Palpitations and Arrhythmias (Case 6)
Arzhang Fallahi MD and Michael Kim MD
Case: A 75-year-old man with a history of hypertension and hyperlipidemia presents to the emergency department complaining of palpitations. He states that for the past day he has felt a strange feeling in his chest, like his heart is racing, and says he is mildly short of breath. He has had similar episodes in the past, but they went away with rest and tended to “come and go.” He reports some light-headedness. He denies any chest pain, cough, or history of thyroid disease. He has never seen a cardiologist before and has never had “serious heart problems.” His only medications are aspirin 81 mg, hydrochlorothiazide 25 mg, and simvastatin 40 mg, all taken once daily. There is no family history of heart disease. His pulse is 135 bpm, and his BP is 145/70 mm Hg. On exam he seems to be in no acute distress but appears anxious. He has clear lung fields bilaterally and an irregular heart rate with no obvious murmurs, rubs, or gallops and no elevated neck veins. He has trace edema in his lower extremities.
An ECG reveals a narrow complex tachycardia at 135 bpm, irregularly irregular with no P waves and no delta waves; aVL shows a QRS complex of 12 little boxes in height; no other ST elevations or depressions are noted.
Differential Diagnosis
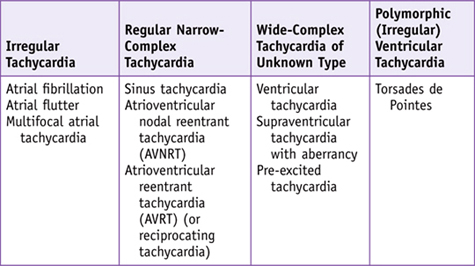
Speaking Intelligently
The approach to a patient with palpitations is as follows:
4. Assess for comorbidities: lung disease (asthma, chronic obstructive pulmonary disease [COPD], pulmonary hypertension, restrictive lung disease), cardiac disease, hypertension, age, diabetes, history of stroke. Comorbidities will help in risk-stratifying the patient, as well as in guiding management.
PATIENT CARE
Clinical Thinking
• The patient is not hypotensive and shows no signs of heart failure. However, given his age and chronic symptoms, it is unclear if his symptoms have been going on for 48 hours or more. Together, this increases his risk for embolization; therefore, electrical cardioversion would not be the best initial option. One must look at comorbidities in determining which pharmacologic agent to use. Atrioventricular (AV) nodal agents such as β-blockers or calcium channel blockers are important for rate control, while amiodarone is one of the main agents used for rhythm control. In patients with severe asthma, β-blockers may exacerbate the condition, and in patients with heart failure and hypotension, calcium channel blockers can result in decompensation. In patients with severely reduced ejection fraction, agents such as digoxin, in combination with AV nodal agents, may be of benefit.
History
Physical Examination
• Vascular auscultation (carotid bruits): to assess atherosclerotic status.
• Neurologic examination: Quickly assess patient’s mental status to determine if there is adequate perfusion of the brain. A screening neurologic exam should be performed to see if there are any focal deficits that might preclude use of anticoagulation therapy.
• Extremities: Check to see if extremities are warm to assess perfusion.
Tests for Consideration
|
$27 |
|
|
$49 |
|
|
$39 |
|
|
$11 |
|
|
• Liver function tests may be helpful to help ascertain if there is a hepatic cause of infection. |
$12 |
|
$15 |
|
|
$21 |
| Clinical Entities | Medical Knowledge |
|
Atrial Fibrillation/Atrial Flutter |
|
|
Pφ |
Atrial fibrillation and atrial flutter are often caused by underlying heart disease (of any etiology), which contributes to heart failure and atrial enlargement, an increase in atrial pressure, or infiltration or inflammation of the atria. Metabolic causes such as hyperthyroidism can also precipitate atrial fibrillation or flutter, as can cardiac surgery. Often episodes of paroxysmal atrial fibrillation are triggered by premature atrial beats, while other episodes are triggered by atrial tachycardia or atrial flutter. Ectopic foci are mostly located in the pulmonary vein in atrial fibrillation, while in atrial flutter the conducting reentrant circuit can be located in the low right atrial isthmus between the orifice of the inferior vena cava and annulus of the tricuspid valve. |
|
TP |
Classically the patient presents with palpitations, dyspnea, dizziness, and reduced exercise capacity and may exhibit chest pain (which may suggest acute coronary syndrome as a precipitant). |
|
Dx |
Diagnosis is made on clinical grounds, but the most informative test is the ECG. Patients in atrial fibrillation will have a complete absence of P waves and irregularly irregular RR intervals with varying amplitude and morphology. Atrial fibrillation is typically a narrow-complex tachycardia unless accompanied by fascicular block, preexcitation via an accessory pathway, or aberration. In atrial flutter, P waves are absent, with F waves or “sawtooth”-appearing flutter waves at a rate most typically 300 bpm. The ventricular response is usually one half the atrial rate (2 : 1 AV nodal conduction). Atrial flutter should always be considered when the ventricular rate is around 150 bpm. |
|
Tx |
