12 Pathology of Lung Transplantation
Lung transplantation may offer longer survival and improved quality of life to patients with end-stage lung disease. Common indications for single lung, bilateral (double) lung, and heart-lung transplantation are listed in Table 12-1. Bilateral lung transplantation is the norm for cystic fibrosis, but of interest, the proportion of bilateral lung transplantation procedures has been rising for other major indications as well.1 Living donor single lobe transplantation may be a viable alternative to cadaveric lung transplantation for selected patients.2 Benchmark survival rates for adult lung transplant recipients are 88% at 3 months, 78% at 1 year, 63% at 3 years, 51% at 5 years, and 28% at 10 years after transplantation.1
Table 12-1 Most Common Indications for Lung Transplant Procedures
| Transplant Procedure | Most Common Indications |
|---|---|
| Adult single lung | Chronic obstructive pulmonary disease Idiopathic pulmonary fibrosis α1-Anti-trypsin deficiency emphysema |
| Adult bilateral/double lung | Cystic fibrosis Chronic obstructive pulmonary disease Idiopathic pulmonary fibrosis α1-Anti-trypsin deficiency emphysema Idiopathic pulmonary arterial hypertension |
| Adult heart-lung | Congenital heart disease Idiopathic pulmonary arterial hypertension Cystic fibrosis |
| Pediatric lung | Cystic fibrosis “Primary pulmonary hypertension” Congenital heart disease “Interstitial pneumonitis” Surfactant protein B deficiency |
Unfortunately, the number of patients who can benefit from lung transplantation is limited by the availability of donor organs. Historically, waiting time was the main determinant of donor lung allocation in the United States. In 2005, a lung allocation score (LAS) was implemented, dramatically changing the way donor lungs are distributed.3 Under the new system, priority for transplantation is determined by medical urgency and expected outcome. Early evaluations of the new system indicate that the waiting time and waitlist mortality rate are decreased, the number of transplants is increased, and the post-transplantation survival is unchanged.4
Complications of lung transplantation may be related to (1) the operation itself (primary graft dysfunction, anastomotic complications), (2) the host’s immunologic response to the allograft (rejection), and (3) the immunosuppressive therapy used to prevent rejection (infection, post-transplantation lymphoproliferative disorders [PTLDs]). Other complications, such as organizing pneumonia and recurrence of the original disease, may also occur. To aid the differential diagnosis, post-transplant time intervals can be divided arbitrarily into immediate (within 4 days), early (4 days to 1 month), and late (beyond 1 month) post-transplantation periods.5 Differential diagnostic possibilities for each of these periods are listed in Table 12-2.
Post-transplantation transbronchial biopsy may be performed for a specific clinical indication or for surveillance of acute rejection. The role of surveillance biopsy in lung transplant patients remains controversial.6,7 At least five pieces of well-expanded alveolated lung parenchyma are required for the assessment of acute rejection.8 The histopathologic findings most commonly encountered in a post-transplantation transbronchial biopsy include acute rejection, cytomegalovirus infection, airway-centered inflammation, pneumonia, bronchiolitis obliterans, harvest injury, invasive aspergillosis, and PTLDs.6,9
Operation-Related Complications
Primary Graft Dysfunction
Despite many advances in organ preservation, surgical technique, and perioperative care, primary graft dysfunction, also known as harvest injury, ischemia-reperfusion injury, early graft dysfunction, and reimplantation response, contributes significantly to both the morbidity and mortality for lung transplantation. Primary graft dysfunction affects an estimated 10% to 25% of pulmonary allografts and can range in clinical severity from transient decrease in oxygenation to complete graft failure.10 The International Society for Heart and Lung Transplantation (ISHLT) has proposed a definition and a grading scheme based on the chest film and Pao2/Fio2 ratio.11
Diagnosis
The diagnosis of primary graft dysfunction is based on the radiographic and Pao2/Fio2 criteria as well as on exclusion of clinically similar conditions such as acute antibody-mediated rejection, venous anastomotic obstruction, cardiogenic pulmonary edema, and pneumonia.11 In selected cases, a lung biopsy may be helpful.12
Pathologic Findings
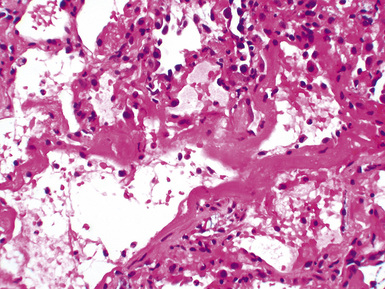
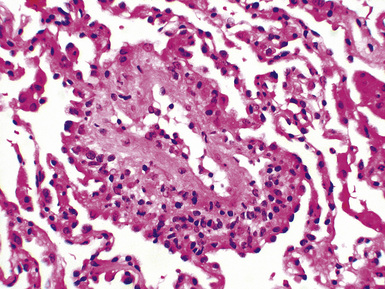
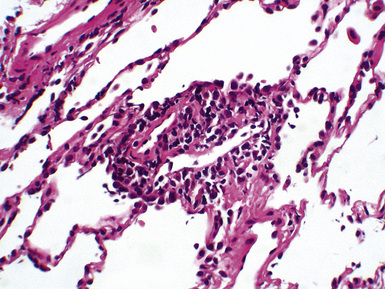
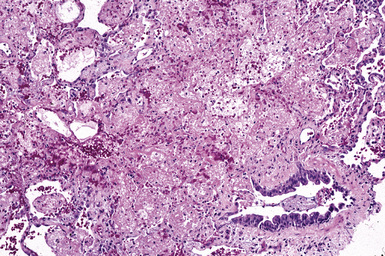
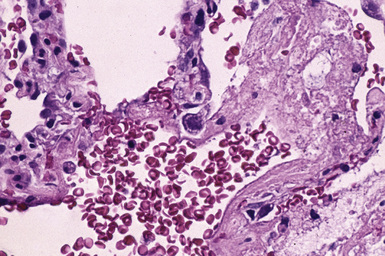
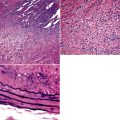
Mild cases may show alveolar and interstitial edema with scattered neutrophils.13 The histologic correlate of severe primary graft dysfunction is diffuse alveolar damage.12 The acute phase of diffuse alveolar damage is characterized by hyaline membranes, interstitial edema, occasional fibrin thrombi, and scattered neutrophils in the alveolar septa (Fig. 12-1). In the organizing phase, hyaline membranes are incorporated into the alveolar septa, which become thickened by fibroblast-rich connective tissue (Fig. 12-2).
Histologic Differential Diagnosis
Diffuse alveolar damage is a nonspecific histologic pattern that can be elicited by various insults in the post-transplantation setting (Box 12-1). Immunofluorescent studies are helpful in separating primary graft dysfunction from acute antibody-mediated rejection. Acute antibody-mediated rejection is characterized by alveolar septal deposits of IgG and complement (particularly C4d), which are absent in primary graft dysfunction. Acute rejection is not a major concern during the immediate post-transplantation period. Any infection can manifest as diffuse alveolar damage in an immunocompromised patient; therefore, it is always prudent to perform special stains to rule out acid-fast bacilli and fungal organisms.
