Chapter 28 Pathogenesis and Classification of Elbow Stiffness
Introduction
Motion of the elbow is important for positioning the hand in space. While the shoulder places the hand on the surface of a sphere of space, the elbow can change the radius of that sphere.1 Forearm motion can help position the hand for the most effective manipulation of the environment and is particularly important for tool use. Unfortunately, shoulder and wrist motion have a limited ability to compensate for stiffness of the elbow and forearm. Morrey et al. demonstrated that most activities of daily living can be performed within a 100° elbow flexion arc from 30° to 130° of flexion and a 100° rotation arc from 50° of supination to 50° of pronation; however, small contractures can affect specific functional tasks.2 The elbow is prone to stiffness after injury, but congenital abnormalities, degenerative and inflammatory diseases, and neuromuscular problems can contribute to loss of mobility.
Pathogenesis
Osseous and articular anatomy
The elbow is one of the most congruent, and constrained synovial joints in the body, providing both intrinsic osseous stability as well as a relatively large range of motion. With the evolution from quadrapedal to bipedal locomotion, enhanced stability of the ulnohumeral articulation has led to the upper limb becoming less adapted for weight-bearing and more adapted for tool use and dexterity. Ultimately, the hominid elbow developed articulations that can tolerate little distortion without losing either stability or motion.3
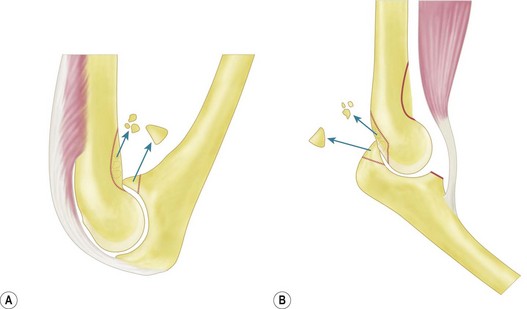
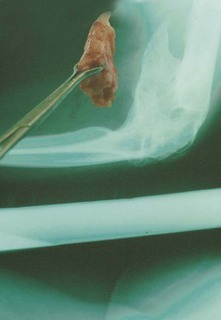
Distortion of the coronoid, radial and olecranon fossae of the distal humerus are common reasons for stiffness. These fossae accommodate the structures for which they are named at the extremes of elbow motion. Implants, scar tissue, fracture callus, malunion, or ectopic bone can obstruct these fossae and thereby limit motion.4 Similarly, malunion of the structures that fit into the fossae can cause stiffness (Fig. 28.1).
Loss of the anterior translation/angulation of the articular surface of the distal humerus can limit elbow flexion. This translation facilitates elbow flexion by allowing clearance of the coronoid with respect to the humeral diaphysis as well as providing space for the muscles and soft tissues when the elbow flexes.1
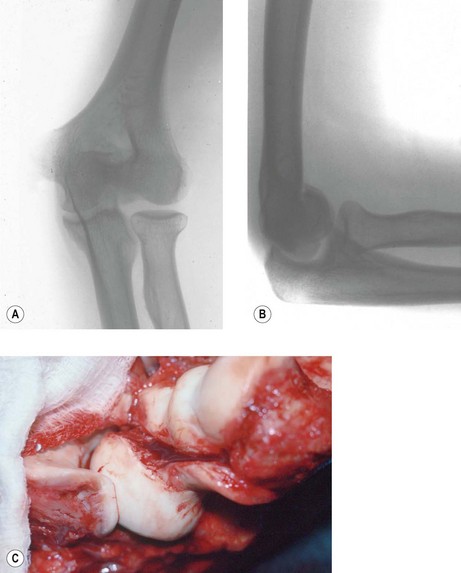
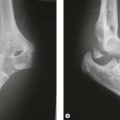
Incongruity of the articular surface (including slight subluxation) is a known contributor to loss of motion via arthrosis in the long term, but can also limit elbow motion acutely. This is particularly true for malunited fractures of the radial head, which can limit forearm rotation and malunited fractures of the capitellum and trochlea, which can limit flexion and extension (Fig. 28.2).4–6 Malunion of the coronoid leads to subluxation of the ulnohumeral joint, and progressive arthrosis and loss of motion.7 Arthrosis is limited after a simple olecranon fracture, perhaps because the fractures typically occur in a relatively non-articular portion between the coronoid and olecranon articular facets.
