Chapter 8 Pathology of Reproductive Endocrine Disorders
UTERUS
Endometrium
Endometrial Dating
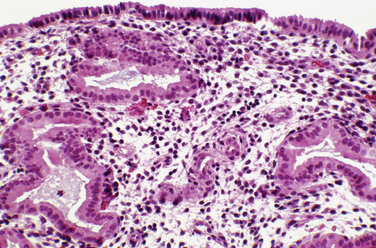
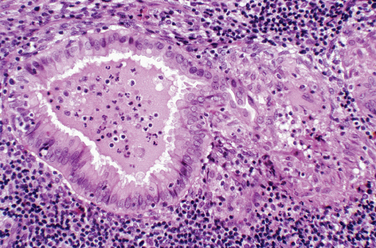
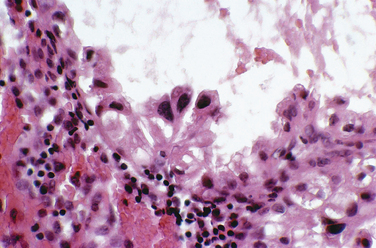
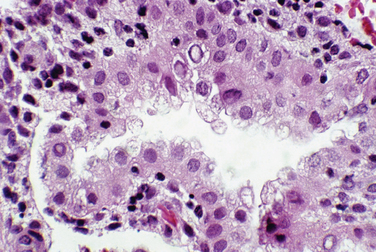
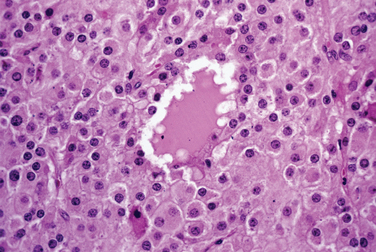
Most clinicians perform endometrial biopsy in the midluteal phase at about the time implantation is thought to occur. However, the original reports on secretory endometrial dating recommend a biopsy 3 days before the expected menses. Histologic criteria are then used to determine where the endometrial response would be in relation to ovulation (Table 8-1 and Figs. 8-1 and 8-2).
| Gland mitosis |
| Pseudostratification of nuclei |
| Subnuclear vacuoles |
| Edema |
| Stromal mitosis |
| Decidual reaction in stroma |
| Leukocyte infiltration |
| Secretion |
Several assumptions in the original concept of dating the endometrium besides the assumption of ovulation on day 14 increased the variation found with this method. For example, another assumption was that the length of the luteal phase is 14 days.1 In reality, there is a normal variation in luteal phase length of several days. In addition, the original description fixed the time of ovulation with the onset of the period after the biopsy. The onset of ovulation can be more accurately determined using current modalities, such as determination of the midcycle urinary luteinizing hormone surge or ultrasonic identification of the collapse of a follicle. The accuracy of the former is approximately 85%; of the latter, 95%. Additional intrinsic inaccuracies of dating resulted from intraobserver and interobserver variation. This variation is typically about 2 days. For these reasons, a 2-day difference between the histologic estimation and the actual interval since ovulation is considered within normal limits.
Recently a detailed analysis on endometrial dating demonstrated that the histologic criteria used are not as temporally distinct as originally thought, and thus do not provide an accurate method to detect a luteal phase defect. In one study, approximately 20% of fertile couples had a delay of more than 2 days.2 A between-cycle variation of more than 2 days was found in 30% to 60% of patients if the biopsy was performed between day 6 and day 13 after ovulation.
Endometrial Response to Exogenous Hormones
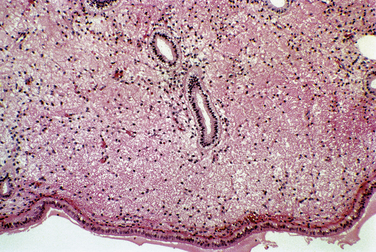
The endometrium responds to exogenous hormones with specific morphologic changes. With oral contraceptives, the progestin effect dominates because progestins decrease estrogen receptors. As a result, endometrial glands atrophy over time. The appearance of the stroma depends on the dose of progestin. Typically, a weak pseudodecidual response will occur (Fig. 8-3).
