CHAPTER 316 Pathology of the Cervicothoracic Junction
Evaluation and Treatment
Anatomically, the cervicothoracic junction has varying definitions; however, it is strictly defined as the functional unit of the C7 and T1 vertebral bodies and the intervening disk space. Many authors define this region as a transition zone and include the distal cervical spine through the T3 disk space to account for the biomechanical properties and anatomic variability.1–3 We define the cervicothoracic junction as the superior end plate of the C7 vertebral body to the T3-4 disk space. This chapter reviews the unique anatomy and biomechanical principles of the cervicothoracic junction, imaging techniques, and surgical management of common pathologic entities involving this region.
History and Physical Examination
Pain, as is often seen in other spinal pathologies, is the most common complaint of patients with cervicothoracic pathology.4 Differentiating between musculoskeletal pain, osteoporotic fractures, pathologic fractures, degenerative disease, and infection can be difficult. For example, patients with a lung malignancy can have complaints of dysesthetic nerve root pain rather than neurological sequelae secondary to cord compression. Isolated axial pain is uncommon. Interestingly, isolated osteoporotic vertebral compression fractures at the cervicothoracic junction are rare because of the decreased load on the anterior column, and such fractures should be lower on the list of differential diagnoses. Therefore, the presence of a compression fracture over this region may suggest the possibility of a pathologic fracture from metastatic disease and should be evaluated as such.
Although not the initial symptom, up to 80% of patients with disorders of the cervicothoracic junction are found to have neurological impairment when first evaluated.1 This high incidence of neurological dysfunction is related to the limited amount of space in the canal as a result of the large spinal cord–to-canal ratio; the smaller caudal cervical and upper thoracic vertebral bodies, which can lead to earlier encroachment on nervous tissue by infection and neoplasm; and theoretically, the tenuous blood supply to the spinal cord over this region.5 Thus, patients commonly exhibit global destruction over the region, whereas isolated radiculopathy and individual nerve root injuries are uncommon findings.6 When patients have signs of neurological dysfunction, they are more often found to have myelopathic findings than patients with pathology in other regions of the spinal column. Patients may initially be seen with or without signs or symptoms in the upper extremities, depending on whether the disease process is affecting the C8 and T1 nerve roots. Myelopathic features encountered include long-tract signs, pathologic reflexes (Babinski’s signs), bowel or bladder dysfunction (or both), lower extremity weakness, and gait dysfunction.7
Patients may also exhibit or are found on examination to have Horner’s syndrome, defined as ptosis, miosis, anhidrosis, and enophthalmos, because of interruption of the sympathetic innervation of the ipsilateral orbit and face.8 Cervicothoracic junction pathology may disrupt either the second-order (damage to preganglionic axons as they approach the stellate ganglion) or third-order sympathetic neurons (damage to postganglionic axons within or on leaving the stellate ganglion).9 Diseases that are destructive or expansile may involve the sympathetic neurons and their associated ganglia. This is typically associated with extension of a primary lung neoplasm, infiltration of an infectious or metastatic process into the vertebral bodies, or iatrogenic injury after anterior spinal approaches.10
Anatomy
The Superior Mediastinum and Associated Vasculature
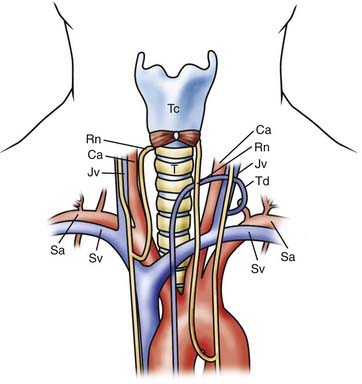
The superior mediastinum extends from the thoracic inlet to the plane between the manubriosternal joint and the T4-5 disk space (Fig. 316-1). Proximally, the thoracic inlet is bounded by the superior aspect of the manubrium, the superior border of the first rib, and the T1 vertebral body.7,11 The manubriosternal joint, also referred to as the angle of Louis, is the junction of the manubrium and body of the sternum and is the point of attachment of the second costal cartilages. An axial line drawn from this joint to the T4-5 disk space defines the inferior margin of the superior mediastinum.
The subclavian artery and accompanying vein traverse the superior aspect of the first rib and are in close proximity to the anterior scalene muscle, which can be used to localize these structures. The subclavian vein passes anterior to the anterior scalene muscle, whereas the subclavian artery passes posteriorly. At the medial border of the anterior scalene muscle, the left subclavian vein joins with the left internal jugular vein at a sharp angle to form the left brachiocephalic vein. The thoracic duct, which returns lymphatic drainage to the circulatory system, drains into this left venous angle in the majority of patients.11,12 The left brachiocephalic vein then courses inferiorly and medially, posterior to the manubrium, until it reaches the first right intercostal space and unites with the right brachiocephalic vein to create the superior vena cava.11
Neural Structures of the Superior Mediastinum
The Vagus and Recurrent Laryngeal Nerves
Two important anatomic relationships exist between the vagus nerves and associated structures. First, the vagus nerves descend in the carotid sheath and pass anterior to the major arterial structures of the superior mediastinum. The right vagus nerve travels ventral to the right subclavian artery, whereas the contralateral vagus nerve descends anterior to the aortic arch. Both vagus nerves then descend posterior to the hilar structures of the lung. However, before reaching the lung hila, both vagus nerves give rise to an RLN. The right RLN descends parallel to the carotid artery and loops beneath the right subclavian artery, typically at the level of T3.13 It then courses medially and superiorly to reenter the neck, hence the name recurrent, and travels along the tracheoesophageal groove. On the left side, the RLN also descends parallel to the carotid; however, because of the presence of the aortic arch, it loops around distally at the level of the ligamentum arteriosum before entering the tracheoesophageal groove. Both RLNs then ascend posterior to the lateral lobes of the thyroid gland and have a variable relationship to the inferior thyroidal artery and veins, potentially crossing either ventral or dorsal to the vessels. In the cervical region, a left-sided approach is believed to be associated with a lower incidence of injury because the left RLN travels distally and more frequently within the tracheoesophageal groove than the right RLN does.13,14
The Phrenic Nerves
The phrenic nerves, which innervate the diaphragm, arise from the motor component of the C3-5 nerve roots and leave the cervical spinal cord to enter the mediastinum posterior to the ventral portion of the first rib. These nerves descend anterior to the aorta on the left and the subclavian artery on the right. Their course through the mediastinum differs from that of the vagus nerves in that they pass anterior to the hila of the lungs.6,11
Viscera of the Superior Mediastinum
The Esophagus, Trachea, and Lungs
The trachea divides into the primary bronchi at the manubriosternal joint. Along with the esophagus, it courses through the mediastinum from C6 to T5.6,11 During dissection, this close association between these structures allows them to be mobilized medially as a single unit.
