Oesophageal cancer
INTRODUCTION
1. The epidemiology of squamous oesophageal carcinoma is the most varied of any tumour, so that in certain geographical regions there is a very high incidence and in other areas it is rare. Squamous cell carcinoma of the oesophagus often shows multicentric occurrence in association with head and neck tumours, suggesting that environmental factors are important. Many such factors have been postulated, including smoking, alcohol, dietary nitrosamines, fungal contamination, pickled vegetables, hot tea, gruel, the habit of chewing betel leaf and areca nut and deficiency of certain vitamins and trace elements. A number of benign diseases predispose to its development, including corrosive alkali burns, achalasia, the Paterson-Kelly-Plummer-Vinson syndrome and a history of irradiation. Studies have suggested a link between enzymatic polymorphism related to alcohol degradation (ALDH2) and the development of squamous cell carcinoma of the oesophagus. Genetic predispositions include the autosomal dominant condition tylosis (Greek: tylos = callus) which results in hyperkeratosis of the skin, papillomas and eventually squamous carcinoma of the oesophagus. On the other hand, Barrett’s oesophagus is known to increase the risk of the development of adenocarcinoma: the incidence of adenocarcinoma of the lower oesophagus and gastric cardia is rapidly increasing in Western countries, although it is not clear if this increase is due to an increase in gastro-oesophageal reflux disease (GORD; gastro-oesophageal, hence GERD in the USA) and Barrett’s oesophagus.
2. The oesophagus runs from the neck through the thorax and into the abdomen. Apart from the cervical region, the oesophagus is divided into upper, middle and lower thirds, although the abdominal segment is sometimes considered separately. Approximately half of all squamous carcinomas develop in the middle third of the thoracic oesophagus. A much larger number of adenocarcinomas develop in the lower third in Western countries.
3. The tumour spreads circumferentially and longitudinally within the mucosa (intraepithelial spread) and in the submucosa and muscle layer, either continuously or separate from the main tumour (intramural metastasis). It may invade the trachea, bronchi, lungs, thoracic duct, recurrent laryngeal nerves, pericardium and aorta. Spread to lymph nodes, both local and distant, is common: detailed Japanese studies have shown that even upper-third carcinomas may be associated with nodal metastases inside the abdomen, while lower-third tumours involve the supraclavicular nodes in a significant proportion of patients. Haematogenous spread is relatively late, and affects the liver, lungs and bones.
4. Because of its insidious development, many patients present with advanced disease, complaining of dysphagia, weight loss, substernal or back pain, aspiration pneumonia or hoarseness. Supraclavicular lymph nodes may be palpable on presentation. The prognosis at this stage is very poor.
5. In Japan, a large number of asymptomatic squamous cell carcinomas are detected through endoscopic examinations at the time of routine health checks, and many of them are treated with endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD). Such local treatment is highly curative as long as the tumour invasion is limited to the epithelium and lamina propria mucosae.
6. Contrast radiography typically demonstrates a long, irregular stricture. However, early lesions produce only slight or even no mucosal irregularities on contrast barium swallow films. The diagnosis is confirmed by endoscopy with biopsy. The technique of Lugol’s iodine staining is useful as abnormal areas of mucosa (including carcinoma, oesophagitis, ectopic gastric mucosa and Barrett’s epithelium) remains unstained. Newer technologies of image enhanced endoscopy such as narrow band imaging (NBI) and flexible spectral imaging colour enhancement (FICE) may make early detection easier. Biopsy specimens should be taken from all suspicious lesions, where there is an irregularity or a colour change. Take a number of biopsy specimens and record the levels at which they are taken. Do not take many biopsy specimens from one lesion, particularly when the lesion may be suitable for endoscopic resection, because biopsies often result in submucosal fibrosis and may make later intervention difficult.
7. If the cytological brushings and biopsy specimens are reported to be normal but you suspect carcinoma, repeat the examination and go on repeating it until you are absolutely sure that you are not missing a cancer. Cancers located in the hypopharynx, cervical and the thoracic inlet oesophagus are the most difficult to detect. They are very often overlooked by otolaryngologists, radiologists and endoscopists. Particular effort should be taken at endoscopy to observe the hypopharynx, including both piriform sinuses, at the beginning of the examination and the oesophageal inlet on removal of the scope to avoid misdiagnosis.
8. Other valuable imaging modalities are computed tomography (CT), endoscopic ultrasonography and magnetic resonance imaging: these provide information on tumour size, invasion of contiguous structures and extent of lymph node involvement. Conventional endoscopic ultrasonography images the normal oesophageal wall as five to seven concentric layers; high-frequency ultrasound machines can visualize a nine-layered structure, allowing the depth of penetration to be estimated with greater accuracy. Ensure that CT scans include the upper abdomen and also the lower half of the neck, since enlarged lymph nodes that are not easily palpable may otherwise be missed. Conventional abdominal and cervical ultrasonography is also useful to detect abdominal and cervical nodal involvement, but remember that lymph node size is not diagnostic of metastasis: micrometastases are common and reactive hypertrophy of lymph nodes without metastasis is also frequent. FDG-PET is becoming routine for preoperative staging as it provides metabolic evidence of occult metastases, albeit with a significant incidence of both false negative and false positive results. Perform bronchoscopy with a flexible instrument in patients with middle- and upper-third advanced tumours to determine if there is invasion of the trachea or bronchi.
9. Preoperative staging should now be undertaken using the TNM classification of the International Union against Cancer (Table 9.1).
10. Carry out a careful assessment of the patient to exclude or confirm the presence of incidental disease, particularly that of the cardiovascular and respiratory systems.
11. Recent advances in the field of chemoradiotherapy (CRT) are rapidly changing the management of this disease. Although surgery remains the standard treatment for lesions where R0 resection is possible an increasing number of patients, particularly those with advanced disease, are treated with chemoradiation as the first mode of treatment.
12. As with many other fields of surgery, oesophagectomy has been influenced by the current trend towards minimally invasive surgery, and nowadays even Japanese-style extended radical oesophagectomy can be performed via Video-assisted thoracic surgery (VATS). Although the operation may not be truly minimally invasive because of the necessity for extended lymphadenectomy, the advantages of video-assisted surgery have been recognized.
Appraise
1. Because oesophageal carcinoma is so often advanced at the time of diagnosis, some surgeons feel that treatment should be palliative, thus depriving patients who might be cured of the opportunity to benefit from a radical approach. Our experience of over 25 years of extensive lymph node dissection proves that even patients with ‘distant’ lymph node metastases regarded as M1 in the TNM system still have a chance of being cured by surgery. With the recent advance of non-surgical treatments, some T4 tumours have also become candidates for radical operation after neoadjuvant therapy.
2. Except in a few areas, oesophageal carcinoma is not a common disease. Unless patients are referred to a specialist centre, the clinicians who see them have relatively little experience on which to base management decisions. It has also been shown that surgeons who regularly perform oesophageal operations achieve lower mortality rates and better results than occasional operators.
3. There is general agreement, at least among surgeons, that radical resection is indicated in otherwise fit patients with tumours at the loco-regional stage (TNM Stage I-III, excluding T4b). However, there are different opinions about what constitutes radical resection.
4. There is more disagreement about the treatment options for patients with advanced tumours that involve adjacent structures (T4, particularly T4b) or have more extensive nodal involvement (N3 or with M1 lymph node metastasis). Some surgeons still apply radical surgery with curative intent together with vigorous multimodality treatments to these diseases as long as R0 resection is possible. Even when only palliative treatment is indicated, resection or bypass surgery remains an important option for achieving the best palliation, especially with regard to dysphagia. Radiotherapy (including concurrent CRT), chemotherapy, stenting, laser therapy and laser-activated photodynamic therapy are all claimed to be beneficial alone or in combination. In recent reports, concurrent CRT has been shown to be more effective than single-modality treatments. A surgeon working alone is unable to evaluate the various claims that are made. For this reason, work closely with your radiotherapy and oncology colleagues or refer your patients to a specialist centre.
5. Adequate resection of oesophageal carcinoma requires a tumour-free margin to avoid the development of recurrent malignant dysphagia. Because of the frequency of intraepithelial spread, intramural metastasis and multicentric tumours, precise preoperative diagnosis is of the utmost importance. Some surgeons send specimens from the upper and lower cut ends for frozen-section histology, but this does not entirely rule out the possibility of a ‘skip’ lesion remaining in the oesophageal remnant.
6. Do not make your decision without first discussing the possibilities and problems with the patient and, when appropriate, with the relatives. You must take into account the physical fitness and the psychological and philosophical attitudes of the patient. If you have obtained your patient’s confidence, you will usually be entrusted with making the decision.
Prepare
1. Discuss the possibilities with the patient, since cooperation is essential to the success of major procedures.
2. Restore the dysphagic patient to a good nutritional state with soft oral foods and an elemental fluid diet, introducing a fine nasogastric tube into the stomach if necessary. Percutaneous endoscopic gastrostomy (PEG) is another option, particularly when neoadjuvant treatment requiring a longer time is planned. PEG is possible if a thin (transnasal) gastroscope can be introduced into the stomach, and it does not present an obstacle to future gastric pull-up. Parenteral intravenous alimentation is a useful option, although enteral feeding is more effective and preferred due to the concept of ‘immuno-nutrition’.
