Chapter 95 Pathogenetic Mechanisms of Retinal Detachment
Major types
Accumulation of subretinal fluid is a feature of all retinal detachments. When the normal physiologic forces that maintain contact between the retina and the RPE (e.g., the metabolic pump of the RPE,1 the osmotic pressure of the choroid,2 and the more minor mechanical forces of the interphotoreceptor matrix) are compromised or overwhelmed, a retinal detachment occurs. Various pathologic conditions can upset the balance of the normal transretinal pressure gradient and result in subretinal fluid accumulation.
Rhegmatogenous retinal detachment
Rhegmatogenous retinal detachments are those arising from one or more full-thickness retinal breaks. The term retinal break refers either to a retinal tear or to a retinal hole. Retinal tears are commonly associated with well-defined vitreoretinal traction either on an attached flap or on the retina that had been adjacent to a now free-floating vitreous operculum. In contrast, retinal holes occur more commonly as a result of localized retinal atrophy or deterioration and are not believed to be associated with vitreoretinal traction.3–5 Clinically, the distinction is not always clear: for instance some operculated retinal breaks have vitreous traction upon nearby retinal vessels and behave as tears with persistent traction upon them.
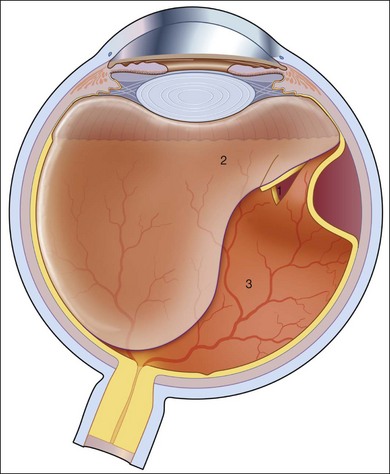
The characteristics of a rhegmatogenous retinal detachment are: (1) the existence of abnormal mobility of partially liquefied vitreous gel; (2) tractional forces that can precipitate a retinal break; and (3) the presence of a retinal break that will allow the passage of liquefied vitreous into the subretinal space (Fig. 95.1). All three factors need to be present to cause a rhegmatogenous retinal detachment. For example, if a tear or hole is present in the absence of tractional forces and liquid vitreous, it is unlikely that the retina will detach. Examination of postmortem eyes indicates that approximately 5–10% of eyes have full-thickness retinal defects without any apparent detachment.6 Subclinical retinal detachments are defined as having less than 1–2 disc diameters of associated subretinal fluid and usually do not progress, if asymptomatic.7 A spontaneous rhegmatogenous retinal detachment is usually preceded by a posterior vitreous detachment (PVD). With age, fragmentation of collagen fibers and an aggregation of proteoglycans around these fragments is believed to be responsible for destabilizing the vitreous gel and leading to liquefaction (syneresis).8 The subsequent reduced volume of the vitreous gel is associated with the collapse and aggregation of the collagen fibrillar network. The liquefied vitreous gel may coalesce into a large lacuna, which mimics a true PVD.9 When the denser posterior vitreous cortex ruptures, the liquefied vitreous can pass into the subhyaloid space and separate the posterior vitreous surface from the internal limiting membrane of the retina producing a true PVD.9 Both the degree of vitreous liquefaction and the prevalence of PVD are age-related.10 Pools of liquefied vitreous can be seen with the slit lamp in more than 90% of patients after the age of 40 years.11 PVD is found in 27% of patients aged 60–69 years and in 63% of patients after age 70.10 Other factors that hasten increased mobility of partially liquified vitreous include cataract extraction, high myopia, inflammation, and trauma.12
In retinal detachment, the normal forces maintaining neurosensory retinal attachment to the RPE are overwhelmed by opposing forces causing retinal detachment. The forces maintaining retinal adhesion are discussed in detail elsewhere in the text and include the Na+/K+-ATPase metabolic pump of the RPE, the osmotic pressure of the choroid, and the weaker forces of the interphotoreceptor matrix. The opposing forces include hydraulic dissection caused by the intraocular fluid swirling through the retinal break held open by vitreoretinal traction.13 The location of the residual vitreoretinal adhesion on the