The lung apices and associated pleura are consistently exposed during posterolateral or ventral approaches to the distal thoracic spine (T8 to T12); however, anterior exposure of the cervicothoracic junction will also expose these structures. Stanescu and coauthors reported that in 46% of cadaveric axial sections through the T1 body, the pleural cavity was directly anterolateral to the vertebral body.3 Thus, even a distal anterior approach, without manubriotomy or sternotomy, will expose the pleura to potential injury.9
The Thoracic Duct
Although a left-sided approach to the cervical spine is reported to have a lower incidence of RLN injury, it does potentially expose the thoracic duct to injury.15,16 The thoracic duct, which returns lymphatic drainage to the systemic circulation, typically empties into the left jugulosubclavian junction located between C7 and T1.15 However, this course is variable and the termination point may extend cranially to C6. The jugulosubclavian junction lies within a triangle bounded by the medially placed esophagus and longus colli muscles, the laterally lying anterior scalene, and the inferiorly based first rib.17 When dissecting through this region, identification and manipulation of the thoracic duct are necessary, and Resnick reported that ligation and division of this structure is well-tolerated.16
The Vertebral Arteries
The vertebral artery typically enters the cervical foramen transversarium at the C6 level, but in up to 5% of patients the vertebral artery enters at the C7 foramen.11,18 The vertebral artery, from its origin at the subclavian to its entrance into the foramen magnum, follows a variable path. The artery may be located anterior to the lateral mass (0% to 14%) or lateral to the pedicle (14% to 100%), or rarely, it may be shifted so far anterior that it is found lateral to the cervical vertebral bodies.3
The Cervicothoracic Spinal Column
Surgical treatment of the cervicothoracic junction can be a challenging endeavor because of obstacles encountered during the anterior and posterior approaches. Posterior decompression and stabilization are technically difficult as a result of the transitional and variable anatomic dimensions of each vertebral segment. Anterior access to the upper thoracic vertebrae is limited by the mediastinal structures and, additionally, by the thoracic kyphosis, which angles the disk spaces away from the surgeon. Distally in the cervical spine, the lateral masses decrease in anteroposterior thickness, thus limiting the area for internal fixation. Pedicle width increases from C5 to C7-T1 but then decreases from T1 to T5.3
To assess the variability in the dimensions of the bony structures in the cervicothoracic junction, Stanescu’s group analyzed 324 cross-sectional spinal segments from nine cadaveric specimens.3 Anteroposterior lateral mass thickness measured in the sagittal plane was found to be greatest at C5 and then successively decreased, with the greatest decrease occurring between C6 and C7.3 Pedicle width measured at the vertebral base progressively increased from C5 to T1 and then decreased from T1 to T5. The pedicle base measurements had a more significant change in width than did the laminar measurements.3,7,14 Pedicle height increases from C5 to T5, whereas medial angulation decreases from C7 to T2 because of the progressive decrease in width of the thoracic lamina.3,7,14
The diameter of the vertebral bodies and spinal canal is also an important dimension to consider when treating disorders of the cervicothoracic junction. Vertebral body sagittal diameter from C5 to T3 remains relatively constant, with a minimal increase in diameter of the superior end plate from 18.9 to 21.4 mm. In the coronal plane, vertebral body height increases from 27.9 mm at C5 to 29 mm at T1 and then decreases to 25.4 mm at T3.3 The spinal canal is relatively narrow through this region and assumes an oval shape averaging 24 by 15 mm, with the widest dimension in the transverse plane. The spinal cord enlarges over C3 to T2 because of the presence of the brachial plexus.6 The combination of diminishing bony dimensions and enlarging neural structures results in two thirds of the spinal canal being occupied by the spinal cord, with little room left for space-occupying pathology. Additionally, the large nerve roots associated with the cervical enlargement occupy two thirds of the intervertebral foramina.
Blood supply to the spinal cord undergoes a transition through this region as the anterior and posterior spinal arteries become less prominent. The caudal cervical spinal cord derives its blood supply from radicular branches of the vertebral, thyrocervical, and costoclavicular arteries, whereas the upper thoracic cord receives branches from the supreme intercostal arteries.7,11
Biomechanical Considerations
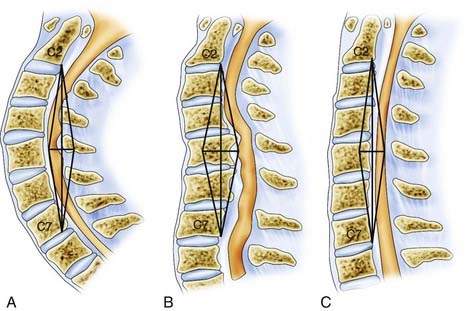
The transition from the cervical to the thoracic spine must be accounted for when treating pathology in this region. The mobile, lordotic cervical spine transitions into the relatively rigid, thoracic kyphosis. Through a wide range of flexion, extension, and rotation of the cervical spine, the head can assume a multitude of positions and maintains overall sagittal balance. The cervical spine has a range of 60 to 75 degrees in flexion-extension.19 Axial rotation distal to C6 is limited to a range of just 4 to 8 degrees.19,20 In addition, lateral bending has a range of 6 to 8 degrees from C5 to T6.19,21 Little bony stability is derived from the osseous cervical spine, and the majority of cervical stabilization is derived from the ligamentous structures. The thoracic spine, through its association with the stabilizing architecture of the chest, including the scapulae, clavicles, ribs, and sternum, is relatively rigid and immobile.22,23 The stark contrast in range of motion between these two adjacent regions subjects the cervicothoracic junction to translational forces, excessive flexion, and a significant transfer of axial loads (Fig. 316-2).5
Trauma, degeneration, infection, and neoplasia can result in instability of the cervicothoracic segment because of compromise of these biomechanical properties. Destabilization of the cervicothoracic junction can result in a progressive kyphotic deformity, translation of the vertebral elements, narrowing of the spinal canal, and compression of the spinal cord and neural elements.24 Failure to account for these unique biomechanical principles when operating in this region may result in construct failure, a worsening deformity, and neurological compromise.
Diagnostic Studies
Plain Radiography
Evaluation of the cervicothoracic junction with plain roentgenographic imaging is frequently limited, and adequate visualization may not be achieved in up to 26% of patients.25 The swimmer’s view displaces the shoulder from its position overlying the spine, yet limiting factors such as coexisting neurological impairment, injuries to the upper extremity or chest, and persistent obscuration by the shoulder commonly render this view inadequate.26 In a series of 100 swimmer’s view radiographs obtained over a 2-year period in trauma patients, Rethnam and colleagues evaluated the images for adequate visualization of the C7 body and the C7-T1 junction.26 Only 55% of the images were adequate, with none of the inadequate radiographs satisfactorily visualizing the C7 body and the C7-T1 junction.26 In 2000, Jelly and coauthors published a prospective study that examined a series of 73 trauma patients with spiral CT, 12 of whom were found to have fractures at the cervicothoracic junction.27 These fractures were demonstrated with conventional radiography in only 5 of the 12 patients (42%).27
Computed Tomography
The use of high-resolution multidetector CT has increased the speed, sensitivity, and accuracy of the diagnosis of fractures of the cervicothoracic junction.28 In Tan and associates’ series of 360 blunt trauma patients in whom the C7-T1 level was not adequately visualized on plain radiographs, CT led to detection of 11 injuries to the cervicothoracic junction.29 Avoidance of the potential sequelae of undiagnosed cervicothoracic junction injuries proved cost-effective, with $9192 saved for each fracture identified, $16,852 for each potentially or definitely unstable fracture identified, and $50,557 for each definitely unstable fracture identified.29
CT of the cervicothoracic junction has proved to be useful in surgical planning because it details the bony and soft tissue relationships in a region of anatomic variability and visualizes compromise of neural structures.1 CT reconstructions provide a three-dimensional image of the osseous spine and allow the surgeon to make measurements for designing constructs preoperatively.
Magnetic Resonance Imaging and Myelography
MRI offers a noninvasive detailed assessment of the soft tissues, neural anatomy, and potential pathology over the cervicothoracic region and is thus used more often than invasive myelography. In traumatic injury, MRI evaluates for the presence of hematomas, ligamentous injury, and disk herniations while also identifying nerve root and spinal cord compression or signal abnormality.1 The addition of a contrast agent (gadolinium) provides detailed enhancement of tumors and abscesses while also improving the visualization of neural tissue.
Preoperative sagittal MRI scout images can be used to evaluate the relationship between the sternal notch and level of pathology to determine whether access without a thoracotomy is possible.30 In patients who have contraindications to MRI (metal prostheses, spinal cord stimulators, implantable defibrillators) or in whom the MRI table will not support their body habitus, myelography with postmyelographic CT is an alternative. Cord compression is most effectively demonstrated in the sagittal plane, whereas axial imaging allows visualization of dye-filling defects in the nerve root sleeve but does not effectively visualize intraspinal pathology.
