Pain management
After reading this chapter the student or therapist will be able to:
1. Describe the pain pathways.
2. Describe how pain is modulated within the nervous system.
3. Identify the causes of acute and chronic pain.
4. List the signs and symptoms of CNS, ANS, and peripheral pain and give an example of each.
5. Perform a comprehensive pain evaluation, including taking a pain history, measuring pain intensity, measuring pain character, and examining the client.
6. Design a comprehensive pain management program that addresses the objective and subjective aspects of the pain experience.
Chronic pain is prevalent. The National Institutes of Health (NIH) estimate that 100 million Americans suffer from chronic pain.1 The prevalence of chronic noncancer pain in patients seen in the primary care setting shows an approximate range of 5% to 33%,2 and a 2006 American Pain Foundation survey found that fewer than 40% of people with chronic noncancer pain reported that their pain was under control.3 Studies of physicians, nurses, and therapists who treat individuals with chronic pain show that most do not have even a basic understanding of the concepts of pain management.4–6 The result is inadequate or inappropriate care6–8 of individuals who report having pain.
The use of the International Classification of Functioning, Disability and Health (ICF) model as a framework for understanding the relationship among impairments, activity limitations, and participation restrictions has been covered previously (refer to Chapter 1 as well as many chapters in the second section of this text). In terms of the application of the ICF model to pain, the clinician must understand that many impairments within various body systems cause pain and can limit activity and participation. The ability to identify appropriate rehabilitation approaches to improve activity and participation depends to a great extent on the clinician’s ability to identify different impairments that cause pain, or the resulting impairments and activity and participation problems caused by pain.
Defining pain
The primary purpose of pain is to protect the body. It occurs whenever there is tissue damage, and it causes the individual to react to remove the painful stimulus. Pain is also a sensation with more than one dimension. To the individual, pain is both an objective and a subjective experience. The objective dimension is the physiological tissue damage causing the pain. The subjective dimensions include the following9:
 A perceptual component: the client’s awareness of the location, quality, intensity, and duration of the pain stimulus
A perceptual component: the client’s awareness of the location, quality, intensity, and duration of the pain stimulus
 An affective component: the psychological factors surrounding the client’s pain experience, including the client’s personality and emotional state
An affective component: the psychological factors surrounding the client’s pain experience, including the client’s personality and emotional state
 A cognitive component: what the client knows and believes about the pain resulting from his or her cultural background and past pain experiences (both personal pain experiences and those of others)
A cognitive component: what the client knows and believes about the pain resulting from his or her cultural background and past pain experiences (both personal pain experiences and those of others)
 A behavioral component: how the client expresses the pain to others through communication and behavior
A behavioral component: how the client expresses the pain to others through communication and behavior
Pain anatomy
Pain arises from the stimulation of specialized peripheral free nerve endings called nociceptors. Injurious stimulation to the skin, muscle, joint, viscera, or tissue can trigger these peripheral terminals, whose cell bodies are located in the dorsal root ganglia and trigeminal ganglia. The density of nociceptors varies between as well as within these tissues. Nociceptors are extremely heterogeneous, differing in the neurotransmitters they contain, the receptors and ion channels they express, their speed of conduction, their response properties to noxious stimuli, and their capacity to be sensitized during inflammation, injury, and disease.10
Nociceptors found in interstitial tissues become excited with extreme mechanical, thermal, and chemical stimulation,11 whereas nociceptors found in vessel walls become excited with these stimuli plus marked constriction and dilation of the vessels.12 These receptors respond directly to some noxious stimuli and indirectly to others by means of one or more chemicals (histamine, potassium, bradykinin) released from cells in the traumatized tissues.13
These three types of nociceptors are broadly distributed in the skin and tissues and may work together. One example would be hitting one’s shin against a table: a sharp “first pain” is felt immediately, followed later by a more prolonged aching, sometimes burning, “second pain.”13 The fast, sharp pain is transmitted by A delta fibers that carry information from thermal and mechanical nociceptors. The slow, dull pain is transmitted by C fibers that are activated by polymodal nociceptors.
Nociceptive input travels on A delta and C fibers into the dorsal horn of the spinal cord, where the gray matter is laminated and organized by cytological features. The first-order A delta and C fibers synapse with second-order neurons in lamina I (marginal layer), II (the substantia gelatinosa [SG]), and V. The second-order neurons do one of three things. A small number synapse with motor neurons, causing reflex movements (e.g., withdrawing the hand from a hot object). Others synapse with autonomic fibers, causing responses such as changes in heart rate and blood pressure and localized vasodilation, piloerection, and sweating. Most, however, travel a multisynaptic route to the higher centers by means of the ascending tracts.11,14
There are four major classes of neurons15 responding to pain in the dorsal horn: low-threshold nociceptive-specific neurons designated class I; wide dynamic range (WDR) neurons designated class II; high-threshold nociceptive neurons designated class III; and a fourth, nonresponder group of neurons that develop spontaneous activity with exposure to endogenous inflammatory cytokines, designated class IV. Nociceptive-specific neurons are most abundant in superficial lamina; their receptor fields are discrete and vary from one to several square centimeters.16 WDR neurons, in contrast, respond to a wide range of stimuli from A delta, A beta, and C fibers in a graded manner (i.e., the rate of firing escalates with increasing intensity of stimulation), can be found in all lamina, and are the most prevalent cells in the dorsal horn.16 Because of their unique response to innocuous or nociceptive input, as well as their larger receptor field, WDR neurons play an important role in the central sensitization and the plasticity of the spinal cord.16
Nociceptive input crosses at the cord level to the anterolateral quadrant of the ascending contralateral spinothalamic tract (Figure 32-1). The axons of the anterolateral quadrant are arranged so that the sacral segments are most lateral, with the lumbar segments more medial and the cervical segments most central. This arrangement may be important clinically in that symptoms may be provoked according to dermatomal maps to some degree.17 Pain dermatomes overlap to several adjacent dorsal roots so boundaries can be less distinct, requiring the clinician to distinguish the pain and dysfunction.
The thalamus processes and relays information to several higher centers.12 Each projection serves a specific purpose. Axons of the spinothalamic tract project information to both the lateral and medial nuclear groups of the thalamus. The lateral nuclear group of the thalamus is where information about the location of an injury is thought to be mediated.13 Injury to the spinothalamic tract and the lateral nuclear group of the thalamus causes central neuropathic pain, which is discussed in further detail later.
Pain transmission
Ascending transmission of pain impulses is mediated by the action of the chemical excitatory neurotransmitter glutamate (A delta and C fibers) and tachykinins such as substance P (C fibers). Glutamate and neuropeptides have distinct actions on postsynaptic neurons, but they act together to regulate the firing properties postsynaptically.13 Tachykinins’ activity is thought to prolong the action of glutamate, as levels are increased in persistent pain conditions.16 The substrates of nociception that exist at the spinal level are complex in that more than 30 different neurotransmitters acting on more than 50 different receptors have been identified in the spinal cord and associated with some pain-related phenomenon.18 Modulation of these substrates will assist in the effectiveness of therapeutic interventions and will be discussed next.
Pain modulation
The gate control theory
The SG contains an ascending gating mechanism to block nociceptive impulses from leaving the dorsal horn of the spinal cord. The first-order neurons for both nociceptive and nonnociceptive information synapse with second-order neurons in the SG. The second-order neurons for both types of information project to specialized neurons named T cells (transmission cells) in lamina V. For pain transmission to occur, T cells must be stimulated while the SG is inhibited. The input from A delta and C fibers stimulates the T cells and inhibits the SG (Figure 32-2). Therefore A delta and C fiber input opens the gate, allowing pain transmission to the higher centers. On the other hand, when the SG and T cells are both stimulated, the T cells are inhibited and the gate is closed to pain transmission. The input from nonnociceptive A beta fibers carrying information from pressoreceptors and mechanoreceptors stimulates both the T cells and the SG. Therefore A beta fiber input closes the gate, blocking pain transmission.17
Descending pain modulation system
There are at least two descending pain modulation systems. One involves the action of neurotransmitters, including serotonin, dopamine, norepinephrine, and substance P. High concentrations of brain serotonin108 and l-dopa (a precursor of dopamine)19 have been found to inhibit nociception, whereas norepinephrine appears to enhance nociception.20–23 The spinal mediators of descending nociceptive inhibitory influences include serotonin, norepinephrine, and acetylcholine (ACh). This may be relevant to the action of antidepressants in relieving pain in the absence of depression. Substance P is thought to be the neurotransmitter for neurons transmitting chronic pain.24
The second descending modulating system is mediated by neuromodulators—chemicals capable of directly affecting pain transmission. The neuromodulators include enkephalin and β-endorphin, which are referred to as endogenous opiates because they have morphine-like actions and are found in areas of the central nervous system (CNS) that correspond to opiate-binding sites. Endogenous opiates are believed to modulate pain by inhibiting the release of substance P. They have been shown to have a profound effect on nociception and mood.25–27 Their levels in the brain and spinal cord rise in response to emotional stress, causing an increase in the pain threshold and providing a possible reason that acute stress decreases acute pain.28,29
Although serotonin is not classified as an endogenous opiate, it exerts a profound effect on analgesia and enhances analgesic drug potency. High concentrations of serotonin lead to decreased pain by inhibiting transmission of nociceptive information within the dorsal horn,30,31 whereas low concentrations result in depression, sleep disturbances, and increased pain.
