99 Parasitic Infections
Parasitic disease causes extensive morbidity and mortality worldwide in children, particularly Plasmodium falciparum, which causes 1 to 2.7 million deaths annually. The morbidity of parasitic disease disproportionately affects the developing world with an estimated 39 million disability-adjusted life years attributable to infections with parasitic worms. Children in the United States are still at risk for parasitic infection. More common infections include giardiasis, pinworm infections, and head lice. Contaminated water and pets can serve as exposures to particular parasites including Giardia lamblia, Cryptosporidium parvum, Toxoplasma gondii, and Toxocara canis. Although covered in Chapter 89, it should be kept in mind that infection with Trichomonas spp. is the most common parasitic infection in the United States with 7.4 million infections each year. A comprehensive review of all parasitic infections in children would exceed the scope of the chapter, so an overview discussion of the most common intestinal parasites, systemic parasites, and specific coverage of malaria is presented.
Intestinal Parasites
Etiology and Pathogenesis
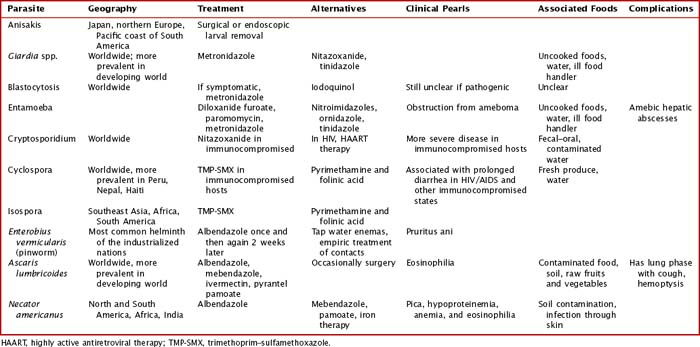
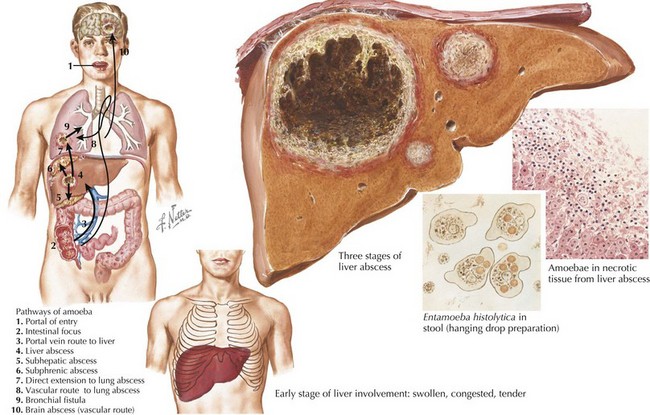
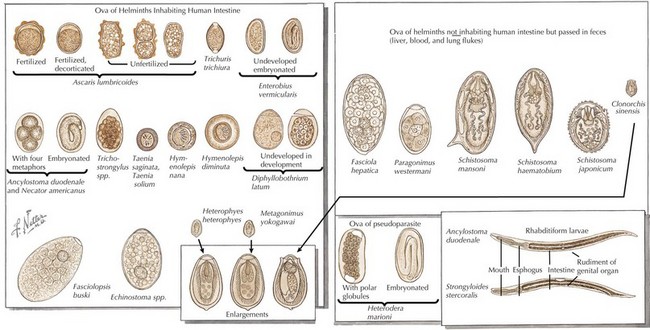
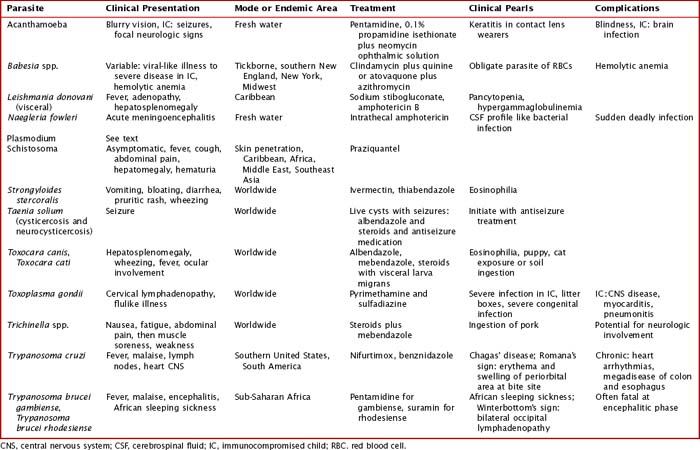
Intestinal parasites infect millions worldwide, particularly in developing countries with poor access to potable water and in patients with comorbidities. Intestinal parasites that are the most common in children in the United States include: G. lamblia, Entamoeba histolytica, and C. parvum. Table 99-1 reviews additional causes of intestinal parasitic infections (Figures 99-1 and 99-2).
