Syndromic tumors: Small in size (usually < 3 cm)
 in the pancreatic tail.
in the pancreatic tail.
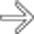
 , with its typical well-circumscribed, noninfiltrative appearance. NETs are often referred to by their main hormonal output (e.g., insulinoma), but pathologists call them NETs because of the electron microscopic finding of neuron-specific enolase.
, with its typical well-circumscribed, noninfiltrative appearance. NETs are often referred to by their main hormonal output (e.g., insulinoma), but pathologists call them NETs because of the electron microscopic finding of neuron-specific enolase.
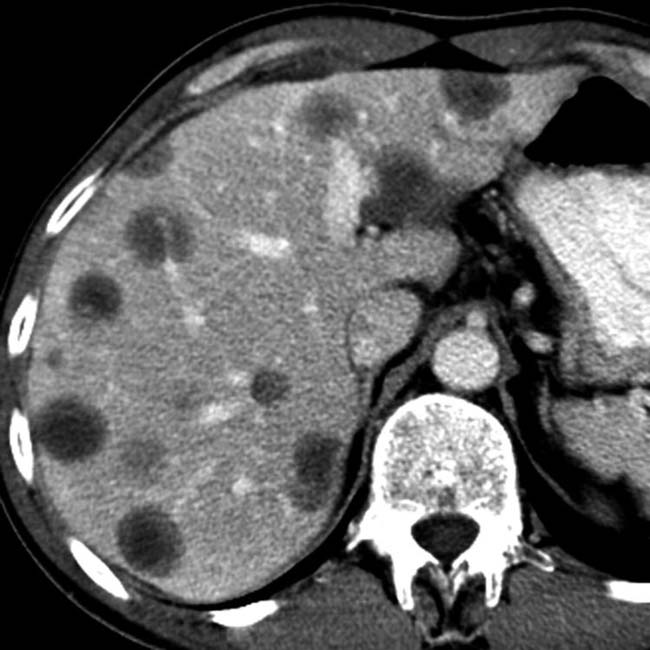
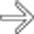
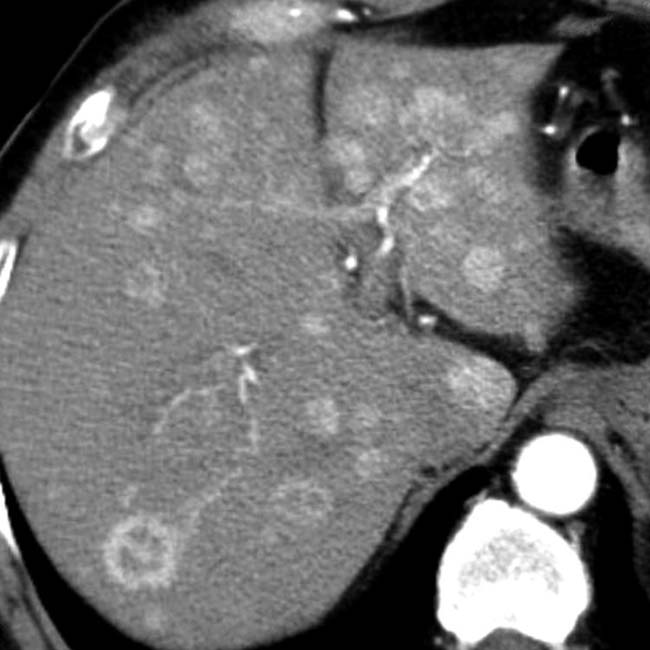
 and additional hepatic metastases
and additional hepatic metastases  . This constellation of findings is typical of a malignant NET of the pancreas, a glucagonoma in this case. Glucagonomas are more commonly malignant than insulinomas.
. This constellation of findings is typical of a malignant NET of the pancreas, a glucagonoma in this case. Glucagonomas are more commonly malignant than insulinomas.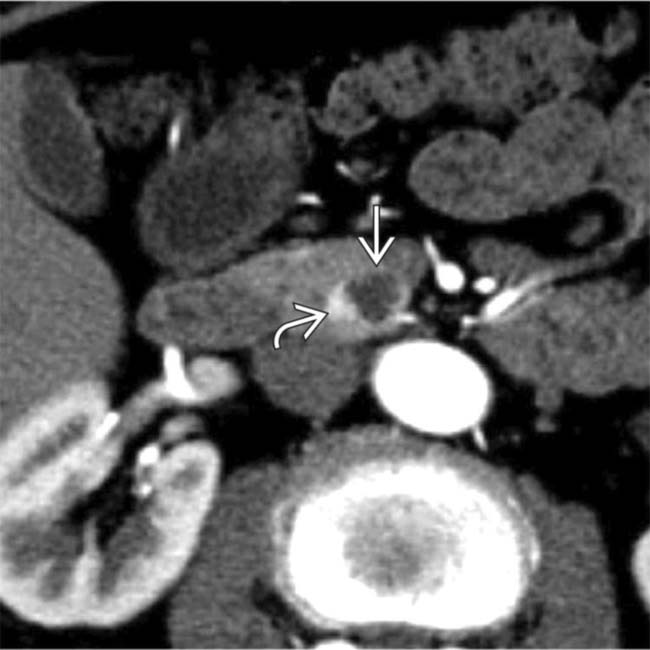
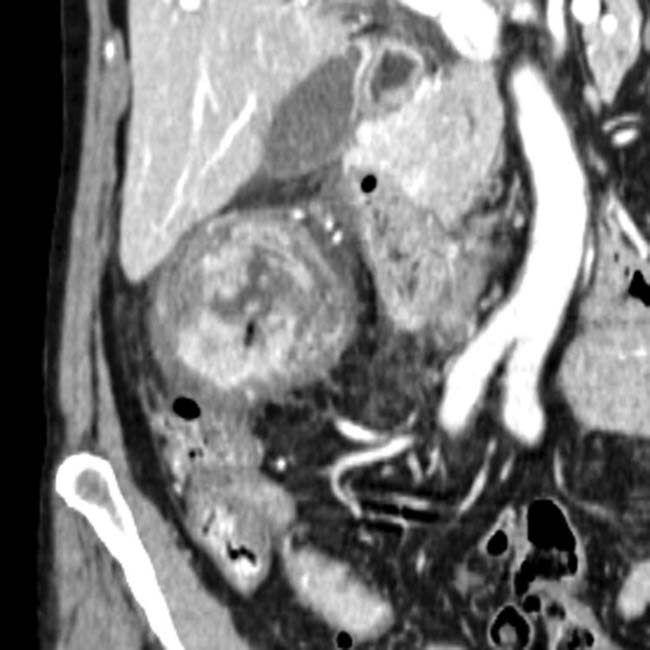
 in the pancreatic uncinate process. The subtle nodular hypervascular rim
in the pancreatic uncinate process. The subtle nodular hypervascular rim  around the lesion strongly suggests that this is a cystic NET.
