Parathyroid
HYPERPARATHYROIDISM AND PARATHYROIDECTOMY
Appraise
1. The multiple symptoms of primary HPT can be summarized by the aphorism ‘bones, stones, groans, and psychic moans’. However, 80% of patients are asymptomatic and present incidentally with the finding of hypercalcaemia on routine blood testing for other medical conditions. Very rarely, patients may present with significant bone disease (osteitis fibrosa cystica), most clearly seen on X-rays of the middle phalanges showing subperiosteal bone resorption. This presentation tends to occur more frequently in severe longstanding disease, parathyroid cancer or secondary and tertiary hyperparathyroidism.
2. The biochemical diagnosis of hyperparathyroidism requires the demonstration of persistently raised levels of serum calcium with an associated high serum parathormone (PTH) level, or a level that is inappropriately normal in the presence of high serum calcium levels. Normocalcaemia does not exclude hyperparathyroidism and some patients with renal stones have been shown to have high PTH levels in the presence of a normal serum calcium. Always consider and exclude other causes of hypercalcaemia. In particular, familial hypercalcaemic hypocalciuria (FHH) can be confused with it since it presents a similar biochemical profile. Order urinary calcium estimations to exclude this condition. Hypocalciuria (less than 2 mmol/day) should prompt the diagnosis of familial hypercalcaemic hypocalciuria.
3. You have a responsibility to ensure that, whatever the referral pathway, potential reversible causes of secondary hypercalcaemia (including lithium therapy and vitamin D deficiency) have been excluded, and hereditary conditions identified. The early diagnosis of familial disease is important since the management differs from primary to secondary HPT. The syndromal acronyms are: FHH, ADMH, NSHPT, MEN1, MEN2a, HPT-JT, HPT-IF. (Refer to the landmark article on these acronyms by Marx et al. 2002.)
4. A serum calcium level in excess of 3 mmol/L is an absolute indication for surgery. Consider symptomatic patients for surgery. However, the majority are asymptomatic patients with the discovery of incidental hypercalcaemia during biochemical analysis. A more liberal approach to surgery for asymptomatic patients is developing (see Proceedings of the 3rd International Workshop JCEM 2009).
5. There is increasing evidence that patients aged 70 years or less have a higher mortality with untreated disease and that this can be reversed by successful parathyroid surgery. Cardiac changes have been shown to be reversed after treatment of asymptomatic disease and symptoms of depression and anxiety may be reversed. Loss of bone mineral content has been shown to be partially reversible, an important factor in postmenopausal women.
6. In secondary hyperparathyroidism, elevated calcium levels can make control of dialysis programmes difficult and can produce significant pathological bone change. It is often accompanied by severe pruritus, muscle weakness and sometimes with soft-tissue calcification. The timing and indications for surgery very much rely on the renal physicians.
7. Once the decision to operate has been made, decide the type of operation needed. In primary HPT, this decision depends upon the patient’s wishes, your competence in the subspecialty, and the success of preoperative localization.
8. The standard operation is a four-gland neck exploration through a cervical collar incision made in a similar way as for exploring the thyroid (see Chapter 20).
9. In 80% of cases, the causative lesion is a solitary parathyroid adenoma, and successful preoperative localization of such a lesion enables you to offer minimally invasive parathyroidectomy using a targeted approach under general or local anaesthesia.
10. In 15–20% of cases, HPT is caused by parathyroid hyperplasia, which may be genetically predetermined. Here, preoperative imaging is far less successful at localizing the causative pathology, and the four-gland exploration is usually more appropriate. Both the targeted approach and four-gland exploration are performed endoscopically in some centres.
11. Explain in preoperative counselling that the same potential risks exist in parathyroid surgery as can occur after thyroid surgery, including recurrent laryngeal nerve injury. There is also the additional risk of failing to find the gland at the first operation, often because it is ectopic.
Prepare
1. An experienced parathyroid surgeon can cure up to 95% of patients without the benefit of preoperative localization, employing the traditional and more extensive four-gland exploration. However, preoperative localization techniques are now commonly used prior to an initial targeted neck exploration. This is in response to the increasing popularity of daycase parathyroidectomy, minimally invasive parathyroidectomy (MIP), and parathyroidectomy under local anaesthesia.
