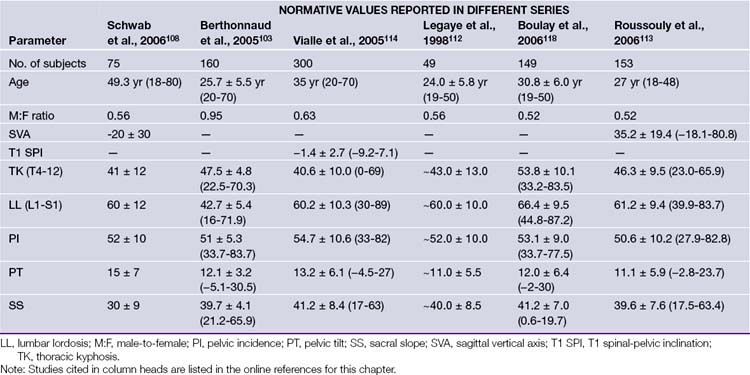
Many clinicians have investigated the regional and global alignment in the normal (asymptomatic) adult population102–108 (Fig. 31.8). These data are helpful in understanding normative ranges but offer limited value in understanding disability related to ASD. However, a study by the Spinal Deformity Study Group (SDSG),109 including 947 adults with spinal deformity, set out to answer this key question. Data from their multicenter prospective database were analyzed for HRQOL/radiographic correlation. All patients had radiographic analysis: frontal Cobb angle, deformity apex, lumbar lordosis, intervertebral subluxation. Health assessment included the Oswestry Disability Index (ODI) and Scoliosis Research Society instrument (SRS). Patients were classified by deformity apex, lordosis (T12-S1), and intervertebral subluxation. The correlation analysis revealed that HRQOL was driven by several key parameters (in the following order): global sagittal alignment (sagittal vertical axis, SVA), loss of lumbar lordosis (LL), intervertebral subluxation. This data set did not include pelvic parameters, which subsequently emerged as being critical to understanding ASD. In a recent investigation by Lafage and co-workers110 radiographic pelvic parameters were established as an integral component in patient-reported function in the setting of spinal deformity.
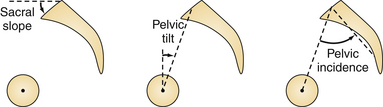
Several authors have described the pelvis as a regulator of the sagittal plane alignment, and extensive studies have been conducted to understand the relationship between pelvic parameters and spinal alignment. Radiographic parameters of pelvic alignment (Fig. 31.9) have now emerged as essential components of the global standing alignment,108,111–114 and their clinical relevance is briefly summarized here:
 Pelvic tilt (PT) is defined by the angle between the vertical and the line through the midpoint of the sacral plate to the femoral head axis; it is commonly reported as a compensatory mechanism: when the spine tilts forward (age-related change, sagittal imbalance, loss of lordosis, increase of kyphosis) the subject will try his or her best to maintain an economic posture and to keep the spine as vertical as possible (i.e., bring the spine over the pelvis). One way to maintain this spinal-pelvic alignment is to retrovert the pelvis (increase of pelvic tilt), which may be seen as a backward rotation of the pelvis around the hips.
Pelvic tilt (PT) is defined by the angle between the vertical and the line through the midpoint of the sacral plate to the femoral head axis; it is commonly reported as a compensatory mechanism: when the spine tilts forward (age-related change, sagittal imbalance, loss of lordosis, increase of kyphosis) the subject will try his or her best to maintain an economic posture and to keep the spine as vertical as possible (i.e., bring the spine over the pelvis). One way to maintain this spinal-pelvic alignment is to retrovert the pelvis (increase of pelvic tilt), which may be seen as a backward rotation of the pelvis around the hips.
 Pelvic incidence (PI) is defined as the angle between the perpendicular line to the sacral plate at its midpoint and the line connecting this point to the femoral head axis. This is a morphological parameter of primary importance because of its correlation with ideal lumbar lordosis. In a simplified formula lumbar lordosis should be within 9 degrees of PI (LL = PI ± 9) degrees.
Pelvic incidence (PI) is defined as the angle between the perpendicular line to the sacral plate at its midpoint and the line connecting this point to the femoral head axis. This is a morphological parameter of primary importance because of its correlation with ideal lumbar lordosis. In a simplified formula lumbar lordosis should be within 9 degrees of PI (LL = PI ± 9) degrees.
 Sacral slope (SS) is defined as the angle between the horizontal and the sacral plate; this last parameter completes the geometric relationship where PI = PT + SS. Normative values and age-related changes of the pelvic parameters are provided (Table 31.2).
Sacral slope (SS) is defined as the angle between the horizontal and the sacral plate; this last parameter completes the geometric relationship where PI = PT + SS. Normative values and age-related changes of the pelvic parameters are provided (Table 31.2).
Classification
To date, plain x-ray films remain the basis for diagnostic approaches to many orthopedic disorders. Although newer imaging modalities have emerged, substantial variation in interpretation of information related to spinal diseases exist. In terms of establishing a classification of adult deformity it may be ideal to base this principally on x-ray image information to remain simple in form and reliable in application.
