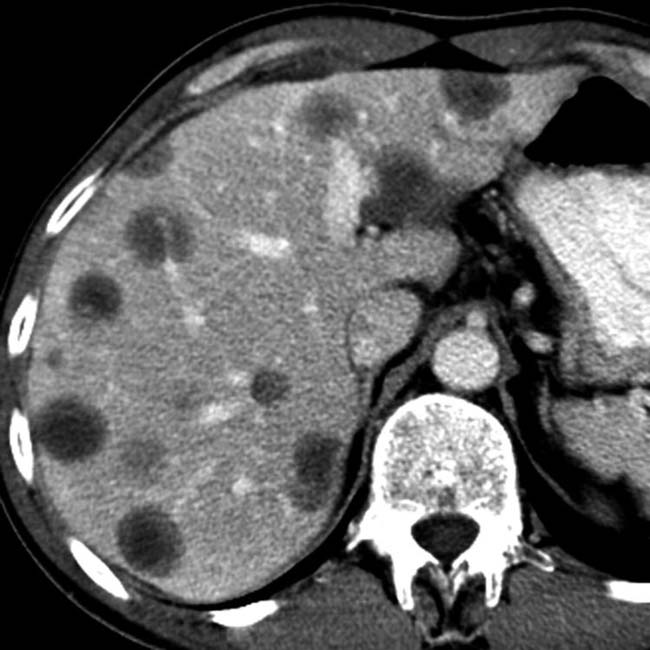
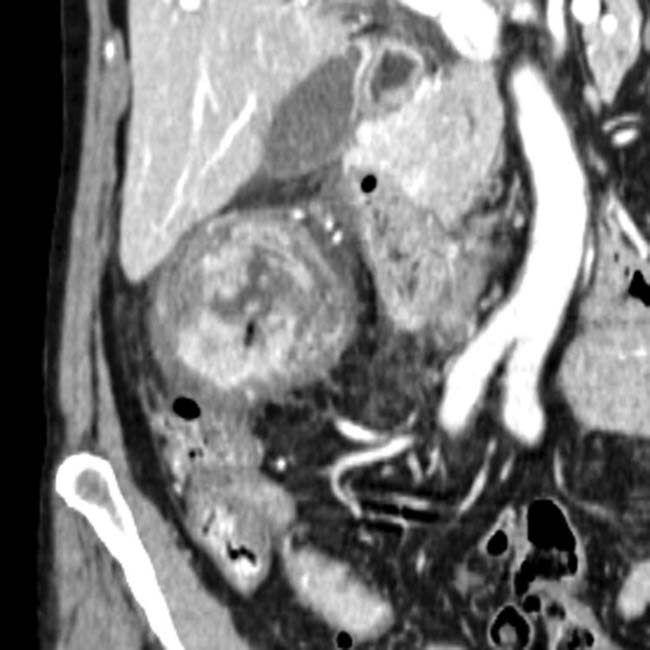
Well-defined cystic lesion with variable morphology: Unilocular, multicystic, or tubular
 Communication with adjacent main pancreatic duct is key to diagnosis (may be more visible on MR than CT)
Communication with adjacent main pancreatic duct is key to diagnosis (may be more visible on MR than CT)CLINICAL ISSUES
• Most patients asymptomatic (incidental imaging finding), but can result in repetitive bouts of pancreatitis
• Risk of transformation into invasive carcinoma, with main duct involvement associated with ↑ risk of malignancy
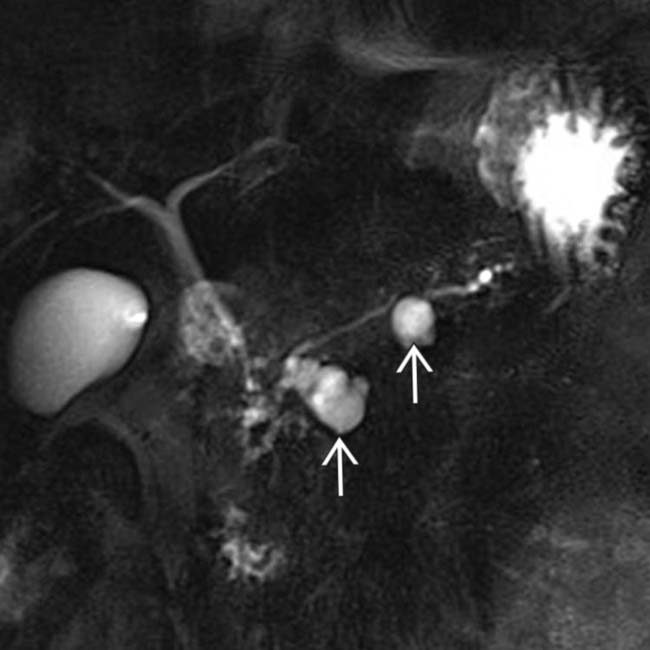
 and their direct connection with the adjacent normal sized pancreatic duct.
and their direct connection with the adjacent normal sized pancreatic duct.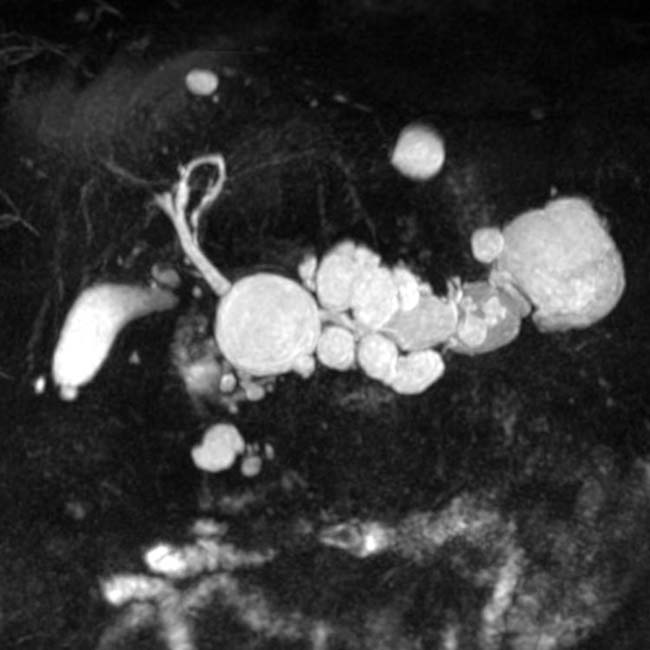
IMAGING
General Features
• Location
CT Findings
• Side branch IPMN
 Well-defined cystic lesion with variable morphology: Unilocular, multicystic (with grape-like clusters or tubes and arcs), or tubular
Well-defined cystic lesion with variable morphology: Unilocular, multicystic (with grape-like clusters or tubes and arcs), or tubular
 Well-defined cystic lesion with variable morphology: Unilocular, multicystic (with grape-like clusters or tubes and arcs), or tubular
Well-defined cystic lesion with variable morphology: Unilocular, multicystic (with grape-like clusters or tubes and arcs), or tubular• Main duct IPMN
• Concerning imaging features based on 2012 International Association of Pancreatology (IAP) guidelines
MR Findings
• Little data directly comparing CT and MR, but MR likely superior for identifying small cysts and multifocal disease, visualizing communication between cyst and main duct, and assessing main duct involvement
• Side branch IPMN typically hyperintense on T2WI and low signal on T1WI, and can appear unilocular, multicystic, tubular, or as grape-like cluster of cysts
Ultrasonographic Findings
• Conventional ultrasound lacks spatial resolution to identify high-risk or worrisome imaging features
PATHOLOGY
General Features
CLINICAL ISSUES
Presentation
Treatment
• Management of IPMN based on 2012 IAP guidelines
 High-risk features (MPD ≥ 1 cm, enhancing mural nodularity, or biliary obstruction) warrant resection
High-risk features (MPD ≥ 1 cm, enhancing mural nodularity, or biliary obstruction) warrant resection
