Chapter 20 Patent Ductus Arteriosus Aortopulmonary Window
In 1593, Giambattista Carcano, Professor of Anatomy in Pavia, an ancient town in northern Italy, described the ductus arteriosus in his book on the great cardiac vessels of the fetus.1 However, Leo Bottali came to be associated with the arterial duct, the duktus arteriosus persisten, even though he misapplied the term to the foramen ovale.1 It was not until Karl von Rokitansky’s handbook of 1844 and his beautifully illustrated monograph of 1852 that patent ductus arteriosus was recognized as a specific congenital malformation.2 The first section of this chapter is concerned with persistent patency of the ductus arteriosus. The second section is devoted to aortopulmonary window, often called aortopulmonary or aorticopulmonary septal defect, an anomaly that is embryologically unrelated to patent ductus but that is physiologically and clinically similar.
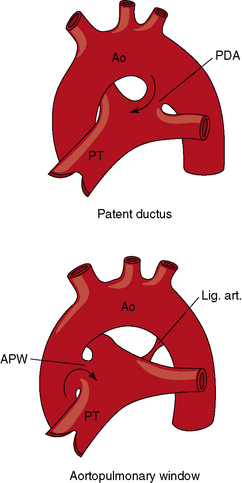
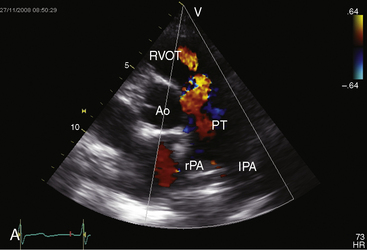
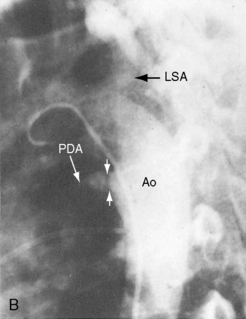
The incidence rate of isolated persistent patency of the ductus has been estimated at 1:2000 to 1:5000 births, or about 10% to 12% of all varieties of congenital heart disease.3 The pulmonary orifice of the ductus is located immediately to the left of the bifurcation of the pulmonary trunk near the origin of its left branch (Figures 20-1 and 20-2). The aortic orifice is located immediately distal to the origin of the left subclavian artery (see Figures 20-1 and 20-2). A patent ductus can be long and narrow or short and wide, with all gradations in between (Figures 20-3, 20-4, and 20-5). Closure consistently begins at the pulmonary arterial end, so the ductus assumes the shape of a truncated cone that is larger at its aortic end (see Figures 20-3 and 20-4).4,5 A widely patent aortic end with a sealed pulmonary end is the substrate for a ductal aneurysm (Figure 20-6).6–9 Patency confined to the pulmonary end is exceptional.10 Anatomic variations include bilateral patent ductus,11,12 left-sided patent ductus with right aortic arch,13 right-sided patent ductus with right aortic arch,14 patent ductus or ligamentum arteriosum as a component of a vascular ring (Figure 20-7),15 and dissection of the aorta with extension into a patent ductus.16
Despite its seeming anatomic simplicity, the ductus arteriosus is a complex structure. The fetal ductus is a major anatomic component of a contiguous intrauterine great arterial system that consists of pulmonary trunk/ductus/aortic continuity that delivers 85% of right ventricular output into the descending aorta.17 Persistent fetal circulation is a designation applied to an intrauterine right-to-left ductal shunt that persists after birth (see Chapter 14).18 Persistent patency of the ductus arteriosus is abnormal and therefore undesirable, although certain forms of congenital heart disease depend for survival on neonatal ductal patency. Ductal dependent circulations include malformations in which a patent ductus is the only source of pulmonary arterial blood flow (pulmonary atresia with intact ventricular septum), the only source of systemic arterial blood flow (aortic atresia or complete interruption of the aortic arch), or the only source of bidirectional blood flow (simple complete transposition of the great arteries; see relevant chapters).
The ductus arteriosus is derived from the sixth aortic arch. By the fourth month of gestation, ductal tissue has become distinctive, differing histologically from pulmonary arterial and aortic tissue.19 At 16 weeks of gestation, the ductus consists of a muscular arterial channel with an endothelium separated by an internal elastic lamina and a thin subendothelial layer.19 The media differs at the aortic and pulmonary ends, so ductal media can be aortic, pulmonary, or mixed.4 As gestation continues, the intima thickens, and the subendothelial layer is invaded by cells from the media that disrupt the internal elastic lamina. At term, the mature ductus harbors conspicuous intimal cushions that protrude into the lumen. The ductus is then is capable of contraction, functional closure, which is followed by anatomic closure that uniformally begins at the pulmonary arterial end (see previous).4 Anatomic closure follows a sequence of immunohistochemical and ultrastructural changes, namely4,19: (1) separation of endothelium from internal elastic lamina; (2) enfolding and ingrowth of endothelial cells; (3) migration of undifferentiated medial smooth muscle cells into the subendothelium; (4) fragmentation of the internal elastic lamina; (5) sealing of the lumen by endothelial cell apposition; (6) accumulation of lipid droplets; and (7) intimal and subendothelial degenerative changes that spread centrally and peripherally and result in disappearance of endothelial cells at luminal apposition lines.4 The normal process of functional closure begins within 10 to 15 hours after birth and is virtually complete (probe patent) by the second week of extrauterine life. The ductus is an anatomically closed ligamentum arteriosum 2 to 3 weeks after birth.5,17,20 When a ductus is destined to remain patent, the intrauterine subendothelial internal elastic lamina lies adjacent to the intimal cushions, endothelial cells adhere to the elastic lamina, and subendothelial edema with enfolding of endothelial cells does not occur.4,19 A ductus that remains patent in full-term infants after 3 months of extrauterine life harbors the histologic features of persistent patency just described. Spontaneous closure is then unlikely.19,21,22
In utero ductal tone is determined by an interplay between the constricting effect of oxygen (relatively weak because of low fetal pO2) and the dilating effect of endogenous prostaglandin E2.23,24 Prostaglandin synthetase inhibitors administered to mammalian fetuses or to pregnant ewes constrict the fetal ductus and deprive the fetal right ventricle of its only outlet.24 As term approaches, the ductus becomes less responsive to prostaglandin E2 and more responsive to oxygen, setting the stage for constriction that begins a few hours after birth in full term infants.25 Functional closure is closely coupled to the increase in extrauterine ambient oxygen tension that exerts a direct constricting effect on the ductal wall (see previous). Oxygen-induced constriction has been related to inhibition of voltage-gated potassium channels.26 Flow through the closing ductus is transiently bidirectional. Left-to-right flow25 then decreases rapidly during the next 12 hours and cannot be detected at 48 hours.27 Anatomic closure is the culmination of morphologic changes accrued during intrauterine ductal maturation (see previous).4,19,28 In addition, apoptosis and smooth muscle cell proliferation have been assigned a role in anatomic closure.29
In healthy preterm infants, delayed closure of the ductus arteriosus is common.30–33 Premature neonates with a gestational age of 30 weeks or more usually experience spontaneous ductal closure within a time frame that corresponds to the closure time in full-term infants.31,32 In full-term infants, spontaneous closure is unlikely after 3 months of age,21,22 and in premature infants, it is unlikely after 1 year of age (Figure 20-8).22 Exceptional examples of spontaneous closure have been documented between 5 and 6 years of age, between 7 and 14 years,34 after 17 years,35 and at age 19 years.
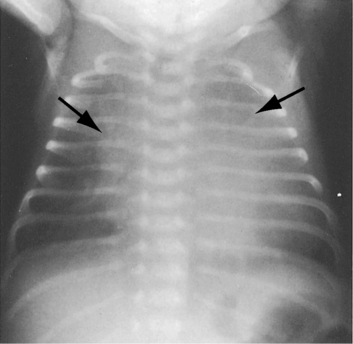
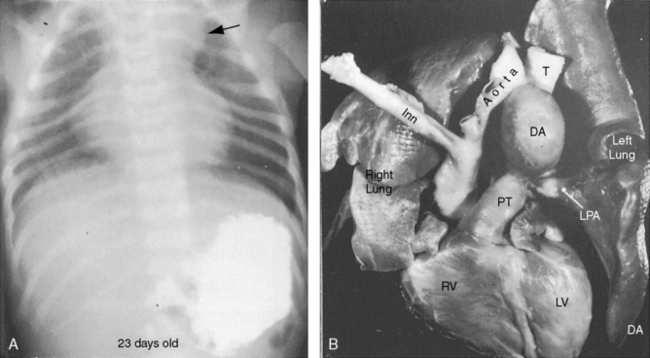
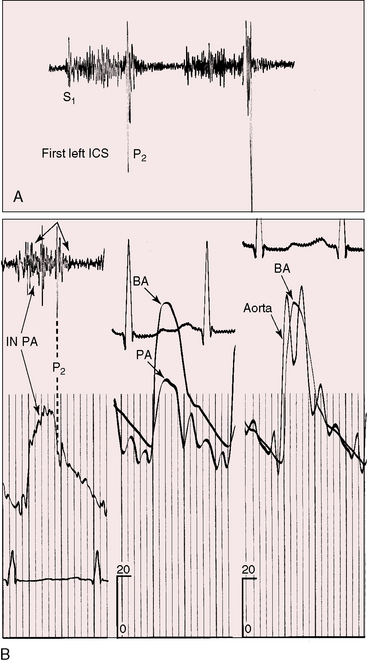
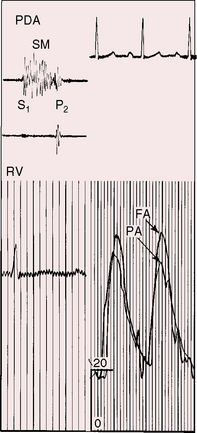
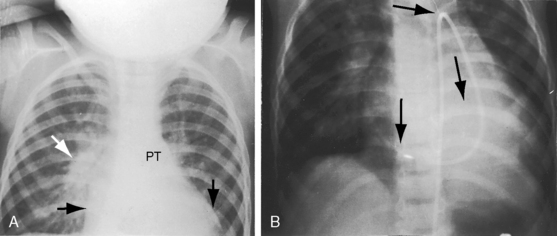
Figure 20-8 X-ray from the 21-day-old premature male with a widely patent ductus arteriosus. The phonocardiogram is shown in Figure 20-10. Pulmonary blood flow is increased, the heart is considerably enlarged, and a thymus (arrows) obscures the base. By age 4 months, the ductus had spontaneously closed, the thymus had disappeared, and the x-ray was virtually normal.
