31 Pain management
Addiction: A chronic, neurobiologic disease characterized by impaired control over drug use, compulsive use, continued use despite harm, and craving.
Multimodal Analgesia: Combinations of drugs with different underlying mechanisms administered to allow lower doses of each of the drugs, reduce the potential for analgesic adverse effects, and provide comparable or greater pain relief than can be achieved with any single analgesic.
Neuropathic Pain: Pain that results from abnormal processing of sensory input by the nervous system because of damage to the peripheral or central nervous systems or both.
Nociceptive Pain: Pain that results from the normal functioning of physiologic systems that leads to the perception of noxious stimuli (tissue injury) as being painful.
Opioid Naive: An individual who has not recently taken enough opioid on a regular enough basis to become tolerant to the effects of an opioid.
Opioid Tolerance: An individual who has taken opioids long enough at doses high enough to develop tolerance to many of the effects of the opioid, including analgesia and sedation; a timeframe for development of tolerance has not been established.
Physical Dependence: Potential for withdrawal symptoms if the opioid is abruptly stopped or an antagonist is administered; not the same as addiction.
Pseudoaddiction: A mistaken diagnosis of addiction in which the patient exhibits behaviors often seen in addictive disease, such as asking for analgesics on time or early, but actually reflect undertreated pain.
Titration: The process of adjusting the amount of the dose of an analgesic.
Tolerance: A process characterized by decreasing effects of a drug at its previous dose, or the need for a higher dose of drug to maintain an effect; not the same as addiction.
Pain is one of the most common reasons people seek health care. Despite an abundance of research and improvements in analgesics and drug delivery technology, pain continues to be undertreated and costly for patients and the health care system in general.1,2 Nurses are experts in assessment, drug administration, and patient education and are the only members of the health care team who are at the patient’s bedside around the clock. These characteristics have led to their distinction as the patient’s primary pain manager.3 Nurses are critical to ensuring that their patients receive the best possible pain relief available.
Definition of pain
The American Pain Society defines pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.”4 This definition describes pain as a complex, multifactoral phenomenon that affects a person’s psychosocial, emotional, and physical functioning. The definition of pain that is applied in the clinical setting reinforces that pain is a highly personal and subjective experience: “Pain is whatever the experiencing person says it is, existing whenever he says it does.”5 All accepted guidelines consider the patient’s report to be the most reliable indicator of pain and the gold standard of pain assessment.2,4
Types and categories of pain
Pain is usually described as being acute or chronic (persistent).6 Acute pain and chronic pain differ from one another primarily in their duration. For example, tissue damage as a result of surgery, trauma, or burns produces acute pain that is expected to have a relatively short duration and to resolve with normal healing. Chronic pain can occur from an underlying medical condition, such as peripheral neuropathy from diabetes, cancer pain from tumor growth, or osteoarthritis pain from joint degeneration, and it can persist throughout the course of a person’s life. Some medical conditions can produce both acute and chronic pain. For example, some patients with cancer have continuous chronic pain and also experience acute exacerbations of pain periodically (called breakthrough pain) or endure repetitive painful procedures during cancer treatment.
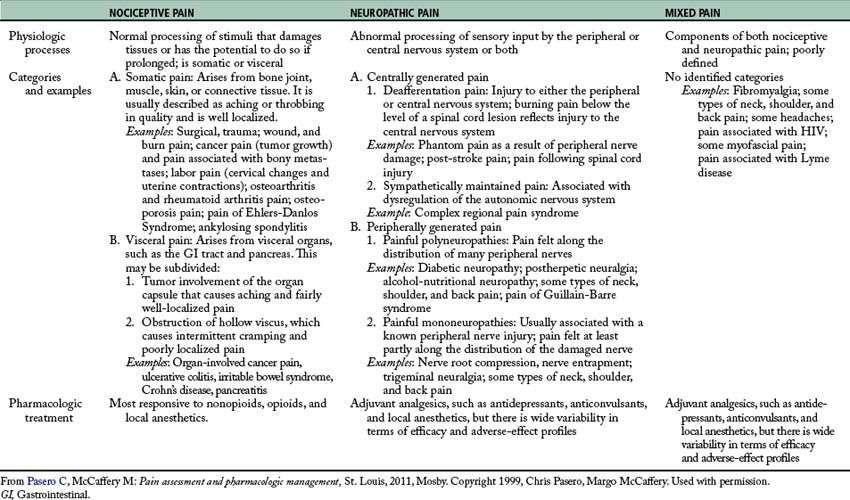
Pain is increasingly classified by its inferred pathology as being either nociceptive pain or neuropathic pain (Table 31-1).6 Nociceptive pain refers to the normal functioning of physiologic systems that leads to the perception of noxious stimuli (tissue injury) as being painful. This explains why nociception is described as “normal” pain transmission. Pain from surgery, trauma, burns, and tumor growth are examples of nociceptive pain. Patients often describe this type of pain as “aching,” “cramping,” or “throbbing.”
Neuropathic pain is pathologic and results from abnormal processing of sensory input by the nervous system as a result of damage to the peripheral nervous system (PNS) or central nervous system (CNS), or both.6 Examples include postherpetic neuralgia, diabetic neuropathy, phantom pain, and post-stroke pain syndrome. Patients with neuropathic pain describe their pain with distinctive words, such as “burning,” “sharp,” and “shooting.”
Nociception and analgesic action sites
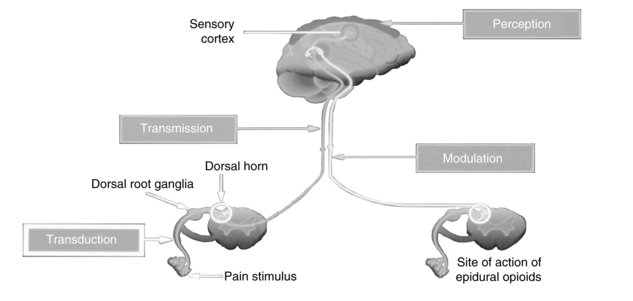
Nociception includes four specific processes: transduction, transmission, perception, and modulation. Figure 31-1 illustrates these processes, and an overview of each follows.
Transduction
Transduction refers to the processes by which noxious stimuli activate primary afferent neurons called nociceptors, which are located throughout the body in the skin, subcutaneous tissue, and visceral and somatic structures (see Fig. 31-1).6 These neurons have the ability to respond selectively to noxious stimuli generated as a result of tissue damage from mechanical (e.g., incision, tumor growth), thermal (e.g., burn, frostbite), chemical (toxins, chemotherapy), and infectious sources.7,8 The noxious stimuli cause the release of a number of excitatory compounds (e.g., serotonin, bradykinin, histamine, substance P, prostaglandins), which facilitate the movement of pain along the pain pathway.6 These substances are collectively referred to as inflammatory soup.8
Prostaglandins are a particularly important group of compounds that accompanies tissue injury and initiates inflammatory responses that increase tissue swelling and pain at the site of injury.9 They are formed when the enzyme phospholipase breaks down phospholipids into arachidonic acid, and arachidonic acid, in turn, is acted upon by the enzyme cyclooxygenase (COX) to produce prostaglandins (Fig. 31-2). The two best characterized isoenzymes of COX are COX-1 and COX-2; they have an important role in producing the effects of the nonopioid analgesics, which act peripherally and centrally to inhibit the COX isoenzymes. Nonsteroidal antiinflammatory drugs (NSAIDs) work primarily by blocking the formation of prostaglandins in the periphery. The nonselective NSAIDs, such as ibuprofen, naproxen, diclofenac, and ketorolac, inhibit both COX-1 and COX-2, and the COX-2 selective NSAIDs, such as celecoxib, inhibit just COX-2. As Fig. 31-2 illustrates, both types of NSAIDs produce antiinflammatory and pain relief through the inhibition of COX-2. Although the exact underlying mechanisms of action of acetaminophen continue to be investigated,10 acetaminophen is a known COX inhibitor that has minimal peripheral effect, is not antiinflammatory, and can both relieve pain and reduce fever by preventing the formation of prostaglandins in the CNS.6
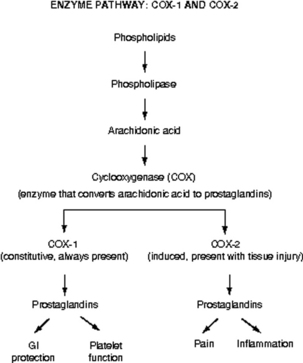
FIG. 31-2 Enzyme pathway: COX-1 and COX-2. GI, Gastrointestinal.
(From Pasero C, McCaffery M: Pain assessment and pharmacologic management, St. Louis, 2011, Mosby. Copyright Pasero C, McCaffery M. Used with permission.)
Other types of analgesics work by partially blocking transduction as well. For example, sodium channels are closed and inactive at rest, but undergo changes in response to membrane depolarization. Transient channel opening leads to an influx of sodium and subsequent nerve conduction.11 Local anesthetics are capable of blocking sodium channels and reducing the nerve’s ability to generate an action potential. Anticonvulsants also affect the flux of other ions, such as calcium and potassium, to reduce transduction and produce pain relief.
Transmission
Transmission is the second process involved in nociception. Effective transduction generates an action potential that is transmitted along the A-delta (δ) and C fibers.6 A-δ fibers are lightly myelinated and faster conducting than the unmyelinated C fibers. The endings of A-δ fibers detect thermal and mechanical injury and allow relatively quick localization of pain and a rapid reflex withdrawal from the painful stimulus. Unmyelinated C fibers are slow conductors and respond to mechanical, thermal, and chemical stimuli. They yield poorly localized, often aching or burning pain. A-beta (β) fibers are the largest of the fibers and do not normally transmit pain, but do respond to touch, movement, and vibration.6
Afferent information passes through the cell body of the dorsal root ganglia (see Fig. 31-1), which lie outside of the spinal cord, to synapse in the dorsal horn of the spinal cord. An action potential is generated and the impulse ascends up to the spinal cord and transmits information to the brain, where pain is perceived. Extensive modulation occurs in the dorsal horn via complex neurochemical mechanisms. The primary A-δ fibers and C fibers release a variety of transmitters including glutamate, neurokinin, and substance P. Glutamate binds to the N-methyl-D-aspartate (NMDA) receptor and promotes pain transmission. Ketamine is an NMDA receptor antagonist that provides pain relief by preventing glutamate from binding to the NMDA receptor sites. Endogenous and therapeutically administered opioids bind to opioid receptor sites in the dorsal horn to block substance P and thereby produce analgesia.6
Perception
The third broad process involved in nociception is perception. Perception, the result of the neural activity associated with transmission of noxious stimuli,6 involves the conscious awareness of pain and requires activation of higher brain structures for the occurrence of awareness, emotions, and drives associated with pain (see Fig. 31-1). The physiology of the perception of pain is poorly understood, but presumably can be targeted by mind-body therapies, such as distraction and imagery, which are based on the belief that brain processes can strongly influence pain perception.6
Modulation
Modulation of afferent input generated in response to noxious stimuli occurs at every level from the periphery to the cortex and involves dozens of neurochemicals.9 For example, serotonin and norepinephrine are central inhibitory neurotransmitters that are released in the spinal cord and brainstem by the descending fibers of the modulatory system (see Fig. 31-1). Some antidepressants provide pain relief by blocking the body’s reuptake of serotonin and norepinephrine, extending their availability to fight pain. Endogenous opioids are located throughout the peripheral and central nervous systems, and like therapeutically administered opioids they inhibit neuronal activity by binding to opioid receptors. As an example, Fig. 31-1 shows that the dorsal horn of the spinal cord, which is densely populated with opioid receptors, is the primary action site of epidural opioids.