The success of several therapeutic modalities, including noxious counterirritation (e.g., brief intense TENS or acupressure) and diversion (including hypnosis), is attributed to raising the level of endogenous opiates in the body.29
Categorizing pain
Acute pain is the normal predicted physiological response and serves as a warning. It alerts the individual that tissues are exposed to damaging or potentially damaging noxious stimuli. Acute pain is localized, occurs in proportion to the intensity of the stimuli, and lasts only as long as the stimuli or the tissue damage exists (1 to 6 months).32 Although acute pain is associated with anxiety and increased autonomic activity (increased muscle tone, heart rate, and blood pressure),33 it is usually relieved by interventions directed at correcting the injury. The pain experience is usually limited to the individual.34
Chronic pain is usually referred to as intractable pain if it persists for 6 or more months. It is defined as pain that continues after the stimulus has been removed or the tissue damage heals. Physiologically, chronic pain is believed to result from hypersensitization of the pain receptors and enlargement of the receptor field in response to the localized inflammation that follows tissue damage.35 Chronic pain is poorly localized, has an ill-defined time of onset, and is strongly associated with the subjective components outlined previously. It does not respond well to interventions directed solely at correcting the injury. Chronic pain patients frequently report other symptoms, such as depression, difficulty sleeping, poor mental and physical function, and fatigue. The effects of the pain experience extend beyond the individual and affect the family, the workplace, and the social sphere of the individual.34
Referred pain is felt at a point other than its origin. Pain can be referred from an internal organ, a joint, a trigger point, or a peripheral nerve to a remote musculoskeletal structure. Referred pain usually follows a specific pattern. For example, cardiac pain is frequently referred to the left arm or jaw; the referral pattern for trigger points is exact enough to be used as a diagnostic tool and is often used by physicians to diagnose pathology. Referred pain is the result of a convergence of the primary afferent neurons from deep structures and muscles to secondary neurons that also have a cutaneous receptive field.36,37
Although it is now recognized that all neuropathic pain results in abnormal activity within the CNS,38 pain initiated or caused by a primary lesion or dysfunction of the CNS39 is referred to as central neuropathic pain. The involvement of the nervous system can be at many levels: nerves, nerve roots, and central pain pathways in the spinal cord and brain. In this circumstance, there is permanent damage to the nervous system (usually a peripheral nerve) and likely anatomical reorganization of spinal terminations of surviving axons or ectopic activity from a neuroma that contributes paroxysmal, persistent input to the spinal cord.40 In addition to anatomical reorganization in the spinal cord, there could be some reorganization in the rostroventral medulla (RVM) as well, but more likely there is prolonged input to the RVM that sustains facilitatory influences that descend to the spinal cord. Less appreciated, descending facilitatory influences on spinal sensory processing could also be important to maintenance of chronic pain conditions, particularly those that persist in the absence of obvious tissue pathology.40
The onset of central neuropathic pain is usually delayed after the occurrence of the initial episode that results in damage to the CNS; onset of pain may occur during the phase of recovery from neurological deficits.41 Pain originating from a cerebrovascular incident and spinal cord injury usually begins weeks or months after the insult, whereas pain originating from tumors may take years to begin.38
Individuals with central neuropathic pain may have difficulty describing their pain and report burning, aching, pricking, squeezing, or cutting pain after cutaneous stimulation, movement, heat, cold, or vibration. A normally nonnoxious stimulus, such as moving clothing across skin, becomes agonizing. In some cases the pain begins spontaneously.42 Pain intensity varies, but it does seem to be associated to some degree with the location of the lesion.38 Allodynia (pain from normally nonnoxious stimuli) and dysesthesia are common, and one of the characteristic features of central neuropathic pain is that the clinical symptoms persist long after the stimulus has been removed.
Central neuropathic pain is topographical. The site of the lesion determines the location of the symptoms. The pain may involve half the body, an entire extremity, or a small portion of one extremity.38 It is frequently migratory. Thalamic pain is the classic example of central neuropathic pain.
Central neuropathic pain is difficult to treat. Surgery is not helpful for most individuals with central neuropathic pain, and medications have not been effective in permanently relieving the symptoms.11 Therefore the treatment of clients with central neuropathic pain stresses coping strategies and prevention of loss of activity and participation. The ideal management of a chronic pain patient is by a multidisciplinary approach, including disciplines such as internal medicine, neurology, anesthesia, nursing, psychology, pharmacy, rehabilitation medicine, physical therapy, occupational therapy, and others. The limitation of this approach is that access to such a wide range of specialists is often available only at large medical centers and special pain clinics, which restricts access to a limited number of patients.
Allodynia is a product of the phenomenon of central sensitization.43 After injury, new axons sprout from the sympathetic efferent neurons. These fire spontaneously and, because they synapse on the cell bodies of the primary afferent neurons, cause them to fire as well. In addition, the dorsal horn neurons themselves become more excitable. They show an enlargement in their receptive field and become more sensitive to mechanical, thermal, and chemical stimulation. The result is an increase in the neuronal barrage into the CNS and the perception of pain with usually nonpainful stimuli.13
Complex regional pain syndrome (CRPS) is an example of pain that arises from abnormal activity within the ANS.44 CRPS has been classified into two distinct types39: CRPS type I (formerly reflex sympathetic dystrophy) follows mild trauma without nerve injury, and CRPS type II (formerly causalgia) follows trauma with nerve injury. CRPS type I generally begins within the month after the injury, whereas CRPS type II can occur any time after the injury.45
The main features of CRPS type I are constant burning pain that fluctuates in intensity and increases with movement, constant stimulation, or stress. There are also allodynia and hyperalgesia, edema, abnormal sweating, abnormal blood flow and trophic changes in the area of pain, and impaired motor function. CRPS type I is relieved by blocking the SNS, indicating that the pain is sympathetically maintained.45
CRPS type II occurs in the region of a limb innervated by an injured nerve. The nerves most commonly involved in CRPS type II are the median, sciatic, tibial, and ulnar; involvement of the radial nerve is rare. Pain is described as spontaneous, constant, and burning and is exacerbated by light touch, stress, temperature change, movement, visual and auditory stimuli, and emotional disturbances. Allodynia and hyperalgesia are common and may involve the distribution of more than one peripheral nerve. As with CRPS type I, edema, abnormal sweating, abnormal blood flow, trophic changes, and impaired motor function occur. The symptoms spread proximally and can involve other areas of the body. Evidence also points to sympathetic involvement in CRPS type II.45
The treatment of CRPS is complex and must be carefully coordinated among members of an interdisciplinary team including the neurologist (medications), psychologist (behavior), anesthesiologist (injections), and therapist (functional recovery). The therapist provides the core treatment to improve function. Therapists need to pay close attention to the following aspects of the disorder: (1) the degree of motor abnormalities, including restricted active range of motion (ROM), abnormal posturing, spasm, tremor, and dystonia; (2) true passive range restriction; (3) hyperesthesia and allodynia; (4) swelling and vasomotor changes; and (5) evidence of osteoporosis by radiograph.46 Please refer to Case Study 32-1 for interventions for clients with CRPS.
Peripheral pain results from noxious irritation of the nociceptors. The character of peripheral pain depends on the location and intensity of the noxious stimulation, as well as which fibers carry the information into the dorsal gray matter. As noted previously, information carried on A delta fibers is sharp and well localized, begins rapidly, and lasts only as long as the stimulus is present, whereas information carried on C fibers is dull and diffuse, has a delayed onset, and lasts longer than the duration of the stimulus. The treatment of peripheral pain is covered in detail in Chapter 18.