Endometritis
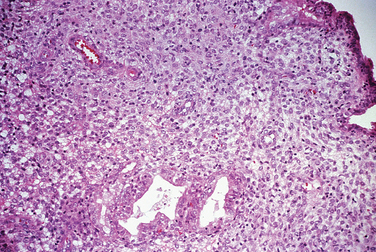
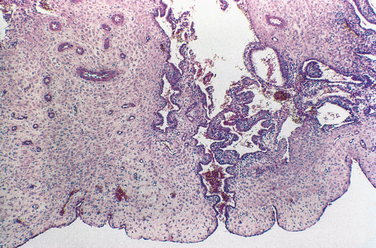
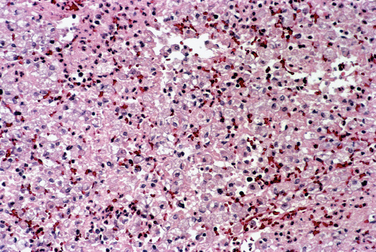
Chronic endometritis is sometimes demonstrated in endometrial biopsies for infertility or recurrent pregnancy loss. This is characterized by infiltration of plasma cells (Fig. 8-4). Chronic pelvic inflammatory disease, intrauterine devices, and postabortion complications are also associated with chronic endometritis. Although rare in the United States, endometrial tuberculosis may sometimes be found during a biopsy for infertility and is characterized by granulomas (Fig. 8-5).
Abnormal Uterine Bleeding
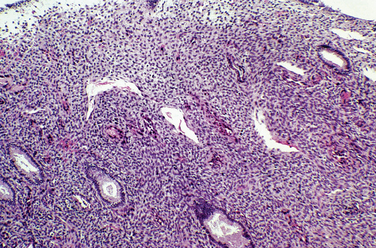
The biopsy of an endometrium for dysfunctional uterine bleeding in women may show a range of abnormalities of the endometrium (Fig. 8-6). In the reproductive age group organic lesions such as polyps and leiomyomas or a normal endometrium are often found. Pregnancy-related endometrial changes or premalignant or malignant changes may be found in this age group.
Pregnancy-related Endometrial Changes
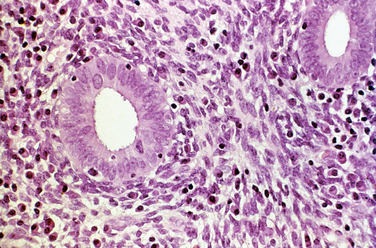
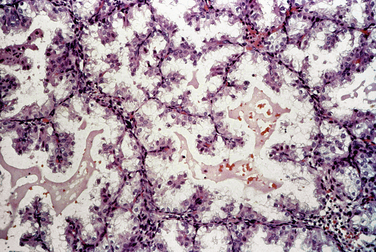
Early pregnancy, both intrauterine and ectopic, is characterized by hypersecretory endometrium (Fig. 8-7). However, hypersecretory endometrium is not specific for pregnancy, and similar changes can be seen with persistent corpus luteum cyst, double corpora lutea, or rarely as a drug effect. By the end of the first trimester, endometrial glands involute and the stroma shows a prominent decidual reaction (Fig. 8-8). Other histologic changes associated with gestation include the Arias-Stella reaction (Fig. 8-9) and optically clear nuclei (Fig. 8-10).
Endometriosis
Endometriosis is defined as the presence of endometrial glands and stroma at an extrauterine site. Although most commonly located in the pelvis, endometriosis can occur at a remarkable variety of extrapelvic sites (Table 8-2). Endometriotic lesions can invade neural tissue. The stromal component can undergo smooth muscle metaplasia. Hyperplasia of smooth muscle, especially the bowel, is characteristic.
Endometriotic lesions are sensitive to sex hormones and undergo cyclic changes. The appearance of endometriosis on visual inspection at laparoscopy or laparotomy is quite variable and ranges from blue nodules to red or white lesions. The microscopic appearance also varies, depending on secondary changes of fibrosis and hemorrhage (Figs. 8-11 and 8-12). Endometriotic lesions should be differentiated microscopically from the benign serous tubules of endosalpingiosis.