Surgical Approaches
Anterior Approaches
The Low Anterior Cervical Approach
Pathology ventral to the cervical spinal cord has traditionally been addressed through the anteromedial or Smith-Robinson approach. Although this approach effectively exposes pathology in the upper cervical spine, it is limited in the caudal cervical and upper thoracic spine, particularly distal to the T2 vertebra. Access to the vertebral elements is restricted by body habitus and the regional anatomy of the manubrium and sternum. In a series of 7 patients, Sharan and coworkers concluded that the upper thoracic vertebrae can be exposed through a suprasternal approach without sternotomy or thoracotomy.30 Midsagittal cervicothoracic MRI was used to identify the thoracic vertebrae above the sternum, thus determining whether the low suprasternal approach was feasible. In a review of MRI of 103 cervicothoracic junctions, Sharan and colleagues reported that the T1-2 disk space could be accessed through a suprasternal route. A direct suprasternal approach to the T2-3 disk space was possible in only 15 of 103 (15%) patients.30 However, if the goal of surgery was corpectomy, exposure down to the T3 vertebral body could be achieved. Corpectomy enlarges the area of exposure because of greater visualization of the thecal sac along a more rostral-caudal trajectory and by eliminating the restricted angulation of the disk space. Pathology below the level of the sternal notch on preoperative imaging was addressed with a lateral extracavitary or transpedicular approach, thereby avoiding sternotomy or manubriectomy (Fig. 316-3).30
A low cervical approach is performed with the patient supine and the neck and head in maximal extension. A longitudinal incision parallel to the sternocleidomastoid muscle is brought down to the sternal notch. The platysma muscle is divided and the sternohyoid and sternothyroid muscles are then mobilized medially. The omohyoid, which often lies in the field when the lower cervical spine is exposed, is divided if necessary. Blunt dissection is carried down to the vertebral bodies, with care taken to maintain the tracheoesophageal structures medially and the carotid sheath laterally.7,15,25 If the plane of dissection is not maintained medial to the carotid sheath or if the carotid is subjected to excessive retraction, the descending limb of the RLN will be placed in jeopardy. Additionally, if the trachea is retracted too vigorously, the transverse or ascending segments of the RLN (or both) may suffer a stretch injury.15
Right versus Left-Sided Approach
The superiority of the left-sided versus the right-sided approach to the cervicothoracic junction is debated in the literature. In 1976, Fielding and Stillwell advocated a right-sided approach to avoid the thoracic duct and gain better control of the innominate vessels.31 In their series of 14 patients, Boockvar and coworkers also favored the right-sided approach because of the more variable course of the left RLN and left-sided thoracic duct.24
Resnick, in a series of 21 patients undergoing anterior cervicothoracic junction corpectomy and plate fixation, preferred a left-sided approach.16 He noted that the right RLN, although more consistent in its path, crosses the operative field obliquely and was more susceptible to injury.16 Additionally, the only case of permanent vocal cord paralysis in his series resulted from a right-sided approach. He reported that with a left-sided approach, identification and retraction of the thoracic duct were achieved routinely and ligation was well tolerated. Ultimately, surgeon preference and familiarity are the determining factors when choosing a right- versus left-sided approach.
Distal Extensions of the Low Cervical Anterior Approach
Fielding and Stillwell noted that with the addition of inferior retraction on the innominate vessels, the low anterior cervical approach can visualize the T4 vertebral body.31 Although visualization of the T4 vertebral body may be achieved, the limited angulation and working space created by the sternum are often an impediment to effective decompression and stabilization down to the T2 level.15 To extend the distal exposure provided by a low anterior cervical approach, numerous procedures have been reported and are reviewed in the following text.
Cauchoix and Binet, in 1957, first described an anterior approach to the cervicothoracic junction through a full median sternotomy as an extension of the cervical approach.32 To expose the ventral aspect of the spine, the authors reported ligating the left brachiocephalic vein and exposing the aortic arch. However, Hodgson and associates reported 40% operative mortality in a series of 10 patients with this technique.33,34 Over the past 50 years, advances in operative and anesthetic technique have led to a significant decrease in operative mortality. Unfortunately, morbidity is high because of persistent pain at the sternotomy site and left upper extremity venous congestion secondary to vein ligation. Additionally, this procedure is limited in that the inferior aspect of the exposure is not used because the heart and structures of the mediastinum lie directly over the spinal column and cannot be retracted or mobilized.
In 1995, Darling and coauthors reported a modified anterior approach to the cervicothoracic junction in a series of four patients with metastatic spinal disease.35 They reported exposure from C3 to T4 through a combined standard anterior cervical approach and a partial median sternotomy. The additional exposure is gained by dividing the sternum from the sternal notch to the level of the second intercostal space. A transverse osteotomy through the synostosis between the manubrium and body of the sternum is then performed to allow the sternum to be divided laterally to the left. Division of the strap muscles at their origin from the sternum connects the partial sternotomy to the already completed anterior cervical approach. Access to the T2 level is consistently achieved with this approach, but visualization and dissection of T3-4 can also be achieved by allowing the surgeon a lower trajectory to the spine. This combined approach minimizes the amount of dissection, eliminates the unnecessary exposure provided by a full sternotomy, and requires little additional operative time when compared with an anterior cervical approach.
Another alternative to a full median sternotomy is partial resection of the manubrium and proximal part of the clavicle. Sundaresan and colleagues reported extending a horizontal incision bilaterally beyond the lateral margins of the sternocleidomastoid muscle at a point 1 cm above the clavicles.36 A T-shaped incision is completed through the midline by extending a ventral incision from the horizontal incision to the midbody of the sternum. The sternocleidomastoid and strap muscles are detached from their insertions on the sternum and proximal clavicle and reflected. The proximal third of the clavicle is divided with a Gigli saw and resected after detachment of the sternoclavicular joint. A central rectangular portion of the manubrium is then resected and the underlying subclavian vein dissected free. The remainder of the exposure proceeds as for the anterior cervical approach and sternotomy previously described. The resected manubrium and clavicle may be used as autologous bone grafting material. Unfortunately, resection of the clavicle places the subclavian vessels at risk, and delayed instability of the shoulder may result from resection of the sternoclavicular joint.
Multiple variations of these techniques have been reported by numerous authors, all of which provide exposure to the vertebral levels from C3 to T4. Kurz and associates modified the Sundaresan approach by making a unilateral transverse incision on the side of the approach and leaving the manubrium intact because manubrial resection did not add to their exposure.37 Four patients underwent decompression and stabilization via this approach for kyphosis and neck pain caused by cervicothoracic junction metastases.37 Three of the four patients exhibited an improvement in neurological function and the fourth remained neurologically intact.37 The approach afforded good visualization, successfully reduced the kyphosis in all four patients, and provided all patients with adequate pain relief.37
Sar and colleagues further modified Sundaresan’s technique because resection of the manubrium and medial part of the clavicle creates a significant bony defect and a predisposition to eventual shoulder dysfunction.38 After decompression and fusion of the ventral spine, these authors advocate replantation of the osteotomized segment of manubrium and clavicle to stabilize the shoulder. Thus, the resected manubrium and clavicle are reattached to the sternum and lateral two thirds of the clavicle with wires at the conclusion of the procedure. Sar and coworkers used this technique and reported adequate visualization of the cervicothoracic junction and successful union of the osteotomy sites.38
An additional technique to prevent the morbidity of destabilizing the shoulder with destruction of the sternoclavicular joint is unilateral or bilateral manubriotomy. The approach is performed through an L-shaped incision for a unilateral manubriotomy or an inverted T-shaped incision when bilateral division of the manubrium is necessary.39 The vertical component of the incision involves extending the midline incision of the standard anteromedial approach down to the level of the manubrium. A midline osteotomy is then made through the manubrium and rostral sternum. The transverse component of the L- or T-shaped incision is extended through the second intercostal space. The unilateral and bilateral manubriotomy approaches provide 4 and 8 cm of exposure, respectively, and permit visualization down to the T4-5 level.38–40
Lesoin and associates noted that detachment of the sternocleidomastoid muscle from the sternum with these approaches results in an alteration in the biomechanics of respiration.41 Therefore, to reduce this morbidity, they proposed a modified approach involving resection of the manubrium and proximal third of the clavicles bilaterally, thus maintaining the attachments of the sternocleidomastoid. The remainder of the approach continues as described earlier. This technique allows the clavicles to be reattached at closure and restores the sternocleidomastoid muscles to their natural anatomic position, where they elevate the thoracic cage during inspiration. In addition, the sternoclavicular joints are maintained, thereby reducing the possibility of shoulder instability.
