Chapter 125 Pain in Spine Disease
Back pain is one of the most common complaints in the general population; more than two thirds of the population have back pain at least once during life.1,2 Low back problems are reported to be the second leading complaint in outpatient consultations and the third complaint in hospital admissions.1,3 Annual back pain prevalence is reported to be 15% to 45%.4 Back pain is the most common cause of activity limitation in younger individuals,5 and it is the third most common cause of surgical procedures in the United States, in particular, fusion surgery.4,6 Although back pain is very common, 60% to 70% of patients with acute back pain are likely to recover in less than 3 months without functional loss. The prognosis worsens significantly when pain becomes persistent for more than 6 months, with less than 50% complete recovery.4 The recurrence rate is also higher in patients with persistent back pain.4 Many variables influence recovery, recurrence rates, and the probability of returning to work. Factors related to increased disability include gender (male), age, unemployment,7 stressful work environment,8 and compensation related to disability.7–10 Psychological factors also influence prognosis.11–15
Although less common than low back pain, neck pain is also a frequent reason for seeking health care. The most common causes of neck pain include musculoskeletal disorders and degenerative disease of the cervical spine.5,16 The lifetime prevalence of chronic neck pain ranges from 35% to 50%, and the cross-sectional prevalence is 10% to 35%.16–18
Most episodes of low back, neck, or related limb pain do not come to medical attention or are likely managed by primary care professionals. Specialized attention is most often required for severe, refractory, and chronic pain. However, even this smaller subsection of patients constitutes a large, imposing significant health-care challenge and burden to social security systems. The direct and indirect costs of chronic back pain have been estimated to be greater than 50 billion dollars in the United States,8 and chronic disability secondary to chronic back pain affects more than 5 million Americans8 (Fig. 125-1). This chapter focuses on chronic pain associated with spine disease, with emphasis on postlaminectomy syndrome and neuromodulatory treatment options.

FIGURE 125-1 Pain has been a subject of interest since ancient times.
(Copyright Cleveland Clinic Foundation.)
Chronic Pain
Definition
The transition from acute to chronic pain can be defined according to time course or healing process. The first criterion is more commonly used, although different cutoff time points have been arbitrarily chosen, ranging from 1 to 6 months.18A Chronic pain can also be defined as pain persisting beyond the expected time of healing for the given injury.19 This criterion avoids the need for an arbitrary cutoff but may not be as practical clinically. In this context, chronic pain is understood as pain that is not associated with tissue injury or illness of equivalent severity. Neuropathic mechanisms may be involved in pain perpetuation as well as the influence of behavioral, social, and cognitive factors.
Nociceptive Pain versus Neuropathic Pain
Nociceptive pain is associated with tissue injury, without compromise of the nervous system itself. It is mostly described as sharp, well-defined pain, localized over the injured area. Neuropathic pain may develop from persistent nociceptive pain secondary to continuous sensitization of the nervous system20 but can also occur as a result of injury directly to the peripheral or central nervous system. It usually consists of a less defined sensation, often described as burning, aching, or electrical shocks, and generally associated with altered stimulus perception, such as allodynia or hyperalgesia.
Pain-Related Pathways
Peripheral Receptors
When excited by a stimulus, the peripheral receptor acts as a transducer, transforming the initial information into chemical signals. Receptor depolarization is mediated by transmembrane potentials, triggered by external stimulation that reaches a specific threshold.20–23 Receptors are located on the endings of sensory axons in the skin and other tissues and are composed of free, partially covered or encapsulated nerve endings. Receptors are stimulus-specific and generally do not depolarize with other types of stimuli at normal intensities. Three main modality-specific receptor subtypes are associated with spinal pain: (1) nociceptors, which respond to tissue damage; (2) mechanoreceptors; and (3) thermoreceptors.21 These receptors include not only free nerve endings, as nociceptors, but also more specialized structures, such as partially covered receptors (Merkel discs, Ruffini endings) and encapsulated endings (Meissner, Pacini, and Golgi corpuscles; neuromuscular spindles; neurotendinous organs).21,23,24
Peripheral Nerve Fibers
Peripheral nerves are formed by the union of the dorsal root, which carries afferent information, and the ventral root, which contains mainly efferent information. The epineurium is formed not only by collagen and vessels but also by sympathetic fibers and polymodal receptors, forming the nervi nervorum.24 These are possibly associated with the occurrence of chronic pain after nerve injury,25,26 promoted by sympathetic sprouting and sensitization.
Nerve fibers are classified according to their myelinization and conduction velocity: Aα fibers are the fastest and are associated with muscle efferents. Aβ fibers are the second fastest type, carrying tactile, pressure, and proprioceptive afferents. These fibers are recruited during inflammation or other injury-related phenomena to participate in mechanisms of nociception, hypersensitivity, and sensitization.27,28 Aδ fibers carry not only cold information but also nociception when associated with polymodal receptors. B fibers are related to autonomic activity, and C, or unmyelinated, fibers are related to nociception transmission and postganglionic autonomic function.
Pain Pathways
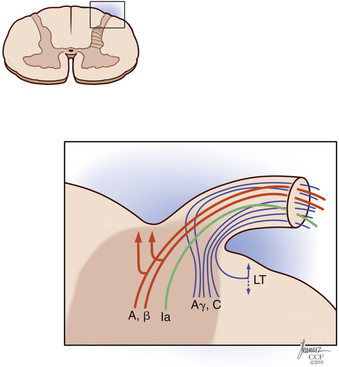
Painful stimuli are transmitted from the peripheral nerve to the dorsal root ganglion, dorsal root, and dorsal horn. At the level of the dorsal root entry zone, most unmyelinated and small myelinated fibers assume a more lateral position to enter the Lissauer tract (Fig. 125-2). In contradiction to the Bell-Majendie law, evidence exists that some of the nociceptive information travels not only through the dorsal root but also through the ventral root.29–33
The Lissauer tract comprises a bundle of longitudinal fibers and, as proposed by Ranson, is part of the pain transmission pathway.34,35 Unmyelinated fibers make up most of the Lissauer tract,35–37 and their central terminations are located mainly in lamina II of Rexed. Aδ fibers have a broader arborization and terminate in laminae I, II, V, and X.35 Although some Aδ fibers terminate in the dorsal horn at the same level they enter, others ascend many levels to terminate in higher segments of the cord.35,38,39
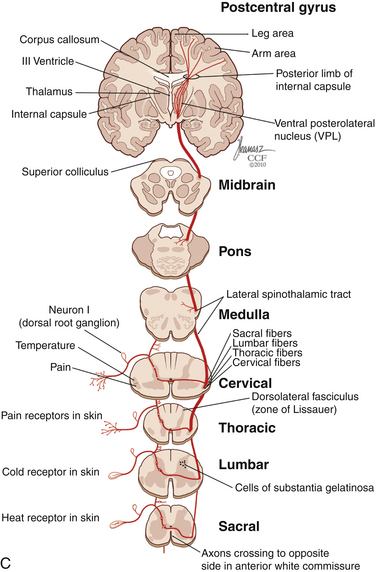
The dorsal horn is divided into ten laminae as defined by Rexed. Lamina I is related specifically to nociceptive and thermal information and is composed mainly of two types of cells: nociceptive-specific neurons, which respond to noxious stimuli, and wide dynamic range (WDR) cells, which respond to both noxious and non-noxious stimuli and are thought to be major contributors in the development of chronic pain.22,40 This lamina contributes to the formation of the spinothalamic tract (STT) (Fig. 125-3) and contains primarily substance P, calcitonin gene–related peptide, and enkephalin and serotonin as neuropeptides.22 Lamina II, also known as the substantia gelatinosa, receives nociceptive, thermoreceptive, and mechanoreceptive input.40 Cells in this lamina project to laminae I, III, and IV41 and contain opioid receptors,40 corroborating the importance of lamina II in modulating nociceptive information.22,42 Lamina III receives inputs from Aβ fibers and mechanoreceptive Aδ fibers.22 The sprouting of the low-threshold terminals present in this layer to the more superficial laminae,40,42 which are generally associated with nociception, suggests a role in chronic pain.5,43 Lamina V is another important component of nociception because of its inputs from Aδ and C fibers and WDR neurons, contributing to the formation of the STT.42,44
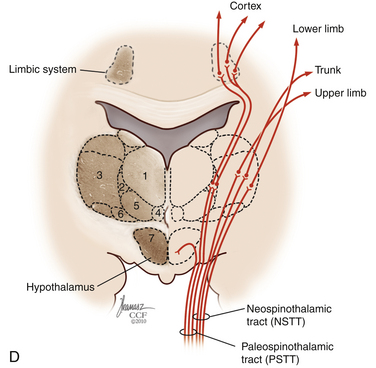
The cell projections of laminae I and V, after crossing the anterior aspect of the central canal, course through the STT in the contralateral ventrolateral column to reach the ventroposterior thalamus.40 Fibers of laminae I, VII, and IX, related to WDR neurons, project to the nonspecific intralaminar nuclei45–47 and to the brainstem reticular formation,48,49 periaqueductal gray matter,50–52 and hypothalamus,53 forming the paleospinothalamic tract22 (see Fig. 125-3). Because the WDR neurons have larger receptive fields and respond to different kinds of stimuli when compared to the specific nociceptive neurons, they are involved in poorly localized and nondiscriminative types of pain, in addition to the transformation of acute pain into chronic pain syndrome.22,52
After thalamic processing, pain information is projected to the primary somatosensory cortex and secondary somatosensory cortex sequentially.22,40,54 The thalamus also projects to the insula20 and the anterior cingular cortex,20 which are primarily related to the motivational and affective spheres of chronic pain22 (see Fig. 125-3).
The role of descending pathways in pain modulation is well established,55 starting in the periaqueductal gray matter, rostral ventromedial medulla, and dorsolateral pontine tegmentum.20,56 The periaqueductal gray matter receives inputs from the dorsal horn, brainstem, diencephalic system, and cortex57,58 and sends inhibitory projections to the dorsal horn.22 It also projects back to the thalamus and orbital frontal cortex,59 possibly exerting an ascending control of nociception. Another important pathway that plays a significant role in spinal pain modulation is the noradrenergic system,60,61 which projects extensively to the dorsal horn (Fig. 125-4). The development of chronic pain is related not only to ascending pain-facilitating mechanisms but also to reduced pain inhibition from descending and ascending modulatory mechanisms.
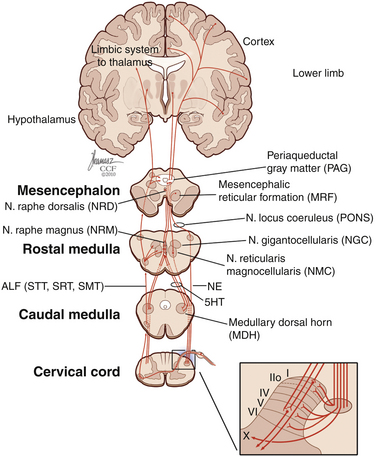
FIGURE 125-4 Diagram of descending inhibitory pain pathways and their respective projections in the dorsal horn.
5HT, serotonin; ALF, anterolateral fasciculus; NE, noradrenaline; SMT, spinomesencephalic tract; SRT, spinoreticular tract; STT, spinothalamic tract. (Copyright Cleveland Clinic Foundation.)