3. Oral hygiene is very important to prevent postoperative pneumonia. Preoperative consultation by a dentist should be performed routinely, and the patient should be trained in vigorous self-oral care.
4. Preoperative consultation by an otolaryngologist is also important. It might reveal unexpected recurrent laryngeal nerve palsy and meso- or hypopharyngeal cancer, which often occurs synchronously. Coincidental hypopharyngeal cancer is, however, more often detected by careful endoscopy.
5. Although enteral feeding can be re-started on the first or second postoperative day, set up a central venous line before surgery commences to secure reliable blood access.
6. Achieve the best possible cardiorespiratory state with physiotherapy, blood replacement and correction of serum protein and electrolytes. Smoking should be stopped as early as possible. Order 2–3 units of cross-matched blood. Collecting 800 ml of the patient’s own blood for autotransfusion is very useful to avoid allogenic blood transfusion. We also prepare cryoprecipitate from the patient’s own blood to apply it to the operative field as fibrin glue.
7. Ensure that the oesophagus, stomach and colon are empty. If the colon may be used for reconstruction, commence 1 or 2 days of low residue diet and administer oral bowel preparation the day before surgery (see Chapter 13).
8. Commence intravenous prophylactic antibiotic cover prior to commencing surgery: second- or third-generation cephalosporins have been used routinely.
9. Institute prophylaxis against deep venous thrombosis. Application of pneumatic cuffs to the lower extremities during and for several days after operation is most effective. Start continuous intravenous administration of heparin (100-150 units/kg/day) from immediately after the operation until the patient is mobile.
10. Undertake urethral catheterization and monitor hourly urine output during and after the operation.
11. Administration of a small dose of a corticosteroid (methylprednisolone, 250 mg) 2 hours prior to surgery is recommended by some authors to prevent the release of cytokines, which may precipitate ALI (acute lung injury) or ARDS (adult respiratory distress syndrome).
RESECTION OF CARCINOMA OF THE LOWER OESOPHAGUS (LEFT THORACO-ABDOMINAL APPROACH)
Appraise
1. Our routine operation for carcinoma of the abdominal oesophagus and the cardia is resection of the lower thoracic oesophagus together with a cuff of the gastric cardia or entire stomach followed by jejunal interposition or Roux-en-Y reconstruction through the left thoracoabdominal approach (Fig. 9.1). Primary carcinoma of the lower thoracic oesophagus needs right thoracotomy for lymph node dissection.
2. You will nearly always find that the resection needs to go higher than you had planned. Technically, you can carry out anastomosis behind the carina through this approach. In doubtful cases, choose the right-sided approach, or bluntly dissect and resect the remaining upper thoracic oesophagus and then make an anastomosis in the neck between the cervical oesophagus and the gastric remnant or colon brought up either retrosternally or through the posterior mediastinum.
3. The VATS procedure is a combination of upper median laparotomy and left thoracoscopic operation assisted by the operator’s left hand inserted transhiatally (Fig. 9.1B). We call the conventional left thoraco-abdominal approach (LTA), and its VATS counterpart hand-assisted left thoracoscopic and abdominal approach (HLTA).
Access
1. Place the anaesthetized, intubated patient on the right side, with the left shoulder rotated back against a support attached to the operating table, or use a self-retaining mat.
2. Open the left upper abdomen obliquely along the line of the sixth or seventh intercostal space, starting in the midline halfway between the umbilicus and the tip of the xiphisternum and extending to the left costal margin. The sixth intercostal space is more suitable when the lesion has substantial extension in the thoracic oesophagus, and mediastinal lymph node dissection is planned. Palpate the liver and the pelvis to rule out distant spread. Determine the fixity of the cardia and feel for extensive lymph node involvement that would make resection useless. Laparoscopic assessment of the abdominal cavity may demonstrate peritoneal dissemination and avoid unnecessary laparotomy.
3. If resection seems feasible, cut across the costal margin and elongate the skin incision toward the posterior axillary line along the planned intercostal space. Open the chest, cutting along the lower attachment of the intercostal muscles. Even in elderly patients with fixed ribs, sufficient separation of the intercostal muscles from the rib will allow adequate access.
4. Cut the diaphragm radially 10–15 cm towards the right crus. Even in very advanced case with direct tumour invasion to the crural muscle, you can achieve a safe surgical margin with circular resection of the muscle around the hiatus. It is not necessary to transect the diaphragm completely from its periphery to the part around the hiatus. If necessary, circumferential resection of the diaphragm around the hiatus can be added.
5. Insert a self-retaining rib retractor and gently open it in stages.
6. Stop all bleeding meticulously.
7. Anchor the edge of the incised diaphragm to the edge of the skin incision so that the left lung will not prolapse and interfere with the operative field during the intra-abdominal procedure.
Assess
1. Determine the extent of spread to the gastric cardia and glands along the left gastric vessels and around the coeliac axis.
2. Even if the stomach and associated glands appear to be uninvolved remove the left gastric basin, including the root of the left gastric artery. If the cardia or upper stomach is widely involved, prefer total gastrectomy.
3. If radical resection is inappropriate because of the extended disease, be willing to carry out an adequate longitudinal resection in a fit patient with a resectable tumour. Treat unresectable lesions by bypass, taking the stomach, jejunum or colon well above the tumour and joining it to the oesophagus in the chest or the neck. In Britain, gastro-oesophageal anastomosis in the neck is associated with the name of Kenneth McKeown of Northallerton. For fixed, extensive, obstructing tumours in high-risk patients, dilatation and insertion of an expandable metallic stent is the treatment of choice. Tube jejunostomy is also recommended for supplementary nutrition.
Resect
Abdominal procedure
1. Open the lesser sac, dissecting the greater omentum from the transverse colon and severing the avascular portion of the gastric lesser omentum.
2. Ligate and divide each artery and vein of the stomach at the root according to the planned resection procedure.
3. Divide the stomach (for proximal gastrectomy) or the duodenal bulb (for total gastrectomy) with a linear stapling device.
4. Remove the anchoring stitch from the diaphragm and pass a tape through the hiatus. Pull the tape down to get good exposure of the left thoracic cavity.
Thoracic procedure
1. Gently free the lower lobe of the left lung, dividing the pulmonary ligament, taking care not to injure the pulmonary vein.
2. Elevate and pull forward the lower lobe of the lung to display the posterior mediastinum. Locate the lower thoracic aorta and incise the mediastinal pleura just anterior to it. Dissect the anterior surface of the aorta. Ligate and divide the proper oesophageal arteries, which are rather rarely found in the lower mediastinum.
3. Incise the mediastinal pleura just posterior to the pericardium. Gently mobilize the lower oesophagus, leaving the mediastinal adipose tissue on the oesophageal side and taking care not to accidentally injure the azygous vein and the thoracic duct on the left side. If necessary, excise the posterior pericardium and a portion of the right pleura.
4. Decide the level of oesophageal transection and do not mobilize too high above this point. Too-extensive mobilization (more than 5 cm) of the oesophagus may result in ischaemia of the oesophageal stump.
5. Gently grasp the oesophagus with Akiyama’s oesophageal clamp or a right-angled vascular clamp and transect the oesophagus below it. Remove the specimen for histological examination. Immediate frozen section examination should be carried out if the upper margin is uncertain.
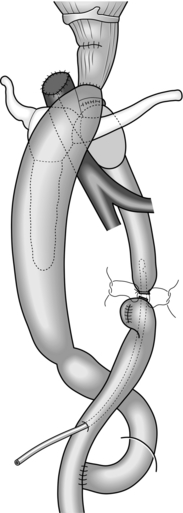
Unite (Fig. 9.2)
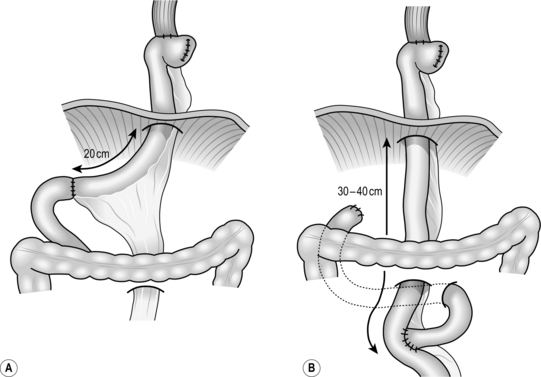
Fig. 9.2 Standard reconstructive methods after resection of lower oesophagus and stomach: (A) jejunal interposition; (B) Roux-en-Y reconstruction. (Modified with permission from Akiyama H 1990 Surgery for cancer of the esophagus. Williams & Wilkins, Baltimore.)