around the lesion strongly suggests that this is a cystic NET.IMAGING
General Features
• General concepts
 Divided into benign (well-differentiated endocrine tumor) or malignant (well/poorly differentiated neuroendocrine carcinoma) based on WHO classification
Divided into benign (well-differentiated endocrine tumor) or malignant (well/poorly differentiated neuroendocrine carcinoma) based on WHO classification
 No longer divided into functioning or nonfunctioning, as all NET are now considered hormonally active
No longer divided into functioning or nonfunctioning, as all NET are now considered hormonally active
 Divided into benign (well-differentiated endocrine tumor) or malignant (well/poorly differentiated neuroendocrine carcinoma) based on WHO classification
Divided into benign (well-differentiated endocrine tumor) or malignant (well/poorly differentiated neuroendocrine carcinoma) based on WHO classification No longer divided into functioning or nonfunctioning, as all NET are now considered hormonally active
No longer divided into functioning or nonfunctioning, as all NET are now considered hormonally activeCT Findings
• Well-circumscribed pancreatic mass with noninfiltrative margins that is usually (but not always) hypervascular and most conspicuous on arterial phase
 Lesions usually hyperenhance to lesser degree on venous phase, making smaller lesions difficult to detect
Lesions usually hyperenhance to lesser degree on venous phase, making smaller lesions difficult to detect
 Lesions usually hyperenhance to lesser degree on venous phase, making smaller lesions difficult to detect
Lesions usually hyperenhance to lesser degree on venous phase, making smaller lesions difficult to detect
• Invasion (rather than encasement) by tumor of mesenteric veins (portal vein or superior mesenteric vein)
CLINICAL ISSUES
Presentation
• Most common signs/symptoms
 Syndromic tumors
Syndromic tumors
 Syndromic tumors
Syndromic tumors
– Insulinoma: Symptoms of hypoglycemia, low glucose (< 50 mg/dL), and relief by IV glucose (Whipple triad)
– Gastrinoma (Zollinger-Ellison syndrome): Severe peptic ulcer disease, increased acidity, and diarrhea
Treatment
• Somatostatin analogs (e.g., Octreotide) for symptom relief for syndromic tumors (except somatostatinoma)
• Locally advanced NET without metastases should be resected if possible, even if negative margins not possible
 in keeping with a pancreatic NET. In this case, the upstream pancreas is markedly atrophic
in keeping with a pancreatic NET. In this case, the upstream pancreas is markedly atrophic  , an atypical feature for NETs, which do not typically obstruct the pancreatic duct or cause parenchymal atrophy.
, an atypical feature for NETs, which do not typically obstruct the pancreatic duct or cause parenchymal atrophy.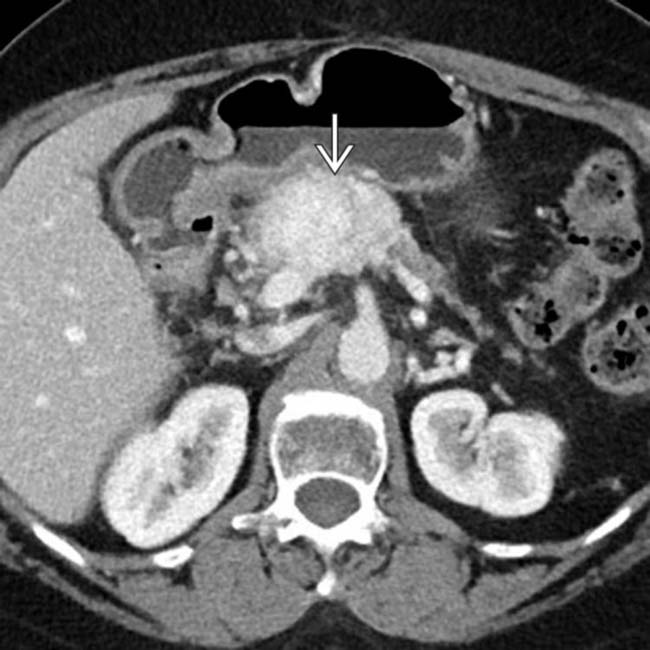
 is still enhancing on this axial venous phase CECT image from the same patient, note that the mass demonstrates a much lesser degree of enhancement compared to the arterial phase image.
is still enhancing on this axial venous phase CECT image from the same patient, note that the mass demonstrates a much lesser degree of enhancement compared to the arterial phase image.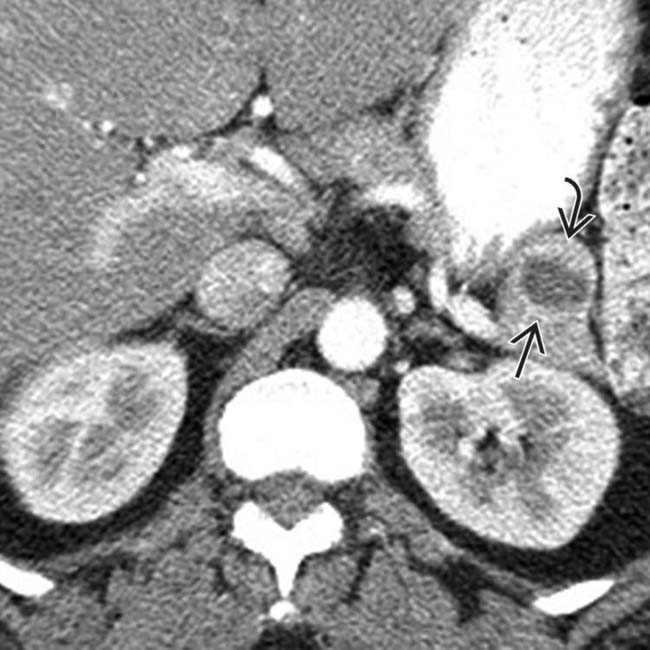
 in the pancreatic tail. While an IPMN or MCN are possibilities, the peripheral enhancement and enhancing soft tissue
in the pancreatic tail. While an IPMN or MCN are possibilities, the peripheral enhancement and enhancing soft tissue  should suggest the correct diagnosis of cystic NET.
should suggest the correct diagnosis of cystic NET.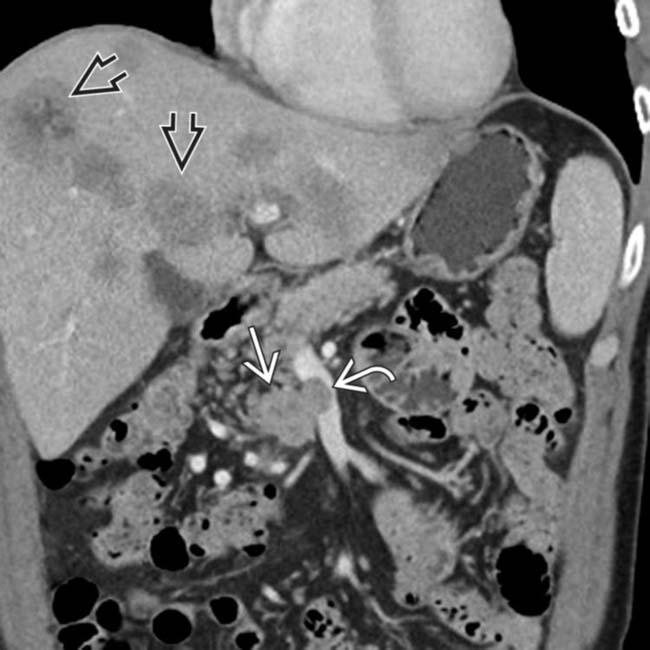
 in the pancreatic uncinate, with multiple liver metastases
in the pancreatic uncinate, with multiple liver metastases  . Note that the lesion is invading the SMV
. Note that the lesion is invading the SMV  , a feature uncommon with adenocarcinoma and more common with NET. This was found to be a NET at resection.