The management of central versus peripheral pain is determined by the type of pain—acute or chronic—and the clinical features present, including clinical localization; time of onset; laboratory study localization; response to analgesics, including narcotics; response to antidepressants; and response to nerve block or neurectomy.41 Differentiation among features will drive the treatment plan, but because some peripheral and central forms can coexist, diagnosis may be difficult.
The multidimensional aspects of chronic pain make it important to evaluate the causes as well as the emotional and cognitive sequelae.47 Persistent pain is now considered to have a psychogenic component.48 The longer an individual has pain, the more a psychological component may become dominant. Many emotional factors can strongly influence pain, such as pain thresholds, past experiences with pain, coping styles, and social roles. The emotional experience that we perceive with pain reflects the interaction of higher brain centers and subcortical regions, such as the amygdala and cingulate gyrus (limbic system).49 Positron emission tomography of patients with chronic neuropathic pain demonstrates a shift of acute pain activity in the sensory cortex to regions such as the anterior cingulate gyrus.50 Understanding the physical limitations imposed by chronic pain is an area that therapists commonly assess; it is the mind-body connection that is often less articulated by the client and more difficult for the practitioner.
Treatment of chronic pain should include a patient-centered approach, given the unique manifestations that occur in an individual’s response to pain. Patient-centered models, such as the ICF model, provide a framework that embraces a multidisciplinary team approach practiced in pain clinics. In such models, chronic pain has been noted to include psychological factors such as feelings of fear, anxiety, and depression,51 which are known to have the ability to modulate and exacerbate the physical pain experience.52 For example, a client with chronic pain who has the fear that movement will increase pain may alter his activity, causing muscular shortening, spasms, and a spiraling course of more pain and disability. The focus in treating clients with chronic pain should be on improving functional physical activity, decreasing peripheral nociception and central facilitation, and providing cognitive and behavioral strategies to help in resuming normal activities.
Examination of the client with pain
Pain history
 Observation: Observation of the client from the moment of entry until (and sometimes beyond) the moment of exit from the clinic. By observing the client outside of the evaluation, the therapist is able to assess the client’s movement. The patient’s nervous system will accurately express itself to the therapist, especially when the patient is asked to focus attention on a topic other than pain and the patient is not aware that movement is being observed.
Observation: Observation of the client from the moment of entry until (and sometimes beyond) the moment of exit from the clinic. By observing the client outside of the evaluation, the therapist is able to assess the client’s movement. The patient’s nervous system will accurately express itself to the therapist, especially when the patient is asked to focus attention on a topic other than pain and the patient is not aware that movement is being observed.
 Origin and onset: Date and circumstances of the onset of pain. How did the pain start? Gradually or suddenly? Was there a precipitating injury? If so, what was the mechanism of injury? If not, can the client correlate the onset to a particular activity or posture?
Origin and onset: Date and circumstances of the onset of pain. How did the pain start? Gradually or suddenly? Was there a precipitating injury? If so, what was the mechanism of injury? If not, can the client correlate the onset to a particular activity or posture?
 Position: Location of the pain. Have the client demonstrate where the pain is located rather than relying on description alone. In addition to being more accurate, demonstration allows another observation of the client’s ability and willingness to move. Clients can also be asked to draw their symptoms on a schematic, such as the pain drawing, which is described later.
Position: Location of the pain. Have the client demonstrate where the pain is located rather than relying on description alone. In addition to being more accurate, demonstration allows another observation of the client’s ability and willingness to move. Clients can also be asked to draw their symptoms on a schematic, such as the pain drawing, which is described later.
 Pattern: Pattern of the pain. Is the pain constant or periodic? Does it travel or radiate? Which activities and postures increase or decrease the pain? Does medication or time of day have any effect on the pain? Have there been any recent changes in the pattern? Does the client believe that the pain is improving, worsening, or remaining the same?
Pattern: Pattern of the pain. Is the pain constant or periodic? Does it travel or radiate? Which activities and postures increase or decrease the pain? Does medication or time of day have any effect on the pain? Have there been any recent changes in the pattern? Does the client believe that the pain is improving, worsening, or remaining the same?
 Quality: Characteristics of the pain. Does the client use adjectives indicating mechanical (pressing, bursting, stabbing), chemical (burning), neural (numb, “pins and needles”), or vascular (throbbing) origin? Two tools for describing pain character are described later.
Quality: Characteristics of the pain. Does the client use adjectives indicating mechanical (pressing, bursting, stabbing), chemical (burning), neural (numb, “pins and needles”), or vascular (throbbing) origin? Two tools for describing pain character are described later.
 Quantity: Intensity of the pain. How has the pain intensity changed since the onset? Several methods that allow for monitoring change in pain intensity are presented later.
Quantity: Intensity of the pain. How has the pain intensity changed since the onset? Several methods that allow for monitoring change in pain intensity are presented later.
 Radiation: Characteristics of pain radiation. Does the pain radiate? What causes the pain to radiate? Can the radiation be reversed? How?
Radiation: Characteristics of pain radiation. Does the pain radiate? What causes the pain to radiate? Can the radiation be reversed? How?
 Signs and symptoms: Functional and psychological components of the pain. Has the pain resulted in any functional limitations? Has it caused any changes in the client’s ability to participate in life, including employment and recreational activities? Does the client’s personality contribute to the pain, or has the pain caused changes in the client’s emotional stability? Does the client benefit from the pain? How? It may be necessary to interview the client’s significant others or family members for an accurate picture.
Signs and symptoms: Functional and psychological components of the pain. Has the pain resulted in any functional limitations? Has it caused any changes in the client’s ability to participate in life, including employment and recreational activities? Does the client’s personality contribute to the pain, or has the pain caused changes in the client’s emotional stability? Does the client benefit from the pain? How? It may be necessary to interview the client’s significant others or family members for an accurate picture.
 Treatment: Previous and current medical and therapeutic treatment and its effectiveness, including medications, home remedies, and recommendations for movement activities. It is also important to determine the client’s attitude and expectations concerning therapy in addition to obtaining a treatment history.
Treatment: Previous and current medical and therapeutic treatment and its effectiveness, including medications, home remedies, and recommendations for movement activities. It is also important to determine the client’s attitude and expectations concerning therapy in addition to obtaining a treatment history.
 Visceral symptoms: Physical symptoms of visceral origin that can accompany and be responsible for the pain (Box 32-1). Visceral causes of pain require referral to the client’s physician for further investigation before the initiation of treatment by a therapist.
Visceral symptoms: Physical symptoms of visceral origin that can accompany and be responsible for the pain (Box 32-1). Visceral causes of pain require referral to the client’s physician for further investigation before the initiation of treatment by a therapist.
Pain measurement
Research has shown that pain memory does not provide an accurate measure of pain intensity.53 Therefore pain measurement tools are designed to provide information about the intensity, location, and character of a client’s symptoms at the time of the evaluation. This information can then be merged with the pain history, the disease or pathology history, and the physical findings to identify the cause of the pain. The disease or pathology management and its pain measurement will be the responsibility of the physician, whereas the movement limitations caused by the pain are the responsibility of the therapist. A number of pain measurement tools are available. These tools are used by professionals whose focus is pathology, as well as professionals whose responsibility is helping the patient to regain functional activities and life participation. The applications and limitations of several are discussed.
Measuring pain intensity
Pain intensity rating tools are scales that have the client rate the current level of pain by marking a continuum or assigning a numerical value to the pain intensity (Figure 32-3).
Each of the first three tools described here has been found to be reliable over time when used to measure pain that is present at the time of the rating. In general, however, clients who are depressed or anxious tend to report higher levels of pain and clients who are not depressed or anxious tend to report lower levels of pain on all three of these scales.54
Visual analog scale.
With the visual analog scale (VAS), the client rates the pain on a continuum that begins with “no pain” and ends with “maximum pain tolerable.” This tool provides an infinite number of points between the extremes, making it sensitive to small changes in pain intensity. However, it has not been found reliable for individuals who have impaired abstract thinking skills55 and may be unable to translate their pain intensity into a corresponding point on a line.
Simple descriptive pain scale.
With a simple descriptive pain scale (SDPS), the client rates the pain on a continuum that is subdivided using descriptors that gradually increase in intensity. Sample descriptors are “no pain,” “mild pain,” “moderate pain,” “severe pain,” and “maximum pain tolerable.” This tool is more useful than the VAS for clients with impaired abstract thinking because it is easier for them to identify with the pain descriptors than with the line found in the VAS. However, clients have been found to favor the points corresponding to each descriptor rather than points between, resulting in a less sensitive tool than the VAS.56
Faces pain scale.
