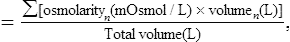7 Parenteral nutrition
Introduction
Malnutrition
Nutrition screening
Routine screening is recommended by the Malnutrition Advisory Group of the British Association of Parenteral and Enteral Nutrition (BAPEN). This group has worked to promote awareness of the clinical significance of malnutrition and has produced guidelines to monitor and manage malnutrition. A range of screening criteria and tools have been developed and refined to assess nutritional status. Examples include the relatively simple and reproducible body mass index tool with consideration of other key factors (Table 7.1), and the BAPEN ‘MUST’ tool (Malnutrition Universal Screening Tool; BAPEN, 2003). Body weight should not be used in isolation; significant weight fluctuations may reflect fluid disturbances, and muscle wasting may be due to immobility rather than undernutrition. More complex anthropometry measurements are sometimes indicated to track changes.
| BMI (kg/m2) | BMI category |
|---|---|
| <18.5 | Underweight |
| 18.5–25 | Ideal BMI |
| 25–29.9 | Overweight |
| >30 | Obese |
Body mass index (BMI) = weight (kg)/height (m)2
Indications for parenteral nutrition (PN)
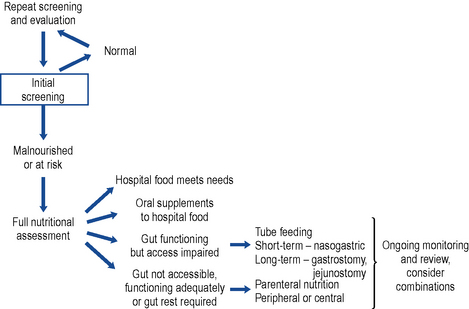
A decision pathway can be followed to guide initial and ongoing nutritional support. While many are published, a locally tailored and regularly updated pathway is favoured. A useful starting point may be found at Fig. 7.1.
Nutrition support teams
A report published by the Kings Fund Report (1992) highlighted the issue of malnutrition both in the hospital and home setting. The findings led to the development of the British Association of Enteral and Parenteral Nutrition (BAPEN) and nutrition support teams throughout the UK. These multidisciplinary nutrition support teams comprise a doctor, nurse, pharmacist and dietitian. They function in a variety of ways, depending on the patient populations and resources. In general, they adopt either a consultative or an authoritative role in nutrition management. Many studies have shown their positive contribution to the total nutritional care of the patient through efficient and appropriate selection and monitoring of feed and route.
Components of a PN regimen
In addition to water, six main groups of nutrients need to be incorporated in a PN regimen (Table 7.2). The aim is to provide appropriate sources and amounts of all the equivalent building blocks in a single daily admixture.
| Oral diet | Parenteral nutrition source |
|---|---|
| Water | Water |
| Protein | L-amino acids mixture |
| Carbohydrate | Glucose |
| Fat with essential fatty acids | Lipid emulsions with essential fatty acids |
| Vitamins | Vitamins |
| Minerals | Trace elements |
| Electrolytes | Electrolytes |
Water volume
Water is the principal component of the body and accounts for approximately 60% and 55% of total body weight in men and women, respectively. Usually, homeostasis maintains appropriate fluid levels and electrolyte balance, and thirst drives the healthy person to drink; however, some patients are not able physically to respond by drinking and so this homeostasis is ineffective. There is risk of over- or underhydration if the range of factors affecting fluid and electrolyte balance is not fully understood and monitored. In general, an adult patient will require 20–40 ml/kg/day fluid; however, Table 7.3 describes other factors that should be considered in tailoring input to needs.
| Consider increasing fluid input | Consider reducing fluid input |
|---|---|
| Signs/symptoms of dehydration | Signs/symptoms of fluid overload |
| Fever: increased insensible losses from lungs in hyperventilation and from skin in sweating. Allow 10–15% extra water per 10°C above normal | High humidity: reduced rate of evaporation |
| Acute anabolic state: increased water required for increased cell generation | Blood transfusion: volume input |
| High environmental temperature or low humidity: increased rate of evaporation | Cardiac failure: may limit tolerated blood volume |
| Drug therapy: assess volume and electrolyte content of infused drug | |
| Abnormal GI loss (vomiting, wounds, ostomies, diarrhoea): consider both volume loss and electrolyte content | |
| Burns or open wound(s): increased water evaporation | |
| Renal failure: fluid may accumulate so reduce input accordingly or provide artificial renal support | |
| Blood loss: assess volume lost and whether replaced by transfusion, colloid, crystalloid |
Amino acids
To balance the patient’s amino acid requirements and the chemical characteristics of the amino acids (solubility, stability and compatibility), a range of commercially available licensed solutions has been formulated containing a range of amino acid profiles (Table 7.4). Aminoplasmal®, Aminoven, Synthamin® and Vamin® are designed for adult patients. The amino acid profiles of Primene® and Vaminolact® are specifically tailored to neonates, infants and children (reflecting the amino acid profile of maternal cord blood and breast milk, respectively).