, a feature uncommon with adenocarcinoma and more common with NET. This was found to be a NET at resection.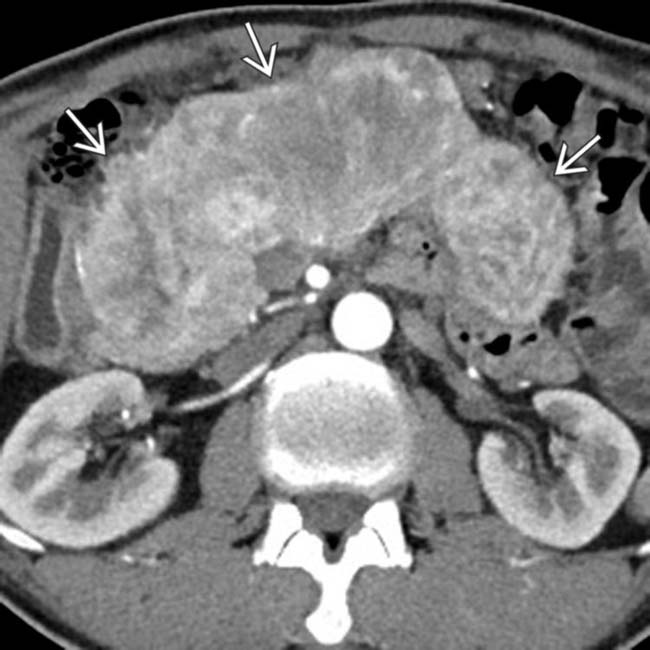
 replacing nearly the entirety of the pancreas. Nonsyndromic tumors, as in this case, are often larger at presentation.
replacing nearly the entirety of the pancreas. Nonsyndromic tumors, as in this case, are often larger at presentation.
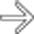
 . While these lesions could easily be mistaken for cysts or hemangiomas, neuroendocrine metastases to the liver are well known for being very T2 hyperintense.
. While these lesions could easily be mistaken for cysts or hemangiomas, neuroendocrine metastases to the liver are well known for being very T2 hyperintense.
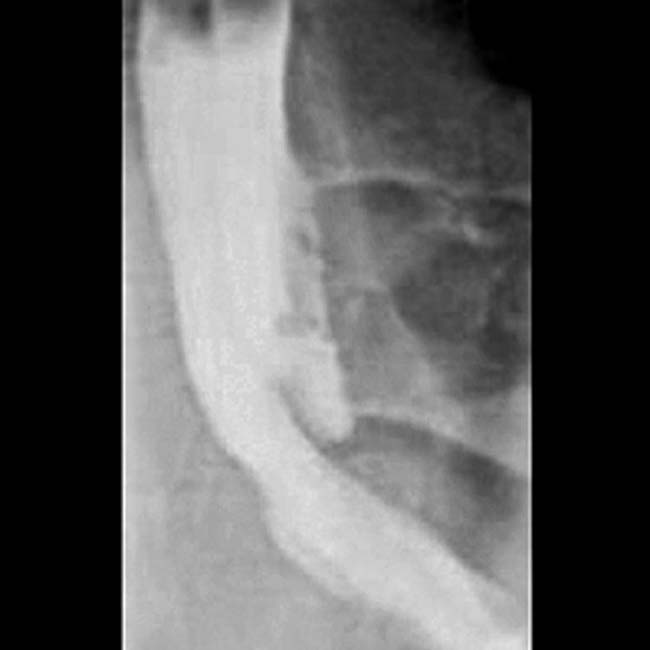
 and wall hyperenhancement in the proximal aspect of the stomach. The more distal stomach demonstrates normal wall thickness
and wall hyperenhancement in the proximal aspect of the stomach. The more distal stomach demonstrates normal wall thickness  . This appearance is typical of Zollinger-Ellison syndrome.
. This appearance is typical of Zollinger-Ellison syndrome.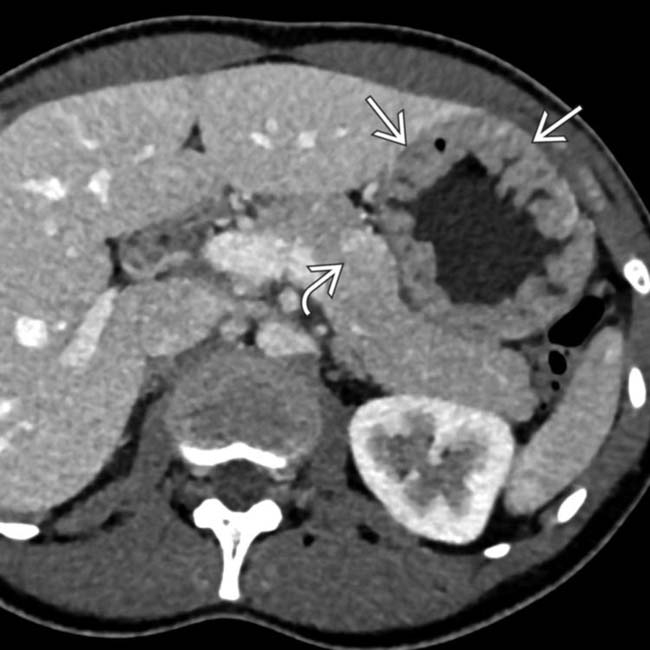
 , with a subtle enhancing mass
, with a subtle enhancing mass  in the pancreatic body representing a gastrinoma. In this case, the mass was not visible on the arterial phase, unusual for NETs.
in the pancreatic body representing a gastrinoma. In this case, the mass was not visible on the arterial phase, unusual for NETs.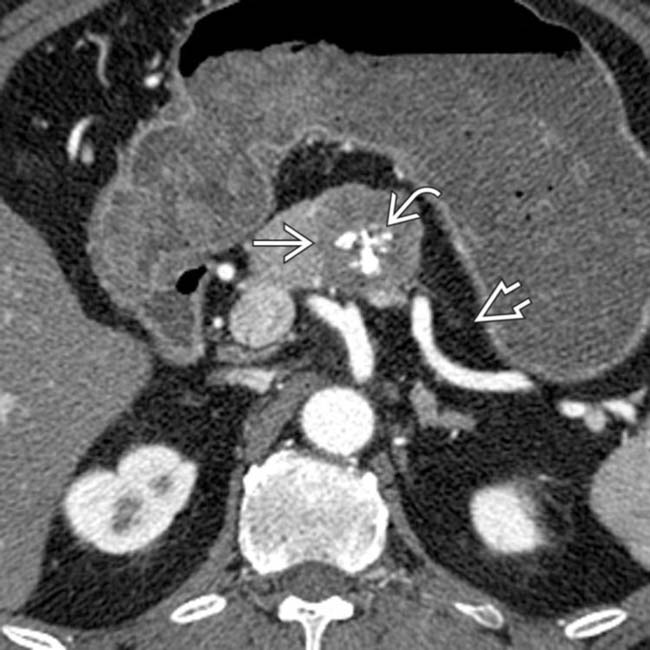
 with central calcifications
with central calcifications  in the pancreatic body. Note the atrophy
in the pancreatic body. Note the atrophy  of the distal pancreas, an unusual feature for NETs. ∼15% of NETs calcify, while pancreatic adenocarcinomas almost never calcify.