Estimates for malignant transformation of endometriosis range from 0.3% to 0.8% for surgical series of ovarian endometriosis.3 In one study almost 80% of endometriosis-associated malignancies arose in the ovary.4 Malignancies arising in endometriosis are usually adenocarcinomas, almost always endometroid or clear cell type. Rarely sarcomas such as endometrial stromal sarcoma or müllerian adenosarcoma occur.5
Adenomyosis
Adenomyosis refers to endometrial glands and stroma located deep within the myometrium. The etiology and pathogenesis of adenomyosis are poorly understood. Smooth muscle cell hyperplasia and hypertrophy are commonly found. Many cases of adenomyosis are associated with leiomyomas, and it is difficult to ascertain which pathology is responsible for the symptoms. Histologically, thickened rims of myometrial smooth muscle surround benign endometrial glands and stroma (Fig. 8-13).
Leiomyoma
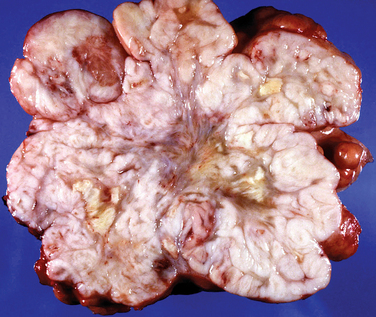
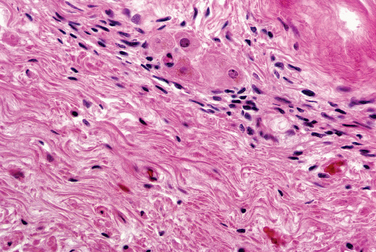
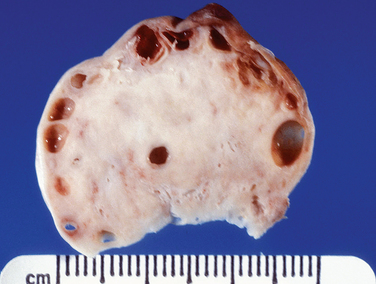
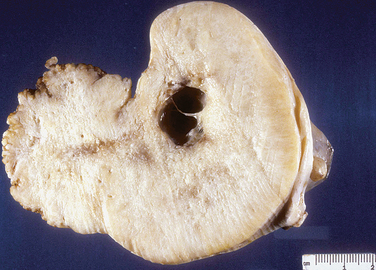
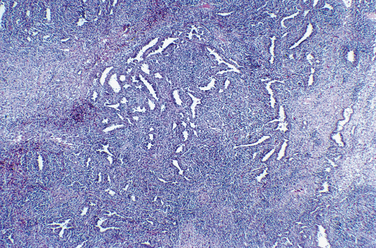
Grossly, leiomyomas appear as circumscribed masses of whorled, bulging, rubbery, usually light tan masses (Fig. 8-14). Microscopically, fascicles of bland spindled cells characterize leiomyomas. Mitotic figures are infrequent, and the nuclei are uniform (Fig. 8-15).
Leiomyoma variants (Table 8-3) include cellular, apoplectic, bizarre (Fig. 8-16), mitotically active, hyaline, cystic, myxoid, and infarcted. All of these variant appearances can occur in benign tumors, but some, especially myxoid, mitotically active, and bizarre, should prompt a thorough search to exclude malignancy.
| Cellular |
| Apoplectic |
| Bizarre |
| Mitotically active |
| Hyaline degeneration |
| Cystic |
| Myxoid |
| Infarcted |
Another treatment modality for symptomatic uterine leiomyomas is arterial embolization. Histopathologic evaluation of the effects of this treatment are obtained from leiomyomas expelled from the uterus after therapy or from hysterectomy specimens. The primary features are thrombosis, necrosis, and presence of embolic foreign material (Fig. 8-17). Acute features are coagulation necrosis and acute inflammation. Chronic features are hyaline necrosis and dystrophic calcification.