Table 7.4 Examples of amino acid and consequential nitrogen content of licensed amino acid solutions available in the UK
| Name | Nitrogen content (g/L) | Electrolytes present |
|---|---|---|
| Aminoplasmal® 5% E | 8 | Potassium, magnesium, sodium and dihydrogen phosphate |
| Aminoplasmal® 10% | 16 | |
| Aminoven® 25 | 25.7 | |
| Glamin® | 22.4 | |
| Hyperamine® 30 | 30 | Sodium |
| Primene® 10% | 15 | |
| Synthamin® 9 | 9.1 | Potassium, magnesium, sodium and acid phosphate |
| Synthamin® 9 EF | 9.1 | |
| Synthamin® 14 | 14 | Potassium, magnesium, sodium and acid phosphate |
| Synthamin® 14 EF | 14 | |
| Synthamin® 17 | 17 | Potassium, magnesium, sodium and acid phosphate |
| Synthamin® 17 EF | 17 | |
| Vamin® 9 Glucose | 9.4 | Potassium, magnesium, sodium and calcium |
| Vamin® 14 | 13.5 | Potassium, magnesium, sodium and calcium |
| Vamin® 14EF | 13.5 | |
| Vamin® 18EF | 18 | |
| Vaminolact® | 9.3 |
Energy
Many factors affect the energy requirement of individual patients and these include age, activity and illness (both severity and stage). Predictive formulae can be applied to estimate the energy requirement, for example, the Harris Benedict equation or the more commonly used Schofield equation, which is shown below in Table 7.5.
| Age (years) | Male | Female |
|---|---|---|
| 15–18 | BMR = 17.6 × weight (kg) + 656 | BMR = 13.3 × weight (kg) + 690 |
| 18–30 | BMR = 15.0 × weight (kg) + 690 | BMR = 14.8 × weight (kg) + 485 |
| 30–60 | BMR = 11.4 × weight (kg) + 870 | BMR = 8.1 × weight (kg) + 842 |
| >60 | BMR = 11.7 × weight (kg) + 585 | BMR = 9.0 × weight (kg) + 656 |
BMR, basal metabolic rate.
Dual energy
While effectively maintaining nitrogen balance, lipid inclusion is seen to confer a number of advantages (Box 7.1). Some patients, notably long-term home patients, do not tolerate daily lipid infusions and need to be managed on an individual basis. Depending on the enteral intake and nutritional needs, lipids are prescribed for a proportion of the days. A trial with the newer generation lipid emulsions may be appropriate.
Glucose
Glucose is the recommended source of carbohydrate (1 g anhydrous glucose provides 4 kcal). Table 7.6 indicates the energy provision and tonicity for a range of concentrations. Glucose 5% is regarded as isotonic with blood. The higher concentrations cause phlebitis if administered directly to peripheral veins and should, therefore, be given by a central vein or in combination with compatible solutions to reduce the tonicity.
Table 7.6 Energy provision and tonicity of glucose solutions
| Concentration (w/v) | Energy content (kcal/L) | Osmolarity (mOsmol/L) |
|---|---|---|
| 5% | 200 | 278 |
| 10% | 400 | 555 |
| 20% | 800 | 1110 |
| 50% | 2000 | 2775 |
| 70% | 2800 | 3885 |
Lipid emulsions
Typically, patients receive up to 2.5 g lipid/kg/day. For practical compounding reasons, and assuming clinical acceptance, this tends to be rounded to 100 g or 50 g. Details of lipid emulsions available within the UK can be found in Table 7.7.
