CHAPTER 43 Patent Ductus Arteriosus
Patent ductus arteriosus (PDA) is defined as incomplete closure and patency of the ductus arteriosus beyond functional closure after birth. The ductus arteriosus is a vessel that extends from the anterolateral aspect of the descending thoracic aorta to the superior aspect of the main pulmonary artery, close to the origin of the left pulmonary artery. Embryologically, it is a remnant of the distal left sixth aortic arch. A normal and vital structure in the fetus, it is necessary for diverting blood flow from the main pulmonary artery to the aorta, thereby bypassing the high-resistance pulmonary circulation.1 This diversion is crucial to normal development of the right ventricle and results in passage of only 10% of the right ventricular cardiac output through the lungs. Premature closure of the ductus arteriosus in the fetus, as seen with maternal use of nonsteroidal anti-inflammatory drugs (NSAIDs), leads to right ventricular failure and fetal hydrops (Fig. 43-1).2
The duct functionally closes in the first 18 hours of life in full-term healthy infants. However, it fully closes several weeks later, after which it is unable to regain its patency. In premature infants, patency can be seen up to 10 days after birth.3,4 Some consider the patency abnormal only after 3 months of age. The patency causes a left to right shunt that increases pulmonary blood flow at the expense of systemic flow.5 If untreated, based on the size of the PDA, pulmonary hypertension and Eisenmenger syndrome develop in children and adults (Fig. 43-2).1
MECHANISM OF PHYSIOLOGIC CLOSURE
Histologically, the wall of the ductus arteriosus is composed of the same three layers as those of the aorta or main pulmonary artery—the intima, media, and adventitia. The media of the ductus arteriosus is different in that the smooth muscle fibers have a spiral and longitudinal orientation, instead of a concentric one, as seen in the aorta and pulmonary artery.1
Relative low oxygen tension and high levels of prostaglandin E2 (PGE2) and prostacyclin (PGI2), produced by the placenta, are responsible for the continued patency of the ductus arteriosus in the fetus. PGE2 and PGI2 interact with specific receptors in the smooth muscle layer of the media, leading to vasodilation.6
The process of closure begins at birth. The sudden increase of oxygen tension leads to influx of calcium and thereby muscle contraction in the smooth muscles of the media. Because of the metabolic breakdown of prostaglandins by the newly functioning lung and the absence of the placenta, the PGE2 and PGI2 levels fall, diminishing their vasodilation effect. Smooth muscle contraction in the ductus arteriosus wall shortens the ductus and causes luminal occlusion. In the following weeks, fibrosis ensues, leading to permanent sealing.1,6 Perinatal hypoxia impedes the natural closure process. Pharmacologic treatment of PDA is based on muscular constriction in the middle of the ductus, leading to segmental ischemia and fibrosis.3,6
PREVALENCE AND EPIDEMIOLOGY
PDA can be isolate or part of a complex cardiac malformation.7 The overall incidence in children born at term is 0.02% to 0.08%, which accounts for approximately 10% of all congenital heart disease. Most cases are sporadic. It is more common in premature and term infants with maternal rubella infection in the first trimester of pregnancy. Maternal use of amphetamine and phenytoin and fetal alcohol syndrome are also associated with PDA.8 PDA is more frequent in certain genetic syndromes such as trisomy 21, trisomy 18, 4p− syndrome, Holt-Oram syndrome, Carpenter syndrome, and Char syndrome.1,9
PDA is twice as common in females when it is isolated and sporadic; however, if associated with prematurity or prenatal infection, the frequency is equal between the genders.9 A study by Mangones and colleagues,10 which compared the prevalence of various congenital cardiovascular malformations by race and ethnicity, showed no racial preference for isolated PDA and an overall less frequency of congenital cardiac malformations in Hispanics. However, a previous study by Chavez and associates11 described an increased prevalence of isolate PDA in African Americans.
ETIOLOGY AND PATHOPHYSIOLOGY
The magnitude of left to right shunting depends on the flow resistance of the ductus arteriosus and the pressure gradient between the aorta and pulmonary artery. The flow resistance depends mainly on the diameter and length of the narrowest portion of the ductus arteriosus. The pressure gradient between the two ends of the PDA depends on the pulmonary and systemic vascular resistance and the cardiac output. The larger the diameter of the narrowest portion of the ductus, the greater the impact of changes in the systemic and pulmonary resistance on the pressure gradient and, therefore, on the magnitude of shunting. If the narrow portion is small in caliber, thereby a restrictive shunt, then the length of the narrow portion is important. A longer length of the ductus is associated with a smaller magnitude of shunting.1,6,8
The left to right shunt causes overcirculation and fluid overload of the pulmonary bed, which lead to decreased lung compliance and increased work of breathing. The shunting also causes left heart volume overload and, if the ductus is moderate to large in size, the increased left atrial and left ventricular end-diastolic pressures can eventually lead to hypertrophy of the left ventricle. The wall tension of the hypertrophied left ventricle causes catecholamine release, which results in tachycardia and therefore a shorter diastolic time. On the other hand, the diastolic blood pressure of the aorta decreases because of blood passage through the ductus during diastole. This decreased diastolic pressure and shorter diastolic time causes decreased coronary perfusion and, when combined with increased myocardial tension and oxygen demand, may result in subendocardial ischemia, which can be detected by elevated troponin levels.12
When the pulmonary arteries are subjected to long-standing increased flow and high pressure, microvascular injury develops, which leads to intimal proliferation and arteriolar medial hypertrophy. This eventually causes fibrosis and obliteration of the pulmonary arterioles and capillaries, and pulmonary arterial hypertension ensues. As the pulmonary vascular resistance gradually increases and approaches or exceeds the systemic vascular resistance, ductal shunting reverses and becomes right to left. This is called Eisenmenger syndrome.1,6,8
Clinical Features
Age of clinical presentation and presence of and type of symptoms at the time of presentation depend on the size of the PDA and pulmonary vascular resistance. The clinical presentation can vary from no symptoms, where the shunt is diagnosed incidentally, to congestive heart failure and Eisenmenger syndrome. Many patients have exercise intolerance or the diagnosis of reactive airway disease.1,8,13 Most patients compensate well, even with a moderate left to right shunt and remain asymptomatic during childhood. A well-tolerated PDA can become clinically significant when combined with effects of acquired conditions such as ischemic heart disease or calcific aortic stenosis. The hallmark physical finding is a machinery murmur, which is a continuous murmur detected at the left upper sternal border. If the shunt is moderate or large, the left ventricular impulse will be prominent and laterally displaced and the pulse pressure will be increased, resulting in a bounding peripheral pulse.