The Hemi-Clamshell Approach
The hemi-clamshell approach was devised to provide greater access to the cervicothoracic junction for resection of primary and metastatic lesions that cannot be accessed with an extension of a low cervical approach. It is a combined approach consisting of a partial median sternotomy, anterior thoracotomy, and neck incision.42 The fourth intercostal space is accessed through an anterolateral thoracotomy incision with the patient positioned supine. The incision is extended from the sternum to the anterior axillary line. The ipsilateral lung is collapsed and the intercostal space is opened to the midclavicular line. The initial skin incision is then extended over the sternum and into the neck and terminates over the anterior border of the ipsilateral sternocleidomastoid muscle. A partial sternotomy is performed from the sternal notch to the fourth intercostal space. The sternotomy exposure is made contiguous with a low anterior cervical exposure, thus connecting all three approaches.
The primary advantage of this approach is that it provides excellent visualization of the spinal cord anteriorly while also affording access to adjacent structures that may be invaded by neoplasm, such as the ribs, associated neurovascular bundles, and thyroid. Additionally, any lung parenchyma invaded by neoplasm may be resected. Furthermore, this approach preserves shoulder stability by avoiding division of the clavicle and disruption of the sternoclavicular joint.43
The hemi-clamshell approach is limited in its ability to access the posterior chest wall and neural foramina, and therefore the approach is used mostly for anterolateral pathology. In addition, some authors have expressed concern over the possibility of a postoperative flail chest and pulmonary complications.42,44 Korst and Burt reported a series of 42 patients in which the invaded structures were resected en bloc with this technique.42 Seven patients underwent successful vertebral body resection and experienced no flail chest postoperatively.42
Transthoracic Approaches
A transthoracic thoracotomy provides access to the cervicothoracic junction through an inferior approach. Typically, it is used routinely to gain access to the mid to lower thoracic spine. When approaching the cervicothoracic junction, the thoracotomy is routinely performed at the level of the third rib but requires mobilization and retraction of the scapula.45 The pleura is incised to expose the lung, which is subsequently retracted. Dissection of the pleura off the vertebral column exposes the anterolateral aspects of the T1 and T2 vertebral bodies. The anatomy of each individual patient may limit thoracotomy and the ability to expose the ventral aspect of the vertebral bodies in this region.
Posterior Approaches
Laminectomy
If the pathology lies posterior to the spinal cord or in the posterior elements, a laminectomy provides sufficient decompression. A vertical midline incision is made and carried down to the spinous processes. Subperiosteal dissection separates the soft tissues off the lamina. Intraoperative lateral radiography is used to confirm the correct vertebral segment, typically by counting from the cervical region. Laminectomy is then carried out with a rongeur or drill. In addition, instrumentation may also be used, depending on the pathology and degree of instability.1 However, Steinmetz and coauthors noted that isolated cervicothoracic laminectomy was a risk factor for construct failure.46
Posterolateral Approaches: Costotransversectomy and the Lateral Extracavitary Approach
To achieve further exposure of the ventral thoracic spinal cord and column through a posterolateral approach, Larson and colleagues first described a lateral extracavitary approach (LECA) for the treatment of osteomyelitis and Pott’s disease.47 With the patient in the prone position, a paramedian or midline incision is made. The erector spinae muscles are identified, dissected free from the ribs, and reflected medially, thereby revealing a larger segment of the proximal rib than possible with costotransversectomy. Proximal rib resection of up to 10 cm reveals the ventral aspect of the vertebral body. When a midline incision is used, the entire vertebral segment, from its ventral aspect to its posterior elements, is exposed.
The ability of the LECA to expose both the ventral and posterior aspects of the spine eliminates the need for a second incision or approach. Lifshutz and coworkers, in their review of the LECA, made the important point that the surgeon is able to work sequentially: first decompressing the anterior elements, then reducing the deformity, and finally, placing posterior instrumentation.48 The approach has been applied to the treatment of degenerative disease, trauma, neoplasia, and infection at the cervicothoracic junction.
Although the LECA offers the advantage of a single incision, the approach is technically challenging, time-consuming, and associated with a high morbidity rate. Resnick and Benzel, in a retrospective review of 33 patients with thoracic or thoracolumbar traumatic injuries treated by the LECA, showed a 55% incidence of adverse events, with the majority being pulmonary complications.49 Eleven patients had chest tubes placed for hemothorax or persistent pleural effusions, and postoperative pneumonia developed in 7. Although no patient suffered neurological deterioration and there were no mortalities, the LECA was found to be associated with excessive morbidity.49 Thus, the advantage gained by a combined ventral and dorsal construct must be weighed against the complications that are inherent in the LECA.47
The Transpedicular Approach
The transpedicular approach requires resection of the facet joint and removal of the pedicle down to its attachment to the vertebral body, followed by ventral and lateral decompression. This approach is used for biopsy of pathology in the vertebral body or for resection of lateral disk herniations in the lower thoracic and lumbar spine.50 At the cervicothoracic junction, where pedicle width is variable and the neural elements lie in close approximation to the pedicle, the procedure may be associated with a risk for injury.51 However, with oncologic lesions, where ventral approaches are limited because of patient comorbidity, this approach may be warranted.
Posterior Cervical Foraminotomy at the Cervicothoracic Junction
As discussed previously, isolated radiculopathies at the cervicothoracic junction are rare, with a reported incidence of just 4% to 7%.52–54 In the case of cervicothoracic disk herniations resulting in radiculopathy, the anterior approach has been the traditional procedure. Harrop and colleagues, in a series of 19 patients, discussed the advantages of a posterior approach to lateral disk herniations via posterior cervical foraminotomy and diskectomy.55 Eight of the 11 patients (73%) with weakness had complete recovery of strength within 1 week of surgery.55 An additional 2 patients experienced delayed recovery past 1 week. Pain was the most common initial symptom, and patients experienced a significant reduction in the radicular component of their pain.55 Multiple studies have shown posterior cervical foraminotomy to be a safe procedure with a complication rate of 0% to 4%.51,53,56–59 The most common complications are wound infections and serous drainage from the incision.54,58 Importantly, posterior cervical foraminotomy avoids complications of the anterior approach, such as esophageal perforation,59 pneumothorax,60 Horner’s syndrome,1,61 and tracheal/laryngeal injury.62 Posterior foraminotomy is an established treatment and should be considered when treating disk herniations at the cervicothoracic junction.52
Construct Failure at the Cervicothoracic Junction
There is limited literature pertaining to failure of anterior and posterior constructs at the cervicothoracic junction. Construct failure can occur for a variety of reasons, including displacement or fracture of the anterior vertebral body or graft, failure of the instrumentation, pseudarthrosis, progressive kyphotic deformity, or translation of the vertebral bodies. As in any region of the spine, pseudarthrosis at the cervicothoracic junction is associated with tobacco use, previous surgery, and correction of deformity at the involved levels.63,64 In a series of 14 patients, Boockvar and colleagues outlined the preoperative risk factors and anterior surgical approaches linked to construct failure.24 Steinmetz and associates evaluated 14 construct failures in a series of 593 anterior and posterior cervicothoracic junction procedures.46 Both reports found laminectomy to be associated with a high rate of construct failure. Furthermore, Steinmetz and associates showed laminectomy across the cervicothoracic junction without supplemental posterior instrumentation to be associated with a 38% failure rate.46 Because uninstrumented laminectomy over this region has a high failure rate, supplementary dorsal instrumentation is advocated.