Physiology of Pain
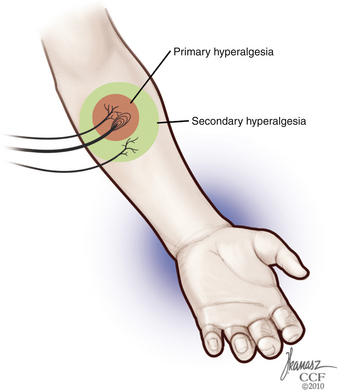
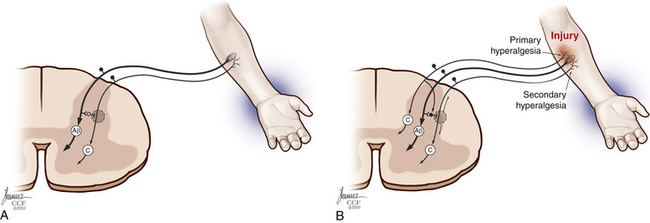
Each nerve fiber has different physiologic response durations, known as adaptation. Because C fibers are usually slowly adapting, and their responses last longer than the stimuli, the occurrence of temporal and spatial summation of painful stimuli during tissue injury may occur.24 Properties of other fibers include a well-defined receptive field and spontaneous discharges, generated without exogenous stimuli. Summation, expansion of the receptive field, and increase in spontaneous discharges are significantly enhanced during inflammation, leading to the development of hyperalgesia and sensitization.62 In addition to this mechanism of primary hyperalgesia, secondary hyperalgesia—increased pain sensitivity and allodynia in the surrounding uninjured area—may also occur, secondary to peripheral and central events, such as increased response to glutamate and central neuronal plasticity63 (Fig. 125-5).
Each sensory cell has specific thresholds to respond to a given stimulus, which is lowered during inflammation. This condition is defined as sensitization, which is divided into peripheral and central according to the mechanisms involved.64 Peripheral sensitization is characterized by a decreased threshold20 and increased response to suprathreshold stimuli,65 spontaneous nociceptive neural activity, and expansion of the receptive fields after tissue injury66,67 (Fig. 125-6). The threshold of nociceptors is decreased as a result of exposed free nerve endings that fire abnormally. Sprouting of nerve terminals also generates ectopic discharges, as seen in neuromas.22
The increase in the number of sodium channels seen in damaged fibers68 may lead to nociceptor hyperexcitability, which is reverted at least partially by sodium channel blockers.69 Sympathetic sensitization is also a contributor,20 with increased nociceptor response to catecholamines of injured70,71 and uninjured neurons.72,73 The sensitivity of polymodal WDR receptors is also increased in response to inflammation, causing activation that is no longer triggered preferentially by nociceptive stimuli but also by other mechanical stimuli.20,63,64,74 Chemomediators are also involved in peripheral sensitization, with the secretion of cytokines interleukin-1β,75 interleukin-6,76 and tumor necrosis factor-α77,78 by lymphocytes, macrophages, and mast cells. Glutamate levels are increased during inflammation via macrophage and epithelial cell release, activating nociceptors via ion channels and metabotropic receptors.79–81
Central sensitization is mediated by short-term and long-term changes in the dorsal horn of the spinal cord.82–85 This mediation is supported by the occurrence of allodynia in association with the recruitment of Aβ fibers and their sprouting from lamina III into lamina II and loss of regulation of nociceptive fibers.22 Repetitive stimulation of C fibers, triggered by tissue injury, can lead to hyperexcitability and overactivity of these fibers and further perpetuation of nociceptive transmission.22,86,87 This perpetuation of nociceptive transmission can result in magnification of the sensory input and consequent expansion of the receptive fields,84,88–90 a phenomenon known as wind-up91–93
A key component in the development of chronic pain is antinociception and the failure of its underlying mechanisms. The inhibitory control of pain pathways is exerted by the spinal cord via several neurotransmitters, in addition to the descending inhibitory system as discussed previously.22 The most common inhibitory neurotransmitter in the spinal cord is gamma-aminobutyric acid, which mediates presynaptic inhibition of afferents,94,95 decreased release of neuropeptide P, and postsynaptic inhibition of the STT.42 Another important inhibitory pathway is the opioid system, with neurotransmitters that bind to three main receptors: mu, the most common opioid receptor in the spinal cord, which has the highest morphine affinity and mediates not only analgesia (μ1) but also respiratory depression (μ2)22,96,97; kappa, which binds to dynorphin; and delta, which binds to enkephalins.42 Opioids exert their action via presynaptic and postsynaptic mechanisms,98,99 and although they are well known for their analgesic effect, the symptoms caused by central sensitization, such as allodynia, usually do not respond well to these substances.
Cognitive and Behavioral Considerations in Chronic Pain
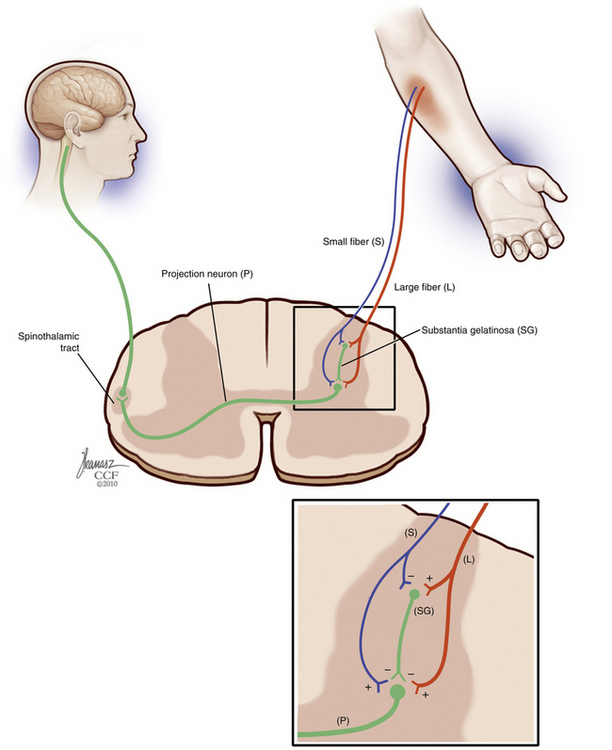
The gate control theory by Melzack and Wall in 1965100 is a landmark in the understanding of chronic and neuropathic pain. The gate control theory suggested that pain is not merely transmitted by the peripheral nervous system to the CNS and proposed instead endogenous modulatory mechanisms. According to this theory, pain transmission is modulated by a gating mechanism in the dorsal horn composed of large-diameter and small-diameter fibers that close (inhibit) and open (facilitate) the pain gate.
In 1999, Melzack101 proposed the neuromatrix theory, refining the gate control theory with additional key elements in pain processing. In the neuromatrix model, pain modulation occurs not only at the spinal level; cerebral mechanisms of pain processing and transmission are also taken into consideration, and cognitive and affective inputs are recognized to influence the final pain experience (Fig. 125-7). Several studies have corroborated further the role of cognitive and limbic systems in central pain processing.102–105 A patient’s beliefs and understanding about his or her pain syndrome, pain sensitivity, fear, anger, depression, anxiety, and catastrophic thinking influence the final pain experience.102,106,107 Patients who are able to develop techniques to cope with pain and reduce the impact of psychological comorbidities generally have a better prognosis.108–114
Based on these advances in understanding of central pain processing, the evaluation of patients with chronic pain should include not only a measure of the sensory component of pain (e.g., verbal, numerical, or visual analogue scales) but also the affective and evaluative components. The McGill Questionnaire has been largely validated as a comprehensive tool for the assessment of various chronic pain conditions, including spine pain.115 Other inventories can also be used, such as the pain disability index.116,117 Quality-of-life measurements such as the European Quality of Life Inventory may also be useful in evaluating the impact of the pain syndrome on the patient’s life and in assessing outcomes after interventions.
Work disability also plays a key role in the perpetuation of chronic pain, and its impact on long-term prognosis cannot be underestimated. Although the American Medical Association defines disability as “an alteration of an individual’s capacity to meet personal, social, or occupational demands…because of an impairment,”118 work disability agencies use more restricted definitions to guide benefit eligibility or ability to work.119 Compensation for disability and unresolved litigation have a complex influence on treatment outcome. Compensation and litigation have been linked to a poor prognosis,9,120–123 higher risk to develop chronic pain and pain behavior,124,125 and a lower likelihood of returning to work.126,127 Waiting for litigation and benefit disputes to resolve before discussing invasive pain interventions may improve outcomes and obviate the need for intervention.121,128 Likewise, patients who show little interest for becoming more active or returning to work may be less likely to benefit from additional intervention.129–131
Postlaminectomy Syndrome
Low back pain and pain of spinal origin are among the most common chronic pain conditions in the population132–135 and are associated with physical and psychosocial dysfunction, disability, and reduced quality of life. Although low back pain is more prevalent, neck pain is also a common reason to seek health care. Although approximately 90% of low back pain cases are nonsurgical,136,137 the proportion of patients undergoing spine surgery has progressively increased,6,138,139 and success rates remain variable (23–83%).140–142 Patient selection is known to be a key factor for successful outcomes, and inappropriate indications are likely associated with a higher frequency of postlaminectomy syndrome.139,143–145
Postlaminectomy syndrome, also known as failed back surgery syndrome (FBSS), is characterized by persistent, recurrent, and chronic back pain with or without radiation to the lower extremities after surgical treatment.20,134,146 The syndrome comprises different clinical etiologies138 and can occur after any surgical procedure, with or without fusion or instrumentation.138 Identification of the etiology of FBSS may assist in directing treatment, which includes medical, rehabilitative, surgical decompression or fusion, and neuromodulatory options.147 FBSS can be associated with numerous etiologies, including ruptured discs and fragments, which are found in approximately 15% to 35% of cases138,147,148; degenerative changes in levels adjacent to instrumentation149–153; extensive fusion associated with flat back syndrome154,155; pseudarthrosis156; and instability caused by facet joint failure after decompression.142,156 Some FBSS etiologies are associated predominantly with persistent leg pain.
Surgical treatment of spinal stenosis has failure rates of 10% to 30%.138,147,148 Accurate diagnosis and meticulous decompression may reduce the risk of FBSS.147 Foraminal stenosis, either residual or worsened by instability,142,155,157 is responsible for a large proportion of FBSS cases, followed by lateral and central stenosis.138,147,158 Recurrent or residual disc herniations causing nerve root compression are another common cause of persistent leg pain, with a highly variable incidence ranging from 10% to 50%.138,147,148 Neuropathic pain caused by prolonged dorsal root ganglion or nerve injury is thought to be less common but may lead to severe and refractory chronic pain.138,147,148 Arachnoiditis and epidural fibrosis, which can be caused not only by surgical manipulation but also by recurrent irritation and instability, may also lead to persistent pain.138,142,147,148
Although there are several alternatives for the management of FBSS, treatment is often challenging. It is common for patients and physicians to be disappointed and frustrated with outcomes. Patients may have already undergone multiple failed interventions, contributing to their psychological distress and reducing the odds that additional treatments will be successful. It is important to discuss treatment expectations beforehand with the patient and family. It is unlikely that additional treatments would be able to resolve completely chronic pain that has been refractory to medical management and surgery. In the setting of chronic back pain, a 50% improvement in pain is usually considered a reasonable outcome.159 Good candidates for any treatment for FBSS should be motivated to return to work and have a physically active lifestyle.159 A multidisciplinary approach has been recommended in the management of these difficult conditions, including physical therapy, rehabilitation programs, pain management, and possibly surgical intervention.160
Medical Management
Defining a logical algorithm is a critical step in chronic pain treatment, and conservative and reversible treatments are generally instituted first. Because medical management already has been covered elsewhere in this book, it is briefly discussed in this chapter. In this first approach, multidisciplinary assessment may allow for addressing the pain and its etiology and the management of comorbidities, such as depression and other psychological aspects of chronic pain, rehabilitation, and pharmacologic options.161 Back pain can be associated with modifications in neuromuscular activity, altering abdominal and back muscle function and contributing to the maintenance of the pain state.162–165 Physical therapy and rehabilitation are mainstays of low back pain treatment, and patients with FBSS should consider these programs, which are aimed at reducing biomechanical deficits and restoring strength and range of motion.162,166–170
The most common medications used for treatment of low back pain are nonsteroidal anti-inflammatory drugs, muscle relaxants, opioids, benzodiazepines, antidepressants, and antiepileptic drugs.171–174 Nonsteroidal anti-inflammatory drugs can be effective for acute and chronic pain,161,171,175,176 although long-term use of these agents is generally limited by side effects.