Leiomyosarcoma
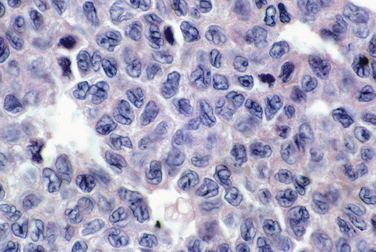
Leiomyosarcomas are encountered in the treatment of presumed leiomyomas in approximately 0.1% of cases. Leiomyosarcomas typically have tumor cell necrosis (Fig. 8-18), many mitoses (almost always >4 mitotic figures/10 high magnification fields [hpf] and usually ≥10/10 hpf), and nuclear atypia (Fig. 8-19). These histologic features are reflected grossly as soft, variegated tumors with necrosis (Fig. 8-20). Table 8-4 summarizes the principal predictors of malignancy.
| Tumor cell necrosis |
| Mitotic activity |
| Nuclear atypia (pleomorphism and nuclear hyperchromatism) |
FALLOPIAN TUBES
Acute Salpingitis
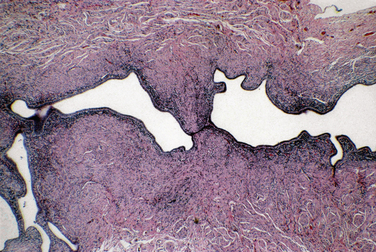
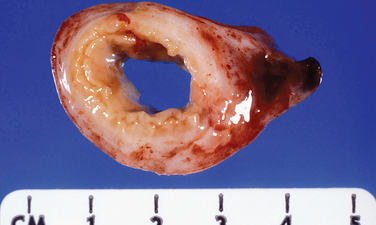
Acute salpingitis is an ascending infection that is usually associated with a sexually transmitted disease. The lumen contains pus (pyosalpinx) and may become distended. In chronic salpingitis there is a marked fibrosis of the tubal wall, usually associated with luminal dilatation as well as tubo-ovarian adhesions (Fig. 8-21). Xanthogranulomatous salpingitis is a histologic variant of chronic salpingitis resulting from necrosis and obstruction (Fig. 8-22).
Hydrosalpinx is thought to be the result of end-stage salpingitis. Grossly, the tube is dilated and often thin-walled (Fig. 8-23). The lumen has a plasma transudate that is embryotoxic. Microscopically, the tubal wall is thin and fibrotic. A flattened epithelium lines the attenuated wall. However, scattered relatively normal-appearing tubal mucosal plicae usually remain.
Ectopic Pregnancy
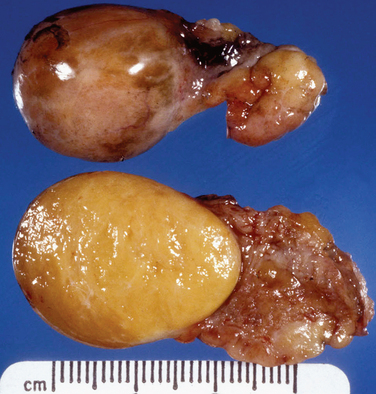
Ectopic pregnancies can occur in several nonuterine locations. The most common site is the uterine tube. Hematosalpinx is the typical gross appearance. Trophoblastic tissue can grow predominantly intraluminally or invade the tubal wall (Fig. 8-24).
Tuberculosis Salpingitis
Tuberculosis can hematogeneously spread to the pelvic organs to cause chronic salpingitis. The majority of cases of tuberculosis of the uterine tubes simultaneously involve the endometrium. Other uncommon causes of granulomatous tubal inflammation are sarcoidosis and Crohn’s disease.
OVARIES
Ovarian Cysts
Gonadal Dysgenesis
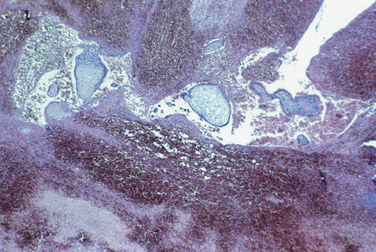
Patients with gonadal dysgenesis usually have streak gonads (Fig. 8-25). Dysgenetic gonad (i.e., abnormally developed gonads) can harbor gonadoblastoma (Fig. 8-26). Gonadoblastomas are mixed germ cell-sex cord stromal tumors characterized by nests of immature sex cord cells surrounding germ cells. Gonadoblastomas almost always affect dysgenetic gonads in patients with a karyotype containing Y chromosome. Importantly, gonadoblastomas can give rise to invasive germ cell neoplasms, most commonly dysgerminoma (Fig. 8-27). Patients with genotypes that include a Y chromosome are at risk to develop a gonadoblastoma. In these patients the gonad should be removed.