Persistent patency of the ductus in premature infants sometimes coincides with respiratory distress, but the distress may not improve with subsequent ductal closure.31,32,36 Patent ductus in preterm infants is associated with reduced cerebral blood flow from a steal effect caused by the aortic-to-pulmonary shunt, rather than by a limited capacity of the preterm left ventricle to achieve adequate cardiac output.37,38
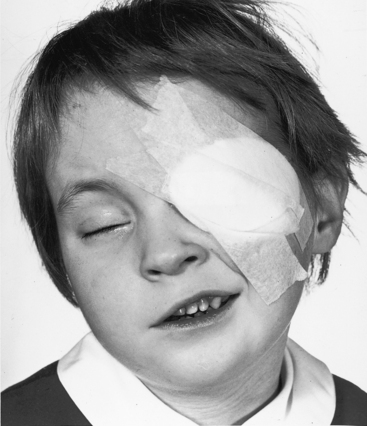
First-trimester maternal rubella with rash carries an 80% incidence rate of intrauterine viral infection39; deafness and cataracts (Figure 20-9). Congenital heart disease affects two thirds of offspring. Patent ductus arteriosus accounts for a third of the congenital malformations22 and is characterized by maturational arrest and an immature ductal wall of the type found at 16 weeks of gestation (see previous).22
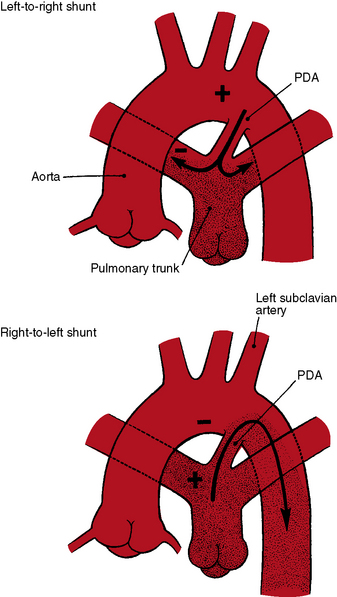
The physiologic consequences of persistent patency of the ductus arteriosus depend on five variables: (1) the size of the ductus; (2) pulmonary vascular resistance; (3) the adaptive response of the left ventricle to volume overload; (4) prematurity; and (5) respiratory distress. When the ductus is restrictive, pulmonary vascular resistance is normal, right ventricular afterload is normal, and the hemodynamic consequences are negligible. When the ductus is moderately restrictive and pulmonary vascular resistance is normal or nearly so, right ventricular afterload is not significantly affected and continuous aortic-to-pulmonary flow imposes only a moderate volume load on the left ventricle. About 95% of isolated patent ductuses are restrictive or moderately restrictive. When the ductus is nonrestrictive, systolic pressure in the aorta and pulmonary trunk equalize at systemic level, so the direction of blood flow depends on the relative resistances in the systemic and pulmonary vascular beds.40 If pulmonary resistance is lower than systemic, a left-to-right shunt is established, imposing volume overload on the left ventricle while right ventricular afterload remains at systemic level (see Figure 20-2, upper). When pulmonary vascular resistance exceeds systemic, the shunt is reversed (see Figure 20-2, lower). Volume overload of the left ventricle is then curtailed, pressure overload of the right ventricle remains at systemic level, and the pulmonary vascular bed exhibits histologic changes similar to primary pulmonary hypertension or Eisenmenger’s syndrome (see Chapters 14 and 17).35,41
history
A newborn is typically pronounced healthy and discharged as a well baby. As neonatal pulmonary vascular resistance falls, a left-to-right shunt is established, the ductus murmur emerges, and the diagnosis becomes apparent. Less commonly, a neonate comes to attention because of low birth weight, systemic hypoperfusion, or congestive heart failure without an incriminating ductus murmur (Figure 20-10).33,42 Absence of a murmur does not necessarily mean that the ductus has closed.17 A ductus can be patent but silent because of the direction of the jet as it enters the pulmonary artery.43 No correlation has been found between the presence of a murmur and the size of the arterial duct.43 Doppler echocardiography occasionally detects a tiny patent ductus in infants without auscultatory signs of its presence,44 or auscultation detects a tiny ductus in adults in whom the diagnosis had been missed (see Figure 20-40). Closure of a patent ductus is occasionally the result of healed infective endocarditis45,46 or thrombotic occlusion.47,48
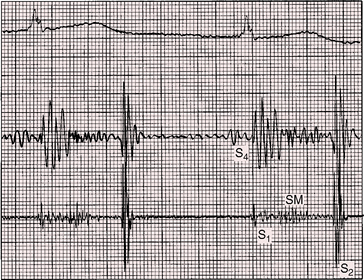
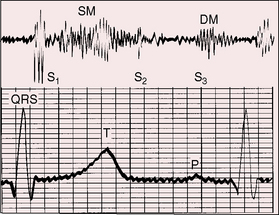
Figure 20-10 Phonocardiograms at the second left intercostal space in a 21-day-old premature male with a nonrestrictive patent ductus arteriosus (see x-ray in Figure 20-8). The low-frequency filter (upper tracing) shows a fourth heart sound (S4). The high-frequency filter (lower tracing) shows a normal first heart sound (S1) and an unimpressive early to midsystolic ductal murmur (SM) that fades before a loud single second heart sound (S2).
In 1561, Vesalius described a valve or membrane in a patent ductus arteriosus.49 A valve-like structure was subsequently found in stillborn human fetuses and in newborn rabbits,49 and in 1903, a necropsy report called attention to a perforated ductal valve.50 Taussig51 confirmed the presence of a membranous valve at the pulmonary end of a ductus and theorized that rupture might account for the sudden appearance of a ductus murmur, an event occasionally witnessed in children or young adults. A continuous murmur intermittently appeared and disappeared in a patient with a veil-like valve at the pulmonary end of a ductus,52 and the abrupt appearance of a loud continuous murmur was described in a 55-year-old man with a ductal membrane.53 Rarely, a closed lumen is reopened by spontaneous intramural dissection of a ductal aneurysm or by propagation of aortic dissection into the ductus.16
Patent ductus arteriosus predominates in females, with a gender ratio of 2 or 3 to 1.35 Female prevalence is even greater in older patients.54 There is a tendency for recurrence of patent ductus in siblings55–57 and in the offspring of parents with patent ductus.55 Familial recurrence has been reported in three generations of a single family.58 Identical twins may both have a patent ductus, or the ductus may be patent in only one twin. Canine patent ductus is more common in females and can be hereditary.59
In offspring of gravida with maternal rubella, patent ductus arteriosus and pulmonary artery stenosis coexist as congenital malformations (see Chapter 11).60–62 Maternal rubella resulted in patent ductus arteriosus in one of a twin pair; the other twin had pulmonary artery stenosis. Low birth weight and failure to thrive are features of the rubella syndrome, even if the ductus is restrictive. A seasonal incidence of patent ductus in late winter and early spring coincided with the peak incidence of rubella.62
Persistent patency of the ductus arteriosus is about six times more prevalent in high-altitude locations than in sea-level locations.63 A predilection for increased pulmonary vascular resistance is a feature of high-altitude births with patent ductus.63 The predilection exists even when the ductus is restrictive.63
Congestive heart failure is the most common cause of death directly related to patent ductus.35 Rarely, death is from dissection or rupture of a ductal aneurysm64 or from rupture of a hypertensive aneurysmal pulmonary trunk.65,66 Aneurysm of a nonpatent ductus (see Figure 20-6) can be complicated by rupture, by spontaneous intramural dissection, by systemic embolism, by infection, by recurrent laryngeal nerve paralysis, by compression of the pulmonary trunk, or by hemorrhagic erosion into the esophagus or tracheobronchial tree.8,9
Infective endarteritis occurs with a restrictive patent ductus because of the high-velocity left-to-right shunt but does not occur with a nonrestrictive ductus and reversed shunt.35 The infection is located at the narrow pulmonary arterial end of the ductus or at the site of an intimal jet lesion in the pulmonary artery opposite the ductus. Susceptibility has not been determined for a tiny clinically silent ductus detected only with Doppler echocardiography (see Figure 20-40; see previous).67
Abnormal patterns of cerebral arterial blood flow in infants, especially preterm neonates with a nonrestrictive patent ductus, predispose to central nervous system ischemia and hemorrhage into the germinal matrix.38,68,69 Increased pulse pressure and major fluctuations of blood flow velocity caused by opening and closing of a ductus may rupture capillaries of the germinal matrix and cause intraventricular hemorrhage. A sharp decrease in diastolic arterial flow velocity can act as a steal from the cerebral circulation.68
After the first year of life, most patients with patent ductus arteriosus are asymptomatic. Beginning with the second decade, the risk of infective endarteritis exceeds the risk of congestive heart failure.35,54,70 In the third decade, more and more patients with a moderately restrictive ductus experience heart failure,35,54 and those with a restrictive ductus remain asymptomatic. A 20-year-old man with a patent ductus had been a cross-country runner, and an active schoolmistress died at the age of 85 years because of gastrointestinal bleeding. A number of reports have called attention to survival beyond age 60 years (see Figure 20-16)35,71–75: an elderly woman presented with biventricular failure in her 81st year,76 and a patient died at 90 years of age.77
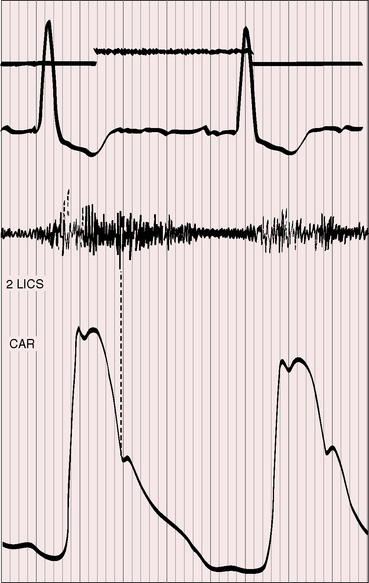
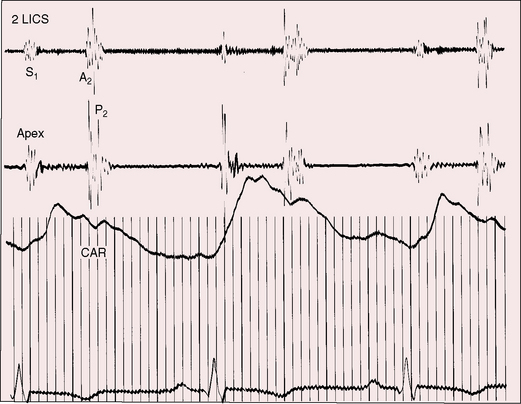
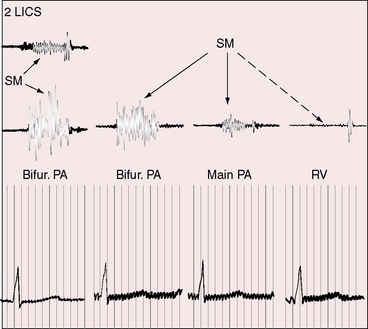
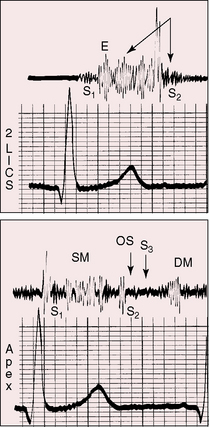
Figure 20-16 Tracings from an 84-year-old woman with a moderately restrictive patent ductus arteriosus (see x-ray in Figure 20-38). The murmur in the second left intercostal space (2 LICS) continues through the second heart sound that was timed by the dicrotic notch of the carotid pulse (CAR). The continuous murmur then faded, rendering late diastole murmur-free.