 Lesions with worrisome features (cyst size ≥ 3 cm, MPD dilatation between 5-9 mm, nonenhancing mural nodularity, abrupt change in main duct caliber with distal pancreatic atrophy) should undergo EUS
Lesions with worrisome features (cyst size ≥ 3 cm, MPD dilatation between 5-9 mm, nonenhancing mural nodularity, abrupt change in main duct caliber with distal pancreatic atrophy) should undergo EUS
 High-risk features (MPD ≥ 1 cm, enhancing mural nodularity, or biliary obstruction) warrant resection
High-risk features (MPD ≥ 1 cm, enhancing mural nodularity, or biliary obstruction) warrant resection Lesions with worrisome features (cyst size ≥ 3 cm, MPD dilatation between 5-9 mm, nonenhancing mural nodularity, abrupt change in main duct caliber with distal pancreatic atrophy) should undergo EUS
Lesions with worrisome features (cyst size ≥ 3 cm, MPD dilatation between 5-9 mm, nonenhancing mural nodularity, abrupt change in main duct caliber with distal pancreatic atrophy) should undergo EUS
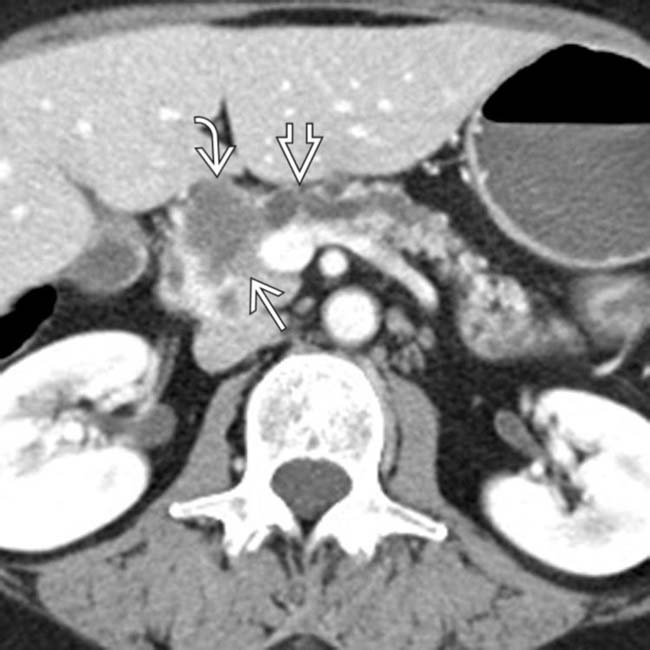
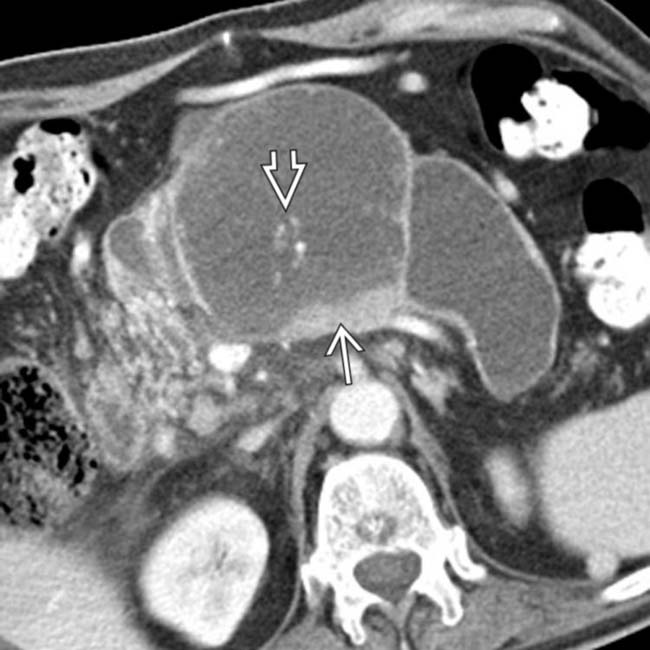
 in the pancreatic head. Note the soft tissue
in the pancreatic head. Note the soft tissue  around the margins of the cyst, as well as its communication with a dilated pancreatic duct
around the margins of the cyst, as well as its communication with a dilated pancreatic duct  . This was found to be an IPMN with invasive carcinoma and main duct involvement at surgery.
. This was found to be an IPMN with invasive carcinoma and main duct involvement at surgery.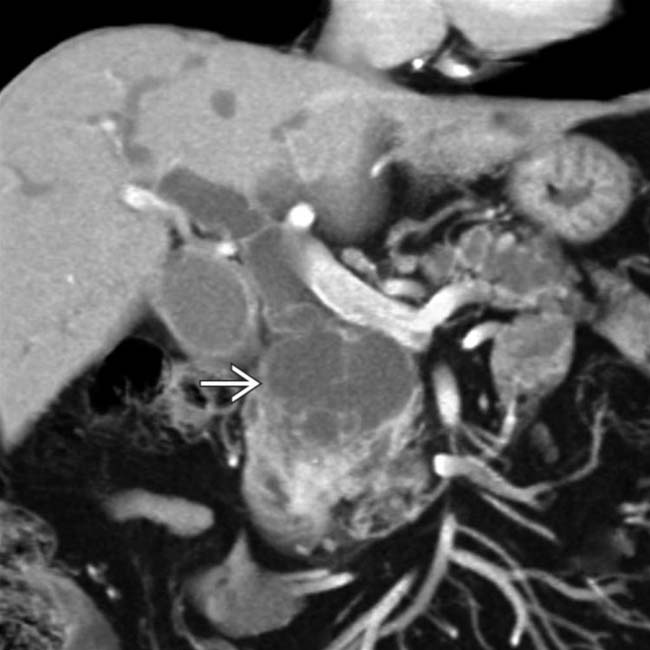
 in the pancreatic head with multiple septations, mural nodularity, and obstruction of the CBD. This was found to be an IPMN with invasive carcinoma.
in the pancreatic head with multiple septations, mural nodularity, and obstruction of the CBD. This was found to be an IPMN with invasive carcinoma.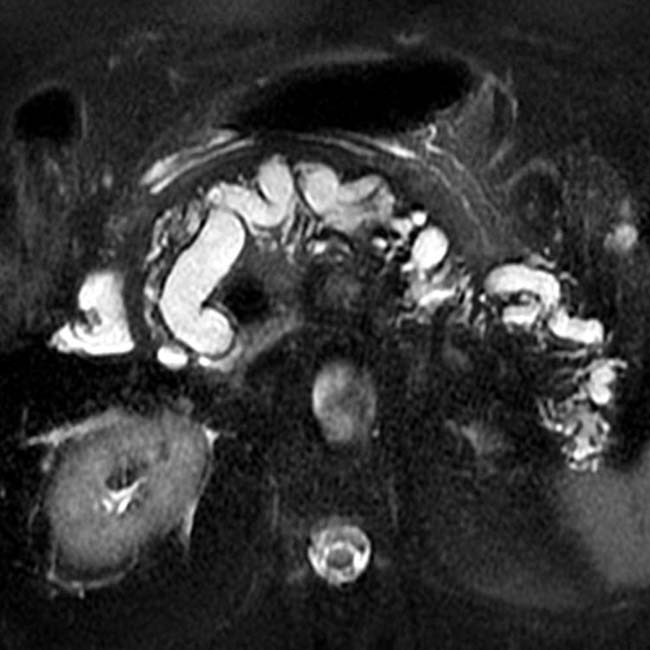
 in the center of the duct and clear enhancing intraductal soft tissue
in the center of the duct and clear enhancing intraductal soft tissue  . The pancreatic parenchyma is severely atrophic.
. The pancreatic parenchyma is severely atrophic.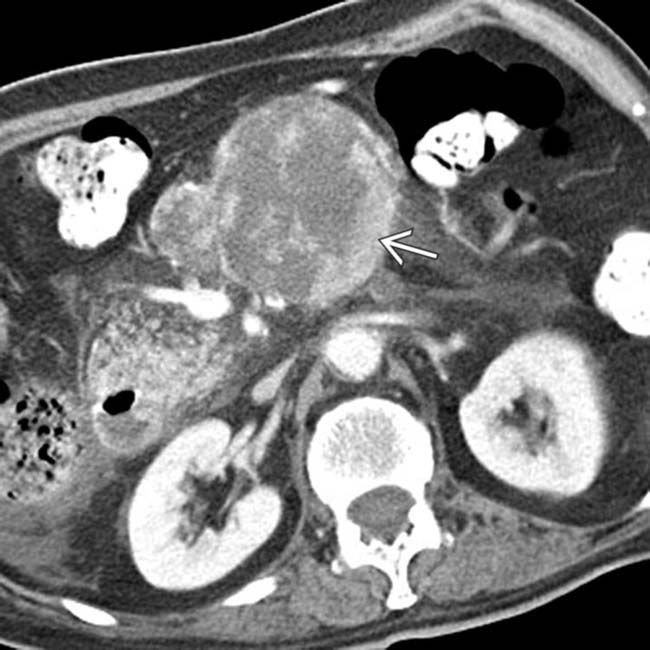
 within the mass. This was found to represent a mixed-type IPMN with invasive colloid carcinoma at surgery.
