Chapter 103 Pancreatic and duodenal injuries
Evolution of the Approach to Pancreatic Trauma
A patient with blunt pancreatic injury was first reported in the Lancet as early as 1827, by Tavers. Although several survivors were subsequently reported after ligation and excision of exteriorized penetrating pancreatic wounds (Otis, 1876), the major advance in the operative treatment came in 1923, when Walton described distal pancreatectomy (see Chapter 62A, Chapter 62B ) as the treatment of choice for pancreatic transection. The dramatic reduction in mortality among patients with pancreatic injuries—from 56% in World War II, mostly due to associated injuries, to 22% in the Korean War—was more a result of improvements in general perioperative care than of specific technical innovations (Sako et al, 1955).
In the final decades of the last century, advances in medical imaging and in surgical technique converged to improve the care of patients with pancreatic trauma. The most important of these was computerized tomography (CT) of the abdomen (see Chapter 16), which for the first time allowed detailed visualization of the pancreas (Udekwu et al, 1996). This enabled surgeons to diagnose hitherto unrecognized pancreatic injuries and to drain peripancreatic fluid collections without the need for a laparotomy.
At approximately the same time, the introduction of endoscopic retrograde cholangiopancreatography (ERCP; see Chapter 18) provided a minimally invasive approach to diagnose leakage from the pancreatic duct after trauma (Buccimazza et al, 2006; Gupta et al, 2004; Lin et al, 2004; Wind et al, 1999; Wong et al, 2002). By contrast, the role of magnetic resonance cholangiopancreatography (MRCP; see Chapter 17) in the evaluation of the injured pancreatic duct remains to be defined (Bhasin et al, 2009; Fulcher et al, 2000).
The presumed adverse effect of pancreatic enzymes on the suture lines of duodenal repairs in patients with injuries to both organs led to the development of the Berne duodenal diverticulization, essentially a Bilroth II gastrectomy combined with biliary diversion (Berne et al, 1968, 1974), to preemptively divert gastric secretions around the repaired duodenum. Later, Jordan introduced the pyloric exclusion procedure (Vaughan et al, 1977), which achieves the same result but with a much simpler operation. Because of its conceptual elegance and technical simplicity, pyloric exclusion rapidly became the buzzword of PDC trauma in the 1990s. This led to very liberal use of the procedure in reported series (Feliciano et al, 1987); however, pyloric exclusion has never been subjected to a prospective randomized trial, and most attempts to compare it retrospectively to simple duodenal repair have demonstrated increased morbidity without benefit (DuBose et al, 2008; Seamon et al, 2007).
Resection of the entire PDC (see Chapter 62A, Chapter 62B ), which is routinely done in elective pancreatic surgery, is very rarely needed in the trauma situation. When complete disruption of the head of the pancreas and surrounding duodenal loop leaves the surgeon with no other choice, a so-called trauma Whipple (TW) may be necessary, but only as a last resort to save an exsanguinating patient. When necessary, the procedure is performed in a staged fashion with resection during the original operation and reconstruction 24 to 48 hours later (Subramanian et al, 2007). This is a reflection of the move toward damage control surgery, the most important paradigm shift in trauma surgery in the last two decades of the last century (Hoey & Schwab, 2002).
Distal pancreatectomy remains the mainstay of treatment of patients with major ductal disruption (Balasegaram & Lumpur, 1976; Heitsch et al, 1976); however, reports of successful stenting of the injured pancreatic duct via ERCP (Lin et al, 2006, 2007) suggest that this minimally invasive intervention may evolve into a safe management option in selected patients. Pancreatic trauma continues to tax the judgment and operative skills of experienced surgeons, even as sophisticated, minimally invasive diagnostic and therapeutic adjuncts continue to evolve.