A significant advance in the classification of scoliotic deformity is attributed to Lenke and colleagues.72 In a 2001 publication, this classification offered an updated approach to AIS which was more comprehensive than the King classification71 and offered sagittal plane consideration. Furthermore, the new AIS classification offered guidelines on surgical fusion levels using segmental fixation.
The classification of adult scoliosis115 is clearly more complex than approaches to AIS. Adult deformity covers a broad range of segmental, regional, and global expression (Fig. 31.10). The treatment is driven by disability and pain and not simply by radiographic appearance. The latter has thus made it difficult to arrive at a clinically relevant x-ray classification. One of the first studies aimed at isolating the pain generator in adult scoliosis was published in 2002.116 This work by Schwab and associates launched an ongoing effort at reconciling HRQOL measures and radiographic presentation of adult deformity. The approach has led to the Schwab-SDSG classification of which the basic structure hinges on the following:
 Basic coronal curve locations and basic patterns (single vs. multiple)
Basic coronal curve locations and basic patterns (single vs. multiple)
 A principally sagittal plane deformity category
A principally sagittal plane deformity category
 Regional and global modifiers with three grades tied to HRQOL
Regional and global modifiers with three grades tied to HRQOL
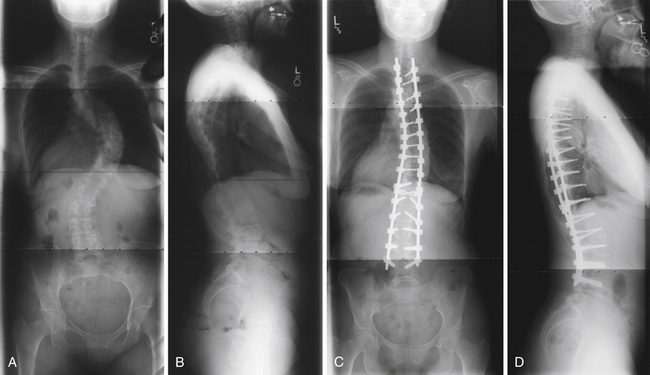
FIGURE 31.10 Adult scoliosis. Preoperative PA (A, B) and lateral views (C, D) show both coronal and sagittal plane deformity.
All aspects of the classification found a basis in HRQOL analysis. The cutoffs between groups within each modifier were determined by the HRQOL measures, splitting the population into discrete groups by clinical impact of each modifier parameter. Description of the classification is summarized in Table 31.3.
TABLE 31.3 Schwab-SDSG Adult Spinal Deformity Classification
| Type | Location of Deformity∗ |
|---|---|
| Type I | Thoracic-only scoliosis (no thoracolumbar or lumbar component) |
| Type II | Upper thoracic major, apex T4-8 (with thoracolumbar or lumbar curve) |
| Type III | Lower thoracic major, apex T9-T10 (with thoracolumbar/lumbar curve) |
| Type IV | Thoracolumbar major curve, apex T11-L1 (with any other minor curve) |
| Type V | Lumbar major curve, apex L2-L4 (with any other minor curve) |
| Type K | Deformity in the sagittal plane only |
| Lordosis Modifier | Sagittal Cobb Angle: T12 to S1 |
| A | Marked lordosis >40 degrees |
| B | Moderate lordosis 0-40 degrees |
| C | No lordosis present <0 degrees |
| Subluxation Modifier | Maximum Value: Frontal or Sagittal Plane (Anterior or Posterior) |
| 0 | No subluxation |
| + | Subluxation 1-6 mm |
| ++ | Subluxation >7 mm |
| Global Balance Modifier† | C7 Offset From Posterior Superior Corner of S1: Sagittal Plane |
| N | 0-4 cm |
| P | 4-9.5 cm |
| VP | >9.5 cm |
SDSG, Spinal Deformity Study Group.
Alignment Goals When Treating Adult Spinal Deformity
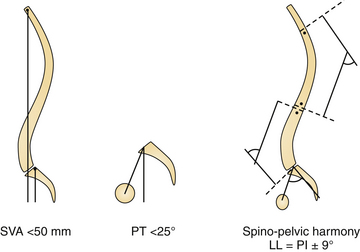
Tailoring patient-specific treatment involves the crucial PI-LL relationship, wherein ideal lumbar lordosis is proportional to pelvic morphology quantified as pelvic incidence (PI). Additionally, alignment parameters driving pain and disability, namely, sagittal vertical axis (SVA) and PT, need to be addressed. The surgical method of realignment (i.e., pedicle subtraction osteotomy versus a Smith Peterson osteotomy, intervertebral spacer, rod contouring) remains of secondary importance to the primary goal: obtaining surgical realignment objectives. As a general concept and pragmatic tool for clinical application, spinal-pelvic realignment objectives involve attention to the following three key parameters117 (Fig. 31.11):
 SVA: Global spinal realignment should attempt to obtain a postoperative SVA less than 50 mm. Restoration of SVA facilitates level gaze and achievement of a physiological standing posture. An SVA less than 50 mm brings the plumbline behind the femoral heads to relieve the complaint of “falling forward.” Clinically, this threshold has been met with better HRQOL scores. Similarly, the reference T1 − SPI < 0 degrees may be used. Both parameters outline the same principle.