2. Nuclear scintigraphy is seen as the most accurate localization technique. Technetium Tc 99 m sestamibi, either with a subtraction technique using radio-iodine (I123) or used alone in a ‘double-phase scan’ where the scan is repeated at 2–3 hours, gives the best results. It can localize solitary adenomata in 95% of cases and multigland disease in 80%. The double-phase method relies on the differential washout rate of sestamibi from parathyroid tissue compared to thyroid tissue as a result of the high metabolic rate of the parathyroids, particularly when adenomatous.
3. Ultrasonography is also frequently employed. Results are operator dependent, but in the best hands solitary adenomas can be identified in 93% of cases. Colour Doppler Ultrasound is of limited value when glands are located in the mediastinum.
4. In the detection of ectopic glands, particularly when other tests have failed or following unsuccessful exploration and especially in the identification of suspected mediastinal glands, other forms of imaging may be valuable. Apart from CT or MRI, three-dimensional imaging techniques can be such as single photon emission computed tomography (SPECT). Positron emission tomography (PET) may be carried out either with (18F)-fluoro-2-deoxy-D-glucose or (11C)-methionine.
5. Venous sampling can be employed if less invasive imaging techniques have failed, when planning revision surgery. This involves central venous catheterization and the subsequent collection of blood samples, measuring intact PTH levels, from the draining veins of the thyroid plexus. Intact PTH assays are measured. Although interpretation of results is hampered somewhat by previous surgery(s) it can be very useful in rough localization of the gland(s) by determining the side, whether it is higher or lower in the neck, or likely to be in the superior mediastinum and thus direct the general area of re-exploration.
6. The patient needs to be well hydrated: if the patient has been admitted in a hypercalcaemic crisis then a period of re-hydration is mandatory before attempting surgery.
STANDARD OPERATION: FOUR-GLAND EXPLORATION
Access
1. With the patient in a supine position, and with approximately 30 degrees of reverse Trendelenberg to decrease venous engorgement, extend the neck where possible by appropriate placement of a head ring and sand bag.
2. Make a standard Kocher incision situated midway between the cricoid cartilage and the jugular notch. In most cases it need only be 5 cm long. Continue it through the platysma muscle to the level of the superficial layer of strap muscles. Raise the superior myocutaneous flap to a level at least 2 cm above the cricoid cartilage.
3. Identify the linea alba then separate the strap muscles. Now peel the strap muscles from the ventral surface of one of the thyroid lobes. Begin the search for the parathyroid glands by dislocating the thyroid lobe medially and anteriorly whilst retracting the strap muscles laterally. It is often necessary to divide the middle thyroid vein. Use blunt dissection with pledgets, mosquito forceps and bipolar diathermy division of fine vessels, to maintain absolute haemostasis. In a series of 547 autopsies Gilmour demonstrated that 6% of patients have three and 6% have five glands. In 80% of cases, the position of both the superior and inferior glands on each side is symmetrical – useful to recall when a gland proves difficult to locate.
4. While searching for the superior parathyroid gland the inferior thyroid artery can be readily identified deep to the carotid sheath, usually around the mid-part of the thyroid lobe. Follow it to the thyroid lobe. Now identify the recurrent laryngeal nerve (see Chapter 20). The superior parathyroid glands usually lie behind the upper pole of the thyroid lobe, close to the cricothyroidius muscle. The majority of superior parathyroids are within 1 cm of the cricothyroid joint, above the main branch of the inferior thyroid artery in a plane deep to the recurrent laryngeal nerve. It may be closely adherent to the recurrent laryngeal nerve.
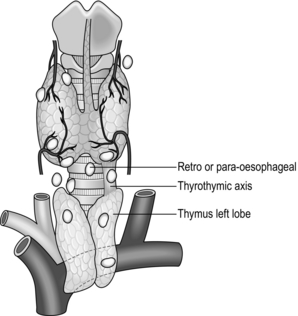
5. The inferior parathyroid glands develop together from the third pouch and descend with the thymus. The majority settle in the neck along this line of descent and separate somewhat from the thymus, which continues into the chest leaving a thyrothymic tract in the neck in which the parathyroid is frequently situated.
6. Mobilize the inferior pole, inspecting particularly in the region of the thyrothymic tract. The gland normally lies on or just below the inferior pole of the thyroid lobe and ventral to the coronal plane of the recurrent nerve. In about 15% of patients it lies distinctly separate from the gland and in the upper pole of the thymus. Its position often allows the excision of an inferior adenoma without recurrent laryngeal nerve identification, provided the dissection proceeds directly on the surface of the gland.