1. Plan the mesenteric incision line under transillumination to obtain the longest jejunal loop. The second or third jejunal artery is usually most suitable as a feeder. Cut the jejunum between two clamps and pull the distal end up retrocolically through the hiatus to the oesophageal stump.
2. Place a running purse-string suture in the oesophageal stump and insert the anvil of a 25 mm circular stapling device. Insert the circular stapler through the open jejunal end to construct an end-to-side (functionally end-to-end) oesophagojejunostomy. Close the open jejunal stump using a linear stapling device. Add seromuscular sutures to bury the staple line. Insert a nasogastric tube though the anastomosis.
3. If the distal stomach is preserved, bury the line of staples on the gastric remnant with seromuscular stitches. Cut the elevated jejunum 20 cm below the hiatus, while preserving the feeding vessels. Make an anastomosis between the distal end of the jejunal segment and the anterior wall of the gastric remnant. Then form another end-to-end jejunojejunal anastomosis below the mesocolon to complete the reconstruction.
4. Following a total gastrectomy, Roux-en-Y reconstruction is a preferable choice.
5. We routinely omit Heineke-Mikulicz pyloroplasty in spite of truncal vagotomy. Although this may result in early-stage gastric stasis, patients have fewer episodes of bile regurgitation on long-term follow-up.
6. Make sure there is no tension on the anastomoses, and no twisting or kinking of the bowel.
Checklist
1. Make sure the intrathoracic anastomosis is perfectly fashioned and there is no evidence of tension or ischaemia. An intrathoracic leak is often fatal.
2. Has all the bleeding stopped?
3. Close all the defects of the mesentery and mesocolon in order to avoid internal herniation. Fix the organ that was elevated for reconstruction to the hiatus to prevent prolapse into the thoracic cavity. Do not tighten the hiatus unless it was widened during the tumour resection. If you perform hiatal narrowing, make sure that the hiatus is still loose afterwards.
Closure
1. Insert an underwater-seal drain into the left pleural cavity through a separate stab incision near the costophrenic angle in the posterior axillary line.
2. Close the diaphragm using a continuous interlocking suture. The last 4–5 cm are closed with interrupted sutures from the abdominal side. Leave them unknotted until the incised intercostal space has been approximated with two thick, absorbable threads through both ribs.
3. Close the abdominal wound in your usual manner.
4. Close the chest wound, including the external oblique muscle and serratus anterior muscle above and below.
OPERATIONS FOR THORACIC OESOPHAGEAL CARCINOMA
INTRODUCTION
1. The two-stage operation of Ivor Lewis is the classic method for dealing with mid-oesophageal carcinoma. Many surgeons still employ this as their standard procedure for all oesophageal carcinomas. Newer and more radical techniques include the extensive (three-field) lymph node dissection popularized by Japanese surgeons.
2. VATS (video-assisted thoracic surgery) oesophagectomy is growing in popularity, as it offers advantages related to magnified view and variable view-point. It is also advantageous in surgical training because all the members of the team share the same view of the operative field and the instructor feels more confident when supervising a trainee performing a critical part of the operation.
3. It is not safe to embark on any of these advanced techniques, whether open or thoracoscopic, after simply reading a description. However, all the advanced techniques were developed as the result of step-by-step revision of older methods, enabling surgeons to make their operations more radical.
4. The more limited procedure of transhiatal oesophagectomy is still employed, combined with adjuvant therapy, and is claimed to be equally effective by its advocates.
IVOR LEWIS RESECTION FOR MID-OESOPHAGEAL CARCINOMA
Appraise
1. This operation is the source of other newer and more radical operations. Therefore, the essential points of the Ivor Lewis operation also apply to newer techniques. Only the general concept of the operation and a few specific points are presented here.
2. The first step is the abdominal operation, in which the whole stomach is mobilized for reconstruction leaving the right gastric and gastroepiploic vessels as feeding vessels. The second step is the thoracic operation, involving oesophageal resection and reconstruction using pulled-up stomach as an oesophageal substitute. An oesophagogastrostomy is made intrathoracically. This is performed with an end-to-end circular stapling device or side-to-side application of the linear stapling device and is rarely performed manually these days.
3. By careful positioning of the patient, it is possible for two surgical teams to work simultaneously, placing the upper part of the patient in the position for right thoracotomy, with the pelvis almost flat on the operating table. The position may thereafter be adjusted by tilting the table.
4. Carry out a full Kocher’s mobilization of the head of pancreas and duodenal loop, because oesophageal reconstruction is usually done after closure of the abdominal incision.
RADICAL CURATIVE SURGERY: EXTENSIVE LYMPH NODE DISSECTION (AKIYAMA TECHNIQUE)
Appraise
1. Japanese surgeons have been performing extensive lymph node dissection for about 25 years, and more recently the technique has been adopted by surgeons in North America and Europe. The operative mortality is satisfactorily low and the 5-year survival rate exceeds 50% for those who undergo a curative (R0) resection. These good results are often attributed to differences in the type of tumour, population screening procedures, the general health and body habitus of the patients and stage migration due to extensive lymph node dissection itself (the Will Rogers phenomenon), but there is no good evidence that such factors play a part.
2. Achieving negative surgical margins for local control and extended lymphadenectomy for regional control are different but equally important targets of R0 resection. Skinner has advocated ‘en bloc dissection’ as a variation of such radical surgery. The essential concept of his operation is wide resection of tissues surrounding the oesophageal tumour, including mediastinal pleura, azygous vein, thoracic duct and pericardium for tumours with infiltrative growth beyond the adventitia. These days, such tumours are usually considered candidates for neoadjuvant chemoradiation.
3. The concept of extensive lymph node dissection was developed following investigation into the extent of lymphatic tumour spread, and made possible by careful attention to detail in planning and performing the operation. We do not take an inflexible attitude towards the extent of either oesophageal or lymph node excision, but try to match the operation to the site and extent of tumour growth and to the likelihood of invasion and spread to the lymph nodes.
4. Reliable preoperative assessment of the tumour by endoscopy, conventional ultrasound of the neck and abdomen, endoscopic ultrasound and CT is mandatory.
5. Although this preoperative assessment is helpful in detecting spread, it does not reveal micrometastases, so radical dissection must encompass what appears to be normal tissue.
6. Modern imaging techniques have made an initial abdominal exploration unnecessary in most patients. If uncertainty exists concerning resectability and curativity in the abdomen, start with the abdominal procedure. In such cases, the cervical and abdominal parts of the operation can be completed first (reconstruction-first approach). The order of mediastinal dissection and reconstruction is interchangeable so this is not a major problem.
7. Video-assisted radical oesophagectomy is now a standard procedure, particularly when the patient has no history of radio- or chemoradiotherapy. It is performed with the patient in a left lateral recumbent position similar to conventional open oesophagectomy. Because the essential operative technique of the two approaches is similar, details of the VATS procedure are appended to descriptions of the open procedure in the following paragraphs as necessary. Some surgeons prefer to perform VATS oesophagectomy in a prone position under positive pressure pneumothorax. Surgeons learning VATS oesophagectomy as part of a new team may find this approach easier, as it is almost ‘solo-surgery’ and no skilled assistant is necessary. However, for an experienced team the left lateral position has the advantage because the operation is performed within a view familiar to them.
THORACIC PROCEDURE
Access
1. Place the anaesthetized, intubated patient on the left side using a self-retaining mat.
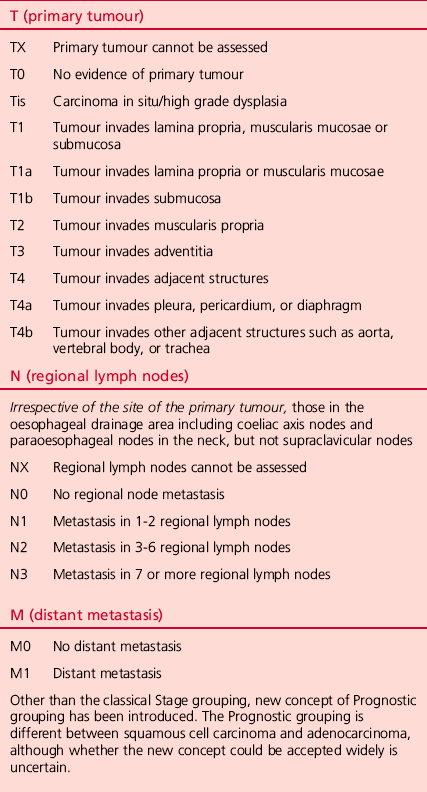
2. Make a right anterolateral thoracotomy incision in the fourth intercostal space preserving the right latissimus dorsi muscle (Fig. 9.3).