retina often determines the location of the retinal break. Specific clinical situations will lead to similar vitreoretinal adhesion and characteristic tears. Aphakia and pseudophakia are commonly associated with peripheral retinal breaks at the posterior edge of the vitreous base near the ora serrata. The retina in this region is relatively thin and less developed. Ocular trauma can result in retinal breaks, three-quarters of which are retinal dialyses. A retinal dialysis following trauma usually occurs in either the superonasal or inferotemporal quadrants. Dialyses, in general and following trauma, are more common in the inferotemporal quadrant although most of the dialyses in the superonasal quadrant are associated with a definite history of preceding trauma.14 Giant retinal tears (circumferential retinal breaks of ≥90°) commonly arise from circumferential vitreous traction in the region of the posterior vitreous base,15 although tears in the anterior vitreous base can occur with Stickler syndrome.16 This inherited condition is associated primarily with the COL2A gene and also with the COL11A1 gene.17 It carries a very high lifetime risk for rhegmatogenous retinal detachments.16,18 Retinal detachments originating from the posterior pole (macula) or the intermediate zone between the posterior pole and the equator are characteristic to high myopia. Posterior pole retinal detachments are very rare, accounting for less than 1% of retinal detachments in the USA, but the prevalence may be much higher in Japan.19 Most of the nontraumatic retinal breaks located in the posterior pole are secondary to macular holes and associated with a posterior staphyloma.20 In some myopic macular holes and detachments, tangential vitreoretinal traction based on epiretinal membranes after incomplete posterior vitreous separation “vitreoschisis,”21,22 are speculated to be pathophysiologic factors.23
However, even when tangential traction is completely released by removing the internal limiting membrane during a vitrectomy, the retinal detachment may still persist. Some authors believe that operative failure in these instances is related to axial elongation and posterior staphyloma formation.24 The incidence of retinal detachment associated with cataract extraction has decreased with the changes in surgical technique over the years, from intracapsular cataract extraction and aphakic correction to phacoemulsification, combined with the placement of a posterior chamber intraocular lens in the presence of an intact posterior lens capsule.25 The reasons for this change are postulated to include: reduced forward shift of the vitreous, reduced shrinkage of the vitreous secondary to hyaluronic acid loss from the eye, and support of the vitreous body by the posterior chamber intraocular lens and posterior lens capsule. Furthermore, in eyes with an intact posterior lens capsule after extracapsular cataract extraction, surgical or Nd:YAG laser posterior capsulotomy is associated with an increased incidence of subsequent retinal detachment.26 Nevertheless, the risk of retinal detachment after cataract extraction continues despite these changes. Rowe et al.27 predicted that 10 years after either phacoemulsification or extracapsular cataract extraction, the cumulative probability of retinal detachment was 5.5 times higher than expected. The question of whether patients have inherent risk factors that are manifested by manipulations during cataract extraction, arises when considering phakic fellow eyes. Sharma et al.28 evaluated fellow eyes of patients experiencing a pseudophakic rhegmatogenous retinal detachment and noted that 7.8% developed a phakic retinal detachment over a mean follow-up period of 57.4 months. Neuhann et al.29 reviewed the risk of pseudophakic retinal detachment in a large series of very high myopic eyes. To the surprise of many they found that pseudophakia means no additional risk. Their explanation is based on the facts that shifting the risk of retinal detachment to early age with increasing axial length of the eye30 