Eisenmenger syndrome presents with cyanosis and clubbing that may spare the fingers because the right to left shunting is distal to the subclavian arteries. This cyanosis may be more profound when systemic vascular resistance is decreased, such as after exercise or hot weather. On auscultation, there may be no murmur, a high-frequency diastolic decrescendo murmur of pulmonary regurgitation, and/or a holosystolic murmur from tricuspid valve regurgitation. Peripheral edema is a late manifestation caused by right ventricular dysfunction.14,15
MANIFESTATIONS OF DISEASE
Clinical Presentation
Grading
In adults, PDA is usually isolated and discovered incidentally on physical examination or during echocardiography screening. Grading of PDA is based on a combination of echocardiographic findings, physical findings on auscultation and examination, and presenting symptoms. In adults, PDA is categorized as four grades—silent, small, moderate, and large. A silent PDA is usually incidentally diagnosed on echocardiography. The patient is asymptomatic and has no audible heart murmur. A small PDA is associated with an audible continuous murmur in the left upper sternal border that radiates to the back. The patient has normal peripheral pulses and no pulmonary hypertension. In moderate PDA, there is an audible continuous murmur, wide, bouncy peripheral pulses caused by aortic regurgitation, and runoff of blood during diastole by the shunt. The left atrium and left ventricle are enlarged; the PDA is associated with pulmonary hypertension that is commonly reversible. A PDA is graded as large when signs of pulmonary hypertension have developed. The typical continuous murmur is absent. Differential cyanosis, in which the oxygen saturation of the feet is lower than that of the right arm, is typically seen. Grade 4 patients are mostly adults with Eisenmenger syndrome.14,15
Complications
The complications of PDA usually present in adulthood, when congestive heart failure presents in the third decade of life. This is caused by chronic left heart volume overload. Heart failure is associated with atrial flutter or fibrillation and is caused by left atrial dilation. This may be the first sign in these patients. Pulmonary hypertension or Eisenmenger syndrome is a manifestation of irreversible pulmonary vascular disease, seen with moderate and large PDA.1,14,16
Infective endarteritis is a significant health issue in countries where there are lower standards of health, inadequate oral hygiene, and less widespread antibiotic use. When endoarteritis develops, it occurs at the pulmonary end of the shunt, which can cause bland and septic emboli to the lungs. No systemic embolization occurs.15,16
The left recurrent laryngeal nerve passes in the triangular space created by the main pulmonary artery, aortic arch, and PDA. When pulmonary hypertension develops, the pulmonary artery becomes enlarged and this space narrows, which may cause impingement of the recurrent laryngeal nerve and development of unilateral vocal cord paralysis and hoarseness. This is a rare complication.1
Imaging Technique and Findings
Ultrasound
The diagnostic modality of choice for PDA is generally echocardiography, in which color Doppler is used to detect a PDA and used to estimate the degree of shunting. Gray-scale and M-mode echocardiography are useful for visualizing the geometry of the ductus, calculating left atrium and ventricle sizes, and quantifying the left ventricle systolic function. If Eisenmenger syndrome is present, there is a low-velocity right to left shunting, which is difficult to detect, even by color Doppler. In this case, secondary signs of septal flattening, right ventricular hypertrophy, and high-velocity pulmonary regurgitation are the findings that warrant a search for a PDA (Fig. 43-3).1,15
Radiography
On standard chest radiography, PDA only becomes evident when there is a moderate PDA. The left atrium and left ventricle are enlarged. In infants and children with a moderate or large PDA, shunt vascularity is seen. In patients who have Eisenmenger syndrome, the central pulmonary arteries are enlarged and there is peripheral pruning of the pulmonary arteries. In older patients, a calcification in the aortopulmonary window indicates a calcified ductus arteriosus or PDA. In such patients, the aorta may be enlarged because of the chronic right to left shunt (Fig. 43-4).17
Computed Tomography
Computed tomography angiography (CTA) is useful for demonstrating the PDA, especially when it is not detectable on echocardiography, but is suspected. CTA also helps in characterizing the morphology of PDA and determining whether it is calcified, both important factors in treatment planning. Other associated vascular anomalies, such as a vascular ring or the presence of other associated congenital heart disease, can be detected by CTA. Multidetector CT (MDCT) involves faster imaging with a smaller contrast bolus, allowing increased use of this modality in the assessment of mediastinal vascular anomalies and acquired disease. The quick scanning results in fewer motion artifacts and the ability to evaluate children and older patients. The higher spatial resolution and presence of multiple rows of detectors along the longitudinal axis of the patient allow better multiplanar-reformatted and surface-shaded three-dimensional images to be created. These are most useful for quickly displaying the anatomy of the vascular abnormality and its relationship with adjacent structures. The ease of use of MDCT and its excellent depiction of anatomy has rendered CTA a common first means of evaluating for vascular anomalies in the thorax. The disadvantage of CTA is the use of intravenous iodinated contrast, contraindicated for patients with renal failure and for those who are allergic to intravenous contrast (Fig. 43-5).17–19