of the distal pancreas, an unusual feature for NETs. ∼15% of NETs calcify, while pancreatic adenocarcinomas almost never calcify.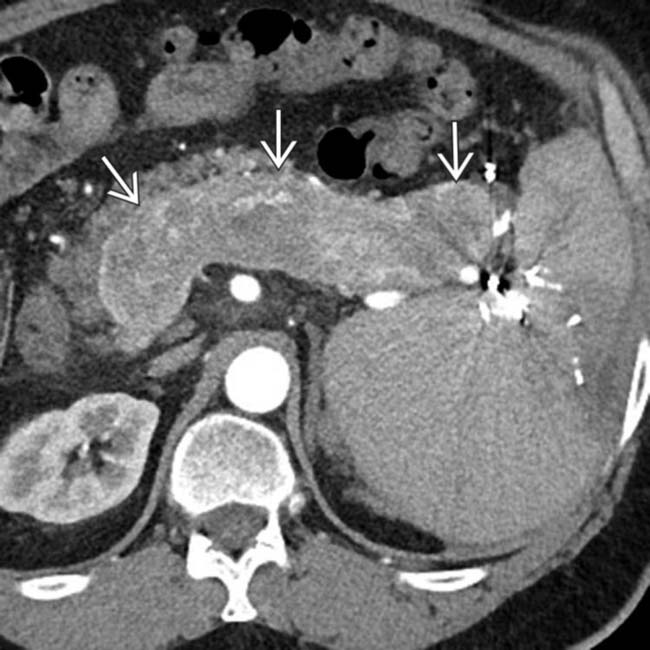
 . NETs, unlike pancreatic adenocarcinomas, do not narrow/occlude the mesenteric veins, but may invade the veins with hypervascular tumor thrombus.
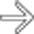
. NETs, unlike pancreatic adenocarcinomas, do not narrow/occlude the mesenteric veins, but may invade the veins with hypervascular tumor thrombus.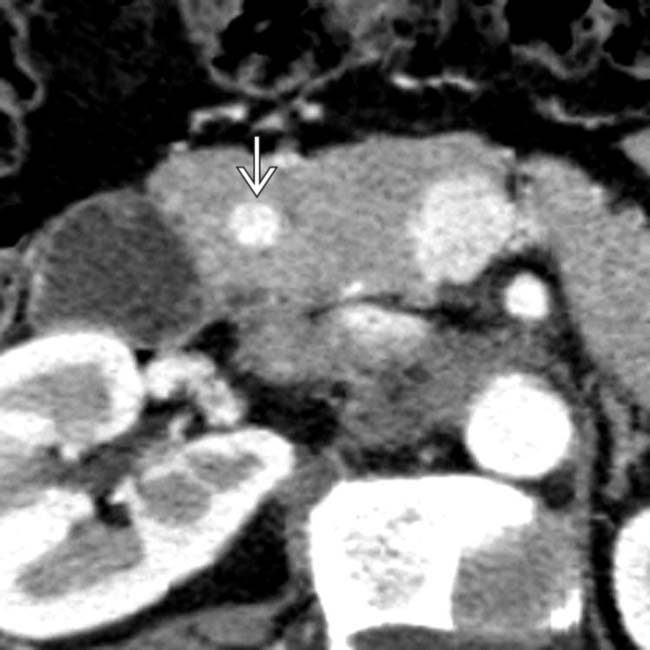
 in the head of the pancreas.
in the head of the pancreas.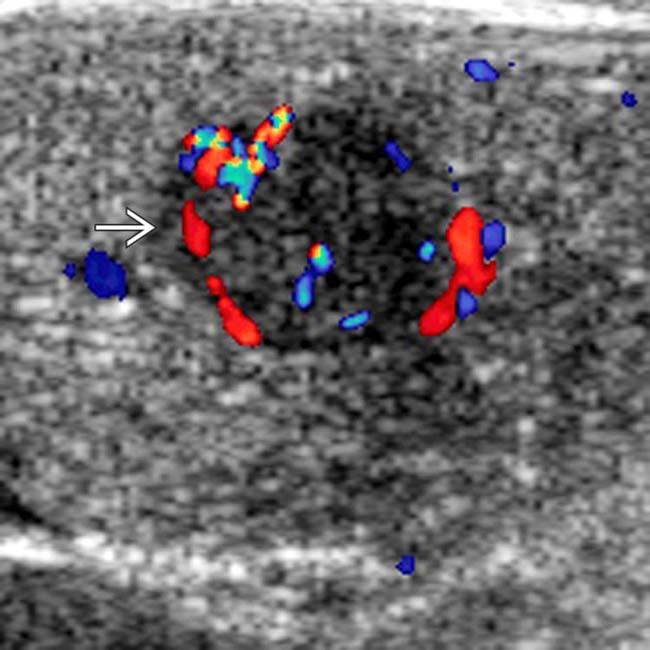
 that was not palpable. The patient underwent a Whipple resection, and an insulinoma was found at pathology. Intraoperative ultrasound is extremely valuable to localize lesions and to guide surgical resection of small NETs.
that was not palpable. The patient underwent a Whipple resection, and an insulinoma was found at pathology. Intraoperative ultrasound is extremely valuable to localize lesions and to guide surgical resection of small NETs.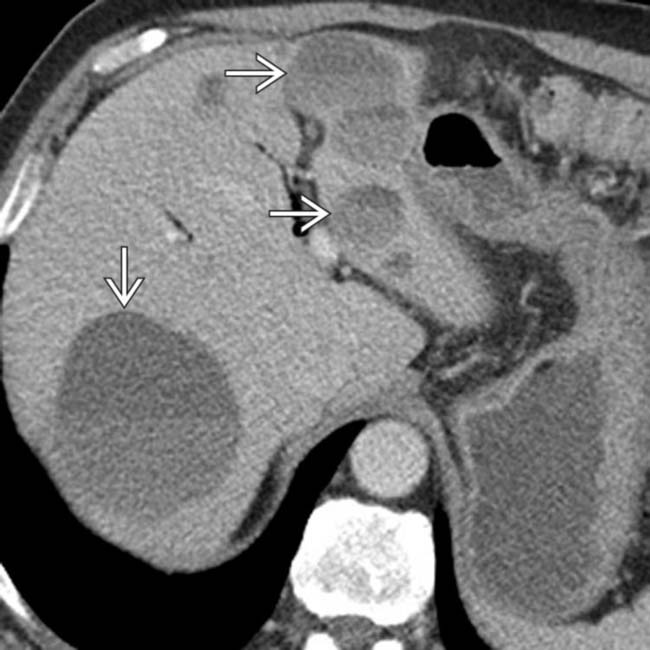
 with fluid-fluid levels. Although uncommon, this feature has been described as specific for neuroendocrine tumor liver metastases.