Treatment, Prognosis, and Prevention
The treatment is supportive and may include mechanical ventilation. A retrospective analysis by Christie and associates showed that in patients with and those without primary graft dysfunction, 30-day mortality rates are 42.1% and 6.1%, respectively.14 Primary graft dysfunction is also associated with an increased risk of obliterative bronchiolitis.15 For prevention of primary graft dysfunction, research studies have focused on improving lung preservation techniques by optimizing the volume, temperature, pressure and components of preservation solutions, and inflation and ventilation parameters of the organs during transport.10 So far, these studies have had modest clinical impact.
Arterial Anastomotic Obstruction
The incidence of pulmonary arterial anastomotic obstruction after lung transplantation is relatively low.16 Causes include narrowed anastomosis, with or without thrombus formation resulting from suboptimal surgical anastomoses and excessive length of donor or recipient pulmonary artery with kinking or torsion of the anastomosis.16
Time Period
Arterial anastomotic obstruction usually occurs during the first week after transplantation.
Venous Anastomotic Obstruction
Minor abnormalities of the pulmonary venous anastomosis are relatively common complications of lung transplantation.17 Occlusive thrombus formation is relatively rare but may have catastrophic consequences, including allograft failure and stroke.
Time Period
Venous anastomotic obstruction usually presents in the immediate post-transplantation period but has been reported to occur as late as the eighth postoperative day.16
Clinical Presentation
Pulmonary venous obstruction after lung transplantation should be suspected in every case of persistent pulmonary edema in the first postoperative days, often associated with a frothy blood-stained secretion from the endotracheal tube.18
Diagnosis
Transesophageal echocardiography with color-flow Doppler imaging is virtually diagnostic, demonstrating a marked reduction of the flow in the affected pulmonary vein.18
Airway Dehiscence
In the early years of lung transplantation, airway dehiscence due to ischemia of the donor bronchus was a major cause of morbidity and death. Improved surgical techniques, reduced immunosuppression, and better allograft preservation have reduced the incidence of airway complications.19 Currently, most centers report a 7% to 18% complication rate with a related mortality rate of 2% to 4%.19
Diagnosis
Ischemia and necrosis of the bronchus can be diagnosed by direct visualization with a bronchoscope.
Large Airway Stenosis
Large airway (bronchial) stenosis is the most common airway complication. The incidence is estimated to be between 1.6% and 32%.19 It is usually seen after necrosis or dehiscence or in healing or treated infections. “Telescoped” anastomosis is associated with a 7% incidence of airway stenosis.
Nonanastomotic large airway stenosis has also been described.20,21 The pathogenesis of this lesion is unclear, but it may represent a response to ischemic damage, alloreactive injury, or infection.
Time Period
Bronchial stenosis usually occurs a few months after the transplantation procedure but has been described as early as 8 days.22
Diagnosis
Bronchoscopic examination provides the diagnosis, with biopsies providing confirmatory histology.
Rejection
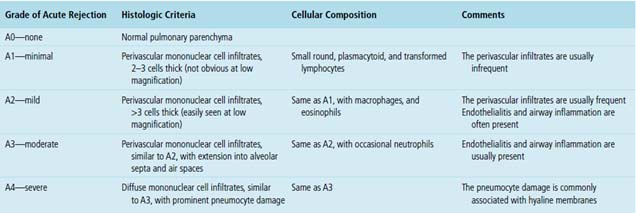
With the exception of monozygotic twins, donors and recipients are genetically different and express different histocompatibility antigens. As a result, allografts are rejected by the recipient’s immune system. Multiple immunologic processes are involved, creating a spectrum of rejection responses. A “working formulation for the classification of pulmonary allograft rejection” was introduced by the ISHLT in 1990.24 The working formulation was first revised in 1996.25 The currently accepted scheme for grading pulmonary allograft rejection was approved by the ISHLT board of directors in 2007 (Box 12-2).8 The differences between the 1996 and 2007 schemes are relatively minor and are related to airway inflammation and chronic airway rejection. Acute antibody-mediated rejection is a controversial subject and is not included in the 2007 working formulation. For the sake of completeness, however, it is discussed at the end of this section.
Box 12-2 2007 Revised Working Formulation for Classification and Grading of Pulmonary Allograft Rejection
Acute (Cellular) Rejection
Diagnosis
Clinical features may suggest acute rejection, but a transbronchial biopsy usually is required to confirm the diagnosis and rule out infection. If biopsy from multiple sites is technically impossible, lower lobe biopsies are preferred because they appear to be more informative.26
Pathologic Findings
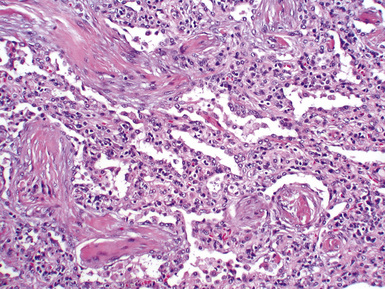
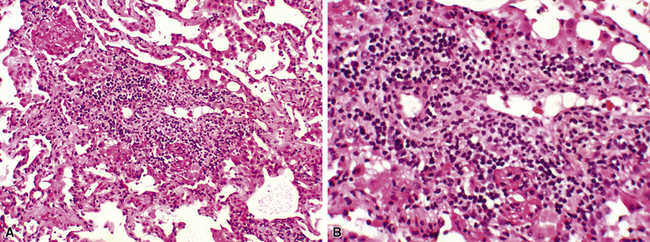
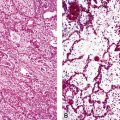
Acute rejection is graded according to the density and extent of the perivascular infiltrates and the presence or absence of secondary pneumocyte damage (Table 12-3). Rejection-type infiltrates usually involve more than one vessel, but a single perivascular infiltrate should be evaluated by the same criteria as for multiple infiltrates, as follows:
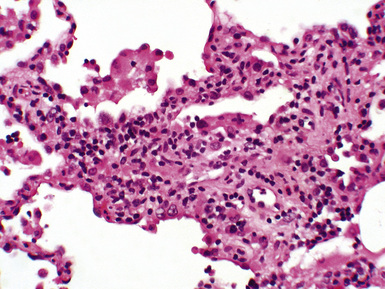
Figure 12-5 In moderate acute rejection (A3), the perivascular infiltrate extends into the alveolar septa.
Histologic Differential Diagnosis
Perivascular and interstitial mononuclear cell infiltrates are not specific for acute rejection.8 Differential diagnostic considerations include infections, especially cytomegalovirus pneumonia and Pneumocystis jiroveci pneumonia,27–29 and PTLDs.30 Some histologic features may favor infection over acute rejection (Table 12-4). Cultures and special stains may be helpful in the diagnosis of mycobacterial, fungal, and Pneumocystis jiroveci infections. Viral pneumonias can be confirmed by cultures as well as serologic, immunohistochemical, or molecular hybridization techniques.
Table 12-4 Histologic Features Favoring Infection over Acute Rejection
| Histologic Features | Infection Favored |
|---|---|
| Predominant alveolar septal infiltrates as compared with perivascular infiltrates | Any infection |
| Abundant neutrophils | Bacterial pneumonia, CMV pneumonia, or candidiasis |
| Abundant eosinophils | Fungal infection |
| Nuclear or cytoplasmic inclusions | Viral pneumonia |
| Multinucleation | Respiratory syncytial virus or parainfluenza virus pneumonia |
| Punctate zones of necrosis | Herpes simplex virus, varicella-zoster virus, or CMV pneumonia |
| Granulomatous inflammation | Mycobacterial, fungal, or Pneumocystis jiroveci infection |
| Frothy intra-alveolar exudates | Pneumocystis jiroveci pneumonia |
CMV, cytomegalovirus.