within the mass. This was found to represent a mixed-type IPMN with invasive colloid carcinoma at surgery.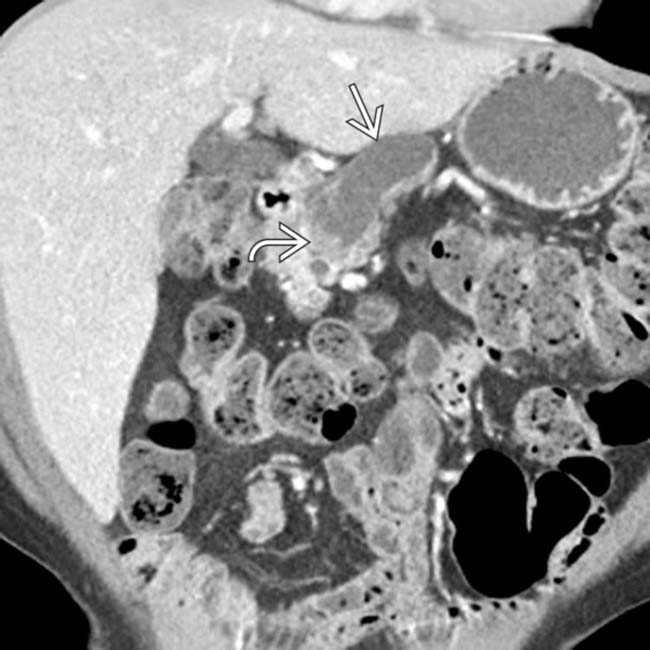
 . Note the presence of subtle enhancing soft tissue
. Note the presence of subtle enhancing soft tissue  within the duct.
within the duct.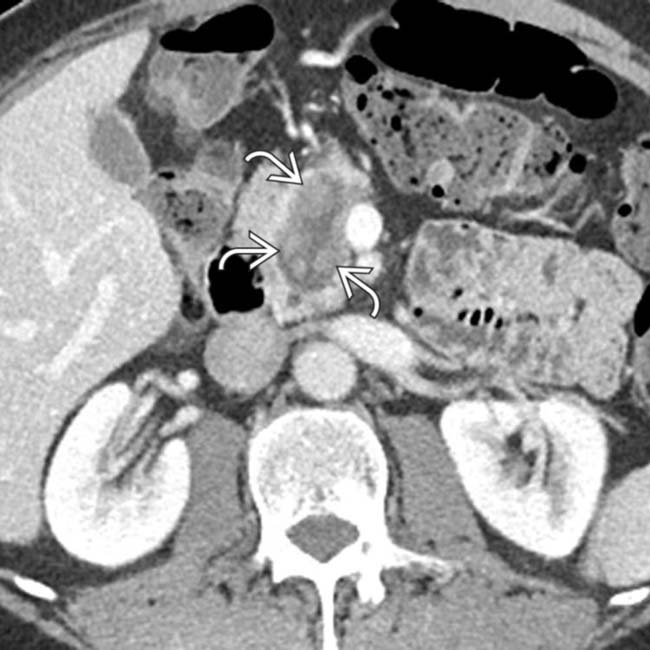
 within the downstream duct, a highly suspicious feature for malignancy. This was found to be a main duct IPMN with invasive carcinoma at surgery.
within the downstream duct, a highly suspicious feature for malignancy. This was found to be a main duct IPMN with invasive carcinoma at surgery.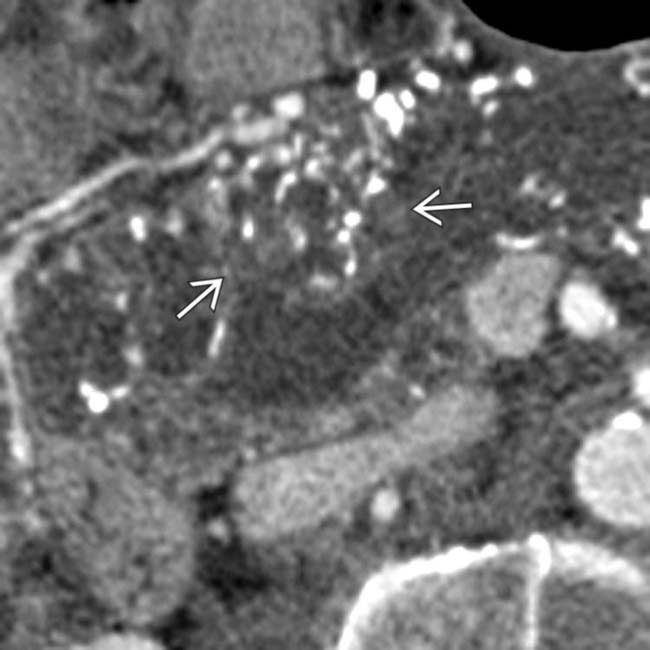
 within the massively dilated pancreatic duct. At surgery the calcifications were due to invasive mucinous adenocarcinoma from a main duct IPMN.
within the massively dilated pancreatic duct. At surgery the calcifications were due to invasive mucinous adenocarcinoma from a main duct IPMN.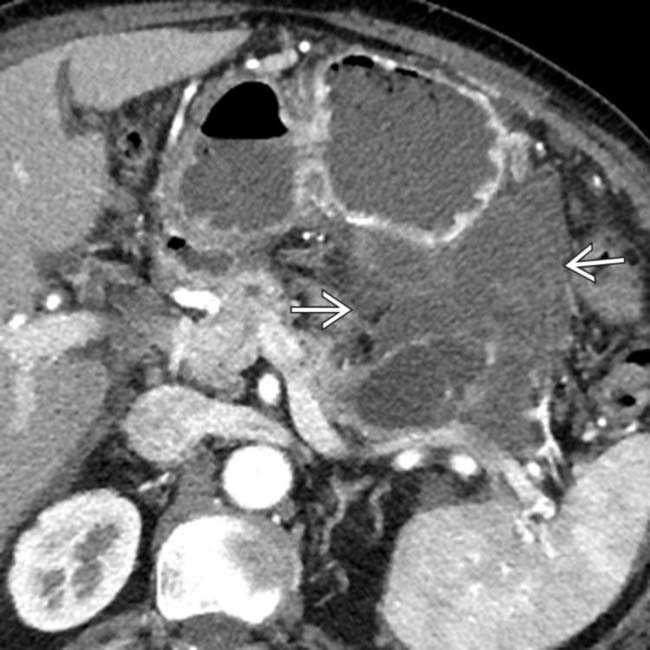
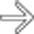
 . At surgery a ruptured IPMN was found with mucinous debris in the lesser sac.
. At surgery a ruptured IPMN was found with mucinous debris in the lesser sac.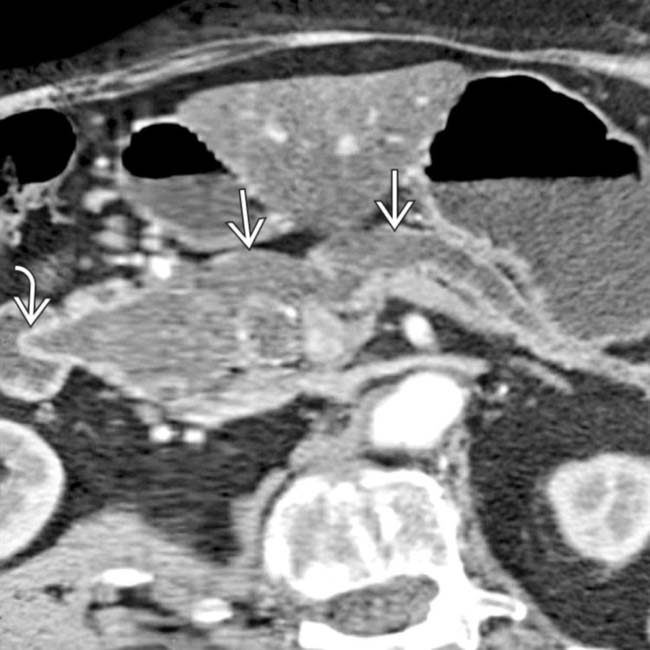
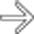
 with a bulging ampulla
with a bulging ampulla  , a characteristic feature of main duct IPMN.