Pregnancy-related Cysts
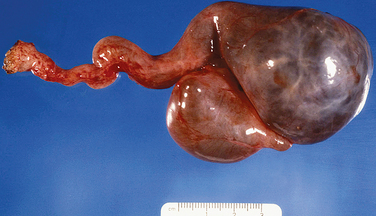
Pregnancy causes prominent luteinization of the theca layer of atretic follicles. Multiple atretic follicles with theca luteinization are designated theca-lutein cysts. Theca-lutein cysts result from high levels of or increased sensitivity to human chorionic gonadotropin. This condition has been called hyperreactio luteinalis. Multiple theca-lutein cysts often occur with hydatidiform mole and choriocarcinoma but occasionally in other clinical settings, including normal single pregnancy. Grossly, the process is typically bilateral with greatly enlarged multicystic ovaries (Fig. 8-28). Pregnancy luteomas are non-neoplastic solid masses of lutein cells occurring in pregnant patients (Fig. 8-29).6 Multiple nodules occur in half of patients, and bilaterality occurs in at least one third of cases. The nodules spontaneously regress postpartum. Pregnancy luteomas are easily confused with sex cord neoplasms.
Polycystic Ovaries
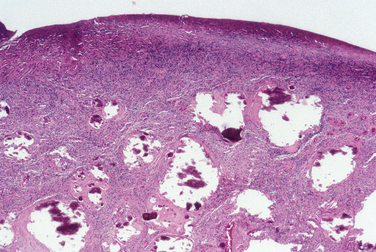
Polycystic ovary syndrome (PCOS) is an endocrine diagnosis rather than a pathologic one. The pathologic findings include characteristic gross and microscopic features. Grossly, the ovaries are referred to as sclerocystic (Fig. 8-30).
Sclerocystic ovaries are enlarged with cysts underlying thickened fibrotic superficial stroma. Stigmata of prior ovulation are inconspicuous or absent. The cysts are relatively small, usually ranging in size from 0.5 to 1.5 cm. Histologically, luteinized follicular cells line the cysts.
Stromal Hyperthecosis
Stromal hyperthecosis is defined histologically as the presence of luteinized cells within the ovarian stroma. Stromal hyperthecosis can cause clinical signs of hyperandrogenism, and with peripheral conversion, hyperestrogenism. These patients are typically postmenopausal. Stromal hyperthecosis also occurs commonly in pregnancy. The ovaries are enlarged bilaterally with no clear appearance of cysts. Microscopically nests and individual lutein cells occur in the stroma (Fig. 8-31). The associated spindle cell ovarian stroma is typically hyperplastic, characterized by increased cellularity and a loss of the normal corticomedullary demarcation.
Sex Cord-Stromal Tumors
Sex cord-stromal tumors represent approximately 5% of ovarian neoplasms. They are usually unilateral and occur in all age groups but are more common in postmenopausal women. Sex cord-stromal tumors often secrete hormones and are thus often associated with endocrine-related symptoms (Table 8-5). Both Leydig and theca cells secrete androgens (Table 8-6). They should be considered as part of the differential diagnosis of severely hyperandrogenic patients. However, many of these tumors have no endocrine manifestations.
Table 8-6 Typical Clinical Features of Sex Cord-Stromal Tumors
| Can occur in any age group |
| May be hormonally active |
| Usually unilateral |
| Solid or cystic solid tumors |
Granulosa Cell Tumors
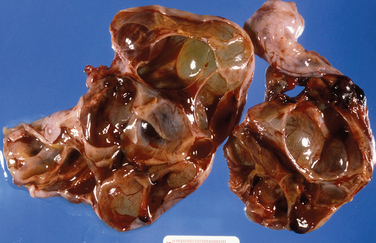
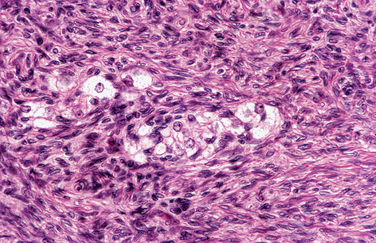
Granulosa cell tumors are usually unilateral and characteristically have both cystic and solid components (Fig. 8-32). Histologically, the tumor architecture varies, but the cells often have “coffee bean” nuclei described as pale, angular, and cleaved (Fig. 8-33).7
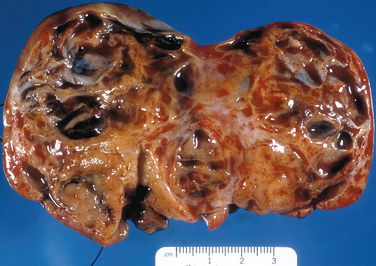
Figure 8-32 This adult-type granulosa cell tumor has a characteristic hemorrhagic cystic and solid gross appearance.