In some cases, histologic features of acute rejection and infection coexist. In these cases, the pathologist should attempt to decide which is dominant and guide the clinician by favoring one over the other. Follow-up biopsy after appropriate antimicrobial therapy is also recommended so that any acute rejection component can be reassessed.8 The differential diagnosis between acute rejection and PTLDs is discussed later.
Treatment and Prognosis
The treatment of acute rejection typically consists of bolus therapy with intravenous steroids, which may be supplemented by temporary increases in the maintenance immunosuppression regimen. In at least 80% of the cases, acute rejection is successfully treated. However, 15% to 20% of acute rejection episodes persist or recur, presenting a particularly difficult management problem for the clinician. When this occurs, intensified immunosuppression with one or more agents is usually attempted. However, it has been shown that patients with persistent, recurrent, or late (occurring at least 3 months after transplantation) acute rejection are at increased risk for developing chronic airway rejection.31 Recent studies have indicated that an increased risk may exist even with minimal acute rejection.32,33
Airway Inflammation: Lymphocytic Bronchiolitis
The 2007 working formulation has collapsed the four previous B grades into two (grade 1R—low grade and grade 2R—high grade) and has retained B0 (no airway inflammation) and BX (ungradable). Another change from the previous working formulation is that the B grade designation applies only to small airways (bronchioles). Airway inflammation may be a harbinger of chronic airway rejection.34,35
Pathologic Findings
Criteria for grading airway inflammation are listed in Table 12-5.
| Grade | Airway Inflammation |
|---|---|
| B0—no airway inflammation | None |
| B1R—low-grade small airway inflammation | Mononuclear cells in the submucosa (can be infrequent and scattered or forming band-like infiltrates) Occasional eosinophils may be seen |
| B2R—high-grade small airway inflammation | Mononuclear cells in the submucosa with greater numbers of eosinophils Epithelial damage and intraepithelial lymphocytic infiltration Ulceration and fibrinopurulent exudates may occur |
| BX—ungradable | Sampling problems, infection, tangential cutting, other problems |
Chronic Airway Rejection: Obliterative Bronchiolitis
Obliterative bronchiolitis is the most significant long-term complication of lung transplantation, with a prevalence of 30% to 50% and an associated mortality rate of 25%.36 The terminology is somewhat confusing, because obliterative bronchiolitis of chronic airway rejection is sometimes referred to as bronchiolitis obliterans or bronchiolitis obliterans syndrome in the clinical lung transplantation literature. It is important to recognize that obliterative bronchiolitis or bronchiolitis obliterans of chronic airway rejection is both clinically and histologically distinct from the (sub)acute lung injury pattern once known as bronchiolitis obliterans organizing pneumonia (BOOP). To make this distinction clear, the nomenclature has been changed, and the currently preferred term for BOOP is organizing pneumonia.37
Time Period
Obliterative bronchiolitis is most frequently diagnosed between 9 and 15 months after transplantation.38 It rarely develops during the first 3 months but has been reported as early as 2 months after transplantation.38
Diagnosis
Transbronchial biopsy is an insensitive method for the detection of obliterative bronchiolitis.8 An ad hoc ISHLT working group has concluded that FEV1 is the most reliable and consistent indicator of chronic airway rejection.39
Pathologic Findings
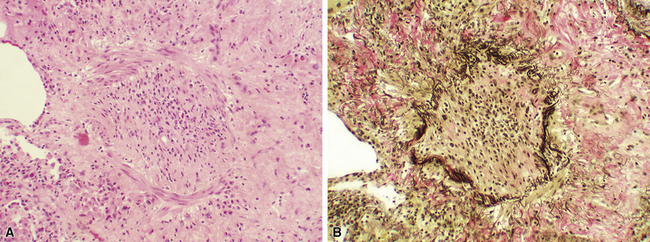
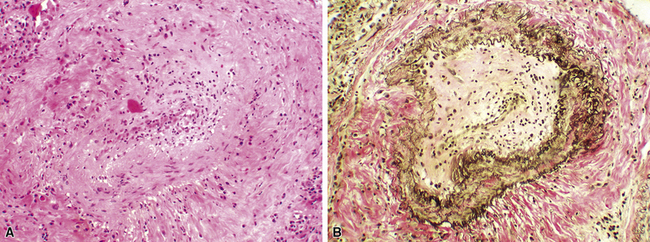
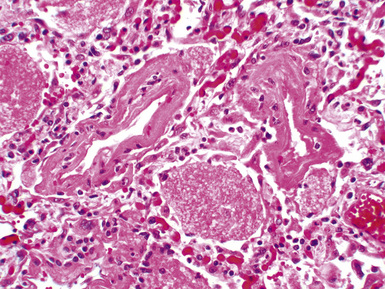
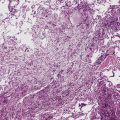
The term obliterative bronchiolitis refers to hyalinized fibrous plaques present in the submucosa of small airways.8,13 They lead to partial or complete luminal compromise (Fig. 12-7). The scar tissue may be concentric or eccentric and may be associated with destruction of the smooth muscle wall. The 1996 working formulation retained the designation of active versus inactive obliterative bronchiolitis, depending on the presence and degree of accompanying inflammation.25 However, the consensus in 2007 was that the distinction between active and inactive is no longer useful, and the condition should be designated merely as C0, indicating a biopsy with no evidence of obliterative bronchiolitis, and C1, indicating that obliterative bronchiolitis is present in the biopsy.8 Obliterative bronchiolitis often produces mucostasis or postobstructive (endogenous lipid) pneumonia.8,13
Histologic Differential Diagnosis
Transplant-related obliterative bronchiolitis involves the small airways. Large airway fibrosis is a nonspecific finding and should not be considered as evidence of chronic airway rejection. Organizing pneumonia pattern is manifested as fibromyxoid connective tissue plugs within the lumina of bronchioles and alveoli.40 These loose edematous airspace-filling plugs should be distinguished from the densely eosinophilic submucosal scars of transplant bronchiolitis obliterans.
Treatment and Prognosis
Augmented immunosuppression appears to be of some benefit in treating bronchiolitis obliterans, but it is far from optimal. Avoiding this complication of lung transplantation may require better use of current immunosuppressive medications, or the development of novel immunosuppressive strategies.38
Chronic Vascular Rejection: Accelerated Graft Vascular Sclerosis
The clinicopathologic significance of chronic vascular rejection is not entirely clear. However, chronic vascular changes may coincide with the presence of obliterative bronchiolitis in lung transplant recipients and with the presence of accelerated coronary artery disease in combined heart-lung transplant recipients.41,42
Diagnosis
Chronic vascular rejection is not applicable with transbronchial biopsies but may be noted in surgical lung samples.8
Acute Antibody-Mediated (Humoral) Rejection
Acute antibody-mediated (humoral) rejection is mediated by antibodies specific for donor antigens, particularly those of the human leukocyte antigen (HLA) system. These antibodies, which may develop before and after transplantation, bind to target antigens and activate the complement system, leading to tissue injury.43,44 Improvements in anti-HLA antibody detection have increased recognition of antibody-mediated rejection following renal, heart, and lung transplantation.43,45,46 Early observations of acute antibody-mediated rejection were based on the phenomenon of hyperacute rejection, in which preexistent antibodies lead to complement activation and rapid graft loss. With improved cross-matching before transplantation, the incidence of hyperacute rejection has decreased.