SVA: Global spinal realignment should attempt to obtain a postoperative SVA less than 50 mm. Restoration of SVA facilitates level gaze and achievement of a physiological standing posture. An SVA less than 50 mm brings the plumbline behind the femoral heads to relieve the complaint of “falling forward.” Clinically, this threshold has been met with better HRQOL scores. Similarly, the reference T1 − SPI < 0 degrees may be used. Both parameters outline the same principle.
 PT: Pelvic realignment should attempt to obtain a postoperative PT less than 25 degrees. Attention to PT, as outlined by clinical data, is necessary to obtain optimal outcomes. Additionally, PT realignment restores appropriate femoral-pelvic-spinal alignment required during efficient ambulation (need an extension reserve to clear the step). Realignment of the SVA less than 50 mm in the setting of an elevated PT means the ASD patient is still compensating for residual structural spinal deformity. This parameter independently has been shown to correlate to impairment in walking tolerance and therefore should be realigned appropriately.
PT: Pelvic realignment should attempt to obtain a postoperative PT less than 25 degrees. Attention to PT, as outlined by clinical data, is necessary to obtain optimal outcomes. Additionally, PT realignment restores appropriate femoral-pelvic-spinal alignment required during efficient ambulation (need an extension reserve to clear the step). Realignment of the SVA less than 50 mm in the setting of an elevated PT means the ASD patient is still compensating for residual structural spinal deformity. This parameter independently has been shown to correlate to impairment in walking tolerance and therefore should be realigned appropriately.
 LL = PI ± 9 degrees: Finally, to achieve patient-specific alignment treatment LL = PI ± 9 degrees may pragmatically be used. Increasing the angulation of the hypolordotic spine to match the patient’s spinal-pelvic morphological type (i.e., PI) assures appropriate lordotic alignment. This chain of correlation has been extensively studied by many authors.108,111,118,119 as it relates to the subject’s morphological type.113
LL = PI ± 9 degrees: Finally, to achieve patient-specific alignment treatment LL = PI ± 9 degrees may pragmatically be used. Increasing the angulation of the hypolordotic spine to match the patient’s spinal-pelvic morphological type (i.e., PI) assures appropriate lordotic alignment. This chain of correlation has been extensively studied by many authors.108,111,118,119 as it relates to the subject’s morphological type.113
Predicting Outcome Related to Surgery for Adult Spinal Deformity
In building predictive models of outcome from surgery it is key to identify what combination of patient characteristics (including Schwab classification modifiers) and treatment options can be used to predict which patients will meet an outcome threshold for success.120 Previous work by the SDSG on minimal clinically important difference (MCID) has identified improvement thresholds for discriminating between patients who report being satisfied with surgical results and those who report being less than satisfied. Based upon those identified thresholds, binary logistic regression was used to examine how adult classification types and surgical strategy combine and interact to predict successful surgical outcomes. A second approach was to employ multiple linear regressions. For both binary and logistic regression, predictor variables included all classification types, all treatment options within surgery, patient age, gender, BMI (body mass index), previous surgery, and baseline health status. Backward and stepwise techniques were used to eliminate redundant predictor variables.
An analysis of patients most and least likely to reach an MCID can be drawn from the predictive models. It was found that in addition to the classification modifiers, baseline HRQOL was associated with higher chance of a poor outcome (Tables 31.4 and 31.5).
TABLE 31.4 Strength of Predictive Models1 Meeting MCID Threshold2∗
| Outcome Score1 | % Correct Classification by Model | Area Under ROC Curve |
|---|---|---|
| SRS pain | 81.10% | 0.864 |
| SRS appearance | 75.40% | 0.838 |
| SRS pain and appearance | 78.10% | 0.845 |
| SF-12v2 PCS | 77.90% | 0.862 |
MCID, minimal clinically important difference; SF-12v2 PCS, Medical Outcomes Study, short-form, version 2, physical component summary; ROC, receiver operating characteristic; SRS, Scoliosis Research Society.1,2
∗ 0.80 and above is considered good discrimination.
TABLE 31.5 Summary of Post–Scoliosis Surgery Treatment Groups Likely to Reach MCID (Success) or Unlikely to Do So (Poor Outcome)
| Groups Less Likely to Reach Threshold Improvement | Groups With Higher Chance of Reaching Threshold Improvement | |
|---|---|---|
| SF-12v2 PCS MCID summary | Apical level III Marked lordosis No subluxation Negative sagittal balance Baseline PCS ≥35 |
Apical level IV Subluxation + or ++ Positive sagittal balance Surgery involved osteotomy Surgery involved fixation to sacrum Baseline PCS <35 |
| SRS combined pain/appearance MCID summary | Apical level III Marked lordosis No subluxation Negative sagittal balance |
No lordosis Subluxation ++ Circumferential surgery Surgery involved osteotomy |
| SRS pain MCID summary | Apical level III Marked lordosis No subluxation Negative sagittal balance Posterior-only surgical approach No fixation to the sacrum |
Apical level IV Subluxation ++ Circumferential surgery Surgery involved osteotomy Baseline PCS <35 |
| SRS appearance MCID summary | Apical level V Surgery involved fixation to sacrum |
No previous surgery Apical level IV Baseline PCS ≥35 |
MCID, minimal clinically important difference; SF-12v2 PCS, Medical Outcomes Study, short-form, version 2, physical component summary; SRS, Scoliosis Research Society.