is a consequence of a concomitant increase in vitreous mobility from premature posterior vitreous separation.31 At the time of cataract extraction, vitreous mobility and risk of retinal detachment are at a summit for some time. Therefore, the clinically relevant risk of pseudophakia in emmetropia and low myopia, becomes irrelevant in very high myopia. Various intraocular inflammatory and infectious conditions can cause vitreous gel liquefaction, PVD, and retinal breaks. Ocular toxoplasmosis,32 ocular toxocariasis,32,33 and pars planitis34 are associated with morphologic vitreoretinal changes that can lead to vitreoretinal traction and retinal breaks. The acute retinal necrosis syndrome and cytomegalovirus retinitis are forms of infectious retinitis that can result in multiple small breaks along the border between atrophic and normal retina and within necrotic retina.4,11,35 Uveitis itself is an overall risk factor for a rhegmatogenous retinal detachment. Patients presenting with a rhegmatogenous retinal detachment from uveitis will also have a higher rate of proliferative vitreoretinopathy (PVR) at presentation and an overall worse prognosis.36 Retinal detachment resulting from retinoschisis is a subcategory of rhegmatogenous retinal detachment. This occurs when there are holes in both the inner and outer walls of the retinoschisis cavity, allowing fluid to enter the subretinal space and hence produce a retinal detachment. The incidence of retinal detachment with retinoschisis was approximately 2.5% in a large series of patients with retinal detachment.37
Traction retinal detachment
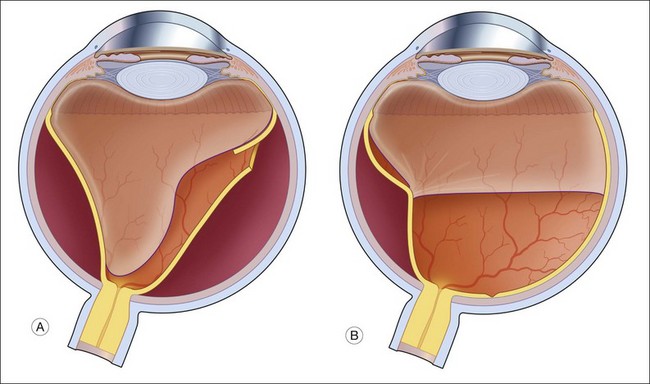
In contrast to the rhegmatogenous retinal detachment, which often has a convex, even bullous, surface, the typical traction retinal detachment has a more concave surface and is likely to be more localized, often not extending to the ora serrata (Fig. 95.2).
Traction retinal detachments are a common feature of diabetic retinopathy.38 Contracture of the vitreous gel often occurs in eyes with proliferative diabetic retinopathy. As the vitreous gel shrinks, the fibrovascular tissue and underlying retina are drawn anteriorly toward the vitreous base. Initially, the retina along the temporal arcades may be the only area to detach, but the detachment can spread both peripherally and centrally toward the macula. Diabetics who develop posterior neovascularization in childhood are prone to this form of traction retinal detachment. However, traction detachments related to diabetes do not inevitably go on to involve the macula. In one series, only 14% progressed to macular detachment within 1 year.39
Retinopathy of prematurity is the leading cause of childhood blindness in the USA, with more than 500 new cases diagnosed each year. Approximately 90% and in some series up to 100% of ROP, stages 1 and 2, regress. However, in the 6% of infants born under 1250 g, ROP develops to a certain threshold of vasoproliferation and the risk of peripheral traction retinal detachments approaches 50%.40 The pathophysiology of this traction detachment is believed to be from endothelial cell proliferation at the junction of the vascularized and avascular retina and migration onto the vitreous scaffold. Contraction of these membranes and the marked adherence of the posterior hyaloid to the retina can produce a complete retinal detachment, if untreated.41
Machemer and Williams have described an uncommon secondary form of traction retinal detachment.42 Retinal vascular diseases such as angiomatosis retinae and Coats’ disease can occasionally cause a fibrous proliferation and membrane formation in the vitreous and a secondary tractional retinal detachment in addition to primarily causing exudative retinal detachments.43
Combined tractional and rhegmatogenous retinal detachment
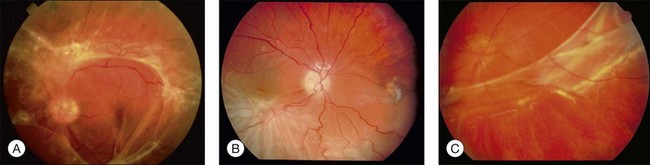
Combined tractional–rhegmatogenous retinal detachments are often seen in proliferative diabetic retinopathy (Fig. 95.3A), PVR, proliferative sickle-cell retinopathy, and penetrating intraocular injuries.