Radiologic Findings
In hyperacute rejection, complete opacification of the pulmonary allograft is seen.
Diagnosis
The presence of donor-specific anti-HLA antibodies in the context of vascular C4d deposition and refractory acute rejection fulfills the criteria for antibody-mediated rejection.47 Assays used for HLA antibody screening and identification include complement-dependent cytotoxicity (CDC) and solid-phase technologies such as enzyme-linked immunosorbent assay (ELISA), flow cytometry, and Luminex analysis.43
Pathologic Findings
The recent ISHLT report remains very cautious in discussing the pathologic appearance of acute antibody-mediated rejection.8 The consensus is that capillaritis, along with immunofluorescent or immunohistochemical staining for C4d, should raise clinical suspicion for acute antibody-mediated rejection.48,49 Histologic features of hyperacute rejection also include diffuse alveolar damage.50–52
Histologic Differential Diagnosis
Capillaritis is a nonspecific histologic finding that may occur in infection, diffuse alveolar damage, and severe acute cellular rejection.8 However, the presence of capillaritis and C4d staining as well as anti-HLA antibodies should be seen as strong evidence for acute antibody-mediated rejection.
Prevention, Treatment, and Prognosis
One of the major goals in donor selection is to avoid HLA antigens against which the potential recipient has preformed antibodies.43
Optimal treatment of acute antibody-mediated rejection remains uncertain. Intravenous immunoglobulin (IVIG) is one of the most common therapies used to decrease antibody-mediated immunity.53 Rituximab, an anti-CD20 monoclonal antibody that causes B cell depletion, has been proved effective in the treatment of presensitized renal transplant recipients in conjunction with IVIG.43 Plasmapheresis has been shown to lead to clinical improvement in lung transplant recipients with pulmonary capillaritis unresponsive to steroids.48 However, it is usually reserved for severe cases of suspected acute antibody-mediated rejection, given the side effects and difficulties of administration.
Infection
Bacterial Infections
Cystic fibrosis patients frequently show airway colonization with gram-negative bacteria both before and after lung transplantation. Recent data suggest that colonization with gram-negative bacteria may play a role in the pathogenesis of chronic airway rejection.54
Bacterial infections of the lower respiratory tract may manifest as acute bronchitis or bronchopneumonia. Gram-negative infections, especially those caused by Pseudomonas species, account for about 75% of bacterial pneumonias. Other reported bacterial pathogens include a wide range of nosocomial organisms. Legionellosis is rarely reported.55
Radiologic Findings
New or increasing infiltrates on chest radiograph are common manifestations of bacterial pneumonias.
Pathologic Findings
In acute bronchitis, neutrophils infiltrate the bronchial mucosa. This pathologic change may be associated with mucosal ulceration and intraluminal neutrophils. As in the normal host, acute pneumonia is recognized by the presence of neutrophils within the alveolar spaces (Fig. 12-9).
Viral Infections
Cytomegalovirus (CMV) infection remains a serious problem in lung transplant recipients. Donor-recipient mismatch, with the donor being seropositive and the recipient seronegative for CMV, poses the highest risk for the development of CMV pneumonia. Seropositive recipients of a seropositive or seronegative donor are at intermediate risk of acquiring active CMV pneumonia, and seronegative recipients of a seronegative donor are at lowest risk. Universal ganciclovir prophylaxis is a strategy aimed at reducing CMV infection and delaying the development of obliterative bronchiolitis. However, the optimal duration of ganciclovir prophylaxis remains unclear. If the prophylaxis is discontinued, the incidence of CMV pneumonia is around 57%.56 A recent study has suggested that indefinite ganciclovir prophylaxis may prevent CMV pneumonia in 98% of lung transplant recipients.56
Other viruses responsible for respiratory infections include adenovirus, respiratory syncytial virus, influenzavirus, parainfluenza virus, and varicella-zoster virus.57,58
Pathologic Findings
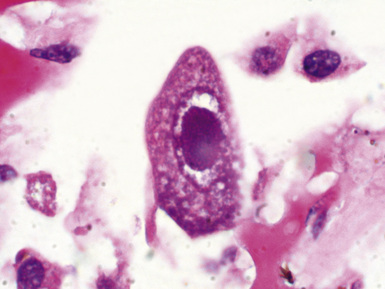
Recognizing tissue responses and cytopathic effects may help in identifying viral infections (see Chapter 6). Tissue responses to viral pathogens range from minimal nonspecific inflammation to diffuse alveolar damage. Most cases of CMV infection show interstitial pneumonia with a mixed lymphocytic and polymorphonuclear cell infiltrate27 (Figs. 12-10 and 12-11). Zonal necrosis may be seen with herpes simplex, varicella-zoster, and CMV pneumonia (Figs. 12-12 and 12-13). CMV may also be associated with neutrophilic microabscesses. Necrotizing bronchiolitis may be a feature of adenovirus, influenzavirus, and respiratory syncytial virus infections. Some characteristic viral cytopathic effects are listed in Table 12-6. These cytopathic effects, however, can be sparse or absent.
| Virus | Cytopathic Effects |
|---|---|
| Cytomegalovirus | Cytomegaly, nuclear and cytoplasmic inclusions |
| Herpes simplex virus | Nuclear inclusions |
| Varicella-zoster virus | Nuclear inclusions |
| Adenovirus | Smudge cells, nuclear inclusions |
| Respiratory syncytial virus | Occasional multinucleation, cytoplasmic inclusions |
| Influenzavirus | None |
| Parainfluenza virus | Occasional multinucleation, cytoplasmic inclusions |
Immunohistochemistry, in situ hybridization, and polymerase chain reaction (PCR) techniques can be used to identify many viruses and have largely replaced electron microscopy in this role (Fig. 12-14).
Histologic Differential Diagnosis
The histologic differential diagnosis of viral pneumonia includes acute rejection.27 Both processes can exhibit perivascular and interstitial mononuclear cell infiltrates. However, perivascular infiltrates predominate in acute rejection, and alveolar septal infiltrates are more prominent in viral infection (see Table 12-4). The presence of CMV inclusions is indicative of CMV pneumonia, but attention to other histologic details is necessary to exclude concurrent acute rejection and bronchiolitis obliterans, which are frequently associated with CMV infection.
Fungal Infections
Fungal infections are less frequent than other infections in the transplant recipient but carry a high mortality rate when they do occur. The fungal species most commonly encountered in lung transplant biopsies include Aspergillus and Candida.59 Cryptococcosis, histoplasmosis, coccidioidomycosis, and mucormycosis have also been reported.59,60 Fungal organisms may colonize the respiratory tract or cause overt infection.60 Prolonged antibiotic therapy predisposes patients to disseminated candidiasis.
Time Period
Fungal infections have a bimodal presentation: early onset between 2 weeks and 2 months after transplantation, secondary to difficult postsurgical periods and prior colonizations; and late onset, primarily secondary to chronic rejection and terminal renal insufficiency.60
Radiologic Findings
Radiographically, pulmonary infiltrates with consolidation or cavitary nodules may be seen.
