CHAPTER 27 Patient Positioning for Spinal Surgery
Equipment
Table
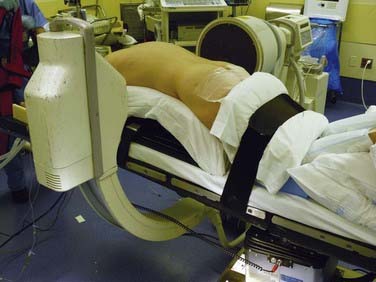
A basic electric operating table may be used for many spinal procedures (Fig. 27-1). Operations in the supine position, such as anterior cervical procedures and anterior lumbar fusions in the distal lumbar spine (L3-S1), are easily performed with such a table. Reversing the table may improve clearance under the table for the fluoroscopic unit. A lateral approach for thoracic, thoracolumbar, and lumbar procedures may also be performed on an electric table. For thoracoabdominal and retroperitoneal flank approaches, it is often helpful to place the level of pathology at the table break and flex the patient laterally.
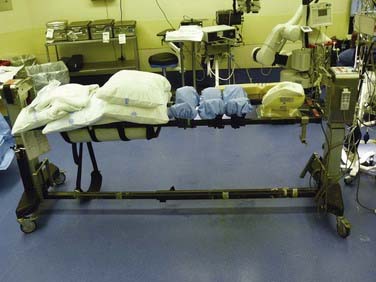
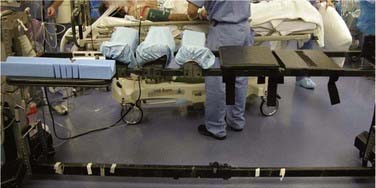
There are many advantages to modular spine-specific operating tables such as the Jackson Spinal table (Mizuho OSI, Union City, CA) (Fig. 27-2). The carbon fiber frame is radiolucent and low profile to allow 360-degree fluoroscopy and the use of intraoperative CT scanners such as the O-arm (Medtronic Navigation, Louisville, CO). Modular components allow customization for each procedure and for a wide range of patient phenotypes. The head may be secured in a foam headrest, in a rigid fixator, or with traction. The legs may be supported in a sling to allow lumbar flexion or on a rigid tabletop to enhance lordosis (Fig. 27-3). Finally, intraoperative repositioning for anterior-posterior or posterior-anterior surgery is facilitated with a rotational capability that obviates the need for moving the patient from one table to another.
Principles of Positioning
Patient Safety and Protection
Neuropathies and Prevention
Ulnar neuropathy, one of the most common postoperative neuropathies, accounts for a third of all nerve injury claims in the American Society of Anesthesiologists Closed Claims Study database.1 Although the etiology of postoperative peripheral nerve injury is not entirely known, it is thought to be related to intraneural capillary ischemia resulting from nerve overstretch or compression, perhaps exacerbated by prolonged intraoperative hypotension. The ulnar nerve appears to be more vulnerable to ischemia than the median and radial nerves, with a reported incidence of 0.04% after noncardiac surgery to 37% in one series of cardiac patients who underwent detailed postoperative sensory testing. The time of onset of ulnar nerve symptoms varies from immediately after surgery to 3 days postoperatively. The duration of symptoms tends to vary across reports, with some completely resolving spontaneously in days and others persisting for years after the initial insult.2 Risk factors for postoperative ulnar neuropathy include diabetes, increased age, and male gender.3
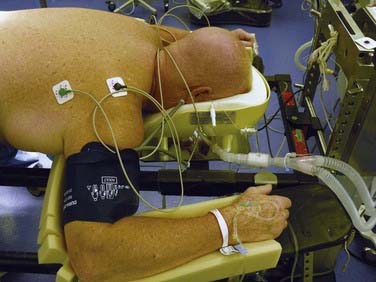
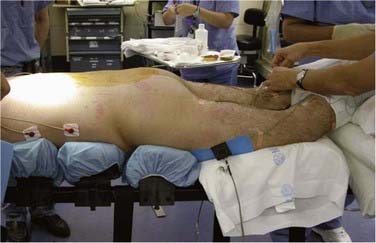
Anatomically, the ulnar nerve appears to be particularly susceptible to direct compression as it courses through the superficial condylar groove at the elbow. Elbow flexion, especially to greater than 110 degrees, can tighten the cubital tunnel retinaculum and directly compress the nerve,4 and external compression in the absence of flexion may compromise the nerve. With the patient in the supine position, direct pressure on the ulnar nerve at the elbow is significantly higher if both forearms are pronated than if they are in a neutral and supinated position (Fig. 27-4).5