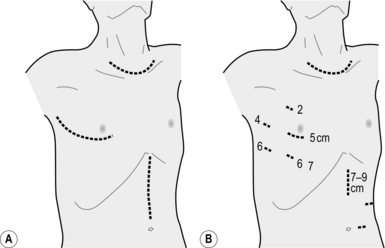
Fig. 9.3 (A) Skin incisions for the oesophagectomy with three-field lymphadenectomy. Thoracic incision is first made in left lateral recumbent position along the 4th intercostal space. The cervical and abdominal incisions are then made in supine position simultaneously by two teams. (B) Skin incisions for the video-assisted oesophagectomy with three-field lymphadenectomy. A minithoracotomy of 5 cm is placed along the 4th intercostal space. The numbers on the chest wall indicate the levels of intercostal spaces for each port for thoracoscopic surgery. A minilaparotomy of 7–9 cm and two port wounds are made for hand-assisted laparoscopic surgery. (Modified with permission from Akiyama H 1990 Surgery for cancer of the esophagus. Williams & Wilkins, Baltimore.)
3. Cut the intercostal muscles with electrocautery along the upper margin of the fifth rib from a point lateral to the internal mammary artery to a point lateral to the sympathetic trunk.
4. Apply two self-retaining rib retractors in a crossed fashion to produce a square window for access to the right thoracic cavity.
5. Free the lower lobe of the lung dividing the right pulmonary ligament.
6. For VATS oesophagectomy, a minithoracotomy of 5 cm in length is placed and four 11-mm ports are inserted, as shown in Figure 9.3B.
Assess
1. Rule out tumour implantation. Any suspicious nodules should be sent to the pathologist for frozen section diagnosis.
2. Check whether the main tumour is fixed to the surrounding organs. If direct invasion is suspected, carefully dissect the tumour from the organs around it. The trachea, left main bronchus, vertebra and descending aorta (those organs involved in T4b disease) are the most important to examine. Lung, pericardium, azygous vein, thoracic duct and diaphragm (organs involved in T4a disease) can easily be resected together with the oesophagus.
Action (Figs 9.4–6)
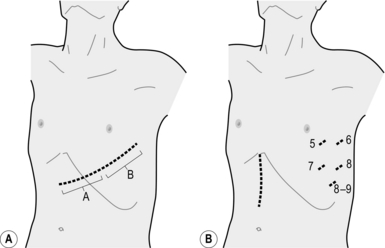
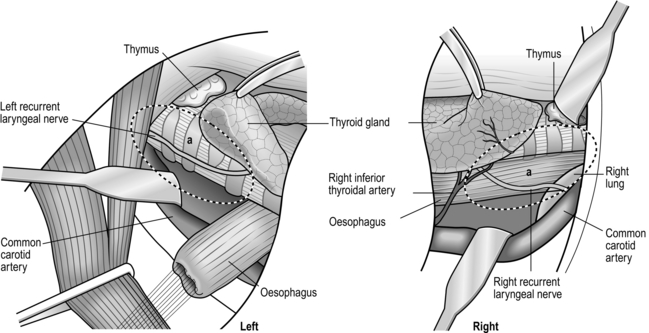
1. Incise the mediastinal pleura vertically over the right vagus nerve on the lateral tracheal wall. Elongate the pleural incision upward to the level of the subclavian artery. Identify the origin of the right recurrent laryngeal nerve and gently dissect it upward. The area behind this is the lower portion of the right recurrent laryngeal lymph node chain, which is a very frequent site of lymph node metastasis. It is possible to dissect this lymph node chain until the lower pole of the right thyroid lobe is encountered, but it is safer to leave the upper half untouched and dissect it from the neck (Figs 9.4, 5, 10). In VATS oesophagectomy a much higher portion of the right recurrent laryngeal lymph node chain can be reached safely under thoracoscopic vision.
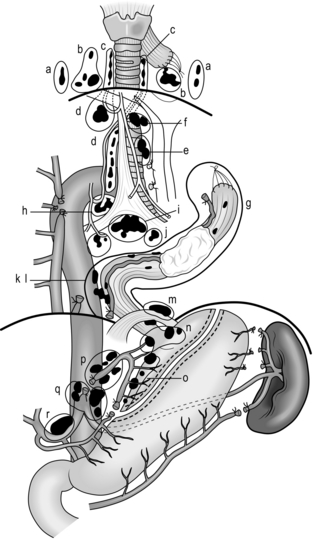
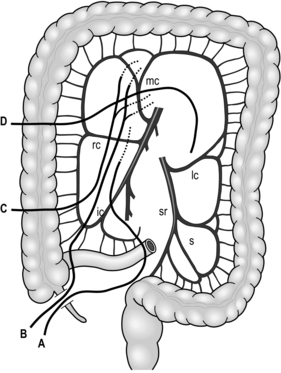
Fig. 9.4 Extent of resection and lymph node dissection of the standard radical oesophagectomy with extensive lymph node dissection for thoracic oesophageal carcinoma. Nodes to be dissected are in solid black. The mediastinal part is the lateral view. a, deep lateral cervical nodes; b, deep jugular nodes; c, cervical portion of recurrent nerve chain; d, thoracic portion of recurrent nerve chain; e, (right) pretracheal nodes; f, brachiocephalic artery nodes; g, upper thoracic para-oesophageal nodes; h, infra-aortic arch (left tracheobronchial) nodes; i, subcarinal nodes; j, main bronchus nodes; k, middle thoracic para-oesophageal nodes; l, lower thoracic para-oesophageal nodes; m, supradiaphragmatic nodes; n, right and left cardiac nodes; o, lesser curvature nodes; p, left gastric artery nodes; q, coeliac trunk nodes (including proximal splenic artery nodes); r, common hepatic artery nodes. Area k and l includes para-aortic nodes and thoracic duct nodes. (Modified with permission from Akiyama H 1990 Surgery for cancer of the esophagus. Williams & Wilkins, Baltimore.)
2. Dissect the anterior and posterior ends of the azygous arch, ligate and divide it. Be sure that the right bronchial artery, which lies below the azygous arch, is not included (Fig. 9.5).
3. Identify the right bronchial artery, which is usually the main branch from the third right intercostal artery. Ligate and cut the (third) intercostal artery peripheral to the origin of the rightbronchial artery so that you can safely retract the mediastinum anteriorly without tension on the bronchial artery (Fig. 9.5).
4. Cut the thin membranous structure containing small sympathetic nerve branches behind the upper oesophagus, and dissect this plane to the left side leaving the thoracic duct and the adipose tissue around attached to the oesophagus. Cut the similar thin membranous structure on the left side, and you will observe the left lung translucently through the left mediastinal pleura. With anterior extension of the dissection along this plane, you will identify the pulsation of the left subclavian artery.
5. Identify the thoracic duct again in the middle and inferior mediastinum. It runs on the surface of the descending aorta anteriorly to the origin of the right intercostal bronchial artery. Dissect it upward and downward for en bloc resection with the oesophagus, denuding the aortic surface. The thoracic duct runs over the vertebral bodies behind the oesophagus, lying between the azygous vein on its right and the aorta on its left in the middle and inferior mediastinum. Preserve the thoracic duct in T1 tumours or when the patient has liver cirrhosis.
6. Dissect the lymph nodes below the bilateral main bronchi and the carina, preserving the peripheral branches of the right bronchial artery and the bilateral pulmonary branches of the vagus nerves. First, identify the plane between the oesophagus and pericardium. Complete the circumferential dissection of the lower oesophagus identifying the bilateral inferior pulmonary veins. Then extend the dissection cephalad. This leads you to the anterior plane of the glands below the carina. Detach the nodes from the tracheobronchial wall beginning from the right end, toward the carina, and then deeply along the left main bronchus. Ligate the branches of other bronchial arteries from the anterior aspect of the subcarinal nodes if they are encountered (Fig. 9.5).
7. Dissect the plane between the membranous portion of the trachea and the upper thoracic oesophagus. Take care not to dissect too close to the tracheal wall in order to maintain the microvascular circulation and prevent tracheobronchial injury.
8. Anchor the upper thoracic oesophagus as high as possible using a tape, and retract the oesophagus posteriorly. Sharply dissect the soft tissue on the left lateral side of the trachea so that the fascia over the left subclavian artery is denuded. This dissection plane meets the space already made from the posterior side of the oesophagus in mobilizing the thoracic duct.
9. Identify the left recurrent laryngeal nerve in the soft tissue dissected from the left lateral side of the trachea. Dissect out the recurrent nerve using sharp dissection and perform en bloc dissection of the left paratracheal nodes (left recurrent laryngeal nerve chain nodes) remaining attached to the oesophagus.
10. Transect the thoracic oesophagus at its upper end manually or with a linear stapling device. Divide and ligate the thoracic duct behind the upper thoracic oesophagus at its upper end in the thorax.
11. Dissect the nodes around the oesophagus, pulling the upper cut end of the oesophagus downwards past the arch of preserved right bronchial artery. Several proper oesophageal arteries that arise from the descending aorta need to be ligated and cut. Completely dissect the nodes in the posterior mediastinum so that the left lung can be observed through the thin left mediastinal pleura and the descending aorta is denuded widely to its left lateral margin (Fig. 9.5).