PVR is a complication of a rhegmatogenous retinal detachment and is the most common cause of failure of surgical repair of these cases, occurring in 7–10% of primary operations and in a higher percentage of reoperations (Fig. 95.3B,C).44 The basic pathologic process in eyes with PVR is growth and contraction of cellular membranes on the inner retinal surface and on the posterior vitreous surface, leading to the reopening of retinal breaks or the creation of new ones.45 Macular pucker has some cellular features in common but is usually not classified as PVR. It is not associated with retinal breaks and usually not complicated by retinal detachment. Macular pucker has a much better overall prognosis compared with PVR, even though it can compromise central vision The formation of abnormal membranes on the outer retinal surface is clinically known as subretinal fibrosis. Subretinal fibrosis can disrupt the normal intercellular relationship between the photoreceptors and RPE, thus preventing the regeneration of photoreceptor outer segments after reattachment.46 (For more details on the pathogenesis of proliferative vitreoretinopathy, see Chapter 97, Pathogenesis of PVR.)
In advanced proliferative diabetic retinopathy, combined tractional–rhegmatogenous retinal detachments generally start as tractional retinal detachments that are secondarily complicated by posterior retinal breaks. At this stage, they often have the appearance of a mobile retina with a convex surface and may extend to the ora serrata. The retinal break is commonly located near an area of fibrovascular proliferation. Moradian et al. report that vitreoretinal traction may be augmented even to the extend of retinal detachment by intravitreal injection of VEGF-blockers.47
Exudative and hemorrhagic retinal detachment
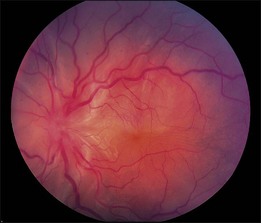
Retinal detachments can occur in the absence of a retinal break or vitreoretinal traction (Fig. 95.4). These detachments are the result of a collection of subretinal fluid secondary to diseases of the choroid and RPE or of the retina itself. The RPE is largely responsible for the absorption of the subretinal fluid. The RPE maintains retinal adherence and absorbs subretinal fluid by means of active transport, creation of an osmotic gradient and, to a lesser degree, hydrostatic forces. Exudative retinal detachments occur when the balance between fluid production and fluid absorption is disrupted, either by damage to the RPE or by excessive fluid production. Inflammatory diseases and neoplastic lesions are the leading causes of exudative (serous) retinal detachments. Fluid leakage from the choroid exceeds the physiologic mechanisms that eliminate the subretinal fluid both by damage to the RPE and by exudation of fluid. Exudative conditions include Vogt–Koyanagi–Harada disease, posterior scleritis, collagen vascular disease, malignant hypertension, sympathetic ophthalmia, and toxemia of pregnancy, and neoplasms such as malignant melanoma, choroidal hemangioma, and metastatic lesions to the choroid. In conditions such as central serous chorioretinopathy (idiopathic central serous choroidopathy), the metabolic activity of the RPE can be diffusely compromised,48 and subretinal fluid is presumably less effectively absorbed. Hyperpermeability of choroidal vessels as demonstrated by indocyanine green angiography are also seen as part of the disease process.49 Exudative retinal detachments can occur when steroids are used in the setting of central serous chorioretinopathy.50 Steroids administered exogenously as well as hypercortisolism were reported as risk factors for central serous chorioretinopathy.51 Retinal vascular diseases, including Coats’ disease and angiomatosis