Surgical Anatomy of the Pancreas and Duodenum: A Trauma Surgeon’s Perspective (See Chapters 1A and 1B)
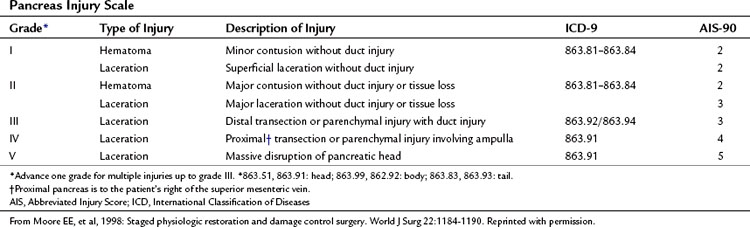
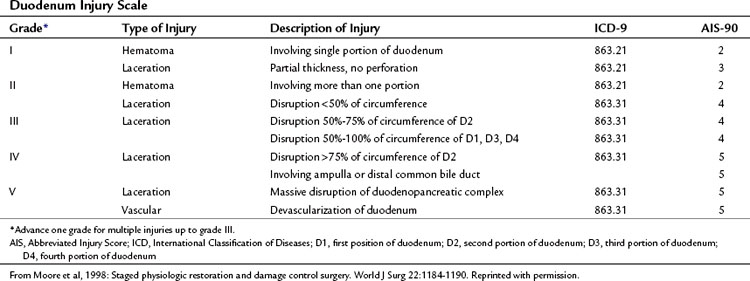
The organ injury scales for the pancreas and duodenum, developed by the American Association for the Surgery of Trauma (AAST) (Tables 103.1 and 103.2; Moore et al, 1990) are used for grading injuries of these two organs. Unfortunately, these scales do not acknowledge the fundamental anatomic differences between the pancreatic body and tail and the PDC, and therefore their clinical usefulness is limited.
For practical purposes, trauma to the pancreas ranges from superficial injuries that require only drainage (Feliciano, 1989) to ductal disruption that mandates a distal pancreatectomy. Low-grade injuries to the duodenum can be repaired the same as any other penetrating injury to the gut; however, high-grade duodenal injuries, and especially those that combine duodenal and pancreatic trauma, require a different approach because of the high risk and unforgiving nature of a duodenal leak. Injuries that destroy the PDC or disrupt the ampulla are rare and carry very high mortality rates because of associated trauma to adjacent major vascular structures. This perspective on pancreatic anatomy forms the basis for a detailed discussion of the management of pancreatic injuries in the forthcoming sections.
Diagnosis of Pancreatic and Duodenal Trauma
Hemodynamically stable patients with injuries to the pancreas and/or duodenum may exhibit only subtle signs because of the retroperitoneal location of these organs. Patients with a history of acceleration/deceleration injury; forceful anterior compression of the abdomen, such as from kicks or handlebar injuries; and lower thoracic and upper lumbar vertebra fractures are at increased risk. A seatbelt sign across the upper and middle abdomen should suggest the possibility of serious intraabdominal injury (Velmahos et al, 1999); however, the physical examination can be remarkably benign: abdominal pain and peritoneal signs sometimes take days to develop. For all of these reasons, a high index of suspicion is essential to avoid missing an injury (Wright & Stanski, 2000).
Routine laboratory tests are not particularly helpful. Obviously, patients with elevations in white blood cell count, amylase, or lipase require further investigation. Unfortunately, many patients are seen with normal values. In fact, as many as 35% of patients with a pancreatic transection have normal amylase levels (Adamson et al, 2003; Jones, 1985; Sriussadaporn, 1994; Takishima et al, 1997).
On abdominal CT scan, injury to the duodenum is usually obvious; however, the initial CT findings of pancreatic injury may be quite subtle (Akhrass et al, 1997; Bradley et al, 1998; Canty & Weinman, 2001; Ilahi et al, 2002) even with a study using thin slices of the pancreatic area. If a definitive diagnosis is not possible, a repeat CT scan 8 to 12 hours later allows time for retroperitoneal edema as a result of leakage of pancreatic secretions, which better delineates the injury. Fluid in the lesser sac, pancreatic hematomas, and pancreatic lacerations all suggest the diagnosis (Subramanian et al, 2007).