With the Faces Pain Scale, the client selects one of seven schematic faces representing gradually increasing pain intensities. The scale begins with a face representing no pain and ends with a face representing the most pain possible. This tool is designed for use with young children who do not have the ability to use any of the three previous tools. The Faces Pain Scale has been found to be valid across cultural lines57 and to have a strong correlation with other pain measures.56 It is simple to use, does not require verbal skills, and requires little instruction. It has been used successfully with children as young as age 3 and with individuals who are limited in verbal expression.
Localizing pain symptoms
Pain drawings.
The client is asked to draw his or her symptoms on a schematic of the human body using a provided list of symbols (Figure 32-4). The result is a diagram describing the nature and location of the client’s pain, which can be compared with the client’s verbal report. In addition to providing a database, the pain drawing has been found to be useful in identifying individuals who have a heavy psychological or emotional component to their pain, making it helpful also in identifying clients who would benefit from further psychological evaluation.58
Describing pain quality
Mcgill pain questionnaire (mpq).
One of the most popular scales to rate pain quality is the McGill Pain Questionnaire (MPQ), which includes 20 categories of descriptive words covering the sensory (numbers 1 to 10), affective (numbers 11 to 15), and evaluative (number 16) properties of pain (Figure 32-5). Sensory properties are measured using temporal, thermal, spatial, and pressure descriptors. Affective properties are measured using fear, tension, and autonomic descriptors. Evaluative properties are measured using pain experience descriptors.59 Each word has a numerical value based on its position within its category.
The client is instructed to “select the word in each category that best describes the pain you have now. If there is no word in the category that describes the pain, skip the category. If there is more than one word that describes the pain, select the word that best describes the pain.”59
The MPQ can provide the following types of information59:
 A pain-rating index based on the sum of the values of all the words selected
A pain-rating index based on the sum of the values of all the words selected
 A pain-rating index based on the sum of the values of all the words in a given category
A pain-rating index based on the sum of the values of all the words in a given category
The MPQ has been studied extensively and found valid for adults with acute and chronic pain as well as for those with a variety of specific pathological states.60–62 It provides clues into the specific cause of pain because it describes the client’s symptoms.
However, the MPQ does pose some disadvantages. It is time-consuming, requiring more time to complete than any of the previously described rating scales. Thus it is not appropriate for quick estimates of pain after treatment. Clients, especially children, are frequently unfamiliar with some of the descriptors and ask the evaluator to assist by defining words. However, reliability and validity of this test are based on examiner objectivity, and care must be taken to avoid the introduction of evaluator bias by helping the client to select appropriate descriptors.59 This issue can be dealt with by telling the client, “If you do not recognize a word, it probably does not apply to you.”
Pediatric verbal descriptor scale.
Because a child’s description of pain is limited by a smaller vocabulary, Wilkie and associates63 have developed a verbal descriptor scale specifically for use with children (Table 32-1). Their list includes 56 words commonly used by children aged 8 to 17 to describe their pain experience. The word list is divided into the four categories found in the MPQ. The evaluators’ research has shown the list to be useful for children with a variety of diagnoses because it is relatively free of gender, ethnic, and developmental bias.
TABLE 32-1 
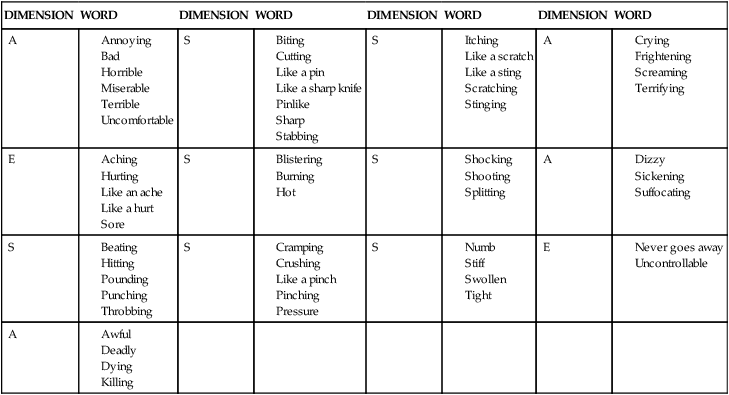
S, Sensory; A, affective; E, evaluative.
Reprinted from Wilkie DJ, Holzemer WL, Tesler MD, et al: Measuring pain quality: validity and reliability of children’s and adolescents’ pain language. Pain 41:151–159, 1990, with permission from Elsevier Science.
Caregiver checklist.
Clients who are unable to communicate verbally because of neurological disabilities may be unable to use any of the just-described pain measurement scales. However, because of pain associated with their medical conditions, extensive and repeated surgery, and behavioral oddities that might limit pain expression, these individuals are at high risk for having their pain go unrecognized. McGrath and colleagues64 have attempted to develop and categorize a checklist of demonstrated pain behaviors identified by caregivers of severely handicapped individuals. Although their list did not pass validity criteria, the researchers propose that clinicians develop a client-specific checklist that could be used to gauge changes in the client’s pain from the information gained during the caregiver interview portion of the evaluation of nonverbal handicapped clients.
Psychosocial assessment
Psychosocial factors are key variables in the comprehensive assessment of chronic pain. Davidson and colleagues65 determined seven factors using a “prototypical” pain assessment battery that included pain and disability, pain description, affective distress, support, positive coping strategies, negative coping strategies, and activity. Irving and Squire66 described two key individual personality features that affect pain: catastrophizing and health-related anxiety. Persons who catastrophically misinterpret innocuous bodily sensations, including pain, are likely to become fearful of pain, which results in pain-related fear. Pain-related fear is associated with avoidance of movement and physical activity, which directly affects recovery of function. Pain-related fear is also associated with increased pain levels and an exacerbated painful experience. Two validated tools to screen for these features include the Pain Catastrophizing Scale (PCS) and the Tampa Scale of Kinesophobia (TKS).67
Examination of the client
 Observation of gait and movement patterns, including the use of assistive devices.
Observation of gait and movement patterns, including the use of assistive devices.
 Notation of body type and anomalies.
Notation of body type and anomalies.
 Examination of sitting and standing posture, including both the normal posture and that assumed because of the pain. (Observe the client during activities, if possible, to differentiate movement patterns altered by intent versus automatic adjustments.)
Examination of sitting and standing posture, including both the normal posture and that assumed because of the pain. (Observe the client during activities, if possible, to differentiate movement patterns altered by intent versus automatic adjustments.)
 Inspection of the skin for pliability, trophic changes, scar tissue, and other abnormalities.
Inspection of the skin for pliability, trophic changes, scar tissue, and other abnormalities.
 Palpation of the soft tissue structures to identify changes in temperature, swelling, tenderness, and areas of discomfort.
Palpation of the soft tissue structures to identify changes in temperature, swelling, tenderness, and areas of discomfort.
 Palpation of the anatomical structures to determine end feel—the sensation felt at the end of the available movement.68
Palpation of the anatomical structures to determine end feel—the sensation felt at the end of the available movement.68
 Bone to bone: hard normal, for example, at the end range of elbow extension.
Bone to bone: hard normal, for example, at the end range of elbow extension.
 Spasm: muscular resistance abnormal.
Spasm: muscular resistance abnormal.
 Capsular feel: rubbery normal at the extreme of full ROM; abnormal when encountered before the end of ROM.
Capsular feel: rubbery normal at the extreme of full ROM; abnormal when encountered before the end of ROM.
 Springy block: rebound abnormal.
Springy block: rebound abnormal.
 Tissue approximation: soft tissue normal at the extremes of full passive flexion.
Tissue approximation: soft tissue normal at the extremes of full passive flexion.
 Empty feel: no physiological resistance, but client resists movement because of pain.
Empty feel: no physiological resistance, but client resists movement because of pain.
 Examination of ROM: active ROM testing is performed to assess the client’s willingness to move and to identify any limitations or painful areas; passive ROM testing is used to further refine the observations.68
Examination of ROM: active ROM testing is performed to assess the client’s willingness to move and to identify any limitations or painful areas; passive ROM testing is used to further refine the observations.68
 When active and passive movements are painful and restricted in the same direction and the pain appears at the limit of motion, the problem is most likely arthrogenic.
When active and passive movements are painful and restricted in the same direction and the pain appears at the limit of motion, the problem is most likely arthrogenic.
 When active and passive movements are painful or restricted in opposite directions, the problem is most likely muscular.
When active and passive movements are painful or restricted in opposite directions, the problem is most likely muscular.
 When there is relative restriction of passive movement in the capsular pattern, the problem may be arthritic in nature.