Magnetic Resonance Imaging
Magnetic resonance imaging has the advantage of providing both anatomic and functional information without the use of ionizing radiation. Flow quantification across the PDA allows one to assess the direction of flow in addition to quantifying the velocity of flow across the ductus arteriosus. The shunt ratio can be quantified by comparing the flow across the pulmonary and aortic valves. MR angiography (MRA) also provides information about flow direction, presence of collateral vasculature, and the presence of a PDA and other vascular anomalies. The disadvantage of MRI and MRA is the inability to use them in patients with pacemakers, the need for sedation in infants and small children, the decreased sensitivity in depicting calcifications, and the long scanning time (Fig. 43-6).17,20
On both CT and MRI, PDA is seen as a tubular connection arising on the undersurface of the aortic arch, approximately 5 to 10 mm distal to the origin of the left subclavian artery.19 It connects to the superior surface of the main pulmonary artery, close to the origin of the left pulmonary artery.17 It is variable in length and shape; some are straight and some are tortuous (Fig. 43-7).19 If the timing of imaging is optimized on CTA to opacify the pulmonary arteries, the left to right shunt can appear as negative contrast of nonopacified blood on the superior surface of the main pulmonary artery. On cine MRI, a signal void in the main pulmonary artery at the origin of the left pulmonary artery suggests the presence of a PDA. Three-dimensional MRA in an oblique sagittal plane can reveal the ductus origin of the dephasing jet (Fig. 43-8).17–19
DIFFERENTIAL DIAGNOSIS
From Clinical Presentation
Aortopulmonary Window
Aortopulmonary window is a rare lesion in which there is a broad-based direct communication between the ascending aorta and pulmonary artery. It is caused by incomplete division of the embryonic common trunk. Clinically, it simulates the symptoms of PDA and radiographically it causes increased pulmonary vascularity on the chest radiographs of newborns who are acyanotic. With imaging, this entity can be distinguished from PDA by its location; the aortopulmonary window involves the ascending aorta and in PDA, it involves the proximal descending aorta at the isthmus.15,21
From Imaging Findings
Other Vascular Entities at the Aortic Isthmus
The differential diagnosis of PDA is composed of vascular lesions at the aortic isthmus, the point where the aortic arch joins the descending aorta (Fig. 43-9).22 These include aortic spindle, ductus diverticulum, ductus aneurysm, diverticulum of Kommerell, traumatic pseudoaneurysm, atherosclerotic aneurysms and penetrating atherosclerotic ulcers, and mycotic or infectious pseudoaneurysms.
Aorta Spindle or Ductus Bump
Aortic spindle or ductus bump is a normal variant in which there is dilation of the descending thoracic aorta, just distal to the isthmus. It involves the aorta circumferentially and is smooth in outline. In newborns, such dilation is more prevalent when there is a normal decreased caliber of the aorta between the origins of the left subclavian artery and the ductus arteriosus, and dilation of the descending aorta distal to it. With closure of the ductus arteriosus and increased flow through the aortic arch, the narrowing of the isthmus resolves and this configuration usually disappears.23 In adults, its frequency has been reported to be approximately 16% in adults and a ductus bump may be more prominent in older patients (Fig. 43-10).24
Ductus Diverticulum
Ductus diverticulum is defined as a focal convexity along the anterior undersurface of the isthmic region of the aortic arch, where the ligamentum arteriosum exists. Its frequency has been reported to be as high as 26%.24 There are two theories about its origin. The first is that it is a remnant of the closed ductus arteriosus, where the unfused, nonatretic aortic end of the ductus creates the ductus diverticulum. The other theory is that it is a remnant of the right dorsal root.25 The ductus diverticulum typically has gentle obtuse angles at its junction with the aortic wall and has smooth contours with symmetric shoulders as it gently slopes from the anteroinferior aorta. In a study of 200 normal aortograms, in which 51 ductus diverticula were found, Morse and coworkers24 reported that the inferior angle of the ductus diverticulum is always obtuse, but in 2% of cases the superior angle is acute. In that study, 75% of the diverticula had an anteromedial location and 25% had an anterolateral location. The main differential diagnosis of this entity is post-traumatic pseudoaneurysm, which is differentiated from a ductus diverticulum by its irregular contour and the acute angles it creates where it meets the anteroinferior aorta (Fig. 43-11).