Management of atrial fibrillation or flutter depends on the clinical presentation. Patients with severe hypotension or shock secondary to their underlying rhythm should undergo immediate cardioversion. In more stable patients, consider whether to use rate control or rhythm control (attempt to convert the patient to normal sinus rhythm), and consider prevention of systemic embolization. Treatment is also guided by the type of atrial fibrillation, whether it is paroxysmal (self-terminating), persistent (fails to self-terminate within 7 days), permanent (lasts for more than 1 year and cardioversion has not been attempted or failed), or lone (which describes paroxysmal, persistent, or permanent atrial fibrillation in patients without structural heart disease). Rate control with chronic anticoagulation is recommended for most patients. The most common agents for rate control are AV nodal agents such as β-blockers and calcium channel blockers (diltiazem or verapamil). In patients with heart failure or hypotension, digoxin may be helpful, although it has a longer onset of action. Amiodarone can also control rate but is not used as a primary therapy. Rhythm control can be divided into external synchronized DC cardioversion and pharmacologic cardioversion. Caution should be exercised in patients with symptoms lasting more than 48 hours, as they are at higher risk of embolization from atrial thrombi, which can form due to turbulent flow in the atria while the patient is in atrial fibrillation; in these patients, anticoagulation is required or thrombi should be excluded with transesophageal echocardiography. In patients with minimal heart disease, agents such as flecainide or propafenone can be used, while amiodarone and dofetilide are preferred for patients with reduced LV ejection fraction or heart failure. |
|
Radiofrequency catheter ablation is another modality to treat atrial fibrillation and atrial flutter. In most cases of atrial fibrillation, the focus is located inside the pulmonary veins, with the left superior vein being the most common site. In patients with atrial flutter, the macroreentrant pathway is located in the right atrium and is generally more amenable to ablation than in atrial fibrillation. The annual risk for stroke in patients can be estimated using the CHADS2 score, which assigns a point for every comorbidity—CHF, hypertension, age over 75 years, diabetes—and 2 points for prior stroke. A higher CHADS2 score means patients would benefit from anticoagulation with agents such as warfarin sodium (Coumadin), although patient comorbidities must be taken into account especially in patients with a history of bleeding. A new class of agents, the direct thrombin inhibitors (e.g., dabigatran), is now available for anticoagulation in patients with atrial fibrillation; use of these agents does not require INR monitoring. See Cecil Essentials 10. |
|
|
Pφ |
In most cases the different P-wave morphologies seen on the ECG suggest that a pacemaker arises in different locations within the atria. Cardiac disease (coronary, valvular, and congestive heart failure) is associated with MAT. Pulmonary disease, especially COPD, is also associated with MAT. Pulmonary arterial hypertension results in increased right atrial stretch, which can result in ectopic atrial activity. Hypokalemia and hypomagnesemia can also contribute to MAT. |
|
TP |
Patients may not have symptoms or may complain of palpitations. One must always consider the underlying cause that is contributing to the arrhythmia. |
|
Dx |
Diagnosis is made by ECG, which reveals P waves with three different morphologies (best seen in leads II, III, and V1), atrial rate over 100 bpm, P waves separated by isoelectric intervals, and PP, PR, and RR intervals that vary. |
|
Tx |
Treatment should be focused the underlying disorder. β-Blockers and calcium channel blockers (i.e., verapamil) have been shown to be effective. Due to risk of bronchospasm in patients with pulmonary disease, verapamil is most commonly used, although in the absence of such comorbidities, agents such as metoprolol can also be effective. Repletion in those with low magnesium (and even in those with normal magnesium) may be of some benefit. Repletion of potassium may also be of benefit. Ablation may be an option in some patients with MAT. See Cecil Essentials 10. |
|
Atrioventricular Nodal Reentrant Tachycardia (Junctional Reciprocating Tachycardia) |
|
|
Pφ |
Structural heart disease is not required for AVNRT, and most times AVNRT develops in patients with otherwise normal hearts, although it can also occur in patients with organic heart disease. While there are usually no precipitating factors, AVNRT can be brought on by nicotine, alcohol, stimulants, or surges in vagal tone. |
|
TP |
Patients typically present with palpitations, a strange feeling in the chest, and possible dizziness. In severe cases, patients may feel dyspnea and chest pain, and possibly fatigue or syncope. |
|
Dx |
Diagnosis is made by ECG. Usually the rate is between 120 and 220 bpm. In most cases, the retrograde atrial activation causes the P wave to be buried or fused with the QRS complex. If the P wave occurs shortly after the QRS complex, a fused waveform can appear as a pseudo-R’ in lead V1 and a pseudo-S wave in the inferior leads. The axis of the P wave, because of retrograde activation, is typically inverted in leads I, II, III, and aVF. |