Soft tissue contracture
The aetiology of soft tissue contracture about the elbow is multifactorial and incompletely understood. The elbow has the largest capsular capacity in 70° of flexion and any intra-articular effusion, some believe, will cause the patient to assume this elbow position to decrease pressure and pain.8 The capsule responds by thickening, further limiting motion9 (Fig. 28.1B).
In animal models, ligament and capsular trauma has been shown to lead to increased levels of transforming growth factor β and increased numbers of myofibroblasts that have intrinsic contractile properties.10,11 Other changes include increased collagen cross-linking and decreased water content that manifests as thickened and less compliant tissue.12
Musculotendinous units can contribute to stiffness by either limiting excursion or adhering to bone. After trauma and immobilization, both the elbow flexor and extensor musculature will co-contract and be less elastic. In rat models, sarcomere shortening was seen after only 1 week of immobilization.13 Additionally, direct muscle injury may play a role by starting the inflammatory response cascade with possible upregulation of a growth factor that contributes to ectopic ossification around the elbow.14,15 Lastly, spasticity of muscles about the elbow can be seen after central nervous system injury. Electromyography (EMG) studies in head injury patients have shown the elbow flexors to be more spastic than the extensors, which might contribute to flexion contracture.16,17 However, despite all of this research data, the exact pathogenesis remains unclear.
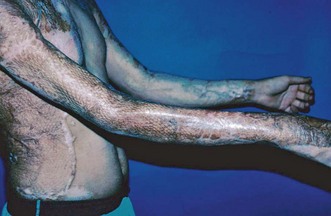
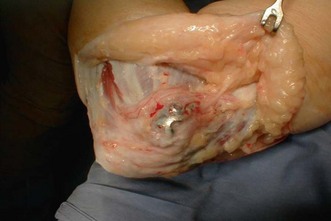
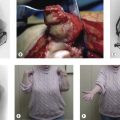
Although contracture of the skin is rarely a problem after closed trauma, it can be a major factor after suffering thermal burns (Fig. 28.3) and may be relevant in congenital aetiologies such as pterygium cubitale where cutaneous webs may create contractures.18 The elbow is the second most common contracture in burn patients, second only to contractures of the shoulder.19
Heterotopic ossification
The elbow is particularly predisposed to HO, but most HO around the elbow does not contribute appreciably to loss of motion. HO is most commonly associated with direct trauma to the elbow and may correlate with its severity: HO is seen in 3% of simple elbow dislocations and up to 20% of fracture elbow dislocations.20,21 Additionally, HO is associated with neural axis trauma with formation seen below the level of spinal cord injury, specifically on the side of hemiplegia.22 The greatest risk, however, is seen in patients with concomitant head and elbow trauma with up to 80% developing HO.23 Thermal injury, organ transplantation, early surgical insult and forced manipulation may also predispose the elbow to HO formation.24
The mechanism of formation of this amorphous bone is believed to involve pluripotent cells that come from the surrounding muscle and differentiate into osteoblasts. Though incompletely understood, trauma and inflammation may start this process. Immature heterotopic bone contains woven bone and mineralized bone surrounded by fibroblasts and skeletal muscle cells.25 Mature heterotopic bone is identical to other lamellar bone but is more metabolically active and does not have a true periosteal layer. This picture is consistent with the hypothesis that myositis ossificans represents fibrous replacement of muscular hematoma, though it has not been reproduced in animal studies.26 Most of the heterotopic bone in the elbow that forms after trauma, severe burns, or central nervous system injury forms between the capsule and muscle.27 Attempts to isolate systemic inductive factors in closed head trauma patients has been unsuccessful to date.1,15