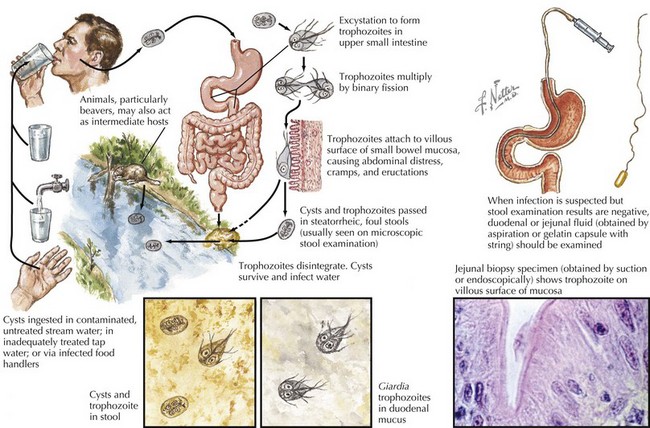
G. lamblia is a flagellated protozoon and the most common cause of parasitic enteritis in the United States. Outbreaks can occur from contaminated water supplies, including pools, because it is resistant to chlorination as well as person to person in daycare centers. The mode of transmission is often fecal–oral or direct person-to-person contact (Figure 99-3).
Clinical Presentation
Intestinal infection with parasites is often initially asymptomatic in a carrier state. Children with intestinal parasites can present with failure to thrive, diarrhea, abdominal pain, a protuberant abdomen, anemia, blood in the stool, and delayed development. A careful history with particular attention to travel and food and water intake is valuable. Additional details about sources and geography can be found in Table 99-1
Extraintestinal Parasites
Etiology and Pathogenesis
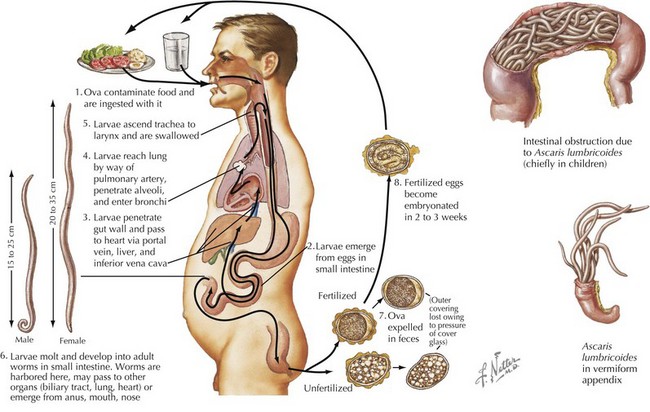
Systemic helminth infections, including Strongyloides stercoralis, Trichinella spiralis, Ascaris lumbricoides, Toxocara canis, and Trichuris trichiura, are spread via fecal contamination with worms penetrating into the toes of barefoot humans or through ingestion of cysts (Figure 99-4). Discussion of individual parasites is included in Table 99-2.
Clinical Presentation
The clinical presentations of many extraintestinal parasites are in Table 99-2. Particular parasites are known for infection within the United States both from zoonotic spread and from international travel. The clinical presentation and burden of disease of Toxoplasma and Toxocara spp. are discussed. The use of clinical testing can aid in the differential diagnosis of parasitic infection. Malaria is covered in a subsequent section in this chapter.
Toxoplasma spp. are estimated to cause 1.5 million new infections each year, with 400 to 4000 cases of congenital toxoplasmosis yearly in the United States. Primary infection during pregnancy can pass Toxoplasma spp. directly to the fetus transplacentally. Potential complications include miscarriage, stillborn birth, or congenital infection. An infant with congenital toxoplasmosis may be asymptomatic at birth but then develop with age vision loss, seizure activity, and developmental delay with intracranial calcifications on imaging. Treatment can be undertaken in the mother as discussed below and in Table 99-2. In addition, more than 1 million people have eye involvement with toxoplasmosis either from congenital or postnatal infection. An inflammatory lesion of the retina caused by direct infection of Toxoplasma spp. can lead to retinal scarring. This infection clinically can present with photophobia, blurred vision, and pain and may over time cause loss of vision, leading to blindness. Toxoplasma spp. are known for more severe infection in immunocompromised hosts either through reactivation of latent infection or new primary infection. In particular, Toxoplasma spp. Have a tropism for cardiac tissue and the brain, so they are of particular concern in heart transplant recipients. Clinical symptoms from toxoplasmosis usually occur 2 to 24 weeks after transplantation often from reactivation of cysts within a graft. Prophylaxis may be achieved with pyrimethamine or trimethoprim–sulfamethoxazole.