|
Tx |
In unstable patients with evidence of hemodynamic collapse, DC cardioversion is the treatment of choice. In the absence of severe symptoms or hemodynamic collapse, vagal maneuvers (carotid massage, cough, Valsalva) can be used to break the rhythm. Adenosine can be used for rapid conversion to sinus rhythm. Non-dihydropyridine calcium channel blockers or β-blockers can also be used. For chronic cases of AVNRT, patients can undergo catheter ablation. See Cecil Essentials 10. |
|
Atrioventricular Reentrant (or Reciprocating) Tachycardia |
|
|
Pφ |
In AVRT a defined circuit exists consisting of two distinct pathways: the normal AV conduction system and an AV accessory pathway, which are linked by proximal tissue (the atria) and distal tissue (the ventricles). The two major forms of this type of arrhythmia are orthodromic AVRT and antidromic AVRT. In antidromic AVRT (AAVRT), the ventricles become activated anterogradely via the AV accessory pathway, which is then followed by retrograde conduction over the AV node/His-Purkinje system, which then completes the reentrant circuit. |
|
TP |
Similar to symptoms in patients with AVNRT. |
|
Dx |
In OAVRT the ECG shows a narrow-complex tachycardia, typically 150 to 250 bpm, with P waves inscribed within the ST-T segment with an RP interval usually less than one-half the tachycardic RR interval. The RP interval remains constant. In AAVRT, the ECG shows a wide QRS complex (retrograde conduction over the AV node/His-Purkinje system), with regular RR intervals and ventricular rates of up to 250 bpm. This wide-complex tachycardia with an up-sloping “delta wave” is called Wolff-Parkinson-White (WPW) syndrome. |
|
Tx |
For OAVRT, acute termination may be achieved with increases in vagal tone, such as carotid sinus massage and the Valsalva maneuver. If these do not terminate the tachycardia, IV verapamil or adenosine may be used and are the preferred agents. Procainamide and β-blockers can be used but are second-line agents. If the etiology of the wide QRS tachycardia is unknown, IV procainamide is the drug of choice. AAVRT must first be distinguished from other wide-complex tachycardias, mainly VT. The drug of choice is procainamide. Unless the diagnosis is certain, β-blockers, calcium channel blockers, adenosine, and digoxin should be avoided to prevent an undiagnosed wide-QRS tachycardia, such as ventricular tachycardia, from degrading into ventricular fibrillation. Patients with WPW who have atrial fibrillation will have preferential conduction via the accessory pathway with these agents, and thus the agents should be avoided. See Cecil Essentials 10. |
|
Ventricular Tachycardia |
|
|
Pφ |
VT occurs within the ventricular myocardium distinct from the normal conduction system. Compared to a normal supraventricular beat, ventricular activation is slower and results in a wide QRS complex. VT is the most common wide-complex tachycardia, especially in patients with a history of cardiac disease. |
|
Symptoms are important for assessing the severity of hemodynamic compromise and may consist of chest pain, syncope, shock, seizure, and cardiac arrest. Comorbidities such as prior cardiac disease may also be a key factor in the typical presentation of VT. |
|
|
Dx |
Wide-complex tachycardias, especially in patients with coronary disease, should be treated as VT unless proven otherwise. VT presents as a wide-complex tachycardia but may also be confused as supraventricular tachycardia [SVT] with aberrancy (an SVT with slow conduction down the AV node or His-Purkinje system). Findings that suggest VT are concordance (QRS complex that is monophasic with the same polarity), AV dissociation (atrial rate slower than the ventricular rate), and the presence of fusion beats (supraventricular beat following a P wave that fuses with a complex originating in the ventricle). |
|
Tx |
In unstable patients, immediate synchronized external cardioversion should be performed. In stable patients with known or presumed VT, external cardioversion may also be used. In refractory or recurrent VT, IV amiodarone (recommended in most settings because it acts on both atrial and ventricular arrhythmias), procainamide or lidocaine may be used. See Cecil Essentials 10. |

Practice-Based Learning and Improvement: Evidence-Based Medicine
Title
A comparison of rate control and rhythm control in patients with atrial fibrillation
Authors
Van Gelder IC, Hagens VE, Bosker HA, et al.; Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study Group
Institution
AFFIRM Clinical Trial Center, Axio Research, 2601 4th Avenue, Suite 200, Seattle, Washington 98121, USA
Reference
N Engl J Med 2002;347:1834–1840
Problem
The treatment of atrial fibrillation has relied on two basic strategies: rhythm and rate control. Rhythm control offers the prospect of maintaining sinus rhythm, while rate control can be achieved with drugs that are generally less toxic. A comparison of these two strategies with respect to mortality before this trial had not been performed.