with fluid-fluid levels. Although uncommon, this feature has been described as specific for neuroendocrine tumor liver metastases.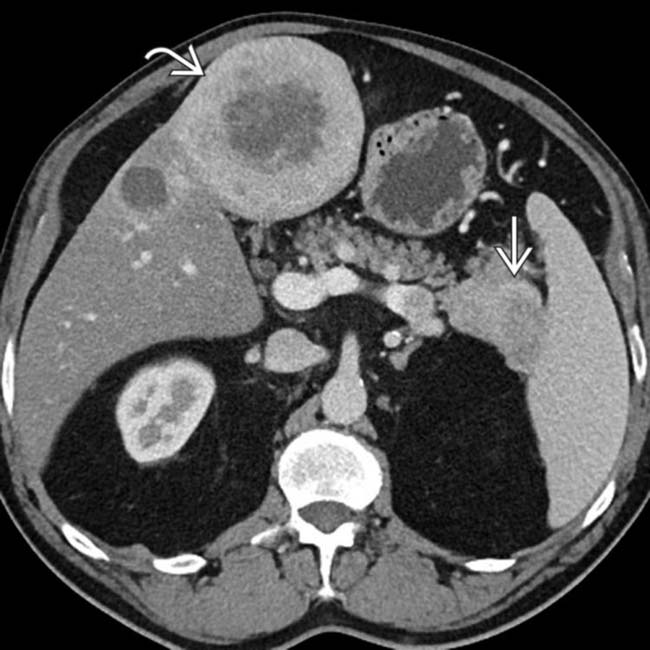
 in the pancreatic tail, compatible with a neuroendocrine tumor. Note that the large metastatic lesion
in the pancreatic tail, compatible with a neuroendocrine tumor. Note that the large metastatic lesion  in the liver demonstrates relatively similar enhancement to the primary pancreatic tumor.
in the liver demonstrates relatively similar enhancement to the primary pancreatic tumor.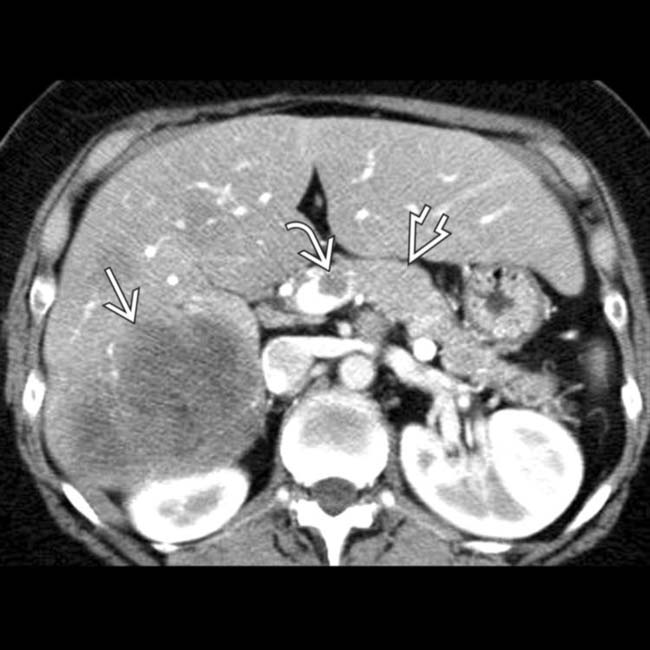
 invading the portal vein
invading the portal vein  with a large liver metastasis
with a large liver metastasis  . Treatment with chemoembolization led to several years of effective palliation. Malignant NETs often invade the portal vein with widespread hepatic metastases.
. Treatment with chemoembolization led to several years of effective palliation. Malignant NETs often invade the portal vein with widespread hepatic metastases.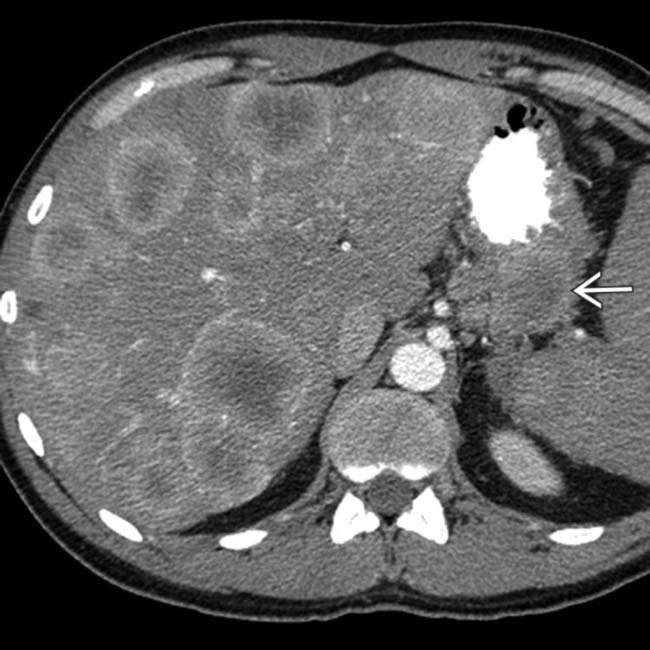
 . As in this case, NET and their metastases are often markedly hypervascular in the arterial phase.
. As in this case, NET and their metastases are often markedly hypervascular in the arterial phase.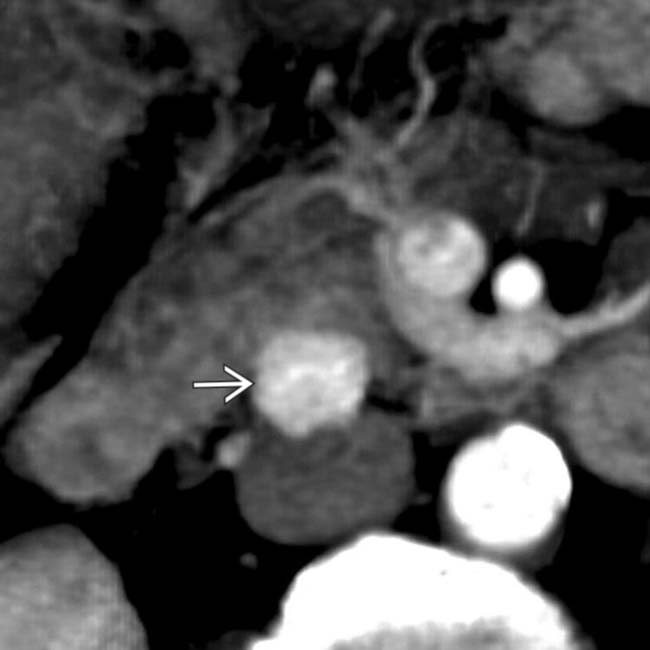
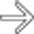 in the head of the pancreas.
in the head of the pancreas.