Pathophysiology of neuropathic pain
Neuropathic pain is sustained by mechanisms that are driven by damage to, or dysfunction of, the PNS or CNS. In contrast to nociceptive pain, neuropathic pain is abnormal processing of stimuli.7,12 Whereas nociceptive pain involves tissue damage or inflammation, neuropathic pain can occur in the absence of either. Neuropathic pain, even when acute, reflects a pathophysiology that serves no useful purpose.6 A discussion of some of the peripheral and central mechanisms that initiate and maintain neuropathic pain follows. Extensive research is ongoing to better define these mechanisms.
Peripheral mechanisms
At any point from the periphery to the CNS, the potential exists for the development of neuropathic pain. For example, when nociceptors are injured, changes in the number and location of ion channels, particularly sodium channels, can abnormally accumulate.6 The threshold for nerve depolarization is then lowered, which leads to an increased response to stimuli and ectopic discharges. Hyperexcitable nerve endings in the periphery can become damaged, leading to abnormal reorganization of the nervous system, an underlying mechanism of some neuropathic pain states.7 Chemically mediated connections can form between nerve fibers and cause abnormal activation of neurons and ultimately pain. These processes lead to a phenomenon called peripheral sensitization, which is thought to contribute to the maintenance of neuropathic pain. Topical local anesthetics, such as lidocaine patch 5%, are an example of analgesics that produce effects in the tissues under the site of application by “dampening” neuropathic pain mechanisms in the peripheral nervous system.13
Central mechanisms
Central mechanisms also have a role in establishing neuropathic pain. Central sensitization is defined as abnormal hyperexcitability of central neurons as a result of complex changes induced by incoming barrages of nociceptors.6 Extensive release and binding of excitatory neurotransmitters, such as glutamate, activate the NMDA receptor and cause an increase in intracellular calcium levels into the neuron, resulting in pain. As noted, the NMDA antagonist ketamine directly antagonizes this activity. An increase in the influx of sodium is thought to lower the threshold for nerve activation, increase response to stimuli, and enlarge the receptive field served by the affected neuron. The accumulation of intracellular ions causes spinal neurons to become highly sensitized and fire rapidly in a process called wind-up.9 As mentioned, local anesthetics and anticonvulsants can block ion channels and inhibit abnormal pain sensation.
As with injured peripheral neurons, synaptic reorganization and anatomic changes can also occur in the CNS. These are thought to be sustained by an increased responsiveness of central neurons to relatively mild peripheral stimuli.12 For example, injury to a nerve route can lead to reorganization in the dorsal horn of the spinal cord. Nerve fibers can invade other areas and create abnormal sensations in the area of the body served by the injured nerve. Allodynia, or pain from a normally nonnoxious stimulus (e.g., touch), is one such type of abnormal sensation and a common feature of neuropathic pain. In patients with allodynia, the mere weight of clothing or bed sheets can be excruciatingly painful. The ability of the nervous system to change structure and function as a result of noxious stimuli is called neuroplasticity.6
Another underlying mechanism called central disinhibition occurs when control mechanisms along inhibitory (modulatory) pathways are lost or suppressed, leading to abnormal excitability of central neurons.6 Possible causes of disinhibition include dysfunction of the gamma-aminobutyric acid (GABA) pathways. GABA is the most abundant neurotransmitter in the CNS and composes a major inhibitory neurotransmitter system. Increased GABA function may help to relieve neuropathic pain. Benzodiazepines, such as midazolam, enhance GABA function, resulting in analgesia for pathologic conditions like muscle spasm.6,13
Harmful effects of unrelieved pain
Literally every system in the body is affected by unrelieved pain; the harmful effects are numerous (Table 31-2). Unrelieved pain triggers and prolongs the stress response causing the release of excessive amounts of hormones, such as cortisol, catecholamines, and glucagon; insulin and testosterone levels decrease.14,15 This increased endocrine activity initiates a number of metabolic processes that can result in weight loss, tachycardia, increased respiratory rate, shock, and even death.14 Persistent unrelieved pain has been linked to increased tumor growth16 and a higher incidence of health care–associated infections.17
| DOMAINS AFFECTED | SPECIFIC RESPONSES TO PAIN |
|---|---|
| Endocrine | ↑ ACTH, ↑ cortisol, ↑ ADH, ↑ epinephrine, ↑ norepinephrine, ↑ GH, ↑ catecholamines, ↑ renin, ↑ angiotensin II, ↑ aldosterone, ↑ glucagon, ↑ interleukin-1, ↓ insulin, ↓ testosterone |
| Metabolic | Gluconeogenesis, hepatic glycogenolysis, hyperglycemia, glucose intolerance, insulin resistance, muscle protein catabolism, ↑ lipolysis |
| Cardiovascular | ↑ Heart rate, ↑ cardiac workload, ↑ peripheral vascular resistance, ↑ systemic vascular resistance, hypertension, ↑ coronary vascular resistance, ↑ myocardial oxygen consumption, hypercoagulation, deep vein thrombosis |
| Respiratory | ↓ Flows and volumes, atelectasis, shunting, hypoxemia, ↓ cough, sputum retention, infection |
| Genitourinary | ↓ Urinary output, urinary retention, fluid overload, hypokalemia |
| Gastrointestinal | ↓ Gastric and bowel motility |
| Musculoskeletal | Muscle spasm, impaired muscle function, fatigue, immobility |
| Cognitive | Reduction in cognitive function, mental confusion |
| Immune | Depression of immune response |
| Developmental | ↑ Behavioral and physiologic responses to pain, altered temperaments, higher somatization, infant distress behavior, possible altered development of the pain system, ↑ vulnerability to stress disorders, addictive behavior, and anxiety states |
| Future pain | Debilitating chronic pain syndromes: postmastectomy pain, postthoracotomy pain, phantom pain, postherpetic neuralgia |
| Quality of life | Sleeplessness, anxiety, fear, hopelessness, ↑ thoughts of suicide |
ACTH, Adrenocorticotrophic hormone; ADH, antidiuretic hormone; down arrow (↓), decreased; GH, growth hormone; up arrow (↑), increased.
From Pasero C, McCaffery M: Pain assessment and pharmacologic management, St. Louis, 2011, Mosby. Copyright Pasero C, McCaffery M. Used with permission.
Effects on the cardiovascular (CV) system include increased postoperative blood loss18 and hypercoagulation,14 which can lead to myocardial infarction and stroke. The respiratory system is affected by small tidal volumes and decreases in functional lung capacity, which can lead to pneumonia, atelectasis, and an increased need for mechanical ventilation.19,20
Every surgical procedure has the potential to produce persistent (chronic) postsurgical pain; however, inguinal hernia repair, amputation, and thoracic, cardiac, and breast surgery are among those identified as high risk for this complication.6,21,22 Multiple factors are thought to contribute to the development of persistent postsurgical pain, including nerve injury from the surgical procedure, preexisting pain, and genetic susceptibility.23 Persistent postsurgical pain may have nociceptive, inflammatory, and neuropathic components indicating a need for a multimodal treatment approach.22 Similar to other complex pain syndromes, it can be difficult to treat and last a lifetime.
Pain assessment: the gold standard
The gold standard for assessing the existence and intensity of pain is the patient’s self report.2 A comprehensive pain assessment includes obtaining the following information from the patient:
• Location of pain: Ask the patient to state or point to the areas of pain on the body.
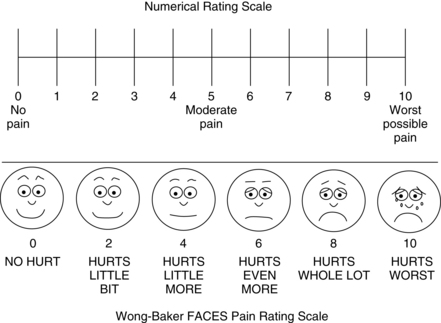
• Intensity: Ask the patient to rate the intensity of the pain using a reliable and valid pain assessment tool. A number of scales in several language translations have been evaluated and made available for use in clinical practice and for educational practice.2 See Box 31-1 for practical tips on using self-report pain rating tools. The most common are:
• Quality: Ask the patient to describe how the pain feels. Descriptors such as sharp, shooting, or burning may help to identify the presence of neuropathic pain.
• Onset and duration: Ask when the pain started and whether it is constant or intermittent.
• Aggravating and relieving factors: Ask the patient what makes the pain worse and what makes it better.
• Effect of pain on function and quality of life: It is particularly important to ask patients with persistent pain about how pain has affected their lives; what could they do before the pain began that they can no longer do, or what do they want to do but cannot do because of the pain?
• Comfort-function (pain) goal: For patients with acute pain, identify short-term functional goals and reinforce the link between good pain control and successful achievement of the goals. For example, surgical patients are told that they will be expected to cough, deep breathe, turn, and ambulate or participate in physical therapy postoperatively. Patients with chronic pain can be asked to identify their unique functional or quality-of-life goals, such as being able to work, walk the dog, or garden. Patients are then asked to identify (using a 0-to-10 scale) a level of pain that will allow accomplishment of the identified functional or quality-of-life goals with reasonable ease. A realistic goal for most patients is 2 or 3, and pain intensity ratings that are consistently above the goal warrant further evaluation and consideration of an intervention and possible adjustment of the treatment plan.2
• Other information: The patient’s culture, past pain experiences, and pertinent medical history, such as comorbidities, laboratory tests, and diagnostic studies, are considered when establishing a treatment plan.28
BOX 31-1 Practical Tips on the Use of Self-Report Pain Rating Scales
• Try using a standard pain assessment tools such as the 0-to-10 numerical rating scale; verbal descriptor scale with simple adjectives such as mild, moderate, severe, and worst possible pain; or the FACES Pain Scale-Revised.