Danielsson A.J., Nachemson A.L. Back pain and function 23 years after fusion for adolescent idiopathic scoliosis: a case-control study-part II. Spine. 2003;28(18):E373-383.
Lenke L.G., Betz R.R., Harms J., et al. Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am. 2001;83-A(8):1169-1181.
Lenke L.G., Kuklo T.R., Ondra S., Polly D.W.Jr. Rationale behind the current state-of-the-art treatment of scoliosis (in the pedicle screw era). Spine. 2008;33(10):1051-1054.
Lonstein J.E., Carlson J.M. The prediction of curve progression in untreated idiopathic scoliosis during growth. J Bone Joint Surg Am. 1984;66(7):1061-1071.
Schwab F., Farcy J.P., Bridwell K., et al. A clinical impact classification of scoliosis in the adult. Spine. 2006;31(18):2109-2114.
Weinstein S.L., Ponseti I.V. Curve progression in idiopathic scoliosis. J Bone Joint Surg Am. 1983;65(4):447-455.
Please go to expertconsult.com to view the complete list of references.
1. Charles Y.P., Dimeglio A., Marcoul M., et al. Influence of idiopathic scoliosis on three-dimensional thoracic growth. Spine. 2008;33(11):1209-1218.
2. Villemure I., Aubin C.E., Grimard G., et al. Progression of vertebral and spinal three-dimensional deformities in adolescent idiopathic scoliosis: a longitudinal study. Spine. 2001;26(20):2244-2250.
3. Bunnell W.P. The natural history of idiopathic scoliosis before skeletal maturity. Spine. 1986;11(8):773-776.
4. Lonstein J.E., Carlson J.M. The prediction of curve progression in untreated idiopathic scoliosis during growth. J Bone Joint Surg Am. 1984;66(7):1061-1071.
5. Weinstein S.L., Ponseti I.V. Curve progression in idiopathic scoliosis. J Bone Joint Surg Am. 1983;65(4):447-455.
6. Weinstein S.L., Zavala D.C., Ponseti I.V. Idiopathic scoliosis: long-term follow-up and prognosis in untreated patients. J Bone Joint Surg Am. 1981;63(5):702-712.
7. Shahcheraghi G.H., Hobbi M.H. Patterns and progression in congenital scoliosis. J Pediatr Orthop. 1999;19(6):766-775.
8. Thompson G.H., Lenke L.G., Akbarnia B.A., et al. Early onset scoliosis: future directions. J Bone Joint Surg Am. 2007;89(Suppl 1):163-166.
9. Mehta M.H. Growth as a corrective force in the early treatment of progressive infantile scoliosis. J Bone Joint Surg Br. 2005;87(9):1237-1247.
10. Sanders J.O., D’Astous J., Fitzgerald M., et al. Derotational casting for progressive infantile scoliosis. J Pediatr Orthop. 2009;29(6):581-587.
11. Mehta M.H. The rib-vertebra angle in the early diagnosis between resolving and progressive infantile scoliosis. J Bone Joint Surg Br. 1972;54(2):230-243.
12. Ward K., Ogilvie J., Argyle V., et al. Polygenic inheritance of adolescent idiopathic scoliosis: a study of extended families in Utah. Am J Med Genet A. 2010;152A(5):1178-1188.
13. Wynne-Davies R. Familial (idiopathic) scoliosis. A family survey. J Bone Joint Surg Br. 1968;50(1):24-30.
14. Riseborough E.J., Wynne-Davies R. A genetic survey of idiopathic scoliosis in Boston, Massachusetts. J Bone Joint Surg Am. 1973;55(5):974-982.
15. Gao X., Gordon D., Zhang D., et al. CHD7 gene polymorphisms are associated with susceptibility to idiopathic scoliosis. Am J Hum Genet. 2007;80(5):957-965.
16. Qiu X.S., Tang N.L., Yeung H.Y., et al. Melatonin receptor 1B (MTNR1B) gene polymorphism is associated with the occurrence of adolescent idiopathic scoliosis. Spine. 2007;32(16):1748-1753.
17. Rogala E.J., Drummond D.S., Gurr J. Scoliosis: incidence and natural history. A prospective epidemiological study. J Bone Joint Surg Am. 1978;60(2):173-176.
18. Bruszewski J., Kamza Z. Incidence of scoliosis based on an analysis of serial radiography. Chir Narzadow Ruchu Ortop Pol. 1957;22(2):115-116.
19. Kane W.J. Scoliosis prevalence: a call for a statement of terms. Clin Orthop Relat Res. 1977(126):43-46.
20. Patynski J., Szczekot J., Szwaluk F. Scoliosis in the light of statistics. Chir Narzadow Ruchu Ortop Pol. 1957;22(2):111-114.
21. Shands A.R.Jr., Eisberg H.B. The incidence of scoliosis in the state of Delaware; a study of 50,000 minifilms of the chest made during a survey for tuberculosis. J Bone Joint Surg Am. 1955;37A(6):1243-1249.
