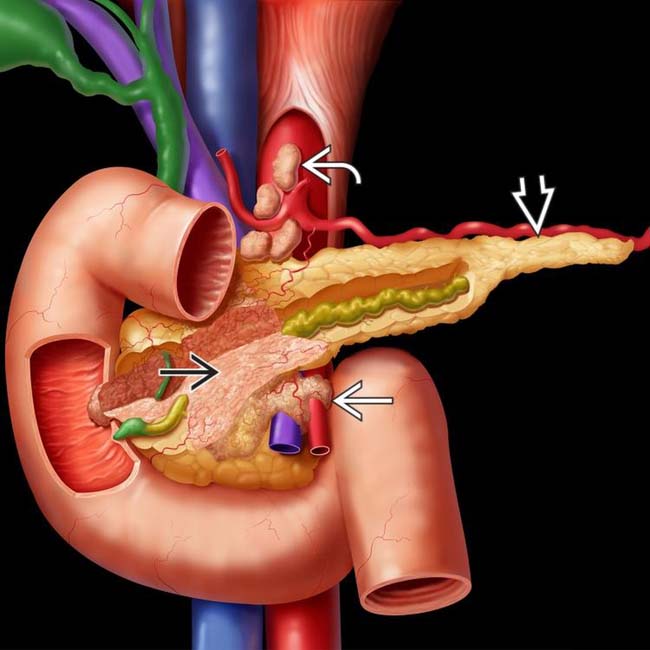
Strong tendency to obstruct pancreatic and common bile ducts with abrupt ductal cutoff at site of obstruction
 Soft tissue infiltration to involve adjacent vessels and organs (e.g., duodenum, bowel, stomach, and adrenals)
Soft tissue infiltration to involve adjacent vessels and organs (e.g., duodenum, bowel, stomach, and adrenals)• MR: Tumor conspicuous on T1WI, appearing low signal and juxtaposed against high signal pancreatic parenchyma
CLINICAL ISSUES
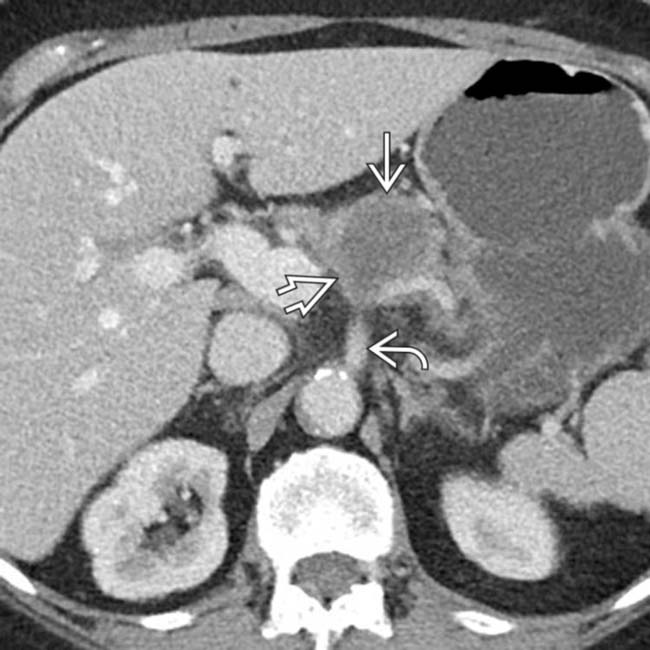
 encasing and obstructing the pancreatic and distal bile ducts. There is encasement of the superior mesenteric vessels
encasing and obstructing the pancreatic and distal bile ducts. There is encasement of the superior mesenteric vessels  and spread to celiac nodes
and spread to celiac nodes  . Note the atrophy of the distal body-tail segments
. Note the atrophy of the distal body-tail segments  .
.
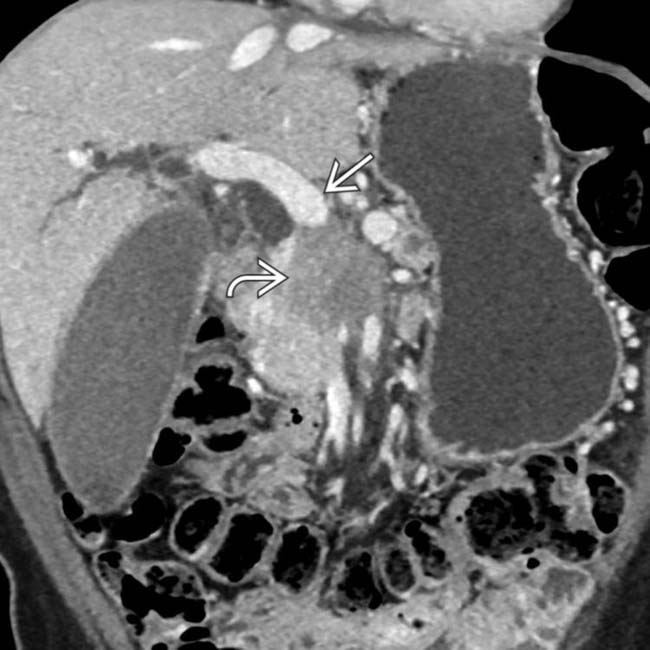
 in the pancreatic body, typical for pancreatic adenocarcinoma. The mass abuts the distal celiac trunk
in the pancreatic body, typical for pancreatic adenocarcinoma. The mass abuts the distal celiac trunk  and the hepatic artery
and the hepatic artery  , with < 180° involvement of each.
, with < 180° involvement of each.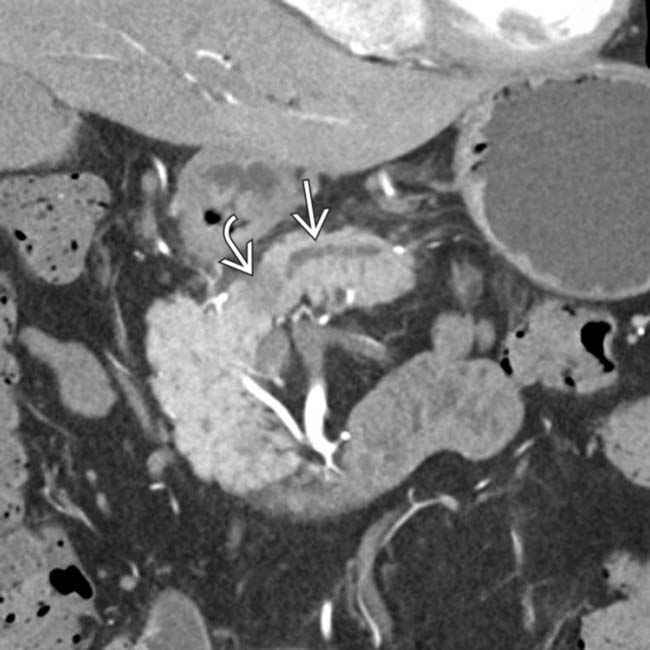
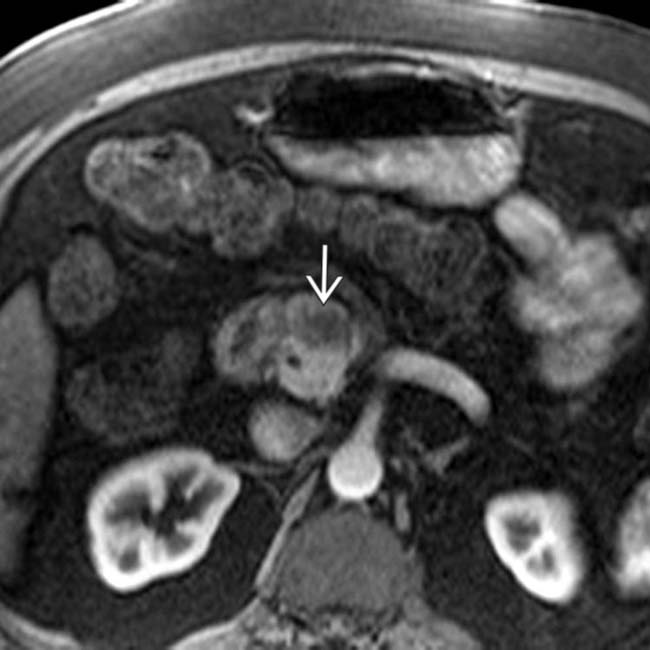
 in the pancreatic head resulting in obstruction and upstream dilatation of the pancreatic duct
in the pancreatic head resulting in obstruction and upstream dilatation of the pancreatic duct  . The presence of pancreatic ductal dilatation and abrupt cut-off should always prompt careful search for a pancreatic mass.
. The presence of pancreatic ductal dilatation and abrupt cut-off should always prompt careful search for a pancreatic mass.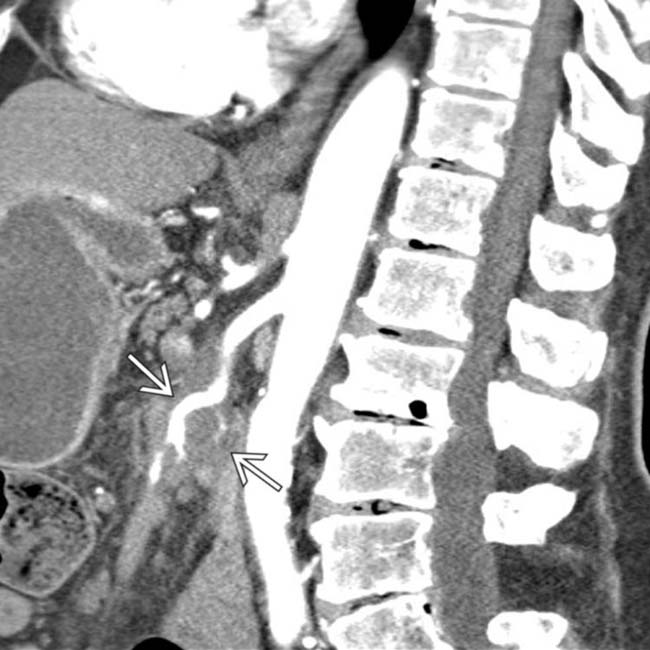
 encasing the SMA, with 360° involvement. This degree of encasement almost certainly makes this tumor unresectable.
encasing the SMA, with 360° involvement. This degree of encasement almost certainly makes this tumor unresectable.IMAGING
General Features
CT Findings
• CT sensitivity for pancreatic cancer is excellent (∼ 97%)
• Poorly marginated, hypodense mass with tendency to infiltrate posteriorly into retroperitoneum
• Secondary signs of tumor
• CT best modality for determining vascular invasion
MR Findings
• MR particularly helpful in identifying small group of tumors that are isodense to normal pancreas on CT
• Tumor conspicuous on T1WI, appearing low signal and juxtaposed against high signal pancreatic parenchyma
• Conspicuity on T1WI C+ similar to CT, with hypovascular tumors often demonstrating progressive delayed enhancement
Nuclear Medicine Findings
• PET/CT
 PET alone (without diagnostic CT) not effective for diagnosis of primary tumor (sensitivity as low as 72%)
PET alone (without diagnostic CT) not effective for diagnosis of primary tumor (sensitivity as low as 72%)
 PET alone (without diagnostic CT) not effective for diagnosis of primary tumor (sensitivity as low as 72%)
PET alone (without diagnostic CT) not effective for diagnosis of primary tumor (sensitivity as low as 72%)
DIFFERENTIAL DIAGNOSIS
Pancreatic Neuroendocrine Tumors
Chronic Pancreatitis
• May be associated with focal fibroinflammatory mass that can be indistinguishable from pancreatic cancer
• Usually other stigmata of chronic pancreatitis, including diffuse atrophy of gland, dilated/beaded pancreatic duct with ductal calculi, and parenchymal calcifications
PATHOLOGY
General Features
• Genetics
 Family history is strong risk factor, as ∼ 10% of patients have 1st degree relative with pancreatic cancer
Family history is strong risk factor, as ∼ 10% of patients have 1st degree relative with pancreatic cancer
 Family history is strong risk factor, as ∼ 10% of patients have 1st degree relative with pancreatic cancer
Family history is strong risk factor, as ∼ 10% of patients have 1st degree relative with pancreatic cancerStaging, Grading, & Classification
• Determination of locoregional resectability (MD Anderson criteria)
 Unequivocally resectable
Unequivocally resectable
 Unresectable
Unresectable
 Unequivocally resectable
Unequivocally resectable
 Unresectable
Unresectable
CLINICAL ISSUES
Presentation
• Most common signs/symptoms
 Most common symptoms are jaundice, weight loss (often severe), abdominal pain (usually epigastric pain radiating to back), and back pain
Most common symptoms are jaundice, weight loss (often severe), abdominal pain (usually epigastric pain radiating to back), and back pain
 Most common symptoms are jaundice, weight loss (often severe), abdominal pain (usually epigastric pain radiating to back), and back pain
Most common symptoms are jaundice, weight loss (often severe), abdominal pain (usually epigastric pain radiating to back), and back pain
– Patients may present with Courvoisier gallbladder (distended palpable gallbladder due to obstruction)
 in the pancreatic head/neck, typical of pancreatic adenocarcinoma. Note that the tumor involves the distal portal vein
in the pancreatic head/neck, typical of pancreatic adenocarcinoma. Note that the tumor involves the distal portal vein  , portal/SMV confluence, and superior SMV.
, portal/SMV confluence, and superior SMV.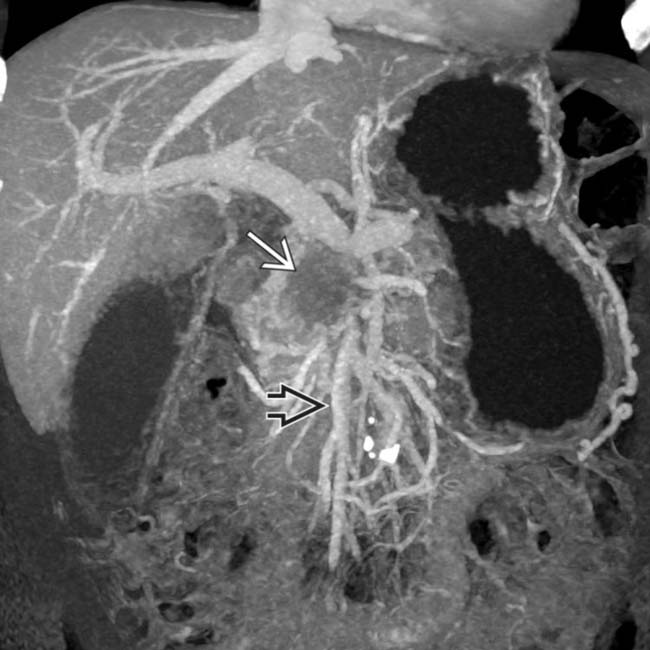
 has occluded the SMV over several centimeters extending from the confluence, with development of multiple collaterals and reconstitution of the SMV more inferiorly
has occluded the SMV over several centimeters extending from the confluence, with development of multiple collaterals and reconstitution of the SMV more inferiorly  .