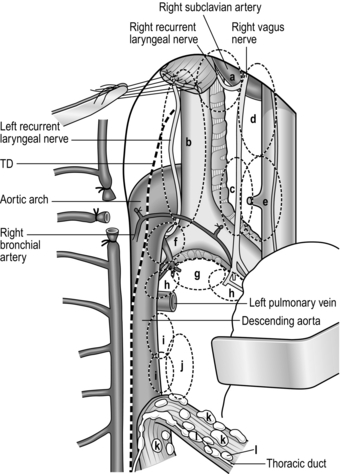
12. Ligate and cut the lower end of the thoracic duct. Dissect the soft tissues around the hiatus and visualize the crural muscles (Fig. 9.6).
13. In VATS oesophagectomy, you should complete the dissection described up to this point before you transect the oesophagus as traction on the oesophageal cut-end will not produce sufficient tension in the tissue to be dissected.
14. Go back to the superior mediastinum and dissect the pretracheal nodes if necessary. Also dissect the left tracheobronchial and subaortic arch nodes (Fig. 9.5).
15. Cover the fully mobilized oesophagus with nodes attached using a rubber sac in order to avoid possible scattering of tumour cells and loss of some nodes when the oesophagus is pulled out later from the thoracic cavity.
Checklist
1. Has all the bleeding stopped? Most bleeding points are on the dissected oesophagus, which has to be left in the thorax for a while.
2. Are the lower end of the thoracic duct and its small branches completely ligated? An unligated small branch can cause postoperative chylothorax.
3. If the left mediastinal pleura is opened, suck out all the fluid that has accumulated in the left pleural cavity during the thoracic operation.
4. If pulmonary injury occurred during the thoracic operation, repair it meticulously with manual suturing, ligation, linear stapling and fibrin glue. Newer synthetic sheets of absorbable fibres are very effective in controlling difficult air leaks and persistent bleeding.
Closure
1. Insert an underwater-seal drain into the right pleural cavity through a separate stab incision in the sixth or seventh intercostal space in the posterior axillary line. The lowest port site can be utilized in VATS.
2. Approximate the incised intercostal space with two thick, absorbable sutures through both ribs.
3. Close the chest wound, including the pectoralis and serratus anterior muscles above and below.
ABDOMINAL AND CERVICAL PROCEDURES
Assess: abdominal procedure
1. Determine the extent of spread to the lymph nodes along the left gastric vessels and around the coeliac axis.
2. Also check for para-aortic lymph node involvement. Peritoneal dissemination, which occurs when tumour seeds from the main lesion or massively metastatic lymph nodes, should also be excluded. Such advanced tumour growth should be suspected as a result of preoperative assessment, and a ‘reconstruction-first’ method should be employed.
Action: abdominal procedure
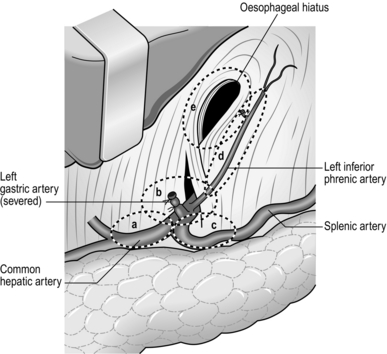
1. The basic manoeuvres applied around the root of the left gastric artery are very similar to the Japanese D2 dissection for gastric cancer. We routinely dissect the nodes on the common hepatic artery, the root of splenic artery and the nodes around the coeliac axis (Fig. 9.7).
2. After complete mobilization of the stomach by dividing the left gastroepiploic, short gastric, posterior gastric and left gastric vessels, while dissecting the nodes mentioned above, cut the phreno-oesophageal ligament and carefully pull out the oesophagus from the thorax with lymph nodes attached. Cover the oesophagus with a towel.
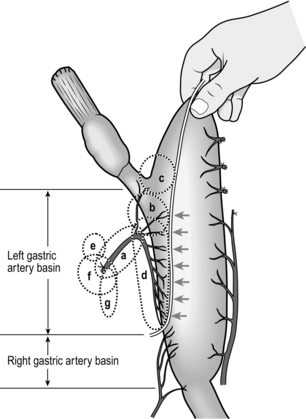
3. Find the highest point by the ‘pinching up’ technique (Fig. 9.8). Design the resection line for the stomach by connecting the points where the vessels from the left gastric artery enter the gastric wall so that the pericardiac nodes and the nodes in the left gastric basin are all removed. Resection is done along this line with two or three applications of a linear stapling device. The suture line is covered with interrupted seromuscular stitches.
Action: cervical procedure
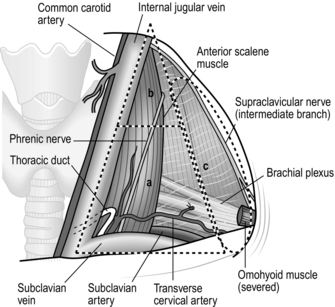
1. Mobilize the bilateral sternomastoid muscles widely. Separate the clavicular part from the sternal part, and place tapes around each muscle. Supraclavicular dissection can be achieved by pulling these tapes laterally and medially without cutting the muscle head (Fig. 9.9).
2. The area to be cleared extends from the cervical oesophagus laterally to the lateral supraclavicular nerve and from the level near the laryngeal promontory down to the subclavian vein and apical pleura (Fig. 9.9). More than 90% of cervical lymph node metastases arising from tumours of the thoracic oesophagus are located within the triangle defined by the bilateral omohyoid muscles and subclavian veins.
3. First, dissected out lymph nodes lateral to the common carotid arteries. Clear away the areolar tissue and lymph nodes from the posterior surface of the platysma, to the anterior, middle and posterior scalene muscles, the thyrocervical trunk and its three branches. Identify and preserve the phrenic and vagus nerves. Sacrifice the omohyoid muscle.
4. Draw up the cut end of the thoracic duct into the neck on the left side and ligate and cut its entry into the junction of the internal jugular and subclavian veins.
5. Now dissect out the lymph nodes along the carefully preserved recurrent laryngeal nerves. They may be very small, but are liable to contain micrometastases. Make sure that the paratrachealdissection plane of the thoracic operation and the cervical operation are completely continuous on both sides (Fig. 9.10).
RECONSTRUCTION
Action
1. Dissect the cervical oesophagus from the surrounding tissue to obtain enough length for anastomosis. Transect the left sternohyoid and sternothyroid muscles with electrocautery near their heads. Then bluntly open the retrosternal space behind the sternal incisura with a finger.
2. In the abdominal operating field, close the oesophageal hiatus with three interrupted sutures. Place the gastric remnant with the suture line on the dorsal side of the patient. Fix two tapes of different colours on each side near the tip with suture-ligatures.
3. Make a retrosternal tunnel using blunt finger dissection and a long, delicately curved, flat, flexible intestinal retractor with a hole at the tip. Ligate the two coloured tapes on the same side of the retractor through the hole at the tip.
4. Then pass the retractor through the retrosternal tunnel again and catch the two tapes in the neck. Bring the tip of the gastric remnant up to the neck by pushing from the abdominal side and gently pulling on the tapes in the neck. Take care to avoid rotation of the gastric remnant in the tunnel, using the coloured tapes as a guide.
5. Oesophagogastrostomy is performed manually in two layers or with Gambee’s technique using 5/0 absorbable monofilament thread. Pass a nasogastric tube for decompression.
6. Place an enteral feeding tube: either a classical Witzel-type jejunostomy or a direct epigastric gastrostomy when the reconstruction route is retrosternal. Alternatively, duodenostomy using a feeding tube through the hepatic falciform ligament is a good choice because it does not increase the risk of postoperative bowel obstruction.
Closure
1. Insert a pair of silicone cylinder drains 5 mm in diameter with multiple holes or longitudinal grooves, through small incisions on the anterior chest surface. Pass them into the dissected supraclavicular fossae through the two heads of sternomastoid muscle on both sides and connect them to the closed low-pressure suction bag.
2. Close the cervical collar incision in the same airtight manner as after a thyroid operation.
3. Close the abdominal wound in your usual manner except that, when the retrosternal route has been chosen for reconstruction, the peritoneal incision line is not sutured at the most cephalic 10 cm so that the elevated stomach is not constricted.
RESECTION OF THE THORACIC OESOPHAGUS WITHOUT THORACOTOMY
Appraise
1. Palliative resection of the oesophagus may be achieved by combined transhiatal abdominal and cervical approaches.
2. We believe that this procedure should be restricted to high-risk patients in whom standard thoracotomy is impossible, or you will deprive some patients with potentially curable disease of the chance to undergo a more radical resection.
3. Transhiatal oesophagectomy also offers a convenient means of providing a gastric conduit following pharyngolaryngectomy. Although a free jejunal graft with microvascular anastomosis is the current standard, the transhiatal procedure is preferable in patients with vascular disease who are unsuitable for microvascular surgery or those with thoracic oesophageal involvement. It may also be of value in patients with widespread or multiple superficial mucosal cancers for which EMR or ESD are impractical, although superficial mucosal cancers are now conventionally treated with chemoradiation therapy. The technique can also occasionally be employed for adenocarcinoma of the cardia and lower oesophagus, intractable benign stricture, incapacitating motility disorders and irrevocable damage following trauma.