Unfortunately, the abdominal CT scan cannot reliably diagnose a laceration of the main pancreatic duct, unless a complete pancreatic transection is visible (Bradley et al, 1998). ERCP (see Chapter 18) is an excellent test for that purpose and can also be used to stent lacerated ducts in selected patients (Bhasin et al, 2009; Silviera et al, 2009); however, the procedure itself has the potential to cause pancreatitis (Cotton et al, 2009; Silviera et al, 2009) and is generally indicated only when diagnosis of a lacerated duct will change the management of the injury. The role of MRCP in the management of pancreatic trauma remains to be determined (Gillams et al, 2006).
Operative Management of Injuries to the Body and Tail of the Pancreas (See Chapter 62A, Chapter 62B )
In assessing the body and tail of the pancreas, the key question that the surgeon must address is whether a pancreatic duct injury is present (Bach & Frey, 1971; Bradley et al, 1998; Tyburski et al, 2001; Voeller et al, 1991). Unless there is an obvious complete transection, it is difficult to diagnose a duct injury by simple inspection. The integrity of the duct can be interrogated by intraoperative imaging, either by amputating the tail and cannulating the distal duct or by cannulating the gallbladder and injecting contrast into the common bile duct. Both these options are cumbersome and do not have high success rates (Berni et al, 1982).
A transduodenal pancreatogram is mentioned here only to be condemned, because it converts a pancreatic injury into a pancreaticoduodenal (PD) injury. Intraoperative ERCP, first reported in 1986, is another option, if it can be organized expeditiously (Laraja et al, 1986). The more practic option in the middle of the night is to simply proceed with empiric distal pancreatectomy, whenever a deep laceration is in the vicinity of the main pancreatic duct, or a large hematoma is found in the center of the gland. An alternative option for nonbleeding pancreatic injuries, particularly in the face of multiple associated injuries, is closed suction drainage of the lesser sac. Patton and colleagues (1997) demonstrated excellent results by simple drainage, regardless of the location of the injury. A disruption of the capsule should be drained without attempting to repair it. Drainage of an injured main pancreatic duct is an effective solution, but it often results in a high output pancreatic fistula, so distal pancreatectomy may be a better option.
The trauma literature of the 1970s and 1980s is replete with descriptions of pancreas-preserving techniques for complete pancreatic transections (Frey, 1982). The most popular of these was anastomosis of the distal pancreatic stump to a Roux-en-Y loop of jejunum to create a pancreatojejunostomy. The proximal stump, which drains into the duodenum, was then oversewn. The rationale for pancreas-preserving approaches is to maintain pancreatic function and to prevent potential long-term complications of exocrine insufficiency and/or diabetes mellitus; however, this elegant technical solution involves a high-risk jejunal anastomosis to a traumatized edematous pancreatic stump in an unstable patient who will not tolerate a leak. So in spite of the fact that it is sometimes technically feasible, preservation of the distal pancreatic stump is not in the best interests of the trauma patient; distal pancreatectomy is a much safer solution (Krige et al, 2005; Steele et al, 1973).
Distal Pancreatectomy
Distal pancreatography (see Chapter 62A, Chapter 62B ) is often performed in unstable patients with associated injuries, which is not the time for splenic preservation (Subramanian et al, 2007). The spleen should be rapidly mobilized to the midline and used as a handle to lift up the body and tail of the pancreas as it is dissected bluntly from the surrounding retroperitoneal fat. The most expedient technique is to use a stapler to divide the gland, followed by selective suture ligation of the splenic artery and vein. If the pancreas is found to be transected already, an effort is made to deploy the stapler immediately to the right of the injury, in healthy tissue. If the pancreatic duct is visible, it should be suture ligated. As an alternative, the free edge of the proximal stump can be oversewn with a 2-0 polypropylene running suture. Closed suction drainage of the pancreatic bed and left upper quadrant is mandatory (Farrell et al, 1996).