Brachial plexus neuropathy may have findings similar to ulnar neuropathy but may additionally be characterized by symptoms such as shoulder pain, scapular winging, and shoulder weakness. The incidence of brachial plexopathy during posterior spinal surgery has been estimated to be between 3.6% and 15%, as compared with 0.02% in a large study of 15,000 general surgical patients. Most patients achieve partial or full functional recovery, although some have persistent symptoms at late (1- to 3-year) follow-up.6,7
Some investigators have suggested intraoperative somatosensory evoked potential (SSEP) monitoring as a way to detect impending nerve injury. One retrospective study of 1000 spinal surgeries determined that the overall incidence of position-related upper extremity SSEP changes was 6.1%, with the lateral decubitus position (7.5%) and prone “superman” position (7.0%) having the highest incidence of position-related upper extremity SSEP changes. In this study, postoperative deficits did not develop in any patient who had positionally reversible SSEP changes.8
Lower extremity neuropathies have not been well studied in spinal surgery because they typically occur in patients undergoing surgery in the lithotomy position and after lower extremity orthopedic procedures. Injury to the common peroneal nerve has been reported more frequently than injury to any other lower extremity peripheral nerve, probably because of its vulnerable anatomic location. The common peroneal nerve is fixed in a superficial location as it traverses the head of the fibula, which leaves it susceptible to direct compression injury by devices that hold the legs in place. The legs should be padded and well protected at the level of the fibular head, particularly for procedures in the lateral decubitus position.9
In the event of a new postoperative neurological deficit, it is important to distinguish peroneal nerve injury from an acute L5 radiculopathy. A peroneal neuropathy is characterized by complete plegia of dorsiflexion and eversion without significant pain complaints, whereas an L5 radiculopathy usually results in dermatomal pain and sensory deficit accompanied by weakness of dorsiflexion, toe extension, and foot inversion.10
The lateral femoral cutaneous nerve (LFCN) originates from the L2-3 nerve roots and travels along the lateral border of the psoas major muscle and across the ilium toward the anterior superior iliac spine (ASIS). Because of its anatomic exit below the ASIS, compression neuropathy of the LFCN by posts or pads that support the pelvis may develop in patients who are placed in the prone position. In patients who sustain perioperative injury to the LFCN, hypoesthesia of the anterolateral aspect of the thigh usually develops, but some experience pain and dysesthesia as well. Few studies have been conducted to assess the incidence of positioning-related LFCN injury, although most estimates are around 20%. One study in particular estimated the incidence of LFCN injury to be 23.8% in patients who underwent prone spinal surgery with use of the Relton-Hall frame. All these patients experienced resolution of symptoms within 1 week to 2 months postoperatively.11
Head Positioning
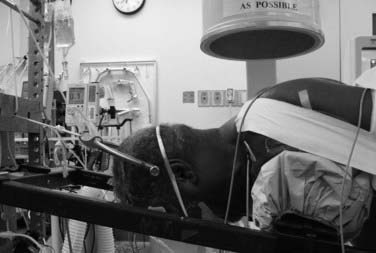
Finally, traction systems can be used to secure the head. They allow some movement of the head and neck during surgery, which can have at least two benefits. First, by setting up dual vectors for traction, alignment of the spine can be altered during surgery by the surgeon while still scrubbed (Fig. 27-5). Second, the small amount of movement produced by the placement of upper thoracic pedicle screws might cause dislodgment of the head from a rigid fixator; a properly adjusted traction system allows a safe amount of movement and eliminates this potential complication.