Table 7.7 Examples of licensed lipid emulsions available in the UK
| Lipid emulsion type | Details of products with kJ per litre |
|---|---|
| Soybean oil | Intralipid® 10% (4600), 20% (8400), 30% (12600) |
| Purified olive oil/soybean oil | ClinOleic® 20% (8360) |
| Medium chain triglycerides/soybean oil | Lipofundin® MCT/LCT 10% (4430), 20% (8000) |
| Purified structured triglycerides | Structolipid® 20% (8200) |
| Omega-3-acid triglycerides/soybean oil/medium chain triglycerides | Lipidem® (7900) |
| Highly refined fish oil | Omegaven® (4700) |
| Fish oil/olive oil/soybean oil/medium chain triglycerides | SMOFLipid® (8400) |
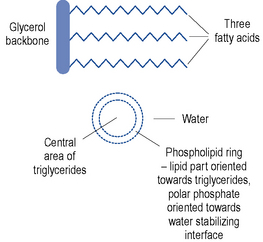
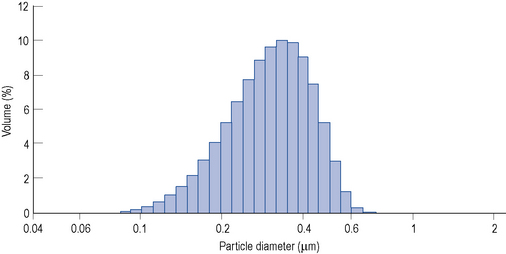
Lipid emulsions are oil-in-water formulations. Figure 7.2 shows the structure of triglycerides (three fatty acids on a glycerol backbone) and a lipid globule, stabilised at the interface by phospholipids. Ionisation of the polar phosphate group of the phospholipid results in a net negative charge of the lipid globule and an electromechanically stable formulation. The lipid globule size distribution is similar to that of the naturally occurring chylomicrons (80–500 nm), as indicated in Fig. 7.3.
Lipid clearance monitoring is particularly important in patients who are at risk of impaired clearance, including those who are hyperlipidaemic, diabetic, septic, have impaired renal or hepatic function or are critically ill (Crook, 2000).
Micronutrients
By the time a patient starts PN, they may have already developed a deficiency of one or more essential nutrients. By the time a specific clinical deficiency is observed, for example, depigmentation of hair in copper deficiency or skin lesions in zinc deficiency, the patient will already have tried to compensate to maintain levels, compromised intracellular enzyme activity and antioxidant systems and expressed non-specific symptoms such as fatigue and impaired immune response. A summary of factors that affect micronutrient needs is presented in Box 7.2.
Box 7.2 Factors affecting micronutrient requirements
Measuring blood levels of vitamins and trace elements in acutely ill patients is of limited value. It is recommended that these are measured every 1–6 months depending on levels, and in patients at home on PN (NICE, 2006). Deficiency states are clinically significant but, with non-specific symptoms, they are often difficult to diagnose.
The micronutrients naturally fall into two groups: the trace elements and vitamins. Micronutrients should be added to all PN infusions under appropriate, controlled, environmental conditions prior to administration (NICE, 2006).
Trace elements
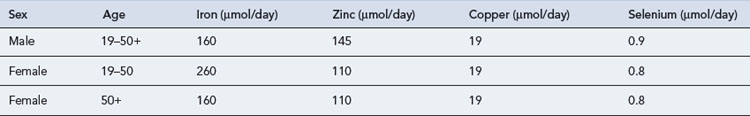
Trace elements are generally maintained at a relatively constant tissue concentration and are present to a level of less than 1 mg/kg body weight. They are essential; deficiency results in structural and physiological disorders which, if identified early enough, can be resolved by re-administration. Ten essential trace elements are known: iron, copper, zinc, fluorine, manganese, iodine, cobalt (or as hydroxycobolamin), selenium, molybdenum and chromium. Adult reference ranges for daily requirements of trace metals can be found in Table 7.8. Currently two preparations are commercially available for adults (Decan®, Additrace®) together with a single paediatric preparation (Peditrace®).
Various recommended baseline doses have been published but no single licensed preparation provides all trace elements at the dose required. Concern over neurotoxicity with accumulated manganese, especially in liver failure, led to a reduction in the advised daily dose and recommendations for plasma monitoring. Recognising the benefits of zinc and selenium on the free radical scavenging system, some specialists advise an increase in the administered dose. Some patients, notably those with burns, renal replacement therapy and/or multiple trauma, as well as long-term patients, may require extra trace elements (Fleming, 1989).