Muscle relaxants and benzodiazepines act predominantly on pain states perpetuated by muscle spasms and increased muscle tone and can be beneficial in the management of acute pain171,173,177; long-term use for chronic pain is not well established.161,171,178 Additional care has to be taken with benzodiazepines because of the risk of aggravating depression further and exacerbating the baseline condition.179,180
The use of opioids in the treatment of chronic nonmalignant pain is controversial and variable.171,181–185 Long-term use of narcotics has been linked to tolerance, addiction, and cognitive decline, which emphasizes the need for cautious use of these medications as a long-term option.184 Their analgesic effects are dose-related and are produced in various levels of the CNS, including the substantia gelatinosa of the spinal cord, the descending antinociceptive system, and the limbic system, altering emotional response and pain behavior.186–188 Neuropathic pain and allodynia are usually not responsive to opioids because of the activation of N-methyl-d-aspartate receptors (increased during central sensitization), leading to phosphorylation and inactivation of the opioid receptor.22 In this context, the use of opioids in the treatment of nonmalignant pain is more likely beneficial for patients with mainly nociceptive pain that did not respond to other medications, without associated major psychosocial comorbidity.189 Major long-term complications of opioid use include physical dependence,190–192 tolerance,184,185,190 addiction,184,193 opioid hyperalgesia,190,194,195 and cognitive dysfunction.184,196–199
Antidepressants are commonly prescribed in the management of chronic pain. Although they provide significant pain relief in various chronic pain states,200–203 studies in chronic low back pain indicate variable efficacy.204–207 The mechanism of action relies on activation of norepinephrine descending brainstem pathways, which is potentiated by serotonin (5-hydroxytryptamine) pathways.208 Tricyclic antidepressants such as amitriptyline and imipramine that block the reuptake of norepinephrine and 5-hydroxytryptamine in addition to H1, adrenergic, and cholinergic receptors tend to be the most efficacious.208–211
Anticonvulsants such as carbamazepine, topiramate, gabapentin, and pregabalin are also frequently tried in the medical management of neuropathic pain.212–215 Improvements in pain scores were seen with the use of these medications,216 mainly when radiculopathy was present,214,217–219 although patients with axial symptoms may also have some benefit.215 Anticonvulsants work primarily via suppression of abnormal discharge at nerve injury sites by Na+ channel block.220,221
Invasive and Surgical Management
Percutaneous Procedures
Patients with FBSS and low back pain caused by ruptured discs or facet joint instability may undergo provocative discography or medial branch block, although the diagnostic usefulness of these methods remains operator-dependent and controversial.222–225 Minimally invasive techniques can also be considered alternatives for the management of chronic pain related to FBSS. Procedures such as intradiscal electrothermal therapy and medial branch lesioning may provide functional improvement.223,226–234 Patients with predominantly leg pain may benefit from nerve root blocks as a guide for subsequent treatments.223,235,236 Pulsed radiofrequency lesioning of dorsal root ganglion has shown promising results in the treatment of radiculopathy associated with low back pain.237–239 Epidural injections, performed alone or in association with spinal endoscopy, have variable long-term efficacy in this population.223,240–245
Reoperation
Reoperation for FBSS in the absence of a clearly defined anatomic cause is a controversial option and may not provide significant improvement of the baseline condition.140,142,144,157,161,246 Success rates of reoperations in patients with FBSS may vary from 22% to 80% and depend on time of follow-up.161 Usual indications to consider additional back surgery include reasonable evidence of a surgically treatable condition (instability, compression of a nerve root, or cauda equina), clinical presentation that is compatible with the anatomic lesion, failure to improve with adequate conservative treatment, and management of surgical complications that may need urgent treatment.144,157,161 Back pain not associated with radicular symptoms, poor outcome after the first procedure, pseudarthrosis, epidural fibrosis, and psychological comorbidities all are factors associated with a worse prognosis.140,144,145,246,247
Intraspinal Delivery Devices
A significant proportion of patients with FBSS can fail to achieve pain relief with the previously discussed treatment options and with combined treatment. The use of long-term intrathecal infusion of pharmacologic agents can be considered an alternative in these cases.248–252 The advantage of this route of administration is in the proximity of the drug to the receptor as it diffuses passively in the dorsal horn, allowing for lower effective doses and consequently a reduced rate of side effects.250,252,253 Commonly used agents used include opioids, ziconotide, local anesthetics, and α2 agonists.250 Clinical trials are under way to evaluate the safety and efficacy of other agents for intrathecal infusion.254–256 Although long-term intrathecal infusion of opioids is already well established for treatment of malignant pain,249,251,257,258 the indications for nonmalignant pain are still controversial.249,259,260 The mechanism of action may include a direct inhibition of dorsal horn cells or a modulatory effect on interneurons, blocking the central transmission of nociceptive information.261–264 Several studies have shown promising results with short-term outcomes, even in patients with severe and refractory pain.249,252,265–270 However, studies with long-term follow-up often describe a decrease in responsiveness over time, without success in recapturing the same extent of early benefits.256,259,271–273
Appropriate patient selection is an essential step for satisfactory results and includes symptoms that are refractory to less invasive therapies, response to oral doses of opioids, significant response to intrathecal opioid trial, absence of addiction history, and favorable psychosocial evaluation.249,260,274–277 The option for intrathecal opioids may be more logical in elderly patients, for whom the goal is to achieve some degree of pain alleviation in the final years of life. Long-term management is more complicated in young patients, who are likely to increase the opioid dosage gradually over decades of use.
The intrathecal trial can be performed with sequential injections of bolus dosing with progressively increasing doses or continuous opioid infusion with an externalized catheter.249 During the trial, the fundamental goals are to assess efficacy in alleviating the chronic pain syndrome, potential side effects, and dosage. Permanent implantation of a pump and catheter system can be considered when a significant improvement is achieved (generally ≥50% reduction in pain) with tolerable side effects.249,250,259,274
Different opioids may be used for intrathecal delivery; these include morphine (approved by the U.S. Food and Drug Administration [FDA]), hydromorphone, fentanyl, and sufentanil.252,269,273,278 Their analgesic potential and side effects are strongly dependent on lipid solubility, and this should be considered when choosing the most appropriate drug for each patient.188 Hydrophilic drugs, such as morphine, may take longer to alleviate the pain, but the concentration remains high in the cerebrospinal fluid for longer periods, allowing it to ascend to supraspinal levels, enhancing the analgesic effects.249 Lipophilic drugs, such as fentanyl and sufentanil, have a rapid onset of action and prolonged duration but do not diffuse easily in the cerebrospinal fluid.249,279
The most common side effects described with intrathecal opioids include nausea, sedation, confusion, pruritus, urinary retention, myoclonus, reduced libido, and respiratory depression,259,271,273,280 which are greater with intrathecal hydrophilic drugs.249 Peripheral edema, usually unresponsive to diuretics, is another side effect of morphine and can be managed by changing to a lipophilic agent.281–283 Granuloma formation is possibly the most serious long-term adverse effect of intrathecal therapy.284 Although different agents have been related to this complication,279,285 it seems to be more common with higher concentrations and doses.279,286 In addition to routine imaging, screening of patients with intrathecal pump systems has been suggested to enable early detection of masses associated with the catheter tip. A panel of experts has recommended that clinicians managing these patients maintain a high index of suspicion and a low threshold for requesting imaging examination aimed at identified granulomas. Imaging should be considered not only for patients with progressive neurologic deficits but also for patients with subjective changes in neurologic status or loss of efficacy to the intrathecal infusion. Treatment options for catheter tip inflammatory masses include replacing the solution for saline and careful observation of neurologic progress. Patients with neurologic deficits or worsening neurologic examination may need surgical intervention aimed at decompression and removal of the hardware.286–289
Ziconotide, a blocking agent of N-calcium channels, is an intrathecal drug approved more recently by the FDA for chronic pain management. Its efficacy has been shown in the literature,290–294 but the high incidence of serious side effects during the dose titration phase may limit its use.279 The most common side effects include memory impairment, confusion, hallucinations, dizziness, and ataxia.290,291,294 Second-line drugs are indicated for opioid-resistant patients and include local anesthetics and α2 agonists, which act mainly on the neuropathic component of pain, enhancing analgesia.256 Bupivacaine and clonidine are generally used in combination with morphine and have been shown to restore pain control and improve quality of life.248,256,295
The major technical complications related to intrathecal pumps include infections involving the implanted hardware, postural headaches, cerebrospinal fluid leak, pseudomeningoceles, seromas associated with the pump reservoir, and device-related complications. Although the pumps have an expected expiration time, catheter complications are more commonly the cause of premature failure of the implanted system.296 Spinal cord injury associated with implantation of the catheter has been reported,297 but its frequency, although thought to be low, has not been established.
Spinal Cord Stimulation
The gate control theory100 led to the development of novel neuromodulation-based therapies for chronic pain. According to the theory, stimulation of large myelinated fibers could modulate nociceptive input.100 Encouraging results were seen with peripheral nerve stimulation, taking advantage of the fact that large myelinated fibers can be stimulated at lower thresholds than small unmyelinated fibers.261,298 In 1967, Shealy et al.299 described stimulation of the dorsal columns to treat refractory lower extremity pain. Initially, the electrodes were implanted in the subdural space, but the occurrence of complications such as fibrosis and cerebrospinal fluid leak limited their use.299,300 The technique was later modified to stimulation with epidural electrodes.301 During the first years of use, spinal cord stimulation (SCS) was attempted in patients with various pain diagnoses, and results were variable.302 More consistent results have been seen in subsequent series with better patient selection and technologic advances.303–313 At the present time, it is estimated that approximately 14,000 SCS devices are implanted yearly worldwide.302 In the United States, the most common indications for SCS are back and leg pain, whether or not associated with FBSS.248,303,314
SCS has been shown in well-conducted studies to be effective in alleviating leg pain associated with degenerative spine disease, improving long-term functional capacity and promoting better quality of life.305,308,309,311,315–317 A systematic review of the literature indicated that successful outcomes after SCS (defined as ≥50% pain improvement) are seen in 59% of cases.318 Results can vary depending on the series, technique, and patient population, ranging from efficacy rates of 12% to 88%.315,317,319 Patients with FBSS undergoing SCS have been shown to have significantly superior pain alleviation, enhancement in quality of life, and functional status compared with patients receiving conventional medical management.306 SCS was also found to be better than reoperation in a selected group of patients with FBSS, with greater pain relief and less crossover to the other arm of the study (reoperation).315,320 Although SCS implants are expensive, imposing a considerably high initial cost to the health-care system, the overall treatment has been shown to be cost-effective in the long term.315,321–328
Patient selection is a major factor determining treatment success. The primary indication for SCS is limb neuropathic pain that is refractory to conservative management, secondary to etiologies such as peripheral nerve or nerve root damage, complex regional syndrome, FBSS, and ischemic pain.300,320,329,330 The decision-making process for permanent implantation of an SCS system should take into account not only factors related to the pain or neurologic syndromes but also the history of previous interventions, psychological comorbidities, narcotic dependence, and outcome expectation. When a trial is performed, the short-term results of SCS can be used to exclude early nonresponders, although a positive response to the trial with greater than 50% pain relief does not guarantee good long-term results.320,330 The trial consists of the temporary implantation of an SCS lead that is externalized for a few days.330
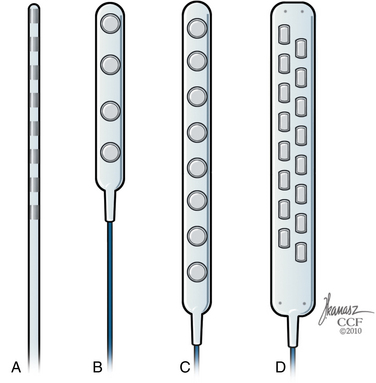
There are several technical alternatives for permanent implantation of the SCS system. Permanent implantation can be performed with cylindrical leads similar to those typically used for the externalized trials or with surgical (or paddle) leads (Fig. 125-8). Cylindrical leads can be implanted with minimally invasive percutaneous techniques but carry a higher risk of migration.331 Surgical leads have a lower risk of migration and can be directly anchored to the spinal elements (e.g., the yellow ligament) but require a laminectomy for placement.331,332 Modern percutaneous leads have 4 to 8 contacts, whereas paddle leads may have 16 contacts built in the lead body. Implantable pulse generators come in various sizes with different programming technologies and can be rechargeable or nonrechargeable devices.