Ductus Aneurysm
Ductal aneurysm is defined as saclike dilation of the ductus arteriosus in the absence of PDA. It is a rare lesion that usually occurs spontaneously. It can sometimes occur after surgical repair of PDA. It is usually diagnosed in infancy because of its complications, when it could cause compression of the esophagus, bronchi, pulmonary arteries, or recurrent laryngeal nerve, thromboembolism, infection, and rupture.26 In cases in which the aneurysm has developed spontaneously, the complication rate has been reported to be 31% in infants younger than 2 months old, 66% in infants and children between the ages of 2 months and 15 years, and 47% in adults. The complication rate is much greater when the aneurysm has developed after PDA repair, resulting in death in more than 90% of cases because of infection and rupture, when giant ductal aneurysms can be associated with aortic wall thinning. Prompt surgical resection of all ductal aneurysms should be considered to avoid potentially fatal complications (Fig. 43-12).27
Aneurysmal Original of Anomalous Right Subclavian Artery (Diverticulum of Kommerell)
Diverticulum of Kommerell is an ectatic infundibulum at the origin of an aberrant right subclavian artery. It is a remnant of the left dorsal aortic root. It arises along the lateral aspect of the distal aortic arch or proximal descending thoracic aorta and connects to the subclavian artery, differentiating it from a PDA, which arises from the anteroinferior aspect of the isthmus of the aorta (Fig. 43-13). This diverticulum can become aneurysmal and atherosclerotic, especially in older adults, causing mass effect on the posterior wall of the trachea and esophagus and causing difficulty swallowing, called dysphagia lusoria (Fig. 43-14).18,22,28
Traumatic Aortic Injury
Blunt chest trauma can cause acute aortic injury, which is commonly located at the aortic isthmus, because of the shearing injury to the aorta caused by its relative fixation by the ligamentum arteriosum. In most cases, traumatic aortic injury results in immediate mortality. Survival of those who have undergone imaging is mostly because of incomplete rupture of the layers of the aortic wall, where the aortic rupture is contained by the adventitia or periadventitial tissues. On cross-sectional imaging and aortography, a focal saccular aneurysm with a narrow neck is seen, usually located medially at the aortic isthmus. This pseudoaneurysm has an irregular shape and margin, creating acute angles with the wall of the aorta. Mediastinal hematoma and fat stranding, in addition to abnormal aortic contour and intimal flaps, are noted on CT and MRI. A pseudocoarctation in which there is abrupt tapering of the diameter of the descending aorta may occur (Fig. 43-15).22,24
Approximately 2.5% of patients who survive the initial trauma develop chronic pseudoaneurysms. These are usually detected incidentally, have rim calcifications and eccentric thrombus, and are located along the inferior aortic arch, at the isthmus. Because of risk of progressive enlargement and rupture, surgical treatment is a consideration (Fig. 43-16).25
Nontraumatic Aneurysm and Pseudoaneurysm
A penetrating atherosclerotic ulcer is caused by erosion of the aortic intima by an ulcerated atheromatous plaque, resulting in a hematoma in the media layer of the aortic wall. It can be complicated by aneurysm, intramural hematoma or, less likely, rupture. It is more common in the older adults and, when present, there is evidence of atherosclerotic disease in the remainder of the arteries. It is more frequently seen in the descending thoracic aorta and abdominal aorta, where atherosclerosis is more prevalent.22
Infectious aneurysms of the aorta are rare and even more unusual in the thoracic aorta. In the chest, they are more common in the ascending aorta. The intima is very resistant to infection, so any condition that damages the aortic wall can predispose a person to infectious aneurysms. These conditions include contiguous bacterial endocarditis, an immunocompromised state, atherosclerosis, drug abuse, and aortic trauma. The infection may also be caused by local spread of an adjacent infection, such as infectious discitis. Because of the high mortality involved with this entity, early diagnosis is crucial. On CT and MRI, findings consist of a saccular aneurysm with eccentric thrombus and adjacent inflammation, fluid collection, abscess, or mediastinal soft tissue thickening or mass. The sudden development and rapid growth of mycotic aneurysms also help differentiate them from other causes of aneurysmal dilation of the aorta. Complications of leakage and periaortic hematoma are also well depicted by cross-sectional imaging. Because of the risk of rupture, mycotic aneurysms are treated by a combination of surgery, usually using endovascular grafts, and adjuvant antibiotic therapy (Fig. 43-17).22,25,29
Coarctation of Aorta
Coarctation and pseudocoarctation are also vascular entities that can occur at the isthmus. Coarctation of aorta can be associated with PDA in situations in which the stenosis is severe. The ductus arteriosus is a crucial pathway for flow in patients with severe postductal stenosis of the descending aorta, where the ductus arteriosus serves as a collateral pathway, resulting in a left to right shunt. Such children are acyanotic and have increased pulmonary vascularity on imaging studies. In newborns with preductal stenosis, the ductus arteriosus of the aorta provides flow to the descending aorta. CTA clearly depicts the vascular communications, aortic caliber, and collateral vasculature. MRI and MRA provide information on the degree of stenosis, for which a shunt gradient can be calculated, and the direction of blood flow through the collateral pathways (Fig. 43-18).18,28
Interrupted Aortic Arch
Interrupted aortic arch is an advanced form of aortic coarctation in which there is absence or atresia of an aortic segment. A patent ductus arteriosus provides flow to the descending aorta. Enlarged systemic arterial collaterals help transfer blood from branches of the ascending aorta to the descending aorta. This anomaly is rare, comprising less than 1% of congenital heart defects. Based on the location of aortic atresia, this anomaly can be classified into three types: (1) type A is when the interruption occurs distal to the left subclavian artery (approximately 33% of cases); (2) in type B (65% of cases), the atresia is between the left carotid artery and left subclavian artery and approximately 50% of these patients have DiGeorge syndrome, especially if there is a right-sided aortic arch; and (3) in type C (<1% of cases), there is an interruption between the innominate artery and left common carotid artery. Based on where the right subclavian artery originates, this anomaly is further classified into subtypes. There is an increased incidence of interrupted aortic arch and ventricular septal defect. CTA and MRA of the chest clearly depict the PDA, the site of aortic interruption, distance between the proximal and distal segments, and narrowest diameter of the left ventricular outflow tract, all information that is needed to plan surgical repair (Fig. 43-19).18,22,28