Vitamins
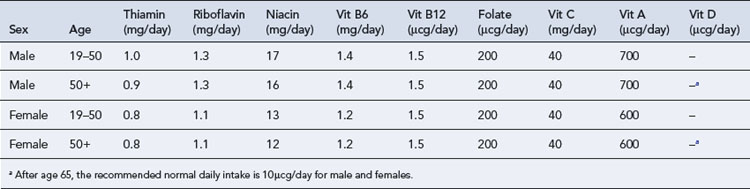
There are two groups of vitamins: the water-soluble vitamins and the fat-soluble vitamins. Fat-soluble vitamins are stored in the body fat, whereas excess water-soluble vitamins are renally cleared; therefore, if there is inadequate provision, deficiency states for the water-soluble vitamins reveal themselves first. The adult reference range for daily requirements of vitamins can be found in Table 7.9. Commercially available preparations are Solivito® N (water soluble), Vitlipid® N Adult/Infant (fat soluble), Cernevit® (water and fat soluble).
In an attempt to reduce the risk of osteoporosis, especially in home PN patients, there have been recent changes to recommended biochemical vitamin D levels (Holick, 2007). Patients on long-term PN should have vitamin D levels measured every 6 months. If low, this should be supplemented in order to help protect against osteoporosis which is a well recognised complication of home PN.
Electrolytes
Electrolytes are included to meet the patient’s needs. Typical daily parenteral requirements are
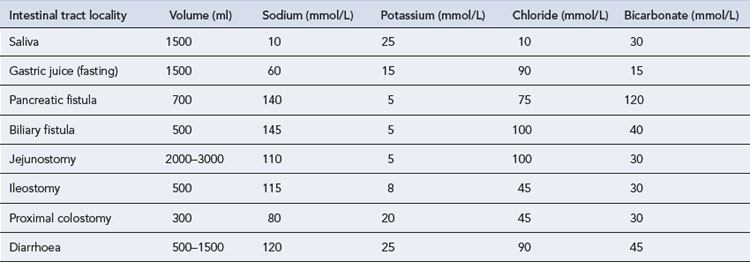
Depending upon the stability of the patient’s clinical state, they are kept relatively constant or adjusted on a near daily basis, reflecting changes in blood biochemistry. Tables, for example, British National Formulary can be used to guide electrolyte replacement if there is excessive gastro-intestinal waste or high losses through burns. Hypophosphataemia should be corrected before starting parenteral or enteral nutrition to avoid the refeeding syndrome. Varying amounts of electrolytes are lost from the different gastro-intestinal secretions. Table 7.10 gives an indication of the content of various gastro-intestinal secretions. This should be taken into account when formulating PN for a patient who may have such losses.
Administration of PN
Routes of administration
PN can be administered peripherally or centrally.
Peripheral route
Some indications and contraindications to the use of the peripheral route are summarised in Box 7.3.
Box 7.3 Indications and contraindications to the use of peripheral parenteral nutrition
Peripheral administration is sometimes complicated or delayed by phlebitis, where an insult to the endothelial vessel wall causes inflammation, redness, pain and possible extravasation. Hot and cold compresses have been used to treat this. A 5 mg glyceryl trinitrate patch placed where the line tip is estimated to be may cause some local vasodilation which is believed to prevent thrombophlebitis (Khawaja and Williams, 1991). Peripheral tolerance can be influenced by a range of factors (Box 7.4).
where n indicates the component.
Standardised formulations
Licensed ready-to-use products
Pharmaceutical issues
Physical stability
Precipitation
Factors affecting calcium phosphate precipitation are shown in Table 7.11. Practical measures can be taken to minimise the risks; these include accurate calculation of the proposed formulation, comparison against professionally defined comprehensive matrices and thorough mixing. Solubility curves and algorithms should be used with extreme caution, even if they are quoted for a specific amino acid source, this is because they do not consider all the factors and do not consistently identify risk. Assuming the sodium content can be tolerated, use of an organic phosphate salt form may be beneficial due to the higher solubility of the sodium glycerophosphate salt form.