, a characteristic feature of main duct IPMN.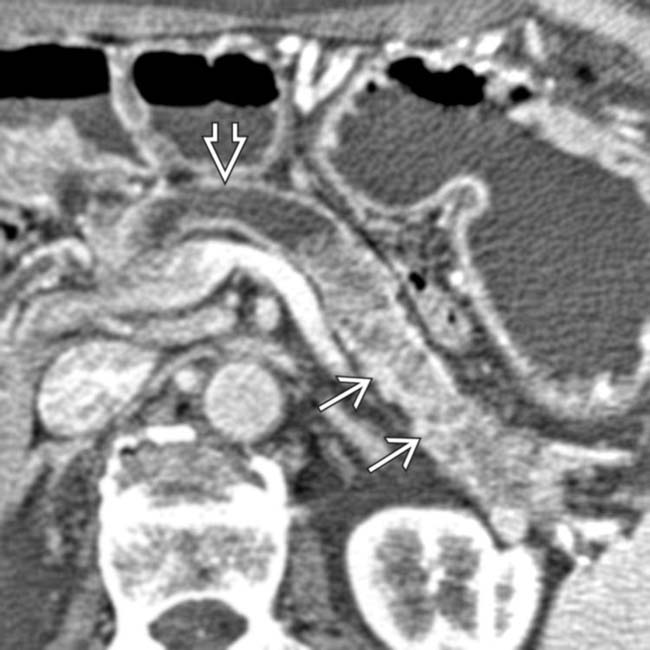
 that contains intraductal solid tissue
that contains intraductal solid tissue  characteristic of a malignant main duct IPMN.
characteristic of a malignant main duct IPMN.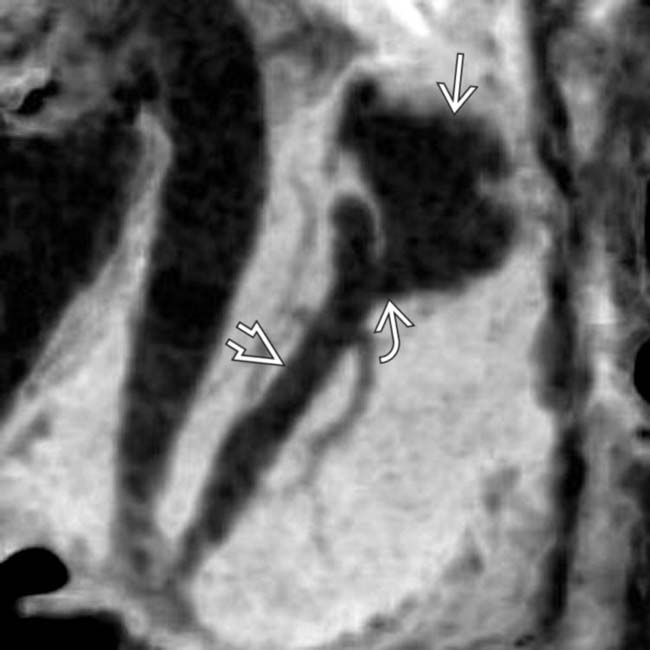
 connected by a side branch
connected by a side branch  to the MPD
to the MPD  . These findings are diagnostic of a side branch IPMN.
. These findings are diagnostic of a side branch IPMN.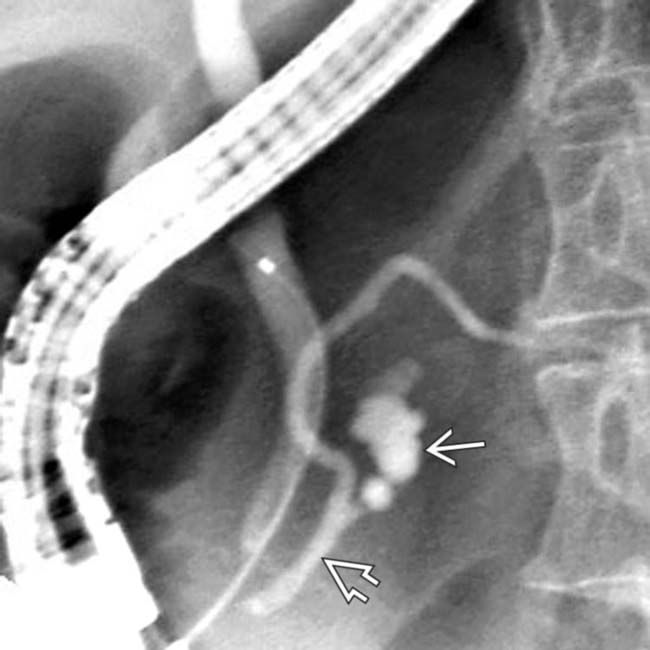
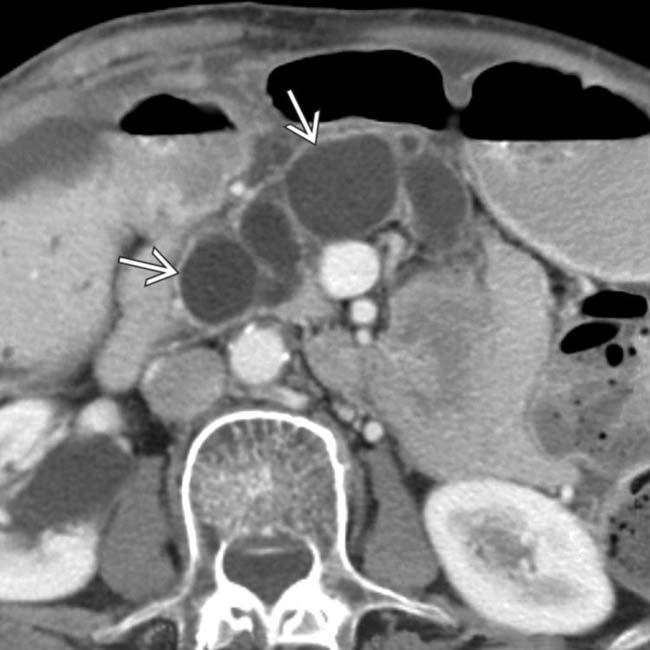
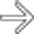 and confirms its communication with the adjacent main pancreatic duct
and confirms its communication with the adjacent main pancreatic duct  .
.
 , with diffuse atrophy of the pancreatic parenchyma, a characteristic constellation of findings for a main duct IPMN.
, with diffuse atrophy of the pancreatic parenchyma, a characteristic constellation of findings for a main duct IPMN.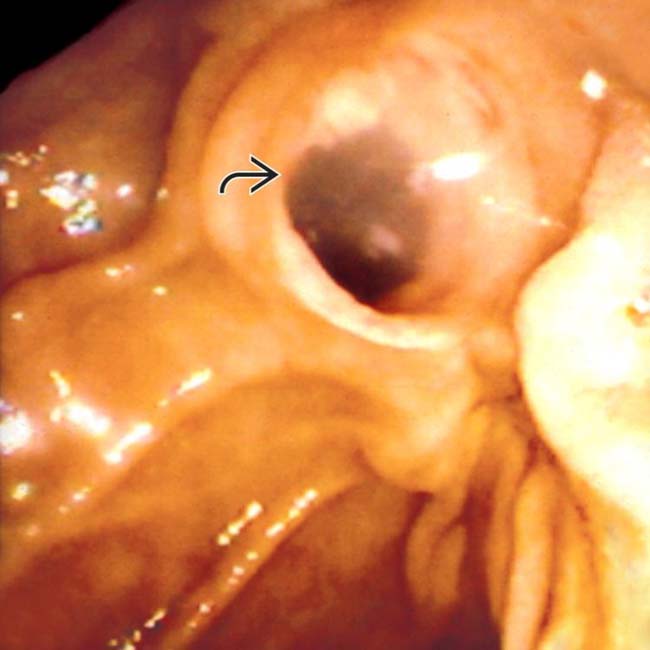
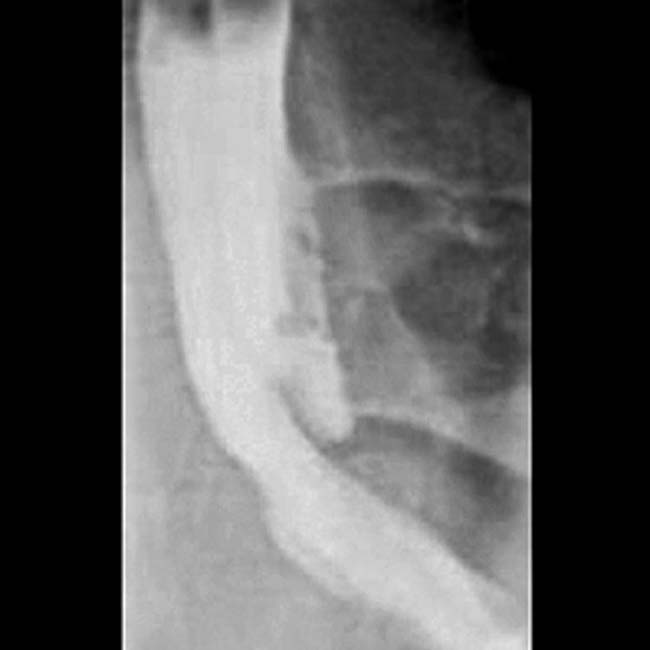
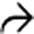 with clear mucin pouring from the orifice, a classic finding in main duct IPMN due to mucin hypersecretion by the tumor.