.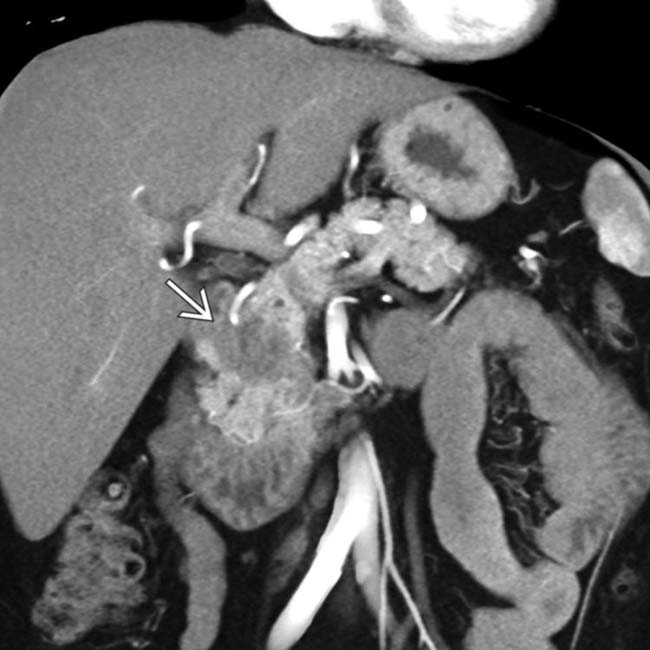
 in the pancreatic head, found to be a pancreatic cancer at resection. The lack of parenchymal atrophy or ductal dilatation is unusual for pancreatic cancer.
in the pancreatic head, found to be a pancreatic cancer at resection. The lack of parenchymal atrophy or ductal dilatation is unusual for pancreatic cancer.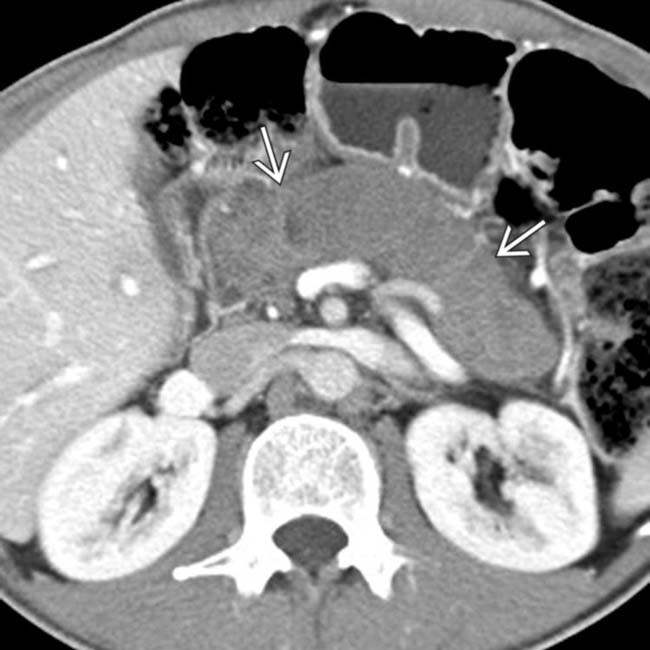
 , representing infiltration by pancreatic adenocarcinoma. Although uncommon, pancreatic cancer can rarely diffusely involve the entire gland, and may be confused for autoimmune pancreatitis.
, representing infiltration by pancreatic adenocarcinoma. Although uncommon, pancreatic cancer can rarely diffusely involve the entire gland, and may be confused for autoimmune pancreatitis.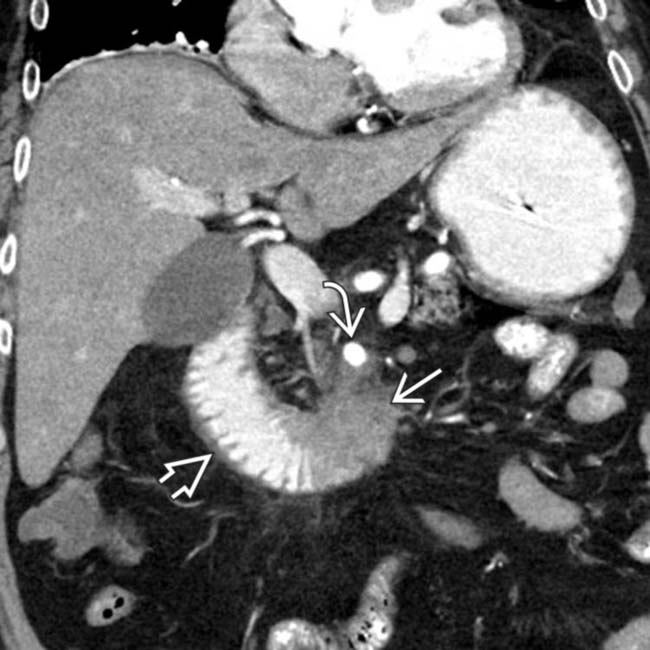
 involving the adjacent duodenum, resulting in duodenal obstruction and dilatation
involving the adjacent duodenum, resulting in duodenal obstruction and dilatation  . Note that the tumor involves roughly a 180° circumference of the SMA
. Note that the tumor involves roughly a 180° circumference of the SMA  .
.
 obstructing the pancreatic duct
obstructing the pancreatic duct  and causing upstream parenchymal atrophy. Note that the mass directly invades the adjacent duodenum
and causing upstream parenchymal atrophy. Note that the mass directly invades the adjacent duodenum  . Multiple liver metastases
. Multiple liver metastases  are present, precluding resection.
are present, precluding resection.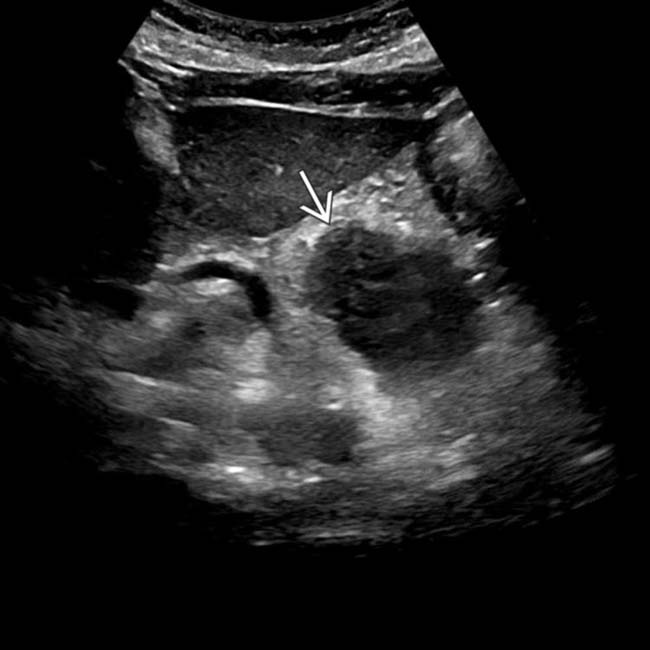
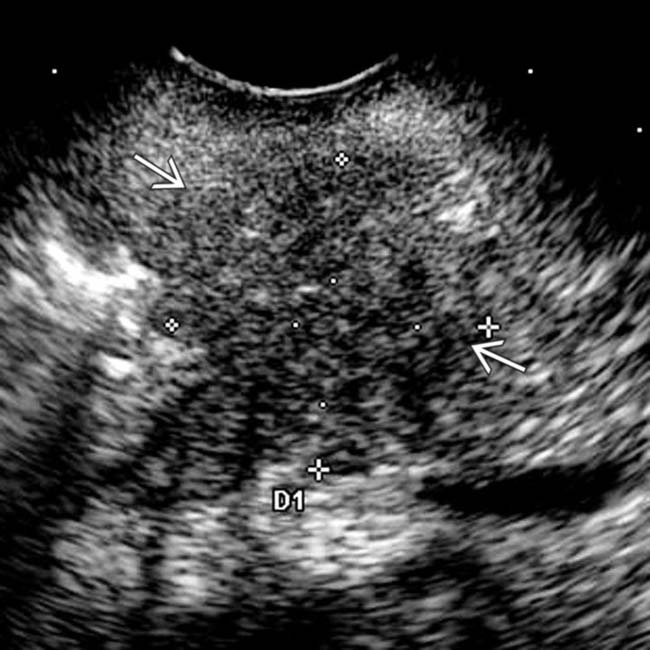
 in the pancreatic body, representing a pancreatic adenocarcinoma. Ultrasound is generally quite limited in assessment of the pancreas due to overlying bowel gas.
in the pancreatic body, representing a pancreatic adenocarcinoma. Ultrasound is generally quite limited in assessment of the pancreas due to overlying bowel gas.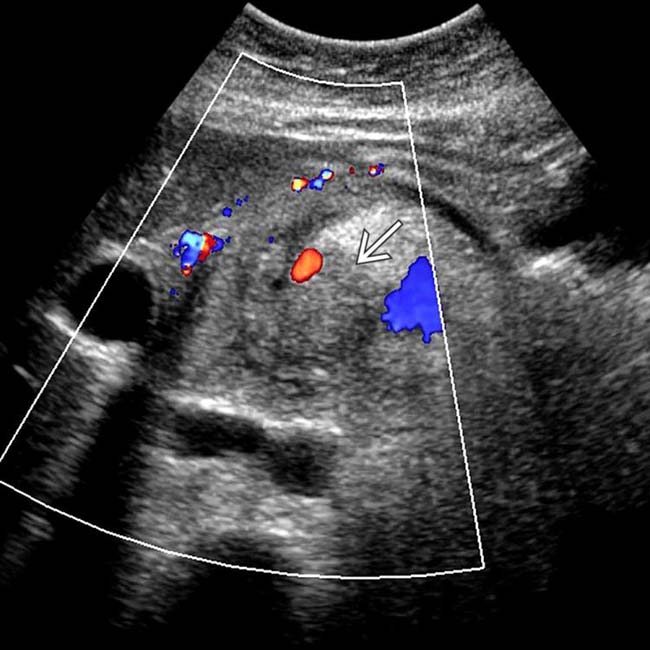
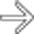
 . Note the relatively minimal internal color flow vascularity within the mass, typical of these lesions on ultrasound.
. Note the relatively minimal internal color flow vascularity within the mass, typical of these lesions on ultrasound.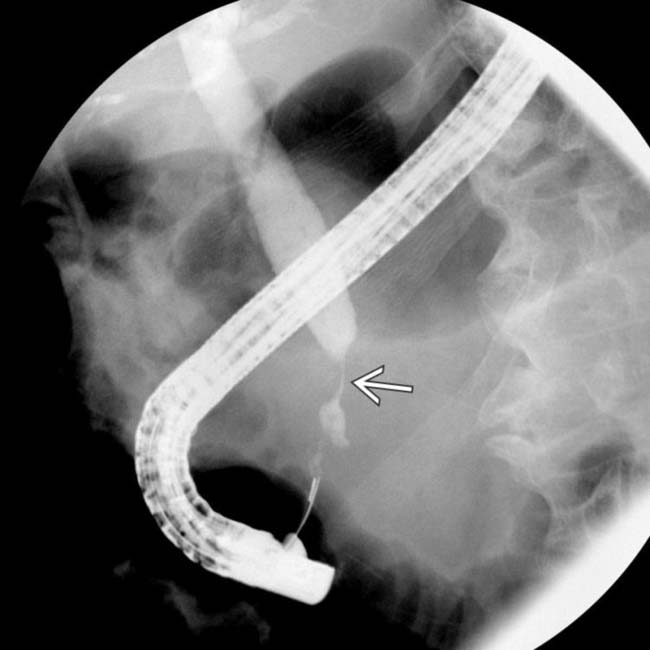
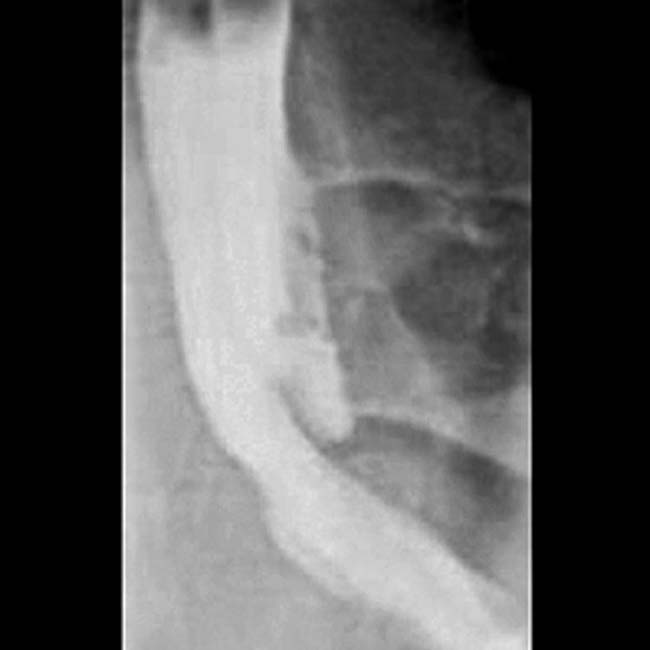
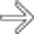
 of the common bile duct. The abrupt, irregular narrowing of the duct at the stricture certainly is a highly suspicious feature for malignancy.