Table 7.11 Factors affecting calcium phosphate precipitation
| Factor | Mechanism and effect |
|---|---|
| pH | Low pH supports solubility, whereas a higher pH supports precipitation. Depending on the amount and buffering capacity of the amino acids, this can be affected by different concentrations and sources of glucose solution and acetate salt forms. |
| Temperature | Higher temperatures associated with greater precipitation, increased availability of free calcium to interact and a shift to the more insoluble salt forms. |
| Amino acids | Buffer pH changes. Complex with calcium so less available to react with phosphate. Both the source of amino acid and the relative content are important. |
| Magnesium | Complex with phosphate forming soluble salts rather than less soluble calcium salts. |
| Calcium salt form | Calcium chloride dissociates more readily than calcium gluconate, releasing it to react with the phosphate. |
| Phosphate salt form | Monobasic salts, for example, dipotassium phosphate, dissociate more readily than dibasic salts, for example, potassium acid phosphate, releasing phosphate to react with the calcium. Organic salts, such as sodium glycerophosphate and glucose-1-phosphate, are more stable. |
| Mixing order | Optimum stability achieved by only permitting calcium and phosphate to come together in a large volume admixture. Agitate between additions to avoid pockets of concentration. |
Lipid destabilisation
The naked eye can identify large-scale destabilisation, as shown in Table 7.12; however, the limitations of this method need to be recognised; clinically significant destabilisation might not be visible to the naked eye. In practice, stability laboratories use specialised technical equipment to determine defined criteria so as to establish the physical stability of a formulation. These tests include assessing changes in lipid globule size distribution with optical microscopes, and variety of particle size analysis instruments against the defined limits of pharmaceutical acceptance. A wide safety margin is applied.
| Description | Visual observation | |
|---|---|---|
| Stable, normal emulsion | Lipid globules equally dispersed. Suitable for administration | Normal emulsion |
| Light creaming | Lipid globules rising to the top of the bag. Slight layering visible. Readily redisperses on inverting the bag. Suitable for administration | Light creaming |
| Heavy creaming, flocculation | Lipid globules coming together but not joining. Rising to the top of the bag. More obvious layering visible. Readily redisperses on inverting the bag. Acceptable for administration | Heavy creaming |
| Coalescence | Lipid globules come together, coalesce to form larger globules and rise to the surface. Larger globules join, releasing free oil. Irreversible destabilisation of the lipid emulsion. Not suitable for administration | Cracked. Oil layer viewed close up |
Chemical stability
Chemical stability takes many forms, notably chemical degradation of the vitamins and amino acids.
Filtration
Precautions taken to minimise the particulate load of the compounded admixture must include:
Guidelines have been published that endorse the use of filters, especially for patients requiring intensive or prolonged parenteral therapy, including home patients, the immunocompromised, neonates and children (Bethune et al., 2001). The filter should be placed as close to the patient as possible and validated for the PN to be used. For 2-in-1 formulations, 0.2μm filters may be used, for 3-in-1 formulations, validated 1.2μm filters may be used.
Nutritional assessment and monitoring
Monitoring
PN monitoring has a number of objectives. It should
Complications
Complications of PN fall into two main categories: catheter- related and metabolic (Box 7.5). Overall, the incidence of such complications has reduced because of increased knowledge and skills together with more successful management (Maroulis and Kalfarentzos, 2000).
Box 7.5 Examples of complications during parenteral nutrition
Refeeding syndrome
Patients should be assessed as to their risk of developing refeeding syndrome (see Table 7.13). Refeeding syndrome can be defined as ‘the potentially fatal shifts in fluids and electrolytes that may occur in malnourished patients receiving nutrition’. Undernourished patients are catabolic and their major sources of energy are fat and muscle. As the PN infusion (which contains glucose) starts, this catabolic state is pushed to anabolic which in turn causes a surge of insulin. As the insulin levels increase, there is an intracellular shift of magnesium, potassium and phosphate, and acute hypomanganesaemia, hypokalaemia and hypophosphataemia result. This can cause cardiac and neurological dysfunction and may be fatal. PN should be gradually increased over a period of 2–7 days depending on the patient’s body mass index and risk of developing refeeding syndrome. Oral thiamine and vitamin B compound strong or full dose intravenous vitamin B preparation may be administered before PN is started and for the first few days of infusion.