with clear mucin pouring from the orifice, a classic finding in main duct IPMN due to mucin hypersecretion by the tumor.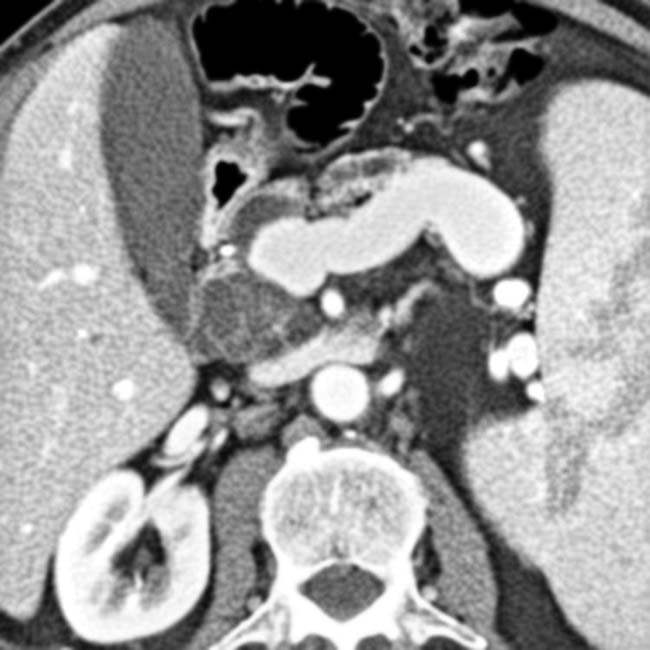
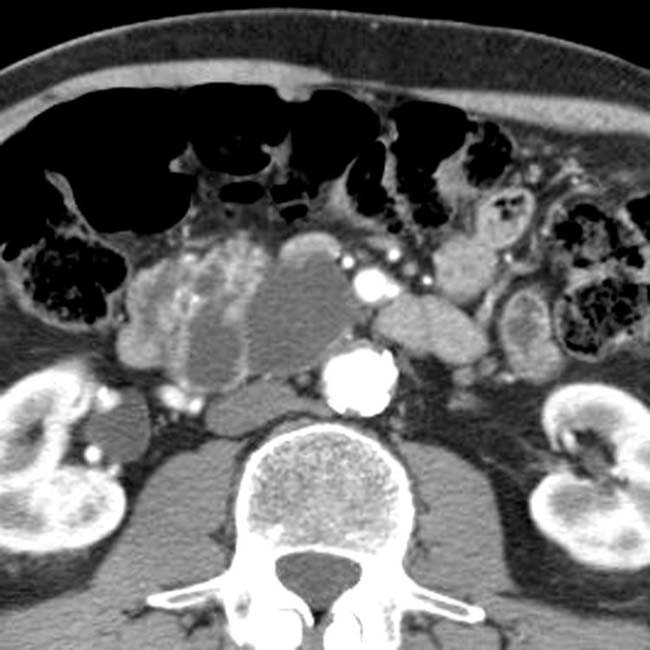
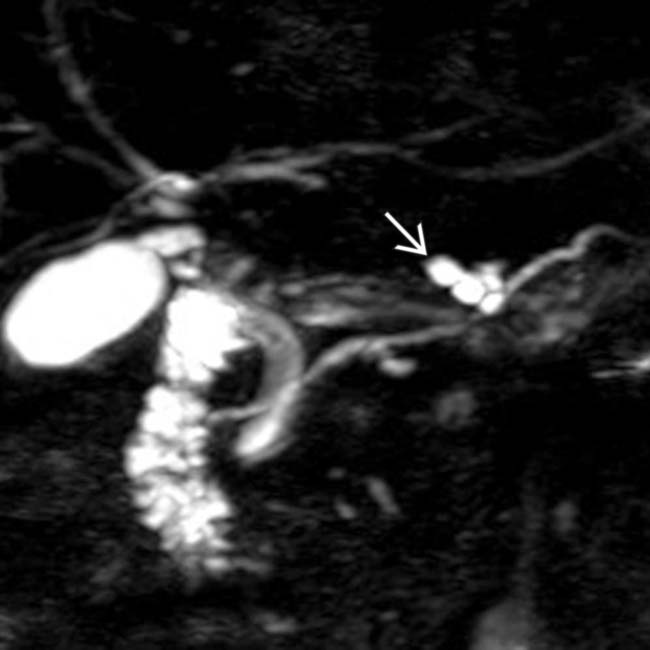
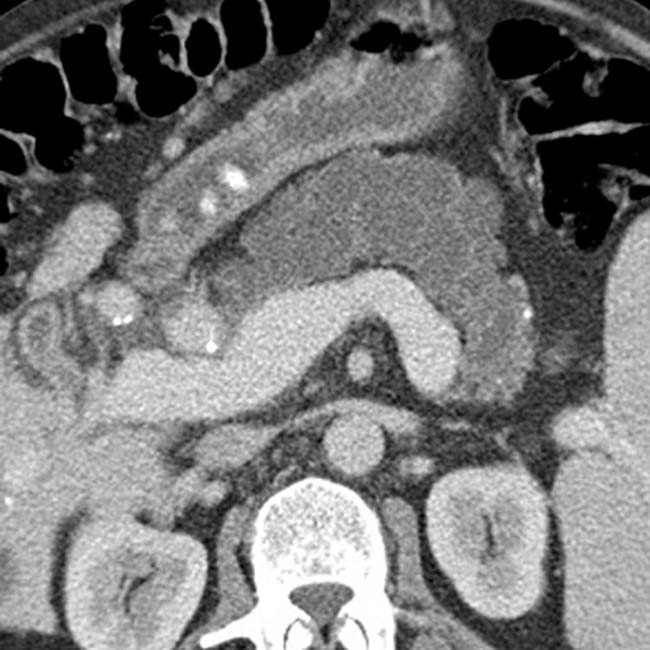
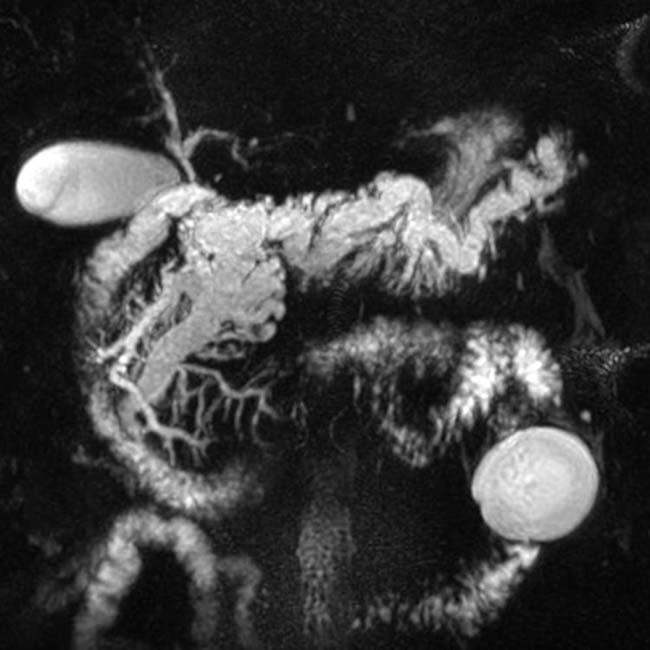
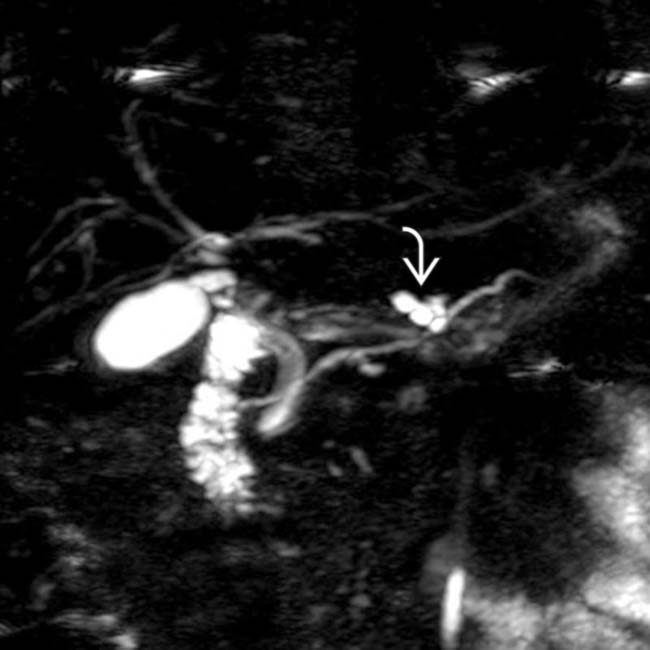
 with a cluster of “grapes” morphology directly communicating with the normal-sized main pancreatic duct, compatible with a side branch IPMN.