• Increase the size of the font and other features of the scale.
• Ensure that eyeglasses and hearing aids are functioning.
• Try using alternative words, such as ache, hurt, and sore when discussing pain.
• Provide a written example of the scale and clear instructions on how to use it.
• Present the tool vertical format (rather than the frequently used horizontal).
• Ask about pain in the present.
• Repeat instructions and questions more than once.
• Allow ample time to respond.
• Ask awake and oriented ventilated patients to point to a number on the numerical scale if they are able.
• Repeat instructions and show the scale each time pain is assessed.
From Pasero C, McCaffery M: Pain assessment and pharmacologic management, St. Louis, 2011, Mosby. Copyright 2008, Pasero C, McCaffery M. Used with permission.
Challenges in assessment
Many patients are unable to provide a report of their pain using the customary self-report pain rating tools, placing them at higher risk for undertreated pain than those who can report.2 These patients are collectively called nonverbal patients29 and include infants, toddlers, and patients who are cognitively impaired, critically ill (intubated, unresponsive), comatose, or imminently dying. Patients who are receiving neuromuscular blocking agents or are sedated from anesthesia and other drugs given during surgery are also among this at-risk population.
When patients are unable to report pain using traditional methods, an alternative approach based on the Hierarchy of Pain Measures is recommended.2,29,30 The key components of the hierarchy are to: (1) attempt to obtain self-report; (2) consider underlying pathology or conditions and procedures that might be painful (e.g., surgery); (3) observe behaviors; (4) evaluate physiologic indicators; and (5) conduct an analgesic trial. See Box 31-2 for detailed information on each component of the Hierarchy of Pain Measures.
BOX 31-2 Hierarchy of Pain Measures
1. Attempt to obtain the patient’s self-report, which is the single most reliable indicator of pain. Do not assume that a patient cannot provide a report of pain; many cognitively impaired patients are able to use a self-report tool, such as the FACES Pain Scale-Revised or Verbal Descriptor Scale.
2. Consider the patient’s condition or exposure to a procedure that is thought to be painful. If appropriate, assume that pain is present and document it when approved by institution policy and procedure.
3. Observe behavioral signs such as facial expressions, crying, restlessness, and changes in activity. There are many behavioral pain assessment tools available that will yield a pain behavior score and may help to determine whether pain is present. However, it is important to remember that a behavioral score is not the same as a pain intensity score. Pain intensity is unknown if the patient is unable to provide it.
4. Evaluate physiologic indicators with the understanding that they are the least sensitive indicators of pain and may signal the existence of conditions other than pain or a lack of it (e.g., hypovolemia, blood loss). Patients may have normal or below normal vital signs in the presence of severe pain. The absence of an elevated blood pressure or heart rate does not mean the absence of pain.
5. Conduct an analgesic trial to confirm the presence of pain and to establish a basis for developing a treatment plan if pain is thought to be present. An analgesic trial involves the administration of a low dose of nonopioid or opioid and observing patient response. The initial low dose may not be enough to illicit a change in behavior and should be increased if the previous dose was tolerated, or another analgesic may be added. If behaviors continue despite optimal analgesic doses, other possible causes should be investigated. In patients who are completely unresponsive, no change in behavior will be evident and the optimized analgesic dose should be continued.
From Pasero C, McCaffery M: Pain assessment and pharmacologic management, St. Louis, 2011, Mosby. Copyright 1999, McCaffery M, Pasero C. Used with permission.
Self report is at the top of the hierarchy and should be attempted, even in patients who present challenges in assessment.2 Many patients with mild to moderate cognitive impairment can provide self-report when clinicians implement fairly simple measures (see Box 31-1).
Several behavioral pain assessment tools exist to facilitate assessment in nonverbal patients; however, patients must be carefully evaluated for their ability to respond with the requisite behaviors in the selected tool.2 For example, tools that require assessment of body movement as a pain indicator should not be used in patients who are unable to move, such as those receiving a neuromuscular blocking agent. According to the hierarchy, pain can be assumed to be present in these patients, justified by research showing that endotracheal intubation, ventilation, and suctioning—all required in patients receiving a neuromuscular blocking agent—are painful.31,32 It is equally important to understand that the score obtained from the use of a behavioral pain assessment tool helps to identify the presence of pain, but the score is a behavioral score and not a pain intensity rating. Simply put, if the patient cannot report the intensity of the pain, the intensity is not known.2,33
Although nurses who care for patients with acute pain often rely on vital signs to assess pain, these physiologic signs are considered poor indicators of pain.2,34,35 Many factors other than pain can influence changes in vital signs, and patients quickly adapt physiologically despite the presence of pain. The primary message is that the absence of an elevated blood pressure or heart rate does not mean the absence of pain.2
Reassessment of pain
Following initiation of the pain management plan, pain is reassessed and documented on a regular basis as a way to evaluate the effectiveness of treatment. At a minimum, pain should be reassessed with each new report of pain and before and after the administration of analgesics.2 The frequency of reassessment depends on the stability of the patient’s pain and is guided by institutional policy. For example, in the postanesthesia care unit (PACU) reassessment may be necessary as often as every 10 minutes when pain is unstable during opioid titration, but can be done every 4 to 8 hours in patients with stable pain 24 hours after surgery.
Pain control on A continuum
The quality of patients’ pain control should be addressed when patients are discharged from one clinical area to another. Many PACUs establish the criterion that patients must achieve a pain rating of 4 on a scale of 0 to 10 or better before discharge; however, the expectation that all patients must be discharged from a given clinical unit with pain ratings less than an arbitrary number is unrealistic and can lead to the unsafe administration of additional opioid doses to patients who are excessively sedated and is widely discouraged.28,36–38 Instead, achieving optimal pain relief is best viewed on a continuum with the primary objective being to provide both effective and safe analgesia.28 Optimal pain relief is the responsibility of every member of the health care team and begins with analgesic titration in the PACU followed by continued prompt assessment and analgesic administration after discharge from the PACU to achieve pain ratings that allow patients to meet their functional goals with relative ease.
Although it may not always be possible to achieve a patient’s pain rating goal within the short time the patient is in an area like the PACU, this goal provides direction for ongoing analgesic care. Important information to give to the nurse assuming care of the patient on the clinical unit is the patient’s pain rating goal, how close the patient is to achieving it, what has been done thus far to achieve it (analgesics and doses), and how well the patient has tolerated analgesic administration (adverse effects).28
Pharmacologic management of pain: multimodal analgesia
Pain is a complex phenomenon involving multiple underlying mechanisms as described earlier in this chapter. This characteristic underscores the importance of using more than one analgesic to manage pain.28 This approach is called multimodal analgesia and is recommended for the treatment of all types of pain.4,28,39,40 A multimodal regimen combines drugs with different underlying mechanisms; this allows lower doses of each of the drugs in the treatment plan, which reduces the potential for each to produce adverse effects.28 Furthermore, multimodal analgesia can result in comparable or greater pain relief than can be achieved with any single analgesic. The use of multimodal analgesia should be the rule, rather than the exception in pain treatment.
The most common analgesics used for postoperative pain management are nonopioid analgesics (e.g., acetaminophen, NSAIDs), opioid analgesics (e.g., morphine, hydromorphone, fentanyl, nd oxycodone), local anesthetics, and anticonvulsants. A multimodal approach in the perioperative setting may combine agents from each of these analgesic groups to provide effective pain relief and help minimize adverse effects. Unless contraindicated, all surgical patients should routinely be given acetaminophen and an NSAID in scheduled doses throughout the postoperative course (preferably initiated preoperatively). Opioid analgesics are added to manage moderate-to-severe postoperative pain in most patients. For some major surgical procedures, a local anesthetic is administered with an opioid epidurally or alone by continuous peripheral nerve block. An anticonvulsant may be added to the treatment plan as well to control severe pain or prevent a chronic postsurgical pain syndrome, such as persistent pain after thoracotomy or mastectomy.6
In a prospective, randomized-controlled study, 80 patients undergoing total knee replacement were assigned to receive morphine via intravenous (IV) patient-controlled analgesia (PCA) alone or as a single 400-mg dose of celecoxib 1 hour before surgery followed by 200 mg of celecoxib every 12 hours for 5 days postoperatively along with IV PCA morphine. Resting pain scores were better, and active range of motion increased significantly in those who received celecoxib compared with those who did not. Celecoxib demonstrated a 40% morphine dose-sparing effect, which resulted in a lower incidence of nausea and vomiting in those who received celecoxib (28%) than in those who did not (43%). Other important findings were that intraoperative and postoperative blood loss was comparable among patients in both groups and celecoxib resulted in no significant increase in the need for blood transfusions.
Source: Huang YM, et al: Perioperative celebrex administration for pain management after total knee arthroplasty—a randomized, controlled study, BMC Musculoskelet Disord 9:77, 2008.
Routes of administration
One principle of pain management is to use the oral route of administration whenever feasible.28 When the oral route is not possible, such as in patients who cannot swallow, can receive nothing my mouth, or are nauseated, other routes of administration are used. In the perioperative setting, the intravenous (IV) route is the first-line route of administration for analgesic delivery; patients are transitioned postoperatively to the oral route as tolerated. Some of the methods used to manage pain are accomplished via catheter techniques, such as intraspinal analgesia and continuous peripheral nerve block infusions. Nurses have an extensive role in the successful management of these therapies, and the American Society for Pain Management Nursing (http://www.aspmn.org) provides guidelines for care.3
Although drugs are rarely administered rectally in adults in the perioperative setting in the United States, this route of analgesic administration has a long history of safety in children undergoing surgery and is an alternative when oral or IV analgesics are not an option.28,41 The rectum allows passive diffusion of medications and absorption into the systemic circulation. This route of administration can be less expensive and does not involve the expertise required of the parenteral route of administration. Drawbacks are that drug absorption can be unreliable and depends on many factors, including rectal tissue health and administrator technique. The rectal route is contraindicated in patients who are neutropenic or thrombocytopenic because of potential rectal bleeding.28 Diarrhea, perianal abscess or fistula, and abdominoperineal resection are also relative contraindications.28
Topical local anesthetics are used for acute procedural pain, but the other second-line routes of administration, such as transdermal and subcutaneous, are generally reserved for the management of chronic pain. The primary disadvantages of transdermal drug delivery are that the skin serves as both a barrier and a reservoir. There is significant lag time before the effects of the drug are felt after transdermal patch application, and the drug continues to enter the systemic circulation for a variable period after the patch is removed.42
Nonopioid analgesics
The nonopioid analgesic group includes acetaminophen and NSAIDs. There are two categories of NSAIDs: the nonselective NSAIDs (e.g., ibuprofen, naproxen, diclofenac, ketorolac), which inhibit both COX-1 and COX-2, and the COX-2 selective NSAIDs (e.g., celecoxib), which inhibit just COX-2 (see Fig. 31-2).