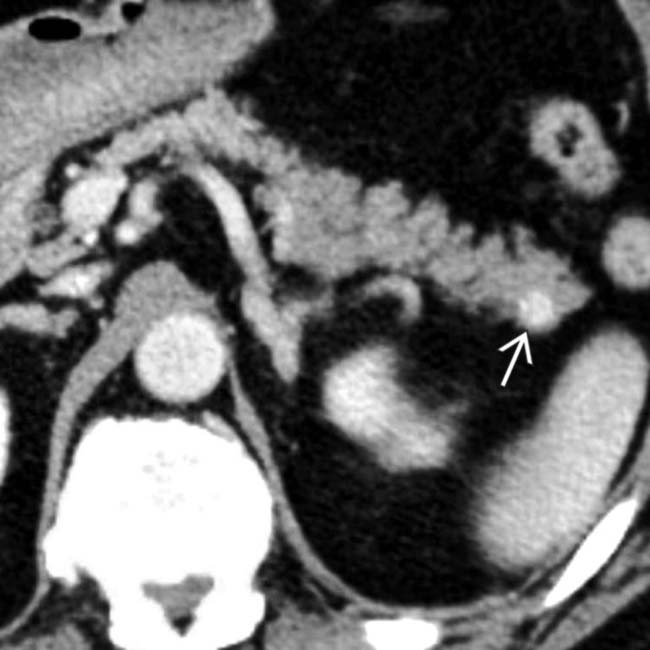
 adjacent to small hypervascular liver metastases
adjacent to small hypervascular liver metastases 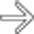 . This focal perilesional steatosis, likely due to the localized effects of insulin from the functioning metastases, is a rare but recognized manifestation of metastatic insulinomas.
. This focal perilesional steatosis, likely due to the localized effects of insulin from the functioning metastases, is a rare but recognized manifestation of metastatic insulinomas.
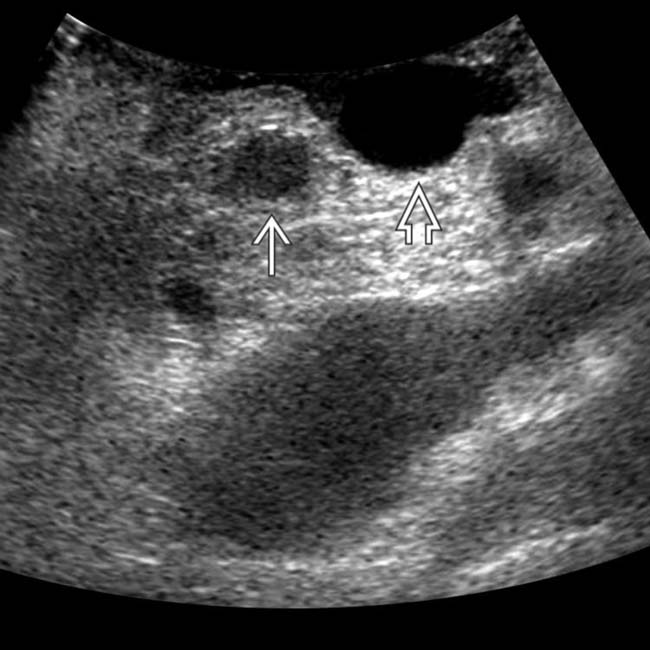
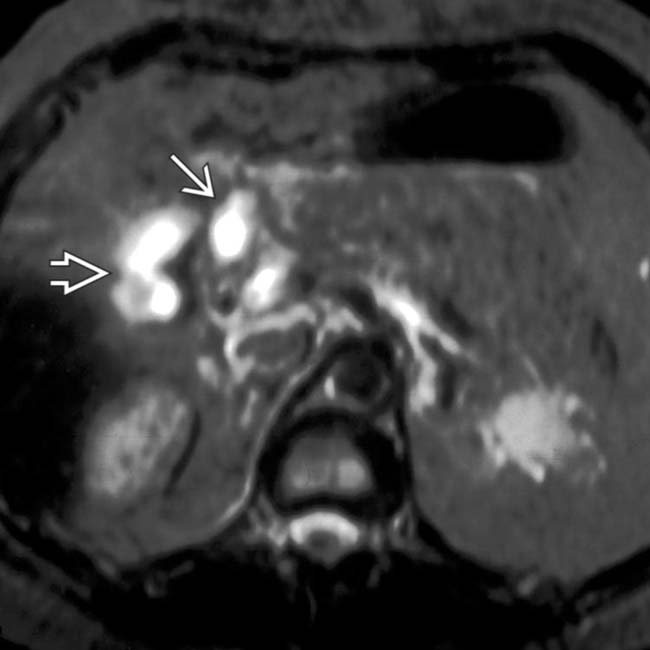
 in the pancreatic head; it was not detected on portal venous phase CT. Note the opacified superior mesenteric artery and unopacified superior mesenteric vein
in the pancreatic head; it was not detected on portal venous phase CT. Note the opacified superior mesenteric artery and unopacified superior mesenteric vein  .
.
 in the pancreatic head, just lateral to the superior mesenteric vein
in the pancreatic head, just lateral to the superior mesenteric vein  .
.
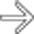 with hypervascular liver metastases.
with hypervascular liver metastases.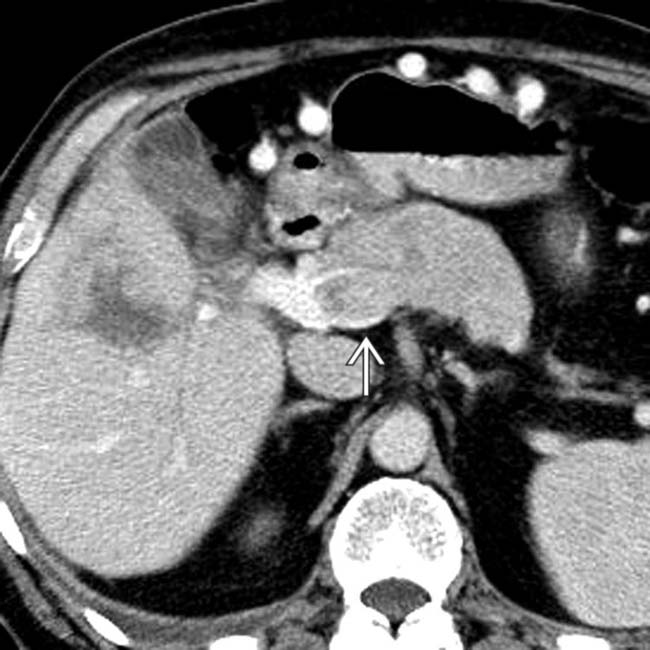
 . Hypodense liver metastases are also seen.
. Hypodense liver metastases are also seen.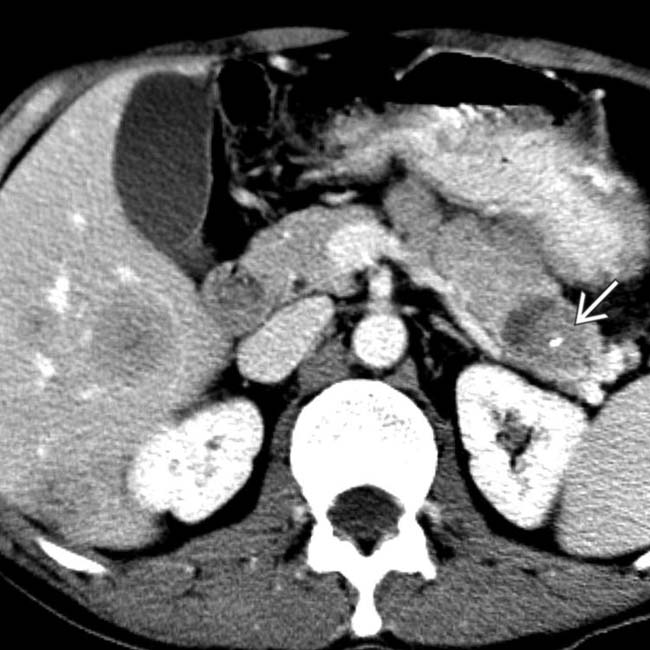
 , which demonstrates liver metastases.