4. Take into account all the advantages and disadvantages before you perform this procedure on oesophageal malignant disease. There are many other options for palliation including bypass surgery, indwelling expandable metallic stents, palliative (chemo-) radiation and simple feeding gastrostomy or enterostomy.
5. Do not embark upon this technique unless you have an intimate knowledge of the mediastinal anatomy and are competent to carry out a formal thoracotomy if necessary. Clumsy and ignorant blunt dissection can lead to calamitous bleeding or tearing of the fragile posterior membrane of the trachea or bronchi, with disastrous results.
6. Orringer and colleagues have claimed that this approach, together with CRT, is an acceptable alternative to other methods of radical oesophagectomy. However, we can see no prognostic benefit in this approach compared with the results obtained by oesophagectomy with extensive lymphadenectomy.
Access
1. Place the anaesthetized, intubated patient supine, with the head turned moderately to the right, and insert a small folded sheet beneath the scapulae to extend the neck.
2. Approach the abdomen through an upper midline incision.
3. Video mediastinoscopy from the hiatus is useful.
4. Make a neck incision along the lower anterior border of the left sternomastoid muscle. Cut platysma, dissect the medial border of sternomastoid muscle and cut the omohyoid muscle and the middle thyroid vein. This gives you entry to the space between the common carotid artery and the left lateral surface of the trachea and the thyroid gland.
Assess
1. Explore the abdomen to assess the intra-abdominal spread and operability. Do not commit yourself to resection until you are sure that the procedure is feasible and you can achieve it safely.
2. Do not forget that this procedure is limited in radicality for thoracic oesophageal cancer with submucosal or deeper infiltration. If the tumour is located low enough to obtain a safe surgical upper margin by resecting the lower thoracic oesophagus through a widened oesophageal hiatus, palliative resection of the main lesion together with proximal or total gastrectomy followed by jejunal interposition or Roux-en-Y reconstruction is a reasonable alternative with the same limited radicality. If the tumour is too advanced or the patient is unfit, consider inserting a stent to relieve dysphagia. In the absence of dysphagia, do not feel under pressure to ‘do something’. Be prepared to simply close the abdomen.
Action
Abdominal procedure
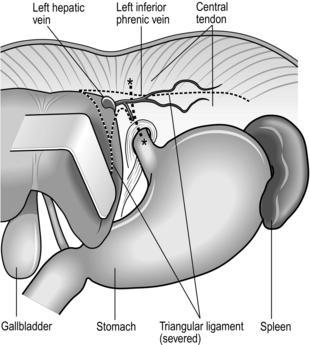
1. If resection is feasible, mobilize the left lobe of the liver and fold it to the right (Fig. 9.11).
2. Incise the peritoneum and the transversalis fascia over the front of the oesophageal hiatus. Insert a finger into the posterior mediastinum and separate the pericardium from the upper surface of the diaphragm. Incise the central tendon of diaphragm forwards for 7–8 cm from the hiatus, while ligating and dividing the inferior phrenic vessels.
3. Assess the extent of the tumour in the lower oesophagus.
4. Mobilize the lower oesophagus keeping well clear of the tumour and excising a cuff of diaphragmatic crus, posterior pericardium, mediastinal pleura and posterior connective tissue as necessary. The oesophagus can be mobilized under vision almost up to the level of the tracheal carina. Carefully avoid excessive compression of the heart, which might cause hypotension, tachyarrhythmia and even cardiac arrest in patients with cardiovascular risk factors.
5. Now proceed close to the oesophagus, carefully separating it from the back of the trachea. Hook the branches of the vagus nerves within the flexed index and middle fingers, and cut them using long scissors within the protection of the fingers.
Cervical procedure
1. Access is increased, if necessary, by transecting the strap muscles on the left. Median sternotomy or excision of the medial clavicle, a portion of the first rib and a half of the manubrium sterni on one side will very efficiently widen the operative field, but it is usually not necessary unless a bulky tumour, primary or metastatic, interferes with the access.
2. Expose the left lateral surface of the cervical oesophagus behind the trachea. The longitudinal fat pad along the posterior margin of the tracheal cartilage contains the left recurrent laryngeal nerve. Sharply dissect this fat pad from the oesophagus and enter the space between the membranous portion of the trachea and the anterior surface of the oesophagus. Widen this space upward and downward. Dissect the posterior surface of the cervical oesophagus from the prevertebral fascia. Place a tape around the oesophagus just on its right lateral surface to avoid injury to the right recurrent laryngeal nerve. Care should also be taken not to compress the left recurrent laryngeal nerve on the left lateral tracheal surface.
3. Continue freeing the oesophagus downwards into the superior mediastinum. Final mobilization is best achieved by inserting the index finger of one hand through the neck and passing the other hand up from the abdomen through the posterior mediastinum.
Resect
1. Remove the nasogastric tube.
2. Apply a ligature (or a linear stapling device) around the cervical oesophagus as low as possible.
3. Transect the oesophagus above the ligature, sterilize the tip and allow the lower ligated cut end to retract into the mediastinum. Connect a tape to the oesophageal stump to guide pulling up the oesophageal substitute later.
4. In the abdomen, transect the upper part of the stomach in the same manner described for extended lymph node dissection (Fig. 9.8) to prevent early recurrence around the elevated stomach. Cover the suture line with seromuscular stitches.
5. Remove the specimen for histology.
6. Insert a basal or apical underwater-seal drain whenever the pleural cavity has been breached.
Unite
1. If the gastric remnant can be used as the conduit, mobilize it as previously described. Ensure that the tip of the gastric remnant will reach the neck by laying it on the chest wall. Bring up the gastric remnant to the neck through one of the three possible routes (usually posterior mediastinal) and unite it to the cervical oesophagus.
2. The alternatives have already been discussed under ‘Assess’.
OESOPHAGEAL BYPASS
1. Resection may not be possible, or may be contraindicated because the tumour is too extensive. Bypass is preferable to stenting in patients with a prognosis of months rather than weeks. Bypass does not preclude radiotherapy as the conduit lies some distance from the field of irradiation.
2. The mobilized stomach may be raised above the unresected tumour when the left thoracoabdominal operation or the Ivor Lewis operation is planned. The anastomosis above the tumour can be fashioned after transection of the oesophagus and closure of the cut lower end or, if there is no fistula, it can be made in the lateral wall of the oesophagus without transection. If possible, leave the lower oesophagus in continuity with the stomach. Sometimes it is necessary to divide the lower oesophagus to free the stomach, so that it can be joined well above the tumour.
3. Kirschner’s operation (total bypass of the thoracic oesophagus with stomach) is preferable when bypassing unresectable middle-third lesions. Mobilize the stomach through the abdomen. You can transect the oesophagus at the cardia and use the whole stomach, but our preference is to prepare the stomach as described for extensive lymph node dissection (Fig. 9.8), but without the ‘pinching up’ technique so as not to pull up the stomach with metastatic lymph nodes. The bypass route can be retrosternal or subcutaneous. For the subcutaneous route elevate the skin at the upper end of the wound. Use long-handled scissors to create a track 7–10-cm wide in the subcutaneous tissue over the sternum and manubrium with a combination of blunt and sharp dissection. Mobilize the cervical oesophagus through an incision in the side of the neck (we prefer the left side) in the same manner as that described for resection of the thoracic oesophagus without thoracotomy (P.139), and use this incision to complete the subcutaneous track into the neck. Grasp the oesophagus with a clamp as low as possible in the mobilized cervical oesophagus. Transect the oesophagus above the grasped level, close the lower cut end, and allow it to retract into the superior mediastinum. Gently manoeuvre the stomach through the subcutaneous track by a combination of pushing and traction. Avoid tension and twisting of the organ. Now anastomose the upper cut end of the oesophagus to the upper surface of the drawn-up stomach.
4. It is often said that it is safe to leave the oesophagus in situ after closing both ends of a segment containing an unresectable tumour. However, this holds true only for a limited period: internal or external (tube) drainage is necessary when continuity of the oesophagus and the lower gastrointestinal tract cannot be maintained unless there is a large fistula with the tracheobronchial tree. This can be achieved by simply inserting a drainage tube from the lower cut end, or anastomosing the lower end to a Roux loop of jejunum. We prefer tube oesophagojejunostomy with Witzel jejunostomy on the anal side (Fig. 9.12).
5. Oesophageal bypass can be accomplished using a long jejunal pedicle taken up subcutaneously or retrosternally to the neck, but mobilization of such a long segment of jejunum is difficult. A segment of colon (see below) taken out of circuit is usually more easily fashioned into a conduit between the cervical oesophagus and the distal stomach.
6. When the tumour is too high to get enough length of cervical oesophagus for anastomosis, often with impending higher airway obstruction or recurrent laryngeal nerve palsy, oesophageal bypass with laryngectomy (Fig. 9.12) is a possible means of palliation.