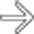
of the common bile duct. The abrupt, irregular narrowing of the duct at the stricture certainly is a highly suspicious feature for malignancy.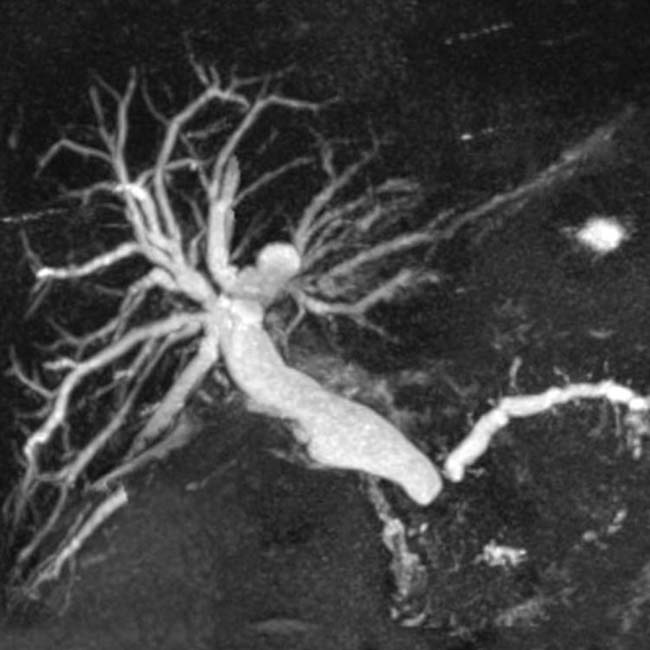
 in the pancreatic head, compatible with pancreatic adenocarcinoma. The appearance of pancreatic cancer on post-gadolinium MR images is comparable to its appearance on CECT.
in the pancreatic head, compatible with pancreatic adenocarcinoma. The appearance of pancreatic cancer on post-gadolinium MR images is comparable to its appearance on CECT.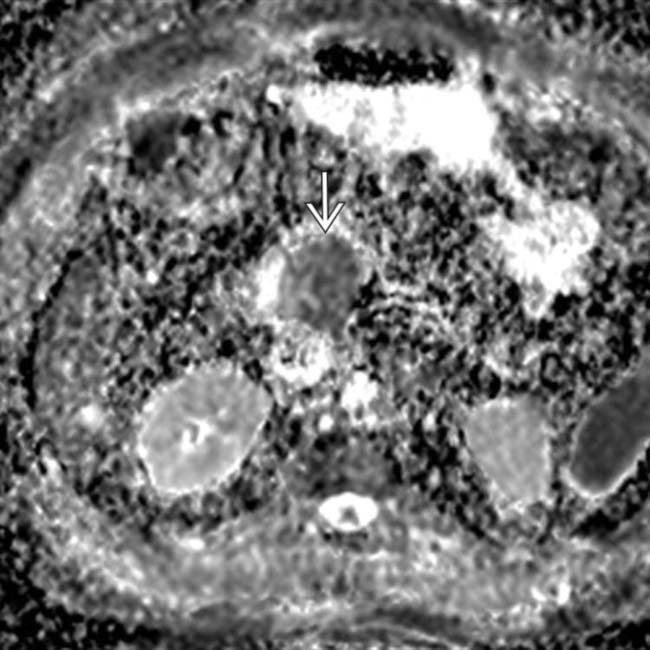
 within the mass. Restricted diffusion can be a valuable tool to help increase the conspicuity of subtle pancreatic cancers.
within the mass. Restricted diffusion can be a valuable tool to help increase the conspicuity of subtle pancreatic cancers.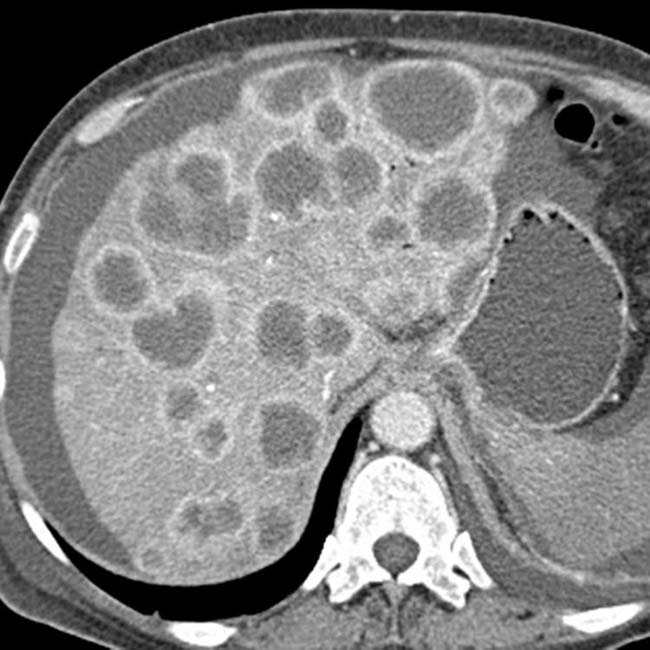
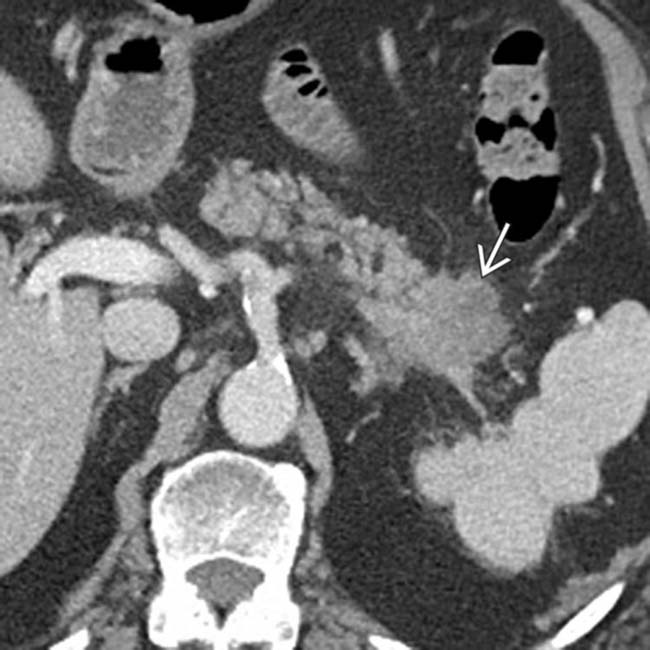
 in the pancreatic tail, in keeping with pancreatic adenocarcinoma. Pancreatic tail cancers typically do not involve vital vasculature and often come to attention later than pancreatic head cancers due to lack of jaundice.
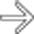
in the pancreatic tail, in keeping with pancreatic adenocarcinoma. Pancreatic tail cancers typically do not involve vital vasculature and often come to attention later than pancreatic head cancers due to lack of jaundice.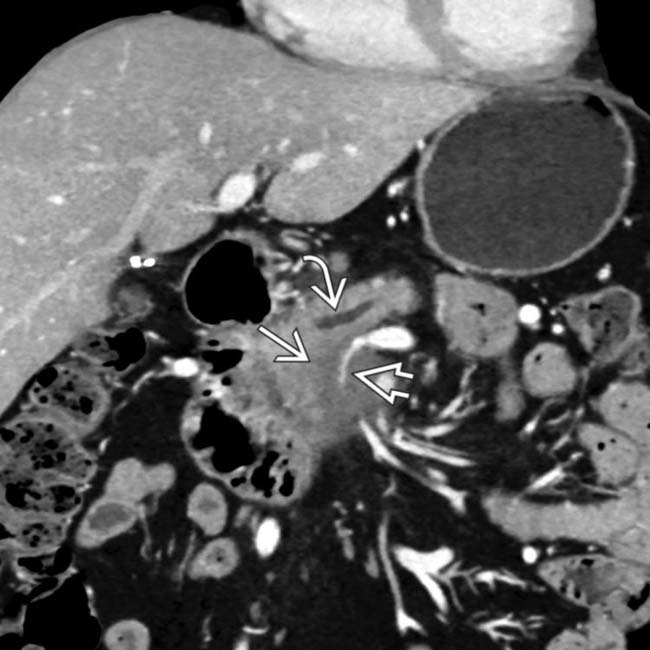
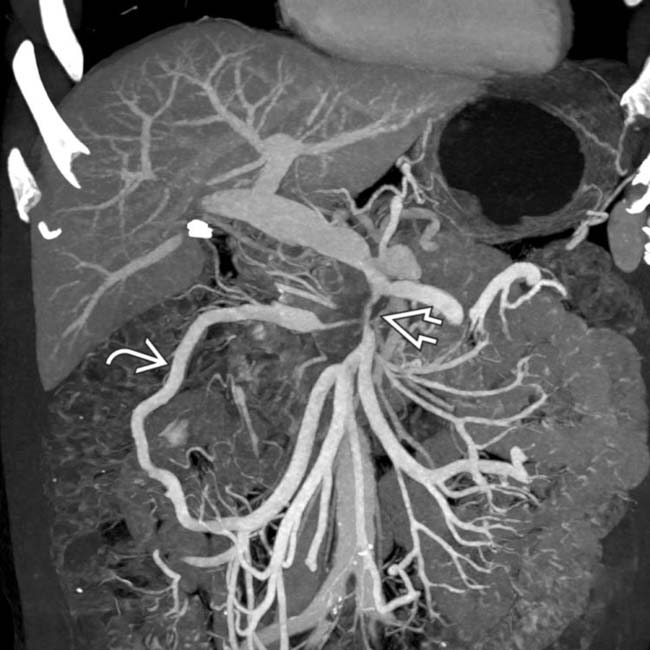
 in the pancreatic head with upstream pancreatic ductal dilatation
in the pancreatic head with upstream pancreatic ductal dilatation  . The mass encases and severely narrows the SMV
. The mass encases and severely narrows the SMV  .
.
 of the SMV, with the development of large venous collaterals
of the SMV, with the development of large venous collaterals  . The presence of venous collaterals in a pancreatic cancer patient should always suggest the presence of venous narrowing or occlusion.
. The presence of venous collaterals in a pancreatic cancer patient should always suggest the presence of venous narrowing or occlusion.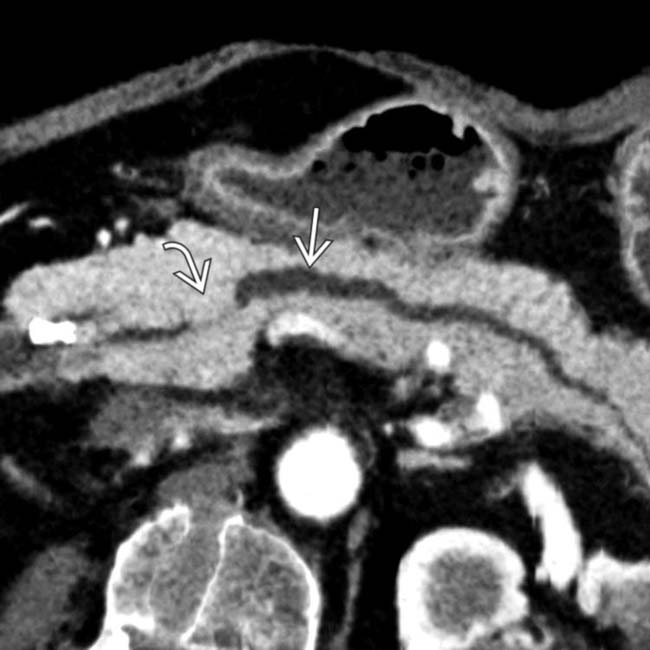
 proximal to a focal structure
proximal to a focal structure  in the pancreatic duct.
in the pancreatic duct.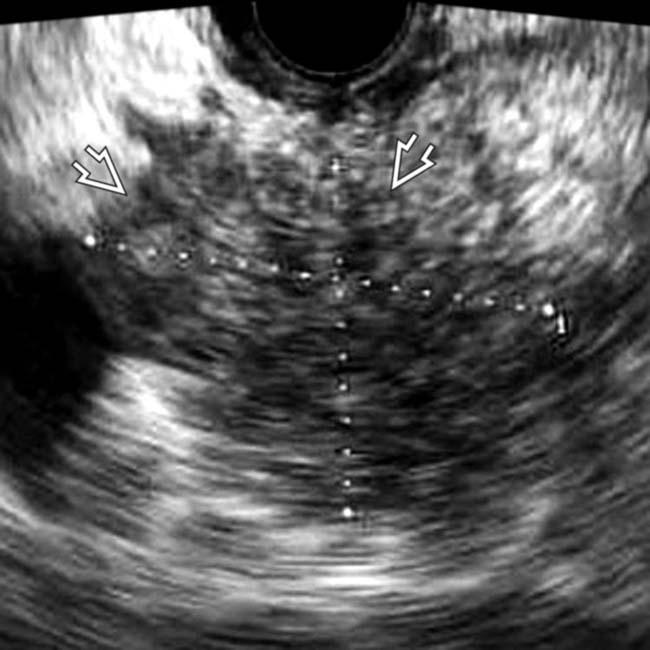
 that was biopsied and shown to be adenocarcinoma. Any focal stricture of the main pancreatic duct should be considered as an isodense carcinoma until proven otherwise, and should lead to EUS interrogation and biopsy.
that was biopsied and shown to be adenocarcinoma. Any focal stricture of the main pancreatic duct should be considered as an isodense carcinoma until proven otherwise, and should lead to EUS interrogation and biopsy.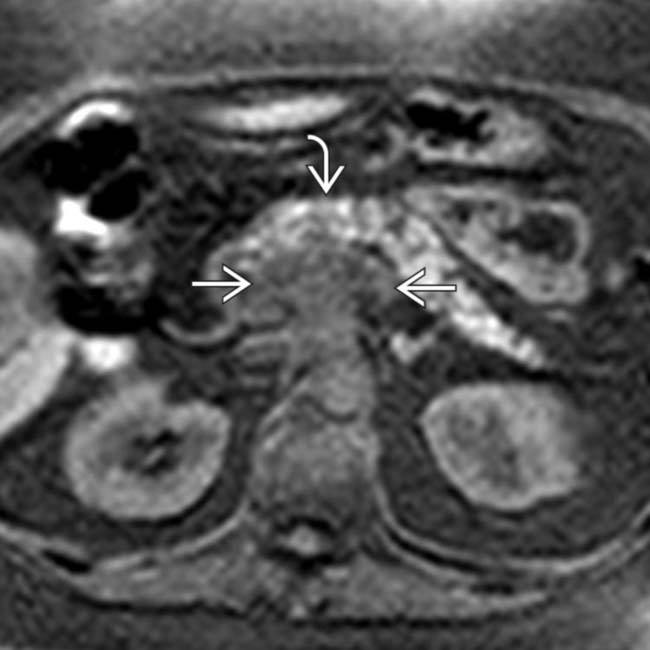
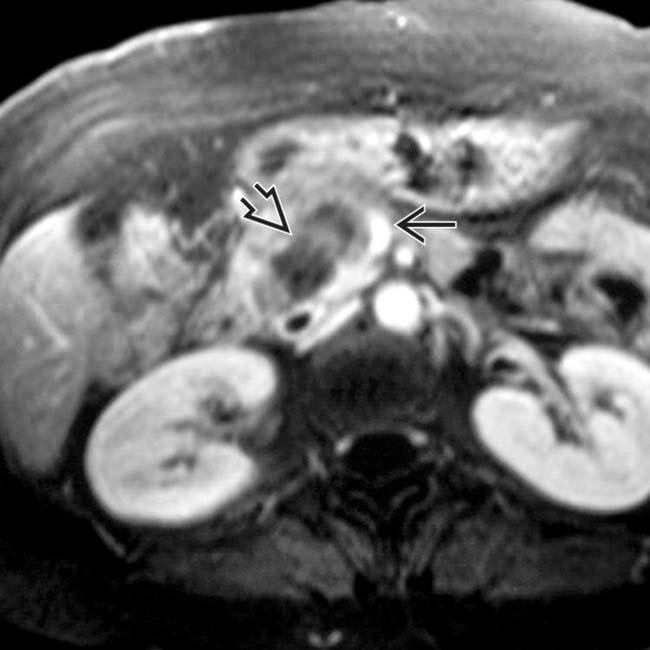
 nicely juxtaposed against the T1 hyperintense normal pancreatic parenchyma
nicely juxtaposed against the T1 hyperintense normal pancreatic parenchyma  . Noncontrast T1 images tend to be more helpful than T2WI for pancreatic adenocarcinoma identification.