7. The parathyroid glands (often pale brown or tan coloured) are enclosed in an envelope of fat that moves separately from the thyroid. There is usually a small feeding vessel which can be cauterized, ensuring that the gland is not in close proximity to the recurrent laryngeal nerve.
8. During the operation, glands judged macroscopically normal are commonly marked with a Ligaclip. Macroscopically abnormal gland(s) are removed and sent for histology. The procedure can be enhanced by intra-operative frozen section and ioPTH, the latter procedure to confirm cure following a successful dissection, or to point to the need for further exploration.
9. An increasingly difficult problem arises in older people with milder disease forms who are now operated on earlier in their disease process than was previously the case. A solitary adenoma and three ‘normal glands’ can be difficult to distinguish from hyperplasia affecting mainly one gland with mild hyperplasia of the other three. This requires great judgment, and in case of doubt elect to remove three and a half glands. Similarly, if more than one gland is clearly involved, or if this is hyperplasia either from primary or secondary hyperparathyroidism, then elect to remove three and a half glands, leaving approximately 30–50 mg of functional tissue. We advocate this approach as the best way to minimize the risk of recurrence whilst preserving parathyroid function. Some authorities advocate the removal of all four glands, then place the patient postoperatively on calcium and vitamin D supplements to minimize recurrence and surgical failure.
10. Further intra-operative localization strategies are available:
 Methylthioninium chloride (methylene blue) is a total-body infusion technique. Methylene blue (5 mg/kg body weight) in 5% dextrose is infused an hour prior to surgery: timing of infusion to surgery is crucial. The dye is preferentially taken up by the parathyroids (> 90%), particularly when adenomatous or hyperplastic, making their identification easier. Warn the anaesthetist that this may affect oxygen saturation but it is not generally significant. Monitor the patient closely, since the infusion occasionally produces adverse reactions. Anaphylaxis is extremely rare and demands orthodox treatment.
Methylthioninium chloride (methylene blue) is a total-body infusion technique. Methylene blue (5 mg/kg body weight) in 5% dextrose is infused an hour prior to surgery: timing of infusion to surgery is crucial. The dye is preferentially taken up by the parathyroids (> 90%), particularly when adenomatous or hyperplastic, making their identification easier. Warn the anaesthetist that this may affect oxygen saturation but it is not generally significant. Monitor the patient closely, since the infusion occasionally produces adverse reactions. Anaphylaxis is extremely rare and demands orthodox treatment.
 Gamma probe identification can be carried out on a radioisotope injected preoperatively and selectively concentrated within parathyroid tissue. Technitium 99m sestamibi is the usual choice. Success depends on strict timing between radioisotope injection and time of surgery. It is not in widespread use but has gained some acceptance in MIP.
Gamma probe identification can be carried out on a radioisotope injected preoperatively and selectively concentrated within parathyroid tissue. Technitium 99m sestamibi is the usual choice. Success depends on strict timing between radioisotope injection and time of surgery. It is not in widespread use but has gained some acceptance in MIP.
 Intra-operative PTH (ioPTH) serum assay. In 1987, the development of an immunochemiluminescent assay for human PTH paved the way for intra-operative PTH monitoring (ioPTH) as a reliable indicator of success during parathyroidectomy. An approximate turnaround time of 10 minutes makes ioPTH monitoring a useful tool, particularly in minimal access, localized and daycase resections. In addition, once an adenoma has been removed, it is unnecessary to biopsy the remaining apparently normal glands to ensure no hypersecreting gland remains, thus avoiding the associated risk of prolonged postoperative hypocalcaemia.
Intra-operative PTH (ioPTH) serum assay. In 1987, the development of an immunochemiluminescent assay for human PTH paved the way for intra-operative PTH monitoring (ioPTH) as a reliable indicator of success during parathyroidectomy. An approximate turnaround time of 10 minutes makes ioPTH monitoring a useful tool, particularly in minimal access, localized and daycase resections. In addition, once an adenoma has been removed, it is unnecessary to biopsy the remaining apparently normal glands to ensure no hypersecreting gland remains, thus avoiding the associated risk of prolonged postoperative hypocalcaemia.
Postoperative
1. The vast majority of patients undergoing parathyroid surgery do not run into problems with hypocalcaemia postoperatively. The small number of patients who do can sometimes be predicted. They are those who have significant bone disease, those who have significant metabolic biochemical disorders and those who start with calcium levels in excess of 3.25 mmol/L.