Postoperative Pancreatic Fistula
Approximately 25% of patients develop a fistula after distal pancreatectomy for trauma (Fahy et al, 2002). In most cases, the fistula is controlled by the drains placed at the time of surgery. Neither the technique of pancreatic closure (Ferrone et al, 2008; Sheehan et al, 2002) nor the use of sealants (Fisher et al, 2008; Suc et al, 2003; Yamamoto et al, 2009) significantly affects the risk of fistula. Nutritional support, preferably via jejunal feeding, is a crucial element of fistula management; however, the presence of a pancreatic fistula does not necessarily require abstinence from oral intake; indeed, depending on the nature and extent of associated injuries, most patients will be able to eat normally during the time it takes for the fistula to close. Endoscopically placed pancreatic stents can reduce the volume and duration of fistula drainage in problem cases (Goasguen et al, 2009; Grobmyer et al, 2009). Failure to identify and drain a contained leak can result in a pancreatic pseudocyst, which may on rare occasion require surgery.
Injuries in the Stable Patient
The first step in the operative management of the injured PDC is wide exposure of the area with a Kocher maneuver. Full mobilization of the PDC from the common bile duct to the superior mesenteric vein (SMV) exposes the head of the pancreas, the second and some of the third portions of the duodenum, and the underlying vena cava. Additional exposure of adjacent structures and an improved workspace can be developed by extending the incision in the posterior peritoneum caudad along the white line of Toldt and mobilizing the right colon (Buscaglia et al, 1969), the so-called extended Kocher maneuver. Complete visualization of the entire third and fourth portions of the duodenum is achieved by carrying the peritoneal incision around the cecum and along the line of fusion of the small bowel mesentery to the posterior peritoneum, all the way up to the ligament of Treitz. This Cattell-Braasch maneuver is a technique for the exposure of the third and fourth portions of the duodenum (Cattell & Braasch, 1960), which allows complete reflection of the midgut onto the chest wall and provides access to the entire duodenum; however, after performing the maneuver, the entire midgut remains suspended on the superior mesenteric vascular pedicle and should be handled with care.
High-grade PDC injuries are an indication for pyloric exclusion (Degiannis & Boffard, 2000), the goal of which is to preemptively divert the gastric effluent around the injured duodenum by temporarily occluding the pylorus. If the duodenal repair leaks, a low-output end-duodenal fistula is much easier to manage than a high-output side fistula.
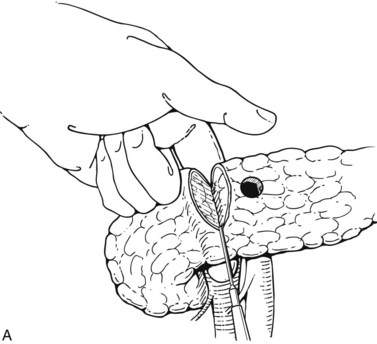
Pyloric exclusion begins with a generous longitudinal gastrotomy in the anterior wall of the antrum, close to the pylorus. The pylorus is grasped with two Babcock clamps, delivered through the gastrotomy and oversewn with size 0 PDS sutures. A side-to-side gastrojejunostomy with a short afferent limb is then constructed at the gastotomy site (Fig. 103.1). Some surgeons prefer to staple across the duodenum just distal to the pylorus, instead of suturing it from the inside. In most cases, the duodenum spontaneously opens several weeks later regardless of the method of closure; we prefer to use an absorbable synthetic suture.
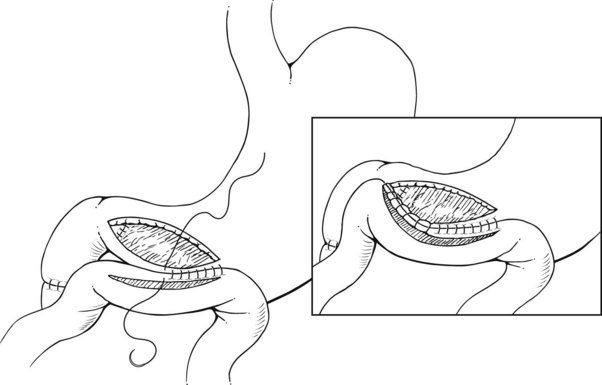
FIGURE 103.1 Pyloric exclusion showing occlusion of the duodenum just distal to the pylorus followed by a gastrojejunostomy.