SYNOPSIS OF TREATMENT OPTIONS
Medical
All patients with PDA require lifelong prophylactic antibiotic treatment for infective endocarditis, even if the PDA is restrictive. Symptoms of congestive heart failure are treated with diuretics and digoxin, and atrial fibrillation and flutter are treated medically or by cardioversion. Adults with atrial fibrillation require lifelong anticoagulation. Before the development of permanent atrial dilation and ventricular failure, permanent closure of the PDA may reverse these symptoms. Patients who have developed pulmonary vascular disease are not candidates for procedures of definitive closure. These patients may benefit from vasodilating agents such as chronic oxygen, PGI2, calcium channel blockers, endothelin antagonists, and phosphodiesterase type V inhibitors. An option for such patients is to close the duct partially by transcatheter techniques or surgery and medically treat with vasodilators. Permanent closure is considered after follow-up assessment reveals a decrease of pulmonary vascular resistance.1,8,16
Surgical and Interventional
Interruption of the left to right shunt is the goal of management. Closure is attempted in neonates by administration of indomethacin and similar NSAIDs. Definitive closure in children and adults is considered if they are symptomatic from the left to right shunting. Asymptomatic patients with moderate to large shunts should also be treated to prevent complications of heart failure and pulmonary hypertension. Because current methods of permanent closure are safe, effective, and associated with little morbidity, any child or young adult with PDA is routinely treated. The value of treatment of an incidentally discovered tiny PDA is not certain, especially for older adults.16
When Eisenmenger syndrome develops, permanent closure of the PDA may cause a hemodynamic strain, which could quickly spiral down to right ventricular failure. This is caused by the reliance on the left to right shunt to provide adequate flow to the pulmonary bed, despite high pulmonary vascular resistance and pulmonary artery pressure. Lung biopsy has been recommended in patients with a vascular resistance higher than 8 HRU/m2 to determine whether such patients are candidates for permanent closure of PDA. Reactivity of pulmonary vessels to pulmonary vasodilating agents, and/or significant reduction of pulmonary artery pressure and resistance during test closure using a balloon catheter, suggest reversibility of the pulmonary vascular disease and positive outcome of PDA closure in patients with right to left shunting through the PDA.15,16
Permanent closure is achieved by transcatheter and surgical methods. Transcatheter occlusion is the treatment of choice for children and young adults. In patients who have increased pulmonary vascular resistance and the ductus arteriosus is calcified, transcatheter closure is preferred to surgical closure, which requires cardiopulmonary bypass and an anterior approach through a median sternotomy.16
Transcatheter closure involves advancing a catheter or delivery sheath across the ductus arteriosus, approaching it from the pulmonary artery or the aorta. A closure device is then positioned to occlude it. Various coils can be used, either those used for general vascular closure or those specific to PDA closure. If the PDA is moderate or large, an Amplatzer Duct Occluder device (AGA Medical, Plymouth, Minn) is preferred. Treatment is effective, so that assessment for complete closure at the time of follow-up has revealed a success rate exceeding 90% to 95%.1,16 The most common complication, although uncommon, is device embolization, which is usually retrieved by an endovascular approach. Other complications are even more rare and constitute flow disturbance in the proximal left pulmonary artery or descending aorta from protrusion of the device, hemolysis from residual high-velocity shunting, and vein thrombosis caused by vascular access (Fig. 43-20).16
Surgical ligation or transection is reserved for patients who have a very large ductus, which may not be adequately occluded by occluder devices. If a large broad-based connection exists, patch closure under cardiopulmonary bypass is the surgical treatment of choice.1,15
SUMMARY
KEY POINTS
 If the ductus remains patent after this initial period, the resulting left-to-right shunt can lead to pulmonary hypertension and Eisenmenger syndrome.
If the ductus remains patent after this initial period, the resulting left-to-right shunt can lead to pulmonary hypertension and Eisenmenger syndrome.1 Schneider DJ, Moore JW. Patent ductus arteriosus. Circulation. 2006;114:1873-1882.
2 Goo HW, Park IS, Ko JK, et al. CT of congenital heart disease: normal anatomy and typical pathologic conditions. Radiographics. 2003;23:S147-S165.
3 Dattilo G, Tulino V, Tulino D, et al. Letter to the editor: Interatrial defect and patent ductus arteriosus. Int J Cardiol. 2009 Apr 28.
4 Mandhan P, Brown S, Kukkady A, et al. Surgical closure of patent ductus arteriosus in preterm low birth weight infants. Congenit Heart Dis. 2009;4:34-37.
5 Tschuppert S, Doell C, Arlettaz-Mieth R, et al. The effect of ductal diameter on surgical and medical closure of patent ductus arteriosus in preterm neonates: size matters. J Thorac Cardiovasc Surg. 2008;135:78-82.
6 Chirvolu A, Jaleel MA. Pathophysiology of patent ductus arteriosus in premature neonates. Early Human Develop. 2009;85:143-146.
7 Tulino V, Dattilo G, Tulino D, et al. Letter to the editor: A recurrent patent ductus arteriosus. Int J Cardiol. 2009 Mar 24.
8 Neisch SR Patent ductus arteriosus eMedicine, July 23, 2009. Available at http://emedicine.medscape.com/article/891096-overview Accessed October 22, 2009
9 Kaneko Y, Kobayashi J, Achiwa I, et al. Cardiac surgery in patients with trisomy 18. Pediatr Cardiol. 2009;30:729-734.
10 Mangones T, Manhas A, Vistainer P, et al. Prevalence of congenital cardiovascular malformations varies by race and ethnicity. Int J Cardiol. 2009 Apr 2.
11 Chávez GF, Cordero JF, Becerra JE. Leading major congenital malformations among minority groups in the United States, 1981-1986. MMWR Morb Mort Wkly Rep. 1988;37:17-24.
12 El-Khuffashaf, Molloy EJ. Influence of a patent ductus arteriosus on cardiac troponin T levels in preterm infants. J Pediatr. 2008;153:350-353.
13 Morgan JM, Gray HH, Miller GA, Oldershaw PJ. The clinical features, management and outcome of persistence of the arterial duct presenting in adult life. Int J Cardiol. 1990;27:193-199.
14 Wiyono SA, Witsenburg M, de Jaegere PPT, et al. Patent ductus arteriosus in adults—case report and review illustrating the spectrum of disease. Nether Heart J. 2008;16:255-259.