When there is relative restriction of passive movement in the capsular pattern, the problem may be arthritic in nature.
 When there is no restriction of passive movement but the client cannot perform the movement actively, the muscle may not be functioning, either from intrinsic problems within the muscle or interruption in the neural pathway (central or peripheral).
When there is no restriction of passive movement but the client cannot perform the movement actively, the muscle may not be functioning, either from intrinsic problems within the muscle or interruption in the neural pathway (central or peripheral).
 Examination of muscle strength68:
Examination of muscle strength68:
 When the movement is strong and painful, there is a minor lesion in the muscle or tendon.
When the movement is strong and painful, there is a minor lesion in the muscle or tendon.
 When the movement is weak and increases the pain, there is a major lesion that needs to be identified with further testing.
When the movement is weak and increases the pain, there is a major lesion that needs to be identified with further testing.
 When the movement is weak but does not increase the pain, there is the possibility of either complete rupture of the muscle or tendon or a neurological disorder.
When the movement is weak but does not increase the pain, there is the possibility of either complete rupture of the muscle or tendon or a neurological disorder.
 When all resisted movements are painful, the pain may be organic or the patient may be emotionally hypersensitive.
When all resisted movements are painful, the pain may be organic or the patient may be emotionally hypersensitive.
 When movement is strong and painless, the test results are normal.
When movement is strong and painless, the test results are normal.
 Assessment of bilateral neurological function:
Assessment of bilateral neurological function:
 Reflexes: peripheral lesions tend to diminish deep tendon reflexes (DTRs). CNS lesions tend to intensify DTRs, and testing frequently elicits a clonic reaction.69 Note any asymmetries in response.
Reflexes: peripheral lesions tend to diminish deep tendon reflexes (DTRs). CNS lesions tend to intensify DTRs, and testing frequently elicits a clonic reaction.69 Note any asymmetries in response.
 Sensation: test light touch, sharp (noxious) touch, and vibration. Pressure on a nerve usually affects conduction on the large, myelinated fibers first. Therefore vibration is the first sensation to be diminished. Where there is decreased perception of touch and noxious stimuli, the lesion is more severe.70 Note any asymmetries in response.
Sensation: test light touch, sharp (noxious) touch, and vibration. Pressure on a nerve usually affects conduction on the large, myelinated fibers first. Therefore vibration is the first sensation to be diminished. Where there is decreased perception of touch and noxious stimuli, the lesion is more severe.70 Note any asymmetries in response.
 Allodynia and hyperalgesia: delineate areas of allodynia and hyperalgesia to touch, hot, and cold. Exact descriptions of these areas, along with areas of decreased perception of vibration, will provide information concerning A beta versus C fiber involvement in the production of pain.70
Allodynia and hyperalgesia: delineate areas of allodynia and hyperalgesia to touch, hot, and cold. Exact descriptions of these areas, along with areas of decreased perception of vibration, will provide information concerning A beta versus C fiber involvement in the production of pain.70
Rehabilitation management of the client with pain
There are three broad avenues of intervention for pain management: physical interventions, cognitive strategies, and behavioral manipulations.71 Each avenue addresses a different aspect of the pain experience, and each requires a different level of participation from the client.
Physical interventions
Thermotherapy
The physiological effects of heat depend on the method of application, the depth of penetration, and the rate and magnitude of temperature change. In terms of the use of thermotherapy to control pain, several mechanisms have been established. Muscle spasm decreases as a result of decreased activity in gamma motor efferents, decreased excitability of muscle spindles, and increased activity of Golgi tendon organs.72,73 This modality will often decrease peripheral pain. Ischemic pain is relieved by the influx of oxygen-rich blood into the dilated vessels, and muscle tension pain is decreased by interruption of the pain-spasm cycle. In addition, the pain threshold itself rises through gating at the spinal cord level.74,75
Several textbooks76–80 on physical agents and rehabilitation discuss the physiological effects, precautions, contraindications, and method of application for these modalities. The reader is advised to refer to the textbooks for details.
Superficial heat can be applied by conduction, convection, or radiation. Conductive heating involves the exchange of heat down a temperature gradient by two objects that are in contact. The depth of penetration with conductive heating is usually 1 cm or less.81 Moist heat packs and paraffin are examples of therapeutic conductive heating. Convective heating involves heat transfer through the flow of hot fluid. Therapeutic convective heating takes place during hydrotherapy and Fluidotherapy (Encore Medical Corporation, Austin, Texas). Molecules with a temperature greater than absolute zero are in an excited state and emit energy, thus creating radiant heat. Objects that are warmed by the energy are heated by radiation. Therapeutic radiant heat is applied with infrared or ultraviolet light. Because of the contraindications of ultraviolet light, this type of radiant heat is seldom used by therapists today in rehabilitation settings.
During shortwave diathermy, the client is placed into an oscillating magnetic field. The systemic ions create friction as they attempt to line up with the continuously reversing current, resulting in an increase in tissue temperature deep within the body. Shortwave diathermy is contraindicated for clients with metal implants because of the potential for the implant to become hot and burn the surrounding tissues. It is also contraindicated for clients with cardiac pacemakers because of the pacemaker’s metal components and because the electromagnetic radiation may interfere with the pacemaker’s operation. Shortwave diathermy should not be used for clients with cancer or multiple sclerosis or who are pregnant, and it should not be used over the eyes, the reproductive organs, or growing epiphyses. Female therapists should avoid prolonged exposure to shortwave diathermy because some research has demonstrated a possible negative effect on pregnancy outcome and fetal development.82
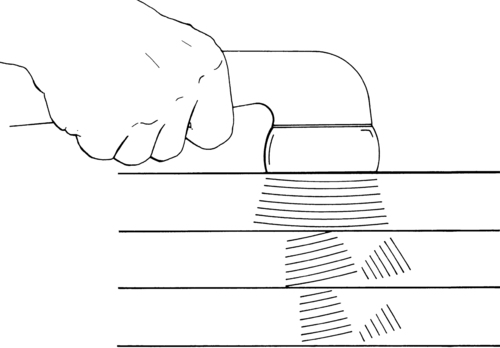
Ultrasound is another modality that heats deep tissues by conversion. As its name implies, ultrasound consists of sound waves delivered at a frequency too high to be perceived by human hearing. Sound waves are repeatedly refracted as they encounter tissues of differing acoustical resistance while traveling through the skin toward the bone (Figure 32-6). Tissues with high collagen content (tendon, ligament, fascia, and joint capsule) are heated more efficiently than tissues with low collagen content (fat, muscle). The extent of the temperature increase is related to the dose of ultrasound energy delivered. As the dose of ultrasound energy is increased by increasing the treatment duration or intensity, more energy is available to the tissues and the heating effect increases.21 Moreover, the higher the frequency of ultrasound delivered, the more superficial the effect. Ultrasound delivered at 1 MHz heats tissues at depths to 5 cm, whereas ultrasound delivered at 3 MHz heats tissues in the upper 2 cm.83
The thermal effects of ultrasound can be used to increase tissue extensibility, cellular metabolic processes, and circulation; decrease pain and muscle spasm; and change nerve conduction velocity. The number of impulses traveling along the nerve decreases at low doses but begins to rise slowly beginning at 1.9 W/cm2. Sounding of C fibers yields pain relief distal to the point of application, whereas sounding of large-diameter A fibers brings relief of spasm by changing gamma fiber activity, making the muscle fibers less sensitive to stretch.84 Because it is impossible to treat C or A fibers selectively, ultrasound provides both pain relief and relief from muscle spasm, making it effective in the treatment of peripheral neuropathies, neuroma, and muscle spasm associated with musculoskeletal pathology, including sprains, strains, and contusions.85
In addition to thermal effects, ultrasound has nonthermal effects that come from the mechanical effects of the ultrasound wave on the tissues. Ultrasound causes cavitation, the development and growth of gas-filled bubbles, in the tissues. Ultrasound also causes tissue fluid to move or stream. The movement of fluid around the gas bubbles formed by cavitation is called microstreaming, and the movement of fluid within the ultrasound delivery area is called acoustic streaming. The nonthermal effects of ultrasound include accelerating metabolic processes, enzyme activity, and the rate of ion exchange, as well as increasing cell membrane permeability and the rate and volume of diffusion across cell membranes. These effects are thought to explain the role of ultrasound in enhancing the healing of soft tissue and bone.86–88 The nonthermal effects of ultrasound can be achieved without raising tissue temperature by applying ultrasound in the pulsed mode.