retinae, may cause excessive production of subretinal fluid and exudates and, consequently, an exudative retinal detachment.43 Idiopathic uveal effusion can produce a serous retinal detachment along with a similar detachment of the choroid, possibly resulting from impaired choroidal venous outflow. This condition when associated with nanophthalmos (mean axial length 16 mm) or high hyperopia (mean +16 diopters) is associated with disorganization of collagen fiber bundles and deposits of proteoglycans in the scleral matrix and can be successfully managed by sclerectomies.52
Subretinal blood may occur with trauma especially after contusion. Drainage of subretinal blood is reported to be beneficial for hemorrhagically elevated retinal tears to allow adequate retinopexy and may help to accomplish long-term anatomic attachment in eyes with massive subretinal hemorrhage or bullous retinal detachment.53 Blood has been proven toxic to the retina and to the retinal pigment epithelium.54 Presumed ocular histoplasmosis syndrome, polypoidal choroidal neovascularization and subretinal neovascular membranes, as seen in age-related macular degeneration may also produce a sometimes massive hemorrhagic retinal detachment.55 For the latter the use of anticoagulant medication is a major risk factor, besides high blood pressure and cardiovascular disease.56
A peripheral localization of subretinal hemorrhage is often misinterpreted as choroidal melanoma (pseudomelanoma).57 Suprachoroidal hemorrhage has been observed after filtering surgery. Of the risk factors reported so far, such as age, myopia, postoperative hypotony, only high preoperative intraocular pressure seems to be strongly associated with suprachoroidal hemorrhage.58
1 Miller SS, Hughes BA, Machen TE. Fluid transport across retinal pigment epithelium is inhibited by cyclic AMP. Proc Natl Acad Sci U S A. 1982;79:2111–2115.
2 Negi A, Marmor MF. The resorption of subretinal fluid after diffuse damage to the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1983;24:1475–1479.
3 Byer NE. Clinical study of retinal breaks. Trans Am Acad Ophthalmol Otolaryngol. 1967;71:461–473.
4 Byer NE. Prognosis of asymptomatic retinal breaks. Arch Ophthalmol. 1974;92:208–210.
5 Byer NE. The natural history of asymptomatic retinal breaks. Ophthalmology. 1982;89:1033–1039.
6 Okun E. Gross and microscopic pathology in autopsy eyes. III. Retinal breaks without detachment. Am J Ophthalmol. 1961;51:369–391.
7 Byer NE. Subclinical retinal detachment resulting from asymptomatic retinal breaks: prognosis for progression and regression. Ophthalmology. 2001;108:1499–1504.
8 Sebag J. Ageing of the vitreous. Eye. 1987;1:254–262.
9 Heller MD, Straatsma BR, Foos RY. Detachment of the posterior vitreous in phakic and aphakic eyes. Mod Probl Ophthalmol. 1972;10:23–36.
10 Steinberg RH. Research update: report from a workshop on cell biology of retinal detachment. Exp Eye Res. 1986;43:695–706.
11 Foos RY, Wheeler NC. Vitreoretinal juncture. Synchysis senilis and posterior vitreous detachment. Ophthalmology. 1982;89:1502–1512.
12 Sebag J, Balazs EA. Human vitreous fibres and vitreoretinal disease. Trans Ophthalmol Soc UK. 1985;104:123–128.
13 Machemer R. The importance of fluid absorption, traction, intraocular currents, and chorioretinal scars in the therapy of rhegmatogenous retinal detachments. XLI Edward Jackson memorial lecture. Am J Ophthalmol. 1984;98:681–693.
14 Tasman W. Peripheral retinal changes following blunt trauma. Trans Am Ophthalmol Soc. 1972;70:190–198.
15 Mehta MC, Hirose T, Schepens CL. Giant retinal tears. In: Schepens CL, Hartnett ME, Hirose T. Schepens’ retinal detachment and allied diseases. 2nd ed. Boston: Butterworth-Heinemann; 2000:421–433.