Complications can be divided into stimulation-related, device-related, and surgical types. Stimulation-related complications include uncomfortable paresthesias or positional changes,317 long-term loss of efficacy, and stimulation in areas not affected by the pain. Higher amplitudes may cause stimulation spread to the thoracic roots, leading to uncomfortable muscle contractions.330,333 Because SCS is a neuromodulatory technique, adverse effects related to electrical stimulation are usually reversible and can be resolved with reprogramming of the system or turning off the stimulation. Device-related complications have been reported to affect approximately 30% of patients.330 The most common problem is migration of electrodes (more common with cylindrical leads than with paddle leads) resulting in loss of efficacy. Although reprogramming can be attempted to recapture the lost benefits, a surgical revision may be necessary.317,333 The main complication related to the surgical procedure is infection, with a rate of approximately 4.5%.311,331,333 Other complications include dural tear with associated spinal headaches or cerebrospinal fluid leak, postoperative hematoma, and neurologic injury, which range from 1% to 9%.320,330
Deer T., Krames E.S., Hassenbusch S., et al. Polyanalgesic Consensus Conference 2007: recommendations for the management of pain by intrathecal (intraspinal) drug delivery: report of an interdisciplinary expert panel. Neuromodulation. 2007;10:300-328.
Dworkin R.H., O’Connor A.B., Backonja M., et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132:237-251.
Hassenbusch S.J., Portenoy R.K., Cousins M., et al. Polyanalgesic Consensus Conference 2003: an update on the management of pain by intraspinal drug delivery—report of an expert panel. J Pain Symptom Manage. 2004;27:540-563.
Kupers R.C., Van den Oever R., Van Houdenhove B., et al. Spinal cord stimulation in Belgium: a nation-wide survey on the incidence, indications and therapeutic efficacy by the health insurer. Pain. 1994;56:211-216.
Mailis-Gagnon A., Furlan A.D., Sandoval J.A., et al. Spinal cord stimulation for chronic pain. Cochrane Database Syst Rev. 2004;3:CD003783.
Raslan A.M., McCartney S., Burchiel K.J. Management of chronic severe pain: spinal neuromodulatory and neuroablative approaches. Acta Neurochir Suppl. 2007;97(Pt 1):33-41.
Turner J.A., Sears J.M., Loeser J.D. Programmable intrathecal opioid delivery systems for chronic noncancer pain: a systematic review of effectiveness and complications. Clin J Pain. 2007;23:180-195.
1. Deyo R.A., Weinstein J.N. Low back pain. N Engl J Med. 2001;344:363-370.
2. Biyani A., Andersson G.B. Low back pain: pathophysiology and management. J Am Acad Orthop Surg. 2004;12:106-115.
3. Irving GA, Wallace MS: Pain management for the practicing physician. New York, 1997, Churchill Livingstone.
4. Andersson G.B. Epidemiological features of chronic low-back pain. Lancet. 1999;354:581-585.
5. Loeser J.D. Low back pain. In: Loeser J.D., editor. Bonica’s management of pain. ed 3. Philadelphia: Lippincott Williams & Wilkins; 2001:1508-1509.
6. Taylor V.M., Deyo R.A., Cherkin D.C., Kreuter W. Low back pain hospitalization: recent United States trends and regional variations. Spine (Phila Pa 1976). 1994;19:1207-1212.
7. Sanderson P.L., Todd B.D., Holt G.R., Getty C.J. Compensation, work status, and disability in low back pain patients. Spine (Phila Pa 1976). 1995;20:554-556.
8. Frymoyer J.W. Predicting disability from low back pain. Clin Orthop Relat Res. 1992;279:101-109.
9. Greenough C.G. Recovery from low back pain: 1–5 year follow-up of 287 injury-related cases. Acta Orthop Scand Suppl. 1993;254:1-34.
10. Sander R.A., Meyers J.E. The relationship of disability to compensation status in railroad workers. Spine (Phila Pa 1976). 1986;11:141-143.
11. van Doorn J.W. Low back disability among self-employed dentists, veterinarians, physicians and physical therapists in The Netherlands: a retrospective study over a 13-year period (N = 1,119) and an early intervention program with 1-year follow-up (N = 134). Acta Orthop Scand Suppl. 1995;263:1-64.
12. Burton A.K., Tillotson K.M., Main C.J., Hollis S. Psychosocial predictors of outcome in acute and subchronic low back trouble. Spine (Phila Pa 1976). 1995;20:722-728.
13. Dionne C.E., Koepsell T.D., Von Korff M., et al. Predicting long-term functional limitations among back pain patients in primary care settings. J Clin Epidemiol. 1997;50:31-43.
14. Murphy K.A., Cornish R.D. Prediction of chronicity in acute low back pain. Arch Phys Med Rehabil. 1984;65:334-337.
15. Main C.J., Wood P.L., Hollis S., et al. The Distress and Risk Assessment Method: a simple patient classification to identify distress and evaluate the risk of poor outcome. Spine (Phila Pa 1976). 1992;17:42-52.
16. Bovim G., Schrader H., Sand T. Neck pain in the general population. Spine (Phila Pa 1976). 1994;19:1307-1309.
17. Makela M., Heliovaara M., Sievers K., et al. Prevalence, determinants, and consequences of chronic neck pain in Finland. Am J Epidemiol. 1991;134:1356-1367.
18. Ghatan S., Goodkin R. Neck pain. In Loeser J.D., editor: Bonica’s management of pain, ed 3, Philadelphia: Lippincott Williams & Wilkins, 2001.
18A. International Association for the Study of Pain: iasp-pain.org, 1994.
19. Turk D.C., Okifuji A. Pain terms and taxonomies of pain. In: Loeser J.D., editor. Bonica’s management of pain. ed 3. Philadelphia: Lippincott Williams & Wilkins; 2001:17-25.
20. McMahon S.B., Koltzenburg M. Wall and Melzack’s textbook of pain, ed 5, Philadelphia: Saunders, 2006.
21. Guyton A.C., Hall J.E., editors. Textbook of medical physiology, ed 9, Philadelphia: Saunders, 1996.
22. Rosenow J.M., Henderson J.M. Anatomy and physiology of chronic pain. Neurosurg Clin North Am. 2003;14:445-462.
23. Parent A. Human neuroanatomy, ed 9, Baltimore: Williams & Wilkins, 1996.
24. Byers M.R., Bonica J.J. Peripheral pain mechanisms and nociceptor plasticity. In: Loeser J.D., editor. Bonica’s management of pain. ed 3. Philadelphia: Lippincott Williams & Wilkins; 2001:26-72.
25. Kruger L. The functional morphology of thin sensory axons: some principles and problems. Prog Brain Res. 1996;113:255-272.
26. Zochodne D.W. Epineurial peptides: a role in neuropathic pain? Can J Neurol Sci. 1993;20:69-72.
27. Neumann S., Doubell T.P., Leslie T., Woolf C.J. Inflammatory pain hypersensitivity mediated by phenotypic switch in myelinated primary sensory neurons. Nature. 1996;384:360-364.
28. Pitcher G.M., Henry J.L. Nociceptive response to innocuous mechanical stimulation is mediated via myelinated afferents and NK-1 receptor activation in a rat model of neuropathic pain. Exp Neurol. 2004;186:173-197.
29. Applebaum M.L., Clifton G.L., Coggeshall R.E., et al. Unmyelinated fibres in the sacral 3 and caudal 1 ventral roots of the cat. J Physiol. 1976;256:557-572.
30. Coggeshall R.E., Applebaum M.L., Fazen M., et al. Unmyelinated axons in human ventral roots, a possible explanation for the failure of dorsal rhizotomy to relieve pain. Brain. 1975;98:157-166.
31. Coggeshall R.E., Maynard C.W., Langford L.A. Unmyelinated sensory and preganglionic fibers in rat L6 and S1 ventral spinal roots. J Comp Neurol. 1980;193:41-47.
32. Sykes M.T., Coggeshall R.E. Unmyelinated fibers in the human L4 and L5 ventral roots. Brain Res. 1973;63:490-495.
33. Wee B.E., Emery D.G., Blanchard J.L. Unmyelinated fibers in the cervical and lumbar ventral roots of the cat. Am J Anat. 1985;172:307-316.
34. Earle K.M. The tract of Lissauer and its possible relation to the pain pathway. J Comp Neurol. 1952;96:93-111.
35. Traub R.J., Mendell L.M. The spinal projection of individual identified A-delta- and C-fibers. J Neurophysiol. 1988;59:41-55.
36. Coggeshall R.E., Chung K., Chung J.M., Langford L.A. Primary afferent axons in the tract of Lissauer in the monkey. J Comp Neurol. 1981;196:431-442.
37. Chung K., Langford L.A., Applebaum A.E., Coggeshall R.E. Primary afferent fibers in the tract of Lissauer in the rat. J Comp Neurol. 1979;184:587-598.
38. Morgan C., Nadelhaft I., de Groat W.C. The distribution of visceral primary afferents from the pelvic nerve to Lissauer’s tract and the spinal gray matter and its relationship to the sacral parasympathetic nucleus. J Comp Neurol. 1981;201:415-440.
39. Traub R.J., Sedivec M.J., Mendell L.M. The rostral projection of small diameter primary afferents in Lissauer’s tract. Brain Res. 1986;399:185-189.
40. Carpenter M.B. Core text of neuroanatomy, ed 4, Baltimore: Williams & Wilkins, 1991.
41. Gobel S. Golgi studies of the neurons in layer II of the dorsal horn of the medulla (trigeminal nucleus caudalis). J Comp Neurol. 1978;180:395-413.
42. Terman G.W., Bonica J.J. Spinal mechanisms and their modulation. In Loeser J.D., editor: Bonica’s management of pain, ed 3, Philadelphia: Lippincott Williams & Wilkins, 2001.
43. Mannion R.J., Doubell T.P., Gill H., Woolf C.J. Deafferentation is insufficient to induce sprouting of A-fibre central terminals in the rat dorsal horn. J Comp Neurol. 1998;393:135-144.
44. Meredith M.A., Stein B.E. Spatial factors determine the activity of multisensory neurons in cat superior colliculus. Brain Res. 1986;365:350-354.
45. Bowsher D., Mallart A., Petit D., Albe-Fessard D. A bulbar relay to the centre median. J Neurophysiol. 1968;31:288-300.
46. Reyes-Vazquez C., Prieto-Gomez B., Dafny N. Noxious and non-noxious responses in the medial thalamus of the rat. Neurol Res. 1989;11:177-180.
47. Krout K.E., Belzer R.E., Loewy A.D. Brainstem projections to midline and intralaminar thalamic nuclei of the rat. J Comp Neurol. 2002;448:53-101.
48. Casey K.L. Reticular formation and pain: toward a unifying concept. Res Publ Assoc Res Nerv Ment Dis. 1980;58:93-105.
49. Bowsher D. Role of the reticular formation in responses to noxious stimulation. Pain. 1976;2:361-378.
50. Oliveras J.L., Woda A., Guilbaud G., Besson J.M. Inhibition of the jaw opening reflex by electrical stimulation of the periaqueductal gray matter in the awake, unrestrained cat. Brain Res. 1974;72:328-331.
51. Mayer D.J., Liebeskind J.C. Pain reduction by focal electrical stimulation of the brain: an anatomical and behavioral analysis. Brain Res. 1974;68:73-93.
52. Willis W.D., Westlund K.N. Neuroanatomy of the pain system and of the pathways that modulate pain. J Clin Neurophysiol. 1997;14:2-31.