with a cluster of “grapes” morphology directly communicating with the normal-sized main pancreatic duct, compatible with a side branch IPMN.
 throughout its length. In addition, a discrete cystic mass
throughout its length. In addition, a discrete cystic mass  is present in the pancreatic head, in direct communication with the duct. The pancreatic parenchyma is atrophic.
is present in the pancreatic head, in direct communication with the duct. The pancreatic parenchyma is atrophic.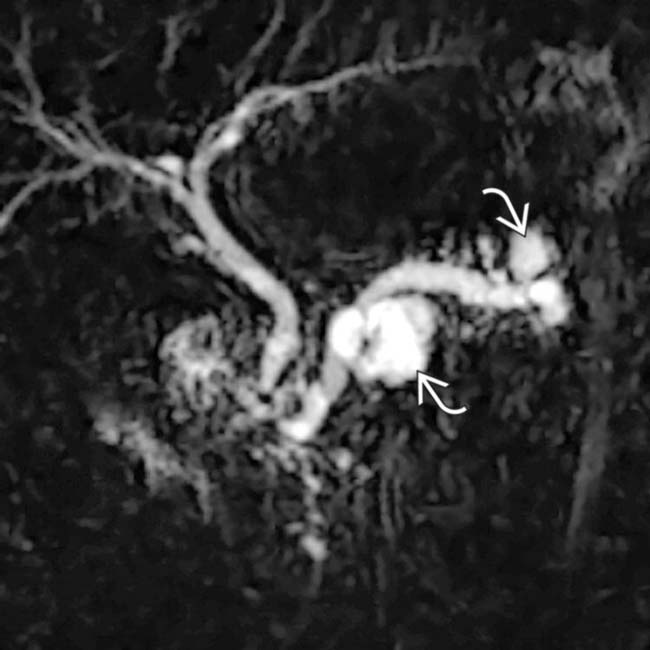
 . MRCP is an excellent noninvasive method to evaluate suspected pancreatic IPMNs.
. MRCP is an excellent noninvasive method to evaluate suspected pancreatic IPMNs.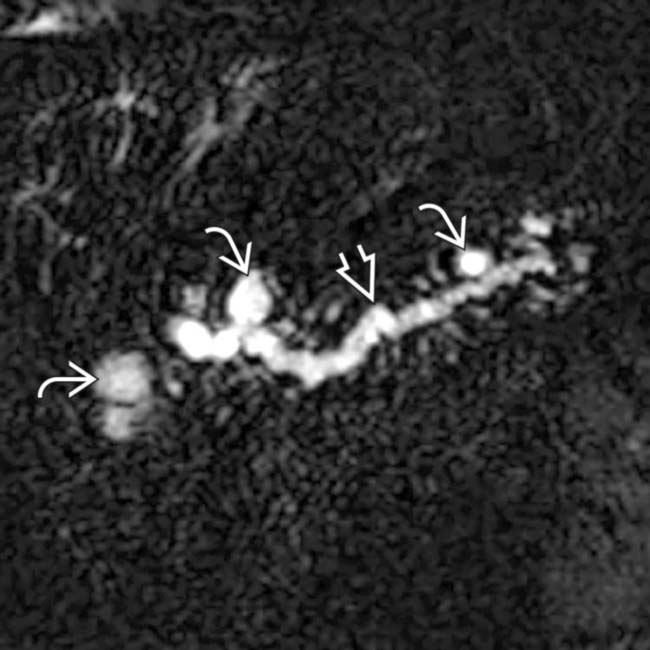
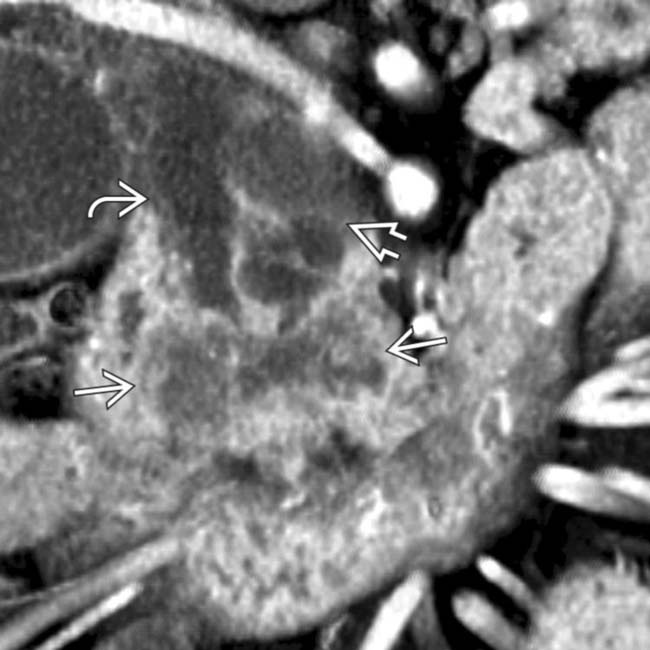
 , as well as the side branches. Note the cyst-like focal dilations of side branches
, as well as the side branches. Note the cyst-like focal dilations of side branches  .
.
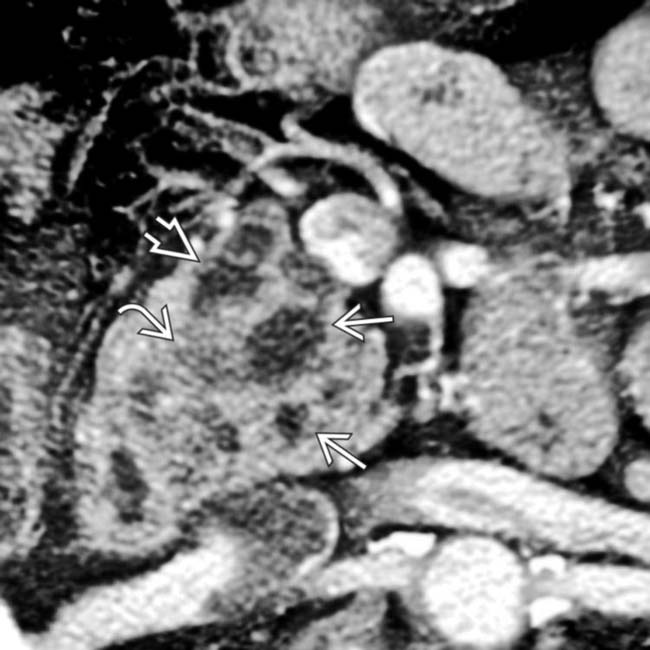
 obstructing the pancreatic
obstructing the pancreatic  and common bile duct
and common bile duct  .
.
 and a hypodense intraductal solid mass
and a hypodense intraductal solid mass  within the pancreatic duct
within the pancreatic duct  . Whipple resection confirmed invasive carcinoma and IPMN.
. Whipple resection confirmed invasive carcinoma and IPMN.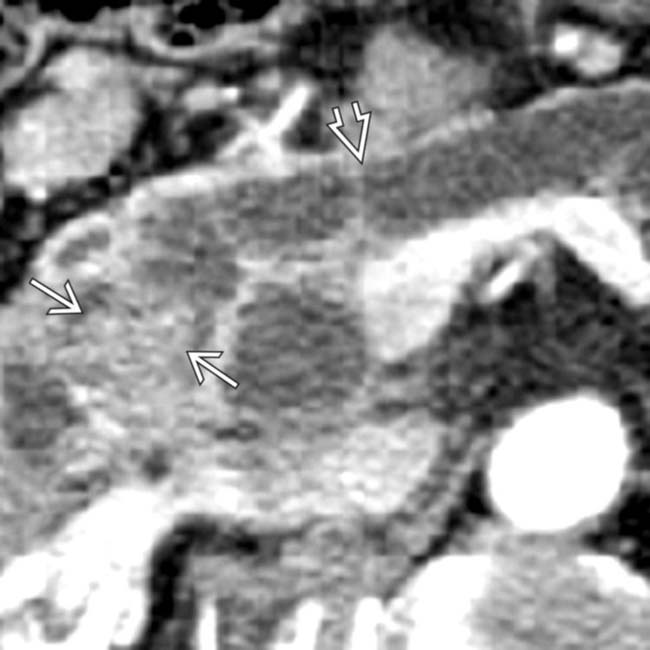
 . Note enhancing soft tissue mass within the duct
. Note enhancing soft tissue mass within the duct 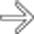 .