Table 7.13 Risk factors for developing refeeding syndrome (NICE, 2006)
| One or more of the following: | Two or more of the following: |
|---|---|
| BMI <16 kg/m2 | BMI <18.5 kg/m2 |
| Unintentional weight loss greater than 15% within the last 3–6 months | Unintentional weight loss greater than 10% within the last 3–6 months |
| Little or no nutritional intake for more than 10 days | Little or no nutritional intake for more than 5 days |
| Low levels of potassium, phosphate or magnesium prior to feeding | A history of alcohol abuse or drugs including insulin, chemotherapy, antacids or diuretics |
Specific disease states
Liver
Although abnormal liver function tests associated with short-term PN are usually benign and transient, liver dysfunction in long-term PN patients is one of the most prevalent and severe complications. Its underlying pathophysiology, however, largely remains to be elucidated. The content of PN should be examined and care should be taken not to overfeed with glucose and/or lipid. Supplementation with taurine in the formulation has been reported to ameliorate PN associated cholestasis through promoted bile flow. Various lipid preparations are now available, including preparations containing fish oils which have been reported to be beneficial in reversing liver disease (De Meijer et al., 2009). Lipid emulsions containing a mix of medium and long chain triglycerides are also available and have an improved liver tolerability. Due to the complexity of liver function, the range of potential disorders and its role in metabolism, the use of PN in liver disease is not without problems. Consensus guidelines for the use of PN in liver disease have been published (Plauth et al., 2009). Nutritional intervention may be essential for recovery, although care must be exercised with amino acid input and the risk of encephalopathy, calorie input and metabolic capacity, and the reduced clearance of trace elements such as copper and manganese (Maroulis and Kalfarentzos, 2000). Low-sodium, low-volume feeds are indicated if there is ascites. Cyclic feeding appears useful, especially in steatosis.
Renal failure
Fluid and electrolyte balance demand close attention, and guidelines for nutrition in adult renal failure are available (Cano et al., 2009). A low volume and poor quality urine output may necessitate a concentrated PN formulation with a reduction in electrolyte content, particularly a reduction in potassium and phosphate. In the polyuric phase or the nephrotic syndrome, a higher volume formulation may be required. If there is fluid retention, ideal body weight should be used for calculating requirements rather than the actual body weight.
Intradialytic PN (IDPN) may be administered at the same time as dialysis; however, this is not without complications. High blood sugars and fluid overload can be a problem and there is uncertainty as to how much of the PN is retained by the body and how much is removed by dialysis. Administration requires local guidelines and monitoring to be in place (Foulks, 1999; Lazarus, 1999).
Pancreatitis
Acute pancreatitis is a metabolic stress that requires high-level nutritional support and pancreatic rest to recover. Guidelines for nutrition in acute pancreatitis are available (Gianotti et al., 2009). While enteral nutrition stimulates the pancreas, PN does not appear to. Hyperglycaemia may occur and require exogenous insulin.
Cancer and palliative care
Standard PN may be useful during prolonged periods of gastro-intestinal toxicity, as in bone marrow transplant patients. The use of PN is not thought to stimulate tumour growth (Nitenberg and Raynard, 2000).
Long-term PN
There are an increasing number of patients at home on PN. Scotland and Wales now have designated networks to care for these patients. Many patients are trained to connect and disconnect their PN and to care for their central lines. Patients are encouraged to lead as active and normal life as possible. Foreign travel is now possible for patients on home PN, as are most other normal daily activities and sports (Staun et al., 2009).
Paediatric PN
Nutritional requirements
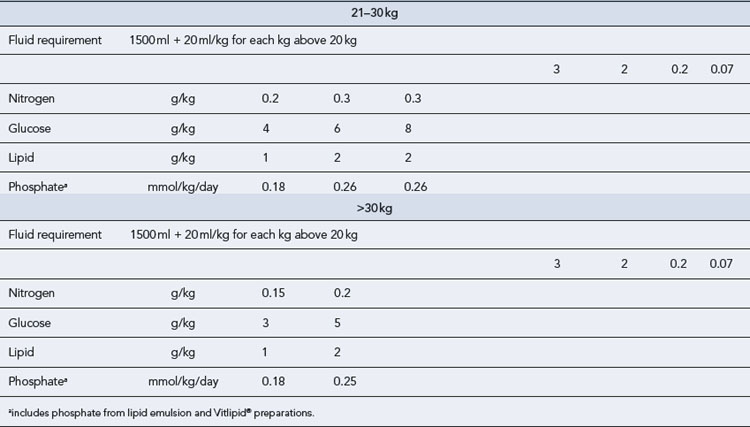
Typical guidelines for average daily requirements of fluid, energy and nitrogen are shown in Table 7.14. The dual-energy approach is favoured in paediatrics. Approximately 30% of the non-protein calories are provided as lipid using a 20% emulsion. Most centres gradually increase the lipid provision from day 1 from 1 g/kg/day to 2 g/kg/day and then 3 g/kg/day, monitoring lipid clearance through the serum triglyceride level. This ensures the essential fatty acid requirements of premature neonates are met.