The gastrojejunostomy is the source of most postoperative morbidity after a pyloric exclusion. This led Ginzburg and colleagues (1997) to attempt pyloric exclusion without gastrojejunostomy, relying on the spontaneous reopening of the sutured pylorus. Pyloric exclusion is, in theory, an ulcerogenic operation, but the incidence of marginal ulcers is unknown (Buck et al, 1992; Feliciano et al, 1987; Martin et al, 1983). Routine vagotomy or delayed takedown of the gastrojejunostomy is not indicated. Routine pyloric exclusion has recently been criticized because of reported increased complications and no effect on outcome (Seamon et al, 2007); however, in our practice, we continue to perform pyloric exclusion in patients with tenuous duodenal closures and in those with high-grade pancreatic injuries associated with a duodenal injury of any grade.
Occasionally, a high-grade injury to the PDC is encountered during a trauma laparotomy with massive blood loss unrelated to the PDC. In this situation, a rapid damage control solution is the only feasible alternative (see Chapter 102). The damage-control option for the injured nonbleeding pancreas is external drainage, regardless of the grade of injury. Similarly, the “bail-out” solution for duodenal injuries is either rapid closure with external drainage or placement of a large Malecot catheter through the hole affixed in place with a purse-string suture to create a controlled fistula.
Management of A Leaking Duodenal Repair
A leak from the duodenal suture line after a PDC injury is unusual but can be devastating (Seamon et al, 2007). Leakage of bile from the drain in a Morison pouch, or any deviation from the patient’s expected clinical trajectory, especially signs of unexplained sepsis, mean a duodenal leak until proven otherwise. A CT scan with contrast in the gastrointestinal tract will demonstrate the leak and establish whether it is controlled. If the leak is uncontrolled—that is, if free fluid is present in the peritoneal cavity—an urgent laparotomy is indicated to achieve source control and effective drainage. Such leaks can be difficult to manage, especially if the pylorus is open; the duodenum cannot be resected or exteriorized. Proximal diversion, if not done during the original procedure, is rarely an option; in addition, the inflamed duodenal wall does not hold sutures well. Inserting an appropriately sized Malecot tube into the hole, combined with wide regional drainage, is often the only feasible option. A creative variation of tube drainage is insertion of a spiral three-channel tube for continuous intraluminal irrigation (Parc et al, 1999). Although we have no personal experience with this method, it nevertheless underscores the technical difficulty of controlling a duodenal leak.
Pancreatoduodenal Injuries in the Unstable Patient
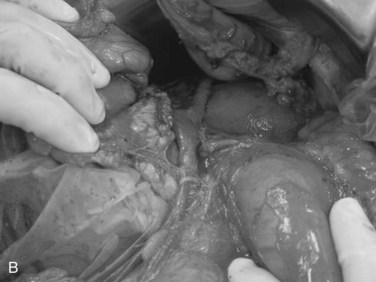
Exsanguinating hemorrhage into the lesser sac is most often due to an injury to the peripancreatic portal vein and is almost always associated with a penetrating injury to the neck of the pancreas. Effective circular compression as described above will dramatically reduce the bleeding, allowing the surgeon to perform a Kocher maneuver and widely open the lesser sac (Fig. 103.2).
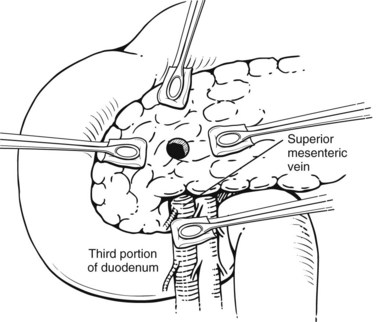
FIGURE 103.2 Sponge-stick control of bleeding from the portal vein. The white circles indicate areas of compression.