.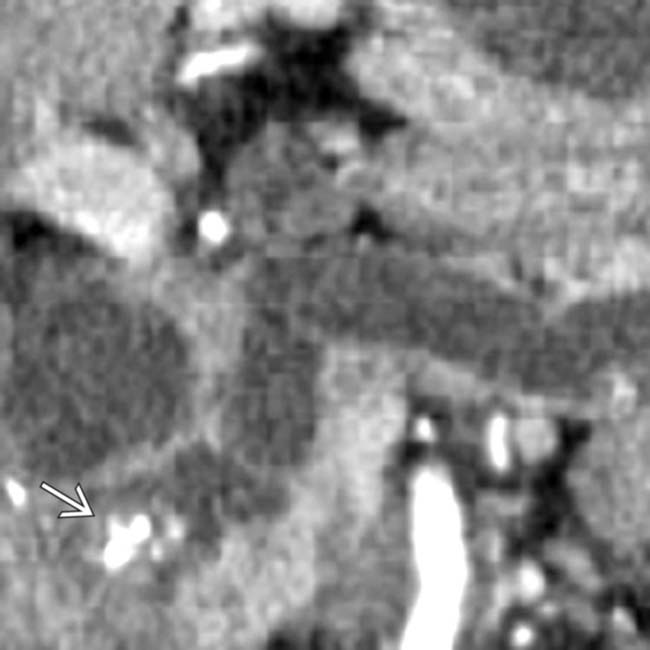
 within the intraductal soft tissue mass. Whipple resection showed a malignant IPMN with invasive carcinoma. Main duct IPMNs have a much higher malignant potential than side branch lesions. A soft tissue mass within the pancreatic duct strongly suggests carcinoma.
within the intraductal soft tissue mass. Whipple resection showed a malignant IPMN with invasive carcinoma. Main duct IPMNs have a much higher malignant potential than side branch lesions. A soft tissue mass within the pancreatic duct strongly suggests carcinoma.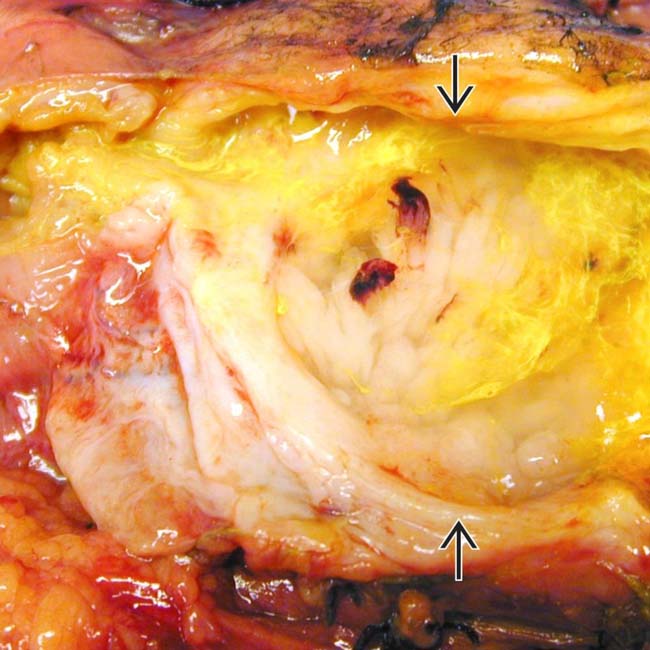
 . Note nodular mucosa with abundant mucin (stained with yellow dye). (Courtesy M. Mino-Kenudson, MD.)
. Note nodular mucosa with abundant mucin (stained with yellow dye). (Courtesy M. Mino-Kenudson, MD.)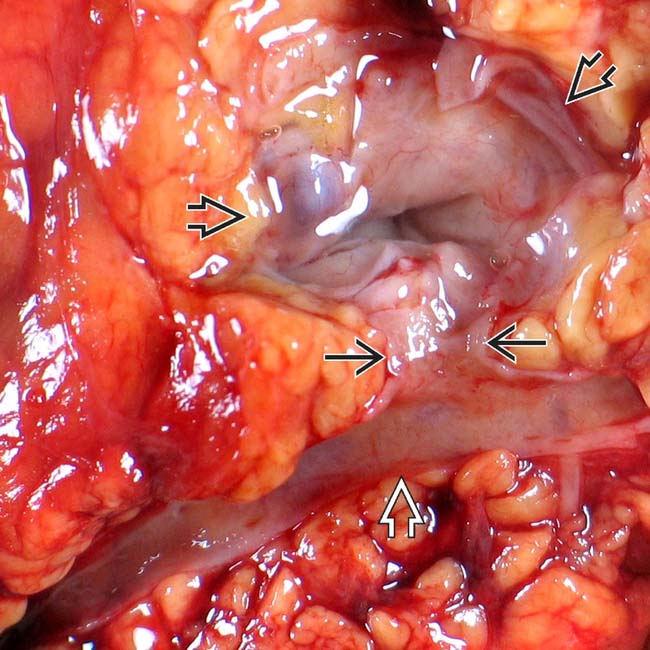
 connected to the main pancreatic duct
connected to the main pancreatic duct  through a dilated branch duct
through a dilated branch duct  . (Courtesy M. Mino-Kenudson, MD.)
. (Courtesy M. Mino-Kenudson, MD.)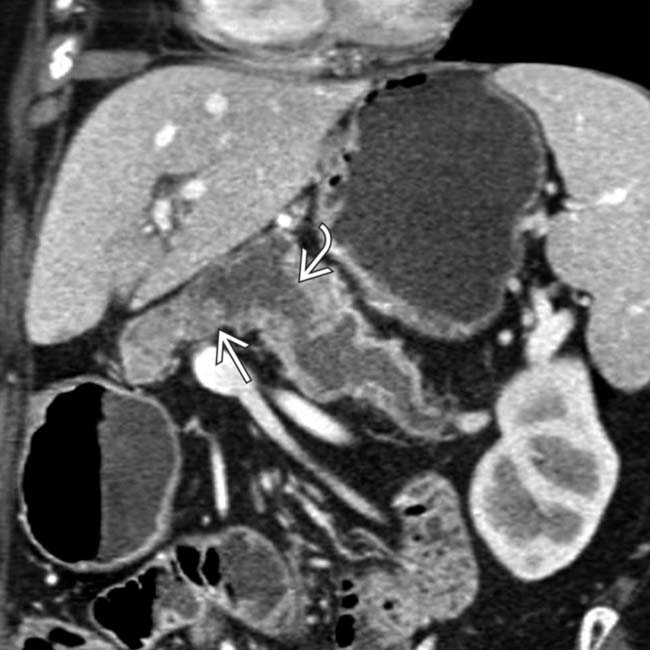
 . Note the subtle soft tissue mural nodularity
. Note the subtle soft tissue mural nodularity  within the duct lumen downstream, a highly suspicious feature for malignancy. This was found to be a main duct IPMN with invasive carcinoma.
within the duct lumen downstream, a highly suspicious feature for malignancy. This was found to be a main duct IPMN with invasive carcinoma.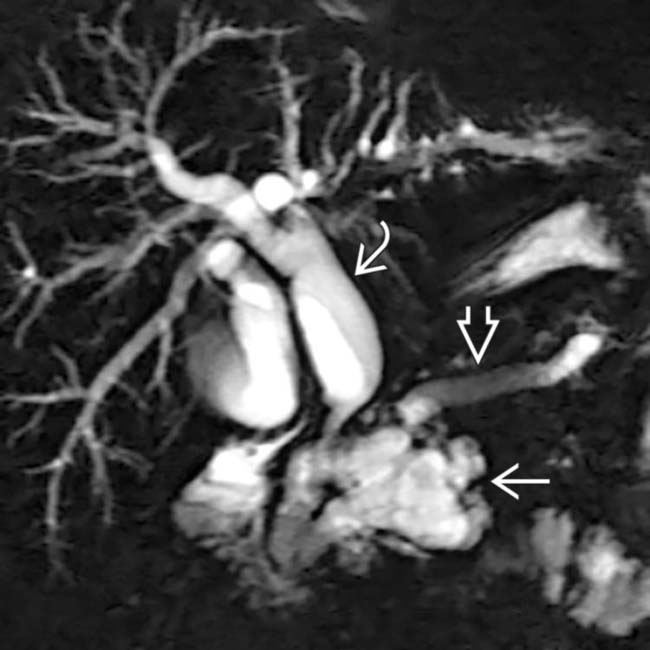
 and common bile duct
and common bile duct  . A cluster of dilated, mucin-filled branch ducts
. A cluster of dilated, mucin-filled branch ducts  in the pancreatic head are seen, simulating a multicystic pancreatic head mass such as serous cystadenoma.