Route of administration
Case 7.1
| Day | Clinical observation/event | PN changes |
|---|---|---|
| 1 | Admitted to gastroenterology ward from clinic for investigation of chronic diarrhoea and weight loss. Current weight 49 kg, height 1.65 m. Usual weight 55 kg, 1.65 m. Estimated current energy requirement 1500 kcal. | |
| 2 | Contrast study revealed intestinal fistula between small bowel and transverse colon. Diarrhoea approx. 1.5 L/day. | |
| 3 | Case discussed with surgeons. For PN for 2–3 weeks prior to surgery to improve nutritional status. | PN prescribed (considering both fluid and electrolytes from other therapies, and potassium loss from diarrhoea of approx. 30–70 mmol/L): |
| Patient made ‘nil by mouth’. Central line inserted for PN use only. | Volume 2 L, nitrogen 9 g, carbohydrate 400 kcal, lipid 550 kcal, Na+ 230 mmol, K+ 80 mmol, Ca2+ 5 mmol, Mg2+ 11 mmol, phosphate 35 mmol and micronutrients added (daily). | |
| 4 | Biochemistry results: Na+ 129 mmol/L (135–145 mmol/L), K+ 3.2 mmol/L (3.4–5.0 mmol/L), urea 6.9 mmol/L (3.1–7.9 mmol/L), Cr 86 μmol/L (75–1550 μmol/L), corr Ca2+ 2.3 mmol/L (2.12–2.60 mmol/L), Mg2+ 0.75 mmol/L (0.7–1.0 mmol/L), phosphate 0.85 mmol/L (0.80–1.44 mmol/L). | Regimen unchanged. |
| Temperature/pulse/respiration normal, diarrhoea losses reduced to 800 mL/day. | ||
| 5 | Biochemistry results: Na+ 132 mmol/L, K+ 3.1 mmol/L, urea 4.3 mmol/L, Cr 85 μmol/L, corr Ca2+ 2.2 mmol/L, Mg2+ 0.50 mmol/L, phosphate 0.60 mmol/L | PN regimen unchanged. Additional 20 mmol Mg2+ prescribed in 500 mL of saline infused over 6 h. |
| 6 | Biochemistry results: Na+ 138 mmol/L, K+ 3.5 mmol/L, urea 3.0 mmol/L, Cr 86 μmol/L, corr Ca 2.2 mmol/L, Mg2+ 0.78 mmol/L, phosphate 0.9 mmol/L. Diarrhoea reduced to 500 mL/day. | PN regimen changed to: Volume 2 L, nitrogen 11 g, carbohydrate 800 kcal, lipid 800 kcal, Na+ 100 mmol, K+ 80 mmol, Ca2+ 5 mmol, Mg2+ 15 mmol, phosphate 40 mmol. |
| 7 | Biochemistry results: Na+ 139 mmol/L, K+ 4.1 mmol/L, urea 2.8 mmol/L, Cr 78 μmol/L, Mg2+ 0.95 mmol/L, phosphate 1.3 mmol/L. | K+ reduced to 60 mmol, Mg2+ reduced to 10 mmol, phosphate reduced to 30 mmol. |
Questions
Answers
Bethune K., Allwood M., Grainger C., et al. Use of filters during the preparation and administration of parenteral nutrition: position paper and guidelines prepared by a British Pharmaceutical Nutrition Group Working Party. Nutrition. 2001;17:403-408.
British Association of Parenteral and Enteral Nutrition. Malnutrition Universal Screening Tool. Available at www.bapen.org.uk/musttoolkit.html, 2003.