Granulosa cell tumors in prepubertal girls and young adults often have microscopic features differing from adult-type granulosa cell tumors. The differences are sufficient to permit recognition of these tumors as a distinctive subset with different behavior that is designated juvenile granulosa cell tumor.8,9
Thecomas
Thecomas are usually unilateral (>90%) and typically occur in postmenopausal women. Thecomas may be hormonally active tumors. Grossly, thecomas typically are yellow and have a solid uniform appearance. Histologically, plump vacuolated spindle cells predominate (Fig. 8-34).
Fibromas
Fibromas are usually unilateral tumors. Large fibromas (>6 cm) may be associated with ascites (40% of cases) or hydrothorax (Meigs’ syndrome). Other ovarian tumors may also cause these clinical signs (pseudo-Meigs’ syndrome). Fibromas are also associated with basal cell nevus syndrome (Gorlin’s syndrome). Fibromas have a uniform, pale, tan, solid gross appearance (Fig. 8-35) and benign spindle cells microscopically.
Sertoli-Leydig Cell Tumors
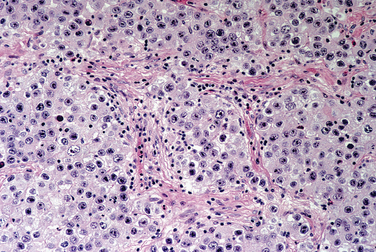
Sertoli-Leydig cell tumors have remarkably diverse histologic appearances.10 The fundamental components are a variable spindle cell stroma and variably immature tubules. Often, an alternating hypercellular and hypocellular zonation characterizes Sertoli-Leydig cell tumors. The presence of heterologous tissue contributes to the array of microscopic appearances. Histologic grading (well, intermediate, and poorly differentiated) correlates well with survival (Fig. 8-36). Other hormonally active tumors occur that have lipid-laden cells associated with hyperandrogenism and are variably referred to as steroid cell tumors.
1 Noyes RW, Herrig AW, Rock J. Dating the endometrial biopsy. Fertil Steril. 1950;1:3-25.
2 Murray AJ, Meyer WR, Zaino RJ, et al. A critical analysis of the accuracy, reproducibility and clinical utility of histologic endometrial dating in fertile women. Fertil Steril. 2004;81:1333-1343.
3 Mostoufizadeh M, Scully RE. Malignant tumors arising in endometriosis. Obstet Gynecol. 1980;23:951-963.
4 Heaps JM, Neiberg RK, Berek JS. Malignant neoplasms arising in endometriosis. Obstet Gynecol. 1990;75:1023.
5 Jelovsek JE, Brainard J, Winans C, Falcone T. Endometriosis of the liver containing Müllerian adenosarcoma. Am J Obstet Gynecol. 2004;191:1725-1777.
6 Norris HJ, Taylor HB. Nodular theca-lutein hyperplasia of pregnancy (so-called “pregnancy luteoma”). Am J Clin Pathol. 1966;47:557-566.
7 Stenwig JT, Hazekamp JT, Beecham JB. Granulosa cell tumors of the ovary: A clinicopathological study of 118 cases with long-term follow-up. Gynecol Onco. 1979;l7:136-152.
8 Young RH, Dickersin GR, Scullyl RF. Juvenile granulosa cell tumors of the ovary: A clinicopathologic analysis of 125 cases. Am J Surg Pathol. 1984;8:575-596.
9 Biscotti DV, Hart WF. Juvenile granulosa cell tumors of the ovary. Arch Pathol Lab Med. 1989;113:40-46.
10 Young RH, Scully RE. Ovarian Sertoli-Leydig cell tumors: A clinicopathologic analysis of 207 cases. Am J Surg Pathol. 1985;9:543-569.