Multilevel corpectomies across the cervicothoracic junction without supplemental posterior fixation were also associated with a high rate of construct failure. Steinmetz and coauthors reported two- and three-level corpectomies involving the vertebral bodies of the cervicothoracic junction to have up to a 16.7% incidence of fusion failure.46 Boockvar and colleagues further replicated these results in their own series.24 However, treatment failure was not reported for one-level corpectomies or diskectomies. Although not statistically significant, the authors noted a trend toward construct failure when the posterior hardware terminated at C7. Therefore, posterior constructs may be extended down to the T1 and T2 pedicles to increase construct stability. Construct failure did not occur in association with combined anterior and posterior stabilization procedures extended to C7 or below. In Steinmetz and associates’ series, 18 of 593 patients underwent combined approaches, none of whom experienced construct failure.46
Pathology at the Cervicothoracic Junction
Neoplasia
The spinal cord and column may be involved with the entire spectrum of neoplastic disease. Benign tumors and primary and metastatic disease can involve the spinal axis and result in pain and neurological sequelae. Neoplastic involvement of T1-4 occurs in 15% of patients with spinal tumors, and the entire cervicothoracic junction has a 10% rate of involvement with spinal metastases.5 No single primary tumor occurs more frequently at the cervicothoracic junction than in other regions of the spine. Adenocarcinoma of the lung and thyroid is commonly involved with the cervicothoracic junction by way of direct extension or metastasis.
Axial pain from bone destruction, a kyphotic deformity, or oncologic burden elsewhere in the body often precedes neurological sequelae. However, the anatomic constraints of the cervicothoracic junction result in a high percentage of patients having symptomatic neurological disease. Radiculopathy is rarely seen, but myelopathy, bowel and bladder dysfunction, gait changes, and spastic paraparesis or tetraparesis (when the pathology extends above T1) are more commonly found because of impingement on the spinal cord.6,7
As in the treatment of all spinal neoplasia, management of tumors involving the cervicothoracic junction requires a multidisciplinary approach. Within the treatment algorithm, operative versus nonoperative management must be based on the presence or absence of neural compression, tumor type, likelihood of construct failure, life expectancy of the patient, and the role of adjuvant therapy.65 Once the decision to operate has been made, selection of the operative approach is based on the goals of surgery.
Metastatic disease is typically centered in the vertebral body, which can present a challenging scenario when addressing malignancy at the cervicothoracic junction. For patients with ventral metastatic disease and neural compression, isolated laminectomy frequently fails to result in sufficient decompression of the neural elements. Additionally, isolated posterior decompression may also result in worsening instability and a progressive kyphotic deformity at the involved levels. Anterior approaches enable resection of the tumor focus, direct neural decompression, correction of deformity, and anterior fusion, but in the setting of postoperative chemotherapy and radiation therapy, there is a significant risk for graft failure.66 Therefore, metastatic disease involving the cervicothoracic junction can be addressed through a combined anterior and posterior approach.
Pancoast’s Tumors
Bronchogenic carcinomas originating in the apex of the lung are known as Pancoast’s tumors (superior sulcus tumors). Because of the close proximity between the upper thoracic vertebrae and the apical pleura, the cervicothoracic junction is commonly involved by direct extension of the tumor. Patients may have Horner’s syndrome (miosis, ptosis, anhidrosis, enophthalmos), chest pain secondary to invasion of the chest wall, hand weakness, and pain radiating to the involved upper extremity. Because of the significant amount of local tumor invasion, difficulty of the surgical approach, and tumor burden commonly found at initial evaluation, patients were traditionally not considered to be surgical candidates. However, with advances in technology, instrumentation, and adjuvant therapy, aggressive surgical treatment of these malignancies has proved promising. Rusch and colleagues reviewed 225 patients over a 24-year period who underwent thoracotomy for the treatment of superior sulcus tumors. The majority of patients (55%) received radiation therapy before surgery; however, a significant number (35%) did not undergo preoperative treatment.67 The ability to achieve complete resection at the time of surgery was found to have a significant impact on overall survival.67
Infection
The cervicothoracic junction is no more susceptible to pyogenic infection than any other region of the spinal column.68 Patients with osteomyelitis, diskitis, and abscesses at these levels are found to have pain, focal tenderness, fever, symptoms related to a mass effect on the trachea and esophagus, or neurological sequelae. The limitations of plain radiography at the cervicothoracic junction may result in delayed diagnosis because of the poor view of the osseous elements. MRI provides the most detailed assessment.
Patients with an abscess at the cervicothoracic junction may have either C8 radiculopathy or spastic paraparesis. The C8 nerve root supplies the majority of the intrinsic hand muscles, and compression of it results in progressive weakness of the affected hand.7,69 Patients with C8 radiculopathy typically describe an inability to perform fine motor tasks such as buttoning a shirt or holding a pen. Sensory abnormalities are most commonly described as diminished sensation in the thumb and second finger in comparison to sensation in the fourth and fifth fingers. Prompt diagnosis of C8 radiculopathy is important because it has the poorest prognosis of all the cervical radiculopathies in terms of regaining motor function.7,69
A unique feature of abscess formation at the cervicothoracic junction is the relatively higher incidence of resulting paralysis than with abscess formation at other spinal levels.70 Neural compression occurs earlier in the course of the disease as a result of the diminished distance between the bony and neural elements in the spinal canal. Additionally, inflammation or disruption of the tenuous blood supply to the spinal cord in this region may result in vascular insult to the cord.7 Finally, anterior column instability secondary to long-standing infection promotes the development of a progressive kyphosis, which can result in further spinal cord compression.
The cervicothoracic junction, because of its close proximity to the lung apices, is particularly vulnerable to infection by tuberculosis and is involved in 13% of all cases of spinal tuberculosis.7,71 The principles of diagnosis, imaging, and treatment are similar to those for pyogenic infection. Prolonged medical treatment has been used with success.71
Trauma
Epidemiology
Multiple series have shown traumatic injury to the cervicothoracic junction to be relatively rare. In a retrospective review of 156 spine injuries, Amin and Saifuddin reported a 4.49% incidence of cervicothoracic fracture-dislocations.72 Nichols and colleagues, in a series of 397 patients, reported 37 (9%) to have injury at the cervicothoracic junction.73 However, the true incidence of traumatic injury to the cervicothoracic junction may not be fully appreciated because of a significant number of delayed or missed diagnoses. Evans reviewed a 27-year period at the Spinal Injuries Unit in Sheffield, England, and reported that nearly two thirds of cervicothoracic junction dislocations were not properly diagnosed on admission.74 In a series of 218 patients with acute injuries to the upper thoracic spine and paralysis, Bohlman noted that there was a delay in diagnosis of 24 injuries to the cervicothoracic junction.75
Multiple factors contribute to the high rate of undiagnosed cervicothoracic junction injuries, but the majority of explanations fall into one of two categories: inadequate imaging or the presence of complicating comorbidity. Motor vehicle accidents account for the majority of injuries to the cervicothoracic junction, and falls from a height represent the second most common mechanism of injury.76,77 Because of the violent nature of these mechanisms, patients frequently have traumatic head injury and multisystem trauma and are under the influence of alcohol or drugs or in a state of altered sensorium. Clinical suspicion of cervicothoracic junction pathology should be heightened in patients with concomitant head or chest injuries, concurrent spinal fractures, or acceleration-deceleration as the mechanism of injury. Additionally, when a fracture is identified at the cervicothoracic junction initially, it is imperative to evaluate for other noncontiguous fractures because they occur at a relatively high frequency.71
Classification Systems
Injuries to both the cervical and thoracic spine are classified under a number of different systems; however, there is currently no system unique to the cervicothoracic junction.78–80 Allen and colleagues’ classification system for subaxial cervical spine injuries may be applied to the cervicothoracic junction.81 It uses six categories based on the mechanism of injury: compressive flexion, vertical compression, distractive flexion, compressive extension, distractive extension, and lateral flexion.81 Vacarro and colleagues proposed a subaxial cervical spine injury classification system to standardize the classification system and provide a more comprehensive evaluation of subaxial cervical spine injury.80 Three categories, injury morphology, diskoligamentous complex, and neurological status, are each assigned a weighted score to define injury severity and guide treatment.80 Finally, the AO (Arbeitsgemeinschaft für Osteosynthesefragen) classification system, which is based on the classification principles for long-bone fracture, uses three fracture types: A (compression injuries), B (distraction injuries), and C (torsion injuries).82,83
Nonoperative Management of Cervicothoracic Injury
Although cervicothoracic compression, a lateral mass, and spinous process fractures may be treated successfully with external orthosis or halo immobilization, more severe injuries have proved difficult to treat with nonoperative management.71 The inadequacy of external orthoses in immobilization of the cervicothoracic junction is due to residual mobility at individual segments of the unstable spine.84 Evans’ series of 14 patients with cervicothoracic injuries treated by manipulation or skeletal traction showed 4 patients to have died of pulmonary embolism within 7 weeks.74 Patients who suffered a complete injury died (4 patients) or never achieved improvement in neurological function (7 patients).74
An’s group noted an attempt at closed reduction for cervicothoracic junction fracture-dislocations. They reported early closed reduction of cervicothoracic junction dislocations with traction weights of up to 140 lb.85 In the event that closed reduction is unsuccessful, immediate open reduction plus decompression is advocated to potentially facilitate the recovery of individual nerve roots and the spinal cord.