, which demonstrates liver metastases.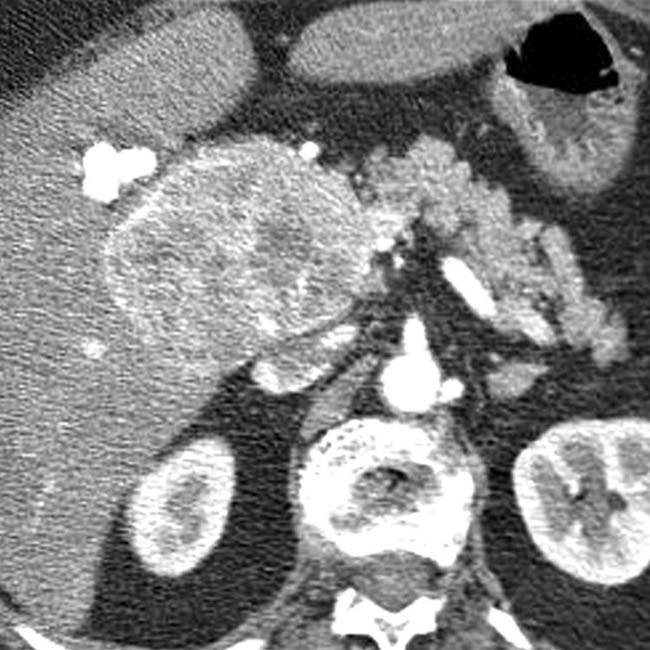
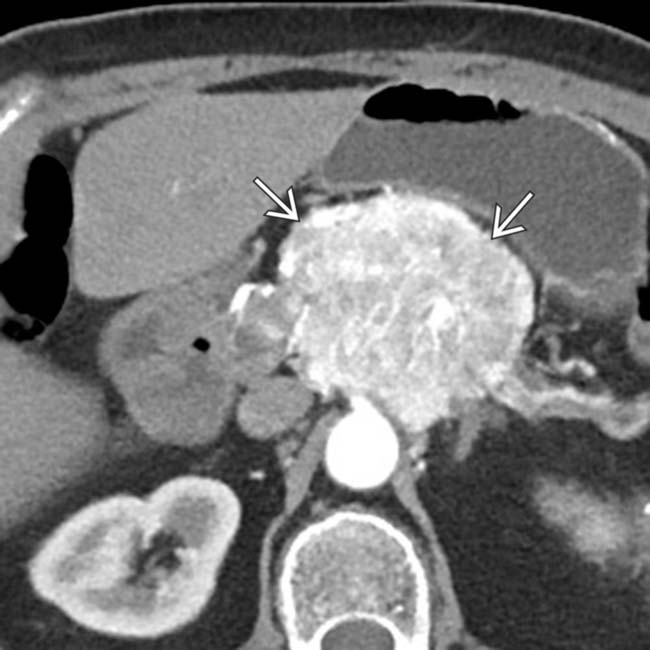
 in the pancreatic head, just medial to the duodenum
in the pancreatic head, just medial to the duodenum  .
.
 , in keeping with a pancreatic neuroendocrine tumor.
, in keeping with a pancreatic neuroendocrine tumor.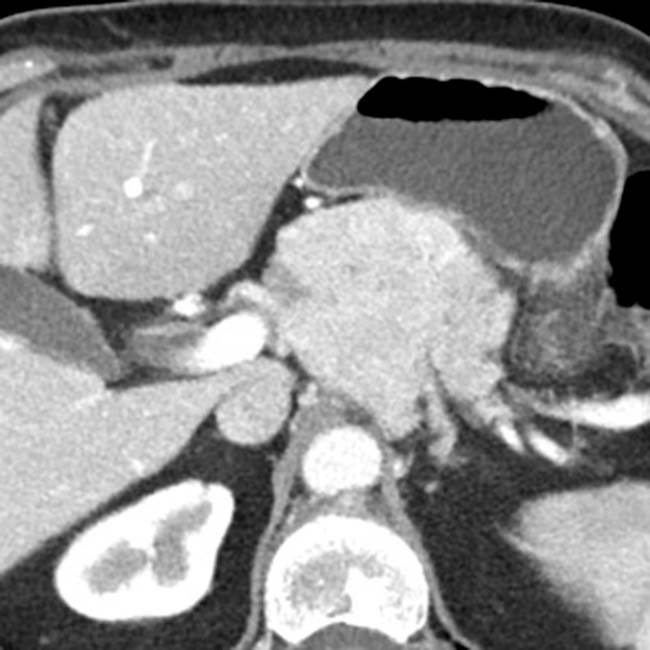
 is still enhancing on this venous phase image from the same patient, note that the mass demonstrates a much lesser degree of enhancement compared to the arterial phase image, very common among most neuroendocrine tumors.
is still enhancing on this venous phase image from the same patient, note that the mass demonstrates a much lesser degree of enhancement compared to the arterial phase image, very common among most neuroendocrine tumors.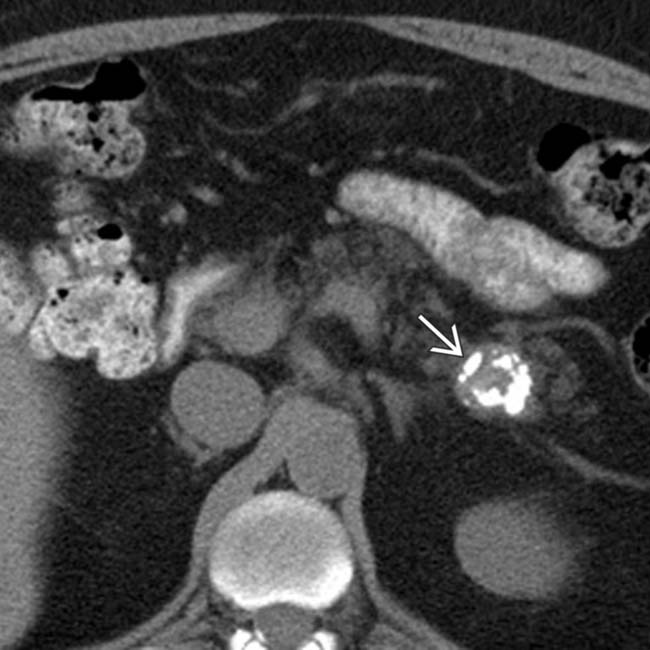
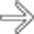
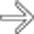
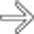
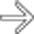 in the pancreatic tail. While one could suggest the possibility of an IPMN at first glance, the cyst’s peripheral enhancement along its margin should suggest the possibility of a cystic neuroendocrine tumor.
in the pancreatic tail. While one could suggest the possibility of an IPMN at first glance, the cyst’s peripheral enhancement along its margin should suggest the possibility of a cystic neuroendocrine tumor.
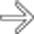 in the pancreatic tail with central dystrophic calcifications. This image was acquired in the venous phase, explaining the relative lack of enhancement shown by the mass, but the presence of calcifications in a solid mass should hint at the possibility of a neuroendocrine tumor. Pancreatic adenocarcinomas do not typically calcify.
in the pancreatic tail with central dystrophic calcifications. This image was acquired in the venous phase, explaining the relative lack of enhancement shown by the mass, but the presence of calcifications in a solid mass should hint at the possibility of a neuroendocrine tumor. Pancreatic adenocarcinomas do not typically calcify.