. Noncontrast T1 images tend to be more helpful than T2WI for pancreatic adenocarcinoma identification.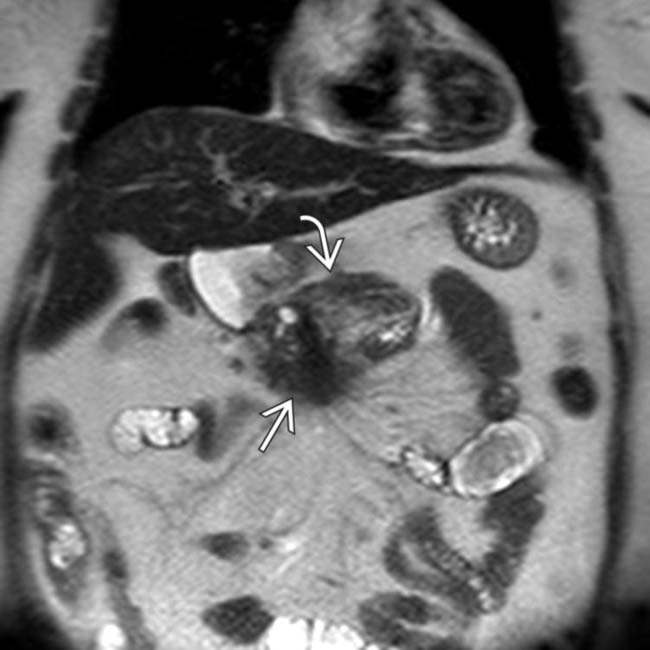
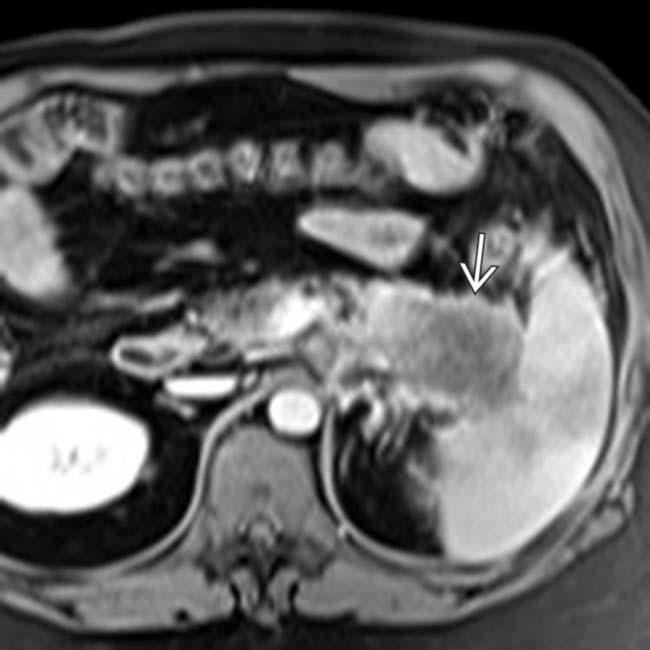
 on T2WI. The lesion is relatively hypointense, and is difficult to differentiate from the adjacent hypointense normal pancreatic parenchyma
on T2WI. The lesion is relatively hypointense, and is difficult to differentiate from the adjacent hypointense normal pancreatic parenchyma  .
.
 in the pancreatic tail extending into the splenic hilum and invading the spleen.
in the pancreatic tail extending into the splenic hilum and invading the spleen.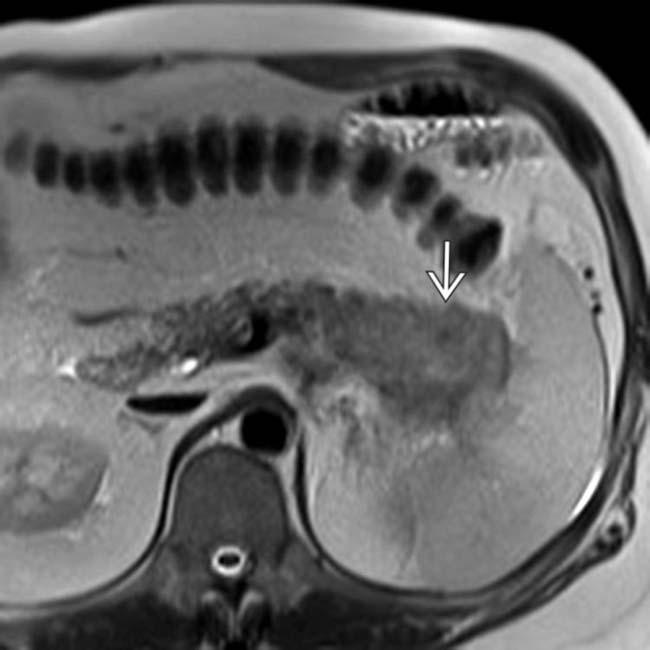
 is mildly T2 hyperintense. In general, pancreatic adenocarcinoma is often isointense or only mildly hyperintense on T2WI, making this pulse sequence unreliable for tumor detection.
is mildly T2 hyperintense. In general, pancreatic adenocarcinoma is often isointense or only mildly hyperintense on T2WI, making this pulse sequence unreliable for tumor detection.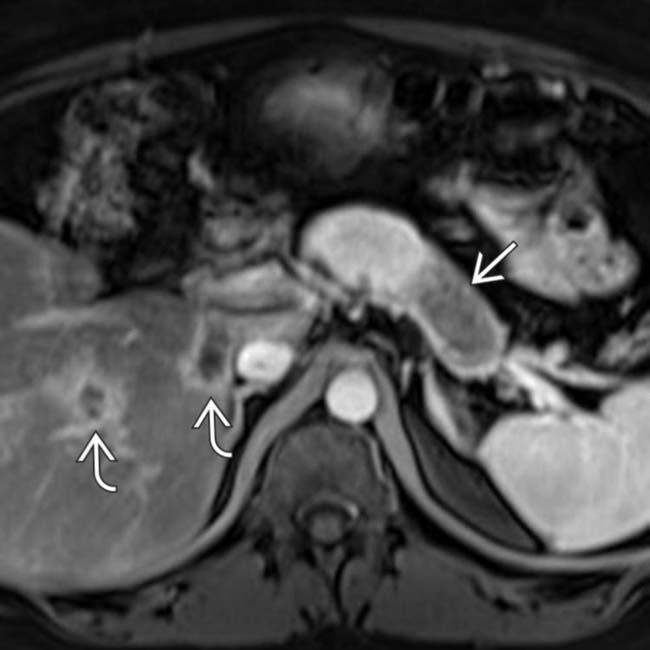
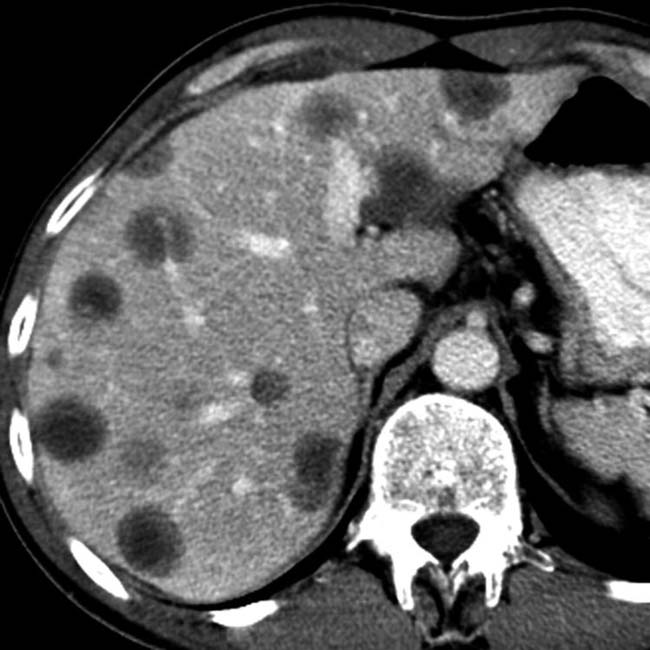
 in the pancreatic tail, compatible with pancreatic adenocarcinoma. Several metastases
in the pancreatic tail, compatible with pancreatic adenocarcinoma. Several metastases  are present in the liver.
are present in the liver.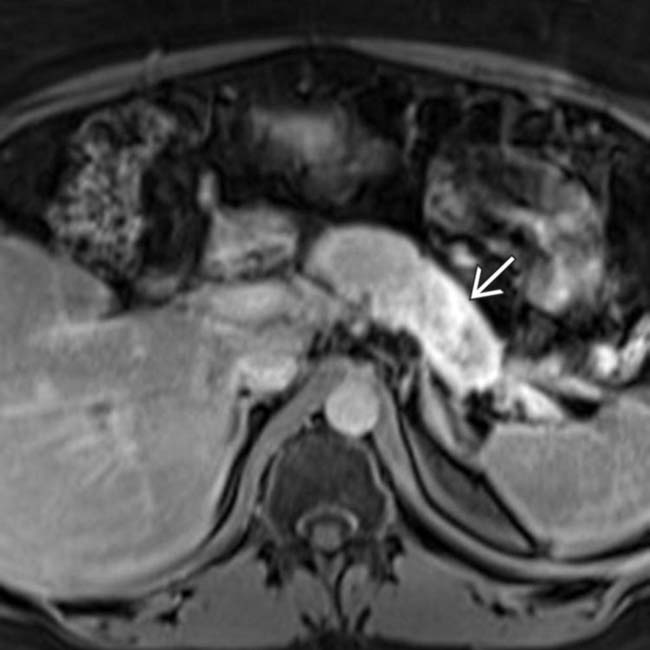
 now demonstrates considerable delayed enhancement. Delayed enhancement, which is much easier to appreciate on MR compared to CT, is a fairly common feature of pancreatic adenocarcinoma.
now demonstrates considerable delayed enhancement. Delayed enhancement, which is much easier to appreciate on MR compared to CT, is a fairly common feature of pancreatic adenocarcinoma.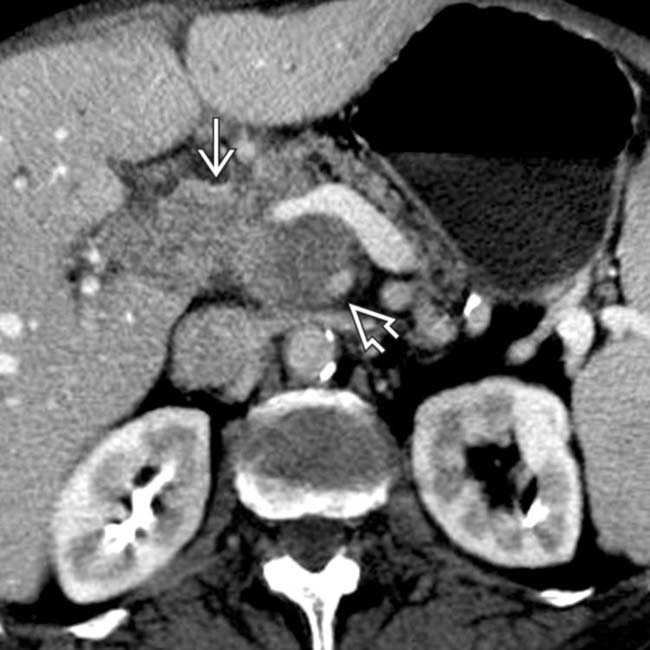
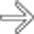
 in the pancreatic head that encases the splenoportal confluence and SMA
in the pancreatic head that encases the splenoportal confluence and SMA  and occludes the SMV. The body and tail are atrophic and the duct is dilated.
and occludes the SMV. The body and tail are atrophic and the duct is dilated.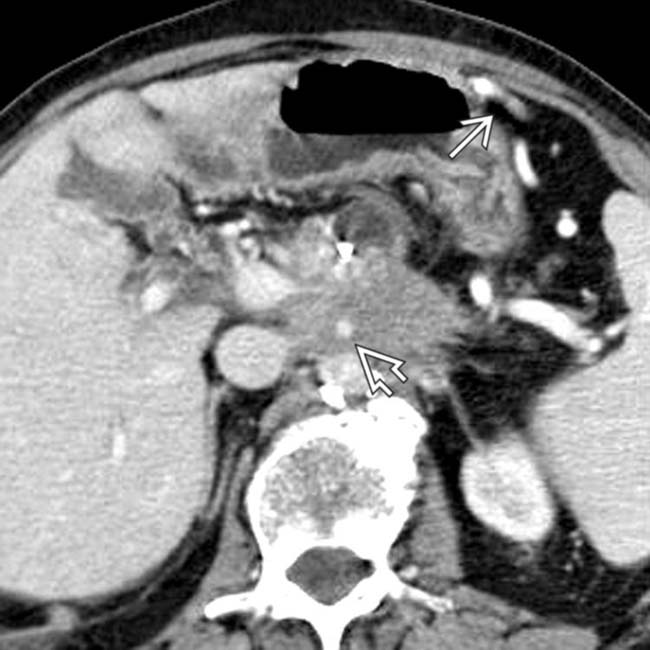
 and splenoportal confluence. Perigastric collaterals
and splenoportal confluence. Perigastric collaterals 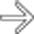 indicate splenic vein occlusion.
indicate splenic vein occlusion.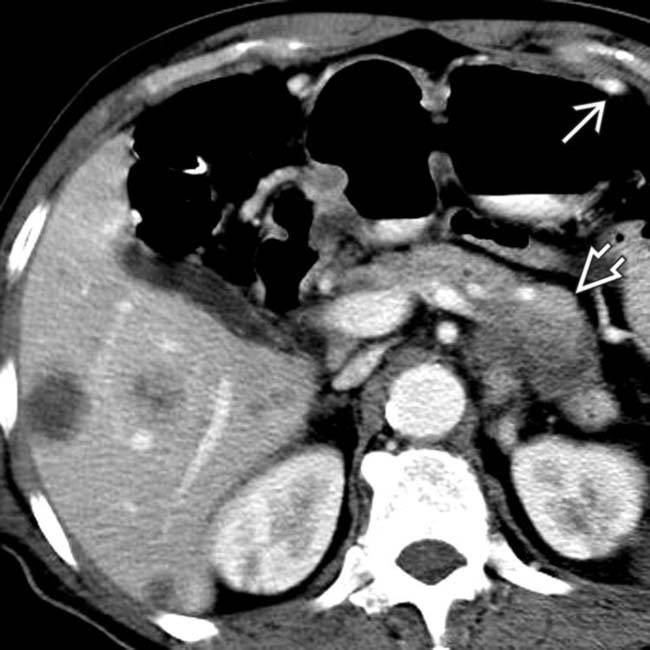
 that occludes the splenic artery and vein, with perigastric varices
that occludes the splenic artery and vein, with perigastric varices  . Note the liver metastases.