A nonrestrictive patent ductus with Eisenmenger’s syndrome is accompanied by the multisystem systemic disorders of cyanotic congenital heart disease (see Chapter 17).78,79 Isotonic exercise with an Eisenmenger’s ductus causes leg fatigue without dyspnea because an exercise-induced increase in right-to-left shunt is channeled into the descending aorta (Figures 20-2 and 20-11) distal to the respiratory center and the carotid body, precluding hypoxia-induced stimulation.78–81 Hypertrophic osteoarthropathy is confined to the lower extremities.82–84 In an Eisenmenger’s ductus, left ventricular failure is absent because volume overload of the left heart is curtailed. A dilated hypertensive pulmonary trunk may cause hoarseness by compressing the recurrent laryngeal nerve. Angina and syncope are not features of nonrestrictive patent ductus with reserved shunt because right ventricular pressure cannot exceed systemic.79 Cyanosis is missed if the feet are not examined (see section Physical Appearance). A young girl came to attention because she noticed that when she sat in a warm bath, her toes were blue but her fingers were pink.
Constriction or closure of the fetal ductus deprives the right ventricle of its only outlet, so neonates present with massive tricuspid regurgitation and right-to-left interatrial shunts.24 Salicylates cause constriction of the fetal ductus, so the history should include enquiries about maternal use of aspirin. Salicylate levels can be determined on umbilical cord blood.85
Physical appearance
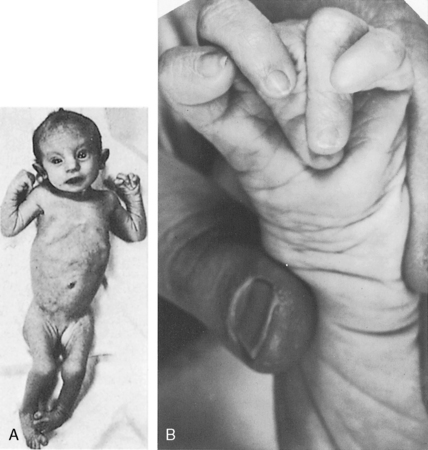
Maternal rubella is characterized by low birth weight and failure to thrive, irrespective of ductal patency, ductal size, or shunt volume.86,87 An underdeveloped child with a patent ductus should therefore be examined for cataracts, deafness, and mental retardation (see Figure 20-9).86,87 Another distinctive phenotype is the clinodactly (overlapping fingers), rocker bottom feet, and lax skin of Trisomy 18 (Figure 20-12).88,89 Char syndrome is an inherited disorder that maps to chromosome 6p12-p21 and is characterized by ptosis, a flat profile, a very short philtrum, patulous duck-bill lips, facial dysmorphism, and abnormalities of the hands.90–93 The recurrence rate in offspring of an affected parent is 50%.92
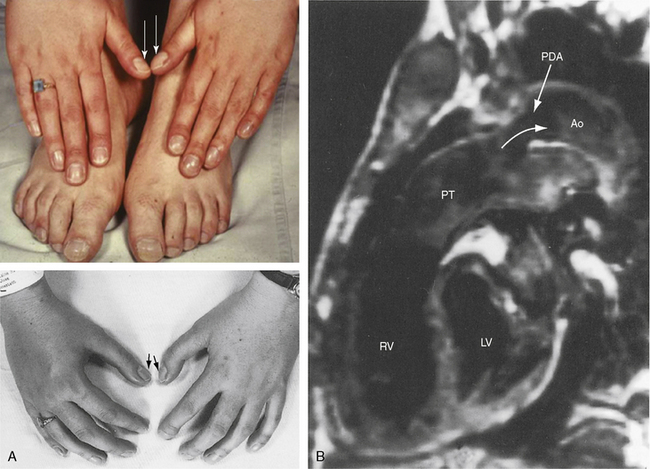
Differential cyanosis and clubbing are important physical signs of patent ductus with reversed shunt (see Figures 20-2 and 20-11; see previous).79,94 The toes are cyanosed and clubbed because unsaturated blood is selectively delivered to the lower extremities. A small amount of unsaturated blood often enters the left subclavian artery, so the digits of the left hand, especially the thumb, are mildly cyanosed and clubbed (see Figure 20-11 A). The fingers of the right hand are normal because unsaturated blood does not reach the right subclavian artery. In the rare presence of bilateral patent ductuses with reversed shunt, the right arm is cyanosed because the right subclavian artery receives desaturated blood from the pulmonary artery via the right ductus arteriosus.12
Patients who are old enough to follow instructions should be examined sitting or squatting with their hands placed alongside their feet or on the dorsum of their feet to facilitate comparison of fingers and toes (see Figure 20-11A). The right and left thumbs should be compared (see Figure 20-11A). Differential cyanosis is exaggerated by isotonic exercise or by warming the hands and feet, which are maneuvers that increase skin blood flow and exaggerate the color differences. In neonates with persistent fetal circulation, the right-to-left ductal shunt may cause distinctive differential cyanosis confined to the head, right shoulder, and right arm with a demarcation line that runs obliquely from above the left shoulder to below the right axilla.18
Arterial pulse
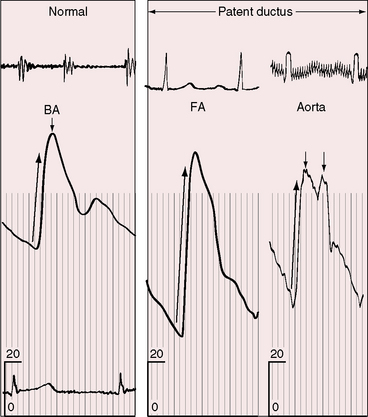
Wide systemic pulse pressure is an important physical sign of patent ductus arteriosus with a large left-to-right shunt.40,94 This sign is especially useful in symptomatic neonates without a ductus murmur. However, the arterial pulse may be weak in preterm infants in whom systemic flow is reduced by the steal effect associated with aortopulmonary shunting (see previous).37,38 The typical pulse is characterized by a brisk rise, a single or bisferiens peak, and a rapid collapse (Figure 20-13). Diastolic flow from aortic root into pulmonary trunk lowers the aortic diastolic pressure, and a large left ventricular stroke volume with forceful left ventricular contraction maintains or elevates aortic systolic pressure. The carotid, brachial, femoral, and even dorsalis pedis pulses can be bounding.94 The superficial palmar arch as it crosses the heads of the metacarpals is sometimes evident as a visible pulsation. In a child with a large patent ductus, an erythematous wheal caused by a mosquito bite blushed and blanched synchronously with the pulse.95
Precordial movement and palpation
George A. Gibson96 wrote, “When the ductus arteriosus is permanently patent, a very distinct thrill is to be felt—a thrill which distinctly follows the systole of the heart and persists until the diastolic phase has existed for some time.”2
Auscultation
In 1900, Gibson96 characterized the murmur of patent ductus arteriosus:“It persists through the second sound and dies away gradually during the long pause. The murmur is rough and thrilling. It begins softly and increases in intensity so as to reach its acme just about, or immediately after the incidence of the second sound, and from that point gradually wanes until its termination.”
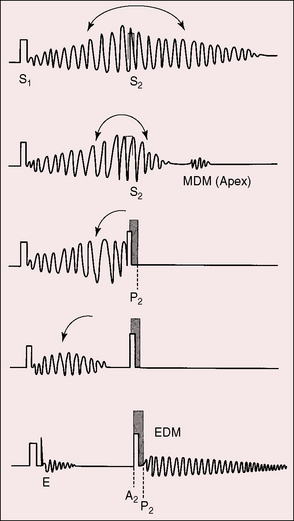
Gibson’s description cannot be improved on. Although he was not the first to describe the continuous murmur of patent ductus, he precisely characterized the murmur and confidently established the clinical diagnosis based on that characterization.2 The classic murmur of uncomplicated patent ductus arteriosus rises to a peak in latter systole; continues without interruption through the second sound, which it envelops; and then declines in intensity during the course of diastole (Figures 20-14, 20-15, and 20-16). The murmur can occupy the entire cardiac cycle (see Figure 20-14), or the end of diastole or early systole can be murmur-free (see Figure 20-15). The term continuous is best applied to the uninterrupted progression of the murmur through the second heart sound and not to the presence of murmur throughout the cardiac cycle.94 The ductus murmur is therefore considered continuous even when late diastole and early systole are murmur-free.
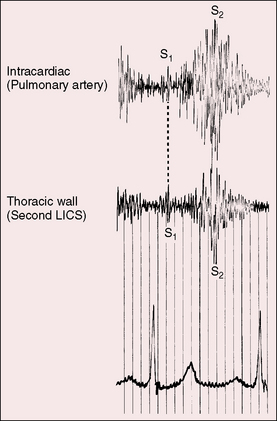
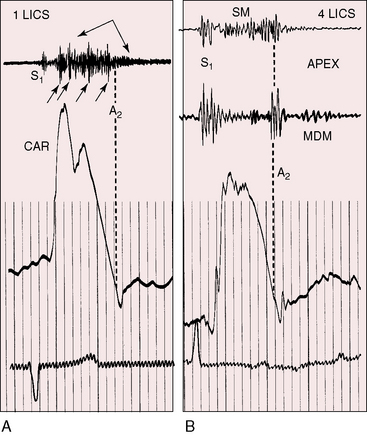
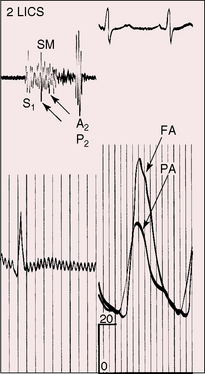
Figure 20-14 Simultaneously recorded phonocardiograms from within the pulmonary artery and on the thoracic wall at the second left intercostal space of a 7-year-old girl with a moderately restrictive patent ductus arteriosus and a 2 to 1 left-to-right shunt. In Gibson’s words, the murmur “begins softly and increases in intensity so as to reach its acme just about, or immediately after the incidence of the second heart sound, and from that point gradually wanes until its termination.”96
High-velocity flow through a restrictive ductus generates a relatively soft high-frequency continuous murmur. A moderately restrictive ductus generates a loud coarse machinery murmur punctuated with eddy sounds that are randomly distributed in the second half of systole and in the first half of diastole (Figure 20-17A).44,94 Intracardiac phonocardiography records the maximal intensity of the ductus murmur in the pulmonary artery at the pulmonary ostium of the ductus (see Figure 20-14). Intraoperative phonocardiograms from the surface of the pulmonary trunk are in accord with this localization,97 and these observations coincide with the chest wall position of the ductus murmur, which is loudest in the first or second intercostal space or beneath the left clavicle as Gibson originally stated.2
Occasionally, a short large ductus is devoid of murmur despite a substantial left-to-right shunt (Figure 20-18). Ductal murmurs are often absent in infants with a nonrestrictive patent ductus and congestive heart failure (see Figure 20-10) and in a significant number of preterm infants with respiratory distress.98 Failure to detect a murmur, a silent but patent ductus, does not necessarily mean that the ductus has closed (see section The History).17,43,99 A relatively rare auscultatory variation is intermittent disappearance and reappearance of an otherwise typical continuous ductal murmur.52,100,101 Interruption of flow has been ascribed to acute angulations of an elongated ductus100,101 or to a valve or veil-like structure within the ductus (see previous).52 A similar mechanism has been proposed for the transient diastolic ductal murmur of the neonate.102 Reappearance of a continuous murmur long after ductal closure has been ascribed to reopening caused by a tear in the valve of the ductus50 or to spontaneous intramural dissection.