15 Brili SV, Toutouzas P. Patent arterial duct and aortopulmonary window. In: Gatzoulis MA, Webb GD, Daubeney PE, editors. Diagnosis and Management of Adult Congenital Heart Disease. New York: Churchill Livingstone; 2003:247-252.
16 Rapacciulo A, Losi MA, Borgia F, et al. Transcatheter closure of patent ductus arteriosus reverses left ventricular dysfunction in a septuagenarian. J Cardiovasc Med. 2009;10:344-348.
17 Goitein O, Fuhrman CR, Lacomis JM. Incidental finding on MDCT of patent ductus arteriosus: use of CT and MRI to assess clinical importance. AJR Am J Roentgenol. 2005;184:1924-1931.
18 Önbas O, Kantarci M, Koplay M, et al. Congenital anomalies of the aorta and vena cava: 16-detector-row CT imaging findings. Diagn Interv Radiol. 2008;13:163-171.
19 Haramati LB, Glickstein JS, Issenberg HJ, et al. MR imaging and CT of vascular anomalies and connections in patients with congenital heart disease: significance in surgical planning. Radiographics. 2002;22:337-349.
20 Debl K, Djavidani B, Buchner S, et al. Quantification of left to right shunting in adult congenital heart disease: phase-contrast cine MRI compared with invasive oximetry. Br J Radiol. 2009;82:386-391.
21 Chaudhari M, Hasan A. Distal aortopulmonary window: a morphological variation. Asian Cardiovasc Thorac Ann. 2009;17:413-414.