Operative Management of Cervicothoracic Injury
The goals of management of traumatic injury to the cervicothoracic junction are neural decompression, stabilization, restoration of anatomic spinal alignment, and early mobilization and rehabilitation. Anterior and posterior approaches to the cervicothoracic junction are complicated procedures because of the anatomic restraints and biomechanical principles inherent in this region.86 There are limited data pertaining to the use of anterior-only decompression and instrumentation for the treatment of fractures and dislocations.
In a retrospective review of 36 patients with instability at the cervicothoracic junction, An and associates advocated an anterior and posterior or combined circumferential fusion because of the anatomic constraints of the region.1 In cases in which the pathology is compressing the neural elements, decompression should be performed with stabilization, and ventral compression should be treated with an anterior approach. If laminectomy is also indicated, supplementation with posterior instrumentation is advised to avoid a progressive kyphotic deformity and translation over the region.
Posterior decompression with instrumentation has become increasingly more popular over the past 20 years as wire-rod techniques have evolved into screw-plate and then screw-rod techniques.87 Although screw-rod instrumentation provides immediate internal fixation, flexible correction of deformity, and high rates of fusion, the literature pertaining to the use of screw-rod systems for cervicothoracic trauma is limited. In biomechanical testing of cadaveric models, Rhee and coworkers demonstrated that a C7 pedicle screw achieves significantly greater stiffness in axial compression, torsion, bending, and flexion than does a lateral mass screw placed at this level.88 However, pedicle screw fixation at C7, T1, and T2 potentially places the neural and vascular structures at greater risk for injury.
In a series of 13 traumatic fractures and dislocations of the lower cervical spine, Abumi and coauthors reported 13 solid fusions and no neurovascular complications.89 In 1997, Kramer and colleagues reported significant variability in pedicle dimensions and topographic landmarks, thus suggesting risk with placement of pedicle screws at the cervicothoracic junction.90 Furthermore, in a cadaveric model, pedicle screws were inserted by one of three techniques: morphometric analysis of data, laminoforaminotomy and palpation, or stereotactic guidance. The percentage of noncritical and critical breaches was lower with laminoforaminotomy and palpation than with the use of just morphometric data, but the lowest incidence of breaches was achieved with stereotactic guidance. In a clinical series of 21 patients, Albert and associates reported safe placement of C7 pedicle screws with laminoforaminotomy and palpation.18 Neurological injury and vascular compromise were not found in any patient after pedicle screw placement.
Albert TJ, Klein GR, Joffe D, et al. Use of cervicothoracic junction pedicle screws for reconstruction of complex cervical spine pathology. Spine. 1998;23:1596-1599.
An HS, Gordin R, Renner K. Anatomic considerations for plate-screw fixation of the cervical spine. Spine. 1991;16:S548-S551.
An HS, Vaccaro A, Cotler JM, et al. Spinal disorders at the cervicothoracic junction. Spine. 1994;19:2557-2564.
An HS, Wise JJ, Xu R. Anatomy of the cervicothoracic junction: a study of cadaveric dissection, cryomicrotomy, and magnetic resonance imaging. J Spinal Disord. 1999;12:519-525.
Bailey AS, Stanescu S, Yeasting RA, et al. Anatomic relationships of the cervicothoracic junction. Spine. 1995;20:1431-1439.
Bohlman HH, Freehafer A, Dejak J. The results of treatment of acute injuries of the upper thoracic spine with paralysis. J Bone Joint Surg Am. 1985;67:360-369.
Boockvar JA, Philips MF, Telfeian AE, et al. Results and risk factors for anterior cervicothoracic junction surgery. J Neurosurg Spine. 2001;94:12-17.
Darling GE, McBroom R, Perrin R. Modified anterior approach to the cervicothoracic junction. Spine. 1995;20:1519-1521.
Gieger M, Roth PA, Wu JK. The anterior cervical approach to the cervicothoracic junction. Neurosurgery. 1995;37:704-710.
Harrop JS, Sharan A, Anderson G, et al. Failure of standard imaging to detect a cervical fracture in a patient with ankylosing spondylitis. Spine. 2005;30:E417-E419.
Harrop JS, Silva MT, Sharan AD, et al. Cervicothoracic radiculopathy treated using posterior cervical foraminotomy/discectomy. J Neurosurg Spine. 2003;98:131-136.
Kurz LT, Pursel SE, Herkowitz HN. Modified anterior approach to the cervicothoracic junction. Spine. 1991;16:S542-S547.
Le H, Balabhadra R, Park J, et al. Surgical treatment of tumors involving the cervicothoracic junction. Neurosurg Focus. 2003;15(5):E3.
Moore KL, Dalley AF. Thorax. In: Clinically Oriented Anatomy. Philadelphia: Lippincott Williams & Wilkins; 1999:59-168.
Nichols CG, Young DH, Schiller WR. Evaluation of cervicothoracic junction injury. Ann Emerg Med. 1987;16:640-642.
Rao R. Pathophysiology, natural history and clinical features of neck pain, cervical radiculopathy and myelopathy. J Bone Joint Surg Am. 2002;10:1872-1881.
Rao RD, Fischgrund JS. Disorders at the cervicothoracic junction. In: Frymoyer JW, Wiesel SW, editors. The Adult and Pediatric Spine. Philadelphia: Lippincott Williams & Wilkins; 2004:731-749.
Resnick DK. Anterior cervicothoracic junction corpectomy and plate fixation without sternotomy. Neurosurg Focus. 2002;12(1):E7.
Resnick DK, Benzel EC. Lateral extracavitary approach for thoracic and thoracolumbar spine trauma: operative complications. Neurosurgery. 1998;43:796-802.
Sar C, Hamzaoglu A, Talu U, et al. An anterior approach to the cervicothoracic junction of the spine (modified osteotomy of manubrium sterni and clavicle). J Spinal Disord. 1999;12:102-106.
Sharan AD, Przybylski GJ, Tartaglino L. Approaching the upper thoracic vertebrae without sternotomy or thoracotomy: a radiographic analysis with clinical application. Spine. 2000;25:910-916.
Steinmetz MP, Miller J, Krishnaney AA, et al. Regional instability following cervicothoracic junction surgery. J Neurosurg Spine. 2006;4:278-284.
Sundaresan N, Shah J, Foley KM, et al. An anterior surgical approach to the upper thoracic vertebrae. J Neurosurg. 1984;6:686-690.
White AA, Panjabi MM. Clinical Biomechanics of the Spine, 2nd ed. Philadelphia: JB Lippincott; 1990.
1 An HS, Vaccaro A, Cotler JM, et al. Spinal disorders at the cervicothoracic junction. Spine. 1994;19:2557-2564.
2 Lakshmanan P, Ahmed SM, Al-Maiyah M, et al. The low anterior cervical approach to the upper thoracic vertebrae: a decision by preoperative MR imaging. Diagn Interv Radiol. 2007;13:30-32.