in the pancreatic head are seen, simulating a multicystic pancreatic head mass such as serous cystadenoma.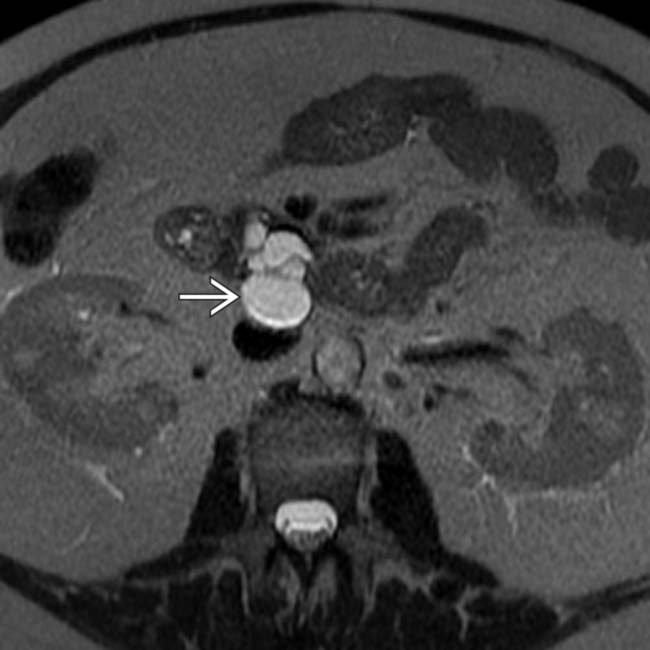
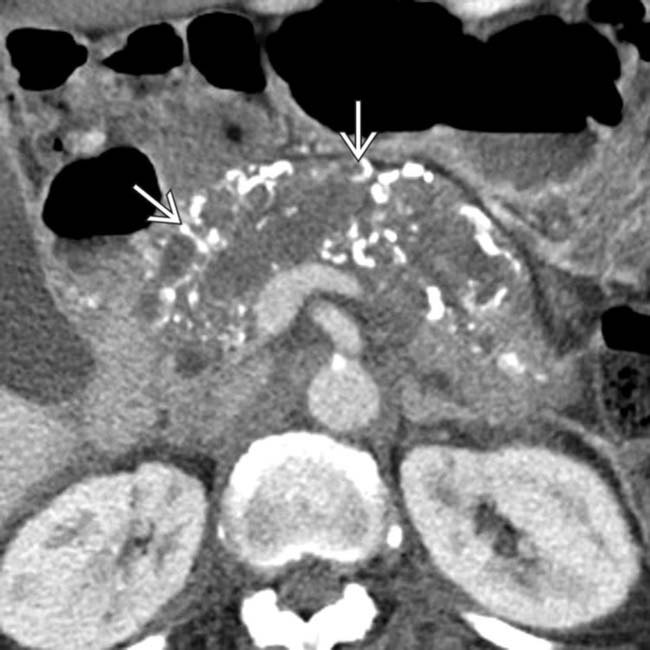
 in the pancreatic uncinate process.
in the pancreatic uncinate process.




































































 .
.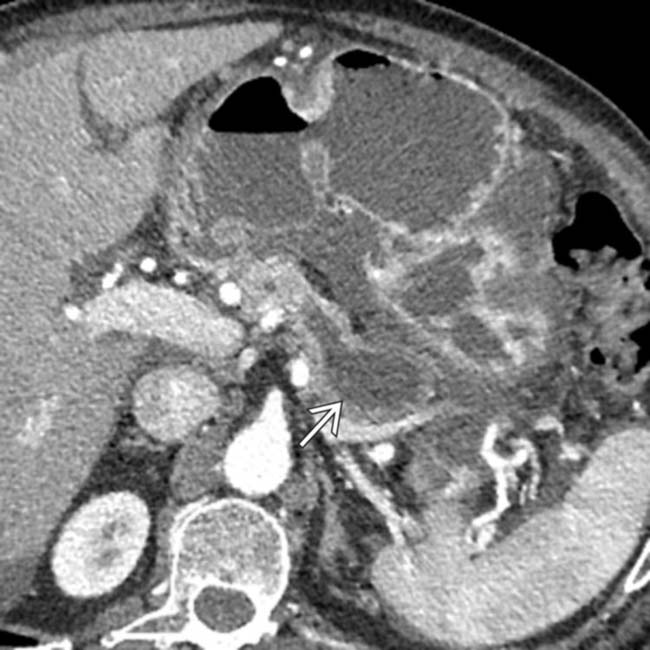
 .
.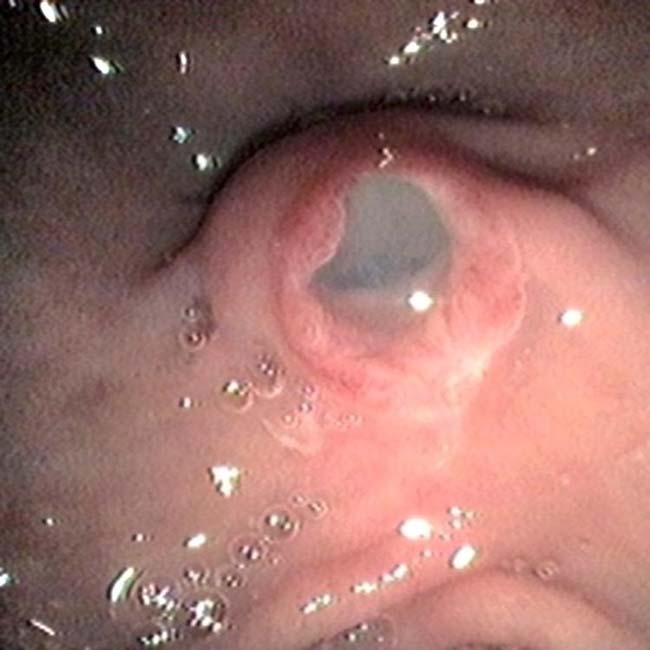
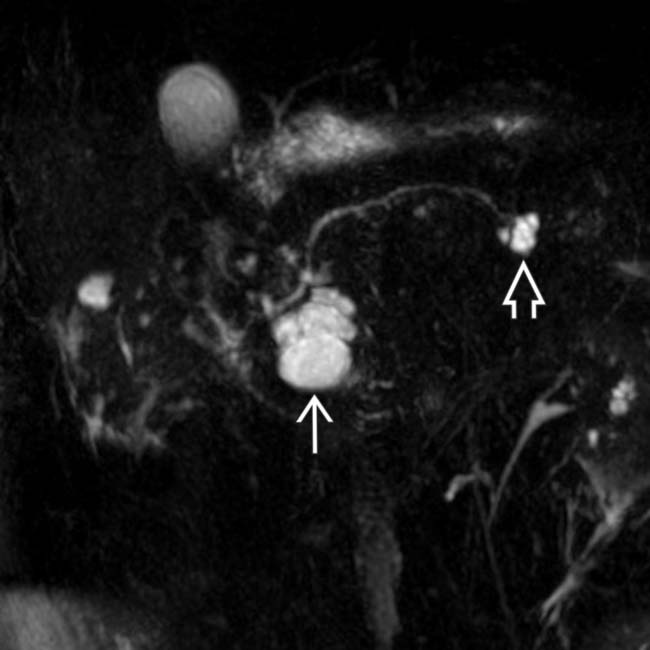
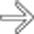
 communicates with the adjacent normal sized pancreatic duct, compatible with a side branch IPMN. There is an additional smaller IPMN in the pancreatic tail
communicates with the adjacent normal sized pancreatic duct, compatible with a side branch IPMN. There is an additional smaller IPMN in the pancreatic tail  .
.
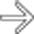
 with a “bunch of grapes” appearance.
with a “bunch of grapes” appearance.