COLONIC REPLACEMENT OR BYPASS OF THE OESOPHAGUS
Appraise
1. The colon usually has a good marginal blood supply (Fig. 9.13) and it makes an excellent oesophageal replacement.
2. An antiperistaltic loop can be created by swinging up the mobilized left colon after dividing the left colic vessels, so that it is supplied through the middle colic vessels. Because it does not always function well, this method should be the last alternative.
3. An isoperistaltic loop of right colon can be swung up, based on the middle colic vessels, after dividing the right colic and ileocolic vessels. The bulky caecum is brought to the neck after closing the terminal ileum and carrying out appendecectomy. The caecal bulk soon diminishes.
4. Our preference is to use an ileocolic segment including 15–20 cm of terminal ileum with the caecum and ascending colon for reconstruction or bypass. There are several advantages in this procedure: the right colic vessels can often be preserved, the bulky colon tends to remain in the thorax and preservation of the ileocaecal valve provides an antireflux mechanism. Perfusion of the ileum should be carefully examined because the vascular arcade between the ileocolic and the right colic vessels is poor in some patients.
5. If the tip of the elevated colon shows poor arterial circulation or bad venous return and microvascular expertise is available, addition of an arterial or venous anastomosis of the sacrificed colonic vessels to a suitable cervical vessel (usually called ‘supercharge’ and ‘superdrainage’) can save this difficult situation.
Action
1. Carefully study the anatomy of the marginal vessels.
2. Any bypass route for a colonic segment, particularly when the right colon is used, should be designed to be wider than for gastric pull-up.
3. Mobilize the caecum, ascending colon and right transverse colon on its primitive mesentery. Check for vascular continuity by inspecting the mobilized mesocolon against a light. In case of doubt, gently apply bulldog clips to the blood vessels at the intended sites of division and an intestinal clamp on the terminal ileum for several minutes, and then check the colour and circulation. Do not commit yourself to colonic bypass with an ischaemic or congested segment of colon. Remember, the venous drainage is as important as the arterial supply.
4. Doubly ligate and divide the ileocaecal vessels near their origin from the superior mesenteric vessels. Do not divide the right colic vessels at this stage, because it is often unnecessary. Then divide the ileum at the point about 20 cm (depending on the pattern of mesenteric vasculature) proximal to the ileocaecal valve with a linear stapling device. Apply seromuscular stitches to the distal cut end of the terminal ileum. Check the length of the ileocolic segment placing it on the anterior chest wall. The right colic vessels can be divided if greater length is required. Swing the mobilized ileocolon upwards and gently push and pull it along the prepared track. Carefully avoid violent traction on the root of the draining vein when you are pulling up any colonic segment. Anastomose the terminal ileum to the cervical oesophagus in an end-to-side fashion.
5. Meticulously divide the marginal vessels for 3 cm at a suitable point on the transverse colon for distal anastomosis whilst avoiding injury to the vascular arcade. Divide the transverse colon at the centre of the devascularized segment. Anastomose the proximal cut end of the colon to a suitable organ (i.e. distal stomach, duodenal bulb or side of the jejunal loop brought up antecolically), depending on the situation. Then anastomose the proximal cut end of the terminal ileum to the distal cut end of the transverse colon.
6. Close the mesenteric window to prevent internal herniation. Close the diaphragm or anterior abdominal wall slackly around the colon so that it is not constricted. Ensure that the bowel and blood vessels are not twisted and are not under tension before closing the incisions.
7. Belsey has described the use of an isoperistaltic loop of transverse colon based on the left colic vessels. If necessary, the left branch or even the right branch of the middle colic artery can be divided.
AFTERCARE (Common to all procedures described)
1. Following major oesophageal surgery, the patient should remain in the intensive care unit for at least 24 hours, so that cardiorespiratory, renal and cerebral functions can be monitored with early correction of any abnormalities. After extensive radical oesophagectomy, it takes much longer for pulmonary function to be restored, so it is advisable to keep the patient in intensive care for 3–4 days. Some patients benefit from initial mechanical ventilation via an endotracheal tube. Arrange for regular chest X-rays to ensure full pulmonary expansion. Order chest physiotherapy and aspiration of bronchial secretions either orally, through the endotracheal tube or using a flexible bronchoscope.
2. Order adequate regular analgesics, and continue antibiotics and prophylaxis for venous thrombosis.
3. Aspirate the visceral tube regularly but, as bowel function recovers, the tube may be used for feeding if its position has been checked radiographically. After 7 days, screen the patient following a swallow of water-soluble contrast to exclude anastomotic leakage. Withdraw the tube and start oral feeding, progressing to solids over a day or two. Then stop parenteral feeding. If a feeding enterostomy was created, start by giving small volumes of dilute fluid on the first postoperative day, gradually increasing to larger volumes of full-strength feeds until oral feeding is established. The enterostomy catheter can be left for several months for supplementary feeding.
4. Remove the chest drains after 48–72 hours unless fluid is being produced. If a drain fails to function and intrapleural fluid or air persists, do not hesitate to insert further suitably placed underwater-sealed drains.
5. Study the pathologist’s report carefully. Is adjuvant radiotherapy or chemotherapy justified and required?
ADJUVANT TREATMENT
1. Although squamous carcinoma, and indeed adenocarcinoma, responds to external beam therapy, the effect of such adjuvant treatment on the prognosis is questionable. Most controlled trials of preoperative radiotherapy have failed to show any prognostic benefit. Radiotherapy has been used instead of surgery in some series and Pearson reported results as good as those obtained with surgery, especially for mid- and upper oesophageal tumours.
2. Brachytherapy (intracavity irradiation with caesium-137 or iridium-192) offers certain advantages, notably that the radiation is concentrated on the tumour. A guidewire is inserted endoscopically or under fluoroscopy and an external applicator tube is passed over the guidewire to straddle the tumour. Pellets of caesium or iridium, stored in a safe source, are then pneumatically transferred into the applicator and are transferred back at the end of treatment. This is an effective way to increase the local control rate of radiotherapy. However, care should be taken as local tissue damage may lead to perforation if the tube positioning was not appropriate.
3. Endoscopic Nd-YAG laser therapy is valuable for palliation of dysphagia but it often has to be repeated to alleviate symptoms and to maintain patency.
4. Photodynamic therapy involves giving sensitizing drugs, usually haematoporphyrin derivatives or phthalylcyanates, which are retained by tumour cells. When exposed to certain light wavelengths, these agents produce cytotoxic substances such as oxygen free radicals. The light is usually delivered via optical fibre, but there is little tissue penetration, so the activity is mainly confined to the surface of lesions.
5. Chemotherapy has been tried alone or in a variety of combinations. Cisplatin has been combined with 5-fluorouracil, and this combination (FP) is still regarded as the standard regimen. Many other drugs such as Adriamycin, etoposide, vindecine and recently paclitaxel, docetaxel and irinotecan have also been tried usually in combination with diamminedichloroplatinum or FP. Postoperative chemotherapy had been thought to lack scientific evidence in survival benefit, but a Japanese multicentric phase III trial with FP successfully showed significant prolongation of disease-free survival. More recently, the same Japanese group (JCOG) reported the superiority of two courses of preoperative chemotherapy with FP over the same chemotherapy given postoperatively. Consequently, neoadjuvant chemotherapy + surgery is currently regarded as standard in Japan.
6. The concept of neoadjuvant treatment was proposed in 1981. Since then, a variety of preoperative treatments have been tried. Among them, concurrent CRT (chemoradiotherapy) has attracted wide attention and most of the trials of neoadjuvant CRT yielded positive results. It is often claimed that neoadjuvant CRT does not increase the risk of a subsequent operation, but this claim remains controversial.
7. Chemoradiotherapy may also be used as a definitive treatment not only in non-resectable advanced tumours but also in earlier stage tumours to preserve the oesophagus. Recent trials suggest that, following a good response to chemoradiation therapy, the addition of surgery results in better local control but no survival benefit. However, the presence or absence of residual tumour after definitive CRT is very difficult to diagnose and its late adverse effects are not fully understood. Salvage operations for recurrent tumour after definitive CRT are not easy and the postoperative quality of life is often poor.
8. The many treatment options for oesophageal cancer now allow individualization of treatment, combining two, three or more modalities. Research is currently geared to rationalizing this individualization, applying techniques of molecular biology and genetics. More experimental treatments such as adoptive immunotherapy and gene therapy are also being tried in several centres.
Ajani, J.A. Resectable esophageal cancer: surgery as primary therapy is not the answer, but then, what is and why? J Clin Oncol. 2010; 28(15):e243–e244.
Akiyama, H., Surgery for carcinoma of the esophagusCurrent problems in surgery. Chicago: Year Book Publishers, 1980.
Akiyama, H. Surgery for cancer of the esophagus. Baltimore: Williams & Wilkins; 1990.
Akiyama, H., Udagawa, H. Total gastrectomy and Roux-en-Y reconstruction. In: Pearson F.G., Cooper J.D., Deslauriers J., et al, eds. Esophageal surgery.. 2nd ed. New York: Churchill Livingstone; 2002:871–879.