Phonophoresis is the use of ultrasound to deliver pain-relieving chemicals to the tissues. Chemicals are delivered to the cells by the ultrasound wave, where they are broken down into ions and taken up into the cells (Figure 32-7). Common pain-relieving chemicals that can be administered with phonophoresis include 5% lidocaine ointment (Xylocaine) for acute conditions in which immediate pain relief is the primary goal, and 10% hydrocortisone cream or ointment for conditions in which pain is the result of inflammation.89
After phonophoresis, measurable quantities of these molecules have been found at tissue depths of up to 2 inches.90 The contraindication to use of any chemical during phonophoresis is having an allergy to that chemical. Clients should be questioned about any adverse reactions to dental local anesthesia (lidocaine) or aspirin.
Cryotherapy
The physiological effects of cold make it superior to heat for acute pain from inflammatory conditions, for the period immediately after tissue trauma, and for treating muscle spasm and abnormal tone. Peripheral nerve conduction velocity in both large myelinated and small unmyelinated fibers decreases 2.4 m per degree centigrade of cooling. As a result, pain perception and muscle contractility diminish.91 Peripheral receptors become less excitable.91 Muscle spindle responsiveness to stretch decreases; as a result, muscle spasm diminishes.84
Local blood flow initially decreases, local edema decreases, the inflammatory response decreases, and hemorrhage is minimized. However, cold application for longer than 15 minutes results in increased local blood flow. Known as the “hunting response,” this protective mechanism brings core temperature blood to the surface and prevents tissue injury resulting from prolonged cooling.81 Cellular metabolic activities slow. The oxygen requirements of the cell decrease.91
As with heat, several precautions must be taken when using cold as a therapeutic modality. Cryotherapy is contraindicated in individuals with Raynaud phenomenon or cold allergy. Cryotherapy should not be used in individuals with rheumatic disease who, with the application of cold, have increased joint pain and stiffness. Cryotherapy should be used with caution in young, frail, or elderly individuals and those with peripheral vascular disease, circulatory pathological processes, or sensory loss.92
Because muscles, tendons, and joints respond differently, the best method of cold application depends on which tissues are causing the pain.93 Acute injuries are best treated with cryotherapy along with rest, compression, and elevation (RICE). Muscle spasm is decreased with cold packs and stretching. Trigger points, irritable foci within muscles, are best treated with vapocoolant spray, deep friction massage, and stretching. Tendinitis responds well to ice massage and exercise. Cold packs are often the only source of pain relief in acute disc pathology. The inflamed joints of rheumatoid arthritis frequently respond to cold packs or ice massage with decreased inflammation, increased function, and long-lasting pain relief.92,94
Transcutaneous electrical nerve stimulation
TENS is the use of electricity to control the perception of pain. It appears that at a high rate TENS selectively stimulates the low-threshold, large-diameter A beta fibers, resulting in presynaptic inhibition within the dorsal horns,95 either directly through the gating mechanism or indirectly through stimulation of the tonic descending pain-inhibiting pathways.96 Research has shown that the neurons in the brain stem fire in synchrony with the TENS stimulation frequency,97 and although the significance of this is not known at this time, it does indicate that the action of high-rate TENS is not limited to the dorsal columns. TENS delivered at a low rate is thought to facilitate elevation of the level of endogenous opiates in the CNS.98
Stimulation frequencies of 1 to 250 pulses per second (pps) decrease pain. Frequencies of 50 to 100 pps have proven most effective for sensory-level (high-rate) TENS, and frequencies of 2 to 3 pps are most effective for motor level (low-rate) TENS.31 Stimulation at exactly 2 pps causes an increase in the pain threshold.53 As the frequency is decreased, more time is needed before the onset of relief, but the effects are more long-lasting.99 Pulse width duration determines which nerves are stimulated. Sensory nerves are stimulated at widths of 20 to 100 ms, and motor nerves at 100 to 600 ms.100
When TENS impulses are generated at a high rate (greater than or equal to 50 pps) with a relatively short duration, the stimulation is referred to as sensory-level or conventional or high-rate TENS. Sensory-level TENS produces mild to moderate paresthesia without muscle contraction throughout the treatment area. Sensory-level TENS is thought to control pain through the gating mechanism in the spinal cord. The onset of relief is fast (seconds to 15 minutes)31 because the gate is closed at the onset of stimulation. The duration of relief after stimulation stops is short-lived (at best up to a few hours). Sensory-level TENS has been found to be beneficial for acute pain syndromes and for some deep, aching chronic pain syndromes. (Refer to Chapter 33.)
Stimulation using high-rate and long-duration impulses is called brief-intense TENS. Brief-intense TENS decreases the conduction velocity of A delta and C fibers, producing a peripheral blockade to transmission.31 Brief-intense TENS is useful in the clinical setting for short-term anesthesia during wound debridement, suture removal, friction massage, joint mobilization, or other painful procedures.
When the impulses are generated at a low rate (less than or equal to 20 pps) and have a relatively long duration (100 to 300 microseconds), the stimulation is referred to as motor-level or acupuncture-like or low-rate TENS. Motor-level TENS produces strong muscle contractions in the treatment area with or without the perception of paresthesia. Motor-level TENS is associated with deployment of endogenous opiates within the CNS. The onset of relief is delayed 20 to 30 minutes, presumably the time it takes to deploy the opiates. Relief frequently lasts hours or days after treatment. Because motor nerves are not stimulated in isolation, sensory fibers are also excited, causing the gating mechanism to come into play.100 Motor-level TENS has been found to be beneficial for chronic pain syndromes and when sensory-level TENS has not been successful.
Stimulation in which the impulses are generated in pulse trains is called burst TENS. Burst TENS is another form of TENS modulation. The stimulator generates low-rate carrier impulses, each of which contains a series of high-rate pulses. Because burst TENS is a combination of high-rate and low-rate TENS, it provides the benefits of each. The low-rate carrier impulse stimulates endorphin release, and the high-rate pulse trains provide an overlay of paresthesia. The advantage to burst TENS is that muscle contractions occur at a lower, more comfortable amplitude, and accommodation does not occur. Burst TENS is beneficial whenever motor-level TENS cannot be tolerated and sensory-level TENS is ineffective because of neural accommodation.101
TENS appears to be of greatest benefit for acute conditions with focal pain, chronic pain syndromes, postoperative incision pain, and during delivery. It has been found least effective with psychogenic pain102 and pain of central origin.103 For additional information on TENS, see Chapter 33.
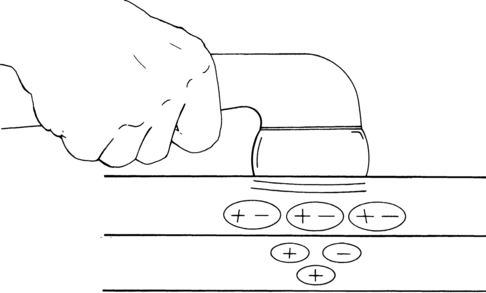
Iontophoresis
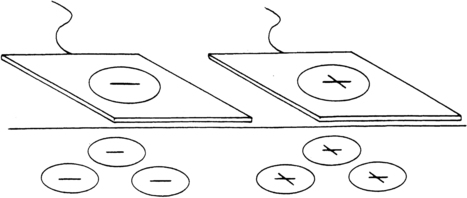
Iontophoresis is a process in which chemical ions are driven through the skin by a small electrical current. Ionizable compounds are placed on the skin under an electrode that, when polarized by a direct (galvanic) current, repels the ion of like charge into the tissues. Once subcutaneous, the ions are free to combine with the physiological ions, resulting in a physiological effect dependent on the characteristics of the ion (Figure 32-8). Ionizable substances that are known to be effective analgesics include the following104:
 Five-percent lidocaine ointment (Xylocaine) administered under the positive electrode for an immediate, although short-lived, decrease in pain. Iontophoresis with lidocaine is recommended before ROM exercises, stretching, and joint mobilization and when immediate relief of acute pain (as in bursitis) is the object of treatment.
Five-percent lidocaine ointment (Xylocaine) administered under the positive electrode for an immediate, although short-lived, decrease in pain. Iontophoresis with lidocaine is recommended before ROM exercises, stretching, and joint mobilization and when immediate relief of acute pain (as in bursitis) is the object of treatment.
 One-percent to 10% hydrocortisone and dexamethasone administered under the positive electrode for relief of inflammatory pain in conditions such as arthritis, bursitis, or entrapment syndromes. Iontophoresis with hydrocortisone has a delayed onset but a prolonged effect, and it frequently eliminates the underlying cause of pain.