. Note the liver metastases.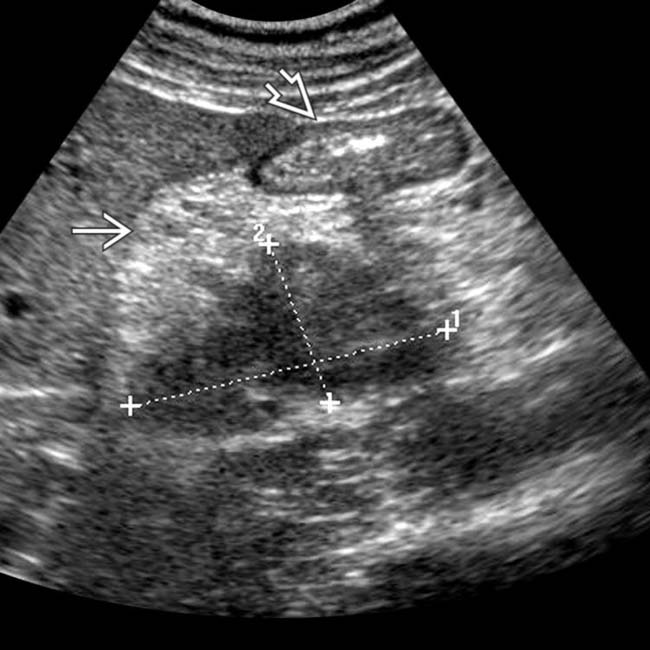
 . Note the gastric antrum
. Note the gastric antrum  .
.
 within the pancreatic head. Note the “teardrop” shape of the SMV
within the pancreatic head. Note the “teardrop” shape of the SMV  , indicating tumor invasion.
, indicating tumor invasion.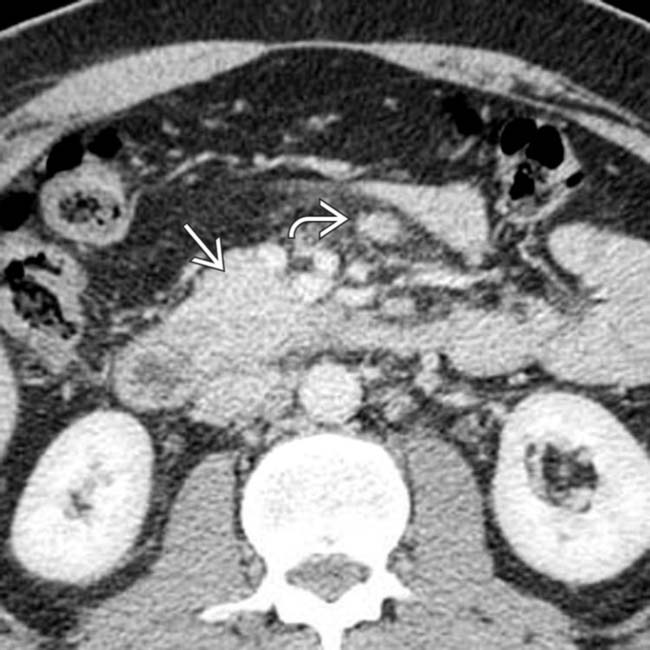
 . Note also the enlarged mesenteric lymph nodes
. Note also the enlarged mesenteric lymph nodes  .
.
 in the head of the pancreas.
in the head of the pancreas.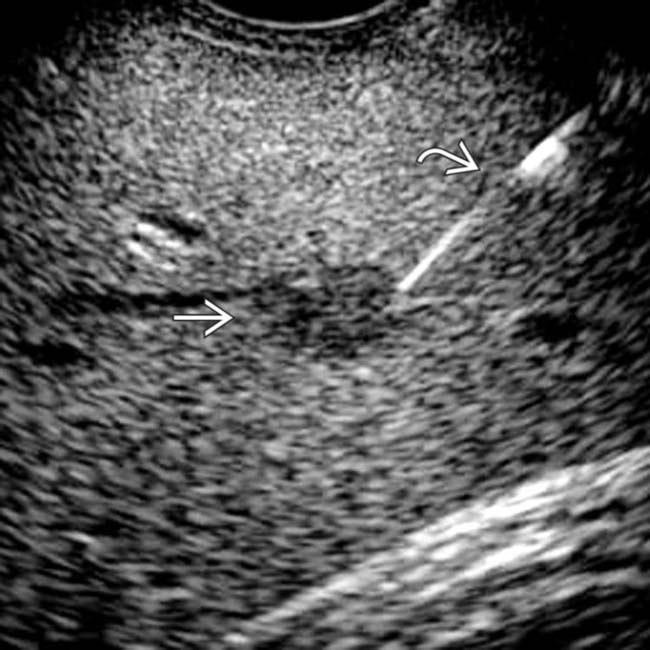
 . A biopsy was performed of the metastatic lesion by EUS
. A biopsy was performed of the metastatic lesion by EUS  . This case illustrates the value of EUS in both diagnosing and staging pancreatic carcinoma.
. This case illustrates the value of EUS in both diagnosing and staging pancreatic carcinoma.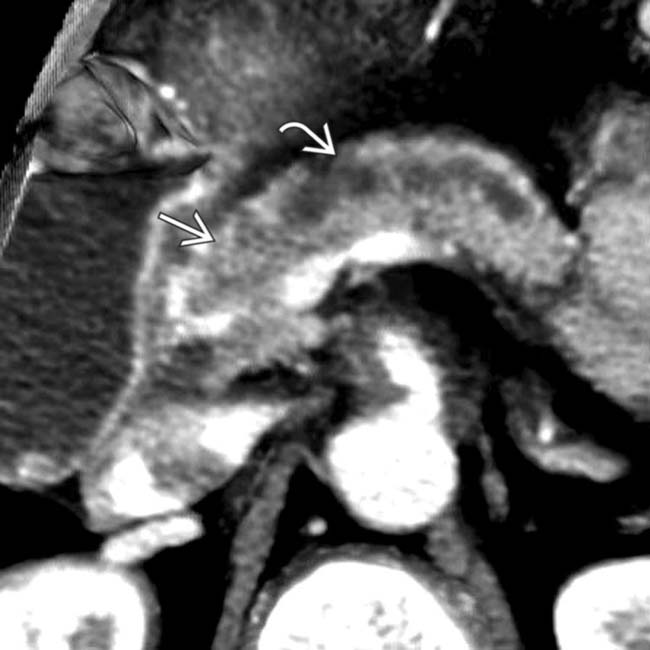
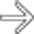
 by a hypodense mass
by a hypodense mass  in the neck of the pancreas.
in the neck of the pancreas.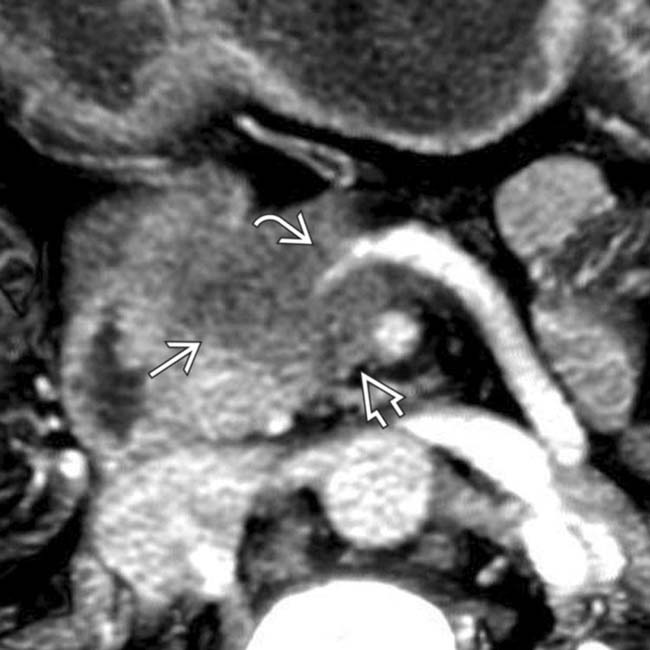
 encasing the splenoportal confluence
encasing the splenoportal confluence  . Note that the tumor extends posterior to the portal vein
. Note that the tumor extends posterior to the portal vein  along a known perineural pathway that leads to the right celiac ganglion. Early extrapancreatic perineural spread of adenocarcinoma is a major contributing factor to the poor prognosis of this disease.
along a known perineural pathway that leads to the right celiac ganglion. Early extrapancreatic perineural spread of adenocarcinoma is a major contributing factor to the poor prognosis of this disease.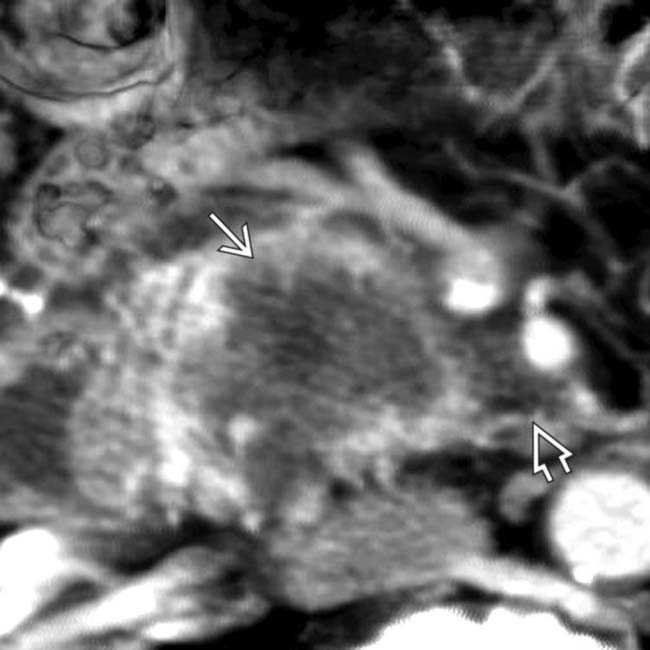
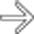 in the head of the pancreas. Note that the lesion infiltrates the fat plane contiguous with the SMA
in the head of the pancreas. Note that the lesion infiltrates the fat plane contiguous with the SMA  , but there is less than 180 degree involvement by tumor.
, but there is less than 180 degree involvement by tumor.
 and extension adjacent to the SMA
and extension adjacent to the SMA  , making this a borderline resectable lesion. A Whipple resection was attempted but the lesion could not be dissected from the SMA.
, making this a borderline resectable lesion. A Whipple resection was attempted but the lesion could not be dissected from the SMA.
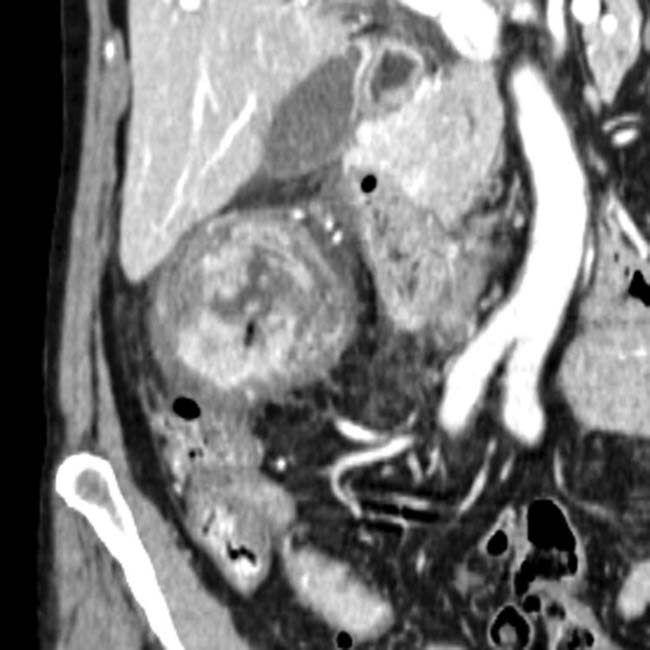
 of the celiac and common hepatic arteries. Note the irregular luminal contour
of the celiac and common hepatic arteries. Note the irregular luminal contour  of the common hepatic artery that results in a “saw-toothed” appearance.
of the common hepatic artery that results in a “saw-toothed” appearance.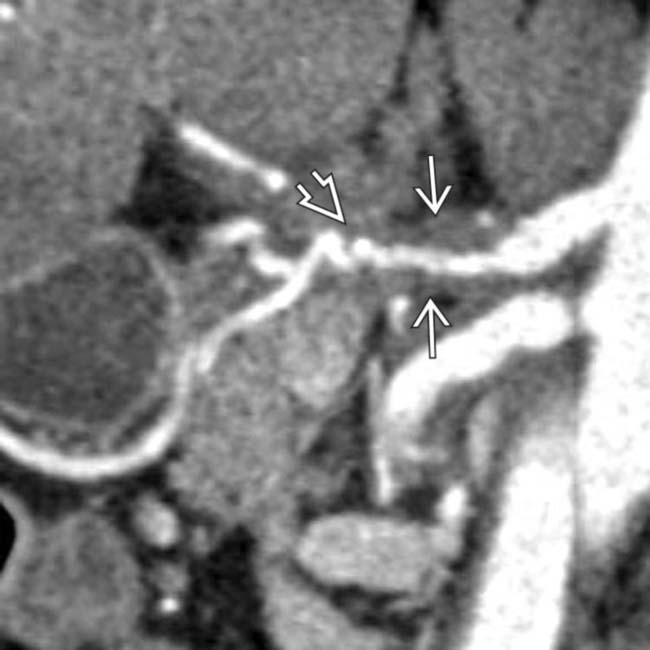
 and irregular saw-toothing of its arterial contour
and irregular saw-toothing of its arterial contour  . This luminal irregularity, or saw-toothing, is a direct sign of adventitial invasion.
. This luminal irregularity, or saw-toothing, is a direct sign of adventitial invasion.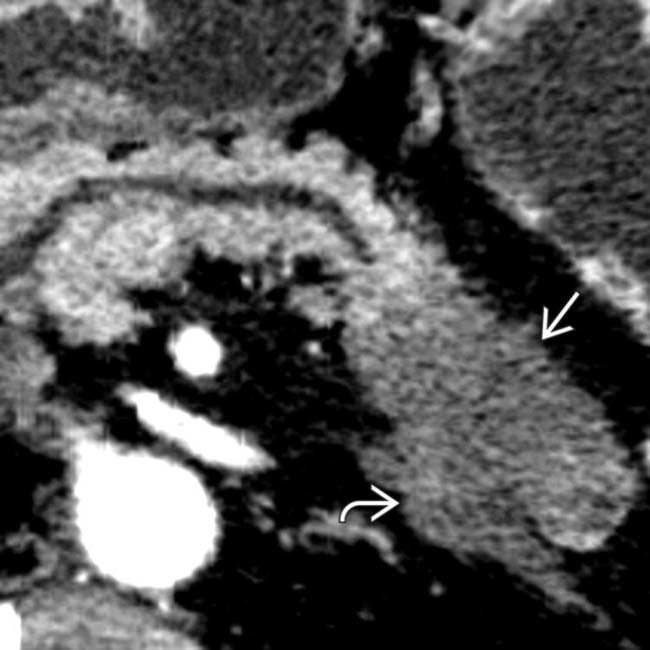
 arising from the tail of the pancreas, directly invading into the retroperitoneum
arising from the tail of the pancreas, directly invading into the retroperitoneum  and into the left adrenal gland.
and into the left adrenal gland.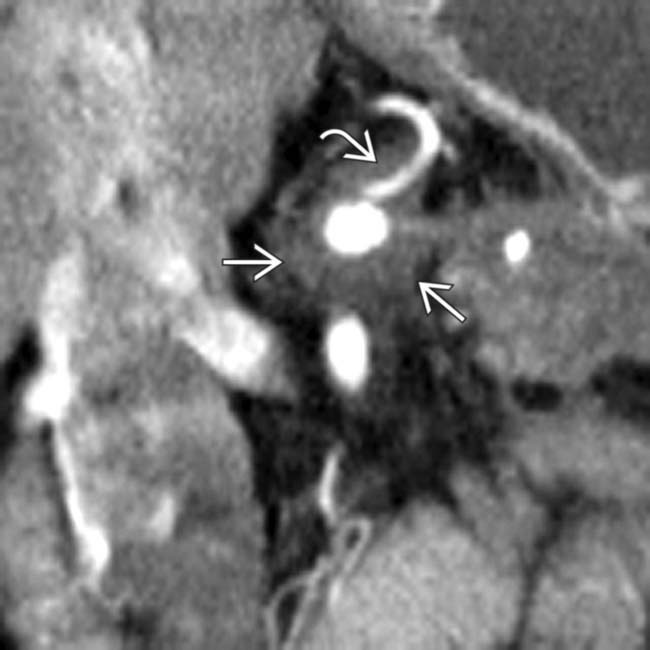
 as well as encasement of the left gastric artery
as well as encasement of the left gastric artery  . As is evident in this patient, tail of pancreas lesions often present clinically at such an advanced stage that they are rarely curable by resection.