Pathologic Findings
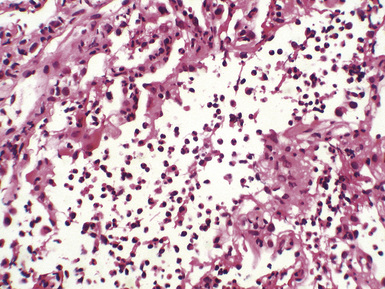
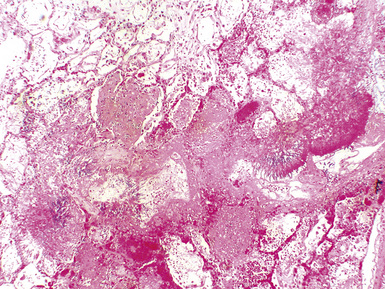
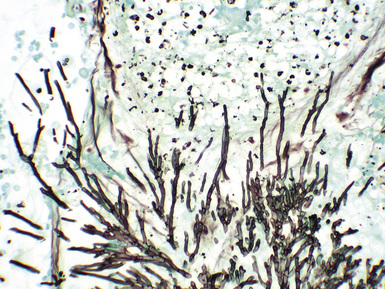
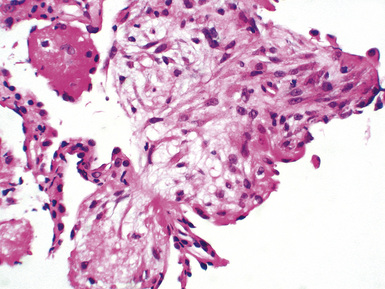
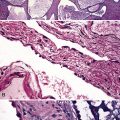
Fungal species may be a source of bronchial anastomotic infections (Figs. 12-15 and 12-16). Aspergillus pneumonia is characterized by hemorrhagic infarction, and sparse inflammatory cell infiltrates (Figs. 12-17 and 12-18). Long, septate hyphae, with 45-degree branching points, invade blood vessels and permeate alveolar septa. Candida infection produces neutrophilic infiltrates and is associated with abscess formation. Clusters of pseudohyphae and yeast forms are often found in the center of abscesses.
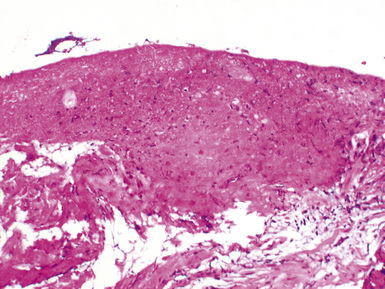
Figure 12-15 Bronchial mucosal necrosis and associated Aspergillus infection. There is no significant inflammation.
Pneumocystis jiroveci Pneumonia
Although recent studies have strongly suggested that Pneumocystis jiroveci (formerly known as Pneumocystis carinii) is a fungus, we discuss Pneumocystis jiroveci pneumonia separately from other fungal infections for didactic purposes. Without prophylaxis, Pneumocystis jiroveci pneumonia occurs in nearly all lung transplant recipients.61 Thanks to the routine use of prophylaxis, it is rarely seen today in this patient population.
Clinical Presentation
The clinical presentation is nonspecific and includes cough, fever, dyspnea, and hypoxemia.
Pathologic Findings
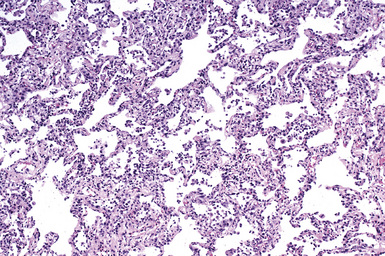
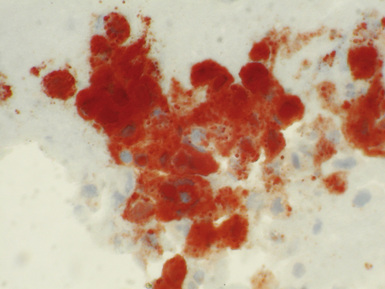
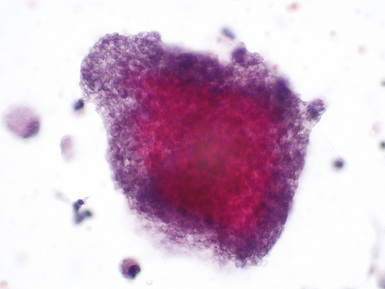
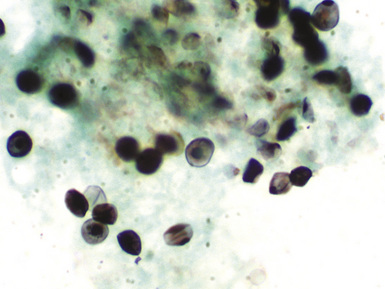
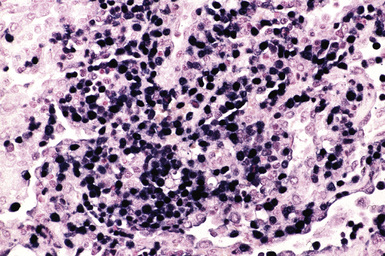
The classic histologic picture of interstitial pneumonia with frothy intra-alveolar exudates, seen in patients with acquired immunodeficiency syndrome, is rarely encountered in lung transplant recipients (Figs. 12-19 and 12-20). In these patients, Pneumocystis jiroveci pneumonia more often manifests as diffuse alveolar damage and the organisms are typically embedded in the prominent hyaline membranes (Fig. 12-21). Granulomatous inflammation is a less common manifestation of infection with Pneumocystis jiroveci.
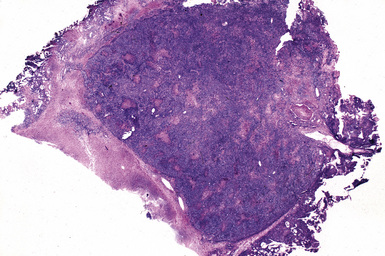
Post-Transplantation Lymphoproliferative Disorders
PTLD lesions are lymphoid or plasmacytic proliferations that develop as a consequence of immunosuppression in an allograft recipient.62 Characteristics of PTLDs vary somewhat with allograft types and with immunosuppressive regimens. PTLDs are relatively more common among pulmonary allograft recipients as a result of higher levels of immunosuppression.30 In this population, the occurrence rate for PTLDs may be as high as 5%.
Radiologic Findings
Thoracic abnormalities are present in most lung transplant recipients with PTLD.64 The most common radiologic finding is multiple pulmonary nodules. Other manifestations include a solitary nodule, multifocal alveolar infiltrates, and hilar or mediastinal adenopathy.
Pathologic Findings
The spectrum of PTLDs ranges from early lesions to polymorphic PTLD to lymphomas.65,66 Several classification schemes have been proposed, but the World Health Organization (WHO) Classification is now widely accepted and is presented in Box 12-3.62
Box 12-3 Classification of Post-Transplantation Lymphoproliferative Disorders (PTLDs)
Specimen evaluation for the diagnosis of PTLD should include routine morphologic examination, immunophenotyping, preservation of tissue for potential molecular genetic studies, and detection of EBV infection.62,67 Flow cytometry or frozen section immunohistochemistry is more useful in determining cell lineage and clonality than paraffin section immunohistochemistry. If immunophenotyping studies show polytypic immunoglobulin, clonality can be further assessed by molecular genetic studies that are capable of identifying polyclonal or monoclonal gene rearrangement. EBV infection can be detected using immunohistochemistry for latent membrane protein (LMP-1), but in situ hybridization for EBV-encoded nuclear RNA (EBER) is considered the gold standard.