One-percent to 10% hydrocortisone and dexamethasone administered under the positive electrode for relief of inflammatory pain in conditions such as arthritis, bursitis, or entrapment syndromes. Iontophoresis with hydrocortisone has a delayed onset but a prolonged effect, and it frequently eliminates the underlying cause of pain.
 Two-percent magnesium (from Epsom salts) administered under the positive electrode for relief of pain from muscle spasm or localized ischemia. High levels of extracellular magnesium inhibit muscle contraction, including the smooth muscle found in the walls of the vessels, leading to localized vasodilation.
Two-percent magnesium (from Epsom salts) administered under the positive electrode for relief of pain from muscle spasm or localized ischemia. High levels of extracellular magnesium inhibit muscle contraction, including the smooth muscle found in the walls of the vessels, leading to localized vasodilation.
 Iodine (from Iodex ointment [Lee Pharmaceuticals, South El Monte, California]) administered under the negative pole for relief of pain caused by adhesions or scar tissue. Iodine “softens” fibrotic, sclerotic tissue, thereby increasing tissue pliability.
Iodine (from Iodex ointment [Lee Pharmaceuticals, South El Monte, California]) administered under the negative pole for relief of pain caused by adhesions or scar tissue. Iodine “softens” fibrotic, sclerotic tissue, thereby increasing tissue pliability.
 Salicylate (from Iodex with Methyl Salicylate [Lee Pharmaceuticals] or Gordogesic Creme [Gordon Laboratories, Upper Darby, Pennsylvania]) administered under the negative pole for relief of pain from inflammation. Salicylate is effective for arthritic joint inflammation, myalgia, and entrapment syndromes.
Salicylate (from Iodex with Methyl Salicylate [Lee Pharmaceuticals] or Gordogesic Creme [Gordon Laboratories, Upper Darby, Pennsylvania]) administered under the negative pole for relief of pain from inflammation. Salicylate is effective for arthritic joint inflammation, myalgia, and entrapment syndromes.
 Two-percent acetic acid administered under the negative pole to dissolve calcium deposits.
Two-percent acetic acid administered under the negative pole to dissolve calcium deposits.
 Two-percent lithium chloride or lithium carbonate administered under the positive pole to dissolve gouty tophi. In both acetic acid and lithium iontophoresis, the insoluble radicals in the deposits are replaced by soluble chemical radicals so the deposits can be broken down through natural processes.
Two-percent lithium chloride or lithium carbonate administered under the positive pole to dissolve gouty tophi. In both acetic acid and lithium iontophoresis, the insoluble radicals in the deposits are replaced by soluble chemical radicals so the deposits can be broken down through natural processes.
Massage
Massage has been recognized as a remedy for pain for at least 3000 years. Evidence of its beneficial effects first appeared in ancient Chinese literature, and then in the writings of the Hindus, Persians, Egyptians, and Greeks. Hippocrates advocated massage for sprains and dislocations as well as for constipation.105
Massage decreases pain through both direct and indirect means. Massage movements increase circulation through mechanical compression of the tissues, resulting in reflex relaxation of muscle tissue and direct relief from ischemic pain. Massage also indirectly stimulates A delta and A beta fibers, causing activation of the gating mechanism and the descending pain-modulating system.34
Massage movements are classified by pressure and the part of the hand that is used.106 The two massage movements that may cause a decrease in pain include stroking (effleurage) and compression (kneading or pétrissage). Stroking involves running the entire hand over large portions of the body. Stroking causes muscle relaxation and elimination of muscle spasm or improved circulation depending on the depth and force of the strokes. Compression is applied with intermittent pressure using lifting, rolling, or pressing movements meant to stretch shortened tissues, loosen adhesions, and assist with circulation.
A specialized massage technique is lymphatic massage, which consists of light-pressure rhythmic strokes to encourage organizational flow of the lymphatic system. This type of massage can be beneficial with clients who have peripheral swelling with or without pain. A popular form of lymph massage called manual lymphatic drainage (MLD) is used after surgical procedures to reduce swelling (for example, mastectomy for breast cancer). Evidence-based studies show conflicting results regarding the efficacy of this technique, and more research needs to be done to validate it.107–109
Myofascial release
Myofascial release (MFR) techniques are used to release the built-in imbalances and restrictions within the fascia and to reintegrate the fascial mechanism. The therapist palpates the various tissue layers, beginning with the most superficial and working systematically toward the deepest, looking for movement restrictions and asymmetry. Areas of altered structure and function are then “normalized” through the systematic application of pressure and stretching applied in specific directions to bring about decreased myofascial tension, myofascial lengthening, and myofascial softening,110 thereby restoring pain-free motion in normal patterns of movement. MFR is useful in treating musculoskeletal injuries, chronic pain, headaches, and adhesions or adherent scars.111 MFR has been shown to be effective in the treatment of chronic prostatitis (CP) and chronic pelvic pain syndrome (CPPS) in conjunction with paradoxical relaxation therapy (PRT).112 More research is needed to provide evidence regarding the efficacy of MFR in pain control.
MFR is contraindicated over areas with infection, diseased skin, thromboembolus, cellulitis, osteomyelitis, and open wounds. In addition, it should not be used with clients who have osteoporosis, advanced degenerative changes, acute circulatory conditions, acute joint pathology, advanced diabetes, obstructive edema, or hypersensitive skin.111 (See Chapter 39 for more in-depth information regarding MFR.)
Joint mobilization
Joint mobilization consists of passive oscillations that restore normal accessory movements.113 In addition, the rhythmical repetition of the motions provides pain relief through the spinal gating mechanism.114
The oscillations involved in joint mobilization are presented in Chapters 9 and 18. Grades I and II oscillations are performed to maintain joint mobility and for pain relief, making them the choice for subacute conditions in which pain and potential loss of motion are the primary considerations. Grades III and IV oscillations are performed to increase joint mobility and are indicated for chronic conditions in which regaining lost motion is the goal. Grade V thrusts are performed to regain full joint mobility.113
Joint mobilization is contraindicated with rheumatoid arthritis, bone disease, advanced osteoporosis, and pregnancy (pelvic mobilization), as well as in the presence of malignancy, vascular disease, or infection in the area to be mobilized.53
Light therapy
Light therapy is described by Bot and Bouter115 as a light source that generates extremely pure infrared light of a single wavelength. When applied to the skin, infrared laser light produces no sensation and it does not burn the skin. Because of the low absorption, it is hypothesized that the energy can penetrate deeply into the tissues, where it is assumed to have a biostimulative effect.77,115,116 It has been suggested that laser therapy may act by stimulating ligament repair,117,118 producing antiinflammatory effects,119 increasing production of endogenous opioids,120 reducing swelling,121 and influencing nerve conduction velocity.122 To promote wound healing and manage pain, rehabilitation centers use lasers with power outputs less than 500 mW at a power density of 50 mW/cm2 and wavelengths ranging from 600 to 1500 nm.77 Contraindications to light therapy include exposing photosensitive areas, hemorrhagic areas, any area that has undergone 4 to 6 months of radiation treatment,116 neoplastic lesions, and unclosed fontanelles in children; the abdomen of pregnant women; areas over the heart, the vagus nerve, or sympathetic innervations routes to the heart of cardiac patients; or, locally, endocrine glands.77,116,123,124 In addition, exposure to the cornea of the eye is contraindicated, so protective eye equipment should be worn by the patient and the therapist. Caution should be used for areas with compromised somatosensation, the epiphyseal plates in children, the gonads, and infected areas and with patients displaying fever, epilepsy, or mental confusion.77,116,123
Therapeutic touch
A description of therapeutic touch can be found in Chapter 39. Therapeutic touch has been effective in treating painful conditions resulting from anxiety and tension. In a report by Keller and Bzdek, 90% of individuals treated with therapeutic touch experienced tension headache relief, and 70% had continued relief for more than 4 hours; only 37% of the placebo group expressed sustained relief.125 A meta-analysis and systematic review on therapeutic touch revealed that the available studies have varying approaches and protocols on therapeutic touch, subject selection, and description. Although most of these studies confirm the efficacy of the technique, several studies also have demonstrated negative or mixed results.126 Therapeutic touch, as well as other approaches, are being more widely accepted; however, the therapist must continue to be diligent in using outcome studies to substantiate the use of any complementary therapy. (See Chapter 39 for additional information.)
Point stimulation
Refer to Chapter 39 for an in-depth discussion of point stimulation. It is interesting to note that acupuncture points frequently correspond in location to trigger points, which are tight, elevated bands of tissue that are extremely sensitive when palpated and have a characteristic pattern of radiation to remote regions of the body. Trigger points appear to be areas of “focal irritability” that are myofascial in origin and are usually the site of small aggregations of nerve fibers that produce continuous afferent input when stimulated.