Although the clinical range of parasitic infections is quite broad and overlaps with other infectious diseases, certain clinical and laboratory presentations are associated with particular parasitic infections. Some of these distinctive features are captured in Table 99-2. With the clinical presentation of pneumonia and peripheral eosinophilia, Ascaris spp., hookworms, Strongyloides spp., and Toxocara spp. should be considered. Isolated eosinophilia can be seen in Strongyloides, hookworm, Schistosomia, and Toxocara infections. Toxocara infections are often associated with hepatosplenomegaly.
Malaria
Etiology and Pathogenesis
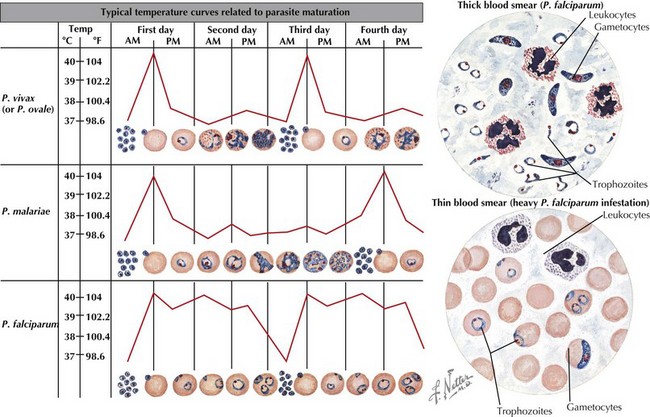
Malaria is caused by protozoa of the genus Plasmodium with four species using humans as the intermediary host. These species include P. falciparum, Plasmodium malariae, Plasmodium ovale, and Plasmodium vivax. The infectious cycle of Plasmodium species can be seen in the attached image. In short, Plasmodium spp. is transmitted by arthropod vector, the Anopheles mosquito, which is active during nighttime hours. The female Anopheles mosquito spreads sporozoites into the human host which then go on to infect liver cells and continue to mature. They then undergo multiplication in the erythrocyte cells, with blood stage parasites causing clinical manifestations of malaria. P. vivax and P. ovale may stay as liver stage parasites for months and then reactivate (Figure 99-5).
Evaluation and Treatment
After infection is identified, treatment should begin immediately. Therapy should be guided by the species of Plasmodium, the clinical status of patient, and drug susceptibility of the region where the infection occurred. The clinical status of the patient is important as uncomplicated malaria can be treated with oral antimalarials, but complicated infection has to be more tailored to parenteral therapy. Exchange transfusion may be considered with parasitemia of 10% or evidence of complications, including cerebral malaria. Treatment in areas of chloroquine resistance includes atovaquone and proguanil. Treatment of P. falciparum infection in nonchloroquine-resistant areas includes quinine sulfate plus doxycycline, tetracycline, or clindamycin. P. vivax (chloroquine resistant) treatment includes quinine sulfate plus doxycycline. Infection with all other Plasmodium spp. may be treated with chloroquine, with parenteral treatment with quinidine. For up-to-date recommendations and dosing, please refer to the Centers for Disease Control and Prevention’s website (www.cdc.gov).
American Academy of Pediatrics’ Committee of Infectious Diseases. Red Book: 2006 Report of the Committee of Infectious Diseases, ed 27. Elk Grove Village, IL: American Academy of Pediatrics; 2006.
Bergelson JM, Shah SS, Zaoutis TE. Pediatric Infectious Diseases: The Requisites in Pediatrics. Philadelphia: Mosby; 2008.
Centers for Disease Control and Prevention. Parasites. Available at http://www.cdc.gov/ncidod/dpd
Freedman DO. Clinical practice: malaria prevention in short-term travelers. N Engl J Med. 2008;359(6):603.
Freedman DO, Weld LH, Kozarsky PE, et al. Spectrum of disease and the relation to place of exposure among returning travelers. N Engl J Med. 2006;354(2):119.
Griffith KS, Lewis LS, Mali S, Parise ME. Treatment of malaria in the United States: a systematic review. JAMA. 2007;297(20):2264.
Long SS, Pickering LK, Prober CG. Principles and Practice of Pediatric Infectious Diseases, ed 3. Philadelphia: Saunders; 2009.
Won K, Kruszon-Moran D, Schantz P, Jones J. National seroprevalence and risk factors for zoonotic Toxocara spp. infection. Philadelphia. Abstracts of the 56th American Society of Tropical Medicine and Hygiene. 2007.