. As is evident in this patient, tail of pancreas lesions often present clinically at such an advanced stage that they are rarely curable by resection.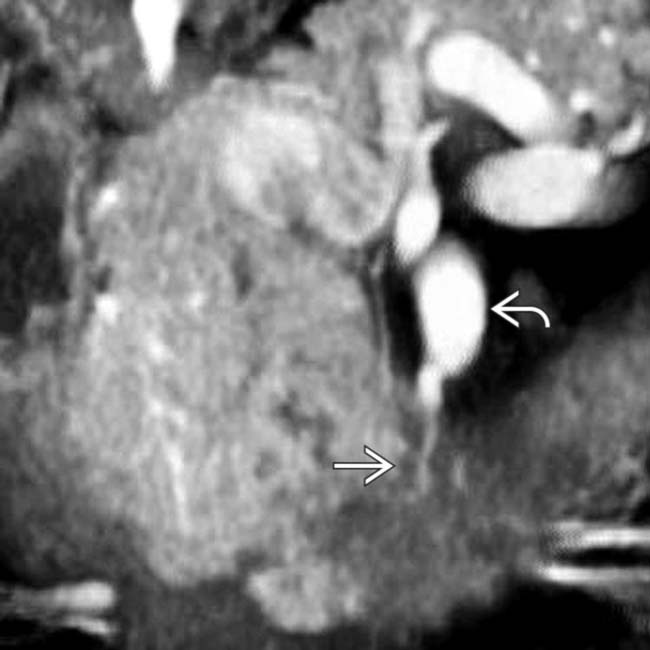
 along the posterior inferior pancreaticoduodenal artery, which is the 1st branch off the SMA
along the posterior inferior pancreaticoduodenal artery, which is the 1st branch off the SMA  . This is a common perineural pathway for extrapancreatic spread, a very characteristic feature of uncinate adenocarcinomas.
. This is a common perineural pathway for extrapancreatic spread, a very characteristic feature of uncinate adenocarcinomas.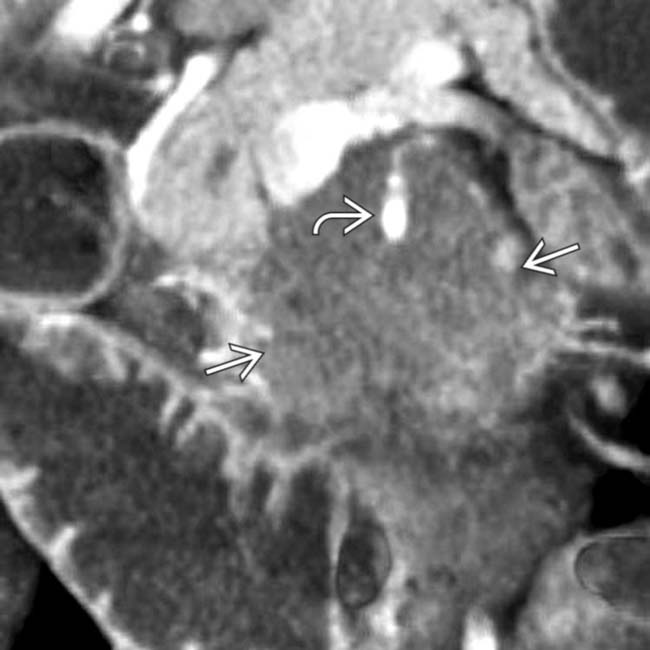
 arising from the uncinate process. Note the extensive soft tissue encasement
arising from the uncinate process. Note the extensive soft tissue encasement  of the SMA.
of the SMA.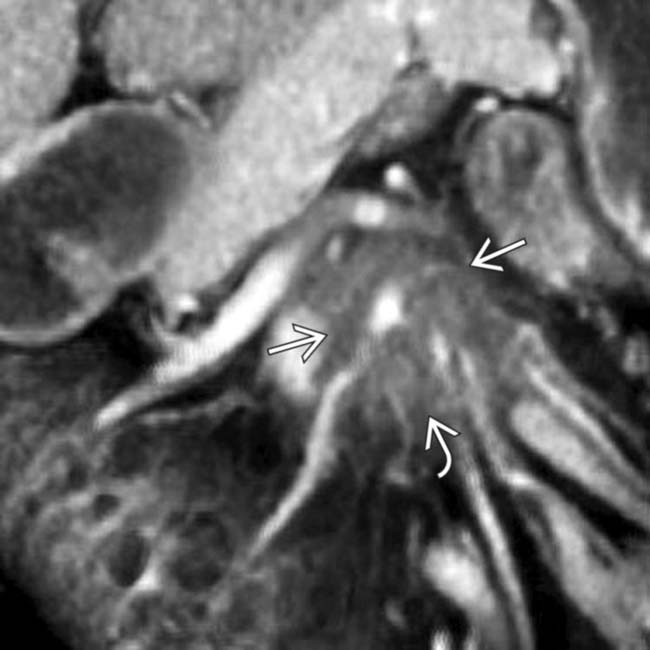
 into the root of the mesentery adjacent to mesenteric veins
into the root of the mesentery adjacent to mesenteric veins  . Arterial encasement and mesenteric invasion make this an unresectable lesion.
. Arterial encasement and mesenteric invasion make this an unresectable lesion.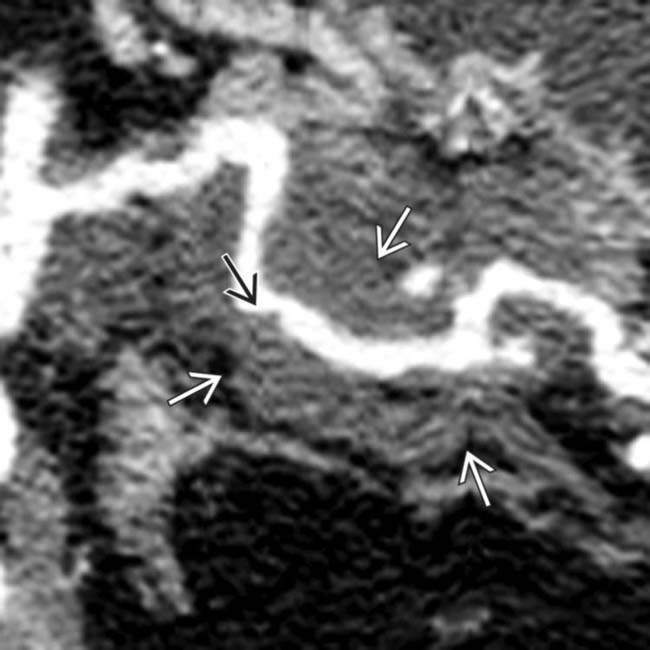
 of the artery with irregular areas of luminal narrowing
of the artery with irregular areas of luminal narrowing  .
.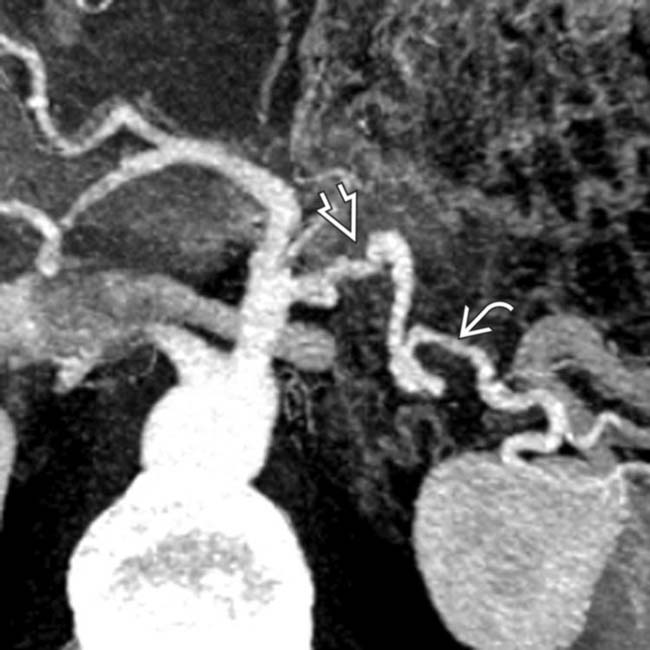
 as well as luminal narrowing
as well as luminal narrowing  . The arterial encasement in this patient makes it unresectable.
. The arterial encasement in this patient makes it unresectable.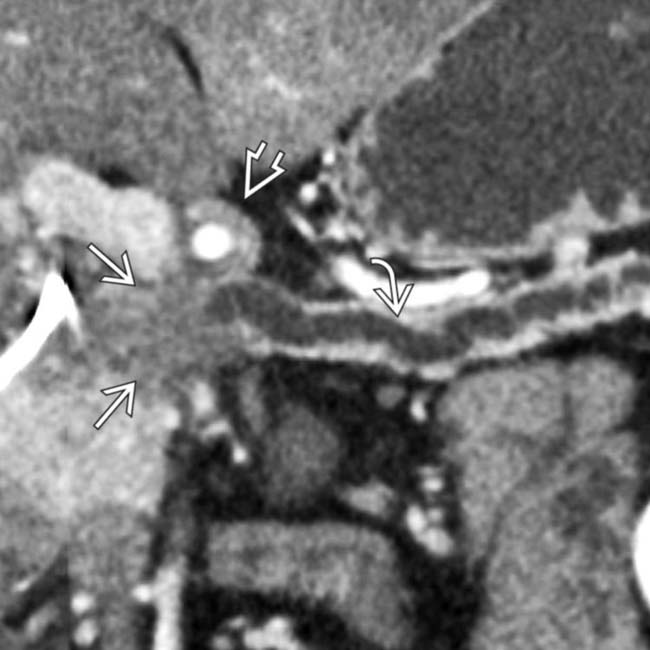
 obstructed by a hypodense mass
obstructed by a hypodense mass  in the head of the pancreas. Note the cuff of soft tissue around the common hepatic artery
in the head of the pancreas. Note the cuff of soft tissue around the common hepatic artery  , indicating arterial encasement.
, indicating arterial encasement.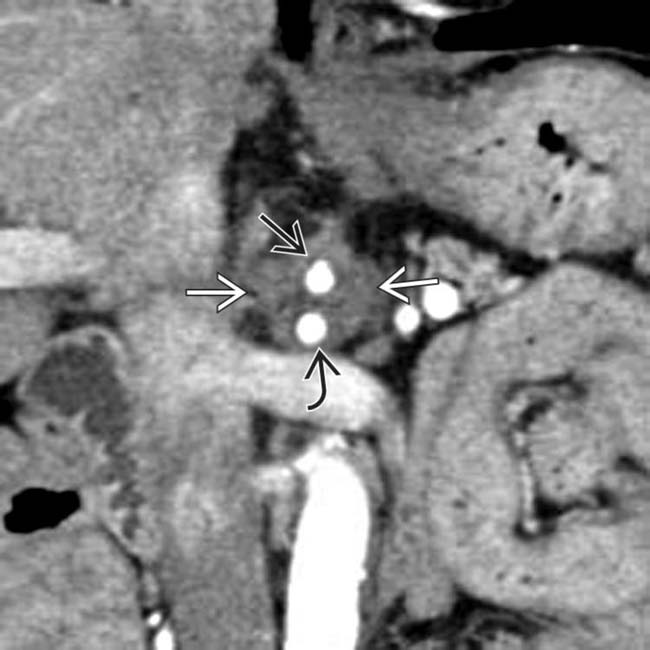
 of the celiac
of the celiac  and SMA
and SMA  . Arterial encasement is a universally accepted criterion for nonresectability and the patient was treated with chemoradiation.
. Arterial encasement is a universally accepted criterion for nonresectability and the patient was treated with chemoradiation.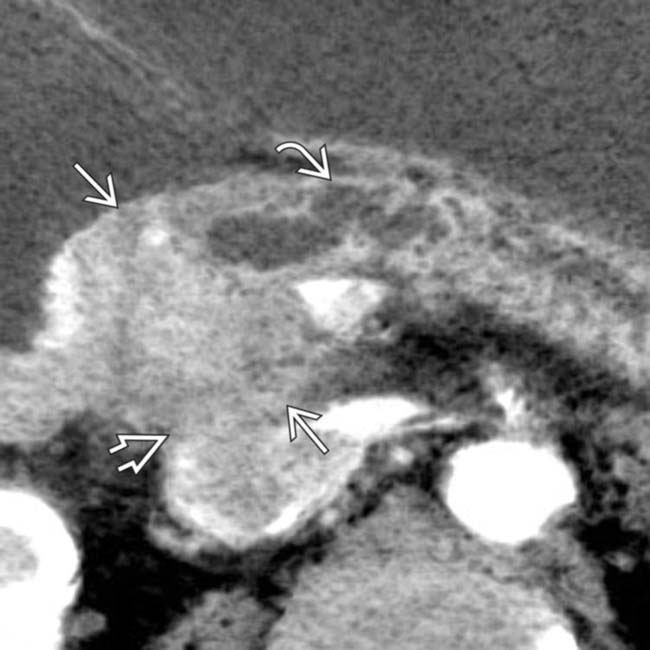
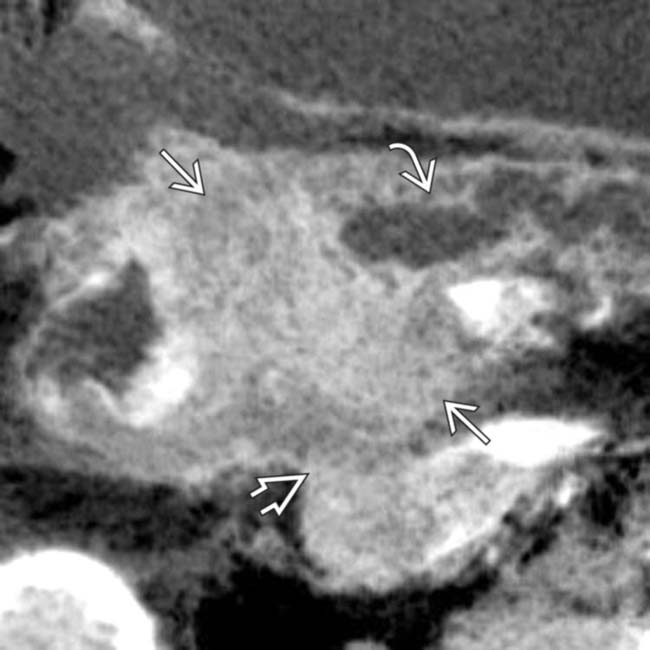
 causing abrupt obstruction of the dilatated pancreatic duct
causing abrupt obstruction of the dilatated pancreatic duct  . Note that the mass extends posteriorly and obliterates the fat plane around the IVC
. Note that the mass extends posteriorly and obliterates the fat plane around the IVC  .