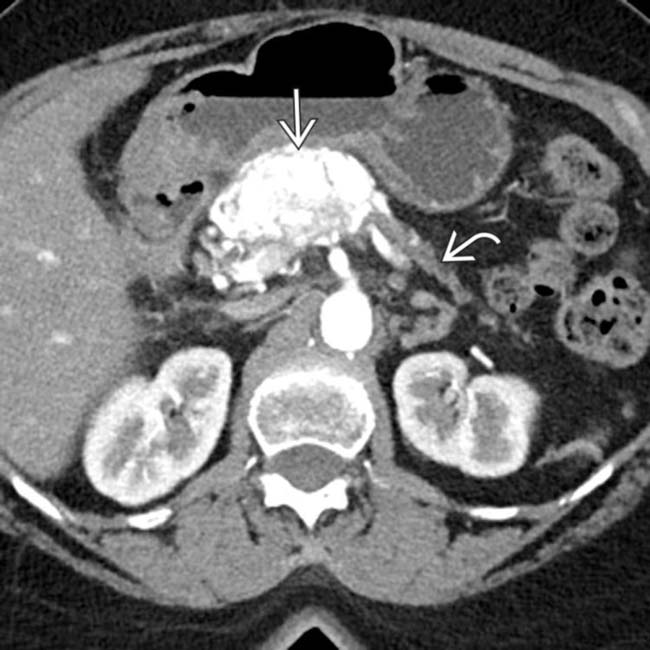
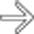
 in the head of the pancreas causing obstruction of the pancreatic duct and marked atrophy of the pancreas and ductal dilation
in the head of the pancreas causing obstruction of the pancreatic duct and marked atrophy of the pancreas and ductal dilation  upstream.
upstream.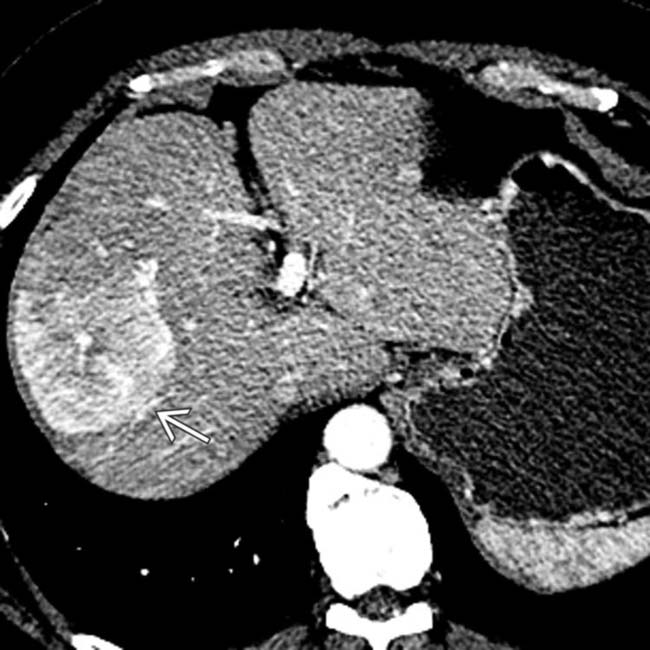
 . Nonsyndromic neuroendocrine tumors often do not obstruct the pancreatic duct, although ductal obstruction can occur with tumors of large size. The large size and hypervascularity of the tumor are characteristic findings in this entity.
. Nonsyndromic neuroendocrine tumors often do not obstruct the pancreatic duct, although ductal obstruction can occur with tumors of large size. The large size and hypervascularity of the tumor are characteristic findings in this entity.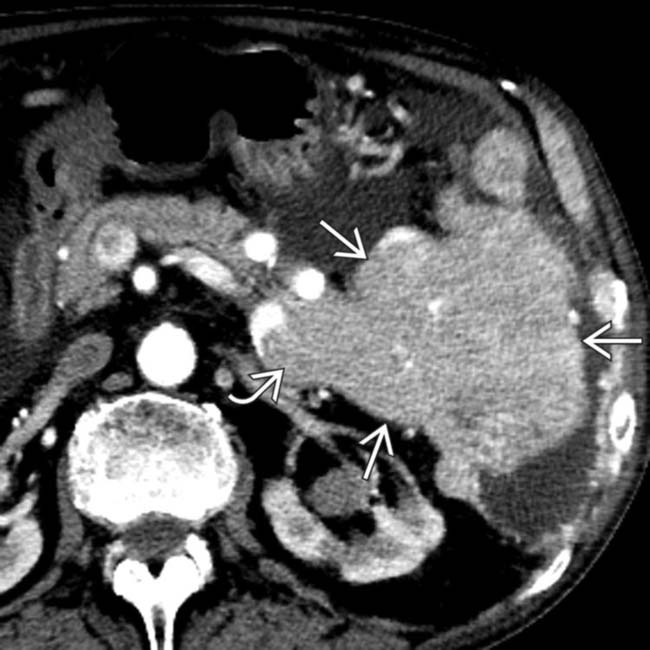
 originating from tail of the pancreas. The mass invades the splenic vein
originating from tail of the pancreas. The mass invades the splenic vein  .
.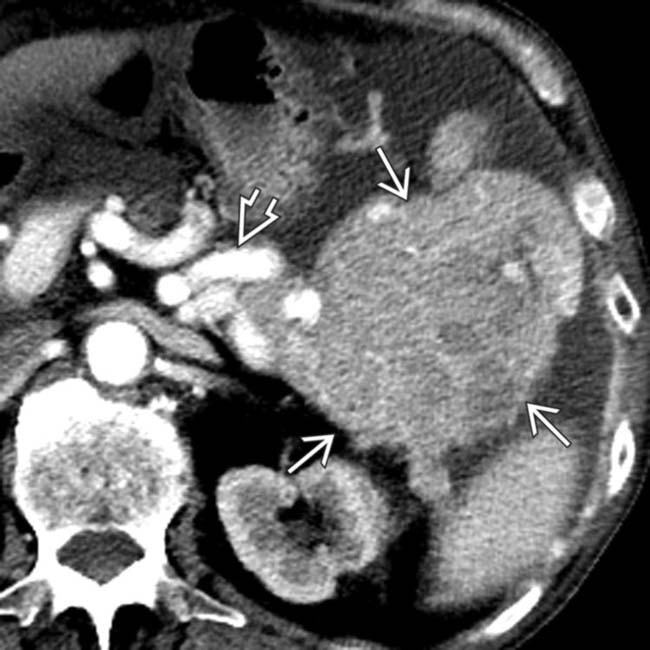
 secondary to splenic vein invasion. This case illustrates a typical nonsyndromic neuroendocrine tumor as a bulky exophytic and hypervascular mass
secondary to splenic vein invasion. This case illustrates a typical nonsyndromic neuroendocrine tumor as a bulky exophytic and hypervascular mass  . Ductal adenocarcinoma often encases and obstructs venous structures rather than causing direct venous invasion.
. Ductal adenocarcinoma often encases and obstructs venous structures rather than causing direct venous invasion.