Nonopioids are flexible analgesics used for a wide spectrum of painful conditions. They are appropriate alone for mild to some moderate nociceptive-type pain (e.g., from surgery or trauma) and are added to opioids, local anesthetics, or anticonvulsants as part of a multimodal analgesic regimen for more severe nociceptive pain.28,43 Acetaminophen and an NSAID can be given concomitantly, and there is no need for staggered doses.43
Acetaminophen is versatile in that it can be given by multiple routes of administration, including oral, rectal, and IV. IV acetaminophen is approved for treatment of pain and fever in adults and children 2 years of age and older and is given by a 15-minute infusion in single or repeated doses. It can be given alone for mild to moderate pain or moderate to severe pain with adjunctive opioid analgesics and has been shown to be well tolerated and to produce a significant opioid dose-sparing effect and superior pain relief compared with placebo.44 The maximum daily dose for IV acetaminophen is the same as for oral acetaminophen (e.g., 1000 mg every 6 hours, for maximum of 4000 mg in adults and adolescents weighing more than 50 kg; 15 mg/kg every 6 hours in adults, adolescents, and children weighing less than 50 kg).
There is evidence that oral NSAIDs are more effective than oral acetaminophen. A randomized controlled trial (n = 151) showed that oral ibuprofen 800 mg taken three times a day provided better pain relief than 1000 mg of oral acetaminophen taken two times a day following anterior cruciate ligament repair.45 For many painful conditions, such as osteoarthritis, oral NSAIDs are tried for mild to moderate pain if oral acetaminophen has been unsuccessful in controlling the pain.43 For surgical pain, an NSAID is added to both acetaminophen and an opioid as part of a multimodal plan.
Ketorolac and ibuprofen are available in IV formulation. An abundance of research has shown ketorolac to be effective for postoperative pain following a wide variety of surgical procedures.43 Although further clinical experience and research are needed with IV ibuprofen, the drug is less COX-1 selective than ketorolac,43 which may result in fewer adverse effects than ketorolac (see Fig. 31-2). A randomized, placebo-controlled study (n = 185) found that orthopedic surgery patients who were given preoperative followed by postoperative IV ibuprofen reported better pain relief, used 31% less morphine, and experienced similar adverse effects and complications, including blood loss, compared with placebo.46
Adverse effects of nonopioids
Acetaminophen is widely considered one of the safest and best tolerated analgesics.47,48 Its most serious complication is hepatotoxicity (liver damage) as a result of overdose. In the healthy adult, a maximum daily dose below 4000 mg is rarely associated with liver toxicity.49 Its lack of effect on platelet aggregation and low incidence of gastrointestinal (GI) adverse effects make acetaminophen the analgesic of choice in individuals with renal insufficiency, advanced chronic kidney disease, and end-stage renal disease.50,51 Acetaminophen has been shown to increase the International Normalized Ratio when administered with warfarin, but the likelihood of surgical bleeding as a result of perioperative acetaminophen intake is thought to be low.52,53
The NSAIDs have significantly more adverse effects than acetaminophen, with gastric toxicity and ulceration being the most common.43 The primary underlying mechanism of NSAID-induced gastric ulceration is the inhibition of COX-1, which leads to a reduction in GI-protective prostaglandins (see Fig. 31-2).47 This effect is systemic, rather than local, and can occur regardless of the route of administration of the NSAID.43 Risk factors include advanced age (older than 60 years), presence of prior ulcer disease, and CV disease and other comorbidities. The use of a COX-2 selective NSAID (e.g., celecoxib) is recommended if not contraindicated by CV risk or the least ulcerogenic nonselective NSAID (e.g., ibuprofen).43 GI adverse effects are also related to the dose and duration of NSAID therapy; the higher the NSAID dose and the longer the duration of NSAID use, the higher the risk of cumulative GI toxicity.43 This fact underscores the importance of administering the lowest dose for the shortest time necessary.
All NSAIDs carry a risk of CV adverse effects through prostaglandin inhibition, and the risk is increased with COX-2 inhibition, whether it is produced by those labeled COX-2 selective NSAIDs (e.g., celecoxib) or those that are nonselective inhibitors of both COX-1 and COX-2 (e.g., ibuprofen, naproxen, ketorolac).43 One proposed underlying mechanism for this adverse effect is that any drug that inhibits COX-2 will have prothrombotic effects, and those that inhibit COX-2 to a much greater extent than COX-1 will promote thrombosis more than others because of a disturbance in the physiologic balance between thromboxane A2, which promotes platelet aggregation, and prostacyclin, which antagonizes platelet aggregation.54,55 Postoperative studies showing elevated CV risk with NSAIDs56,57 led to a recommendation from the U.S. Food and Drug Administration against the use of any NSAIDs after high-risk open heart surgery.58
Most nonselective NSAIDs increase bleeding time though inhibition of COX-1. This is both drug and dose-related; therefore the lowest dose of nonopioids with minimal or no effect on bleeding time should be used in patients or procedures with high risk for surgical bleeding. Options include acetaminophen, celecoxib, choline magnesium trisalicylate, salsalate, and nabumetone.43 There is abundant research showing the safety of both preoperative and postoperative administration of nonopioids with minimal effect on bleeding time.43,59,60
NSAID-induced renal toxicity is relatively rare in otherwise healthy adults who are given NSAIDs during the short-term perioperative period.43 A Cochrane Collaboration Review could find no cases of renal failure or serious kidney problems in individuals with normal preoperative kidney function who were given NSAIDs after surgery in any of the 23 trials (1459 patients) reviewed.61
In contrast, individuals with acute or chronic volume depletion or hypotension rely on prostaglandin synthesis to maintain adequate renal blood flow, and NSAID inhibition of prostaglandin synthesis in such patients can cause acute renal failure (ARF).62 Patients at increased risk for ARF include those with cardiac failure, liver cirrhosis, ascites, diabetes, preexisting hypertension, preexisting renal impairment, advanced age, or left ventricular dysfunction, and those being treated with ACE inhibitors.62 It is generally recommended that NSAIDs are avoided in patients with chronic renal failure and in any patient with a creatinine clearance less than 30 mL/min.50 ARF can develop with the first NSAID dose in patients with elevated risk, and higher doses carry greater risk.49,50 Older adults and anyone with risk factors for ARF should be assessed frequently for adverse renal effects during perioperative NSAID therapy.43 Acetaminophen and opioids (e.g., fentanyl) would be better choices in patients with significant risk.43
The inflammatory process is initiated when bone is fractured, just as it is with any other tissue trauma. Prostaglandins have a central role in bone healing, providing a balance between bone formation and resorption.62 Despite the safe use of NSAIDs for decades to control pain associated with fracture, concerns have been raised about their use under these circumstances.62 Unfortunately, there are few well-designed studies that examine the effects of NSAIDs on bone healing in humans.43 The studies that have been done are retrospective in design and present conflicting findings. There have also been no adequate studies evaluating NSAIDs and spinal fusion. In an effort to offer a balanced appraisal of the existing, limited data, several researchers have agreed that short-term use of an NSAID after skeletal surgery, for a period less than 2 weeks, probably is safe and might be considered an option unless a patient has a comorbid condition that could negatively affect fracture healing, such as smoking, glucocorticoid use, or metabolic bone disease.62–64
Opioid analgesics
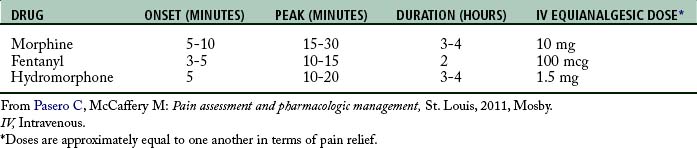
The first-line opioids for the treatment of immediate postoperative pain are morphine, hydromorphone, and fentanyl. At steady state (when equal amounts of a drug are entering and exiting the body), all opioids have similar characteristics; however, differences are noted when administration is by bolus technique (Table 31-3).28 For example, fentanyl is a lipophilic drug (soluble in fatty tissue), which allows it to move rapidly through membranes, a characteristic that produces a relatively fast onset and peak and short duration of action. By bolus technique, blood levels decline rapidly with fentanyl, but with repeated, regular dosing the drug can accumulate and redistribute from fatty tissue back into the blood. Under these circumstances, the terminal half-life of fentanyl (3 to 4 hours) can be extended by as much as fivefold.65 Morphine is a hydrophilic drug (soluble in water) and has a slower onset and peak and longer duration compared with other first-line opioids. Hydromorphone is less hydrophilic than morphine but less lipophilic than fentanyl, which produces pharmacokinetics intermediate between morphine and fentanyl.
Patient characteristics must be considered when selecting the appropriate opioid for pain treatment. For example, fentanyl is the recommended opioid for patients with end-stage organ failure because it has no clinically relevant metabolites.66 It also produces minimal hemodynamic adverse effects66 and is often preferred in patients who are hemodynamically unstable, such as the critically ill.28
The goals of care are also considered when selecting an opioid. A general principle of initial titration in patients with acute pain is to keep in mind the patient’s ongoing pain treatment plan. As an example, consider the patient who will have hydromorphone by intermittent IV boluses for ongoing postoperative pain management after discharge from the PACU. Unless the patient has severe, rapidly escalating pain on admission to the PACU, it makes sense to begin titration with hydromorphone so that the effects (both pain relief and adverse effects) of the drug that will be used for the next day or so can be evaluated more easily.28 The fast action of fentanyl makes the IV route the best choice when it is necessary to control quickly escalating, severe pain on admission to the PACU. In ambulatory surgery where the goal is to initiate oral analgesia as soon as possible to facilitate a rapid discharge to home, an oral opioid is often administered preoperatively followed by IV fentanyl for immediate pain relief in the PACU. The oral opioid that will be taken in the home setting can be administered before discharge to help ensure adequate pain relief during the ride home.