53. Dado R.J., Katter J.T., Giesler G.J.Jr. Spinothalamic and spinohypothalamic tract neurons in the cervical enlargement of rats. II. Responses to innocuous and noxious mechanical and thermal stimuli. J Neurophysiol. 1994;71:981-1002.
54. Tran T.D., Inui K., Hoshiyama M., et al. Cerebral activation by the signals ascending through unmyelinated C-fibers in humans: a magnetoencephalographic study. Neuroscience. 2002;113:375-386.
55. Reynolds D.V. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science. 1969;164:444-445.
56. Chudler E.H., Bonica J.J. Supraspinal mechanisms of pain and nociception. In: Loeser J.D., editor. Bonica’s management of pain. ed 3. Philadelphia: Lippincott Williams & Wilkins; 2001:153-179.
57. Bandler R., Keay K.A. Columnar organization in the midbrain periaqueductal gray and the integration of emotional expression. Prog Brain Res. 1996;107:285-300.
58. Rhodes D.L., Liebeskind J.C. Analgesia from rostral brain stem stimulation in the rat. Brain Res. 1978;143:521-532.
59. Cameron A.A., Khan I.A., Westlund K.N., et al. The efferent projections of the periaqueductal gray in the rat: a Phaseolus vulgaris-leucoagglutinin study. I. Ascending projections. J Comp Neurol. 1995;351:568-584.
60. Yeomans D.C., Proudfit H.K. Antinociception induced by microinjection of substance P into the A7 catecholamine cell group in the rat. Neuroscience. 1992;49:681-691.
61. Yeomans D.C., Clark F.M., Paice J.A., Proudfit H.K. Antinociception induced by electrical stimulation of spinally projecting noradrenergic neurons in the A7 catecholamine cell group of the rat. Pain. 1992;48:449-461.
62. Amir R., Devor M. Chemically mediated cross-excitation in rat dorsal root ganglia. J Neurosci. 1996;16:4733-4741.
63. Treede R.D., Meyer R.A., Raja S.N., Campbell J.N. Peripheral and central mechanisms of cutaneous hyperalgesia. Prog Neurobiol. 1992;38:397-421.
64. Perl E.R. Cutaneous polymodal receptors: characteristics and plasticity. Prog Brain Res. 1996;113:21-37.
65. Cooper B., Ahlquist M., Friedman R.M., Labanc J. Properties of high-threshold mechanoreceptors in the goat oral mucosa. II. Dynamic and static reactivity in carrageenan-inflamed mucosa. J Neurophysiol. 1991;66:1280-1290.
66. Reeh P.W., Bayer J., Kocher L., Handwerker H.O. Sensitization of nociceptive cutaneous nerve fibers from the rat’s tail by noxious mechanical stimulation. Exp Brain Res. 1987;65:505-512.
67. Thalhammer J.G., LaMotte R.H. Spatial properties of nociceptor sensitization following heat injury of the skin. Brain Res. 1982;231:257-265.
68. England J.D., Happel L.T., Kline D.G., et al. Sodium channel accumulation in humans with painful neuromas. Neurology. 1996;47:272-276.
69. Matzner O., Devor M. Hyperexcitability at sites of nerve injury depends on voltage-sensitive Na+ channels. J Neurophysiol. 1994;72:349-359.
70. Chen Y., Michaelis M., Janig W., Devor M. Adrenoreceptor subtype mediating sympathetic-sensory coupling in injured sensory neurons. J Neurophysiol. 1996;76:3721-3730.
71. Habler H.J., Janig W., Koltzenburg M. Activation of unmyelinated afferents in chronically lesioned nerves by adrenaline and excitation of sympathetic efferents in the cat. Neurosci Lett. 1987;82:35-40.
72. Ali Z., Ringkamp M., Hartke T.V., et al. Uninjured C-fiber nociceptors develop spontaneous activity and alpha-adrenergic sensitivity following L6 spinal nerve ligation in monkey. J Neurophysiol. 1999;81:455-466.
73. Sato J., Perl E.R. Adrenergic excitation of cutaneous pain receptors induced by peripheral nerve injury. Science. 1991;251:1608-1610.
74. Schmidt R.F. The articular polymodal nociceptor in health and disease. Prog Brain Res. 1996;113:53-81.
75. Fukuoka H., Kawatani M., Hisamitsu T., Takeshige C. Cutaneous hyperalgesia induced by peripheral injection of interleukin-1 beta in the rat. Brain Res. 1994;657:133-140.
76. Obreja O., Schmelz M., Poole S., Kress M. Interleukin-6 in combination with its soluble IL-6 receptor sensitises rat skin nociceptors to heat, in vivo. Pain. 2002;96:57-62.
77. Shafer D.M., Assael L., White L.B., Rossomando E.F. Tumor necrosis factor-alpha as a biochemical marker of pain and outcome in temporomandibular joints with internal derangements. J Oral Maxillofac Surg. 1994;52:786-791.
78. Sorkin L.S., Xiao W.H., Wagner R., Myers R.R. Tumour necrosis factor-alpha induces ectopic activity in nociceptive primary afferent fibres. Neuroscience. 1997;81:255-262.
79. Carlton S.M. Peripheral excitatory amino acids. Curr Opin Pharmacol. 2001;1:52-56.
80. Bhave G., Karim F., Carlton S.M., Gereau R.W.4th. Peripheral group I metabotropic glutamate receptors modulate nociception in mice. Nat Neurosci. 2001;4:417-423.
81. Zhou S., Komak S., Du J., Carlton S.M. Metabotropic glutamate 1alpha receptors on peripheral primary afferent fibers: their role in nociception. Brain Res. 2001;913:18-26.
82. Hardy J.D., Wolff H.G., Goodell H. Experimental evidence on the nature of cutaneous hyperalgesia. J Clin Invest. 1950;29:115-140.
83. Mendell L.M. Physiological properties of unmyelinated fiber projection to the spinal cord. Exp Neurol. 1966;16:316-332.
84. Woolf C.J. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306:686-688.
85. Devor M., Wall P.D. Plasticity in the spinal cord sensory map following peripheral nerve injury in rats. J Neurosci. 1981;1:679-684.
86. Woolf C.J., Wall P.D. Relative effectiveness of C primary afferent fibers of different origins in evoking a prolonged facilitation of the flexor reflex in the rat. J Neurosci. 1986;6:1433-1442.
87. Cook A.J., Woolf C.J., Wall P.D. Prolonged C-fibre mediated facilitation of the flexion reflex in the rat is not due to changes in afferent terminal or motoneurone excitability. Neurosci Lett. 1986;70:91-96.
88. Hylden J.L., Nahin R.L., Traub R.J., Dubner R. Expansion of receptive fields of spinal lamina I projection neurons in rats with unilateral adjuvant-induced inflammation: the contribution of dorsal horn mechanisms. Pain. 1989;37:229-243.
89. Cook A.J., Woolf C.J., Wall P.D., McMahon S.B. Dynamic receptive field plasticity in rat spinal cord dorsal horn following C-primary afferent input. Nature. 1987;325:151-153.
90. Devor M., Wall P.D. Effect of peripheral nerve injury on receptive fields of cells in the cat spinal cord. J Comp Neurol. 1981;199:277-291.
91. Suzuki R., Dickenson A.H. Neuropathic pain: nerves bursting with excitement. Neuroreport. 2000;11:R17-R21.
92. Dickenson A.H. Gate control theory of pain stands the test of time. Br J Anaesth. 2002;88:755-757.
93. Dickenson A.H. Spinal cord pharmacology of pain. Br J Anaesth. 1995;75:193-200.
94. MacDermott A.B., Role L.W., Siegelbaum S.A. Presynaptic ionotropic receptors and the control of transmitter release. Annu Rev Neurosci. 1999;22:443-485.
95. Willis W.D.Jr. Dorsal root potentials and dorsal root reflexes: a double-edged sword. Exp Brain Res. 1999;124:395-421.
96. Pasternak G.W., Childers S.R., Snyder S.H. Naloxazone, a long-acting opiate antagonist: effects on analgesia in intact animals and on opiate receptor binding in vitro. J Pharmacol Exp Ther. 1980;214:455-462.
97. Pasternak G.W., Childers S.R., Snyder S.H. Opiate analgesia: evidence for mediation by a subpopulation of opiate receptors. Science. 1980;208:514-516.
98. Hori Y., Endo K., Takahashi T. Presynaptic inhibitory action of enkephalin on excitatory transmission in superficial dorsal horn of rat spinal cord. J Physiol. 1992;450:673-685.
99. Schneider S.P., Eckert W.A.3rd, Light A.R. Opioid-activated postsynaptic, inward rectifying potassium currents in whole cell recordings in substantia gelatinosa neurons. J Neurophysiol. 1998;80:2954-2962.
100. Melzack R., Wall P.D. Pain mechanisms: a new theory. Science. 1965;150:971-979.
101. Melzack R. From the gate to the neuromatrix. Pain. 1999;Suppl 6:S121-S126.
102. Villemure C., Bushnell M.C. Cognitive modulation of pain: how do attention and emotion influence pain processing? Pain. 2002;95:195-199.
103. Tasker R.A., Choiniere M., Libman S.M., Melzack R. Analgesia produced by injection of lidocaine into the lateral hypothalamus. Pain. 1987;31:237-248.
104. Vaccarino A.L., Melzack R. Analgesia produced by injection of lidocaine into the anterior cingulum bundle of the rat. Pain. 1989;39:213-219.
105. Casey K.L. Concepts of pain mechanisms: the contribution of functional imaging of the human brain. Prog Brain Res. 2000;129:277-287.
106. Haythornthwaite J.A., Benrud-Larson L.M. Psychological aspects of neuropathic pain. Clin J Pain. 2000;16(Suppl 2):S101-S105.
107. Keogh E., Ellery D., Hunt C., Hannent I. Selective attentional bias for pain-related stimuli amongst pain fearful individuals. Pain. 2001;91:91-100.
108. Jensen M.P., Turner J.A., Romano J.M., Karoly P. Coping with chronic pain: a critical review of the literature. Pain. 1991;47:249-283.
109. Jensen M.P., Turner J.A., Romano J.M. Changes in beliefs, catastrophizing, and coping are associated with improvement in multidisciplinary pain treatment. J Consult Clin Psychol. 2001;69:655-662.
110. Keefe F.J., Kashikar-Zuck S., Robinson E., et al. Pain coping strategies that predict patients’ and spouses’ ratings of patients’ self-efficacy. Pain. 1997;73:191-199.
111. Cui J., Matsushima E., Aso K., et al. Psychological features and coping styles in patients with chronic pain. Psychiatry Clin Neurosci. 2009;63:147-152.
112. Flor H., Turk D.C. Chronic back pain and rheumatoid arthritis: predicting pain and disability from cognitive variables. J Behav Med. 1988;11:251-265.
113. Jensen M.P., Karoly P. Control beliefs, coping efforts, and adjustment to chronic pain. J Consult Clin Psychol. 1991;59:431-438.
114. Turner J.A., Clancy S. Strategies for coping with chronic low back pain: relationship to pain and disability. Pain. 1986;24:355-364.
115. Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1:277-299.
116. Chibnall J.T., Tait R.C. The Pain Disability Index: factor structure and normative data. Arch Phys Med Rehabil. 1994;75:1082-1086.
117. Pollard C.A. Preliminary validity study of the pain disability index. Percept Mot Skills. 1984;59:974.
118. American Medical Association. Guides to the evaluation of permanent impairment, ed 4, Chicago: American Medical Association, 1993.
119. Social Security Administration Office of Research Evaluation and Statistics. Social Security programs in the United States. Washington, D.C., 1997.
120. Aronoff G.M., Livengood J.M. Pain: psychiatric aspects of impairment and disability. Curr Pain Headache Rep. 2003;7:105-115.
121. Aronoff G.M., Mandel S., Genovese E., et al. Evaluating malingering in contested injury or illness. Pain Pract. 2007;7:178-204.