Early Lesions
Early lesions of PTLD include plasmacytic hyperplasia and infectious mononucleosis–like lesions. These lesions usually arise in lymph nodes or Waldeyer’s ring and only rarely involve true extranodal sites such as the lung. They are characterized by some degree of architectural preservation of the involved tissue,62 but they differ from typical reactive follicular hyperplasia in having a diffuse proliferation of plasma cells. Plasmacytic hyperplasia is distinguished by numerous plasma cells and rare immunoblasts, and infectious mononucleosis–like lesion has the typical morphologic features of infectious mononucleosis in the lymph node, namely paracortical expansion and numerous immunoblasts in a background of T cells and plasma cells.
Polymorphic PTLDs
Polymorphic PTLDs are destructive lesions that efface the architecture of lymph nodes or form destructive extranodal masses.62 In contrast with most lymphomas, polymorphic PTLDs show the full extent of B cell maturation and are composed of immunoblasts, plasma cells, small and intermediate-sized lymphocytes, and centrocyte-like cells. Scattered large, bizarre cells (atypical immunoblasts) and areas of necrosis may also be present. Polymorphic PTLDs were at one time subdivided into “polymorphic B cell hyperplasia” and “polymorphic B cell lymphoma.” Today this separation is not deemed necessary, because both have similar clinical features. Immunophenotyping studies typically show a mixture of B and T cells. Most of the cases are monoclonal, at least by molecular genetic studies. EBV-positive immunoblasts are present in a majority of the cases.
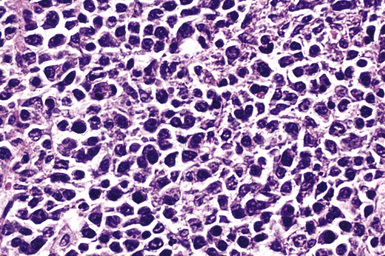
Monomorphic B Cell PTLDs
Monomorphic B cell PTLDs are characterized by nodal architectural effacement or tumoral growth in extranodal sites, with confluent sheets of large, transformed cells.62 These tumors should be diagnosed as B cell lymphomas and should be classified according to lymphoma classification guidelines. However, PTLD should also appear in the differential diagnosis. A majority of B cell PTLDs have morphologic features of diffuse large B cell lymphoma (Figs. 12-22 to 12-24). A minority may be classified as Burkitt lymphoma, plasma cell myeloma, or plasmacytoma-like lesions. Immunophenotyping studies of monomorphic B cell PTLD show B cell–associated antigen expression (CD19, CD20, CD79a). Many cases co-express antigens usually associated with T cells (CD43, CD45RO). A majority of cases are monoclonal and EBV-positive. Monomorphic B cell PTLDs often contain oncogene or tumor suppressor gene alterations (N-ras gene codon 61 point mutation, p53 gene mutation, or c-myc gene rearrangement).68,69
Classic Hodgkin Lymphoma–Type PTLD
Classic Hodgkin lymphoma–type PTLD is the least common form of PTLD.62 The diagnosis is based on classic morphologic and immunophenotypic features, preferably including both CD15 and CD30 expression. This type of PTLD is almost always EBV-positive. Because Reed-Sternberg–like cells may also be seen in some polymorphic and monomorphic PTLDs, the distinction between classic Hodgkin lymphoma–type PTLD and Hodgkin lymphoma–like PTLD may be difficult in some cases. However, the latter are better categorized as either polymorphic or monomorphic PTLDs.
Histologic Differential Diagnosis
Acute rejection may be considered in the differential diagnosis for PTLDs, especially in small biopsy samples. Detection of EBV infection is very helpful in this respect. A sheet-like monomorphous infiltrate with a mononuclear composition of more than 25% B cells and more than 30% large lymphoid cells also favors PTLD over acute rejection.70
Treatment and Prognosis
Therapy of PTLD must be tailored to the individual patient. Newer modalities such as anti-CD20 monoclonal antibody therapy (with rituximab) complement the standard stepwise approach that begins with a reduction of immunosuppression.71 The role of chemotherapy continues to be defined, and in some cases early recourse to this approach may be desirable. Survival varies by age and extent of disease, with pediatric patients and those with localized disease tending to fare better.
Other Complications
Cryptogenic Organizing Pneumonia
Cryptogenic organizing pneumonia, previously known as idiopathic BOOP, occurs as a response to acute lung injury. In lung transplant recipients, it is commonly associated with aspiration, infection, and acute rejection.40,72–74 However, organizing pneumonia is not a component of, and does not necessarily predispose to, chronic airway rejection (obliterative bronchiolitis).
Radiologic Findings
The chest film may be normal in appearance or show localized or diffuse infiltrates.
Pathologic Findings
Fibromyxoid plugs of granulation tissue are seen within small airways and airspaces, typically in a patchy distribution (Fig. 12-25).
Histologic Differential Diagnosis
In organizing diffuse alveolar damage, the fibroblastic proliferation involves the interstitium rather than the airspaces and remnants of hyaline membranes may be seen.40 However, organizing pneumonia and diffuse alveolar damage are both acute lung injury patterns and features of both may be present in a given case. Airspace fibromyxoid tissue can also be seen in organizing infectious pneumonia and healing rejection, especially higher-grade rejection following steroid therapy. Separation of organizing pneumonia from transplant obliterative bronchiolitis has been discussed earlier.
Recurrence of the Primary Disease
A relatively small percentage of transplant patients are at risk for recurrence of their primary disease following lung transplantation. Sarcoidosis is the most common disease to recur.75 Other reported cases include recurrence of lymphangioleiomyomatosis,76–78 diffuse panbronchiolitis,79 giant cell interstitial pneumonia,80 desquamative interstitial pneumonia,72 intravenous talc granulomatosis,81 and bronchioloalveolar carcinoma.82
1 Christie J.D., Edwards L.B., Aurora P., et al. Registry of the International Society for Heart and Lung Transplantation: Twenty-fifth official adult lung and heart/lung transplantation report—2008. J Heart Lung Transplant. 2008;27(9):957-969.
2 Yamane M., Date H., Okazaki M., et al. Long-term improvement in pulmonary function after living donor lobar lung transplantation. J Heart Lung Transplant. 2007;26(7):687-692.
3 Egan T.M., Murray S., Bustami R.T., et al. Development of the new lung allocation system in the United States. Am J Transplant. 2006;6(5 Pt 2):1212-1227.
4 Hachem R.R., Trulock E.P. The new lung allocation system and its impact on waitlist characteristics and post-transplant outcomes. Semin Thorac Cardiovasc Surg. 2008;20(2):139-142.
5 Nizami I., Frost A.E. Clinical diagnosis of transplant-related problems. In: Cagle P.T., editor. Diagnostic Pulmonary Pathology. New York: Marcel Dekker; 2000:485-499.
6 McWilliams T.J., Williams T.J., Whitford H.M., Snell G.I. Surveillance bronchoscopy in lung transplant recipients: Risk versus benefit. J Heart Lung Transplant. 2008;27(11):1203-1209.
7 Valentine V.G., Gupta M.R., Weill D., et al. Single-institution study evaluating the utility of surveillance bronchoscopy after lung transplantation. J Heart Lung Transplant. 2009;28(1):14-20.