Visual Loss and Its Prevention
Postoperative visual loss (POVL) is an infrequently recognized but devastating complication of spinal surgery. Given that the estimated incidence of POVL has varied considerably across the surgical literature and was thought to be escalating in the mid-1990s, the American Society of Anesthesiologists created a POVL registry in 1999. Interim analyses of the POVL registry suggested a frequency of 0.0008% in noncardiac surgical patients undergoing procedures as disparate as hip arthroplasty, thoracotomy, and neck dissection. POVL in spinal surgery appears to be up to 100 times more frequent, with an incidence of roughly 0.08%.12
The most common cause of POVL in spinal surgery is ischemic optic neuropathy (ION) (89% of registry cases), which is more frequently unilateral than bilateral. POVL may also be attributed to central retinal artery occlusion (11% of registry cases). Although the precise etiology of ION in spinal surgery remains unclear, the leading hypothesis attributes ION to compromised blood flow in the optic nerve as a result of increased venous pressure and interstitial edema. Seventy-two percent of all ION cases were associated with prone spinal surgery; it occurred both in patients whose heads were maintained in facial supporters and in those for whom Mayfield pins alone were used for the entirety of the procedure. These data demonstrate that ION occurs independent of external pressure on the globe. Early data also suggested a relationship between POVL and prolonged anesthetic duration (94% of procedures had an anesthetic duration of 6 hours or longer), as well as between POVL and significant blood loss (82% of patients had estimated blood loss of 1 L or greater).13
Spinal Alignment
When performing instrumentation and arthrodesis of the subaxial cervical spine, the surgeon must attend to the restoration or preservation of normal cervical lordosis. Patients who are fixed in a straight alignment are likely to complain about their head and neck position or pain, or both. To facilitate laminectomies, foraminotomies, and placement of lateral mass fixation, we prefer an intraoperative position of relative neck flexion. By using the dual-vector traction system described earlier, we can easily place the patient into cervical lordosis before rod placement and grafting. Although well-placed lateral mass screws can tolerate modest amounts of corrective force during rod placement, their relatively low pullout strength and the lack of a good salvage fixation option in the event of pullout has led us to try to achieve the final alignment through patient positioning and to use the fixation to maintain rather than achieve the final lordotic alignment (see Fig. 27-5).
Finally, the importance of restoring or maintaining adequate lumbar lordosis has received much attention—and rightly so. Lumbar flexion facilitates decompressive lumbar laminectomy and lumbar microdiskectomy but should be assiduously avoided if an arthrodesis is to be performed (Fig. 27-6). Adequate lumbar lordosis can most easily be achieved with maximal hip extension. Use of the Wilson frame and a leg sling should generally be avoided in procedures that involve lumbar arthrodesis. Instead, we use modular hip and thigh pads on a spine table and a flat leg rest padded with pillows (Fig. 27-7).
Specific Procedures
Cooper DE, Jenkins RS, Bready L, et al. The prevention of injuries of the brachial plexus secondary to malposition of the patient during surgery. Clin Orthop Relat Res. 1988;228:33-41.
Heitz JW, Audu PB. Asymmetric postoperative visual loss after spine surgery in the lateral decubitus position. Br J Anaesth. 2008;101:380-382.
Kamel IR, Drum ET, Koch SA, et al. The use of somatosensory evoked potentials to determine the relationship between patient positioning and impending upper extremity nerve injury during spine surgery: a retrospective analysis. Anesth Analg. 2006;102:1538-1542.
Lee LA, Roth S, Posner KL, et al. The American Society of Anesthesiologists Postoperative Visual Loss Registry: analysis of 93 spine surgery cases with postoperative visual loss. Anesthesiology. 2006;105:652-659.