22 Yu T, Zhu X, Tang L, et al. Review of CT angiography of aorta. Radiol Clin North Am. 2007;45:461-483.
23 Fisher RG, Sanchez-Torres M, Whigham CJ, et al. “Lumps” and “bumps” that mimic acute aortic and brachiocephalic vessel injury. Radiographics. 1997;17:825-834.
24 Morse SS, Glickman MG, Greenwood LH, et al. Traumatic aortic rupture: false-positive aortographic diagnosis due to atypical ductus diverticulum. AJR Am J Roentgenol. 1988;150:793-796.
25 Agarwal PP, Chughtai A, Matzinger FRK, et al. Multidetector CT of thoracic aortic aneurysms. Radiographics. 2009;29:537-552.
26 Sachdeva R, Smith C, Greenberg BS, et al. Giant ductal aneurysm in an asymptomatic 4-year-old girl. Ann Thorac Surg. 2009;87:946-948.
27 Lund JT, Jensen MB, Hjelms E. Aneurysm of the ductus arteriosus. A review of the literature and the surgical implications. Eur J Cardiothorac Surg. 1991;5:566-570.
28 Fitoz S, Nuray Unsal N, Tekin M, et al. Contrast-enhanced MR angiography of thoracic vascular malformations in children. Int J Cardiol. 2007;123:3-11.
29 Van der Wal H, Van Geel PP, De Boer RA. Mycotic aneurysm of the aorta as an unusual complication of coronary angiography. Eur J Vasc Endovasc Surg. 2008;36:178-181.