8 Stewart S., Fishbein M.C., Snell G.I., et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26(12):1229-1242.
9 Hopkins P.M., Aboyoun C.L., Chhajed P.N., et al. Prospective analysis of 1,235 transbronchial lung biopsies in lung transplant recipients. J Heart Lung Transplant. 2002;21(10):1062-1067.
10 Lee J.C., Christie J.D. Primary graft dysfunction. Proc Am Thorac Soc. 2009;6(1):39-46.
11 Christie J.D., Carby M., Bag R., et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: Definition. A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2005;24(10):1454-1459.
12 Meyers B.F., de la Morena M., Sweet S.C., et al. Primary graft dysfunction and other selected complications of lung transplantation: a single-center experience of 983 patients. J Thorac Cardiovasc Surg. 2005;129(6):1421-1429.
13 Tazelaar H.D., Yousem S.A. The pathology of combined heart-lung transplantation: an autopsy study. Hum Pathol. 1988;19(12):1403-1416.
14 Christie J.D., Kotloff R.M., Ahya V.N., et al. The effect of primary graft dysfunction on survival after lung transplantation. Am J Respir Crit Care Med. 2005;171(11):1312-1316.
15 Daud S.A., Yusen R.D., Meyers B.F., et al. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2007;175(5):507-513.
16 Clark S.C., Levine A.J., Hasan A., et al. Vascular complications of lung transplantation. Ann Thorac Surg. 1996;61(4):1079-1082.
17 Schulman L.L., Anandarangam T., Leibowitz D.W., et al. Four-year prospective study of pulmonary venous thrombosis after lung transplantation. J Am Soc Echocardiogr. 2001;14(8):806-812.
18 Ruffini E., Maggi G., Actis-Dato G., et al. Successful bilobectomy for pulmonary venous obstruction after bilateral lung transplantation. J Thorac Cardiovasc Surg. 1998;116(4):648-649.
19 Santacruz J.F., Mehta A.C. Airway complications and management after lung transplantation: ischemia, dehiscence, and stenosis. Proc Am Thorac Soc. 2009;6(1):79-93.
20 Hasegawa T., Iacono A.T., Orons P.D., Yousem S.A. Segmental nonanastomotic bronchial stenosis after lung transplantation. Ann Thorac Surg. 2000;69(4):1020-1024.
21 Yousem S.A., Paradis I.L., Dauber J.A., et al. Large airway inflammation in heart-lung transplant recipients—its significance and prognostic implications. Transplantation. 1990;49(3):654-656.
22 Griffith B.P., Magee M.J., Gonzalez I.F., et al. Anastomotic pitfalls in lung transplantation. J Thorac Cardiovasc Surg. 1994;107(3):743-753. discussion 753–754
23 Kapoor B.S., May B., Panu N., et al. Endobronchial stent placement for the management of airway complications after lung transplantation. J Vasc Interv Radiol. 2007;18(5):629-632.
24 Berry G.J., Brunt E.M., Chamberlain D., et al. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: Lung Rejection Study Group. The International Society for Heart Transplantation. J Heart Transplant. 1990;9(6):593-601.
25 Yousem S.A., Berry G.J., Cagle P.T., et al. Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: Lung Rejection Study Group. J Heart Lung Transplant. 1996;15(1 Pt 1):1-15.
26 Hasegawa T., Iacono A.T., Yousem S.A. The anatomic distribution of acute cellular rejection in the allograft lung. Ann Thorac Surg. 2000;69(5):1529-1531.
27 Nakhleh R.E., Bolman R.M.3rd, Henke C.A., Hertz M.I. Lung transplant pathology. A comparative study of pulmonary acute rejection and cytomegaloviral infection. Am J Surg Pathol. 1991;15(12):1197-1201.
28 Stewart S. Pulmonary infections in transplantation pathology. Arch Pathol Lab Med. 2007;131(8):1219-1231.
29 Tazelaar H.D. Perivascular inflammation in pulmonary infections: implications for the diagnosis of lung rejection. J Heart Lung Transplant. 1991;10(3):437-441.
30 Randhawa P.S., Yousem S.A., Paradis I.L., et al. The clinical spectrum, pathology, and clonal analysis of Epstein-Barr virus–associated lymphoproliferative disorders in heart-lung transplant recipients. Am J Clin Pathol. 1989;92(2):177-185.
31 Valentine V.G., Robbins R.C., Wehner J.H., et al. Total lymphoid irradiation for refractory acute rejection in heart-lung and lung allografts. Chest. 1996;109(5):1184-1189.
32 Hachem R.R., Khalifah A.P., Chakinala M.M., et al. The significance of a single episode of minimal acute rejection after lung transplantation. Transplantation. 2005;80(10):1406-1413.
33 Khalifah A.P., Hachem R.R., Chakinala M.M., et al. Minimal acute rejection after lung transplantation: a risk for bronchiolitis obliterans syndrome. Am J Transplant. 2005;5(8):2022-2030.
34 Yousem S.A. Lymphocytic bronchitis/bronchiolitis in lung allograft recipients. Am J Surg Pathol. 1993;17(5):491-496.
35 Girnita A.L., Duquesnoy R., Yousem S.A., et al. HLA-specific antibodies are risk factors for lymphocytic bronchiolitis and chronic lung allograft dysfunction. Am J Transplant. 2005;5(1):131-138.
36 Bando K., Paradis I.L., Similo S., et al. Obliterative bronchiolitis after lung and heart-lung transplantation. An analysis of risk factors and management. J Thorac Cardiovasc Surg. 1995;110(1):4-13.
37 American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165(2):277-304.
38 Paradis I., Yousem S., Griffith B. Airway obstruction and bronchiolitis obliterans after lung transplantation. Clin Chest Med. 1993;14(4):751-763.
39 Cooper J.D., Billingham M., Egan T., et al. A working formulation for the standardization of nomenclature and for clinical staging of chronic dysfunction in lung allografts. International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 1993;12(5):713-716.
40 Yousem S.A., Duncan S.R., Griffith B.P. Interstitial and airspace granulation tissue reactions in lung transplant recipients. Am J Surg Pathol. 1992;16(9):877-884.
41 Yousem S.A., Paradis I.L., Dauber J.H., et al. Pulmonary arteriosclerosis in long-term human heart-lung transplant recipients. Transplantation. 1989;47(3):564-569.
42 Martinu T., Howell D.N., Davis R.D., et al. Pathologic correlates of bronchiolitis obliterans syndrome in pulmonary retransplant recipients. Chest. 2006;129(4):1016-1023.
43 Martinu T., Chen D.F., Palmer S.M. Acute rejection and humoral sensitization in lung transplant recipients. Proc Am Thorac Soc. 2009;6(1):54-65.
44 Morrell M.R., Patterson G.A., Trulock E.P., Hachem R.R. Acute antibody-mediated rejection after lung transplantation. J Heart Lung Transplant. 2009;28(1):96-100.
45 Gloor J., Cosio F., Lager D.J., Stegall M.D. The spectrum of antibody-mediated renal allograft injury: implications for treatment. Am J Transplant. 2008;8(7):1367-1373.
46 Reed E.F., Demetris A.J., Hammond E., et al. Acute antibody-mediated rejection of cardiac transplants. J Heart Lung Transplant. 2006;25(2):153-159.