22. Weinstein S.L. Adolescent idiopathic scoliosis: prevalence and natural history. Instr Course Lect. 1989;38:115-128.
23. Renshaw T.S. Screening school children for scoliosis. Clin Orthop Relat Res. 1988;(229):26-33.
24. Pehrsson K., Larsson S., Oden A., Nachemson A. Long-term follow-up of patients with untreated scoliosis. A study of mortality, causes of death, and symptoms. Spine. 1992;17(9):1091-1096.
25. Aaro S., Ohlund C. Scoliosis and pulmonary function. Spine. 1984;9(2):220-222.
26. Sanders J.O., Khoury J.G., Kishan S., et al. Predicting scoliosis progression from skeletal maturity: a simplified classification during adolescence. J Bone Joint Surg Am. 2008;90(3):540-553.
27. Sanders J.O., Browne R.H., McConnell S.J., et al. Maturity assessment and curve progression in girls with idiopathic scoliosis. J Bone Joint Surg Am. 2007;89(1):64-73.
28. Song K.M., Little D.G. Peak height velocity as a maturity indicator for males with idiopathic scoliosis. J Pediatr Orthop. 2000;20(3):286-288.
29. Little D.G., Song K.M., Katz D., Herring J.A. Relationship of peak height velocity to other maturity indicators in idiopathic scoliosis in girls. J Bone Joint Surg Am. 2000;82(5):685-693.
30. Charles Y.P., Dimeglio A., Canavese F., Daures J.P. Skeletal age assessment from the olecranon for idiopathic scoliosis at Risser grade 0. J Bone Joint Surg Am. 2007;89(12):2737-2744.
31. Ward K., Ogilvie J.W., Singleton M.V., et al. Validation of DNA-based prognostic testing to predict spinal curve progression in adolescent idiopathic scoliosis. Spine. 2010;35(25):E1455-E1464.
32. Edgar M.A., Mehta M.H. Long-term follow-up of fused and unfused idiopathic scoliosis. J Bone Joint Surg Br. 1988;70(5):712-716.
33. Richards B.S., Bernstein R.M., D’Amato C.R., Thompson G.H. Standardization of criteria for adolescent idiopathic scoliosis brace studies: SRS Committee on Bracing and Nonoperative Management. Spine. 2005;30(18):2068-2075. discussion 2076-2077
34. Price C.T., Scott D.S., Reed F.E.Jr., Riddick M.F. Nighttime bracing for adolescent idiopathic scoliosis with the Charleston bending brace. Preliminary report. Spine. 1990;15(12):1294-1299.
35. Katz D.E., Richards B.S., Browne R.H., Herring J.A. A comparison between the Boston brace and the Charleston bending brace in adolescent idiopathic scoliosis. Spine. 1997;22(12):1302-1312.
36. Gammon S.R., Mehlman C.T., Chan W., et al. A comparison of thoracolumbosacral orthoses and SpineCor treatment of adolescent idiopathic scoliosis patients using the Scoliosis Research Society standardized criteria. J Pediatr Orthop. 2010;30(6):531-538.
37. Lonstein J.E., Winter R.B. The Milwaukee brace for the treatment of adolescent idiopathic scoliosis. A review of one thousand and twenty patients. J Bone Joint Surg Am. 1994;76(8):1207-1221.
38. D’Amato C.R., Griggs S., McCoy B. Nighttime bracing with the Providence brace in adolescent girls with idiopathic scoliosis. Spine. 2001;26(18):2006-2012.
39. Katz D.E., Herring J.A., Browne R.H., et al. Brace wear control of curve progression in adolescent idiopathic scoliosis. J Bone Joint Surg Am. 2010;92(6):1343-1352.
40. Rahman T., Bowen J.R., Takemitsu M., Scott C. The association between brace compliance and outcome for patients with idiopathic scoliosis. J Pediatr Orthop. 2005;25(4):420-422.
41. Mullaji A.B., Upadhyay S.S., Luk K.D., Leong J.C. Vertebral growth after posterior spinal fusion for idiopathic scoliosis in skeletally immature adolescents. The effect of growth on spinal deformity. J Bone Joint Surg Br. 1994;76(6):870-876.
42. Sanders J.O., Little D.G., Richards B.S. Prediction of the crankshaft phenomenon by peak height velocity. Spine. 1997;22(12):1352-1356. discussion 1356-1357
43. Dohin B., Dubousset J.F. Prevention of the crankshaft phenomenon with anterior spinal epiphysiodesis in surgical treatment of severe scoliosis of the younger patient. Eur Spine J. 1994;3(3):165-168.
44. Dubousset J., Herring J.A., Shufflebarger H. The crankshaft phenomenon. J Pediatr Orthop. 1989;9(5):541-550.
45. Dobbs M.B., Lenke L.G., Kim Y.J., et al. Anterior/posterior spinal instrumentation versus posterior instrumentation alone for the treatment of adolescent idiopathic scoliotic curves more than 90 degrees. Spine. 2006;31(20):2386-2391.
46. Kim Y.J., Lenke L.G., Bridwell K.H., et al. Pulmonary function in adolescent idiopathic scoliosis relative to the surgical procedure. J Bone Joint Surg Am. 2005;87(7):1534-1541.