Intervention
A total of 4060 patients with atrial fibrillation were randomized to receive either rate control (with β-blockers, calcium channel blockers, digoxin, or combinations of these drugs) or rhythm control (amiodarone, sotalol, propafenone, procainamide, quinidine, flecainide, disopyramide, moricizine, dofetilide, or combinations of these drugs). Average length of follow-up was 3.5 years.
Outcome/effect
The primary end point was overall mortality. The composite secondary end points were death, disabling stroke, disabling anoxic encephalopathy, major bleeding, and cardiac arrest. The overall mortality between the two groups was not statistically significant. The rates of composite end points were also similar in both groups. The rhythm control group did have higher rates of bradycardic arrest and TdP.
Historical significance/comments
This was an important trial that showed that rhythm and rate control are associated with no difference in mortality. Since the rhythm control group had more complications and used drugs with higher potential toxicity, this trial was instrumental in physicians adopting a rate control strategy initially in most patients with atrial fibrillation.
Interpersonal and Communication Skills
Assist Patients in Making Informed Decisions
In patients diagnosed with atrial fibrillation, use of anticoagulation raises many issues of balancing the benefits and risks. The patient should understand that the reason for use of anticoagulation is to prevent stroke, but must also be made to realize that with this comes a risk of bleeding and the need for careful monitoring. These concerns should be factored into the decision to choose this therapy. Patients must be given complete information to make informed decisions about this treatment modality, and their decisions must be respected.
Professionalism
Ensure Access to Care for the Medically Vulnerable
Management of cardiac arrhythmias requires long-term follow-up with either a primary-care physician or cardiologist (or both). When patients with atrial fibrillation are started on a rate control strategy, they need to be followed to determine how effectively the medication is working and how well they are tolerating it. Furthermore, with the need for anticoagulation, patients must be closely monitored to be sure they are anticoagulated at the appropriate level (INR of 2–3 if on warfarin) and to be monitored for signs of bleeding. Access to health care and to proper follow-up is an important issue in these patients and should be a major concern for physicians.
Systems-Based Practice
Anticoagulation: Coordinating Care in the Outpatient Setting to Reduce Costs and Complications
The decision to begin anticoagulation for a patient with atrial fibrillation has important considerations at the outset of therapy. Many patients are started on anticoagulation as part of an inpatient hospital admission. Traditionally, these patients had remained in the hospital until their INRs were therapeutic, at times adding multiple days to an inpatient stay without the acuity to warrant continued hospitalization. Most hospitals now have instituted pathways to bridge patients at home until they are fully anticoagulated. These programs involve the coordinated use of subcutaneous injections of a low-molecular-weight heparin in conjunction with oral warfarin. INRs are monitored at home through a visiting nurse or home care agency. Cost and access to both the subcutaneous injections and the home care services may be a barrier for some patients. Many hospital-based programs, however, have now rectified this by providing the medication to the patients from hospital funds. The cost of care from the hospital perspective is reduced when the patient is discharged home with appropriate follow-up, and the risk of hospital-related health complications is decreased. Newer anticoagulants (such as dabigatran) may also help to reduce unnecessary length of stay for some patients by eliminating the need for continuous blood draws to assess the efficacy of oral anticoagulation.
Suggested Readings
Arnsdorf MF. Treatment of multifocal atrial tachycardia. Article in UpToDate version 17.2 last updated January 11, 2006.
Arnsdorf MF, Ganz LI. Approach to the diagnosis of narrow QRS complex tachycardias. Article in UpToDate version 17.2 last updated February 14, 2008.
Arnsdorf MF, Podrid PJ. Tachyarrhythmias associated with accessory pathways. Article in UpToDate version 17.2 last updated April 29, 2005.
Blomström-Lundqvist C, Scheinman MM, Aliot EM, et al. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients with Supraventricular Arrhythmias). J Am Coll Cardiol 2003;42:1493–1531.
Fuster V, Rydén LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients with Atrial Fibrillation). J Am Coll Cardiol 2006;48:149–246.
Podrid PJ. Overview of the acute management of tachyarrhythmias. Article in UpToDate version 17.2 last updated February 10, 2008.
Podrid PJ, Ganz LI. Approach to the diagnosis and treatment of wide QRS complex tachycardia. Article in UpToDate version 17.2 last updated March 31, 2009.