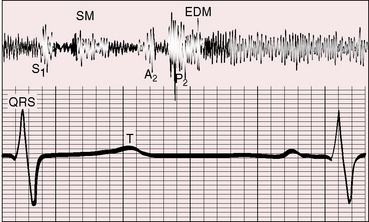
The shape, length, and timing of the murmur of patent ductus arteriosus depend on instantaneous differences in pressure and flow between the aorta and pulmonary trunk (see Figure 20-15).103,104 At the beginning of systole, flow into the pulmonary artery is derived from the right ventricle rather than the ductus. During the course of systole, the flow contribution from the ductus progressively increases, and during diastole, flow into the pulmonary artery is from the ductus alone.94,105 Systolic reinforcement of the ductus murmur described by Skoda occurs because flow from aorta into pulmonary trunk is greater in systole, especially when the systemic pulse pressure is wide, and because systolic flow from right ventricle into pulmonary artery is reinforced by simultaneous flow from the ductus, whereas diastolic flow is derived from the ductus alone. As pulmonary vascular resistance rises, the pulmonary arterial and aortic diastolic pressures equalize (Figure 20-19) and diastolic ductal flow diminishes and finally vanishes, so the diastolic portion of the continuous murmur disappears, leaving a holosystolic murmur (Figures 20-15, 20-20, and 20-21). With a further increase in pulmonary vascular resistance, the systolic portion of the ductus murmur shortens (see Figures 20-15 and 20-21) and ultimately disappears altogether (see Figure 20-15). The ductus is then silent because right-to-left ductal flow, a reversed shunt, does not generate a murmur.105 The classic Gibson murmur is then replaced by auscultatory signs of pulmonary hypertension: namely, a short pulmonary midsystolic murmur introduced by an ejection sound, a single or closely split second heart sound, a loud pulmonary component, and the Graham Steell murmur of hypertensive pulmonary regurgitation (Figures 20-15 and 20-22).105 The diagnosis of patent ductus arteriosus cannot be based on auscultatory signs but can confidently be based on differential cyanosis (see Figure 20-11; see section Physical Appearance).
In the newborn, a transient soft crescendo systolic murmur from left-to-right flow through the ductus is sometimes detected before normal physiologic closure.106–108 The murmur ends with the second sound or continues just beyond it.106 These harmless transient neonatal murmurs are physiologically analogous to the not so harmless murmurs that appear when patent ductus arteriosus is subsequently accompanied by a rise in pulmonary vascular resistance. Even when a ductus is destined to remain patent, the neonatal ductal murmur is initially systolic and becomes continuous only after pulmonary vascular resistance has fallen sufficiently to permit both systolic and diastolic ductal flow.
When the left-to-right shunt is large, apical mid-diastolic murmurs are generated by increased flow across the mitral valve.105 These murmurs cannot not heard unless the diastolic portion of the continuous murmur is attenuated by an increase in pulmonary vascular resistance (Figures 20-15, 20-17, and 20-23).105 In patent ductus arteriosus with reversed shunt, a mid-diastolic murmur has been attributed to a right-sided Austin Flint mechanism associated with pulmonary regurgitation.109 To-and-fro murmurs over the cranium of infants with a nonrestrictive patent ductus arteriosus have been ascribed to accelerated forward flow followed by rapid diastolic runoff.
The second heart sound is occasionally paradoxically split when the left-to-right shunt is large.110 Prolonged left ventricular ejection and short right ventricular ejection are held responsible.40,110 A loud continuous murmur punctuated by eddy sounds obscures the second heart sound. An increase in pulmonary vascular resistance renders the second sound audible because the diastolic portion of the continuous murmur softens or disappears (see Figures 20-15, 20-17, and 20-19). When the shunt is reversed, the second sound is single or closely split, and the pulmonary component is loud (see Figure 20-15). The second sound is widely split when depressed right ventricular contractility and prolonged right ventricular ejection delay the pulmonary component (see Figures 20-22 and 20-23).
Electrocardiogram
A moderately restrictive patent ductus arteriosus with increased pulmonary blood flow is accompanied by a prolonged bifid left atrial P wave in one or more limb leads and in right precordial leads (Figures 20-24 and 20-25). The PR interval is prolonged in 10% to 20% of cases (see Figure 20-24).111 Atrial fibrillation occurs in older patients. The QRS axis is usually normal, but an occasional infant has right axis deviation, especially neonates with respiratory distress. Rare examples of left axis deviation have been reported.112 The rubella syndrome may be associated with an unusually superior QRS axis directed upward and either to the left or right.113
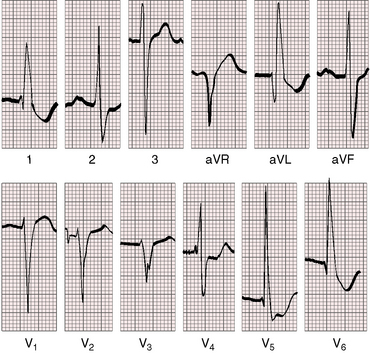
Figure 20-24 Electrocardiogram from a 37-year-old woman with a moderately restrictive patent ductus arteriosus, a 2.8 to 1 left-to-right shunt, and a pulmonary arterial pressure of 48/18 mm Hg (see x-ray in Figure 20-32). There is a broad bifid left atrial P wave in lead 2. The PR interval is 200 msec. The QRS is prolonged, and the axis is horizontal. Left ventricular volume overload is manifested by prominent q waves in leads aVL and V6 and by tall R waves and ST-T wave changes in lead aVL and in leads V4-6.
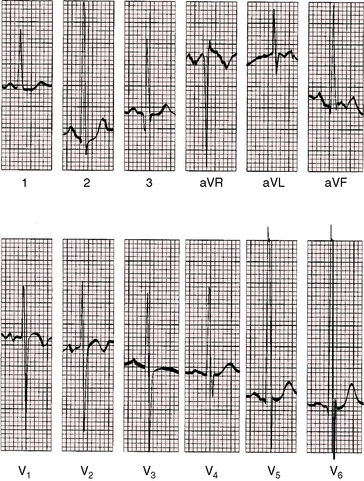
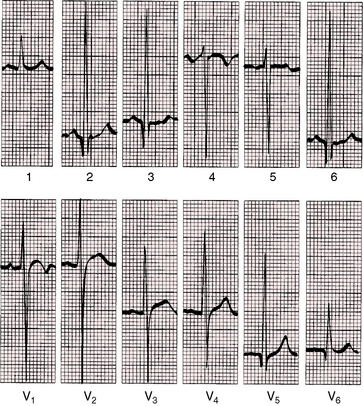
Figure 20-25 Electrocardiogram from a 19-month-old male with a moderately restrictive patent ductus, a 3 to 1 left-to-right shunt, and a pulmonary arterial pressure of 45/22 mm Hg (see x-ray in Figure 20-31). There are biphasic left atrial P waves in leads V1-2. Because the QRS axis is normal, the tall R waves of left ventricular volume overload appear in leads 2 and aVF. Volume overload of the left ventricle is also manifested by tall R waves and upright T waves in leads V5-6. Right ventricular hypertrophy is manifested by prominent S waves in leads V5-6 and a prominent R wave in lead V1. Leads V2-3 are half standardized and exhibit large RS complexes of biventricular hypertrophy.
A nonrestrictive patent ductus with low pulmonary vascular resistance is associated with biatrial P waves and combined ventricular hypertrophy. Large equidiphasic RS complexes appear in most if not all precordial leads, with tall R waves and prominent S waves in leads V5-6 (Figures 20-25 and 20-26). Volume overload of the left ventricle is responsible for tall R waves, prominent q waves, and tall peaked T waves in leads V5-6 (see Figures 20-25 and 20-26).
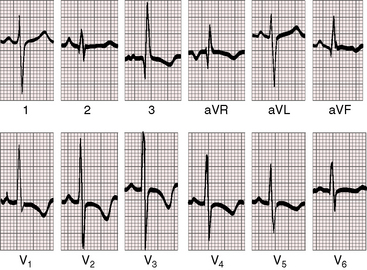
Patent ductus with pulmonary vascular disease and reversed shunt is accompanied by peaked right atrial P waves in leads 2, 3, and V1 (Figure 20-27). Right ventricular hypertrophy is manifested by right axis deviation, tall R waves in lead V1, and inverted right precordial T waves and is prominent in left precordial leads S waves (see Figure 20-27). R waves in leads V5-6 imply that a left-to-right shunt previously existed (see Figure 20-27).
X-ray
The ductus itself is occasionally seen in the frontal projection as an inconspicuous soft convexity between the aortic knob and the pulmonary artery segment (Figures 20-5A and 20-28).114 A striking exaggeration of this inconspicuous shadow is an aneurysm of a nonpatent ductus (see Figure 20-6). Calcium appears in the ductus of older patients (Figure 20-29; see Figure 20-38A).115
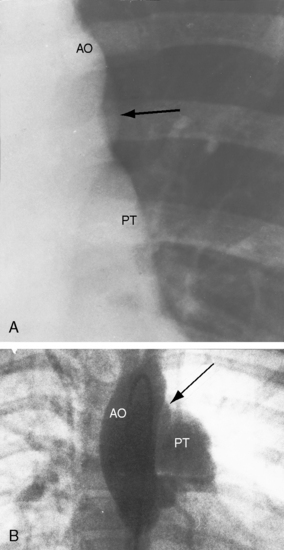
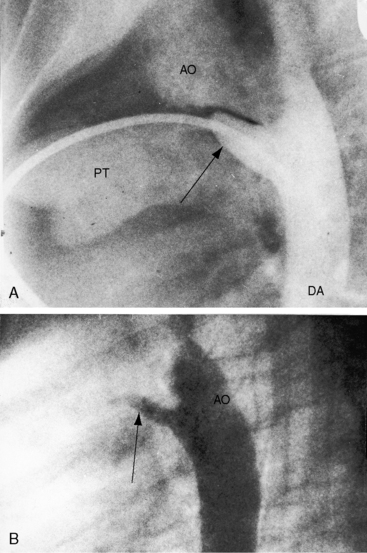
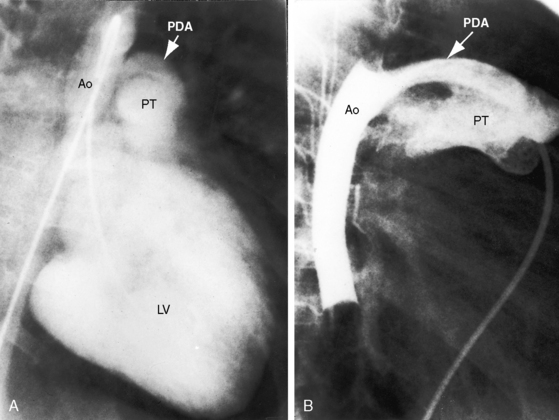
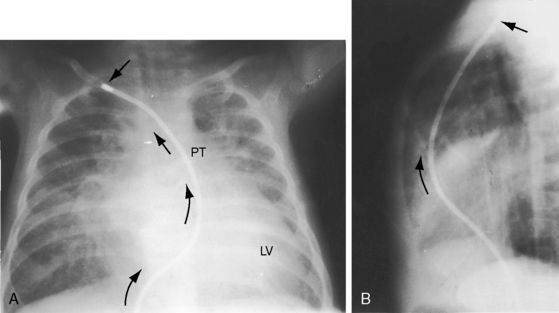
Figure 20-28 A, Close-up of a portion of the chest x-ray in the 14-year-old girl whose aortogram is shown in Figure 20-3. The soft convex shadow of the patent ductus (arrow) is located between the aortic knuckle (AO) and pulmonary trunk (PT). The convex shadow represents the dilated aortic end of the conical ductus shown in the aortogram of Figure 20-3. B, Aortogram in a 5-year-old girl. The convex shadow of a conical ductus (arrow) located between the aortic knuckle (AO) and the pulmonary trunk (PT). Compare with Figure 20-5.