.
 invading the anterior margin of the IVC
invading the anterior margin of the IVC  and the dilated pancreatic duct
and the dilated pancreatic duct  . At surgery, the mass was adherent to the IVC and could not be resected.
. At surgery, the mass was adherent to the IVC and could not be resected.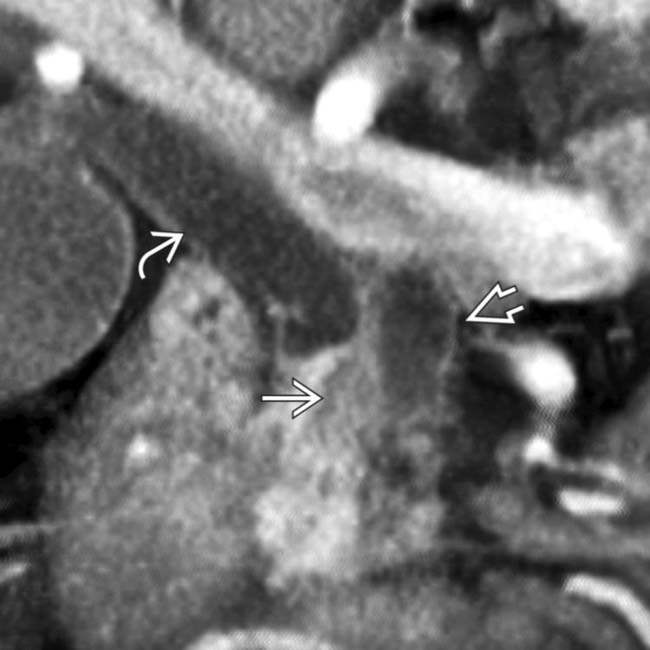
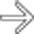
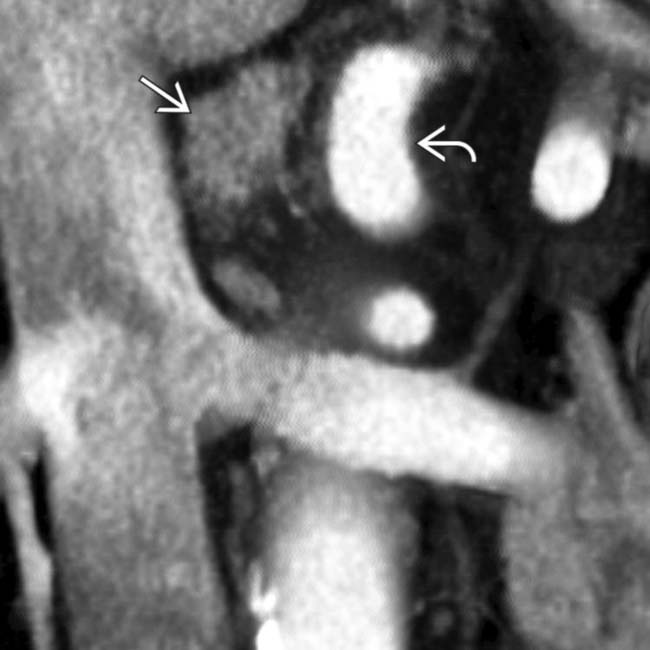
 obstructing both the common bile duct
obstructing both the common bile duct  and the pancreatic duct
and the pancreatic duct  .
.
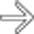
 adjacent to the celiac artery
adjacent to the celiac artery  . At surgery, the celiac node was positive for metastatic adenocarcinoma and the surgery was abandoned. An enlarged lymph node outside the anatomic boundary of a Whipple resection should prompt biopsy of the node to avoid unnecessary surgery.
. At surgery, the celiac node was positive for metastatic adenocarcinoma and the surgery was abandoned. An enlarged lymph node outside the anatomic boundary of a Whipple resection should prompt biopsy of the node to avoid unnecessary surgery.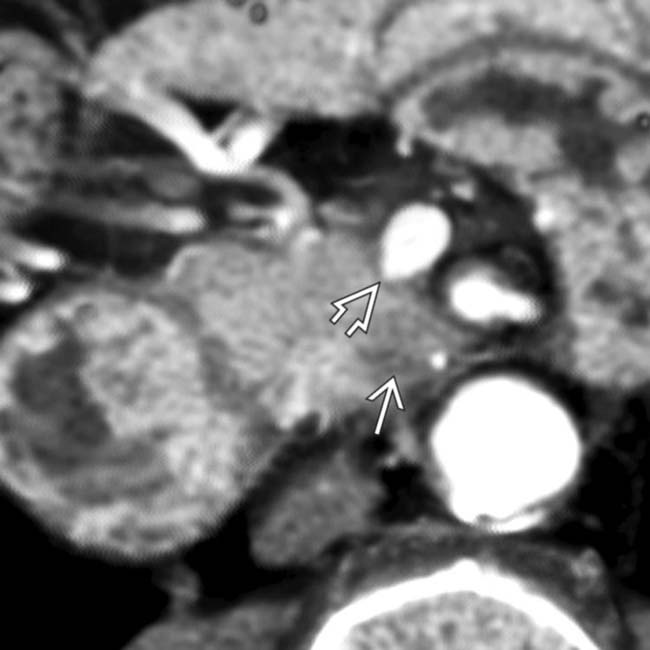
 . Note that the superior mesenteric vein has a pointed or “teardrop” configuration
. Note that the superior mesenteric vein has a pointed or “teardrop” configuration  rather than its normal rounded appearance.
rather than its normal rounded appearance.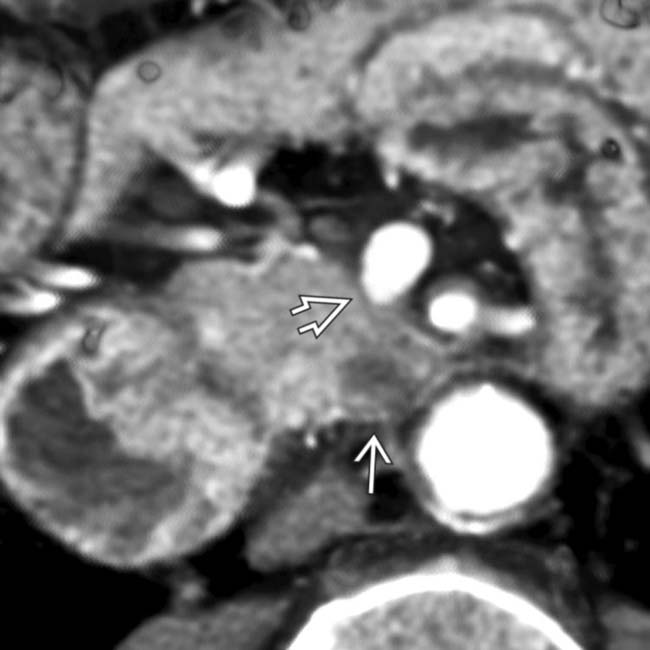
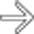 and the “teardrop” sign
and the “teardrop” sign  , indicating adventitial invasion of the wall of the superior mesenteric vein. In these cases, surgical resection with negative margins can only be accomplished with a venous bypass graft.
, indicating adventitial invasion of the wall of the superior mesenteric vein. In these cases, surgical resection with negative margins can only be accomplished with a venous bypass graft.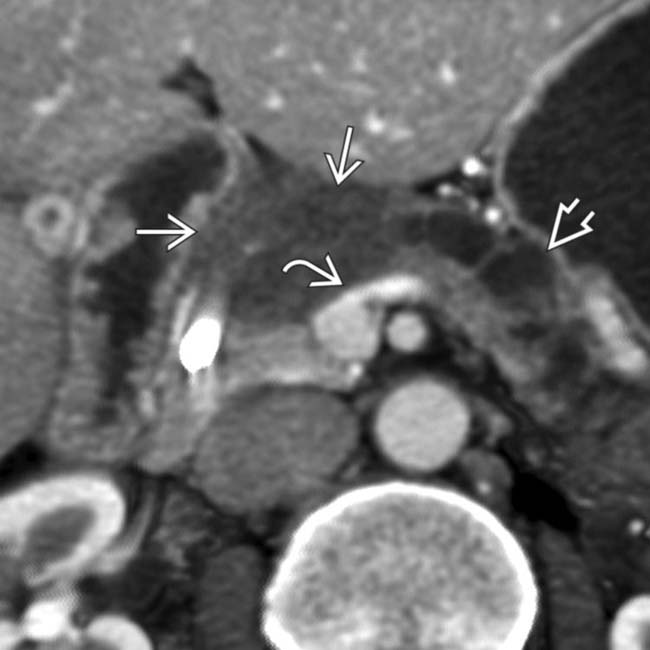
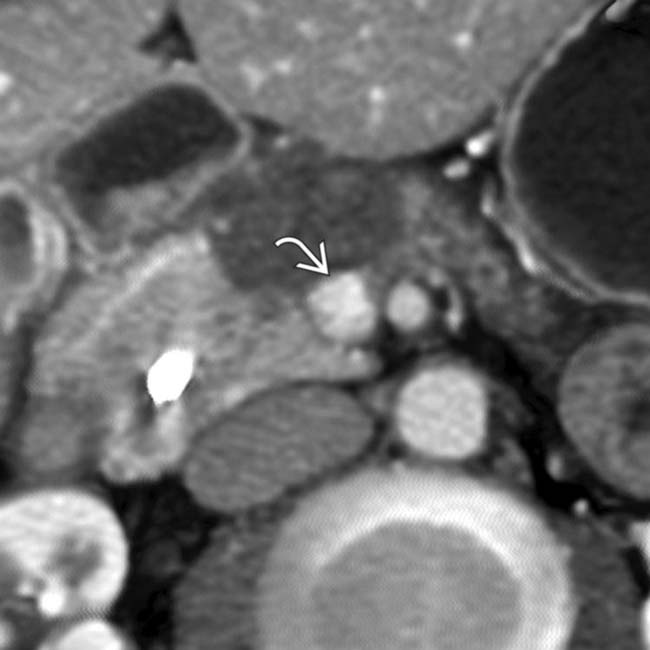
 causing focal flattening
causing focal flattening  of the superior mesenteric vein and splenoportal confluence. Note upstream pancreatic ductal dilatation
of the superior mesenteric vein and splenoportal confluence. Note upstream pancreatic ductal dilatation  .
.
 without circumferential encasement. This tumor was considered to be borderline for resectability. At surgery, the tumor was not able to be dissected free of the splenoportal confluence.
without circumferential encasement. This tumor was considered to be borderline for resectability. At surgery, the tumor was not able to be dissected free of the splenoportal confluence.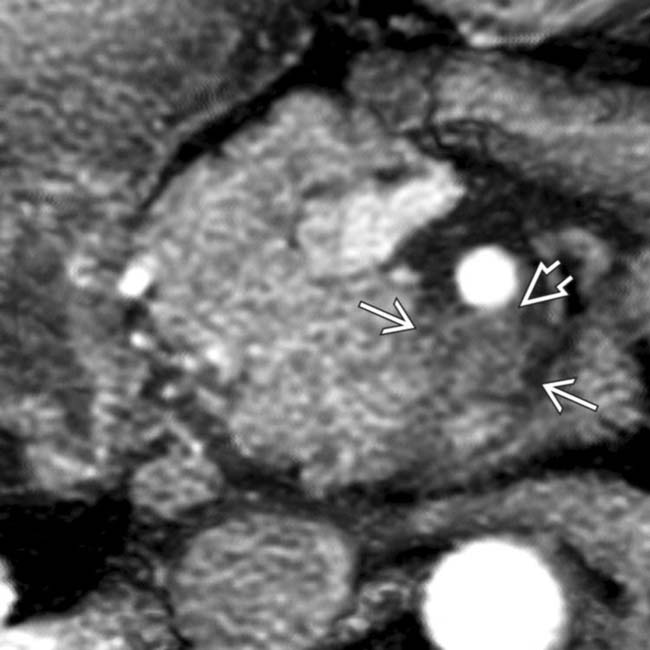
 in the uncinate process. Note that the mass abuts the posterior margin
in the uncinate process. Note that the mass abuts the posterior margin  of the superior mesenteric artery but does not encase or exceed 1/2 of the circumference of the SMA.
of the superior mesenteric artery but does not encase or exceed 1/2 of the circumference of the SMA.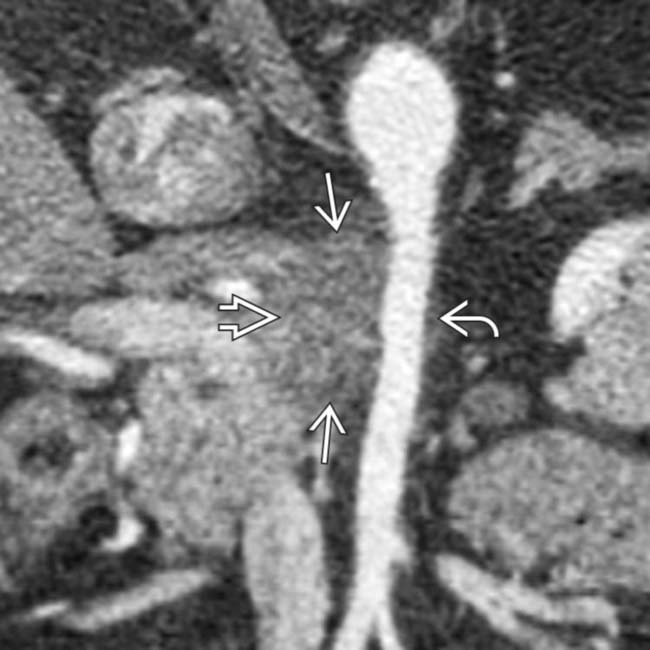
 in the same patient shows the carcinoma
in the same patient shows the carcinoma  with 2 cm length of contact
with 2 cm length of contact  with the lateral margin of the SMA, indicating a borderline resectable lesion. At surgery, the lesion was adherent to the SMA and was unresectable.