One of the authors (A.H.) always performs a Cattell-Braasch maneuver in these situations to gain maximal exposure of the infrarenal retroperitoneum. Careful dissection along the inferior border of the mobilized duodenal loop is carried all the way to the point at which the SMV dives behind the pancreatic neck. The portal vein is then identified in the hepatoduodenal ligament along the upper border of the first portion of the duodenum. Bluntly developing the plane between the common bile duct and the portal vein from above allows the surgeon to pass a finger behind the pancreatic neck, divide it, and repair the portal vein directly from the front (Fig. 103.3). An early decision to transect the pancreas limits blood loss. A distal pancreatectomy is performed after the vascular repair, and the proximal stump is oversewn. In a damage-control situation, the SMV and the portal vein may be ligated (Sheldon, 1985); however, repair is preferable whenever possible, because massive bowel edema inevitably ensues that complicates fluid and wound management. The early fluid requirements during the first 24 hours after ligation of the SMV or portal vein can exceed 20 L. A second-look laparotomy is mandatory approximately 24 hours after the original operation to assess bowel viability.
Trauma Whipple
Complete disruption of the PDC, usually in association with major hemorrhage from the vena cava and/or portal vein, is the only indication for PD in trauma—the TW. A popular saying among trauma surgeons is that a PD is indicated only when the gunshot has done the dissection for you. The paradox of the TW is that the patients who need it are too unstable to tolerate it, and those who are stable enough to tolerate it probably do not need it (Hirshberg & Mattox, 2006). In the 1970s and 1980s, these operations were reported with enthusiasm and applauded as heroic achievements. Unfortunately, patients rarely survived the combined insults of the external injury and the operative trauma. In the era of damage-control surgery, the TW is viewed as a weapon of last resort that is very rarely indicated. In fact, one of the authors (W.S.), during a career in trauma surgery spanning some 30 years, has never found himself in a situation in which a TW was necessary.
Adamson WT, et al. Serum amylase and lipase alone are not cost-effective screening methods for pediatric pancreatic trauma. J Pediatr Surg. 2003;38:354-357. discussion 354-357
Akhrass R, et al. Pancreatic trauma: a ten-year multi-institutional experience. Am Surg. 1997;63:598-604.
Bach RD, Frey CF. Diagnosis and treatment of pancreatic trauma. Am J Surg. 1971;121:20-29.
Balasegaram M, Lumpur K. Surgical management of pancreatic trauma. Am J Surg. 1976;131:536-540.
Berne CJ, et al. Combined duodenal pancreatc trauma: the role of end-to-side gastrojejunostomy. Arch Surg. 1968;96:712-722.
Berne CJ, et al. Duodenal “diverticulization” for duodenal and pancreatic injury. Am J Surg. 1974;127:503-507.
Berni GA, et al. Role of intraoperative pancreatography in patients with injury to the pancreas. Am J Surg. 1982;143:602-605.
Bhasin DK, et al. Endoscopic retrograde pancreatography in pancreatic trauma: need to break the mental barrier. J Gastroenterol Hepatol. 2009;24:720-728.
Bradley EL3rd, et al. Diagnosis and initial management of blunt pancreatic trauma: guidelines from a multiinstitutional review. Ann Surg. 1998;227:861-869.
Buccimazza I, et al. Isolated main pancreatic duct injuries spectrum and management. Am J Surg. 2006;191:448-452.
Buck JR, et al. Severe pancreatico-duodenal injuries: the effectiveness of pyloric exclusion with vagotomy. Am Surg. 1992;58:557-560. discussion 561
Buscaglia LC, et al. Penetrating abdominal vascular injuries. Arch Surg. 1969;99:764-769.
Canty TGSr, Weinman D. Treatment of pancreatic duct disruption in children by an endoscopically placed stent. J Pediatr Surg. 2001;36:345-348.