122. Meyers J.E., Millis S.R., Volkert K. A validity index for the MMPI-2. Arch Clin Neuropsychol. 2002;17:157-169.
123. Larrabee G.J. Exaggerated pain report in litigants with malingered neurocognitive dysfunction. Clin Neuropsychol. 2003;17:395-401.
124. Scuderi G.J., Sherman A.L., Brusovanik G.V., et al. Symptomatic cervical disc herniation following a motor vehicle collision: return to work comparative study of workers’ compensation versus personal injury insurance status. Spine J. 2005;5:639-644.
125. Aronoff G.M. Chronic pain and the disability epidemic. Clin J Pain. 1991;7:330-338.
126. Turner J.A., Franklin G., Fulton-Kehoe D., et al. Prediction of chronic disability in work-related musculoskeletal disorders: a prospective, population-based study. BMC Musculoskelet Disord. 2004;5:14.
127. Crook J., Moldofsky H. The probability of recovery and return to work from work disability as a function of time. Qual Life Res. 1994;3(Suppl 1):S97-S109.
128. Fishbain D.A. Secondary gain concept: definition, problems, and its abuse in medical practice. Am Pain Soc J. 1994;3:264-273.
129. Beals R.K. Compensation and recovery from injury. West J Med. 1984;140:233-237.
130. Herron L.D., Turner J.A., Novell L.A., Kreif S.L. Patient selection for lumbar discectomy with a revised objective rating system. Clin Orthop Relat Res. 1996;325:148-155.
131. Klinger R., Geiger F., Schiltenwolf M. [Can failed back surgery be prevented? Psychological risk factors for postoperative pain after back surgery. Orthopade. 2008;37:1000, 1002-1006.
132. Guo H.R. Working hours spent on repeated activities and prevalence of back pain. Occup Environ Med. 2002;59:680-688.
133. Guo H.R., Tanaka S., Halperin W.E., Cameron L.L. Back pain prevalence in US industry and estimates of lost workdays. Am J Public Health. 1999;89:1029-1035.
134. Boswell M.V., Trescot A.M., Datta S., et al. Interventional techniques: evidence-based practice guidelines in the management of chronic spinal pain. Pain Physician. 2007;10:7-111.
135. Lawrence R.C., Helmick C.G., Arnett F.C., et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778-799.
136. Bueff H.U., Van der Reis W. Low back pain. Prim Care. 1996;23:345-364.
137. Carey T.S., Garrett J., Jackman A., et al. The outcomes and costs of care for acute low back pain among patients seen by primary care practitioners, chiropractors, and orthopedic surgeons. The North Carolina Back Pain Project. N Engl J Med. 1995;333:913-917.
138. Slipman C.W., Shin C.H., Patel R.K., et al. Etiologies of failed back surgery syndrome. Pain Med. 2002;3:200-214.
139. Burton C.V. Causes of failure of surgery on the lumbar spine: ten-year follow-up. Mt Sinai J Med. 1991;58:183-187.
140. Skaf G., Bouclaous C., Alaraj A., Chamoun R. Clinical outcome of surgical treatment of failed back surgery syndrome. Surg Neurol. 2005;64:483-488.
141. North R.B., Kidd D.H., Lee M.S., Piantodosi S. A prospective, randomized study of spinal cord stimulation versus reoperation for failed back surgery syndrome: initial results. Stereotact Funct Neurosurg. 1994;62:267-272.
142. Fritsch E.W., Heisel J., Rupp S. The failed back surgery syndrome: reasons, intraoperative findings, and long-term results: a report of 182 operative treatments. Spine (Phila Pa 1976). 1996;21:626-633.
143. Long D.M., Filtzer D.L., BenDebba M., Hendler N.H. Clinical features of the failed-back syndrome. J Neurosurg. 1988;69:61-71.
144. North R.B., Campbell J.N., James C.S., et al. Failed back surgery syndrome: 5-year follow-up in 102 patients undergoing repeated operation. Neurosurgery. 1991;28:685-690.
145. Spengler D.M., Freeman C., Westbrook R., Miller J.W. Low-back pain following multiple lumbar spine procedures: failure of initial selection? Spine (Phila Pa 1976). 1980;5:356-360.
146. Anderson V.C., Israel Z. Failed back surgery syndrome. Curr Rev Pain. 2000;4:105-111.
147. Waguespack A., Schofferman J., Slosar P., Reynolds J. Etiology of long-term failures of lumbar spine surgery. Pain Med. 2002;3:18-22.
148. Bernard T.N.Jr. Repeat lumbar spine surgery: Factors influencing outcome. Spine (Phila Pa 1976). 1993;18:2196-2200.
149. Hsu C.J., Chou W.Y., Chang W.N., Wong C.Y. Clinical follow up after instrumentation-augmented lumbar spinal surgery in patients with unsatisfactory outcomes. J Neurosurg Spine. 2006;5:281-286.
150. Elsawaf A., Mastronardi L., Roperto R., et al. Effect of cervical dynamics on adjacent segment degeneration after anterior cervical fusion with cages. Neurosurg Rev. 2009;32:215-224.
151. Hilibrand A.S., Carlson G.D., Palumbo M.A., et al. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg [Am]. 1999;81:519-528.
152. Chou W.Y., Hsu C.J., Chang W.N., Wong C.Y. Adjacent segment degeneration after lumbar spinal posterolateral fusion with instrumentation in elderly patients. Arch Orthop Trauma Surg. 2002;122:39-43.
153. Booth K.C., Bridwell K.H., Lenke L.G., et al. Complications and predictive factors for the successful treatment of flatback deformity (fixed sagittal imbalance). Spine (Phila Pa 1976). 1999;24:1712-1720.
154. Jang J.S., Lee S.H., Min J.H., et al. Surgical treatment of failed back surgery syndrome due to sagittal imbalance. Spine (Phila Pa 1976). 2007;32:3081-3087.
155. Lagrone M.O., Bradford D.S., Moe J.H., et al. Treatment of symptomatic flatback after spinal fusion. J Bone Joint Surg [Am]. 1988;70:569-580.
156. Wetzel F.T., LaRocca H. The failed posterior lumbar interbody fusion. Spine (Phila Pa 1976). 1991;16:839-845.
157. Onesti S.T. Failed back syndrome. Neurologist. 2004;10:259-264.
158. Burton C.V., Kirkaldy-Willis W.H., Yong-Hing K., Heithoff K.B. Causes of failure of surgery on the lumbar spine. Clin Orthop Relat Res. 1981;157:191-199.
159. Oaklander A.L., North R.B. Failed back surgery syndrome. In Loeser J.D., editor: Bonica’s management of pain, ed 3, Philadelphia: Lippincott Williams &Wilkins, 2001.
160. Long D.M. Failed back surgery syndrome. Neurosurg Clin North Am. 1991;2:899-919.
161. Ragab A., Deshazo R.D. Management of back pain in patients with previous back surgery. Am J Med. 2008;121:272-278.
162. Cairns M.C., Foster N.E., Wright C. Randomized controlled trial of specific spinal stabilization exercises and conventional physiotherapy for recurrent low back pain. Spine (Phila Pa 1976). 2006;31:E670-E681.
163. Hides J.A., Richardson C.A., Jull G.A. Multifidus muscle recovery is not automatic after resolution of acute, first-episode low back pain. Spine (Phila Pa 1976). 1996;21:2763-2769.
164. Hodges P.W., Richardson C.A. Inefficient muscular stabilization of the lumbar spine associated with low back pain: a motor control evaluation of transversus abdominis. Spine (Phila Pa 1976). 1996;21:2640-2650.
165. Moseley G.L., Nicholas M.K., Hodges P.W. Pain differs from non-painful attention-demanding or stressful tasks in its effect on postural control patterns of trunk muscles. Exp Brain Res. 2004;156:64-71.
166. Hayden J.A., van Tulder M.W., Malmivaara A., Koes B.W. Exercise therapy for treatment of non-specific low back pain. Cochrane Database Syst Rev. 2005;3:CD000335.
167. O’Sullivan P.B., Phyty G.D., Twomey L.T., Allison G.T. Evaluation of specific stabilizing exercise in the treatment of chronic low back pain with radiologic diagnosis of spondylolysis or spondylolisthesis. Spine (Phila Pa 1976). 1997;22:2959-2967.
168. Stuge B., Veierod M.B., Laerum E., Vollestad N. The efficacy of a treatment program focusing on specific stabilizing exercises for pelvic girdle pain after pregnancy: a two-year follow-up of a randomized clinical trial. Spine (Phila Pa 1976). 2004;29:E197-E203.
169. Hides J.A., Jull G.A., Richardson C.A. Long-term effects of specific stabilizing exercises for first-episode low back pain. Spine (Phila Pa 1976). 2001;26:E243-E248.
170. Hayden J.A., van Tulder M.W., Tomlinson G. Systematic review: strategies for using exercise therapy to improve outcomes in chronic low back pain. Ann Intern Med. 2005;142:776-785.
171. Chou R., Huffman L.H. Medications for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:505-514.
172. Luo X., Pietrobon R., Curtis L.H., Hey L.A. Prescription of nonsteroidal anti-inflammatory drugs and muscle relaxants for back pain in the United States. Spine (Phila Pa 1976). 2004;29:E531-E537.
173. Bernstein E., Carey T.S., Garrett J.M. The use of muscle relaxant medications in acute low back pain. Spine (Phila Pa 1976). 2004;29:1346-1351.
174. Luo X., Pietrobon R., Hey L. Patterns and trends in opioid use among individuals with back pain in the United States. Spine (Phila Pa 1976). 2004;29:884-890.
175. van Tulder M.W., Scholten R.J., Koes B.W., Deyo R.A. Nonsteroidal anti-inflammatory drugs for low back pain: a systematic review within the framework of the Cochrane Collaboration Back Review Group. Spine (Phila Pa 1976). 2000;25:2501-2513.
176. Evans D.P., Burke M.S., Newcombe R.G. Medicines of choice in low back pain. Curr Med Res Opin. 1980;6:540-547.
177. van Tulder M.W., Koes B.W., Bouter L.M. Conservative treatment of acute and chronic nonspecific low back pain: a systematic review of randomized controlled trials of the most common interventions. Spine (Phila Pa 1976). 1997;22:2128-2156.
178. Browning R., Jackson J.L., O’Malley P.G. Cyclobenzaprine and back pain: a meta-analysis. Arch Intern Med. 2001;161:1613-1620.
179. Hennes H.M., Wagner V., Bonadio W.A., et al. The effect of oral midazolam on anxiety of preschool children during laceration repair. Ann Emerg Med. 1990;19:1006-1009.
180. Dellemijn P.L., Fields H.L. Do benzodiazepines have a role in chronic pain management? Pain. 1994;57:137-152.
181. Chao J. Retrospective analysis of Kadian (morphine sulfate sustained-release capsules) in patients with chronic, nonmalignant pain. Pain Med. 2005;6:262-265.
182. Dickinson B.D., Altman R.D., Nielsen N.H., Williams M.A. Use of opioids to treat chronic, noncancer pain. West J Med. 2000;172:107-115.
183. Hale M.E., Dvergsten C., Gimbel J. Efficacy and safety of oxymorphone extended release in chronic low back pain: results of a randomized, double-blind, placebo- and active-controlled phase III study. J Pain. 2005;6:21-28.
184. Hojsted J., Sjogren P. An update on the role of opioids in the management of chronic pain of nonmalignant origin. Curr Opin Anaesthesiol. 2007;20:451-455.
185. Sandkuhler J., Kress H.G. Opioids for chronic nonmalignant and neuropathic pain. Eur J Pain. 2005;9:99-100.
186. Duggan A.W., North R.A. Electrophysiology of opioids. Pharmacol Rev. 1983;35:219-281.
187. Benedetti C. Neuroanatomy and biochemistry of antinociception. Bonica J.J., Ventrafridda V., editors. Advances in pain research and therapy. New York: Raven Press. 1979;Vol 2:31-44.