2. Take baseline calcium and PTH levels at the completion of parathyroid surgery. Take further tests according to patient symptoms. Generally take a further sample the following morning either as an outpatient or inpatient. Where there is the possibility of permanent hypoparathyroidism, check serum calcium and possibly PTH again, approximately twice daily. The trend in serum calcium indicates the likely course of action. The serum calcium levels fall within the first few hours but the time of maximum fall of calcium levels is on the 5th postoperative day, by which time most patients have been discharged. Warn patients of symptoms of tingling in the hands, or of tetany (Greek: teinein = to stretch; muscle twitching, spasm, cramps). Advise them to report back to hospital if they suffer from these symptoms.
3. Asymptomatic hypocalcaemia to a level of about 2 mmol/L does not, as a rule, need any specific treatment and usually spontaneously corrects within 24–48 hours. Calcium levels below 2 mmol/L, or where there are symptoms of hypocalcaemia such as tetany and tingling, are usually treated acutely with a combination of intravenous infusion of 10% calcium gluconate plus oral calcium supplements. In mild cases, oral calcium supplementation alone resolves symptoms and maintains acceptable serum calcium level, although more severe cases require One-Alpha calciferol in addition. Resulting from the advent of short-stay surgery, a downward trend is best managed with supplementation for 1 week or longer with twice-weekly calcium checks. Treatment can be with oral calcium alone (1g tds or 2g bd) if the calcium level hovers around 2 mmol/L and above, otherwise add One-Alpha calciferol; 0.25 μg bd controls most, but some patients require 0.5 μg bd. Patients can safely be discharged on this; then, at follow-up within 1 week or 2 in outpatients, monitor their calcium levels and attempt to withdraw the supplements. Temporary hypoparathyroidism usually lasts only for days or weeks. Consider hypoparathyroidism to be permanent when calcium and/or vitamin D supplementation are still required at 3 months (or at 6 months by some authors).
4. In the case of solitary adenomas it is usual to see patients once more at about 3 months, when they can safely be discharged. Keep under review patients who have hyperplasia, because after some years there is a tendency for the remnant half gland to enlarge and become reactive.
RE-OPERATION
The reasons for surgical failure in order of frequency are: inadequate cervical exploration, failure to diagnose or adequately resect multigland disease, gland ectopia (Fig. 21.1) and the wrong diagnosis. When failure occurs, the first step is to reconfirm the diagnosis. Other possible diagnoses, in particular familial hypercalcaemic hypocalciuria, pseudohyperparathyroidism and sarcoidosis must be excluded. Secondly, the requirement for curative surgery must be re-evaluated and its necessity reconfirmed. Patients without bony or renal complications, or normocalcaemic patients with an elevated PTH, for example, may opt to be observed rather than to undergo further surgery with increased complication rates and decreased cure rates. If re-exploration is judged appropriate, the histological specimens and operative notes from the first exploration should be examined, as these will often help in pointing to the likely location of the missing gland.
Bilezikian, J.P., Khan, A.A., Potts, J.T., et al. Guidelines for the management of asymptomatic primary hyperparathyroidism: Summary Statement from the Third International Workshop. J Clin Endocrinol Metab. 2009; 94:335–339.
British Association of Endocrine and Thyroid Surgeons. Guidelines for the surgical treatment of endocrine disease and training requirements for endocrine surgery. Available at www.baets.org.uk. Accessed Dec 2012.
Marx, S.J., Simonds, W.F., Agarwal, S.K., et al. Hyperparathyroidism in hereditary syndromes: special expressions and special managements. J Bone Miner Res. 2002; 17(2):N37–43.
Miccoli, P., Berti, P., Materazzi, G., et al. Results of video-assisted parathyroidectomy: single institution’s six-year experience. World J Surg. 2004; 28:1216–1218.
Palazzo, F.F., Sadler, G.P. Minimally invasive parathyroidectomy. Br Med J. 2004; 328:849–850.
Palestro, C.J., Tomas, M.B., Tronco, G.G. Radionuclide imaging of the parathyroid glands. Semin Nucl Med. 2005; 35:266–276.
Irvin, G.L., Molinari, A.S., Figueroa, C. Improved success rate in reoperative parathyroidectomy with intraoperative PTH assay. Ann Surg. 1999; 229:874–878.
Udelsman, R., Pasieka, J.L., Sturgeon, C., et al. Surgery for Asymptomatic Primary Hyperparathyroidism: Proceedings of the Third International Workshop. J Clin Endocrinol Metab. 2009; 94:366–372.