Cano N.J.M., Aparicio M., Brunori G., et al. ESPEN guidelines on parenteral nutrition: acute renal failure. Clin. Nutr.. 2009;28:401-414.
Crook M.A. Lipid clearance and total parenteral nutrition: the importance of monitoring of plasma lipids. Nutrition. 2000;16:774-775.
De Meijer V.E., Gura K.M., Le H.D. Fish oil-based emulsions prevent and reverse parenteral nutrition associated liver disease: the Boston experience. J. Parent. Ent. Nutr.. 2009;33:541-547.
Department of Health. Dietary Reference Values for Food Energy and Nutrients for the United Kingdom. London: Report of the panel on dietary reference values of the committee on medical aspects of food policy. DH; 1991.
Fleming C.R. Trace element metabolism in adult patients requiring total parenteral nutrition. Am. J. Clin. Nutr.. 1989;49:573-579.
Foulks C.J. An evidence-based evaluation of intradialytic parenteral nutrition. Am. J. Kidney Dis.. 1999;33:186-192.
Gianotti L., Meier R., Lobo D., et al. ESPEN guidelines on nutrition in pancreatitis. Clin. Nutr.. 2009;28:428-435.
Holick M. Vitamin D deficiency. N. Engl. J. Med.. 2007;357:266-281.
Khawaja H.T., Williams J.D. Transdermal glyceryl trinitrate to allow peripheral total parenteral nutrition: a double blind placebo controlled feasibility study. J. R. Soc. Med.. 1991;84:69-72.
Kings Fund Report. A Positive Approach to Nutrition as Treatment. London: Kings Fund; 1992.
Koletzko B., Goulet O., Hunt J. Guidelines on paediatric parenteral nutrition of the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the European Society for Clinical Nutrition and Metabolism (ESPEN), supported by the European Society of Paediatric Research (ESPR). J. Pediatr. Gastroenterol. Nutr.. 2005;41:S1-S4. Available at http://espghan.med.up.pt/joomla/position_papers/con_22.pdf
Lazarus J.M. Recommended criteria for initiating and discontinuing intradialytic parenteral nutrition. Am. J. Kidney Dis.. 1999;33:211-216.
Maroulis J., Kalfarentzos F. Complications of parenteral nutrition at the end of the century. Clin. Nutr.. 2000;19:299-304.
National Institute for Health and Clinical Excellence. Nutrition Support in Adults CG32. London: NICE; 2006. Available at http://www.nice.org.uk/guidance/CG32
Nitenberg G., Raynard B. Nutritional support of the cancer patient: issues and dilemmas. Crit. Rev. Oncol./Haematology. 2000;34:137-168.
Plauth M., Cabre E., Canpillo B., et al. ESPEN guidelines on parenteral nutrition: hepatology. Clin. Nutr.. 2009;28:436-444.
Staun M., Pironi L., Bozzetti F., et al. ESPEN guidelines on parenteral nutrition: home parenteral nutrition (HPN) in adult patients. Clin. Nutr.. 2009;28:467-479.
Austin P., Stroud M., editors. Prescribing Adult Intravenous Nutrition. London: Pharmaceutical Press, 2007.
Ainsworth S.B., Clerihew L., McGuire W. Percutaneous central venous catheters versus peripheral cannulae for delivery of parenteral nutrition in neonates. Cochrane Database Syst. Rev.. (3):2007. Art. No.: CD004219. doi:10.1002/14651858.CD004219.pub3
Baskin J.L., Pui C.H., Reiss U., et al. Management of occlusion and thrombosis with long-term indwelling central venous catheters. Lancet. 2009;374:159-169.
Goulet O. Some new insights in intestinal failure-associated liver disease. Curr. Opin. Org. Transplant.. 2009;14:256-261.
Lloyd D.A., Gabe S.M. Managing liver dysfunction in parenteral nutrition. Proc. Nutr. Soc.. 2007;66:530-538.
Misra S., Kirby D.F. Micronutrient and trace element monitoring in adult nutrition support. Nutr. Clin. Pract.. 2000;15:120-126.
Schofield. Equations for estimating basal metabolic rate (BMR). Clin. Nutr.. 1985;39C:5-41.
Shenkin A. Trace elements and inflammatory response: implications for nutritional support. Nutrition. 1995;11:100-105.