16 Stickler GB, Hughes W, Houchin P. Clinical features of hereditary progressive arthro-ophthalmopathy (Stickler syndrome): a survey. Genet Med. 2001;3:192–196.
17 Snead MP, Yates JR. Clinical and molecular genetics of Stickler syndrome. J Med Genet. 1999;36:353–359.
18 Fivgas GD, Capone A, Jr. Pediatric rhegmatogenous retinal detachment. Retina. 2001;21:101–106.
19 Kuriyama S, Matsumura M, Harada T, et al. Surgical techniques and reattachment rates in retinal detachment due to macular hole. Arch Ophthalmol. 1990;108:1559–1561.
20 Leaver PK, Cleary PE. Macular hole and retinal detachment. Trans Ophthalmol Soc UK. 1975;95:145–147.
21 Sebag J. Vitreoschisis. Graefes Arch Clin Exp Ophthalmol. 2008;246:329–332.
22 Spaide RF, Fisher Y. Removal of adherent cortical vitreous plaques without removing the internal limiting membrane in the repair of macular detachments in highly myopic eyes. Retina. 2005;25:290–295.
23 Ikuno Y, Sayanagi Y, Oshima T, et al. Optical coherence tomographic findings of macular holes and retinal detachment after vitrectomy in highly myopic eyes. Am J Ophthalmol. 2003;136:477–481.
24 Ichibe M, Yoshizawa T, Morakami K, et al. Surgical management of retinal detachment associated with myopic macular hole: anatomic and functional status of the macula. Am J Ophthalmol. 2003;136:277–284.
25 Wilkinson CP. Retinal detachment following intraocular lens implantation. Graefes Arch Clin Exp Ophthalmol. 1986;224:64–66.
26 McPherson AR, O’Malley RE, Bravo J. Retinal detachment following late posterior capsulotomy. Am J Ophthalmol. 1983;95:593–597.
27 Rowe JA, Erie JC, Baratz KH, et al. Retinal detachment in Olmsted County, Minnesota, 1976 through 1995. Ophthalmology. 1999;106:154–159.
28 Sharma MC, Chan P, Kim RU, et al. Rhegmatogenous retinal detachment in the fellow phakic eyes of patients with pseudophakic rhegmatogenous retinal detachment. Retina. 2003;23:37–40.
29 Neuhann IM, Neuhann TF, Heimann H, et al. Retinal detachment after phacoemulsification in high myopia: analysis of 2356 cases. J Cataract Refract Surg. 2008;34:1644–1657.
30 Ye J, Zhou C, Du H, et al. Study on the posterior vitreous detachment in patients with high myopia. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 1999;21:472–477.
31 Burton TC. The influence of refractive error and lattice degeneration on the incidence of retinal detachment. Trans Am Ophthalmol Soc. 1989;87:143–155.
32 Hagler WS, Jarret WH, Chang M. Rhegmatogenous retinal detachment following chorioretinal inflammatory disease. Am J Ophthalmol. 1978;86:373–379.
33 Shields JA. Ocular toxocariasis. A review. Surv Ophthalmol. 1984;28:361–381.
34 Smith RE, Godfrey WA, Kimura SJ. Chronic cyclitis. I. Course and visual prognosis. Trans Am Acad Ophthalmol Otolaryngol. 1973;77:OP760–OP768.
35 Ahmadieh H, Soheilian M, Azarmina M, et al. Surgical management of retinal detachment secondary to acute retinal necrosis: clinical features, surgical techniques, and long-term results. Jpn J Ophthalmol. 2003;47:484–491.
36 Kerkhoff FT, Lamberts QJ, van den Biesen PR, et al. Rhegmatogenous retinal detachment and uveitis. Ophthalmology. 2003;110:427–431.
37 Malagola R, Contestabile MT, Villani GM, et al. Outer layer breaks and asymptomatic schisis detachment: clinical considerations. Ophthalm Surg Lasers. 2002;33:368–372.