The nonopioid-opioid analgesics, such as acetaminophen or ibuprofen in combination oxycodone or hydrocodone, are highly popular for the treatment of mild to moderate acute pain and are the most common choice after invasive pain management therapies are discontinued and for pain treatment after discharge. However, it is important to remember that these combination drugs are not appropriate for severe pain because the maximum daily dose of the nonopioid limits the escalation of the opioid dose.28
Safe opioid titration
Research has shown that the relationship between pain intensity scores and dose requirements during and after titration in postoperative patients is not linear, suggesting that there is no specific dose that will relieve pain of a specific intensity.67 Many factors, such as sedation level, respiratory status, and previous analgesic and sedative intake, in addition to pain intensity must be considered when selecting an opioid dose. Some institutions have guidelines that require dosing based on a specific pain intensity (e.g., set orders that mandate the administration of 2 mg of IV morphine for pain ratings of 1 to 3 on a scale of 0 to 10; 4 mg for pain ratings of 4 to 6; and 6 mg for pain ratings of 7 to 10). This practice can be extremely dangerous and is strongly discouraged.28,38
The goal of titration is to use the smallest dose that provides satisfactory analgesia with the fewest adverse effects.28 At all times, nurses must strive to achieve a balance between pain relief and adverse effects. In opioid-naive patients (those who do not take regular daily doses of opioids) with moderate to severe pain, recommended starting IV doses are given, for example, 2 to 3 mg of morphine, 0.3 to 0.5 mg of hydromorphone, or 25 to 50 mcg of fentanyl.4,28 When an increase in the opioid dose is necessary and safe, this can be done by percentages. When a slight improvement in analgesia is needed, a 25% increase in the opioid dose may be sufficient; a 50% increase for moderate improvement; and a 100% increase may be indicated for strong improvement, such as when treating severe pain.28 The time when the dose can be increased is determined by the onset or peak effect of the opioid. The frequency of IV opioid doses during initial titration may be as often as every 5 to 15 minutes. Doses should not be increased in patients who are excessively sedated (e.g., unable to keep eyes open and falling asleep midsentence). In such cases, nonopioid analgesics should be added or increased (e.g., full doses of an NSAID and acetaminophen). Safe pain management is a primary objective.
Selected opioid therapies
The oral route for analgesic administration is generally reserved for later in the postoperative course when patients can tolerate oral intake; however, there has been a trend over the past few years to use modified-release (long-acting) oxycodone, initiated preoperatively, as a component in multimodal postoperative pain treatment plans.28 One study randomized 40 patients to receive either 20 mg of modified-release oxycodone or placebo preoperatively and every 12 hours postoperatively, in addition to IV morphine via PCA plus IV acetaminophen (1000 mg) for two days following lumbar discectomy.68 Those who received oxycodone consumed significantly less morphine; had significantly lower pain scores during rest, when coughing, and with movement; experienced less nausea and vomiting and earlier recovery of bowel function; and reported higher satisfaction with their pain treatment than those who received placebo.
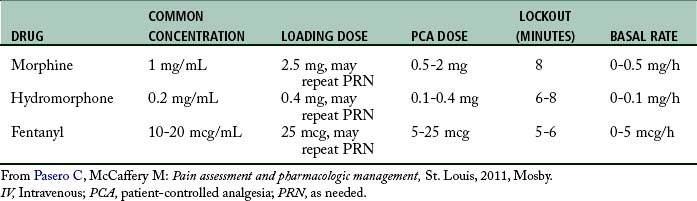
One of the most common methods for administering opioid analgesia in the postoperative setting is via IV patient-controlled analgesia (PCA), whereby patients manage their own pain by pressing a button attached to an infusion pump to deliver a preset bolus dose of pain medication. The concept of PCA recognizes that only the patient can feel the pain and only the patient knows how much analgesic will relieve it.28 This concept underscores the importance of telling family members and friends that PCA is for patient use only. Patients who use PCA must be able to understand the relationships between pain, pushing the PCA button, and pain relief. They must also be able to cognitively and physically use the PCA equipment.28 Table 31-4 provides starting IV PCA prescription ranges in opioid-naive adults.
The use of a basal rate (continuous infusion) during IV PCA is controversial in opioid-naive patients.4,28 The primary safeguard in PCA therapy is that a patient must be awake to self-administer a PCA dose. Patients who are excessively sedated are likely to drop the PCA button, thereby preventing further sedation and clinically significant respiratory depression. However, with a basal rate, the patient has no control over the delivery of a continuous infusion, and the built-in safeguard is gone. As a result, the American Pain Society recommends extreme caution in using basal rates for acute pain management in opioid-naive individuals.4 Absolutely essential to the safe use of a basal rate with PCA is close monitoring by nurses of sedation and respiratory status and prompt decreases in opioid dose (e.g., discontinue basal rate) if increased sedation is detected.28,69,70
Intraspinal analgesia has a long history of safety and effectiveness for major surgical procedures, and extensive guidelines for the nursing care of patients receiving this therapy are available.3,28 Delivery of analgesics via the intraspinal route is accomplished by inserting a needle into the subarachnoid space (for intrathecal analgesia) or the epidural space and injecting the analgesic (usually opioid and local anesthetic), or by threading a catheter through the needle and taping it in place temporarily for bolus dosing or continuous administration. Intrathecal catheters for acute pain management are used more often for providing anesthesia or a single analgesic bolus dose. Temporary epidural catheters for acute pain management are removed after 2 to 4 days.28 Epidural analgesia is administered by clinician-administered bolus, continuous infusion (basal rate), and patient-controlled epidural analgesia. The most common opioids administered intraspinally are morphine, fentanyl, and hydromorphone. These opioids are usually combined with a local anesthetic, most often ropivacaine or bupivacaine, to improve analgesia and produce an opioid dose-sparing effect.
Transition to oral analgesics
Providing adequate oral analgesia and avoiding gaps in pain control after invasive therapies are discontinued are keys to optimizing patient outcomes. Equianalgesic dose charts are widely available4,28 and helpful when switching from one drug or route of administration to another to ensure that patients receive approximately the same pain relief that they were receiving before the switch. Table 31-5 provides equianalgesic dosing guidelines when transitioning a patient from IV to oral analgesia.
| DAILY DOSE IV ANALGESIA | ||
|---|---|---|
| MORPHINE (MG) | HYDROMORPHONE (DILAUDID; MG) | SUGGESTED DAILY ORAL DOSE (MG)* |
| 15 | 2.2 | Hydrocodone 5/APAP 500 (Vicodin) 1 tab every 4 hours (8 tabs maximum)† |
| 20 | 3 | |
NOTE: Recommend a laxative to patients receiving daily opioids.
This table provides a guideline to help ensure approximately the same pain relief when switching from IV to oral opioid analgesia. It can be posted in clinical units where opioid orders are written for the clinician’s quick reference.
* In calculating the oral dose, acetaminophen is given the following opioid values: acetaminophen 325 mg = hydrocodone 2.5 mg or oxycodone 1.5 mg; acetaminophen 650 mg = hydrocodone 5 mg or oxycodone 3 mg. For example, Vicodin (hydrocodone 5 mg/acetaminophen 500 mg) = hydrocodone 7.5 to 8 mg.
† Maximum acetaminophen dosage is 4000 mg/day. For some patients, such as older adults or heavy consumers of alcohol, the dose should be 2000 mg or less per day.
From Pasero C, McCaffery M: Pain assessment and pharmacologic management, St. Louis, 2011, Mosby. Copyright 2003, McCaffery M. May be duplicated for use in clinical practice. APAP, Acetaminophen; PRN, as needed.
Adverse effects of opioid analgesics
The most common adverse effects of opioid analgesics are nausea, vomiting, constipation, pruritus, and sedation.28 Respiratory depression is less common but the most feared of the opioid adverse effects. In surgical patients, postoperative ileus can become a major complication as well. A common perception is that opioids cause hypotension, but research shows that the opioid doses commonly used for pain management rarely cause this adverse effect.71 Many other factors, including pain, can cause hypotension, underscoring the importance of promptly addressing unrelieved pain. When hypotension is a concern, it can be minimized by administering the opioid slowly, keeping the patient supine, and optimizing intravascular volume.71
There is great individual variation in the development of opioid adverse effects, which is why most must be managed with an individualized approach. Prevention rather than treatment of opioid adverse effects is a key principle of pain management.28 Most opioid adverse effects are dose related. Therefore a practical approach includes the use of nonsedating analgesics that have an opioid dose-sparing effect, such as nonopioids and local anesthetics, so that the lowest effective opioid dose can be given. For many patients, simply decreasing the opioid dose is sufficient to eliminate or make an adverse effect tolerable.28
Postoperative nausea and vomiting (PONV) are among the most unpleasant of the adverse effects associated with surgery, and it can have a negative effect on patient outcomes and increase the burden on nursing staff.72 Consensus guidelines have identified opioids as a primary risk factor for the PONV.73–75 Other risks are female sex, nonsmoking status, and history of PONV or motion sickness. Guidelines recommend that all patients are evaluated for risk, baseline risk factors are reduced if possible, multimodal analgesia is provided so that no opioid or the lowest effective opioid dose can be given, and prophylactic treatment (e.g., dexamethasone and a serotonin receptor antagonist such as ondansetron at the end of surgery) is given to patients with moderate risk.73–75 More aggressive interventions should be used in patients with high risk.28
Postoperative ileus is the temporary impairment of GI motility following surgery. It has been described as part of the normal pathophysiologic response to surgical injury, characterized by delayed gastric emptying and inability to pass gas or stool and exacerbated by opioid consumption.76 Unresolved ileus is a postoperative complication that can cause significant discomfort and patient morbidity.76 The incidence is particularly high with colorectal surgery. A major advance in the management of postoperative ileus is the approval of alvimopan for the acceleration of time to upper and lower GI recovery after partial large or small bowel resection.28
Constipation is an almost universal opioid adverse effect (i.e., tolerance rarely develops) and requires a preventive approach and aggressive management if symptoms are detected. Risk is elevated with opioid use, advanced age, and immobility.28 The usual initial daily therapy is a combination of stool softener and mild peristaltic stimulant, such as senna. Patients should be instructed to continue this regimen as long as they are taking opioids.