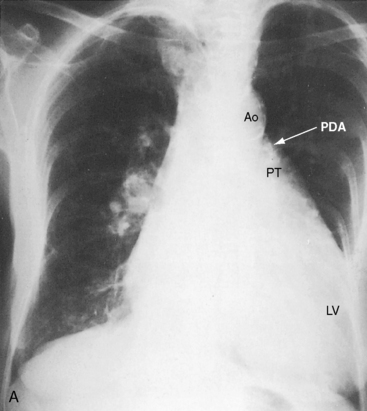
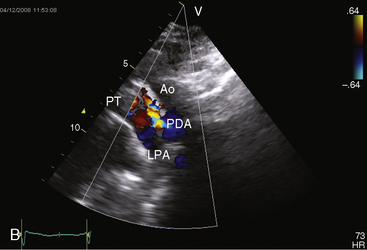
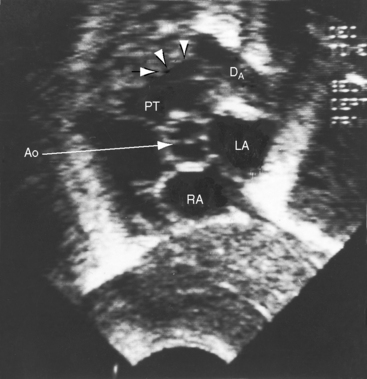
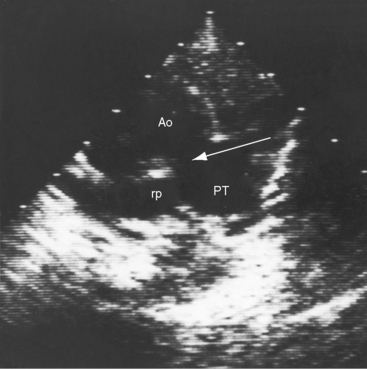
Figure 20-38 A, X-ray from the 84-year-old woman with a moderately restrictive patent ductus arteriosus (PDA) whose phonocardiogram is shown in Figure 20-16. The pulmonary trunk (PT) and its right branch are dilated. A thin rim of calcium appears in the transverse aorta (Ao). An enlarged left ventricle (LV) occupies the apex, and an enlarged right atrium occupies the lower right cardiac border. B, Notch view of ductal flow going from aorta (Ao) to pulmonary trunk (PT) (Video 20-1). (LPA = left pulmonary artery.)
A moderately restrictive patent ductus with low pulmonary vascular resistance results in increased pulmonary arterial vascularity, enlargement of the pulmonary trunk and its proximal branches, and enlargement of the left atrium and left ventricle (Figures 20-30, 20-31 and 20-32).114 Asymmetric pulmonary vascularity is occasionally represented by a hyperlucent left lung.116 In infants, the ascending aorta is inconspicuous because intrauterine ductal flow does not traverse the aorta (see Figures 20-5 and 20-30). After birth, left-to-right ductal flow recirculates through the aortic root, so the ascending aorta becomes prominent (see Figure 20-3).117 In infants and children with a nonrestrictive patent ductus and low pulmonary vascular resistance, pulmonary arterial vascularity is markedly increased and all four cardiac chambers are enlarged (Figure 20-33).
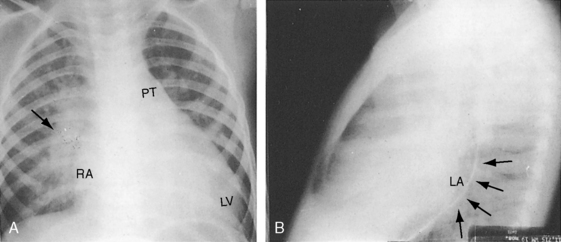
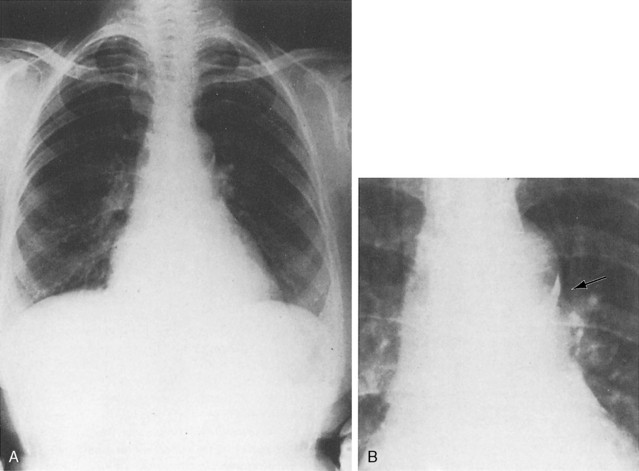
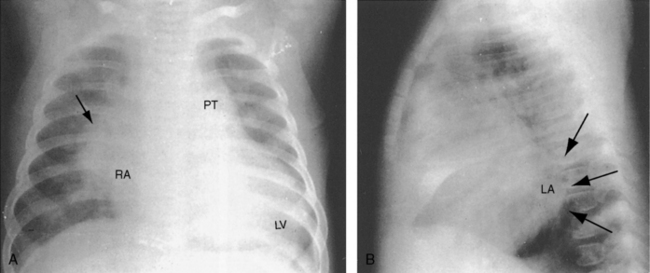
Figure 20-31 X-rays from a 19-month-old male with a nonrestrictive patent ductus and a 3 to 1 left-to-right shunt. The electrocardiogram is shown in Figure 20-25. A, The increased pulmonary vascularity is both arterial and venous. The pulmonary trunk (PT) and its right branch (arrow) are dilated. The apex is formed by an enlarged left ventricle (LV), and the right cardiac border is formed by a dilated right atrium (RA). B, The lateral barium esophagram outlines a moderately enlarged left atrium (LA).
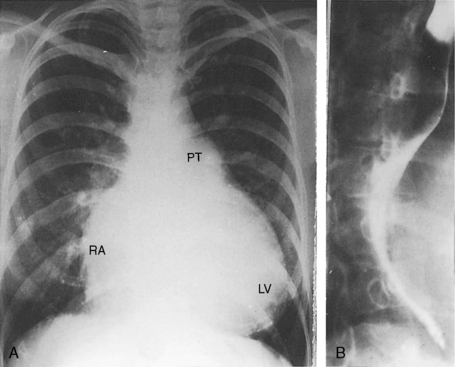
Figure 20-32 A, X-rays from a 37-year-old woman with a moderately restrictive patent ductus and a 2.8 to 1 left-to-right shunt. The electrocardiogram is shown in Figure 20-24. Pulmonary vascularity is increased, the pulmonary trunk (PT) is prominent, the apex is occupied by a dilated convex left ventricle (LV), and the right cardiac border is formed by a dilated right atrium (RA). B, Lateral barium esophagram outlines an enlarged left atrium.
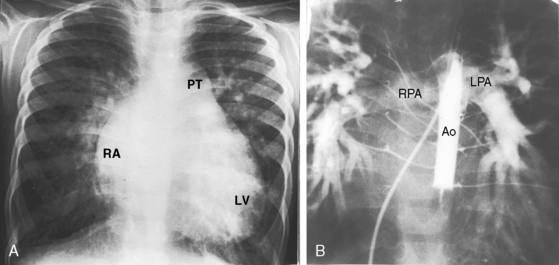
Figure 20-33 A, X-ray from a 3-year-old boy with a nonrestrictive patent ductus, low pulmonary vascular resistance, and a 3 to 1 left-to-right shunt (see Figure 20-5 for angiocardiograms). Pulmonary blood flow is increased, the pulmonary trunk (PT) is dilated, an enlarged left ventricle (LV) occupies the apex, and an enlarged right atrium (RA) forms the right cardiac border. B, The right pulmonary artery (RPA) and left pulmonary artery (LPA) are visualized through the patent ductus after contrast material was injected into the balloon-occluded descending aorta (Ao). The intrapulmonary arteries are strikingly enlarged. Compare with the increased pulmonary vascularity in A.
The x-ray of a nonrestrictive patent ductus with reversed shunt exhibits reduced pulmonary vascularity, dilation of the pulmonary trunk and its proximal branches, a normal or near normal left ventricle and left atrium, and an hypertrophied but not significantly dilated right ventricle (Figure 20-34).
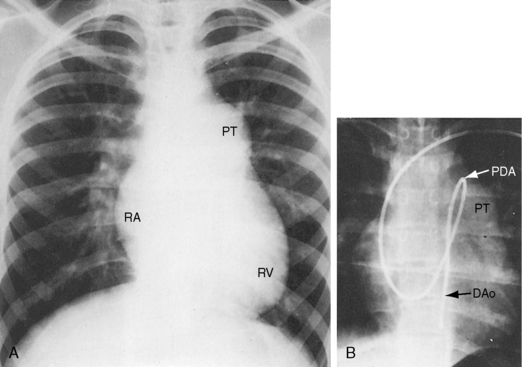
Figure 20-34 X-rays from a 14-year-old boy with a nonrestrictive patent ductus, suprasystemic pulmonary vascular resistance, and reversed shunt. A, Pulmonary vascularity is decreased, the pulmonary trunk (PT) and its proximal branches are enlarged, the right atrium (RA) is prominent, and the hypertrophied right ventricle (RV) forms an acute angle with the left hemidiaphragm. B, A catheter from the left median basilic vein entered the pulmonary trunk (PT), crossed the ductus, and came to rest in the descending aorta (DAo). The course of the catheter represents the pathway taken by unoxygenated blood through a reversed ductal shunt (see Figure 20-2,lower, and Figure 20-11A).
Echocardiogram
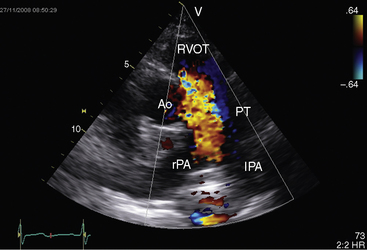
Echocardiography with color flow imaging and Doppler interrogation establishes the size of the patent ductus (Figure 20-35), the flow dynamics through the ductus, and the physiologic consequences of persistent ductal patency.103,104,118 Transesophageal echocardiography improves diagnostic accuracy.119 When the shunt is entirely left-to-right, the flow disturbance within the ductus is continuous, with the peak velocity reinforced in latter systole as forward flow from the right ventricle coincides with shunt flow through the ductus (Figure 20-36).103,104 Systolic forward flow in the aorta distal to the orifice of the ductus is followed by reversed diastolic flow (Figure 20-37). In the presence of large ductal flow, the velocities across the aortic isthmus and in the descending aorta are substantially increased.120 The color flow pattern in the pulmonary trunk consists of a ductal jet that adheres the lateral wall, travels toward the pulmonary valve, and then reverses itself to travel up the medial wall (Figure 20-38 and Video 20-1). Alternatively, a jet directed toward the pulmonary valve adheres to the medial wall of the pulmonary trunk (Figure 20-39).