38 Kaiser PK, Riemann CD, Sears JE, et al. Macular traction detachment and diabetic macular edema associated with posterior hyaloidal traction. Am J Ophthalmol. 2001;131:44–49.
39 Charles S, Flinn CE. The natural history of diabetic extramacular traction retinal detachment. Arch Ophthalmol. 1981;99:66–68.
40 Palmer EA, Flynn JT, Hardy RJ, et al. Incidence and early course of retinopathy of prematurity. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmology. 1991;98:1628–1640.
41 Charles S. Vitreous microsurgery, 2nd ed. Baltimore: Williams & Wilkins; 1987.
42 Machemer R, Williams JM, Sr. Pathogenesis and therapy of traction detachment in various retinal vascular diseases. Am J Ophthalmol. 1988;105:170–181. [Erratum in: Am J Ophthalmol 1988;105:714.]
43 Shields JA, Shields CL, Honavar SG, et al. Clinical variations and complications of Coats disease in 150 cases: the 2000 Sanford Gifford memorial lecture. Am J Ophthalmol. 2001;131:561–571.
44 Rachal WF, Burton TC. Changing concepts of failures after retinal detachment surgery. Arch Ophthalmol. 1979;97:480–483.
45 Machemer R, van Horn D, Aaberg TM. Pigment epithelial proliferation in human retinal detachment with massive periretinal proliferation. Am J Ophthalmol. 1978;85:181–191.
46 Lewis GP, Sethi CS, Linberg KA, et al. Experimental retinal reattachment: a new perspective. Mol Neurobiol. 2003;28:159–175.
47 Moradian S, Ahmadieh H, Malihi M, et al. Intravitreal bevacizumab in active progressive proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2008;246:1699–1705.
48 Chen SJ, Lee AF, Lee FL, et al. Indocyanine green angiography of central serous chorioretinopathy. Zhonghua Yi Xue Za Zhi (Taipei). 1999;62:605–613.
49 Guyer DR, Yannuzzi LA, Slakter JS, et al. Indocyanine green videoangiography of central serous chorioretinopathy. Arch Ophthalmol. 1994;112:1057–1062.
50 Gass JD, Little H. Bilateral bullous exudative retinal detachment complicating idiopathic central serous chorioretinopathy during systemic corticosteroid therapy. Ophthalmology. 1995;102:737–747.
51 Carvalho-Recchia CA, Yannuzzi LA, Negrao S, et al. Corticosteroids and central serous chorioretinopathy. Ophthalmology. 2002;109:1834–1837.
52 Uyama M, Takahashi K, Kozaki J, et al. Uveal effusion syndrome: clinical features, surgical treatment, histologic examination of the sclera, and pathophysiology. Ophthalmology. 2000;107:441–449.
53 Han DP, Mieler WF, Schwartz DM, et al. Management of traumatic hemorrhagic retinal detachment with pars plana vitrectomy. Arch Ophthalmol. 1990;108:1281–1286.
54 Glatt H, Machemer R. Experimental subretinal hemorrhage in rabbits. Am J Ophthalmol. 1982;94:762–773.
55 el Baba F, Jarrett WH, 2nd., Harbin TS, Jr., et al. Massive hemorrhage complicating age-related macular degeneration. Clinicopathologic correlation and role of anticoagulants. Ophthalmology. 1986;93:1581–1592.
56 Tilanus MA, Vaandrager W, Cuypers MH, et al. Relationship between anticoagulant medication and massive intraocular hemorrhage in age-related macular degeneration. Graefes Arch. 2000;238:482–485.
57 Shield CA, Slazar PF, Mashayekhi A, et al. Peripheral exudative hemorrhagic chorioretinopathy simulating choroidal melanoma in 173 eyes. Ophthalmology. 2009;116:529–535.
58 The Fluorouracil Filtering Surgery Study Group. Risk factors for suprachoroidal hemorrhage after filtering surgery. Am J Ophthalmol. 1992;113:501–507.