Classification of heterotopic ossification
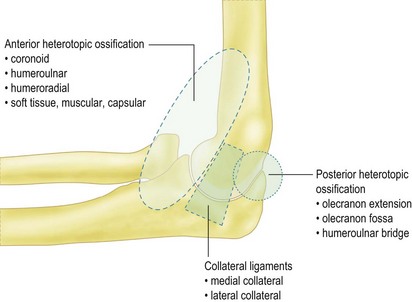
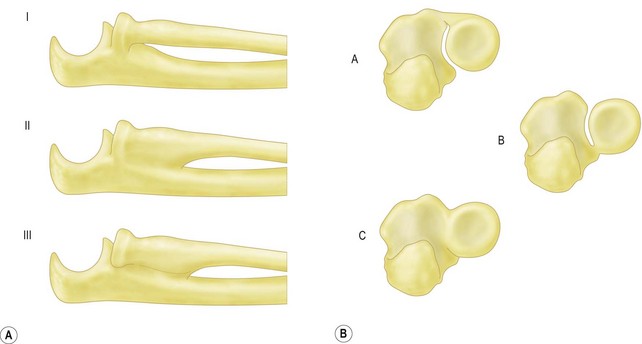
HO can be classified by either its anatomical appearance or its functional effect (Fig. 28.4). In our experience, HO is most commonly seen posteromedially in burn patients and sometimes encases the ulnar nerve. HO can, however be seen in all anatomical distributions about the elbow in these patients and in patients with central nervous system injury.
Hastings and Graham developed a classification based on function and motion. Class I patients have radiographic HO, but no functional limitations. Class II patients have HO that creates some functional limitation but does not cause ankylosis. Class III patients are characterized by ankylosis (Fig. 28.5). Both classification systems have further subclassifications based on the type of motion restriction: flexion–extension (A), pronation–supination (B), or both (C) (Table 28.1).28
Table 28.1 Functional classification of elbow heterotopic ossification
| Class I | Radiographic heterotopic ossification without functional limitations |
| Class II | Radiographic heterotopic ossification with subtotal functional limitations |
| IIA: Limited flexion–extension | |
| IIB: Limited pronation–supination | |
| IIIC: Limited in both planes | |
| Class III | Radiographic and functional ankylosis |
| IIA: Ankylosis in flexion–extension | |
| IIB: Ankylosis in pronation–supination | |
| IIIC: Ankylosis in both planes |
Viola and Hastings classified proximal radioulnar joint (PRUJ) synostosis on the anatomy and surgical approach needed to remove the bone (Fig. 28.6). Type I includes ossification proximally in the PRUJ; type II extends to the bicipital tuberosity; type III is ossification distal in the PRUJ at the level of the bicipital tuberosity. Type I ossification is subtyped into IA, IB and IC based on the need for an anterior or posterior approach, or radial head excision, respectively.27
Patient factors
The results of elbow contracture release seem less predictable in paediatric patients.18 Maturity, direction, motivation and cooperation with postoperative physical therapy may play a role.
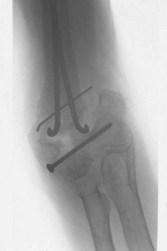
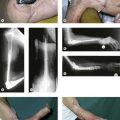
A patient’s intuition or automatic thoughts in response to the pain associated with motion and stretching exercises are an important contributor to elbow stiffness. Patients often feel vulnerable when doing painful stretches after injury or contracture release and those that can adopt the more appropriate mindset of the athletic or healthy stretch seem to do better. It is important to be patient as it can take months to make this transition, and the motion can improve once a patient becomes confident with exercises even if they have been stiff for many months. Entrapment or sensitivity of the ulnar nerve, or injury to a nerve from the initial trauma or surgery can make the exercises even more unpleasant and unsettling and can make painful stretching exercises even more counterintuitive. If a correctable nerve problem – such as ulnar neuropathy exacerbated with flexion exercises – can be identified, operative treatment may be useful (Fig. 28.7).29
Classification
Morrey anatomical classification30
The most commonly used classification system is Morrey’s separation according to factors outside the joint (extrinsic) and factors inside the joint (intrinsic), or both (mixed) (Table 28.2). This simple and anatomically based system is both inclusive and helpful in determining optimal management.