Cattell RB, Braasch JW. A technique for the exposure of the third and fourth portions of the duodenum. Surg Gynecol Obstet. 1960;111:378-379.
Cotton PB, et al. Risk factors for complications after ERCP: a multivariate analysis of 11,497 procedures over 12 years. Gastrointest Endosc. 2009;70:80-88.
Degiannis E, Boffard K. Duodenal injuries. Br J Surg. 2000;87:1473-1479.
DuBose JJ, et al. Pyloric exclusion in the treatment of severe duodenal injuries: results from the National Trauma Data Bank. Am Surg. 2008;74:925-929.
Fahy BN, et al. Morbidity, mortality, and technical factors of distal pancreatectomy. Am J Surg. 2002;183:237-241.
Farrell RJ, et al. Operative strategies in pancreatic trauma. Br J Surg. 1996;83:934-937.
Feliciano DV. Abdominal trauma. In: Schwartz, SI, Ellis, H. Maingot’s Abdominal Operations. Norwalk, CT: Appleton & Lange; 1989:457-512.
Feliciano DV, et al. Management of combined pancreatoduodenal injuries. Ann Surg. 1987;205:673-680.
Ferrone CR, et al. Pancreatic fistula rates after 462 distal pancreatectomies: staplers do not decrease fistula rates. J Gastrointest Surg. 2008;12:1691-1697. discussion 1697-1698
Fisher WE, et al. Effect of BioGlue on the incidence of pancreatic fistula following pancreas resection. J Gastrointest Surg. 2008;12:882-890.
Frey CF. Trauma to the pancreas and duodenum. In: Trunkey, DD, Blaisdell, FW. Abdominal Trauma. New York: Thieme-Stratton; 1982:87-122.
Fulcher AS, et al. Magnetic resonance cholangiopancreatography (MRCP) in the assessment of pancreatic duct trauma and its sequelae: preliminary findings. J Trauma. 2000;48:1001-1007.
Gillams AR, et al. Diagnosis of duct disruption and assessment of pancreatic leak with dynamic secretin-stimulated MR cholangiopancreatography. AJR Am J Roentgenol. 2006;186:499-506.
Ginzburg E, et al. Pyloric exclusion in the management of duodenal trauma: is concomitant gastrojejunostomy necessary? Am Surg. 1997;63:964-966.
Goasguen N, et al. Endoscopic management of pancreatic fistula after distal pancreatectomy and enucleation. Am J Surg. 2009;197:715-720.
Grobmyer SR, et al. Pancreatic stent placement is associated with resolution of refractory grade C pancreatic fistula after left-sided pancreatectomy. Am Surg. 2009;75:654-657. discussion 657-658
Gupta A, et al. Blunt trauma of the pancreas and biliary tract: a multimodality imaging approach to diagnosis. Radiographics. 2004;24:1381-1395.
Heitsch RC, et al. Delineation of critical factors in the treatment of pancreatic trauma. Surgery. 1976;80:523-529.
Hirshberg A, Mattox KL. Top Knife: The Art and Craft of Trauma Surgery. New York: Springer-Verlag; 2006. p 127
Hoey BA, Schwab CW. Damage control surgery. Scand J Surg. 2002;91:92-103.
Ilahi O, et al. Efficacy of computed tomography in the diagnosis of pancreatic injury in adult blunt trauma patients: a single-institutional study. Am Surg. 2002;68:704-707. discussion 707-708
Jones RC. Management of pancreatic trauma. Am J Surg. 1985;150:698-704.
Krige JE, et al. The management of complex pancreatic injuries. S Afr J Surg. 2005;43:92-102.
Laraja RD, et al. Intraoperative endoscopic retrograde cholangiopancreatography (ERCP) in penetrating trauma of the pancreas. J Trauma. 1986;26:1146-1147.
Lin BC, et al. Management of blunt major pancreatic injury. J Trauma. 2004;56:774-778.
Lin BC, et al. Long-term results of endoscopic stent in the management of blunt major pancreatic duct injury. Surg Endosc. 2006;20:1551-1555.