Pruritus is an adverse effect, not an allergic reaction to opioids.71 It is one of the most common adverse effects when opioids are delivered by intraspinal routes.77 Numerous pharmacologic strategies have been tried for relief of pruritus. IV serotonin receptor antagonists (e.g., ondansetron, dolasetron) are effective for the prevention of pruritus from intraspinal opioids.78 Although antihistamines such as diphenhydramine are commonly used, there is no strong evidence that they relieve opioid-induced pruritus.79 Patients may report being less bothered by itching after taking an antihistamine, but this is likely the result of sedating effects.71 Sedation can be problematic in those already at risk for excessive sedation, such as postoperative patients, as this can lead to life-threatening respiratory depression.80 A common clinical observation is that patients with postoperative opioid-induced pruritus usually have well-controlled pain.28 This may be because painful stimuli can inhibit itching and inhibition of pain processing may enhance itching.71 This helps to explain why the single most effective, safest, and least expensive treatment for pruritus is opioid dose reduction. Opioid orders should include the expectation that the opioid dose will be decreased by 25% before or in conjunction with pharmacologic management.28
Sedation and respiratory depression
Most patients experience sedation at the beginning of opioid therapy and whenever the opioid dose is increased significantly.28 In addition to adversely affecting the patient’s ability to participate in the postoperative recovery process, if left untreated, excessive sedation can progress to clinically significant respiratory depression.28,69 The observation that excessive sedation precedes opioid-induced sedation indicates that systematic sedation assessment is an essential aspect of the care of opioid-naive patients receiving opioid therapy.69,70 Nursing assessment of sedation is convenient and inexpensive and takes minimal time to perform. A simple, easy-to-understand and communicate sedation scale, developed for assessment of unwanted sedation and that includes what should be done at each level of sedation is widely recommended to enhance accuracy and consistency of assessment, monitor trends, and communicate effectively between members of the health care team (Box 31-3).28,69,70 The use of scales that include agitation assessment and other parameters are appropriate when sedation is desirable but are not appropriate for assessment when sedation is undesirable, such as during opioid administration for pain management.28,69 Many hospitals adopt two scales and distinguish their use according to these goals of care. All opioid orders and titration protocols should include the expectation that nurses will stop titration or decrease the opioid dose immediately if excessive sedation is detected.
BOX 31-3 Pasero Opioid-Induced Sedation Scale (POSS) with Interventions
2 = Slightly drowsy, easily aroused
3 = Frequently drowsy, arousable, drifts off to sleep during conversation
4 = Somnolent, minimal or no response to verbal and physical stimulation
NSAID, Nonsteroidal antiinflammatory drug.
From Pasero C, McCaffery M: Pain assessment and pharmacologic management, St. Louis, 2011, Mosby. Copyright 1994, Pasero C. Used with permission.
Respiratory depression is assessed on the basis of what is normal for a particular individual and is usually described as clinically significant when there is a decrease in the rate, depth, and regularity of respirations from baseline, rather than just by a specific number of respirations per minute.24,69 There are many risk factors for opioid-induced respiratory depression including opioid naivety, older age (65 years or older), obesity, obstructive sleep apnea, and preexisting pulmonary disease or dysfunction or other comorbidities.28 Risk is elevated during the first 24 hours following surgery and in patients who require a high dose of opioid in a short period of time (e.g., greater than 10 mg of IV morphine or equivalent in the PACU).
Clinically significant opioid-induced respiratory depression can be prevented by careful titration and close nurse monitoring of sedation and respiratory status.28 Administration of the lowest effective opioid dose is critical, and this is best accomplished with the use of a multimodal analgesic approach that includes nonopioid analgesics. A comprehensive respiratory assessment constitutes more than counting a patient’s respiratory rate.69 A proper assessment requires the nurse or nurse technician to watch the rise and fall of the patient’s chest to determine rate, depth, and regularity of respirations. Listening to the sound of the patient’s respiration is critical as well.69 Snoring indicates airway obstruction and must be addressed promptly with repositioning, and depending on severity, a request for respiratory therapy consultation and further evaluation.81
Hospital protocols and opioid orders should include the expectation that nurses will administer the opioid antagonist naloxone intravenously for the treatment of clinically significant respiratory depression.28 The goal is to reverse just the sedation and respiratory depressant effects of the opioid. To this end, it should be diluted and titrated slowly to prevent severe pain and other adverse effects, which can include hypertension, tachycardia, ventricular dysrhythmias, pulmonary edema, and cardiac arrest (see the third footnote in Box 31-3 for correct technique).4,28 Sometimes more than one dose of naloxone is necessary, because naloxone has a shorter duration of action (1 hour in most patients) than most opioids.28
Addiction, physical dependence, and tolerance
The terms physical dependence and tolerance often are confused with addiction; therefore clarification of definitions is important.28 The definitions proposed in a 2001 consensus statement by the American Academy of Pain Medicine, the American Pain Society, and the American Society of Addiction Medicine are82:
• Physical dependence is a normal response that occurs with repeated administration of the opioid for more than two weeks and cannot be equated with addictive disease. It is manifested by the occurrence of withdrawal symptoms when the opioid is suddenly stopped or rapidly reduced or when an antagonist such as naloxone is given. Withdrawal symptoms may be suppressed by the natural, gradual reduction of the opioid as pain decreases or by gradual, systematic reduction, referred to as tapering.82
• Tolerance is also a normal response that occurs with regular administration of an opioid and consists of a decrease in one or more effects of the opioid (e.g., decreased analgesia, sedation, respiratory depression). Tolerance cannot be equated with addictive disease. Tolerance to analgesia usually occurs in the first days to 2 weeks of opioid therapy, but is uncommon after that. It may be treated with increases in dose. However, disease progression, not tolerance to analgesia, appears to be the reason for most dose escalations. Stable pain usually results in stable opioid doses; therefore tolerance poses few clinical problems.82
• Opioid addiction, or addictive disease, is a chronic neurologic and biologic disease. The development and characteristics of addiction are influenced by genetic, psychosocial, and environmental factors. No single cause of addiction, such as taking an opioid for pain relief, has been found. Addiction is characterized by behaviors that include one or more of the following: impaired control over drug use, compulsive use, continued use despite harm, and craving.82 This statement reinforces that taking opioids for pain relief is not addiction, no matter how long a person takes opioids or at what doses.
• Pseudoaddiction is a mistaken diagnosis of addictive disease.83 When a patient’s pain is not well controlled, the patient may begin to manifest symptoms suggestive of addictive disease. In an effort to obtain adequate pain relief, the patient may respond with demanding behavior, escalating demands for more or different medications, and repeated requests for opioids on time or before the prescribed interval between doses has elapsed. Pain relief typically eliminates these behaviors and is often accomplished by increasing opioid doses or decreasing intervals between doses.
The incidence of addiction as a result of taking an opioid for therapeutic reasons, such as postoperative pain management, is thought to be rare.84,85 An evidence-based review of all available studies on the development of addiction and aberrant drug-related behaviors in patients with persistent non–cancer-related pain being treated with opioids calculated the percentage of abuse and addiction to be 0.19%.86 These data suggest that patients with no past or present history of abuse or addiction usually remain responsible medication users over time. Similarly, a registry study of 227 patients who were treated with modified-release oxycodone and followed for up to 3 years after participating in a clinical trial also showed a low occurrence of problematic drug-related behavior—there were just six cases of misuse and no cases of new addiction.87 Again, this number is reassuring in terms of the rate of iatrogenic addiction among those with no history of abuse.
Opioid administration to patients with addictive disease
Opioids, if they are appropriate, should not be withheld from patients with pain who also have addictive disease.28,88 The perioperative period is not the optimal time to attempt detoxification or rehabilitation of a patient who is abusing opioids or other substances.88 Furthermore, there is no research showing that providing opioid analgesics to a person with addictive disease will worsen the disease, or that withholding opioid analgesics when needed will increase the likelihood of recovery.28 In fact, withholding opioids in this situation may cause significant pain, which can increase the patient’s stress level and lead to increased craving for drugs of abuse. Clearly, providing pain relief to the patient with addictive disease, even when it includes opioids, is preferable to withholding opioids.28
Management of the opioid-dependent surgical patient with chronic pain
The management of postoperative pain in patients with underlying chronic pain can be extremely challenging. The key to success in the postoperative period is optimizing the management of the chronic pain before the surgical procedure.28 If preexisting pain is poorly controlled preoperatively, the primary care provider or anesthesia provider should be contacted for evaluation and appropriate orders.
A multimodal postoperative pain treatment plan, initiated preoperatively whenever possible, is essential in patients with underlying chronic pain. NSAIDs that do not affect bleeding time (see earlier in chapter) do not need to be discontinued preoperatively.52 Anticonvulsants and antidepressants, which are often administered for treatment of persistent neuropathic pain, should also be initiated or continued if taken preoperatively.52 Guidelines recommend the continuation of opioid analgesics to prevent opioid withdrawal syndrome in patients who are taking them preoperatively for preexisting pain.28,52,89
Patients who have been taking opioids on a long-term basis preoperatively are likely to be opioid tolerant and may require higher postoperative opioid doses in the postoperative period compared with opioid-naive patients. Unfortunately there are no evidence-based guidelines for predicting postoperative opioid requirements on the basis of the opioid dose consumed before surgery. One suggestion is to expect opioid requirements postoperatively in the opioid-tolerant patient to be twofold to fourfold the dose required in an opioid-naive person90; however, individualization of care is essential.28 Occasionally patients are underdosed by clinicians who are fearful of the high doses often required by patients who are opioid tolerant. Tolerance to the adverse effects of opioids develops more rapidly than to analgesia, which means that opioids can be safely titrated to relatively high doses to provide adequate analgesia.89 It is reassuring to know that, although respiratory depression can occur in opioid tolerant patients, the occurrence is rare when doses are carefully titrated and the patient is monitored appropriately for effect.