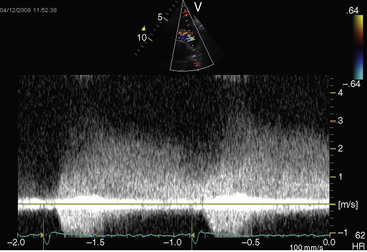
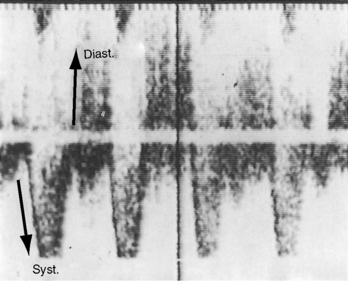
Figure 20-36 Continuous-wave Doppler scan from a 7-month-old female with a moderately restrictive patent ductus.
Color flow imaging with Doppler interrogation identifies a clinically silent nonrestrictive patent ductus in premature infants33,42 and detects the tiny clinically silent ductus in adults (Figure 20-40 and Video 20-2).67 In utero ductal closure can be recognized with fetal echocardiography.121,122 The reversed shunt in a nonrestrictive patent ductus with suprasystemic pulmonary vascular resistance can be identified with Doppler interrogation103 and contrast echocardiography.123 Right ventricular systolic pressure and pulmonary arterial diastolic pressure are estimated with continuous-wave Doppler interrogation of the jets of tricuspid and pulmonary regurgitation and with Doppler velocities across the ductus.
Aortopulmonary window
In a lecture delivered in 1830 at the St Thomas Hospital, London, Professor John Elliotson124 described the first known case of aortopulmonary window, often called aortopulmonary or aorticopulmonary septal defect. This uncommon malformation consists of a communication, usually nonrestrictive, between adjacent walls of the ascending aorta and pulmonary trunk. During early embryogenesis, two opposing proximal truncal cushions rapidly enlarge and fuse to form the truncal septum that partitions the truncus arteriosus into aortic and pulmonary channels.125–127 The distal truncoaortic sac is then divided by the aortopulmonary septum. Maldevelopment of the truncal and aortopulmonary septum results in three morphologic types of aortopulmonary window, namely125: (1) nonfusion of the embryonic aortopulmonary septum and the truncal septum that results in a moderate-sized circular defect located about midway between the great arterial valves and the bifurcation of the pulmonary trunk; (2) malalignment of the embryonic aortopulmonary septum and truncal septum that results in a defect similarly located but helical-shaped; and (3) complete absence of the embryonic aortopulmonary septum that results in a nonrestrictive defect. The morphogenesis of an aortopulmonary window is unrelated to the morphogenesis of a patent ductus arteriosus, but the physiologic consequences and clinical manifestations of the two malformations are similar if not identical,128,129 so inclusion in this chapter is appropriate.
Because an aortopulmonary window tends to be nonrestrictive, it is often mistaken for a nonrestrictive patent ductus, which is the most frequent coexisting anomaly, estimated to have an incidence rate of 12% (Figure 20-41).129 When pulmonary vascular resistance is suprasystemic and the shunt is reversed, the clinical picture is indistinguishable from a nonrestrictive ventricular septal defect with Eisenmenger’s syndrome (see Chapter 17).79 An aortopulmonary window and a ventricular septal defect can coexist, albeit rarely.130
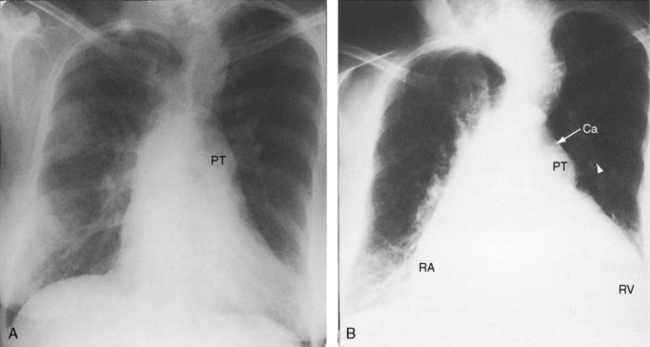
Aortopulmonary window is somewhat more frequent in males in contrast to patent ductus.128 Also in contrast to patent ductus is the rarity of infective endocarditis.128 An appreciable percentage of infants with a nonrestrictive aortopulmonary window die of congestive heart failure in infancy or early childhood.128 Only a minority reach teenage or young adulthood, but occasional survivals have been reported in the fourth or fifth decade.128,131 Survival improves when the shunt is limited by a restrictive defect or curtailed by a rise in pulmonary vascular resistance. The patient referred to in Figure 20-41 lived to age 58 years with a nonrestrictive aortopulmonary window and Eisenmenger’s syndrome.
A bounding arterial pulse and a wide pulse pressure are analogous to nonrestrictive patent ductus with low pulmonary vascular resistance (Figure 20-42). When an aortopulmonary window is accompanied by suprasystemic pulmonary vascular resistance and a reversed shunt, unoxygenated blood enters the ascending aorta so differential cyanosis does not occur. The clinical picture is then indistinguishable from nonrestrictive ventricular septal defect with Eisenmenger’s syndrome (see Chapter 17).
A moderately restrictive aortopulmonary window generates a continuous murmur indistinguishable from a moderately restrictive patent ductus arteriosus.128,132 The physiologic mechanisms responsible for variations in the murmur of a nonrestrictive aortopulmonary window are analogous to those that apply to a nonrestrictive patent ductus arteriosus (see Figure 20-15). In 80% of patients, the murmur is systolic rather than continuous (seeFigure 20-42)128 and is punctuated by eddy sounds (see Figure 20-42). When the murmur is continuous, its diastolic portion is likely to be shortened (Figure 20-43). A nonrestrictive aortopulmonary window with suprasystemic pulmonary vascular resistance and reversed shunt is accompanied by auscultatory signs of pulmonary hypertension. No murmur is generated across the defect. A Graham Steell murmur may appear before the shunt is reversed because of dilation of the hypertensive pulmonary trunk.
Systolic or continuous murmurs generated by an aortopulmonary window are typically maximal in the third left intercostal space.131 A prominent systolic murmur at the mid to lower left sternal border invites the mistaken diagnosis of ventricular septal defect, but eddy sounds and a bounding arterial pulse should prevent error (see Figure 20-42). Apical mid-diastolic murmurs represent increased flow across the mitral valve (see Figure 20-43).
The electrocardiogram of a nonrestrictive aortopulmonary window with low pulmonary vascular resistance reflects combined ventricular hypertrophy in response to volume overload of the left ventricle and pressure overload of the right ventricle, analogous to a nonrestrictive patent ductus with low pulmonary vascular resistance (see Figure 20-25). When pulmonary vascular resistance is suprasystemic and the shunt is reversed, the electrocardiogram is analogous to nonrestrictive patent ductus with Eisenmenger’s syndrome (see Figure 20-27).
The x-ray cannot distinguish a nonrestrictive aortopulmonary window with low pulmonary vascular resistance from a nonrestrictive patent ductus with large left-to-right shunt (Figures 20-44 and 20-45). When the shunt is reversed (see Figure 20-41), the x-ray resembles a nonrestrictive patent ductus with Eisenmenger’s syndrome (seeFigure 20-34).
Echocardiography localizes the aortopulmonary window between the ascending aorta and pulmonary trunk just proximal to the bifurcation (Figure 20-46 and Video 20-3)129,133,134 and determines whether a patent ductus coexists. Color flow imaging identifies the shunt as left-to-right, right-to-left (Figure 20-47 and Video 20-3), or bidirectional.
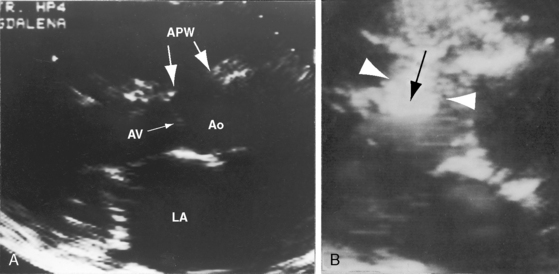
Figure 20-47 A, Echocardiogram (parasternal long-axis) from the 51-year-old patient whose x-rays are shown in Figure 20-41. Paired arrows identify the aortopulmonary window (APW). (Ao = aorta; AV = aortic valve; LA = left atrium.) B, Black-and-white print of a parasternal long-axis color flow image. Large white arrowheads bracket a right-to-left shunt (black arrow) through the aortopulmonary window (Video 20-3).
1 Castiglioni A. A history of medicine. New York: Alfred A. Knopf; 1947.
2 Marquis R.M. The continuous murmur of persistence of the ductus arteriosus—an historical review. Eur Heart J. 1980;1:465-478.
3 Carlgren L.E. The incidence of congenital heart disease in children born in Gothenburg 1941–1950. Br Heart J. 1959;21:40-50.
4 Gittenberger-De Groot A.C., Strengers J.L., Mentink M., Poelmann R.E., Patterson D.F. Histologic studies on normal and persistent ductus arteriosus in the dog. J Am Coll Cardiol. 1985;6:394-404.
5 Jager B.V., Wollenman O.J. An anatomical study of the closure of the ductus arteriosus. Am J Pathol. 1942;18:595-613.
6 Dyamenahalli U., Smallhorn J.F., Geva T., et al. Isolated ductus arteriosus aneurysm in the fetus and infant: a multi-institutional experience. J Am Coll Cardiol. 2000;36:262-269.
7 Acherman R.J., Siassi B., Wells W., et al. Aneurysm of the ductus arteriosus: a congenital lesion. Am J Perinatol. 1998;15:653-659.
8 Sachdeva R., Smith C., Greenberg B.S., Jaquiss R.D.B. Giant ductal aneurysm in an asymptomatic 4-year-old girl. Ann Thorac Surg. 2009;87:946-948.
9 Siu B.L., Kovalchin J.P., Kearney D.L., Fraser C.D., Fenrich A.L. Aneurysmal dilatation of the ductus arteriosus in a neonate. Pediatr Cardiol. 2001;22:403-405.
10 Quiroga C. Partial persistence of the ductus arteriosus. Acta Radiol. 1961;55:103-108.
11 Freedom R.M., Moes C.A., Pelech A., et al. Bilateral ductus arteriosus (or remnant): an analysis of 27 patients. Am J Cardiol. 1984;53:884-891.
12 Keagy K.S., Schall S.A., Herrington R.T. Selective cyanosis of the right arm. Isolation of right subclavian artery from aorta with bilateral ductus arteriosus and pulmonary hypertension. Pediatr Cardiol. 1982;3:301-303.
13 Spencer H., Dworken H.J. Congenital aortic septal defect with communication between aorta and pulmonary artery. Circulation. 1950;2:880-885.