47. Sucato D.J., Erken Y.H., Davis S., et al. Prone thoracoscopic release does not adversely affect pulmonary function when added to a posterior spinal fusion for severe spine deformity. Spine. 2009;34(8):771-778.
48. Lenke L.G., Newton P.O., Marks M.C., et al. Prospective pulmonary function comparison of open versus endoscopic anterior fusion combined with posterior fusion in adolescent idiopathic scoliosis. Spine. 2004;29(18):2055-2060.
49. Gill J.B., Levin A., Burd T., Longley M. Corrective osteotomies in spine surgery. J Bone Joint Surg Am. 2008;90(11):2509-2520.
50. Dorward I.G., Lenke L.G. Osteotomies in the posterior-only treatment of complex adult spinal deformity: a comparative review. Neurosurg Focus. 2010;28(3):E4.
51. Lenke L.G., O’Leary P.T., Bridwell K.H., et al. Posterior vertebral column resection for severe pediatric deformity: minimum two-year follow-up of thirty-five consecutive patients. Spine. 2009;34(20):2213-2221.
52. Sarwark J., Sarwahi V. New strategies and decision making in the management of neuromuscular scoliosis. Orthop Clin North Am. 2007;38(4):485-496.
53. Saito N., Ebara S., Ohotsuka K., et al. Natural history of scoliosis in spastic cerebral palsy. Lancet. 1998;351(9117):1687-1692.
54. Thometz J.G., Simon S.R. Progression of scoliosis after skeletal maturity in institutionalized adults who have cerebral palsy. J Bone Joint Surg Am. 1988;70(9):1290-1296.
55. Velasco M.V., Colin A.A., Zurakowski D., et al. Posterior spinal fusion for scoliosis in Duchenne muscular dystrophy diminishes the rate of respiratory decline. Spine. 2007;32(4):459-465.
56. Dooley J.M., Gordon K.E., MacSween J.M. Impact of steroids on surgical experiences of patients with Duchenne muscular dystrophy. Pediatr Neurol. 2010;43(3):173-176.
57. Akbarnia B.A., Breakwell L.M., Marks D.S., et al. Dual growing rod technique followed for three to eleven years until final fusion: the effect of frequency of lengthening. Spine. 2008;33(9):984-990.
58. Smith J.R., Samdani A.F., Pahys J., et al. The role of bracing, casting, and vertical expandable prosthetic titanium rib for the treatment of infantile idiopathic scoliosis: a single-institution experience with 31 consecutive patients. Clinical article. J Neurosurg Spine. 2009;11(1):3-8.
59. Blakemore L.C., Scoles P.V., Poe-Kochert C., Thompson G.H. Submuscular Isola rod with or without limited apical fusion in the management of severe spinal deformities in young children: preliminary report. Spine. 2001;26(18):2044-2048.
60. Akbarnia B.A., Marks D.S., Boachie-Adjei O., et al. Dual growing rod technique for the treatment of progressive early-onset scoliosis: a multicenter study. Spine. 2005;30(Suppl 17):S46-S57.
61. Campbell R.M.Jr., Smith M.D., Mayes T.C., et al. The effect of opening wedge thoracostomy on thoracic insufficiency syndrome associated with fused ribs and congenital scoliosis. J Bone Joint Surg Am. 2004;86A(8):1659-1674.
62. Campbell R.M.Jr., Hell-Vocke A.K. Growth of the thoracic spine in congenital scoliosis after expansion thoracoplasty. J Bone Joint Surg Am. 2003;85A(3):409-420.
63. Thompson G.H., Akbarnia B.A., Kostial P., et al. Comparison of single and dual growing rod techniques followed through definitive surgery: a preliminary study. Spine. 2005;30(18):2039-2044.
64. Davids J.R., Chamberlin E., Blackhurst D.W. Indications for magnetic resonance imaging in presumed adolescent idiopathic scoliosis. J Bone Joint Surg Am. 2004;86A(10):2187-2195.
65. Nakahara D., Yonezawa I., Kobanawa K., et al. Magnetic resonance imaging evaluation of patients with idiopathic scoliosis: a prospective study of four hundred seventy-two outpatients. Spine. 2010;36(7):E482-E485.
66. Inoue M., Minami S., Nakata Y., et al. Preoperative MRI analysis of patients with idiopathic scoliosis: a prospective study. Spine. 2005;30(1):108-114.
67. Gupta P., Lenke L.G., Bridwell K.H. Incidence of neural axis abnormalities in infantile and juvenile patients with spinal deformity. Is a magnetic resonance image screening necessary? Spine. 1998;23(2):206-210.
68. Wu L., Qiu Y., Wang B., et al. The left thoracic curve pattern: a strong predictor for neural axis abnormalities in patients with “idiopathic” scoliosis. Spine. 2010;35(2):182-185.
69. Mohammad D., Landman Z., Lubicky J., et al. Use and outcome of MRI in the surgical treatment of adolescent idiopathic scoliosis. Spine. 2011;36(8):667-671.
70. Dobbs M.B., Lenke L.G., Szymanski D.A., et al. Prevalence of neural axis abnormalities in patients with infantile idiopathic scoliosis. J Bone Joint Surg Am. 2002;84A(12):2230-2234.