O’Driscoll SW, Horii E, Carmichael SW, et al. The cubital tunnel and ulnar neuropathy. J Bone Joint Surg Br. 1991;73:613-617.
Prielipp RC, Morell RC, Butterworth J. Ulnar nerve injury and perioperative arm positioning. Anesthesiol Clin North Am. 2002;20:589-603.
Prielipp RC, Morell RC, Walker FO, et al. Influence of arm position and relationship to somatosensory evoked potential. Anesthesiology. 1999;91:345-354.
Schwartz DM, Drummond DS, Hahn M, et al. Prevention of positional brachial plexopathy during surgical correction of scoliosis. J Spinal Disord. 2000;13:178-182.
Seyfer AE, Grammer NY, Bogumill GP, et al. Upper extremity neuropathies after cardiac surgery. J Hand Surg Am. 1985;10:16-19.
Vazquez-Jimenez JF, Krebs G, Schiefer J, et al. Injury of the common peroneal nerve after cardiothoracic operations. Ann Thorac Surg. 2002;73:119-122.
Warner MA. Perioperative neuropathies. Mayo Clin Proc. 1998;73:567-574.
Warner MA, Warner ME, Martin JT. Ulnar neuropathy: incidence, outcome, and risk factors in sedated or anesthetized patients. Anesthesiology. 1994;81:1332-1340.
Yang SH, Wu CC, Chen PQ. Postoperative meralgia paresthetica after posterior spine surgery: incidence, risk factors, and clinical outcomes. Spine. 2005;30:E547-E550.
1 Prielipp RC, Morell RC, Walker FO, et al. Influence of arm position and relationship to somatosensory evoked potential. Anesthesiology. 1999;91:345-354.
2 Seyfer AE, Grammer NY, Bogumill GP, et al. Upper extremity neuropathies after cardiac surgery. J Hand Surg Am. 1985;10:16-19.
3 Warner MA, Warner ME, Martin JT. Ulnar neuropathy: incidence, outcome, and risk factors in sedated or anesthetized patients. Anesthesiology. 1994;81:1332-1340.
4 O’Driscoll SW, Horii E, Carmichael SW, et al. The cubital tunnel and ulnar neuropathy. J Bone Joint Surg Br. 1991;73:613-617.
5 Prielipp RC, Morell RC, Butterworth J. Ulnar nerve injury and perioperative arm positioning. Anesthesiol Clin North Am. 2002;20:589-603.
6 Cooper DE, Jenkins RS, Bready L, et al. The prevention of injuries of the brachial plexus secondary to malposition of the patient during surgery. Clin Orthop Relat Res. 1988;228:33-41.
7 Schwartz DM, Drummond DS, Hahn M, et al. Prevention of positional brachial plexopathy during surgical correction of scoliosis. J Spinal Disord. 2000;13:178-182.
8 Kamel IR, Drum ET, Koch SA, et al. The use of somatosensory evoked potentials to determine the relationship between patient positioning and impending upper extremity nerve injury during spine surgery: a retrospective analysis. Anesth Analg. 2006;102:1538-1542.
9 Warner MA. Perioperative neuropathies. Mayo Clin Proc. 1998;73:567-574.
10 Vazquez-Jimenez JF, Krebs G, Schiefer J, et al. Injury of the common peroneal nerve after cardiothoracic operations. Ann Thorac Surg. 2002;73:119-122.
11 Yang SH, Wu CC, Chen PQ. Postoperative meralgia paresthetica after posterior spine surgery: incidence, risk factors, and clinical outcomes. Spine. 2005;30:E547-E550.
12 Heitz JW, Audu PB. Asymmetric postoperative visual loss after spine surgery in the lateral decubitus position. Br J Anaesth. 2008;101:380-382.
13 Lee LA, Roth S, Posner KL, et al. The American Society of Anesthesiologists Postoperative Visual Loss Registry: analysis of 93 spine surgery cases with postoperative visual loss. Anesthesiology. 2006;105:652-659.