Table 28.2 Morrey’s anatomical classification of elbow stiffness
| Extrinsic causes | Skin |
| Muscle | |
| Capsule (anterior/posterior) | |
| Bone | |
| Non-union | |
| Malunion | |
| Heterotopic ossification | |
| Intrinsic causes | Articular malunion/non-union |
| Adhesions | |
| Loose bodies | |
| Articular malunion/non-union | |
| Mixed |
Extrinsic factors
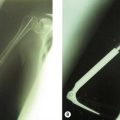
Skin, muscle, capsule, ligament, extra-articular malunions and non-unions, and HO are extrinsic contributors to elbow stiffness. Specifically in the case of a non-union (Fig. 28.8), substantial motion may occur through the fracture site, allowing for capsular and ligament contracture of the adjacent articulation.
Kay classification31
According to the Kay classification of elbow stiffness (Table 28.3), type 1 is soft tissue contracture alone as seen in a burn patient with skin contracture at the antecubital fossa; type 2 combines soft tissue contracture with ossification, but no apparent bony elbow trauma as seen in patients with HO after head injury; type 3 stiff elbows are seen in the setting of a minor articular fracture with resulting soft tissue contracture, which is most commonly seen after a simple radial head fracture; type 4 elbows have displaced intra-articular fractures with soft tissue contracture and ectopic ossification; and type 5 elbows have posttraumatic ossific bars that typically result in ankylosis.
| Type 1 | Soft tissue contracture |
| Type 2 | Soft tissue contracture with ossification |
| Type 3 | Non-displaced articular fracture with soft tissue contracture |
| Type 4 | Displaced articular fracture with soft tissue contracture |
| Type 5 | Posttraumatic bony bar formation |
Simple versus complex stiff elbows
Jupiter et al distinguished the ‘simple’ stiff elbow as characterized by mild to moderate contracture (<80° arc of motion), with no or minimal prior surgery, no prior nerve transposition, no internal fixation, minimal HO and well preserved bony anatomy from the ‘complex’ stiff elbow, that requires more expertise and has a higher surgical complication rate. Most patients with osteoarthritis, rheumatoid arthritis, and osteochondral defects are simple cases of elbow stiffness.32 (Table 28.4).
| Simple elbow contracture | Less than 80° of elbow motion |
| No or minimal prior surgery | |
| No prior nerve transposition | |
| No internal fixation | |
| Minimal heterotopic ossification | |
| Well-preserved bony anatomy | |
| Complex elbow contracture | Multiple prior surgeries |
| Ulnar nerve transposition or dysfunction | |
| Prior internal fixation | |
| Significant eterotopic ossification | |
| Distorted bony anatomy |
1 Kapandji IA, editor. The Physiology of the Joints, 5th ed, Edinburgh: Churchill Livingstone, 1982.
2 Morrey BF, Askew LJ, Chao EY. A biomechanical study of normal functional elbow motion. J Bone Joint Surg (Am). 1981;63(6):872-877.
3 Gordon H. Embryology. In: Morrey BF, editor. The Elbow and its Disorders. Philadelphia: WB Saunders; 2000:1-5.
4 McKee M, Jupiter J, Toh CL, et al. Reconstruction after malunion and nonunion of intra-articular fractures of the distal humerus. Methods and results in 13 adults. J Bone Joint Surg (Br). 1994;76(4):614-621.
5 Shore BJ, Mozzon JB, MacDermid JC, et al. Chronic posttraumatic elbow disorders treated with metallic radial head arthroplasty. J Bone Joint Surg (Am). 2008;90(2):271-280.
6 Broberg MA, Morrey BF. Results of delayed excision of the radial head after fracture. J Bone Joint Surg (Am). 1986;68(5):669-674.
7 Ring D, Hannouche D, Jupiter JB. Surgical treatment of persistent dislocation or subluxation of the ulnohumeral joint after fracture-dislocation of the elbow. J Hand Surg (Am). 2004;29(3):470-480.
8 Gallay SH, Richards RR, O’Driscoll SW. Intraarticular capacity and compliance of stiff and normal elbows. Arthroscopy. 1993;9(1):9-13.