71. King H.A., Moe J.H., Bradford D.S., Winter R.B. The selection of fusion levels in thoracic idiopathic scoliosis. J Bone Joint Surg Am. 1983;65(9):1302-1313.
72. Lenke L.G., Betz R.R., Harms J., et al. Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am. 2001;83A(8):1169-1181.
73. Niemeyer T., Wolf A., Kluba S., et al. Interobserver and intraobserver agreement of Lenke and King classifications for idiopathic scoliosis and the influence of level of professional training. Spine. 2006;31(18):2103-2107. discussion 2108
74. Richards B.S., Sucato D.J., Konigsberg D.E., Ouellet J.A. Comparison of reliability between the Lenke and King classification systems for adolescent idiopathic scoliosis using radiographs that were not premeasured. Spine. 2003;28(11):1148-1156. discussion 1156-1157
75. Morrissy R.T., Goldsmith G.S., Hall E.C., et al. Measurement of the Cobb angle on radiographs of patients who have scoliosis. Evaluation of intrinsic error. J Bone Joint Surg Am. 1990;72(3):320-327.
76. Mehta S.S., Modi H.N., Srinivasalu S., et al. Interobserver and intraobserver reliability of Cobb angle measurement: endplate versus pedicle as bony landmarks for measurement: a statistical analysis. J Pediatr Orthop. 2009;29(7):749-754.
77. Bertrand S.L., Drvaric D.M., Lange N., et al. Electrical stimulation for idiopathic scoliosis. Clin Orthop Relat Res. 1992(276):176-181.
78. Durham J.W., Moskowitz A., Whitney J. Surface electrical stimulation versus brace in treatment of idiopathic scoliosis. Spine. 1990;15(9):888-892.
79. O’Donnell C.S., Bunnell W.P., Betz R.R., et al. Electrical stimulation in the treatment of idiopathic scoliosis. Clin Orthop Relat Res. 1988(229):107-113.
80. Mooney V., Gulick J., Pozos R. A preliminary report on the effect of measured strength training in adolescent idiopathic scoliosis. J Spinal Disord. 2000;13(2):102-107.
81. Robitaille M., Aubin C.E., Labelle H. Intra- and interobserver variability of preoperative planning for surgical instrumentation in adolescent idiopathic scoliosis. Eur Spine J. 2007;16(10):1604-1614.
82. Lenke L.G., Kuklo T.R., Ondra S., Polly D.W.Jr. Rationale behind the current state-of-the-art treatment of scoliosis (in the pedicle screw era). Spine. 2008;33(10):1051-1054.
83. Kim Y.J., Lenke L.G., Cho S.K., et al. Comparative analysis of pedicle screw versus hook instrumentation in posterior spinal fusion of adolescent idiopathic scoliosis. Spine. 2004;29(18):2040-2048.
84. Polly D.W.Jr., Potter B.K., Kuklo T., et al. Volumetric spinal canal intrusion: a comparison between thoracic pedicle screws and thoracic hooks. Spine. 2004;29(1):63-69.
85. Kim Y.J., Lenke L.G., Kim J., et al. Comparative analysis of pedicle screw versus hybrid instrumentation in posterior spinal fusion of adolescent idiopathic scoliosis. Spine. 2006;31(3):291-298.
86. Kosmopoulos V., Schizas C. Pedicle screw placement accuracy: a meta-analysis. Spine. 2007;32(3):E111-E120.
87. Cheh G., Bridwell K.H., Lenke L.G., et al. Adjacent segment disease following lumbar/thoracolumbar fusion with pedicle screw instrumentation: a minimum 5-year follow-up. Spine. 2007;32(20):2253-2257.
88. Cho K.J., Lenke L.G., Bridwell K.H., et al. Selection of the optimal distal fusion level in posterior instrumentation and fusion for thoracic hyperkyphosis: the sagittal stable vertebra concept. Spine. 2009;34(8):765-770.
89. Nault M.L., Labelle H., Aubin C.E., et al. Fuzzy-logic-assisted surgical planning in adolescent idiopathic scoliosis. J Spinal Disord Tech. 2009;22(4):263-269.
90. Carreon L.Y., Puno R.M., Lenke L.G., et al. Non-neurologic complications following surgery for adolescent idiopathic scoliosis. J Bone Joint Surg Am. 2007;89(11):2427-2432.
91. Girardi F.P., Boachie-Adjei O., Burke S.W., Rawlins B.A. Surgical treatment of adolescent idiopathic scoliosis: a comparative study of two segmental instrumentation systems. J Spinal Disord. 2001;14(1):46-53.
92. Flynn J.M., Betz R.R., O’Brien M.F., Newton P.O. Radiographic classification of complications of instrumentation in adolescent idiopathic scoliosis. Clin Orthop Relat Res. 2010;468(3):665-669.
93. Master D.L., Son-Hing J.P., Poe-Kochert C., et al. Risk factors for major complications after surgery for neuromuscular scoliosis. Spine. 2011;36(7):564-571.
94. Mohamad F., Parent S., Pawelek J., et al. Perioperative complications after surgical correction in neuromuscular scoliosis. J Pediatr Orthop. 2007;27(4):392-397.
95. Shapiro F., Zurakowski D., Sethna N.F. Tranexamic acid diminishes intraoperative blood loss and transfusion in spinal fusions for Duchenne muscular dystrophy scoliosis. Spine. 2007;32(20):2278-2283.