14 Fu M., Hung J.S., Liao P.K., Chang C.H. Isolated right-sided patent ductus arteriosus in right-sided aortic arch. Report of two cases. Chest. 1987;91:623-625.
15 Garti I.J., Aygen M.M., Vidne B., Levy M.J. Right aortic arch with mirror-image branching causing vascular ring. A new classification of the right aortic arch patterns. Br J Radiol. 1973;46:115-119.
16 Festic E., Steiner R.M., Spatz E. Aortic dissection with extension to a patent ductus arteriosus. Int J Cardiovasc Imaging. 2005;21:459-462.
17 Mahoney L.T., Coryell K.G., Lauer R.M. The newborn transitional circulation: a two-dimensional Doppler echocardiographic study. J Am Coll Cardiol. 1985;6:623-629.
18 Gersony W.M. Persistence of the fetal circulation: a commentary. J Pediatr. 1973;82:1103-1106.
19 Slomp J., Van Munsteren J.C., Poelmann R.E., De Reeder E.G., Bogers A.J., Gittenberger-De Groot A.C. Formation of intimal cushions in the ductus arteriosus as a model for vascular intimal thickening. An immunohistochemical study of changes in extracellular matrix components. Atherosclerosis. 1992;93:25-39.
20 Wilson R.R. Post-mortem observations on contraction of the human ductus arteriosus. Br Med J. 1958;1:810-812.
21 Gittenberger-De Groot A.C. Persistent ductus arteriosus: most probably a primary congenital malformation. Br Heart J. 1977;39:610-618.
22 Gittenberger-De Groot A.C., Moulaert A.J., Hitchcock J.F. Histology of the persistent ductus arteriosus in cases of congenital rubella. Circulation. 1980;62:183-186.
23 Heymann M.A., Berman W.Jr, Rudolph A.M., Whitman V. Dilatation of the ductus arteriosus by prostaglandin E1 in aortic arch abnormalities. Circulation. 1979;59:169-173.
24 Levin D.L., Mills L.J., Weinberg A.G. Hemodynamic, pulmonary vascular, and myocardial abnormalities secondary to pharmacologic constriction of the fetal ductus arteriosus. A possible mechanism for persistent pulmonary hypertension and transient tricuspid insufficiency in the newborn infant. Circulation. 1979;60:360-364.
25 Moss A.J., Emmanouilides G., Duffie E.R.Jr. Closure of the ductus arteriosus in the newborn infant. Pediatrics. 1963;32:25-30.
26 Michelakis E., Rebeyka I., Bateson J., Olley P., Puttagunta L., Archer S. Voltage-gated potassium channels in human ductus arteriosus. Lancet. 2000;356:134-137.
27 Hirsimaki H., Kero P., Saraste M., Ekblad H., Korvenranta H., Wanne O. Grading of left-to-right shunting ductus arteriosus in neonates with bedside pulsed Doppler ultrasound. Am J Perinatol. 1991;8:247-250.
28 Gittenberger-De Groot A.C., Van Ertbruggen I., Moulaert A.J., Harinck E. The ductus arteriosus in the preterm infant: histologic and clinical observations. J Pediatr. 1980;96:88-93.
29 Tananari Y., Maeno Y., Takagishi T., Sasaguri Y., Morimatsu M., Kato H. Role of apoptosis in the closure of neonatal ductus arteriosus. Jpn Circ J. 2000;64:684-688.
30 Danilowicz D., Rudolph A.M., Hoffman J.I. Delayed closure of the ductus arteriosus in premature infants. Pediatrics. 1966;37:74-78.
31 Reller M.D., Colasurdo M.A., Rice M.J., Mcdonald R.W. The timing of spontaneous closure of the ductus arteriosus in infants with respiratory distress syndrome. Am J Cardiol. 1990;66:75-78.
32 Reller M.D., Ziegler M.L., Rice M.J., Solin R.C., Mcdonald R.W. Duration of ductal shunting in healthy preterm infants: an echocardiographic color flow Doppler study. J Pediatr. 1988;112:441-446.
33 Van De Bor M., Verloove-Vanhorick S.P., Brand R., Ruys J.H. Patent ductus arteriosus in a cohort of 1338 preterm infants: a collaborative study. Paediatr Perinat Epidemiol. 1988;2:328-336.
34 Bishop R.C. Delayed closure of the ductus arteriosus. Am Heart J. 1952;44:639-644.
35 Campbell M. Natural history of persistent ductus arteriosus. Br Heart J. 1968;30:4-13.
36 Carboni M.P., Ringel R.E. Ductus arteriosus in premature infants beyond the second week of life. Pediatr Cardiol. 1997;18:372-375.
37 Alverson D.C., Eldridge M.W., Johnson J.D., et al. Effect of patent ductus arteriosus on left ventricular output in premature infants. J Pediatr. 1983;102:754-757.
38 Martin C.G., Snider A.R., Katz S.M., Peabody J.L., Brady J.P. Abnormal cerebral blood flow patterns in preterm infants with a large patent ductus arteriosus. J Pediatr. 1982;101:587-593.
39 Miller E., Cradock-Watson J.E., Pollock T.M. Consequences of confirmed maternal rubella at successive stages of pregnancy. Lancet. 1982;2:781-784.
40 Rudolph A.M., Scarpelli E.M., Golinko R.J., Gootman N.L. Hemodynamic basis for clinical manifestations of patent ductus arteriosus. Am Heart J. 1964;68:447-458.
41 Whitaker W., Heath D., Brown J.W. Patent ductus arteriosus with pulmonary hypertension. Br Heart J. 1955;17:121-137.
42 Hammerman C., Strates E., Valaitis S. The silent ductus: its precursors and its aftermath. Pediatr Cardiol. 1986;7:121-127.
43 Bennhagen R.G., Benson L.N. Silent and audible persistent ductus arteriosus: an angiographic study. Pediatr Cardiol. 2003;24:27-30.
44 Hubbard T.F., Neis D.D. The sounds at the base of the heart in cases of patent ductus arteriosus. Am Heart J. 1960;59:807-815.
45 Chiles N.H., Smith H.L., Christensen N.A., Geraci J.E. Spontaneous healing of subacute bacterial endarteritis with closure of patent ductus arteriosus. Proc Staff Meet Mayo Clin. 1953;28:520-525.
46 Gibb W.T. Acute bacterial endarteritis of a patent ductus arteriosus. N Y State J Med. 1941;41:1861-1863.
47 Foulis J. On a case of patent ductus arteriosus with aneurysm of the pulmonary artery. Edinburgh Med J. 1884;29:1117.
48 Jager B.V. Noninfectious thrombosis of a patent ductus arteriosus: report of a case, with autopsy. Am Heart J. 1940;20:236-243.
49 Fay J.E., Travill A. The “valve” of the ductus arteriosus–an enigma. Can Med Assoc J. 1967;97:78-80.
50 Wagner O. Beitrag zur pathologie des ductus arteriosus (Botalli). Dtsch Arch Klin Med. 1903;79:90.
51 Taussig H.B. Congenital Malformations of the Heart. New York: The Commonwealth Fund; 1947.
52 Keith T.R., Sagarminaga J. Spontaneously disappearing murmur of patent ductus arteriosus. A case report. Circulation. 1961;24:1235-1238.
53 Umebayashi Y., Taira A., Morishita Y., Arikawa K. Abrupt onset of patient ductus arteriosus in a 55-year-old man. Am Heart J. 1989;118:1067-1069.
54 Ng A.S., Vlietstra R.E., Danielson G.K., Smith H.C., Puga F.J. Patent ductus arteriosus in patients more than 50 years old. Int J Cardiol. 1986;11:277-285.
55 Wilkins J.L. Risks of offspring of patients with patent ductus arteriosus. J Med Genet. 1969;6:1-4.
56 Lamy M., De Grouchy J., Schweisguth O. Genetic and non-genetic factors in the etiology of congenital heart disease: a study of 1188 cases. Am J Hum Genet. 1957;9:17-41.
57 Lynch H.T., Grissom R.L., Magnuson C.R., Krush A. Patent ductus arteriosus. Study of two families. JAMA. 1965;194:135-138.
58 Martin R.P., Banner N.R., Radley-Smith R. Familial persistent ductus arteriosus. Arch Dis Child. 1986;61:906-907.
59 Patterson D.F., Detweiler D.K. Hereditary transmission of patent ductus arteriosus in the dog. Am Heart J. 1967;74:289-290.
60 Emmanouilides G.C., Linde L.M., Crittenden I.H. Pulmonary artery stenosis associated with ductus arteriosus following maternal rubella. Circulation. 1964;29(suppl):514-522.
61 Gregg N.M. Congenital cataract following German measles in the mother. Trans Ophthalmol Soc Aust. 1941;3:35-46.
62 Rutstein D.D., Nickerson R.J., Heald F.P. Seasonal incidence of patent ductus arteriosus and maternal rubella. AMA J Dis Child. 1952;84:199-213.
63 Alzamora-Castro V., Battilana G., Abugattas R., Sialer S. Patent ductus arteriosus and high altitude. Am J Cardiol. 1960;5:761-763.
64 Hays J.T. Spontaneous aneurysm of a patent ductus arteriosus in an elderly patient. Chest. 1985;88:918-920.
65 Jayakrishnan A.G., Loftus B., Kelly P., Luke D.A. Spontaneous post-partum rupture of a patent ductus arteriosus. Histopathology. 1992;21:383-384.
66 Sardesai S.H., Marshall R.J., Farrow R., Mourant A.J. Dissecting aneurysm of the pulmonary artery in a case of unoperated patent ductus arteriosus. Eur Heart J. 1990;11:670-673.
67 Houston A.B., Gnanapragasam J.P., Lim M.K., Doig W.B., Coleman E.N. Doppler ultrasound and the silent ductus arteriosus. Br Heart J. 1991;65:97-99.
68 Bejar R., Merritt T.A., Coen R.W., Mannino F., Gluck L. Pulsatility index, patent ductus arteriosus, and brain damage. Pediatrics. 1982;69:818-822.
69 Lipman B., Serwer G.A., Brazy J.E. Abnormal cerebral hemodynamics in preterm infants with patent ductus arteriosus. Pediatrics. 1982;69:778-781.
70 Hay J.D. Population and clinic studies of congenital heart disease in Liverpool. Br Med J. 1966;2:661.
71 Aiken J.E., Bifulco E., Sullivan J.J.Jr. Patent ductus arteriosus in the aged. Report of this disease in a 74-year-old female. JAMA. 1961;177:330-331.
72 Boe J., Humerfelt S. Patent ductus arteriosus Botalli in an octogenarian followed for fifty years. Acta Med Scand. 1960;167:73-75.
73 Hornsten T.R., Hellerstein H.K., Ankeney J.L. Patent ductus arteriosus in a 72-year-old woman. Successful corrective surgery. JAMA. 1967;199:580-582.
74 Woodruff W.W.3rd, Gabliani G., Grant A.O. Patent ductus arteriosus in the elderly. South Med J. 1983;76:1436-1437.
75 Zarich S., Leonardi H., Pippin J., Tuthill J., Lewis S. Patent ductus arteriosus in the elderly. Chest. 1988;94:1103-1105.