Needling therapies include trigger point injections and trigger point dry needling and are used in myofascial pain conditions. Trigger point injections are usually restricted to medical doctors and their professional support staff.127 Trigger point dry needling consists of superficial and deep dry needling, and the exact mechanism of pain relief is not known. It is thought that needling and injections may trigger changes in the end plate cholinesterase and ACh receptors,127 may involve central pain mechanisms, and may activate enkephalinergic, serotonergic, and noradrenergic inhibitory systems in association with A delta fibers through segmental inhibition.127 Acupressure (i.e., finger pressure applied to acupuncture or trigger points) is thought to decrease their sensitivity through the same mechanism. The therapist applies deep pressure in a circular motion to each point for 1 to 5 minutes, until the sensitivity subsides. Pressure must be applied directly to each point for the treatment to be effective. Acupressure can be accompanied by the use of a vapocoolant spray to provide additional sensory stimulation.
Points that are most sensitive to stimulation are beneficial sites for TENS electrode placement. When point stimulation alone does not provide sufficient pain relief, TENS can be used between sessions for continuous stimulation for more prolonged relief. (See Chapter 39 for additional information on electrical acupuncture.)
Cognitive strategies, including cognitive behavioral therapy
The extent to which an individual perceives and expresses pain is a result of his or her emotional state, expectations, personality, and cognitive view. Each individual feels and responds to pain differently. Melzack and Wall114 identified the following three nonphysical components of pain that interact and determine how an individual will respond to pain:
 The individual’s sensory and discriminative interpretation of the pain
The individual’s sensory and discriminative interpretation of the pain
 The individual’s motivation and attitudes relating to the pain
The individual’s motivation and attitudes relating to the pain
 The individual’s cognitive and evaluative thoughts and beliefs concerning the pain experience
The individual’s cognitive and evaluative thoughts and beliefs concerning the pain experience
As mentioned previously, current research has given the medical community a much deeper understanding of chronic pain. Many new intervention approaches have been developed based on these new theories, and physical rehabilitation clinicians play a significant role in these new interventions. Of particular importance is the increasing role of clinicians in using cognitive behavioral therapy (CBT) in the management of individuals with chronic pain.128 CBT for pain management involves the integration of cognitive, affective, and behavioral factors into the case conceptualization and treatment.128 It is thought that a person’s beliefs about pain are associated with various functional outcomes129,130 and that changes in patients’ beliefs about pain are related to changes in functioning.131,132 Techniques potentially used may include coping skills, education and rationale about the course of an illness, relaxation, imagery, goal setting, pacing, distraction, and cognitive restructuring as well as homework assignments. A thorough discussion of this approach as it applies to physical rehabilitation is beyond the scope of this text. The work of Butler and Moseley provides clinicians a great resource on how to better explain pain to patients and clients, as well as to use the most current evidence on pain science and chronic pain management in treating individuals with chronic pain.133,134
Relaxation exercises
People who are in pain experience stress. Chronic stress can trigger increased pain. Both pain and stress cause an increase in SNS activity, including increased muscle tension. Relaxation exercises can bring about muscle relaxation and a generalized parasympathetic response.135 Benson136 has named this effect the relaxation response and reports that it is accompanied by an increase in alpha brain waves.
Relaxation reduces ischemic pain by normalizing blood flow to the muscles by making way for more oxygen to be delivered to the tissues. In addition, relaxation reduces muscle tension, resulting in an interruption in the pain-spasm cycle.137
Relaxation exercises all have two elements in common: a single focus and a passive attitude toward intruding thoughts and distractions. The end product of relaxation is a lowered arousal of the SNS and a lessening of the symptoms caused by or worsened by stress.138
Attention diversion works by activating the relaxation response and by diverting the individual’s attention from the pain. However, attention diversion also has been found to activate the higher brain centers and may have an inhibitory effect on pain through the spinal gating mechanisms.44,71 Lautenbacher and colleagues139 found that individuals who used attention diversion for pain management reported decreased intensity and unpleasantness of their pain.
Body scanning
Clients with chronic pain frequently become one with their suffering; they do not view themselves as individuals with pain, but rather as painful individuals. Body scanning is a technique that endeavors to separate the individual from the pain.140
Individuals who practice this technique report new levels of insight and understanding concerning their pain experience. They separate the pain experience into the following three parts140:
 An awareness of the pain sensation and their thoughts and feelings about it
An awareness of the pain sensation and their thoughts and feelings about it
 An awareness of a separation between the pain sensation and their thoughts and feelings about it
An awareness of a separation between the pain sensation and their thoughts and feelings about it
 An awareness of a separation between themselves and their pain, because they are able to examine objectively the sensation and their thoughts and feelings about it
An awareness of a separation between themselves and their pain, because they are able to examine objectively the sensation and their thoughts and feelings about it
Studies of chronic pain patients at the Stress Reduction Clinic at the University of Massachusetts Medical Center revealed that 72% of patients who used body scanning along with traditional medical interventions experienced at least a 33% reduction on their McGill-Melzack Pain Rating Index score.140 In addition, at the end of an 8-week training period, the individuals perceived their bodies in a more positive light, experienced an increase in positive mood states, and reported major improvements in anxiety, depression, hostility, and the tendency to be overly occupied with their bodily sensations.
Humor
Ever since Cousins141 reported in his book Anatomy of an Illness that he used humor to manage pain and enhance sleep during his illness, the role of humor in healing has been well studied. Humor has been found beneficial for both acute and chronic pain management.96,142
Laughter increases blood oxygen content by increasing ventilation. It helps to exercise the heart muscle by speeding up the heart rate and enhancing arterial and venous circulation, resulting in more oxygen and nutrients being delivered to the tissues.143 Laughter decreases serum cortisol levels (cortisol levels increase with stress and are thought to have a negative effect on the immune response)144,145 and increases the concentration of circulating antibodies.143 As little as 10 minutes of belly laughter a day has been found to decrease the erythrocyte sedimentation rate and provide 2 hours of pain-free sleep.144 Finally, laughter releases energy and emotional tension and is followed by generalized muscle relaxation.143,146
Therapeutic humor should not be used with individuals who do poorly with humor. This includes individuals who despise or misunderstand humor, individuals who find joy threatening or guilt inducing, and narcoleptic individuals who become cataleptic with laughter.147
General conditioning through exercise
All exercise has an analgesic effect through the gating mechanism by stimulation of the A delta neurons and a pain-modulating effect through activation of the descending systems. Exercise of sufficient intensity has been known to increase circulating β-endorphin levels, but exercise-induced β-endorphin alterations are related to the type of exercise and special populations tested and may differ in individuals with health problems.148,149
Operant conditioning
Operant conditioning addresses the learned (or conditioned) aspects of pain.150 Operant conditioning involves unlearning or separating the behavior and the response from the pain experience. If the goal of treatment is to lessen social reinforcement of the client’s pain behaviors (and thus extinguish those behaviors), the client and the family or other involved individuals are shown how their behaviors and responses provide social reinforcement for the client’s reaction to pain. The involved individuals are provided with specific new responses to the client’s behavior. Family members might be instructed to ignore wincing, groaning, or the verbal report of pain. They might be told not to perform activities that are the client’s responsibility just because the client reports pain. In time, the client will become conditioned to the new response, and pain in those situations will diminish.
Hypnosis
Hypnosis is a state in which the body and conscious mind are deeply relaxed while the subconscious mind remains alert, focused, and open to suggestion.138 This has been demonstrated physiologically by electroencephalography (EEG), which shows an increase in the number of theta waves, which are associated with enhanced attention.151 When a hypnotized individual is given a suggestion that is in alignment with his or her existing belief system, it is accepted by the subconscious mind as reality. The suggestion is not filtered through the conscious mind, which is critical and judgmental. Hypnosis allows the individual to bypass her or his critical beliefs.152 For example, if the individual believes that a certain activity will cause pain (critical belief), that activity is sure to cause pain. If, however, during hypnosis, the individual accepts the suggestion that the activity does not cause pain, the pain may decrease and even disappear.
Biofeedback
Muscle tension, pulse rate, blood pressure, skin temperature, and electromyography (EMG) and EEG readings are some of the physiological processes that can be consciously modified with biofeedback.135
Biofeedback is proving to be an effective pain management tool for headaches, muscle spasms, and other physical dysfunction that leads to or increases chronic pain (see Chapters 33 and 39).