3 Bailey AS, Stanescu S, Yeasting RA, et al. Anatomic relationships of the cervicothoracic junction. Spine. 1995;20:1431-1439.
4 Sapkas G, Papadakis S, Katonis P, et al. Operative treatment of unstable injuries of the cervicothoracic junction. Eur Spine J. 1999;8:279-283.
5 Le H, Balabhadra R, Park J, et al. Surgical treatment of tumors involving the cervicothoracic junction. Neurosurg Focus. 2003;15(5):E3.
6 Rao R. Pathophysiology, natural history and clinical features of neck pain, cervical radiculopathy and myelopathy. J Bone Joint Surg Am. 2002;10:1872-1881.
7 Rao RD, Fischgrund JS. Disorders at the cervicothoracic junction. In: Frymoyer JW, Wiesel SW, editors. The Adult and Pediatric Spine. Philadelphia: Lippincott Williams & Wilkins; 2004:731-749.
8 Ebraheim NA, Lu J, Yang H, et al. Vulnerability of the sympathetic trunk during the anterior approach to the lower cervical spine. Spine. 2000;25:1603-1606.
9 Digre KB, Smoker WR, et al. Selective MR imaging approach for evaluation of patients with Horner’s syndrome. AJNR Am J Neuroradiol. 1992;13:223-227.
10 Lloyd TV, Johnson JC, Paul DJ, et al. Horner’s syndrome secondary to herniated disc at T1-T2. AJR Am J Roentgenol. 1980;134:184-185.
11 Moore KL, Dalley AF. Thorax. In: Clinically Oriented Anatomy. Philadelphia: Lippincott Williams & Wilkins; 1999:59-168.
12 Ammar K, Tubbs RS, Smyth MD, et al. Anatomic landmarks for the cervical portion of the thoracic duct. Neurosurgery. 2003;53:1385-1387. discussion 1387-1388
13 Steinberg JL, Khane GJ, Fernandez CM, et al. Anatomy of the recurrent laryngeal nerve: a redescription. J Laryngol Otol. 1986;100:919-927.
14 An HS, Wise JJ, Xu R. Anatomy of the cervicothoracic junction: a study of cadaveric dissection, cryomicrotomy, and magnetic resonance imaging. J Spinal Disord. 1999;12:519-525.
15 Gieger M, Roth PA, Wu JK. The anterior cervical approach to the cervicothoracic junction. Neurosurgery. 1995;37:704-710.
16 Resnick DK. Anterior cervicothoracic junction corpectomy and plate fixation without sternotomy. Neurosurg Focus. 2002;12(1):E7.
17 Rouvier H. Terminal collecting ducts of the lymphatic system. In: Anatomy of the Human Lymphatic System. Ann Arbor, MI: Edwards; 1938:240-250.
18 Albert TJ, Klein GR, Joffe D, et al. Use of cervicothoracic junction pedicle screws for reconstruction of complex cervical spine pathology. Spine. 1998;23:1596-1599.
19 Maiman DJ, Pintar FA, Groff MW, et al. Concepts and mechanisms of biomechanics. In: Sonntag VK, Vollmer DG, editors. Youmans Neurological Surgery. Philadelphia: Elsevier; 2004:4181-4201.
20 Yoganandan N, Haffner M, Maiman D, et al. Epidemiology and injury biomechanics of motor vehicle related trauma to human spine. SAE Trans. 1990;98:1790-1807.
21 White AA, Panjabi MM. Clinical Biomechanics of the Spine, 2nd ed. Philadelphia: JB Lippincott; 1990.
22 Andriacchi T, Shultz A, Belytschko T, et al. A model for studies of mechanical interactions between the human spine and rib cage. J Biomech. 1974;7:497-507.
23 Pal GP, Routal RV. A study of weight transmission through the cervical and upper thoracic regions of the vertebral column in man. J Anat. 1986;148:245-261.
24 Boockvar JA, Philips MF, Telfeian AE, et al. Results and risk factors for anterior cervicothoracic junction surgery. J Neurosurg. 2001;94(1 suppl):12-17.
25 Kaneriya PP, Schweitzer ME, Spetell C, et al. The cost effectiveness of oblique radiography in the exclusion of C7-T1 injury in trauma patients. AJR Am J Roentgenol. 1998;171:959-962.
26 Rethnam U, Yesupalan RS, Bastawrous SS, et al. The swimmer’s view: does it really show what it is supposed to show? A retrospective study. BMC Med Imaging. 2008;8:2.
27 Jelly LM, Evans DR, Easty MJ, et al. Radiography versus spiral CT in the evaluation of cervicothoracic junction injuries in polytrauma patients who have undergone intubation. Radiographics. 2000;20:S251-S259.
28 Harrop JS, Sharan A, Anderson G, et al. Failure of standard imaging to detect a cervical fracture in a patient with ankylosing spondylitis. Spine. 2005;30:E417-E419.
29 Tan E, Schweitzer ME, Vaccaro L, et al. Is computed tomography of nonvisualized C7-T1 cost-effective? J Spinal Disord Tech. 1999;12:472-476.
30 Sharan AD, Przybylski GJ, Tartaglino L. Approaching the upper thoracic vertebrae without sternotomy or thoracotomy: a radiographic analysis with clinical application. Spine. 2000;25:910-916.
31 Fielding JW, Stillwell WT. Anterior cervical approach to the upper thoracic spine. Spine. 1976;1:158-161.
32 Cauchoix J, Binet JP. Anterior surgical approaches to the spine. Ann R Coll Surg Engl. 1957;21:234-243.
33 Hodgson AR, Stock FE. Anterior spinal fusion: a preliminary communication on the radical treatment of Pott’s paraplegia. Br J Surg. 1956;44:266-275.
34 Hodgson AR, Stock FE, Fang HY, et al. Anterior spinal fusion: the operative approach and pathologic findings in 412 patients with Pott’s disease of the spine. Br J Surg. 1960;48:172-178.
35 Darling GE, McBroom R, Perrin R. Modified anterior approach to the cervicothoracic junction. Spine. 1995;20:1519-1521.
36 Sundaresan N, Shah J, Foley KM, et al. An anterior surgical approach to the upper thoracic vertebrae. J Neurosurg. 1984;6:686-690.
37 Kurz LT, Pursel SE, Herkowitz HN. Modified anterior approach to the cervicothoracic junction. Spine. 1991;16:S542-S547.
38 Sar C, Hamzaoglu A, Talu U, et al. An anterior approach to the cervicothoracic junction of the spine (modified osteotomy of manubrium sterni and clavicle). J Spinal Disord. 1999;12:102-106.
39 Luk KD, Cheung KM, Leong JC. Anterior approach to the cervicothoracic junction by unilateral or bilateral manubriotomy. J Bone Joint Surg Am. 2002;84:1013-1017.
40 McDonald P, Letts M, Sutherland G, et al. Aneurysmal bone cyst of the upper thoracic spine. An operative approach through a manubrial sternotomy. Clin Orthop Relat Res. 1992;279:127-132.
41 Lesoin F, Thomas CE, Autricque A, et al. A transsternal biclavicular approach to the upper anterior thoracic spine. Surg Neurol. 1986;26:253-256.
42 Korst RJ, Burt ME. Cervicothoracic tumors: results of resection by the “hemi-clamshell” approach. J Thorac Cardiovasc Surg. 1998;115:286-295.
43 Grunenwald D, Spaggiari L. Transmanubrial osteomuscular sparing approach for apical chest tumors. Ann Thorac Surg. 1997;63:563-566.
44 Masaoka A, Ito Y, Yasumitsu T. Anterior approach for tumor of the superior sulcus. J Thorac Cardiovasc Surg. 1979;78:413-415.
45 Micheli LJ, Hood RW. Anterior exposure of the cervicothoracic spine using a combined cervical and thoracic approach. J Bone Joint Surg Am. 1983;7:992-997.
46 Steinmetz MP, Miller J, Krishnaney AA, et al. Regional instability following cervicothoracic junction surgery. J Neurosurg Spine. 2006;4:278-284.