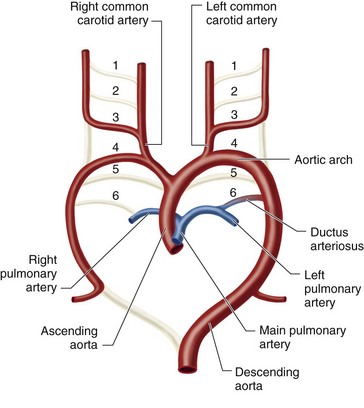
 FIGURE 43-1
FIGURE 43-1
 FIGURE 43-2
FIGURE 43-2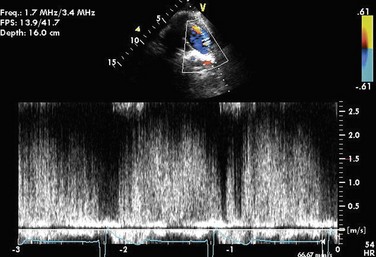
 FIGURE 43-3
FIGURE 43-3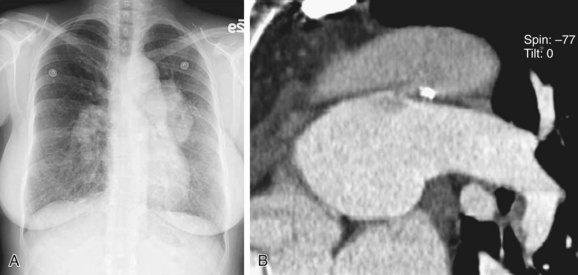
 FIGURE 43-4
FIGURE 43-4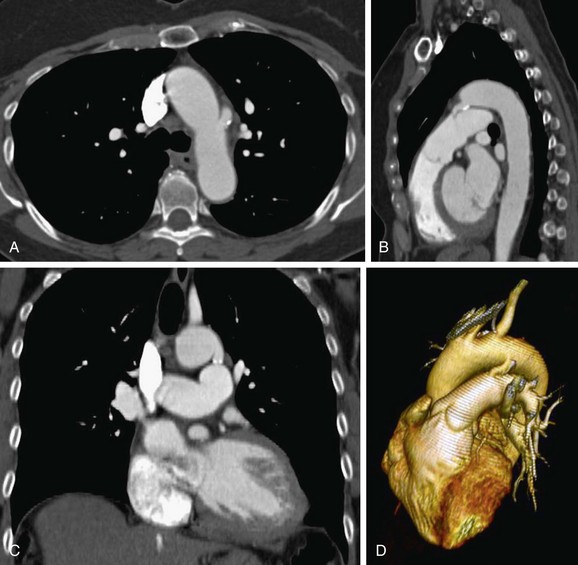
 FIGURE 43-5
FIGURE 43-5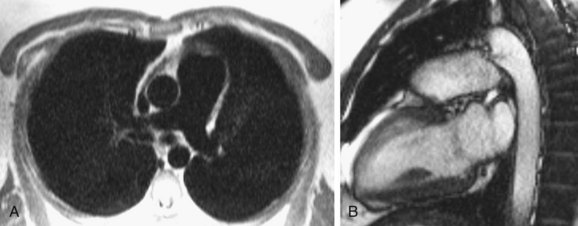
 FIGURE 43-6
FIGURE 43-6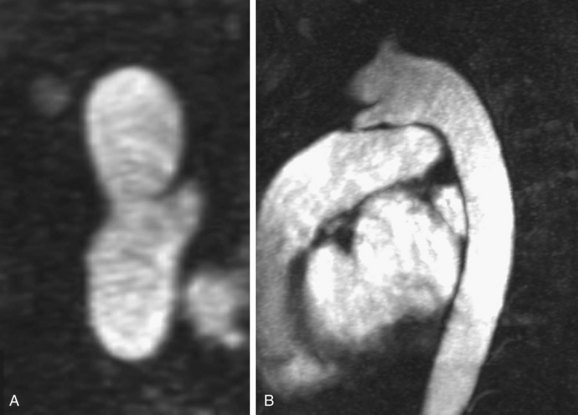
 FIGURE 43-7
FIGURE 43-7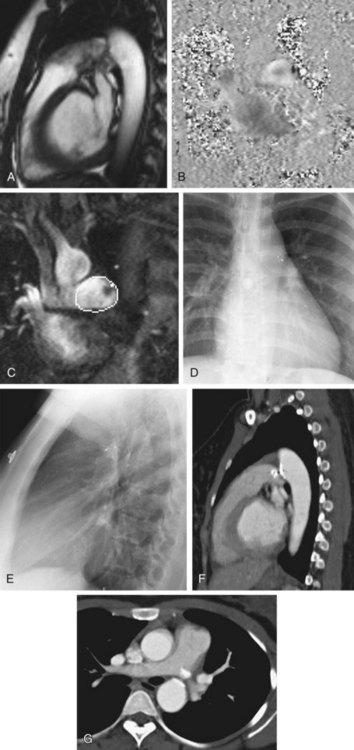
 FIGURE 43-8
FIGURE 43-8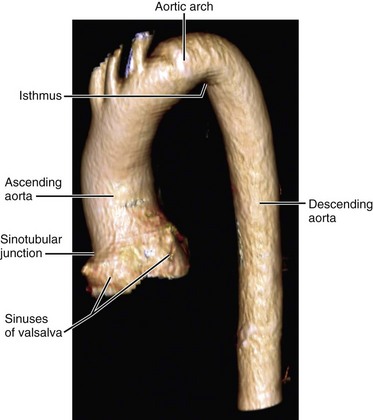
 FIGURE 43-9
FIGURE 43-9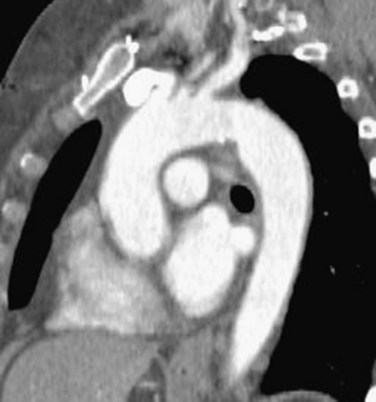
 FIGURE 43-10
FIGURE 43-10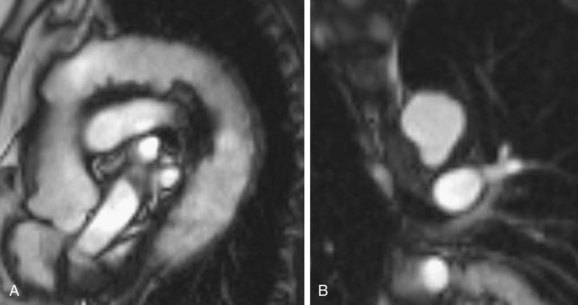
 FIGURE 43-11
FIGURE 43-11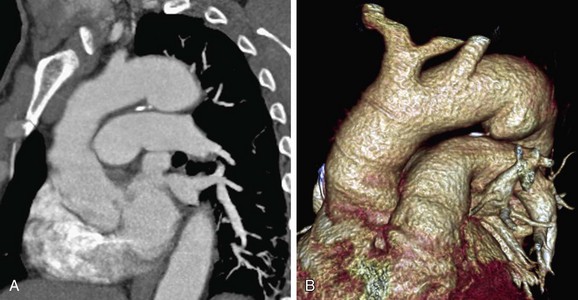
 FIGURE 43-12
FIGURE 43-12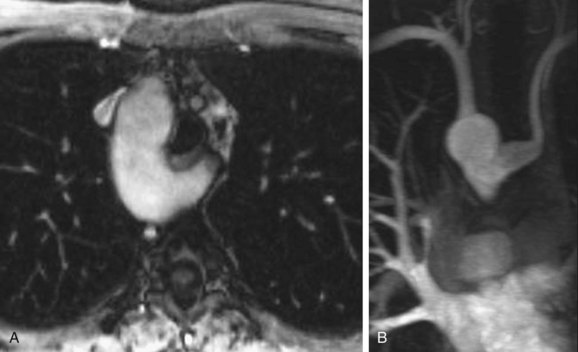
 FIGURE 43-13
FIGURE 43-13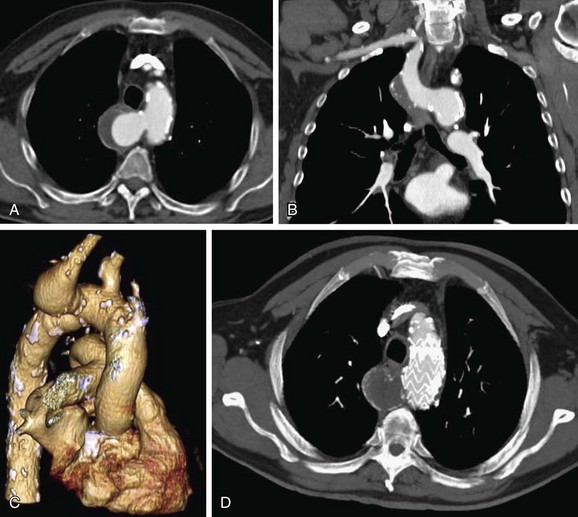
 FIGURE 43-14
FIGURE 43-14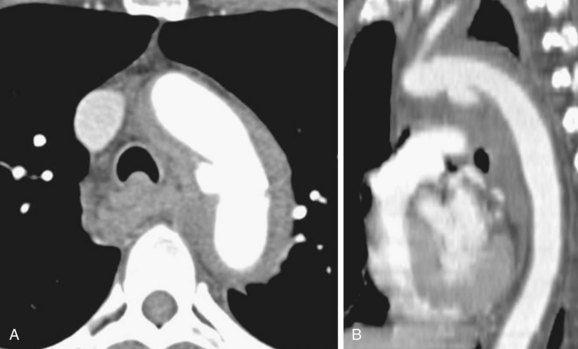
 FIGURE 43-15
FIGURE 43-15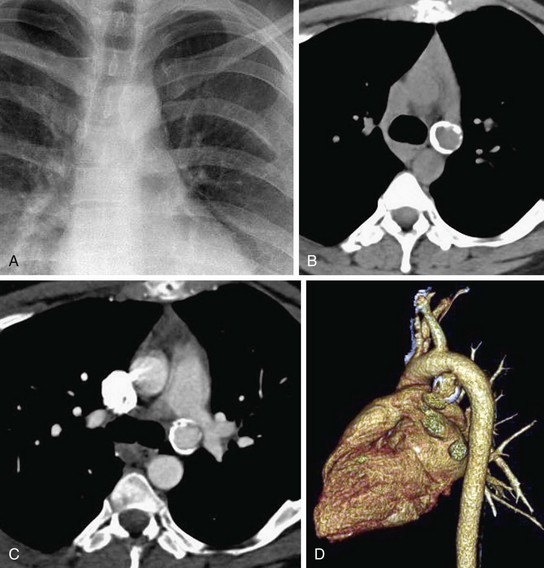
 FIGURE 43-16
FIGURE 43-16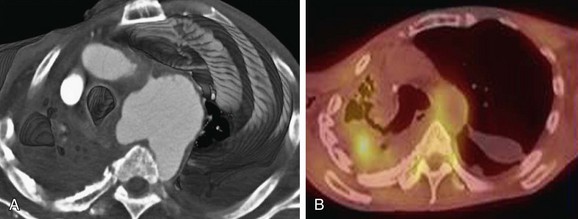
 FIGURE 43-17
FIGURE 43-17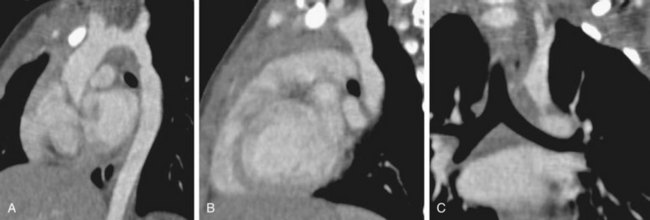
 FIGURE 43-18
FIGURE 43-18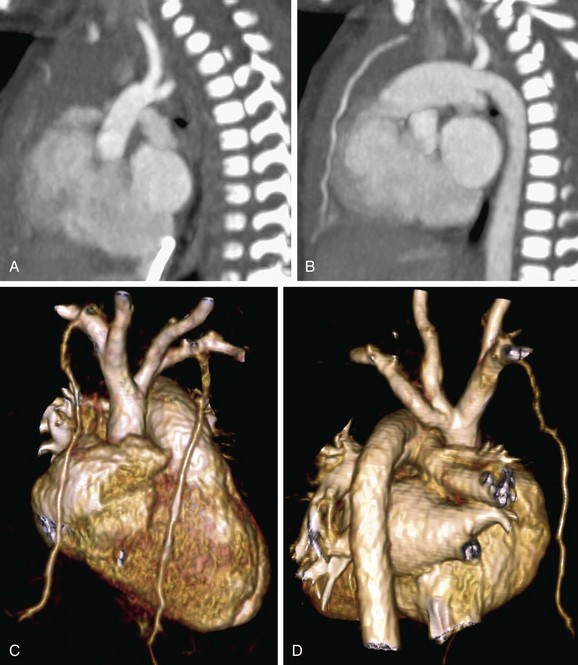
 FIGURE 43-19
FIGURE 43-19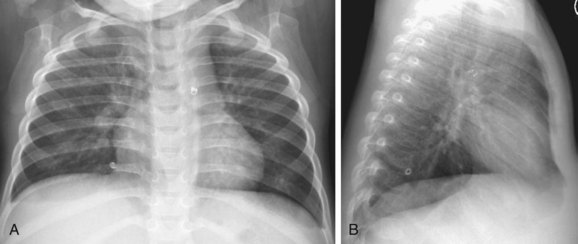
 FIGURE 43-20
FIGURE 43-20