Local anesthetics
Local anesthetics have a long history of safe and effective use for acute pain management. They are given by a variety of routes of administration for both procedural and postoperative pain treatment and are generally well tolerated by most individuals.13
For many years regional anesthesia has been administered via single injection peripheral nerve blocks to target a specific nerve or nerve plexus. Although extremely effective, this method has limited usefulness for postoperative pain treatment because of a short duration of action (4 to 12 hours for bupivacaine and ropivacaine).13 Continuous peripheral nerve block is a relatively new pain management technique that offers an alternative. It involves establishment of an initial block followed by placement of a catheter through which an infusion of local anesthetic is administered continuously, with or without PCA capability. Advances in technology have allowed the expansion of this technique to the outpatient setting where catheters can be removed by patients or families and small disposable pumps can be discarded easily at the end of therapy.13 Another therapy, continuous local anesthetic wound infusion, involves the surgeon’s placement of a catheter subcutaneously into the surgical wound at the end of the surgical procedure to be used for infusion of a local anesthetic to control postoperative pain.13 There is extensive research supporting both continuous peripheral nerve blocks and wound infusions as primary postoperative analgesic strategies following a wide variety of surgical procedures.13,91,92 Guidelines for the nursing care of patients receiving these therapies are available.3,13
IV lidocaine is occasionally used for acute pain that has been refractory to first-line treatment approaches. One of its major benefits is its ability to reduce postoperative GI complications93 A metaanalysis of eight studies involving patients who underwent abdominal surgery revealed that IV lidocaine compared with placebo was associated with better pain control as well as decreases in the duration of ileus, nausea, and vomiting and length of hospital stay.94 Guidelines for the use of IV lidocaine for postoperative pain are provided elsewhere.13
Anticonvulsants
The anticonvulsants gabapentin and pregabalin are first-line analgesics for neuropathic pain and are increasingly being added to postoperative pain treatment plans to address the neuropathic component of surgical pain. Although further research is needed,95 anticonvulsants have been shown to improve analgesia, allow lower doses of other analgesics, and help to prevent persistent neuropathic postsurgical pain syndromes.96 There is no consensus on an optimal dosing regimen; both single and multiple dosing have been described.97–99 Generally, preoperative doses range between 400 and 1200 mg, and postoperative doses are typically 400 to 600 mg every 6 to 8 hours.13 Treatment varies from 24 hours to several days. The most concerning adverse effect of anticonvulsants is sedation; therefore sedation levels should be watched closely during therapy (see Box 31-3).
Nonpharmacologic methods
Research shows that most individuals want to be offered alternative self-management strategies for their health care.100,101 Nonpharmacologic methods that are used to provide comfort and pain relief include the body-based (physical) modalities, such as massage, acupuncture, and application of heat and cold, and the mind-body (cognitive-behavioral) methods, such as relaxation breathing, imagery, and meditation. There are also biologically-based therapies that involve the use of herbs and vitamins, as well as energy therapies such as reiki and tai chi.100 Biologically based and energy therapies are used most often in the outpatient setting.
Nonpharmacologic methods may be effective alone for mild to some moderate-intensity pain and are used to complement, but not replace, pharmacologic therapies for more severe pain.102 The effectiveness of nonpharmacologic methods can be unpredictable, and although not all have been shown to relieve pain, they offer many benefits to patients with pain. For example, researchers have demonstrated that nonpharmacologic methods can facilitate relaxation and reduce anxiety and stress.102–104 Many patients find that the use of nonpharmacologic methods helps them cope better with their pain and feel greater control over the pain experience.
Although time is limited in the acute care setting for implementation of nonpharmacologic interventions,102 nurses have an important role in providing them and teaching patients about their use.105 The following list includes some examples of nonpharmacologic interventions for acute pain that are relatively easy to incorporate into daily clinical practice; they can be used individually or in combination with other nondrug therapies.
• Proper body alignment achieved through optimal positioning and regular repositioning can help to prevent or relieve pain. Pillows can be used to maintain the position and support the patient’s back and extremities.
• Thermal measures such as the application of localized, superficial heat and cooling may relieve pain and provide comfort by decreasing sensitivity to pain and muscle spasms and alleviating joint and muscle aches. The two can be used interchangeably.
• Mind-body therapies are designed to enhance the mind’s capacity to affect bodily function and symptoms and include music therapy, distraction techniques, meditation, prayer, hypnosis, guided imagery, relaxation techniques, and pet therapy.
1. Abouleish AE, Ranganathan G. Economics and costs: a primer for acute pain management specialists. Sinatra RS, et al. Acute pain management. New York: Cambridge University Press, 2009.
2. McCaffery M, et al. Assessment. In: Pasero C, McCaffery M. Pain assessment and pharmacologic management. St. Louis: Mosby, 2011.
3. Pasero C, et al. The nurse’s perspective on acute pain management. In: Sinatra RS, et al. Acute pain management. New York: Cambridge University Press, 2009.
4. American Pain Society (APS): Principles of analgesic use in the treatment of acute and cancer pain. ed 6. Glenview, IL: APS; 2008.
5. McCaffery M. Nursing practice theories related to cognition, bodily pain, and man-environment interactions. Los Angeles: University of California, Los Angeles; 1968.
6. Pasero C, Portenoy RK. Neurophysiology of pain and analgesia and the pathophysiology of neuropathic pain. In: Pasero C, McCaffery M. Pain assessment and pharmacologic management. St. Louis: Mosby, 2011.
7. Argoff CE, et al. Multimodal analgesia for chronic pain: rationale and future directions. Pain Med.2009;10(Suppl 2):S53–S66.
8. Marchand S. The physiology of pain mechanisms: from the periphery to the brain. Rheum Dis Clin N Am.2008;34(2):285–309.
9. Vadivelu N, et al. Pain pathways and acute pain processing. In: Sinatra RS, et al. Acute pain management. New York: Cambridge University Press, 2009.
10. Pasero C, et al. Nonopioid analgesics. In: Pasero C, McCaffery M. Pain assessment and pharmacologic management. St. Louis: Mosby, 2011.
11. Dib-Jajj SD, et al. Voltage-gated sodium channels: targets for pain. Pain Med.2009;10(7):1260–1269.
12. Adler JE, et al. Modulation of neuropathic pain by a glial-driven factor. Pain Med. 2009;10(7):1229–1236.
13. Pasero C, et al. Adjuvant analgesics. In: Pasero C, McCaffery M, et al. Pain assessment and pharmacologic management. St. Louis: Mosby, 2011.
14. Ghouri MK, et al. Pathophysiology of acute pain. Sinatra RS, et al. Acute pain management. New York: Cambridge University Press; 2009.
15. Beilin B, et al. The effects of postoperative pain management on immune response to surgery. Anesth Analg. 2003;97:822–827.
16. Page GG. Acute pain and immune impairment. Pain Clinical Updates. 2005;12(1):1–4.
17. Weatherstone KB, et al. Are there opportunities to decrease nosocomial infection by choice of analgesic regimen. Arch Pediatr Adolesc Med. 2003;157:1108–1114.
18. Guay J. Postoperative pain significantly influences postoperative blood loss in patients undergoing total knee replacement. Pain Med. 2006;7(6):476–482.
19. Erb J, et al. Interactions between pulmonary performance and movement-evoked pain in the immediate postsurgical period: implications for perioperative research and treatment. Reg Anesth Pain Med.2008;33(4):312–319.
20. Shea RA, et al. Pain intensity and postoperative pulmonary complications among the elderly after abdominal surgery. Heart Lung. 2002;31:440–449.
21. Hanley MA, et al. Chronic pain associated with upper-limb loss. Am J Phys Med Rehabil. 2009;88(9):742–751.
22. Kehlet H, et al. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367(9522):1618–1625.
23. Hanley MA, et al. Preamputation pain and acute pain predict chronic pain after lower extremity amputation. J Pain. 2007;8(2):102–109.
24. Spagrud LJ, et al. Children’s self-report of pain intensity. The faces pain scale—revised. Am J Nurs. 2003;103(12):62–64. www.painsourcebook.ca. Scale is available for free for clinical and research use
25. Ware L, et al. Evaluation of the revised faces pain scale, verbal descriptor scale, numeric rating scale and Iowa pain thermometer in older minority adults. Pain Manage Nurs. 2006;71:117–125.
26. Herr KA, et al. Pain intensity assessment in older adults: use of experimental pain to compare psychometric properties and usability of selected pain scales with younger adults. Clin J Pain.2004;20:207–219.
27. Li L, et al. Postoperative pain intensity assessment: a comparison of four scales in Chinese adult. Pain Med.2007;8(3):223–234.
28. Pasero C. Opioid analgesics. In: Pasero C, McCaffery M, et al. Pain assessment and pharmacologic management. St. Louis: Mosby, 2011.
29. Herr K, et al. Pain assessment in the nonverbal patient: position statement with clinical practice recommendations. Pain Manag Nurs.2006;7(2):44–52.
30. Pasero C. Challenges in pain assessment. J Perianesth Nurs. 2009;24(1):50–54.
31. Puntillo KA, et al. Patient’s perceptions and responses to procedural pain: results from Thunder Project II. Am J Crit Care.2001;10(4):238–251.
32. Stanik-Hutt JA, et al. Pain experiences of traumatically injured patients in a critical care setting. Am J Crit Care. 2001;10(4):252–259.
33. Pasero C, McCaffery M. No self report means no pain intensity. Am J Nurs. 2005;105(10):50–53.
34. Gelinas C, Arbour C. Behavioral and physiologic indicators during a nociceptive procedure in conscious and unconscious mechanically ventilated patients: similar or different. J Crit Care.2009;24(4):628e.7–17.
35. Arbour C, Gelinas C. Are vital signs valid indicators for the assessment of pain in postoperative cardiac surgery ICU adults. Intensive Crit Care Nurs. 2010;26(2):83–90.
36. Blumstein HA, Moore D. Visual analog pain scores do not define desire for analgesia in paitents with acute pain. Acad Emerg Med. 2003;10(3):211–214.
37. Lucas CE, et al. Kindness kills: the negative impact of pain as the fifth vital sign. J Am Coll Surg.2007;205(1):101–107.
38. Vila H, et al. The efficacy and safety of pain management before and after implementation of hospital-wide pain management standards: is patient safety compromised by treatment based solely on numerical pain ratings. Anesth Analg.2005;101(2):474–480.
39. Ashburn MA, et al. Practice guidelines for acute pain management in the perioperative setting. An updated report by the American Society of Anesthesiologists task force on acute pain management. Anesthesiology. 2004;100(6):1573–1581.
40. Dworkin RH, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain.2007;132(3):237–251.
41. Pasero C. Perioperative rectal administration of nonopioid analgesics. J Perianesth Nurs. 2010;25(1):5–6.
42. Kaestli LZ, et al. Use of transdermal drug formulations in the elderly. Drugs Aging. 2008;25(4):269–280.
43. Pasero C. Nonopioid analgesics. In: Pasero C, McCaffery M, et al. Pain assessment and pharmacologic management. St. Louis: Mosby, 2011.