96. Vitale M.G., Moore D.W., Matsumoto H., et al. Risk factors for spinal cord injury during surgery for spinal deformity. J Bone Joint Surg Am. 2010;92(1):64-71.
97. Buchowski J.M., Bridwell K.H., Lenke L.G., Good C.R. Epidural spinal cord compression with neurologic deficit associated with intrapedicular application of hemostatic gelatin matrix during pedicle screw insertion. Spine. 2009;34(13):E473-E477.
98. Stockl B., Wimmer C., Innerhofer P., et al. Delayed anterior spinal artery syndrome following posterior scoliosis correction. Eur Spine J. 2005;14(9):906-909.
99. Schwab F., Dubey A., Gamez L., et al. Adult scoliosis: prevalence, SF-36, and nutritional parameters in an elderly volunteer population. Spine. 2005;30(9):1082-1085.
100. Bradford D.S., Tay B.K., Hu S.S. Adult scoliosis: surgical indications, operative management, complications, and outcomes. Spine. 1999;24(24):2617-2629.
101. Schwab F., Dubey A., Pagala M., et al. Adult scoliosis: a health assessment analysis by SF-36. Spine. 2003;28(6):602-606.
102. Bernhardt M., Bridwell K.H. Segmental analysis of the sagittal plane alignment of the normal thoracic and lumbar spines and thoracolumbar junction. Spine. 1989;14(7):717-721.
103. Berthonnaud E., Dimnet J., Roussouly P., Labelle H. Analysis of the sagittal balance of the spine and pelvis using shape and orientation parameters. J Spinal Disord Tech. 2005;18(1):40-47.
104. During J., Goudfrooij H., Keessen W., et al. Toward standards for posture. Postural characteristics of the lower back system in normal and pathologic conditions. Spine. 1985;10(1):83-87.
105. Gelb D.E., Lenke L.G., Bridwell K.H., et al. An analysis of sagittal spinal alignment in 100 asymptomatic middle and older aged volunteers. Spine. 1995;20(12):1351-1358.
106. Jackson R.P., Kanemura T., Kawakami N., Hales C. Lumbopelvic lordosis and pelvic balance on repeated standing lateral radiographs of adult volunteers and untreated patients with constant low back pain. Spine. 2000;25(5):575-586.
107. Vaz G., Roussouly P., Berthonnaud E., Dimnet J. Sagittal morphology and equilibrium of pelvis and spine. Eur Spine J. 2002;11(1):80-87.
108. Schwab F., Lafage V., Boyce R., et al. Gravity line analysis in adult volunteers: age-related correlation with spinal parameters, pelvic parameters, and foot position. Spine. 2006;31(25):E959-E967.
109. Schwab F., Farcy J.P., Bridwell K., et al. A clinical impact classification of scoliosis in the adult. Spine. 2006;31(18):2109-2114.
110. Lafage V., Schwab F., Patel A., et al. Pelvic tilt and truncal inclination: two key radiographic parameters in the setting of adults with spinal deformity. Spine. 2009;34(17):E599-E606.
111. Duval-Beaupere G., Marty C., Barthel F., et al. Sagittal profile of the spine prominent part of the pelvis. Stud Health Technol Inform. 2002;88:47-64.
112. Legaye J., Duval-Beaupere G., Hecquet J., Marty C. Pelvic incidence: a fundamental pelvic parameter for three-dimensional regulation of spinal sagittal curves. Eur Spine J. 1998;7(2):99-103.
113. Roussouly P., Gollogly S., Berthonnaud E., Dimnet J. Classification of the normal variation in the sagittal alignment of the human lumbar spine and pelvis in the standing position. Spine. 2005;30(3):346-353.
114. Vialle R., Levassor N., Rillardon L., et al. Radiographic analysis of the sagittal alignment and balance of the spine in asymptomatic subjects. J Bone Joint Surg Am. 2005;87(2):260-267.
115. Lowe T., Berven S.H., Schwab F.J., Bridwell K.H. The SRS classification for adult spinal deformity: building on the King/Moe and Lenke classification systems. Spine. 2006;31(Suppl 19):S119-125.
116. Schwab F.J., Smith V.A., Biserni M., et al. Adult scoliosis: a quantitative radiographic and clinical analysis. Spine. 2002;27(4):387-392.
117. Schwab F., Patel A., Ungar B., et al. Adult spinal deformity-postoperative standing imbalance: how much can you tolerate? An overview of key parameters in assessing alignment and planning corrective surgery. Spine. 2010;35(25):2224-2231.
118. Boulay C., Tardieu C., Hecquet J., et al. Sagittal alignment of spine and pelvis regulated by pelvic incidence: standard values and prediction of lordosis. Eur Spine J. 2006;15(4):415-422.
119. Mac-Thiong J.M., Berthonnaud E., Dimar J.R.2nd, et al. Sagittal alignment of the spine and pelvis during growth. Spine. 2004;29(15):1642-1647.
120. Schwab F.J., Lafage V., Farcy J.P., et al. Predicting outcome and complications in the surgical treatment of adult scoliosis. Spine. 2008;33(20):2243-2247.