47 Larson SJ, Holst RA, Hemmy DC, et al. Lateral extracavitary approach to traumatic lesions of the thoracic and lumbar spine. J Neurosurg. 1976;45:628-637.
48 Lifshutz J, Lidar Z, Maiman D. Evolution of the lateral extracavitary approach to the spine. Neurosurg Focus. 2004;16(1):E12.
49 Resnick DK, Benzel EC. Lateral extracavitary approach for thoracic and thoracolumbar spine trauma: operative complications. Neurosurgery. 1998;43:796-802.
50 Bilsky MH. Transpedicular approach for thoracic disc herniations. Neurosurg Focus. 2000;9(4):E3.
51 An HS, Gordin R, Renner K. Anatomic considerations for plate-screw fixation of the cervical spine. Spine. 1991;16:S548-S551.
52 Adamson TE. Microendoscopic posterior cervical laminoforaminotomy for unilateral radiculopathy: results of a new technology in 100 cases. J Neurosurg Spine. 2001;95:51-57.
53 Scoville WB, Dohrmann GJ, Corkill G. Late results of cervical disc surgery. J Neurosurg. 1976;45:203-210.
54 Woertgen C, Holzschuh M, Rothoerl RD, et al. Prognostic factors of posterior cervical disc surgery: a prospective, consecutive study of 54 patients. Neurosurgery. 1997;40:724-729.
55 Harrop JS, Silva MT, Sharan AD, et al. Cervicothoracic radiculopathy treated using posterior cervical foraminotomy/discectomy. J Neurosurg Spine. 2003;98:131-136.
56 Aldrich F. Posterolateral microdiscectomy for cervical monoradiculopathy caused by posterolateral soft disc sequestration. J Neurosurg. 1990;72:370-377.
57 Henderson CM, Hennessy RG, Shuey HMJr, et al. Posterior-lateral foraminotomy as an exclusive operative technique for cervical radiculopathy: a review of 846 consecutively operated cases. Neurosurgery. 1983;13:504-512.
58 Herkowitz HN, Kurz LT, Overholt DP. Surgical management of cervical soft disc herniation. A comparison between the anterior and posterior approach. Spine. 1990;15:1026-1030.
59 Newhouse KE, Lindsey RW, Clark CR. Esophageal perforation following anterior cervical spine surgery. Spine. 1989;14:1051-1053.
60 Cloward RB. Complications of anterior cervical disc operation and their treatment. Surgery. 1971;69:175-182.
61 Ebraheim NA, Lu J, Yang H, et al. Vulnerability of the sympathetic trunk during the anterior approach to the lower cervical spine. Spine. 2000;25:1603-1606.
62 Apfelbaum RI, Kriskovich MD, Haller JD. On the incidence, cause, and prevention of recurrent laryngeal nerve palsies during anterior cervical spine surgery. Spine. 2000;25:2906-2912.
63 Bose B. Anterior cervical instrumentation enhances fusion rates in multilevel reconstruction in smokers. J Spinal Disord. 2001;14:3-9.
64 Arnold PM, Klemp JA. Assessment of malunion in spinal fusion. Neurosurg Q. 2005;15:239-247.
65 Bell GR. Surgical treatment of spinal tumors. Clin Orthop Relat Res. 1997;335:54-63.
66 Harrington KD. The use of methylmethacrylate for vertebral body replacement and anterior stabilization of pathological fracture-dislocations of the spine due to metastatic malignant disease. J Bone Joint Surg Am. 1981;63:36-46.
67 Rusch VW, Parekh KR, Leon L, et al. Factors determining outcome after surgical resection of T3 and T4 lung cancers of the superior sulcus. J Thorac Cardiovasc Surg. 2000;119:1147-1153.
68 Malawski SK, Lukawski S. Pyogenic infections of the spine. Clin Orthop Relat Res. 1991;272:58-66.
69 Durrant DH, True JM. Classic signs and symptoms of radiculopathy. In: Durant DH, True JM, editors. Myelopathy, Radiculopathy, and Entrapment Syndromes. Boca Raton, FL: CRC Press; 2002:201-247.
70 Eismont FJ, Bohlman HH, Soni PL, et al. Pyogenic and fungal vertebral osteomyelitis with paralysis. J Bone Joint Surg Am. 1983;65:19-29.
71 Park DW, Sohn JW, Kim EH, et al. Outcome and management of spinal tuberculosis according to the severity of the disease: a retrospective study of 137 adult patients at Korean teaching hospitals. Spine. 2007;32:E130-E135.
72 Amin A, Saifuddin A. Fractures and dislocations of the cervicothoracic junction. J Spinal Disord Tech. 2005;18:499-505.
73 Nichols CG, Young DH, Schiller WR. Evaluation of cervicothoracic junction injury. Ann Emerg Med. 1987;16:640-642.
74 Evans DK. Dislocations at the cervicothoracic junction. J Bone J Surg Br. 1983;65:124-127.
75 Bohlman HH, Freehafer A, Dejak J. The results of treatment of acute injuries of the upper thoracic spine with paralysis. J Bone Joint Surg Am. 1985;67:360-369.
76 Chapman JR, Anderson PA, Pepin C, et al. Posterior instrumentation of the unstable cervical spine. J Neurosurg. 1996;84:552-558.
77 Lenoir T, Hoffman E, Thevenin-Lemoine C, et al. Neurological and functional outcome after unstable cervicothoracic junction injury treated by posterior reduction and synthesis. Spine. 2006;6:507-513.
78 Dvorak MF, Fisher CG, Fehlings MG, et al. The surgical approach to subaxial cervical spine injury injuries: an evidence-based algorithm based on the SLIC classification system. Spine. 2007;32:2620-2629.
79 Mirza SK, Mirza AJ, Chapman JR, et al. Classifications of thoracic and lumbar fractures: rationale and supporting data. J Am Acad Orthop Surg. 2002;10:364-377.
80 Vaccaro AR, Hulbert RJ, Patel AA, et al. The subaxial cervical spine injury classification system: a novel approach to recognize the importance of morphology, neurology, and integrity of the disco-ligamentous complex. Spine. 2007;32:2365-2374.
81 Allen BLJr, Ferguson RL, Lehmann TR, et al. A mechanistic classification of closed, indirect fractures and dislocations of the lower cervical spine. Spine. 1982;7:1-27.
82 Gertzbein SD. Scoliosis Research Society. Multicenter spine fracture study. Spine. 1992;17:528-540.
83 Magerl F, Aebi M, Gertzbein SD, et al. A comprehensive classification of thoracic and lumbar injuries. Eur Spine J. 1994;3:184-201.
84 Chapman JR, Anderson PA, Pepin C, et al. Posterior instrumentation of the unstable cervicothoracic spine. J Neurosurg. 1996;84:552-558.
85 Cotler J, Herbison GJ, Nasuti JF, et al. Closed reduction of traumatic cervical spine dislocation using traction weights up to 140 pounds. Spine. 1993;13:386-390.
86 Lee GY, Massicotte EM, Rampersaud YR. Clinical accuracy of cervicothoracic pedicle screw placement: a comparison of the “open” lamino-foraminotomy and computer-assisted techniques. J Spinal Disord Tech. 2007;20:25-32.
87 Smucker JD, Sasso RC. The evolution of spinal instrumentation for the management of occipital cervical and cervicothoracic junctional injuries. Spine. 2006;31:S44-S52.
88 Rhee JM, Kraiwattanapong C, Hutton WC. A comparison of pedicle and lateral mass screw construct stiffnesses at the cervicothoracic junction: a biomechanical study. Spine. 2005;30:E636-E640.
89 Abumi K, Itoh H, Taneichi H, et al. Transpedicular screw fixation for traumatic lesions of the middle and lower cervical spine: description of the techniques and preliminary report. J Spinal Disord. 1994;7:19-28.
90 Kramer DL, Ludwig SC, Balderston RA, et al. Placement of pedicle screws in the cervical spine: comparative accuracy of cervical pedicle screw fixation using three techniques [abstract]. Orthop Trans. 1997;21:496.