76 Kong M.H., Corey G.R., Bashore T., Harrison J.K. Clinical problem-solving. A key miscommunication—an 81-year-old woman presented to the emergency department with increasing abdominal distention, nausea, and vomiting. N Engl J Med. 2008;358:1054-1059.
77 White P.D., Mazurkie S.J., Boschetti A.E. Patency of the ductus rteriosus at 90. N Engl J Med. 1969;280:146-147.
78 Perloff J.K. Cyanotic congenital heart disease: a multisystem systemic disorder. In Perloff J.K., Child J.S., Aboulhosn J., editors: Congenital heart disease in adults, 3rd ed, Philadelphia: Saunders/Elsevier, 2008.
79 Wood P. The Eisenmenger syndrome or pulmonary hypertension with reversed central shunt. I. Br Med J. 1958;2:701-709.
80 Adams P.Jr, Anderson R.C., Varco R.L. Patent ductus arteriosus with reversal of flow; clinical study of ten children. Pediatrics. 1956;18:410-423.
81 Sietsema K.E., Perloff J.K. Cyanotic congenital heart disease: dynamics of oxygen uptake and control of ventilation during exercise. In Perloff J.K., Child J.S., editors: Congenital heart disease in adults, 2nd ed, Philadelphia: W.B. Saunders Company, 1998.
82 Dailey F.H., Genovese P.D., Behnke R.H. Patent ductus arteriosus with reversal of flow in adults. Ann Intern Med. 1962;56:865.
83 Martinez-Lavin M., Bobadilla M., Casanova J., Attie F., Martinez M. Hypertrophic osteoarthropathy in cyanotic congenital heart disease: its prevalence and relationship to bypass of the lung. Arthritis Rheum. 1982;25:1186-1193.
84 Williams B., Ling J.T., Leight L., Mc G.C. Patent ductus arteriosus and osteoarthropathy. Arch Intern Med. 1963;111:346-350.
85 Arcilla R.A., Thilenius O.G., Ranniger K. Congestive heart failure from suspected ductal closure in utero. J Pediatr. 1969;75:74-78.
86 Korones S.B., Ainger L.E., Monif G.R., Roane J., Sever J.L., Fuste F. Congenital rubella syndrome: study of 22 infants. Myocardial damage and other new clinical aspects. Am J Dis Child. 1965;110:434-440.
87 Robertson S.E., Featherstone D.A., Gacic-Dobo M., Hersh B.S. Rubella and congenital rubella syndrome: global update. Rev Panam Salud Publica. 2003;14:306-315.
88 Lin A.E., Perloff J.K. Upper limb malformations associated with congenital heart disease. Am J Cardiol. 1985;55:1576-1583.
89 Rohde R.A., Hodgman J.E., Cleland R.S. Multiple congenital anomalies in the E1-trisomy (group 16–18) syndrome. Pediatrics. 1964;33:258-270.
90 Satoda M., Pierpont M.E., Diaz G.A., Bornemeier R.A., Gelb B.D. Char syndrome, an inherited disorder with patent ductus arteriosus, maps to chromosome 6p12-p21. Circulation. 1999;99:3036-3042.
91 Satoda M., Zhao F., Diaz G.A., et al. Mutations in TFAP2B cause Char syndrome, a familial form of patent ductus arteriosus. Nat Genet. 2000;25:42-46.
92 Bertola D.R., Kim C.A., Sugayama S.M., Utagawa C.Y., Albano L.M., Gonzalez C.H. Further delineation of Char syndrome. Pediatr Int. 2000;42:85-88.
93 Trip J., Van Stuijvenberg M., Dikkers F.G., Pijnenburg M.W.H. Unilateral charge association. Eur J Pediatr. 2002;161:78-80.
94 Perloff J.K. Physical examination of the heart and circulation, 4th ed. Shelton, Connecticut: People’s Medical Publishing House; 2009.
95 Holden J.D., Jones R.C., Akers W.A. Patent ductus arteriosus diagnosed by a mosquito bite or the cutis Quincke. Arch Derm. 1966;94:742.
96 Gibson G.A. Persistence of the arterial duct and its diagnosis. Edinburgh Med J. 1900;8:1.
97 Magri G., Jona E., Messina D., Actisdato A. Direct recording of heart sounds and murmurs from the epicardial surface of the exposed human heart. Am Heart J. 1959;57:449-459.
98 Baylen B., Meyer R.A., Korfhagen J., Benzing G.3rd, Bubb M.E., Kaplan S. Left ventricular performance in the critically ill premature infant with patent ductus arteriosus and pulmonary disease. Circulation. 1977;55:182-188.
99 Urquhart D.S., Nicholl R.M. How good is clinical examination at detecting a significant patent ductus arteriosus in the preterm neonate? Arch Dis Child. 2003;88:85-86.
100 Kohler C.M., Mcnamara D.G. Elongated patent ductus arteriosus with intermittent shunting. Pediatrics. 1967;39:446-448.
101 Shapiro W., Said S.I., Nova P.L. Intermittent disappearance of the murmur of patent ductus arteriosus. Circulation. 1960;22:226-231.
102 Papadopoulos G.S., Folger G.M.Jr. Diastolic murmurs in the newborn of benign nature. Int J Cardiol. 1983;3:107-109.
103 Hiraishi S., Horiguchi Y., Misawa H., et al. Noninvasive Doppler echocardiographic evaluation of shunt flow dynamics of the ductus arteriosus. Circulation. 1987;75:1146-1153.
104 Liao P.K., Su W.J., Hung J.S. Doppler echocardiographic flow characteristics of isolated patent ductus arteriosus: better delineation by Doppler color flow mapping. J Am Coll Cardiol. 1988;12:1285-1291.
105 Perloff J.K. Auscultatory and phonocardiographic manifestations of pulmonary hypertension. Prog Cardiovasc Dis. 1967;9:303-340.
106 Braudo M., Rowe R.D. Auscultation of the heart: early neonatal period. Am J Dis Child. 1961;101:575-586.
107 Burnard E.D. A murmur from the ductus arteriosus in the newborn baby. Br Med J. 1958;1:806-810.
108 Hallidie-Smith K.A. Murmur of persistent ductus arteriosus in premature infants. Arch Dis Child. 1972;47:725-730.
109 Green E.W., Agruss N.S., Adolph R.J. Right-sided Austin Flint murmur. Documentation by intracardiac phonocardiography, echocardiography and postmortem findings. Am J Cardiol. 1973;32:370-374.
110 Gray I.R. Paradoxical splitting of the second heart sound. Br Heart J. 1956;18:21-28.
111 Mirowski M., Arevalo F., Medrano G.A., Cisneros F.A. Conduction disturbances in patent ductus arteriosus. A study of 20 cases before and after surgery with determination of the P-R index. Circulation. 1962;25:807-813.
112 Cruze K., Elliott L.P., Schiebler G.L., Wheat M.W.Jr. Unusual manifestations of patent ductus arteriosus in infancy. Dis Chest. 1963;43:563-571.
113 Halloran K.H., Sanyal S.K., Gardner T.H. Superiorly oriented electrocardiographic axis in infants with the rubella syndrome. Am Heart J. 1966;72:600-606.
114 Steinberg I. Roentgenography of patent ductus arteriosus. Am J Cardiol. 1964;13:698-707.
115 Currarino G., Jackson J.H. Calcification of the ductus arteriosus and ligamentum botalli. Radiology. 1970;94:139-142.
116 Sang Oh K., Bowen A.D., Park S.C., Galvis A.G., Young L.W. Patent ductus arteriosus: its occurrence with unequal pulmonary vascularity and hyperlucent left lung. Am J Dis Child. 1981;135:637-639.
117 Castellanos A., Hernandez F.A. Size of ascending aorta in congenital cardiac lesions and other heart diseases. Acta Radiol Diagn (Stockh). 1967;6:49-64.
118 Vick G.W.3rd, Huhta J.C., Gutgesell H.P. Assessment of the ductus arteriosus in preterm infants utilizing suprasternal two-dimensional/Doppler echocardiography. J Am Coll Cardiol. 1985;5:973-977.
119 Shyu K.G., Lai L.P., Lin S.C., Chang H., Chen J.J. Diagnostic accuracy of transesophageal echocardiography for detecting patent ductus arteriosus in adolescents and adults. Chest. 1995;108:1201-1205.
120 Guntheroth W.G., Forster F.K. Large ductal flow may cause high velocity in the descending aorta without coarctation: improved diagnosis using the continuity equation. Am J Cardiol. 2001;87:493-495. A498
121 Mielke G., Steil E., Breuer J., Goelz R. Circulatory changes following intrauterine closure of the ductus arteriosus in the human fetus and newborn. Prenat Diagn. 1998;18:139-145.
122 Leal S.D., Cavalle-Garrido T., Ryan G., Farine D., Heilbut M., Smallhorn J.F. Isolated ductal closure in utero diagnosed by fetal echocardiography. Am J Perinatol. 1997;14:205-210.
123 Sohn D.W., Kim Y.J., Zo J.H., et al. The value of contrast echocardiography in the diagnosis of patent ductus arteriosus with Eisenmenger’s syndrome. J Am Soc Echocardiogr. 2001;14:57-59.
124 Elliotson J. Case of malformation of the pulmonary artery and aorta. Lancet. 1830;1:247-251.
125 Kutsche L.M., Van Mierop L.H. Anatomy and pathogenesis of aorticopulmonary septal defect. Am J Cardiol. 1987;59:443-447.
126 Richardson J.V., Doty D.B., Rossi N.P., Ehrenhaft J.L. The spectrum of anomalies of aortopulmonary septation. J Thorac Cardiovasc Surg. 1979;78:21-27.
127 Van Praagh R., Van Praagh S. The anatomy of common aorticopulmonary trunk (truncus arteriosus communis) and its embryologic implications. A study of 57 necropsy cases. Am J Cardiol. 1965;16:406-425.
128 Morrow A.G., Greenfield L.J., Braunwald E. Congenital aortopulmonary septal defect. Clinical and hemodynamic findings, surgical technic, and results of operative correction. Circulation. 1962;25:463-476.
129 Rice M.J., Seward J.B., Hagler D.J., Mair D.D., Tajik A.J. Visualization of aortopulmonary window by two-dimensional echocardiography. Mayo Clinic Proc. 1982;57:482-487.
130 Tandon R., Da Silva C.L., Moller J.H., Edwards J.E. Aorticopulmonary septal defect coexisting with ventricular septal defect. Circulation. 1974;50:188-191.
131 Downing D.F. Congenital aortic septal defect. Am Heart J. 1950;40:285-292.
132 Shepherd S.G., Park F.R., Kitchell J.R. A case of aorto-pulmonic communication incident to a congenital aortic septal defect: discussion of embryologic changes involved. Am Heart J. 1944;27:733-738.
133 Garver K.A., Hernandez R.J., Vermilion R.P., Martin Goble M. Images in cardiovascular medicine. Correlative imaging of aortopulmonary window: demonstration with echocardiography, angiography, and MRI. Circulation. 1997;96:1036-1037.
134 Satomi G., Nakamura K., Imai Y., Takao A. Two-dimensional echocardiographic diagnosis of aorticopulmonary window. Br Heart J. 1980;43:351-356.