47 Girnita A.L., McCurry K.R., Yousem S.A., et al. Antibody-mediated rejection in lung transplantation: case reports. Clin Transpl. 2006:508-510.
48 Astor T.L., Weill D., Cool C., et al. Pulmonary capillaritis in lung transplant recipients: treatment and effect on allograft function. J Heart Lung Transplant. 2005;24(12):2091-2097.
49 Badesch D.B., Zamora M., Fullerton D., et al. Pulmonary capillaritis: a possible histologic form of acute pulmonary allograft rejection. J Heart Lung Transplant. 1998;17(4):415-422.
50 Choi J.K., Kearns J., Palevsky H.I., et al. Hyperacute rejection of a pulmonary allograft. Immediate clinical and pathologic findings. Am J Respir Crit Care Med. 1999;160(3):1015-1018.
51 Frost A.E., Jammal C.T., Cagle P.T. Hyperacute rejection following lung transplantation. Chest. 1996;110(2):559-562.
52 Masson E., Stern M., Chabod J., et al. Hyperacute rejection after lung transplantation caused by undetected low-titer anti-HLA antibodies. J Heart Lung Transplant. 2007;26(6):642-645.
53 Appel J.Z.3rd, Hartwig M.G., Davis R.D., Reinsmoen N.L. Utility of peritransplant and rescue intravenous immunoglobulin and extracorporeal immunoadsorption in lung transplant recipients sensitized to HLA antigens. Hum Immunol. 2005;66(4):378-386.
54 Gottlieb J., Mattner F., Weissbrodt H., et al. Impact of graft colonization with gram-negative bacteria after lung transplantation on the development of bronchiolitis obliterans syndrome in recipients with cystic fibrosis. Respir Med. 2009;103(5):743-749.
55 Marchevsky A., Hartman G., Walts A., et al. Lung transplantation: the pathologic diagnosis of pulmonary complications. Mod Pathol. 1991;4(2):133-138.
56 Valentine V.G., Weill D., Gupta M.R., et al. Ganciclovir for cytomegalovirus: a call for indefinite prophylaxis in lung transplantation. J Heart Lung Transplant. 2008;27(8):875-881.
57 Holt N.D., Gould F.K., Taylor C.E., et al. Incidence and significance of noncytomegalovirus viral respiratory infection after adult lung transplantation. J Heart Lung Transplant. 1997;16(4):416-419.
58 Ohori N.P., Michaels M.G., Jaffe R., et al. Adenovirus pneumonia in lung transplant recipients. Hum Pathol. 1995;26(10):1073-1079.
59 Kramer M.R., Marshall S.E., Starnes V.A., et al. Infectious complications in heart-lung transplantation. Analysis of 200 episodes. Arch Intern Med. 1993;153(17):2010-2016.
60 Sole A., Salavert M. Fungal infections after lung transplantation. Curr Opin Pulm Med. 2009;15(3):243-253.
61 Gryzan S., Paradis I.L., Zeevi A., et al. Unexpectedly high incidence of Pneumocystis carinii infection after lung-heart transplantation. Implications for lung defense and allograft survival. Am Rev Respir Dis. 1988;137(6):1268-1274.
62 Swerdlow S.H. Post-transplant lymphoproliferative disorders. In: Swerdlow S.H., Campo E., Harris N.L., et al, editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2008:343-349.
63 Paranjothi S., Yusen R.D., Kraus M.D., et al. Lymphoproliferative disease after lung transplantation: comparison of presentation and outcome of early and late cases. J Heart Lung Transplant. 2001;20(10):1054-1063.
64 Borhani A.A., Hosseinzadeh K., Almusa O., et al. Imaging of posttransplantation lymphoproliferative disorder after solid organ transplantation. Radiographics. 2009;29(4):981-1000. discussion 1000–1002
65 Lewin K.J. Post-transplant lymphoproliferative disorders. Pathol Oncol Res. 1997;3(3):177-182.
66 Tsao L., Hsi E.D. The clinicopathologic spectrum of posttransplantation lymphoproliferative disorders. Arch Pathol Lab Med. 2007;131(8):1209-1218.
67 Harris N.L., Ferry J.A., Swerdlow S.H. Posttransplant lymphoproliferative disorders: summary of Society for Hematopathology Workshop. Semin Diagn Pathol. 1997;14(1):8-14.
68 Chadburn A., Cesarman E., Knowles D.M. Molecular pathology of posttransplantation lymphoproliferative disorders. Semin Diagn Pathol. 1997;14(1):15-26.
69 Knowles D.M., Cesarman E., Chadburn A., et al. Correlative morphologic and molecular genetic analysis demonstrates three distinct categories of posttransplantation lymphoproliferative disorders. Blood. 1995;85(2):552-565.
70 Rosendale B., Yousem S.A. Discrimination of Epstein-Barr virus–related posttransplant lymphoproliferations from acute rejection in lung allograft recipients. Arch Pathol Lab Med. 1995;119(5):418-423.
71 Nalesnik M.A. Clinicopathologic characteristics of post-transplant lymphoproliferative disorders. Recent Results Cancer Res. 2002;159:9-18.
72 Keller C.A., Cagle P.T., Brown R.W., et al. Bronchiolitis obliterans in recipients of single, double, and heart-lung transplantation. Chest. 1995;107(4):973-980.
73 Chaparro C., Chamberlain D., Maurer J., et al. Bronchiolitis obliterans organizing pneumonia (BOOP) in lung transplant recipients. Chest. 1996;110(5):1150-1154.
74 Siddiqui M.T., Garrity E.R., Husain A.N. Bronchiolitis obliterans organizing pneumonia-like reactions: a nonspecific response or an atypical form of rejection or infection in lung allograft recipients? Hum Pathol. 1996;27(7):714-719.
75 Collins J., Hartman M.J., Warner T.F., et al. Frequency and CT findings of recurrent disease after lung transplantation. Radiology. 2001;219(2):503-509.
76 O’Brien J.D., Lium J.H., Parosa J.F., et al. Lymphangiomyomatosis recurrence in the allograft after single-lung transplantation. Am J Respir Crit Care Med. 1995;151(6):2033-2036.
77 Collins J., Müller N.L., Kazerooni E.A., et al. Lung transplantation for lymphangioleiomyomatosis: Role of imaging in the assessment of complications related to the underlying disease. Radiology. 1999;210(2):325-332.
78 Chen F., Omasa M., Kondo N., et al. Sirolimus treatment for recurrent lymphangioleiomyomatosis after lung transplantation. Ann Thorac Surg. 2009;87(1):6-7.
79 Baz M.A., Kussin P.S., Van Trigt P., et al. Recurrence of diffuse panbronchiolitis after lung transplantation. Am J Respir Crit Care Med. 1995;151(3 Pt 1):895-898.
80 Frost A.E., Keller C.A., Brown R.W., et al. Giant cell interstitial pneumonitis. Disease recurrence in the transplanted lung. Am Rev Respir Dis. 1993;148(5):1401-1404.
81 Cook R.C., Fradet G., English J.C., et al. Recurrence of intravenous talc granulomatosis following single lung transplantation. Can Respir J. 1998;5(6):511-514.
82 Garver R.I.Jr, Zorn G.L., Wu X., et al. Recurrence of bronchioloalveolar carcinoma in transplanted lungs. N Engl J Med. 1999;340(14):1071-1074.