188. Miyoshi H.R., Leckband S.G. Systemic opioid analgesics. In: Loeser J.D., editor. Bonica’s management of pain. ed 3. Philadelphia: Lippincott Williams & Wilkins; 2001:1682-1709.
189. Breivik H. Opioids in chronic non-cancer pain, indications and controversies. Eur J Pain. 2005;9:127-130.
190. Chang G., Chen L., Mao J. Opioid tolerance and hyperalgesia. Med Clin North Am. 2007;91:199-211.
191. Hojsted J., Nielsen P.R., Eriksen J., et al. Breakthrough pain in opioid-treated chronic non-malignant pain patients referred to a multidisciplinary pain centre: a preliminary study. Acta Anaesthesiol Scand. 2006;50:1290-1296.
192. Savage S.R. Opioid therapy of chronic pain: assessment of consequences. Acta Anaesthesiol Scand. 1999;43:909-917.
193. Portenoy R.K. Chronic opioid therapy in nonmalignant pain. J Pain Symptom Manage. 1990;5(Suppl 1):S46-S62.
194. Mao J. Opioid-induced abnormal pain sensitivity. Curr Pain Headache Rep. 2006;10:67-70.
195. Celerier E., Laulin J.P., Corcuff J.B., et al. Progressive enhancement of delayed hyperalgesia induced by repeated heroin administration: a sensitization process. J Neurosci. 2001;21:4074-4080.
196. Belgrade M.J. Opioids for chronic nonmalignant pain: choosing suitable candidates for long-term therapy. Postgrad Med. 1999;106:115-116. 119-124
197. Lorenz J., Beck H., Bromm B. Cognitive performance, mood and experimental pain before and during morphine-induced analgesia in patients with chronic non-malignant pain. Pain. 1997;73:369-375.
198. Sjogren P., Thomsen A.B., Olsen A.K. Impaired neuropsychological performance in chronic nonmalignant pain patients receiving long-term oral opioid therapy. J Pain Symptom Manage. 2000;19:100-108.
199. Sjogren P., Christrup L.L., Petersen M.A., Hojsted J. Neuropsychological assessment of chronic non-malignant pain patients treated in a multidisciplinary pain centre. Eur J Pain. 2005;9:453-462.
200. Bendtsen L., Jensen R., Olesen J. A non-selective (amitriptyline), but not a selective (citalopram), serotonin reuptake inhibitor is effective in the prophylactic treatment of chronic tension-type headache. J Neurol Neurosurg Psychiatry. 1996;61:285-290.
201. Kishore-Kumar R., Max M.B., Schafer S.C., et al. Desipramine relieves postherpetic neuralgia. Clin Pharmacol Ther. 1990;47:305-312.
202. Max M.B., Culnane M., Schafer S.C., et al. Amitriptyline relieves diabetic neuropathy pain in patients with normal or depressed mood. Neurology. 1987;37:589-596.
203. Sindrup S.H., Bjerre U., Dejgaard A., et al. The selective serotonin reuptake inhibitor citalopram relieves the symptoms of diabetic neuropathy. Clin Pharmacol Ther. 1992;52:547-552.
204. Turner J.A., Denny M.C. Do antidepressant medications relieve chronic low back pain? J Fam Pract. 1993;37:545-553.
205. Salerno S.M., Browning R., Jackson J.L. The effect of antidepressant treatment on chronic back pain: a meta-analysis. Arch Intern Med. 2002;162:19-24.
206. Staiger T.O., Gaster B., Sullivan M.D., Deyo R.A. Systematic review of antidepressants in the treatment of chronic low back pain. Spine (Phila Pa 1976). 2003;28:2540-2545.
207. Atkinson J.H., Slater M.A., Williams R.A., et al. A placebo-controlled randomized clinical trial of nortriptyline for chronic low back pain. Pain. 1998;76:287-296.
208. Max M.B., Gilron I.H. Antidepressants, muscle relaxants and N-methyl-D-aspartate receptor antagonists. In Loeser J.D., editor: Bonica’s management of pain, ed 3, Philadelphia: Lippincott Williams & Wilkins, 2001.
209. Max M.B., Lynch S.A., Muir J., et al. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992;326:1250-1256.
210. Watson C.P., Chipman M., Reed K., et al. Amitriptyline versus maprotiline in postherpetic neuralgia: a randomized, double-blind, crossover trial. Pain. 1992;48:29-36.
211. Watson C.P., Evans R.J. A comparative trial of amitriptyline and zimelidine in post-herpetic neuralgia. Pain. 1985;23:387-394.
212. Rull J.A., Quibrera R., Gonzalez-Millan H., Lozano Castaneda O. Symptomatic treatment of peripheral diabetic neuropathy with carbamazepine (Tegretol): double blind crossover trial. Diabetologia. 1969;5:215-218.
213. Swerdlow M., Cundill J.G. Anticonvulsant drugs used in the treatment of lancinating pain: a comparison. Anaesthesia. 1981;36:1129-1132.
214. Khoromi S., Patsalides A., Parada S., et al. Topiramate in chronic lumbar radicular pain. J Pain. 2005;6:829-836.
215. Muehlbacher M., Nickel M.K., Kettler C., et al. Topiramate in treatment of patients with chronic low back pain: a randomized, double-blind, placebo-controlled study. Clin J Pain. 2006;22:526-531.
216. Dworkin R.H., O’Connor A.B., Backonja M., et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132:237-251.
217. McCleane G.J. Lamotrigine in the management of neuropathic pain: a review of the literature. Clin J Pain. 2000;16:321-326.
218. McCleane G.J. Does gabapentin have an analgesic effect on background, movement and referred pain? A randomised, double-blind, placebo controlled study. Pain Clin. 2001;13:103-107.
219. Yildirim K., Sisecioglu M., Karatay S., et al. The effectiveness of gabapentin in patients with chronic radiculopathy. Pain Clin. 2003;15:213-218.
220. Burchiel K.J. Carbamazepine inhibits spontaneous activity in experimental neuromas. Exp Neurol. 1988;102:249-253.
221. Yaari Y., Devor M. Phenytoin suppresses spontaneous ectopic discharge in rat sciatic nerve neuromas. Neurosci Lett. 1985;58:117-122.
222. Walsh T.R., Weinstein J.N., Spratt K.F., et al. Lumbar discography in normal subjects: a controlled, prospective study. J Bone Joint Surg [Am]. 1990;72:1081-1088.
223. Mavrocordatos P., Cahana A. Minimally invasive procedures for the treatment of failed back surgery syndrome. Adv Tech Stand Neurosurg. 2006;31:221-252.
224. Kaplan M., Dreyfuss P., Halbrook B., Bogduk N. The ability of lumbar medial branch blocks to anesthetize the zygapophysial joint: a physiologic challenge. Spine (Phila Pa 1976). 1998;23:1847-1852.
225. Bogduk N. International Spinal Injection Society guidelines for the performance of spinal injection procedures. Part 1: Zygapophysial joint blocks. Clin J Pain. 1997;13:285-302.
226. Helm S., Hayek S.M., Benyamin R.M., Manchikanti L. Systematic review of the effectiveness of thermal annular procedures in treating discogenic low back pain. Pain Physician. 2009;12:207-232.
227. Saal J.A., Saal J.S. Intradiscal electrothermal treatment for chronic discogenic low back pain: prospective outcome study with a minimum 2-year follow-up. Spine (Phila Pa 1976). 2002;27:966-973.
228. Saal J.A., Saal J.S. Intradiscal electrothermal treatment for chronic discogenic low back pain: a prospective outcome study with minimum 1-year follow-up. Spine (Phila Pa 1976). 2000;25:2622-2627.
229. Karasek M., Bogduk N. Twelve-month follow-up of a controlled trial of intradiscal thermal anuloplasty for back pain due to internal disc disruption. Spine (Phila Pa 1976). 2000;25:2601-2607.
230. van Kleef M., Barendse G.A., Kessels A., et al. Randomized trial of radiofrequency lumbar facet denervation for chronic low back pain. Spine (Phila Pa 1976). 1999;24:1937-1942.
231. Burnham R.S., Holitski S., Dinu I. A prospective outcome study on the effects of facet joint radiofrequency denervation on pain, analgesic intake, disability, satisfaction, cost, and employment. Arch Phys Med Rehabil. 2009;90:201-205.
232. Cohen S.P., Stojanovic M.P., Crooks M., et al. Lumbar zygapophysial (facet) joint radiofrequency denervation success as a function of pain relief during diagnostic medial branch blocks: a multicenter analysis. Spine J. 2008;8:498-504.
233. Hooten W.M., Martin D.P., Huntoon M.A. Radiofrequency neurotomy for low back pain: evidence-based procedural guidelines. Pain Med. 2005;6:129-138.
234. Leclaire R., Fortin L., Lambert R., et al. Radiofrequency facet joint denervation in the treatment of low back pain: a placebo-controlled clinical trial to assess efficacy. Spine (Phila Pa 1976). 2001;26:1411-1416.
235. Nachemson A.L. Newest knowledge of low back pain: a critical look. Clin Orthop Relat Res. 1992;279:8-20.
236. Dooley J.F., McBroom R.J., Taguchi T., et al. Nerve root infiltration in the diagnosis of radicular pain. Spine (Phila Pa 1976). 1988;13:79-83.
237. Simopoulos T.T., Kraemer J., Nagda J.V., et al. Response to pulsed and continuous radiofrequency lesioning of the dorsal root ganglion and segmental nerves in patients with chronic lumbar radicular pain. Pain Physician. 2008;11:137-144.
238. Slappendel R., Crul B.J., Braak G.J., et al. The efficacy of radiofrequency lesioning of the cervical spinal dorsal root ganglion in a double blinded randomized study: no difference between 40 degrees C and 67 degrees C treatments. Pain. 1997;73:159-163.
239. Martin D.C., Willis M.L., Mullinax L.A., et al. Pulsed radiofrequency application in the treatment of chronic pain. Pain Pract. 2007;7:31-35.
240. Revel M., Auleley G.R., Alaoui S., et al. Forceful epidural injections for the treatment of lumbosciatic pain with post-operative lumbar spinal fibrosis. Rev Rhum Engl Ed. 1996;63:270-277.
241. Meadeb J., Rozenberg S., Duquesnoy B., et al. Forceful sacrococcygeal injections in the treatment of postdiscectomy sciatica: a controlled study versus glucocorticoid injections. Joint Bone Spine. 2001;68:43-49.
242. Avellanal M., Diaz-Reganon G. Interlaminar approach for epiduroscopy in patients with failed back surgery syndrome. Br J Anaesth. 2008;101:244-249.
243. Igarashi T., Hirabayashi Y., Seo N., et al. Lysis of adhesions and epidural injection of steroid/local anaesthetic during epiduroscopy potentially alleviate low back and leg pain in elderly patients with lumbar spinal stenosis. Br J Anaesth. 2004;93:181-187.
244. Fai K.R., Engleback M., Norman J.B., Griffiths R. Interlaminar approach for epiduroscopy in patients with failed back surgery syndrome. Br J Anaesth. 2008;101:244-249.
245. Boswell M.V., Hansen H.C., Trescot A.M., Hirsch J.A. Epidural steroids in the management of chronic spinal pain and radiculopathy. Pain Physician. 2003;6:319-334.
246. Waddell G., Kummel E.G., Lotto W.N., et al. Failed lumbar disc surgery and repeat surgery following industrial injuries. J Bone Joint Surg [Am]. 1979;61:201-207.
247. Finnegan W.J., Fenlin J.M., Marvel J.P., et al. Results of surgical intervention in the symptomatic multiply-operated back patient: analysis of sixty-seven cases followed for three to seven years. J Bone Joint Surg [Am]. 1979;61:1077-1082.
248. Deer T.R., Caraway D.L., Kim C.K., et al. Clinical experience with intrathecal bupivacaine in combination with opioid for the treatment of chronic pain related to failed back surgery syndrome and metastatic cancer pain of the spine. Spine J. 2002;2:274-278.