9 Lindenhovius AL, Jupiter JB. The posttraumatic stiff elbow: a review of the literature. J Hand Surg (Am). 2007;32(10):1605-1623.
10 Hildebrand KA, Zhang M, Hart DA. Myofibroblast upregulators are elevated in joint capsules in posttraumatic contractures. Clin Orthop Relat Res. 2007;456:85-91.
11 Hildebrand KA, Zhang M, van Snellenberg W, et al. Myofibroblast numbers are elevated in human elbow capsules after trauma. Clin Orthop Relat Res. 2004;419:189-197.
12 Akeson WH, Amiel D, Abel MF, et al. Effects of immobilization on joints. Clin Orthop Relat Res. 1987;219:28-37.
13 Baker JH, Matsumoto DE. Adaptation of skeletal muscle to immobilization in a shortened position. Muscle Nerve. 1988;11(3):231-244.
14 Hannallah D, Peng H, Young B, et al. Retroviral delivery of Noggin inhibits the formation of heterotopic ossification induced by BMP-4, demineralized bone matrix, and trauma in an animal model. J Bone Joint Surg (Am). 2004;86(1):80-91.
15 Kaplan FS, Glaser DL, Hebela N, et al. Heterotopic ossification. J Am Acad Orthop Surg. 2004;12(2):116-125.
16 Page C, Backus SI, Lenhoff MW. Electromyographic activity in stiff and normal elbows during elbow flexion and extension. J Hand Ther. 2003;16(1):5-11.
17 Wu YN, Park HS, Ren Y, et al. Measurement of elbow spasticity in stroke patients using a manual spasticity evaluator. Conf Proc IEEE Eng Med Biol Soc. 2006;1:3974-3977.
18 Wang AA, Hutchinson DT. Use of the elbow compass universal hinge in pediatric patients. J Pediatr Orthop. 2006;26(1):58-60.
19 Schneider JC, Holavanahalli R, Helm P, et al. Contractures in burn injury: defining the problem. J Burn Care Res. 2006;27(4):508-514.
20 Roberts PH. Dislocation of the elbow. Br J Surg. 1969;56(11):806-815.
21 Thompson HC3rd, Garcia A. Myositis ossificans: aftermath of elbow injuries. Clin Orthop Relat Res. 1967;50:129-134.
22 Stover SL, Hataway CJ, Zeiger HE. Heterotopic ossification in spinal cord-injured patients. Arch Phys Med Rehabil. 1975;56(5):199-204.
23 Garland DE. A clinical perspective on common forms of acquired heterotopic ossification. Clin Orthop Relat Res. 1991;263:13-29.
24 Holguin PH, Rico AA, Garcia JP, et al. Elbow ankylosis due to postburn heterotopic ossification. J Burn Care Rehabil. 1996;17(2):150-154.
25 Friedenstein AJ, Chailakhyan RK, Gerasimov UV. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987;20(3):263-272.
26 Cotran RS, Kumar V, Robbins SL. Traumatic myositis ossificans. In: Cotran RS, Kumar V, Robbins SL, editors. Robbins Pathologic Basis of Disease. Philadelphia: WB Saunders; 1989:1381-1382.
27 Viola RW, Hastings H2nd. Treatment of ectopic ossification about the elbow. Clin Orthop Relat Res. 2000;370:65-86.
28 Hastings H2nd, Graham TJ. The classification and treatment of heterotopic ossification about the elbow and forearm. Hand Clin. 1994;10(3):417-437.
29 Faierman E, Wang J, Jupiter JB. Secondary ulnar nerve palsy in adults after elbow trauma: a report of two cases. J Hand Surg (Am). 2001;26(4):675-678.
30 Morrey BF. Post-traumatic contracture of the elbow. Operative treatment, including distraction arthroplasty. J Bone Joint Surg (Am). 1990;72(4):601-618.
31 Kay NR. Artholysis of the post-traumatic stiff elbow. In: Stanley D, Kay NR, editors. Surgery of the elbow. London: Arnold; 1998:228-234.
32 Jupiter JB, O’Driscoll SW, Cohen MS. The assessment and management of the stiff elbow. Instr Course Lect. 2003;52:93-111.