with the lateral margin of the SMA, indicating a borderline resectable lesion. At surgery, the lesion was adherent to the SMA and was unresectable.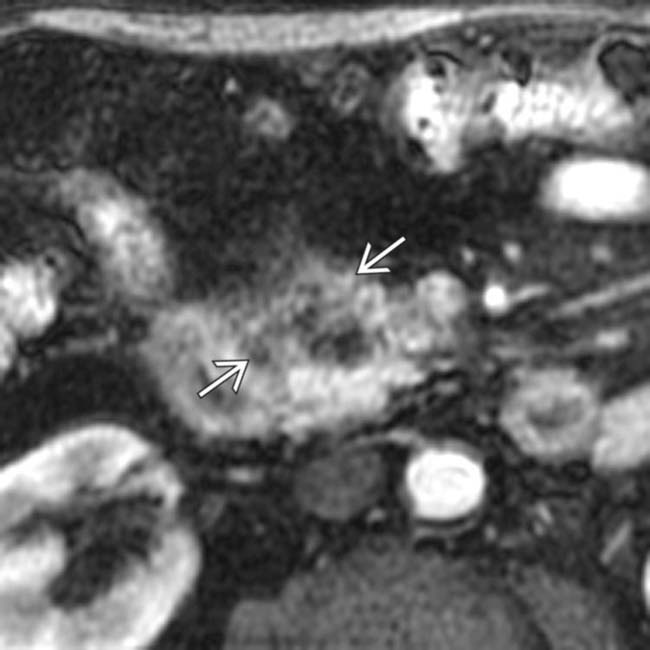
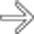 in the head of the pancreas.
in the head of the pancreas.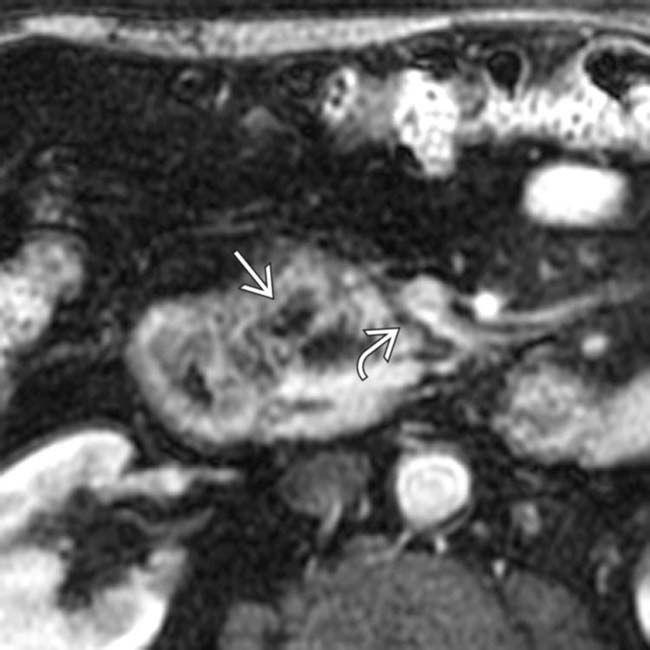
 does not involve the fat planes
does not involve the fat planes  around the superior mesenteric vein, indicating a resectable lesion. The patient underwent a successful Whipple resection with negative margins (R0 resection).
around the superior mesenteric vein, indicating a resectable lesion. The patient underwent a successful Whipple resection with negative margins (R0 resection).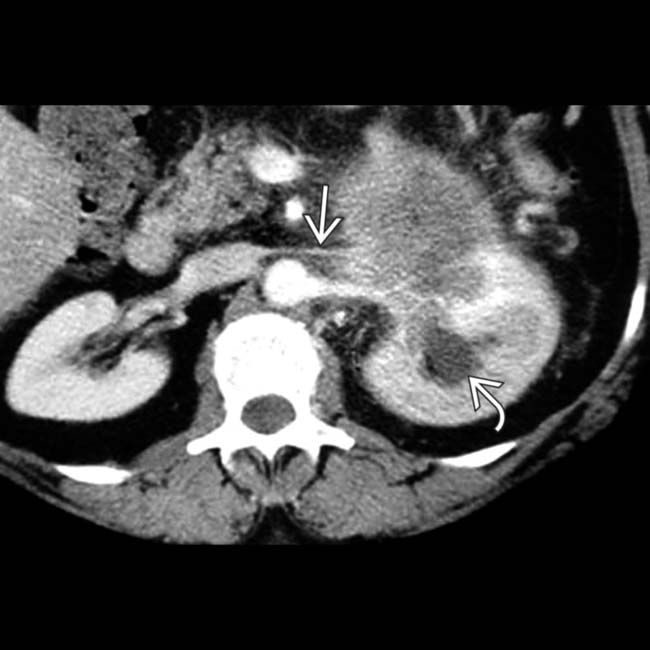
 and the left kidney, resulting in hydronephrosis
and the left kidney, resulting in hydronephrosis  and a delayed nephrogram. Posterior invasion into the retroperitoneum is a characteristic pattern of spread for locally invasive ductal adenocarcinoma.
and a delayed nephrogram. Posterior invasion into the retroperitoneum is a characteristic pattern of spread for locally invasive ductal adenocarcinoma.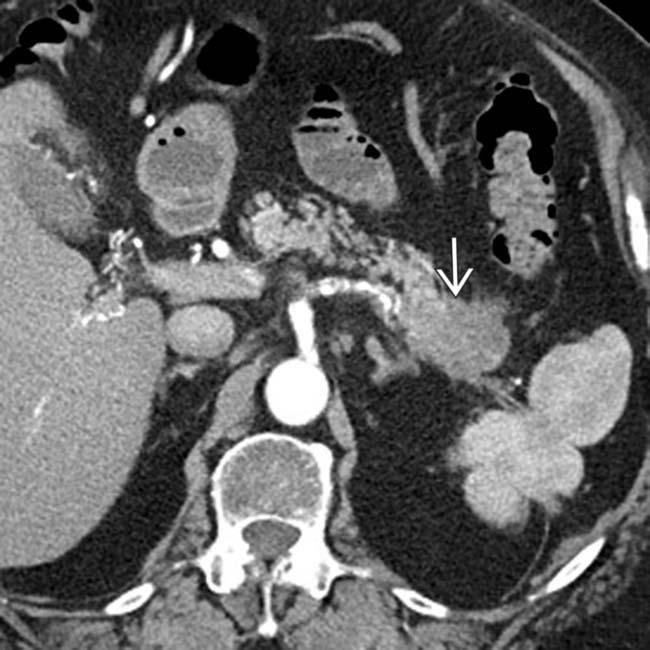
 . The mass involves no major vasculature and would be theoretically resectable.
. The mass involves no major vasculature and would be theoretically resectable.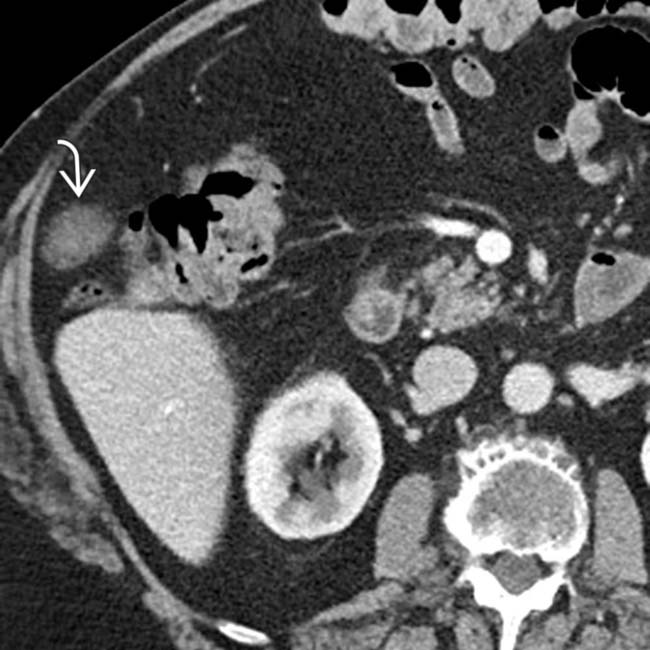
 in the right omentum. The nodule was biopsied and found to be a tumor implant, thus precluding resection. Other than the liver, the peritoneum is one of the more common sites of distant metastatic disease.
in the right omentum. The nodule was biopsied and found to be a tumor implant, thus precluding resection. Other than the liver, the peritoneum is one of the more common sites of distant metastatic disease.


















































































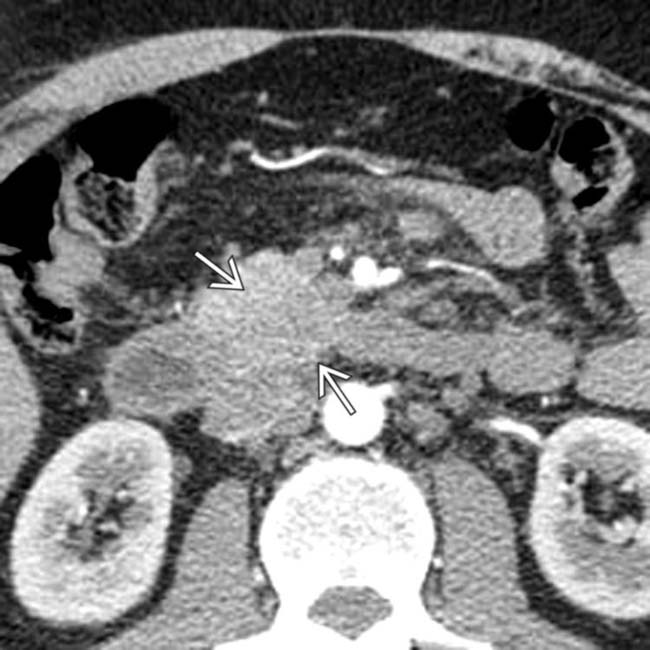
 in the head of the pancreas.
in the head of the pancreas.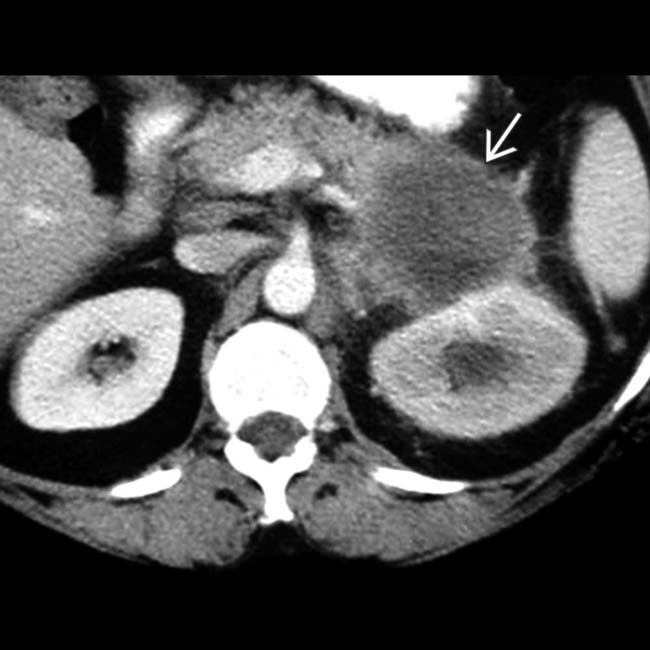
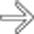
 arising from the body/tail segment of the pancreas.
arising from the body/tail segment of the pancreas.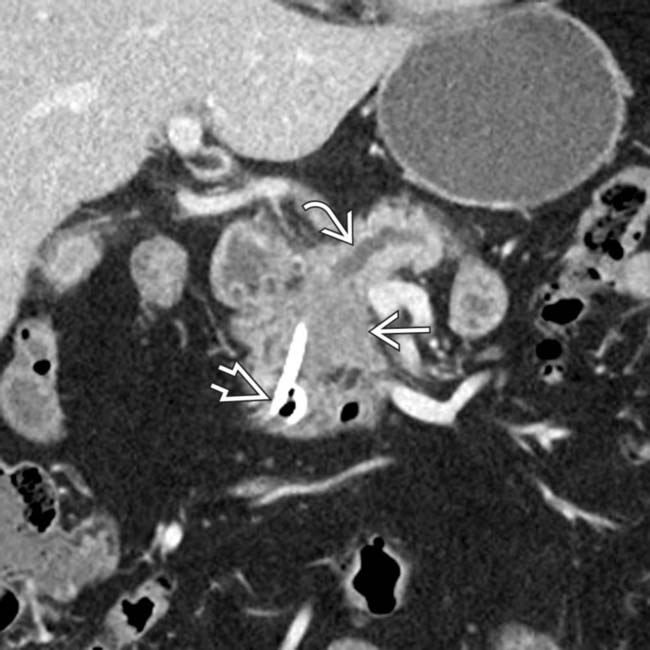
 in the pancreatic head resulting in abrupt occlusion and upstream dilatation of the pancreatic duct
in the pancreatic head resulting in abrupt occlusion and upstream dilatation of the pancreatic duct  . A biliary stent
. A biliary stent  has been placed to relieve the patient’s jaundice.
has been placed to relieve the patient’s jaundice.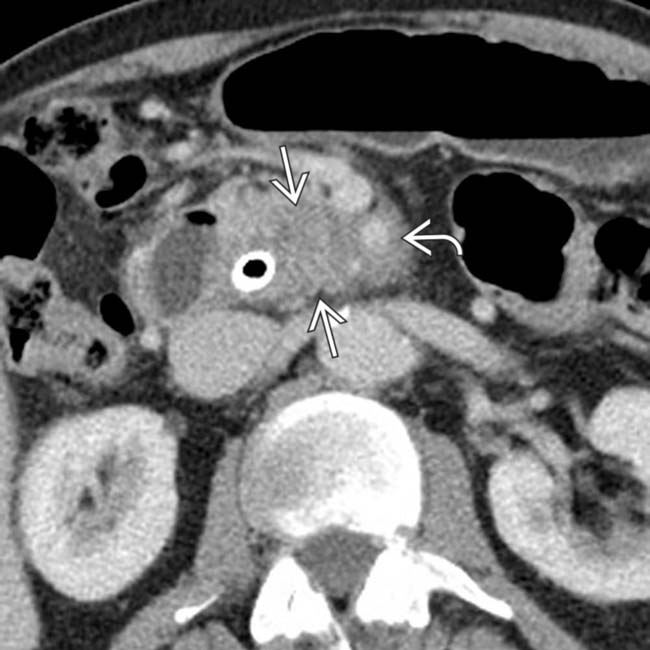
 in the pancreatic head, typical for pancreatic adenocarcinoma. Note that the mass completely encases the adjacent SMA
in the pancreatic head, typical for pancreatic adenocarcinoma. Note that the mass completely encases the adjacent SMA  , precluding this patient from undergoing resection.
, precluding this patient from undergoing resection.