Lin BC, et al. Blunt pancreatic trauma and pseudocyst: management of major pancreatic duct injury. Injury. 2007;38:588-593.
Martin TD, et al. Severe duodenal injuries: treatment with pyloric exclusion and gastrojejunostomy. Arch Surg. 1983;118:631-635.
Moore EE, et al. Organ injury scaling, II: pancreas, duodenum, small bowel, colon, and rectum. J Trauma. 1990;30:1427-1429.
Otis GA, editor. The Medical and Surgical History of the War of Rebellion: part II, vol II. Washington, DC: U.S. Government Printing Office. 1876:158-161. chapter VI
Patton JHJr, et al. Pancreatic trauma: a simplified management guideline. J Trauma. 1997;43:234-239.
Parc Y, et al. Postoperative peritonitis originating from the duodenum: operative management by intubation and continuous intraluminal irrigation. Br J Surg. 1999;86:1207-1212.
Sako Y, et al. A survey of evacuation, resuscitation, and mortality in a forward surgical hospital. Surgery. 1955;37:602-611.
Seamon MJ, et al. A ten-year retrospective review: does pyloric exclusion improve clinical outcome after penetrating duodenal and combined pancreaticoduodenal injuries? J Trauma. 2007;62:829-833.
Sheehan MK, et al. Distal pancreatectomy: does the method of closure influence fistula formation? Am Surg. 2002;68:264-267. discussion 267-268
Sheldon GF, et al. Management of injuries to the porta hepatis. Ann Surg. 1985;202:539-545.
Silviera ML, et al. Complications related to endoscopic retrograde cholangiopancreatography: a comprehensive clinical review. J Gastrointest Liver Dis. 2009;18:73-82.
Sriussadaporn S. Management of pancreatic injuries. J Med Assoc Thai. 1994;77:580-587.
Steele M, et al. Pancreatic injuries: methods of management. Arch Surg. 1973;106:544-549.
Subramanian A, et al. The management of pancreatic trauma in the modern era. Surg Clin North Am. 2007;87:1515-1532. x
Suc B, et al. Temporary fibrin glue occlusion of the main pancreatic duct in the prevention of intra-abdominal complications after pancreatic resection: prospective randomized trial. Ann Surg. 2003;237:57-65.
Takishima T, et al. Serum amylase level on admission in the diagnosis of blunt injury to the pancreas: its significance and limitations. Ann Surg. 1997;226:70-76.
Tavers B. Rupture of the pancreas. Lancet. 1827;12:384.
Tyburski JG, et al. Infectious complications following duodenal and/or pancreatic trauma. Am Surg. 2001;67:227-230. discussion 230-221
Udekwu PO, et al. The use of computed tomography in blunt abdominal injuries. Am Surg. 1996;62:56-59.
Vaughan GD3rd, et al. The use of pyloric exclusion in the management of severe duodenal injuries. Am J Surg. 1977;134:785-790.
Velmahos GC, et al. The “seat belt mark” sign: a call for increased vigilance among physicians treating victims of motor vehicle accidents. Am Surg. 1999;65:181-185.
Voeller GR, et al. The effect of a trauma system on the outcome of patients with pancreatic trauma. Arch Surg. 1991;126:578-580.
Walton A. A textbook of surgical dyspepsias. London: Edward, Arnold; 1923.
Wind P, et al. Contribution of endoscopic retrograde pancreatography in management of complications following distal pancreatic trauma. Am Surg. 1999;65:777-783.
Wong YC, et al. Perisplenic extravasation of contrast medium on enhanced helical computed tomography: a reliable indicator for early surgical management in blunt splenic injuries. Chang Gung Med J. 2002;25:381-387.
Wright MJ, Stanski C. Blunt pancreatic trauma: a difficult injury. South Med J. 2000;93:383-385.
Yamamoto M, et al. Use of Seamguard to prevent pancreatic leak following distal pancreatectomy. Arch Surg. 2009;144:894-899.