Akiyama, H., Tsurumaru, M., Ono, Y., et al. Transoral esophagectomy. Surg, Gynecology Obstet. 1991; 173:399–400.
Akiyama, H., Tsurumaru, M., Udagawa, H., et al, Esophageal cancerCurrent problems in surgery. St Louis: Mosby, 1997.
Allum, W.H., Stenning, S.P., Bancewicz, J., et al. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009; 27:5062–5067.
Ando, N., Iizuka, T., Ide, H., et al. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study—JCOG9204. J Clin Oncol. 2003; 21:4592–4596.
Ando, N., Kato, H., Igaki, H., et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. 2012; 19(1):68–74.
Bedenne, L., Michel, P., Bouche, O., et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007; 25:1160–1168.
Boonstra, J.J., Koppert, L.B., Wijnhoven, B.P., et al. Chemotherapy followed by surgery in patients with carcinoma of the distal esophagus and celiac lymph node involvement. J Surg Oncol.. 2009; 100(5):407–413.
Cooper, J.S., Guo, M.D., Herskovic, A., et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999; 281:1623–1627.
Cuschieri, A. Invited introduction. Treatment of carcinoma of the oesophagus. Ann R Coll Surg Engl. 1991; 73:1–3.
D’Journo, X.B., Doddoli, C., Michelet, P., et al. Transthoracic esophagectomy for adenocarcinoma of the oesophagus: standard versus extended two-field mediastinal lymphadenectomy? Eur J Cardiothorac Surg. 2005; 27(4):697–704.
Dimick, J.B., Pronovost, P.J., Cowan, J.A., Lipsett, P.A. Surgical volume and quality of care for esophageal resection: do high-volume hospitals have fewer complications? Ann Thorac Surg. 2003; 75:337–341.
Earlam, R. An MRC prospective randomized trial of radiotherapy versus surgery for operable squamous cell carcinoma of the oesophagus. Ann R Coll Surg Engl. 1991; 73:8–12.
Fujita, H., Kakegawa, T., Yamana, H., et al. Mortality and morbidity rates, postoperative course, quality of life, and prognosis after extended radical lymphadenectomy for esophageal cancer Comparison of three-field lymphadenectomy with two-field lymphadenectomy. Ann Surg. 1995; 222:654–662.
Gebski, V., Burmeister, B., Smithers, B.M., et al. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol. 2007; 8:226–234.
Ilson, D.H., Bains, M., Ginsberg, R.J., et al. Neoadjuvant therapy of esophageal cancer. Surg Oncol Clin N Am. 1997; 6:723–740.
Ishikura, S., Nihei, K., Ohtsu, A., et al. Long-term toxicity after definitive chemo-radiotherapy for squamous cell carcinoma of the thoracic esophagus. J Clin Oncol. 2003; 21:2697–2702.
Isono, K., Sato, H., Nakayama, K. Results of a nationwide study on the three-field lymph node dissection of esophageal cancer. Oncology. 1991; 48:411–420.
Jamieson, G.G. Surgery of the oesophagus. Edinburgh: Churchill Livingstone; 1988.
Japan Esophageal Society. Japanese classification of esophageal cancer, tenth edition: part I. Esophagus. 2009; 6:1–25.
Japan Esophageal Society. Japanese classification of esophageal cancer, tenth edition: part II and III. Esophagus. 2009; 6:71–94.
Kelsen, D.P., Winter, K.A., Gunderson, L.L., et al. Long-term results of RTOG trial 8911 (USA Intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J Clin Oncol. 2007; 25:3719–3725.
Khoury, G.A. Squamous cell carcinoma of the oesophagus: 10 years on. Ann R Coll Surg Engl. 1991; 73:4–7.
Kitagawa, Y., Fujii, H., Mukai, M., et al. Intraoperative lymphatic mapping and sentinel lymph node sampling in esophageal and gastric cancer. Surg Oncol Clin N Am. 2002; 11:293–304.
Kodama, M., Kakegawa, T. Treatment of superficial cancer of the esophagus: a summary of responses to a questionnaire on superficial cancer of the esophagus in Japan. Surgery. 1998; 123:432–439.
Krasna, M.J., Tepper, J. The role of multimodality therapy for esophageal cancer. Chest Surg Clin N Am. 2000; 10:591–603.
Mathisen, D.J. Ivor Lewis procedure. In: Pearson F.G., Deslauriers J., Ginsberg R.J., et al, eds. Esophageal surgery. New York: Churchill Livingstone; 1995:669–676.
Matthews, H.R., Powell, D.J., McConkey, C.C. Effect of surgical experience on the results of resection for oesophageal carcinoma. Br J Surg. 1986; 73:621–623.
Minsky, B.D., Pajak, T.F., Ginsberg, R.J., et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. 2002; 20:1167–1174.
Murata, Y., Suzuki, S., Ohta, M., et al. Small ultrasonic probes for determination of the depth of superficial esophageal cancer. Gastrointest Endosc. 1996; 44:23–28.
Orringer, M.B. Transhiatal esophagectomy without thoracotomy. In: Pearson F.G., Deslauriers J., Ginsberg R.J., et al, eds. Esophageal surgery. New York: Churchill Livingstone; 1995:683–701.
Osugi, H., Takemura, M., Higashino, M., et al. Learning curve of video-assisted thoracoscopic esophagectomy and extended lymphadenectomy for squamous cell cancer of the thoracic esophagus and results. Surg Endosc. 2003; 17:515–519.
Palanivelu, C., Prakash, A., Senthilkumar, R., et al. Minimally invasive esophagectomy: thoracoscopic mobilization of the esophagus and mediastinal lymphadenectomy in prone position–experience of 130 patients. J Am Coll Surg. 2006; 203:7–16.
Raijman, I., Siddique, I., Ajani, J., et al. Palliation of malignant dysphagia and fistulae with coated expandable metal stents: experience with 101 patients. Gastrointest Endosc. 1998; 48:172–179.
Rice, T.W., Blackstone, E.H., Rusch, V.W. A cancer staging primer: esophagus and esophagogastric junction. J Thorac Cardiovasc Surg. 2010; 139:527–529.
Rizk, N.P., Ishwaran, H., Rice, T.W., et al. Optimum lymphadenectomy for esophageal cancer. Ann Surg. 2010; 251:46–50.
Rutgeerts, P., Vantrappen, G., Broeckaert, L., et al. Palliative Nd:YAG laser therapy for cancer of the esophagus and gastroesophageal junction: impact on the quality of remaining life. Gastrointest Endosc. 1988; 34:87.
Shimada, H., Okazumi, S. Matsubara H, et al., Effect of steroid therapy on postoperative course and survival of patients with thoracic esophageal carcinoma. Esophagus. 2004; 1:89–94.
Skinner, D.B. En bloc resection for neoplasms of the esophagus and cardia. J Thorac Cardiovasc Surg. 1983; 85:59–71.
Sobin L.H., Gospodarowicz M.K., Witteind C.h., eds. International Union Against Cancer. 7th edn. TNM classification of malignant tumors. Wiley, 2009:66–72.
Stahl, M., Walz, M.K., Stuschke, M., et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol. 2009; 27:851–856.
Tepper, J., Krasna, M.J., Niedzwiecki, D., et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008; 26:1086–1892.
Thomas, R.J., Abbott, M., Bhathal, P.S., et al. High-dose photoirradiation of esophageal cancer. Ann Surg. 1987; 206:193.
Udagawa, H. Esophageal bypass with laryngectomy: a method of palliation for nonresectable upper esophageal carcinoma. Dis Esophagus. 1991; 4:63–70.
Udagawa, H., Akiyama, H. Surgical treatment of esophageal cancer: Tokyo experience of the three-field technique. Dis Esophagus. 2001; 14:110–114.
Udagawa, H., Ueno, M., Shinohara, H., et al. The importance of grouping of lymph node stations and rationale of three-field lymphoadenectomy for thoracic esophageal cancer. J Surg Oncol. 2012; 106(6):742–747.
Udagawa, H., Ueno, M., Kinoshita, Y. Rationale for video-assisted radical esophagectomy. General Thoracic and Cardiovascular Surgery. 2009; 57:127–131.
Urschel, J.D., Vasan, H. A meta-analysis of randomized controlled trials that compared neoadjuvant chemoradiation and surgery to surgery alone for resectable esophageal cancer. Am J Surg. 2003; 185:538–543.
Urschel, J.D., Ashiku, S., Thurer, R., et al. Salvage or planned esophagectomy after chemoradiation therapy for locally advanced esophageal cancer: a review. Dis Esophagus. 2003; 16:60–65.
Yokoyama, A., Omori, T. Genetic polymorphisms of alcohol and aldehyde dehydrogenases and risk for esophageal and head and neck cancers. Jpn J Clin Oncol. 2003; 33(3):111–121.
Yokoyama, A., Hirota, T., Omori, T., et al. Development of squamous neoplasia in esophageal iodine-unstained lesions and the alcohol and aldehyde dehydrogenase genotypes of Japanese alcoholic men. Int J Cancer. 2012; 130(12):2949–2960.