44. Sinatra RS, et al. Efficacy and safety of single and repeated administration of 1 gram intravenous acetaminophen injection (paracetamol) for pain management after major orthopedic surgery. Anesthesiology. 2005;102(4):822–831.
45. Dahl V, et al. Ibuprofen vs. acetaminophen after arthroscopically assisted anterior cruciate ligament reconstruction. Eur J Anaesthesiol. 2004;21(6):471–475.
46. Singla N, et al. A multi-center, randomized, double-blind placebo-controlled trial of intravenous-ibuprofen (IV-ibuprofen) for treatment of pain in post-operative orthopedic adult patients. Pain Med. 2010;11(8):1284–1292.
47. Burke A, et al. Analgesic-antipyretic agents: pharmacotherapy of gout. Brunton LL, et al. Goodman & Gilman’s the pharmacological basis of therapeutics, ed 11, New York: McGraw-Hill, 2006.
48. Schug SA, Manopas A. Update on the role of non-opioids for postoperative pain treatment. Best Prac Res Clin Anesth. 2007;21(1):15–30.
49. Laine L, et al. COX-2 selective inhibitors in the treatment of osteoarthritis. Semin Arthritis Rheum. 2008;38(3):165–187.
50. Launay-Vacher V, et al. Treatment of pain in patients with renal insufficiency: the World Health Organization three-step ladder adapted. J Pain.2005;6(3):137–148.
51. Leo RJ. Safe analgesic use in patients with renal dysfunction. Pract Pain Manage. 2008;12:22–27.
52. Ashraf W, et al. Guidelines for preoperative administration of patients’ home medications. J Perianesth Nurs. 2004;19(4):228–233.
53. Munsterhjelm E, et al. Dose-dependent inhibition of platelet function by acetaminophen in healthy volunteer. Anesthesiology. 2005;103(4):712–717.
54. FitzGerald GA. Coxibs and cardiovascular disease. N Engl J Med. 2004;351(17):1709–1711.
55. Scheiman JM. Cardiovascular risks associated with cyclooxygenase-2 selective inhibitors and traditional nonsteroidal antiinflammatory drugs, 2006. available at www.medscape.com/viewarticle/543804_1, February 20, 2011. Accessed
56. Nussmeier NA, et al. Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med. 2005;352(11):1081–1091.
57. Ott E, et al. Efficacy and safety of the cyclooxygenase 2 inhibitors parecoxib and valdecoxib in patients undergoing coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2003;125(6):1481–1492.
58. United States Food and Drug Administration (FDA): Medication guide for non-steroidal anti-inflammatory drugs (NSAIDs), 2007. available at: www.fda.gov/cder/drug/infopage/COX2/NSAIDmedguide.htm, February 10, 2011. Accessed
59. Dorr LD, et al. Multimodal analgesia without parenteral narcotics for total knee arthroplasty. J Arthrop. 2008;23(4):502–508.
60. Sun T, et al. Perioperative versus postoperative celecoxib on patient outcomes after major plastic surgery procedures. Anesth Analg. 2008;106(3):950–958.
61. Lee A, et al. Effects of nonsteroidal anti-inflammatory drugs on postoperative renal function in adults with normal renal function. Cochrane Database Syst Rev. 2007;18(2). (CD002765)
62. Helstrom J, Rosow CE. Nonsteroidal anti-inflammatory drugs in postoperative pain. Shorten G, et al. Postoperative pain management: an evidence-based guide to practice. Philadelphia: Saunders; 2006.
63. Einhorn TA. Cox-2 inhibitors and fracture healing: arguments for such an effect and a suggestion about caution in use. J Bone Miner Res. 2003;18(3):584.
64. Langford RM, Mehta V. Selective cyclooxygenase inhibition: its role in pain and anaesthesia. Biomed Pharmacother.2006;60(7):323–328.
65. Liu LL, Gropper MA. Postoperative analgesia and sedation in the adult intensive care unit: a guide to drug selection. Drugs.2003;63(8):755–767.
66. Fukuda K. Intravenous opioid anesthetics. Miller RD, ed. Miller’s anesthesia, ed 6, St. Louis: Churchill Livingstone, 2005.
67. Aubrun F, Riou B. In reply to correspondence. Anesthesiology. 2004;100(3):745.
68. Blumenthal S, et al. Postoperative intravenous morphine consumption, pain scores, and side effects with perioperative oral controlled-release oxycodone after lumbar discectomy. Anesth Analg. 2007;105(1):233–237.
69. Pasero C. Assessment of sedation during opioid administration for pain management. J Perianesth Nurs. 2009;24(3):186–190.
70. Nisbet AT, Mooney-Cotter F. Selected scales for reporting opioid-induced sedation. Pain Manage Nurs. 2009;10(3):154–164.
71. Ho KT, Gan TJ. Opioid-related adverse effects and treatment options. In: Sinatra RS, et al. Acute pain management. New York: Cambridge University Press, 2009.
72. Miaskowski C. A review of the incidence, causes, consequences, and management of gastrointestinal effects associated with postoperative opioid administration. J Perianesth Nurs. 2009;24(4):222–228.
73. American Society of PeriAnesthesia Nurses (ASPAN): ASPAN’s evidence-based clinical practice guideline for the prevention and/or management of PONV/PDNV. J Perianesth Nurs.2006;21(4):230–250.
74. Gan TJ, et al. Consensus guidelines for managing postoperative nausea and vomiting. Anesth Analg. 2003;97(1):62–71.
75. Gan TJ, et al. Society for Ambulatory Anesthesia guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2007;105(6):1615–1628.
76. Kehlet H. Preventive measures to minimize or avoid postoperative ileus. Sem Colon Rec Surg. 2005;16(4):203–206.
77. Ganesh A, Maxwell LG. Pathophysiology and management of opioid-induced pruritus. Drugs. 2007;67(6):2323–2333.
78. Iatrou CA, et al. Prophylactic intravenous ondansetron and dolasetron in intrathecal morphine-induced pruritus: a randomized, double-blinded, placebo-controlled study. Anesth Analg.2005;101(5):1516–1520.
79. Grape S, Schug SA. Epidural and spinal analgesia. Macintyre PE, et al. Clinical pain management: acute pain. ed 2. London: Hodder Arnold; 2008.
80. Anwari JS, Iqbal S. Antihistamines and potentiation of opioid induced sedation and respiratory depression. Anaesthesia. 2003;58(5):494–495.
81. American Society of Anesthesiologists: Practice guidelines for the perioperative management of patients with obstructive sleep apnea. Anesthesiology.2006;104(5):1081–1093.
82. American Society of Addiction Medicine: Definitions related to the use of opioids for the treatment of pain, 2001. available at: www.painmed.org/pdf/definition.pdf., March 10, 2009. Accessed
83. Weissman DE, Haddox JD. Opioid pseudoaddiction—an iatrogenic syndrom. Pain. 1989;36(3):363–366.
84. Jackson KC. Opioid pharmacology. In: Smith HS, ed. Current therapy in pain. Philadelphia: Saunders, 2009.
85. Rowbotham DJ, et al. Clinical pharmacology: opioids. Macintyre PE, et al. Clinical pain management: acute pain. ed 2. London: Hodder Arnold; 2009.
86. Fishbain DA, et al. What percentage of chronic nonmalignant pain patients exposed to chronic opioid analgesic therapy develop abuse/addiction and/or aberrant drug-related behaviors? A structured evidence-based review. Pain Med. 2008;9(4):444–459.
87. Portenoy RK, et al. Long-term use of controlled-release oxycodone for noncancer pain: results of a 3-year registry study. Clin J Pain.2007;23(4):287–299.
88. Mitra S, Sinatra RS. Patients with opioid dependence and substance abuse. In: Sinatra RS, et al. Acute pain management. New York: Cambridge University Press, 2009.
89. Mehta V, Langford RM. Acute pain management for opioid dependent patients. Anaesthesia. 2006;61(3):269–272.
90. Carroll IR, et al. Management of perioperative pain in patients chronically consuming opioids. Reg Anesth Pain Med. 2004;29(6):576–591.
91. Richman JM, et al. Does continuous peripheral nerve block provide superior pain control to opioids? A meta-analysis. Anesth Analg. 2006;102(1):248–257.
92. Liu SS, et al. Efficacy of continuous wound catheters delivering local anesthetic for postoperative analgesia: a quantitative and qualitative systematic review of randomized controlled trials. J Am Coll Surg. 2006;203(6):915–932.
93. Wright JL, et al. A brief review of innovative uses for local anesthetics. Curr Opin Anaesthesiol. 2008;21(5):651–656.
94. Marret E, et al. Meta-analysis of intravenous lidocaine and postoperative recovery after abdominal surgery. Br J Surg. 2008;95(11):1331–1338.
95. Wiffen PJ, et al. Anticonvulsant drugs for acute and chronic pain. Cochrane Database Syst Rev. 3, 2005. (CD001133)
96. Dauri M, et al. Gabapentin and pregabalin for the acute post-operative pain management. A systematic-narrative review of the recent clinical evidences. Curr Drug Targets. 2009;10(8):716–733.
97. Ho KY, et al. Gabapentin and postoperative pain—a systematic review of randomized controlled trials. Pain. 2006;126(1–3):91–101.
98. Peng PWH, et al. Use of gabapentin for perioperative pain control—a meta-analysis. Pain Res Manage. 2007;12(2):85–91.
99. Tiippana EM, et al. Do surgical patients benefit from perioperative gabapentin/pregabalin? A systematic review of efficacy and safety. Anesth Analg. 2007;104(6):1545–1556.
100. Bruckenthal P. Integrating nonpharmacologic and alternative strategies into a comprehensive management approach for older adults with pain. Pain Manage Nurs. 2010;11(2):S23–S31.
101. National Center for Complementary and Alternative Medicine (NCCAM): What is CAM. available at: nccam.nih.gov/health/whatiscam/overview.htm, February 23, 2010. Accessed
102. McCaffery M. What is the role of nondrug methods in the nursing care of patients with acute pain. Pain Manage Nurs. 2002;3(3):77–80.
103. Kwekkeboom KL, et al. Mind-body treatments for the pain-fatigue-sleep disturbance symptom cluster in persons with cancer. J Pain Symptom Manage. 2010;39:126.
104. Allred KD, et al. The effect of music on postoperative pain and anxiety. Pain Manage Nurs.2010;11:15.
105. Gatlin CG, Schulmeister L. When medication is not enough: nonpharmacologic management of pain. Clin J Onc Nurs.2007;11:699.