249. Krames E.S. Intraspinal opioid therapy for chronic nonmalignant pain: current practice and clinical guidelines. J Pain Symptom Manage. 1996;11:333-352.
250. Koulousakis A., Kuchta J., Bayarassou A., Sturm V. Intrathecal opioids for intractable pain syndromes. Acta Neurochir Suppl. 2007;97(Pt 1):43-48.
251. Krames E.S., Gershow J., Glassberg A., et al. Continuous infusion of spinally administered narcotics for the relief of pain due to malignant disorders. Cancer. 1985;56:696-702.
252. Krames E.S., Lanning R.M. Intrathecal infusional analgesia for nonmalignant pain: analgesic efficacy of intrathecal opioid with or without bupivacaine. J Pain Symptom Manage. 1993;8:539-548.
253. Dahm P., Nitescu P., Appelgren L., Curelaru I. Efficacy and technical complications of long-term continuous intraspinal infusions of opioid and/or bupivacaine in refractory nonmalignant pain: a comparison between the epidural and the intrathecal approach with externalized or implanted catheters and infusion pumps. Clin J Pain. 1998;14:4-16.
254. Hassenbusch S.J., Portenoy R.K., Cousins M., et al. Polyanalgesic Consensus Conference 2003: an update on the management of pain by intraspinal drug delivery—report of an expert panel. J Pain Symptom Manage. 2004;27:540-563.
255. Sator-Katzenschlager S., Deusch E., Maier P., et al. The long-term antinociceptive effect of intrathecal S(+)-ketamine in a patient with established morphine tolerance. Anesth Analg. 2001;93:1032-1034.
256. Rainov N.G., Heidecke V., Burkert W. Long-term intrathecal infusion of drug combinations for chronic back and leg pain. J Pain Symptom Manage. 2001;22:862-871.
257. Coombs D.W., Saunders R.L., Gaylor M.S., et al. Relief of continuous chronic pain by intraspinal narcotics infusion via an implanted reservoir. JAMA. 1983;250:2336-2339.
258. Dennis G.C., DeWitty R. Management of intractable pain in cancer patients by implantable morphine infusion systems. J Natl Med Assoc. 1987;79:939-944.
259. Turner J.A., Sears J.M., Loeser J.D. Programmable intrathecal opioid delivery systems for chronic noncancer pain: a systematic review of effectiveness and complications. Clin J Pain. 2007;23:180-195.
260. Raphael J.H., Southall J.L., Gnanadurai T.V., et al. Long-term experience with implanted intrathecal drug administration systems for failed back syndrome and chronic mechanical low back pain. BMC Musculoskelet Disord. 2002;3:17.
261. Raslan A.M., McCartney S., Burchiel K.J. Management of chronic severe pain: spinal neuromodulatory and neuroablative approaches. Acta Neurochir Suppl. 2007;97(Pt 1):33-41.
262. Yaksh T.L., Al-Rodhan N.R., Jensen T.S. Sites of action of opiates in production of analgesia. Prog Brain Res. 1988;77:371-394.
263. Yaksh T.L., Rudy T.A. Studies on the direct spinal action of narcotics in the production of analgesia in the rat. J Pharmacol Exp Ther. 1977;202:411-428.
264. Yaksh T.L., Rudy T.A. Analgesia mediated by a direct spinal action of narcotics. Science. 1976;192:1357-1358.
265. Auld A.W., Maki-Jokela A., Murdoch D.M. Intraspinal narcotic analgesia in the treatment of chronic pain. Spine (Phila Pa 1976). 1985;10:777-781.
266. Lamb S.A., Hosobuchi Y. Intrathecal morphine sulfate for chronic benign pain, delivered by implanted pump delivery systems. Paper presented at the International Association for the Study of Pain, Adelaide, Australia, 1990; 1990.
267. Auld A.W., Murdoch D.M., O’Laughlin K.A. Intraspinal narcotic analgesia: pain management in the failed laminectomy syndrome. Spine (Phila Pa 1976). 1987;12:953-954.
268. Maron J., Loeser J.D. Spinal opioid infusions in the treatment of chronic pain of nonmalignant origin. Clin J Pain. 1996;12:174-179.
269. Penn R.D., Paice J.A. Chronic intrathecal morphine for intractable pain. J Neurosurg. 1987;67:182-186.
270. Hassenbusch S.J., Pillay P.K., Magdinec M., et al. Constant infusion of morphine for intractable cancer pain using an implanted pump. J Neurosurg. 1990;73:405-409.
271. Kumar K., Kelly M., Pirlot T. Continuous intrathecal morphine treatment for chronic pain of nonmalignant etiology: long-term benefits and efficacy. Surg Neurol. 2001;55:79-86.
272. Anderson V.C., Burchiel K.J. A prospective study of long-term intrathecal morphine in the management of chronic nonmalignant pain. Neurosurgery. 1999;44:289-300.
273. Tutak U., Doleys D.M. Intrathecal infusion systems for treatment of chronic low back and leg pain of noncancer origin. South Med J. 1996;89:295-300.
274. Levy R.M. Quantitative, crossover, double-blind trial paradigm for patient screening for chronic intraspinal narcotic administration. Neurosurg Focus. 1997;2:e2.
275. Arner S., Meyerson B.A. Lack of analgesic effect of opioids on neuropathic and idiopathic forms of pain. Pain. 1988;33:11-23.
276. Vecht C.J. Nociceptive nerve pain and neuropathic pain. Pain. 1989;39:243-246.
277. Jadad A.R., Carroll D., Glynn C.J., et al. Morphine responsiveness of chronic pain: double-blind randomised crossover study with patient-controlled analgesia. Lancet. 1992;339:1367-1371.
278. Murphy T.M., Hinds S., Cherry D. Intraspinal narcotics: non-malignant pain. Acta Anaesthesiol Scand Suppl. 1987;85:75-76.
279. Deer T., Krames E.S., Hassenbusch S., et al. Polyanalgesic Consensus Conference 2007: Recommendations for the management of pain by intrathecal (intraspinal) drug delivery: report of an interdisciplinary expert panel. Neuromodulation. 2007;10:300-328.
280. Angel I.F., Gould H.J.Jr., Carey M.E. Intrathecal morphine pump as a treatment option in chronic pain of nonmalignant origin. Surg Neurol. 1998;49:92-98.
281. Leon-Casasola O, Rauck RL: Intrathecal therapy for the management of cancer and noncancer pain. Anesthesiology News, Pain Medicine News, 2009.
282. Aldrete J.A., Couto da Silva J.M. Leg edema from intrathecal opiate infusions. Eur J Pain. 2000;4:361-365.
283. Anderson V.C., Cooke B., Burchiel K.J. Intrathecal hydromorphone for chronic nonmalignant pain: a retrospective study. Pain Med. 2001;2:287-297.
284. Cabbell K.L., Taren J.A., Sagher O. Spinal cord compression by catheter granulomas in high-dose intrathecal morphine therapy: case report. Neurosurgery. 1998;42:1176-1180.
285. Waara-Wolleat K.L., Hildebrand K.R., Stewart G.R. A review of intrathecal fentanyl and sufentanil for the treatment of chronic pain. Pain Med. 2006;7:251-259.
286. Hassenbusch S., Burchiel K., Coffey R.J., et al. Management of intrathecal catheter-tip inflammatory masses: a consensus statement. Pain Med. 2002;3:313-323.
287. Allen J.W., Horais K.A., Tozier N.A., et al. Time course and role of morphine dose and concentration in intrathecal granuloma formation in dogs: a combined magnetic resonance imaging and histopathology investigation. Anesthesiology. 2006;105:581-589.
288. Allen J.W., Horais K.A., Tozier N.A., Yaksh T.L. Opiate pharmacology of intrathecal granulomas. Anesthesiology. 2006;105:590-598.
289. Miele V.J., Price K.O., Bloomfield S., et al. A review of intrathecal morphine therapy related granulomas. Eur J Pain. 2006;10:251-261.
290. Webster L.R., Fisher R., Charapata S., Wallace M.S. Long-term intrathecal ziconotide for chronic pain: an open-label study. J Pain Symptom Manage. 2009;37:363-372.
291. Rauck R.L., Wallace M.S., Leong M.S., et al. A randomized, double-blind, placebo-controlled study of intrathecal ziconotide in adults with severe chronic pain. J Pain Symptom Manage. 2006;31:393-406.
292. Staats P.S., Yearwood T., Charapata S.G., et al. Intrathecal ziconotide in the treatment of refractory pain in patients with cancer or AIDS: a randomized controlled trial. JAMA. 2004;291:63-70.
293. Wallace M.S. Ziconotide: a new nonopioid intrathecal analgesic for the treatment of chronic pain. Expert Rev Neurother. 2006;6:1423-1428.
294. Wallace M.S., Rauck R., Fisher R., et al. Intrathecal ziconotide for severe chronic pain: safety and tolerability results of an open-label, long-term trial. Anesth Analg. 2008;106:628-637.
295. Kumar K., Bodani V., Bishop S., Tracey S. Use of intrathecal bupivacaine in refractory chronic nonmalignant pain. Pain Med. 2009;10:819-828.
296. Coffey J.R., Cahill D., Steers W., et al. Intrathecal baclofen for intractable spasticity of spinal origin: results of a long-term multicenter study. J Neurosurg. 1993;78:226-232.
297. Harney D., Victor R. Traumatic syrinx after implantation of an intrathecal catheter. Reg Anesth Pain Med. 2004;29:606-609.
298. Wall P.D., Sweet W.H. Temporary abolition of pain in man. Science. 1967;155:108-109.
299. Shealy C.N., Mortimer J.T., Reswick J.B. Electrical inhibition of pain by stimulation of the dorsal columns: preliminary clinical report. Anesth Analg. 1967;46:489-491.
300. North R.B., Wetzel F.T. Spinal cord stimulation for chronic pain of spinal origin: a valuable long-term solution. Spine (Phila Pa 1976). 2002;27:2584-2591.
301. Burton C. Dorsal column stimulation: optimization of application. Surg Neurol. 1975;4:171-179.
302. Meyerson B.A., Linderoth B. Spinal cord stimulation. In: Loeser J.D., editor. Bonica’s management of pain. ed 3. Philadelphia: Lippincott Williams & Wilkins; 2001:1857-1876.
303. Taylor R.S., Van Buyten J.P., Buchser E. Spinal cord stimulation for chronic back and leg pain and failed back surgery syndrome: a systematic review and analysis of prognostic factors. Spine (Phila Pa 1976). 2005;30:152-160.
304. Mailis-Gagnon A., Furlan A.D., Sandoval J.A., Taylor R. Spinal cord stimulation for chronic pain. Cochrane Database Syst Rev. 2004;3:CD003783.
305. Kumar K., Taylor R.S., Jacques L., et al. The effects of spinal cord stimulation in neuropathic pain are sustained: a 24-month follow-up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation. Neurosurgery. 2008;63:762-770.
306. Kumar K., Taylor R.S., Jacques L., et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain. 2007;132:179-188.
307. Kumar K., Malik S., Demeria D. Treatment of chronic pain with spinal cord stimulation versus alternative therapies: cost-effectiveness analysis. Neurosurgery. 2002;51:106-115.
308. Kumar K., Nath R., Wyant G.M. Treatment of chronic pain by epidural spinal cord stimulation: a 10-year experience. J Neurosurg. 1991;75:402-407.
309. Kumar K., Hunter G., Demeria D. Spinal cord stimulation in treatment of chronic benign pain: challenges in treatment planning and present status, a 22-year experience. Neurosurgery. 2006;58:481-496.
310. Long D.M. The current status of electrical stimulation of the nervous system for the relief of chronic pain. Surg Neurol. 1998;49:142-144.
311. North R.B., Kidd D.H., Zahurak M., et al. Spinal cord stimulation for chronic, intractable pain: experience over two decades. Neurosurgery. 1993;32:384-394.
312. Ray C.D. Spinal epidural electrical stimulation for pain control: practical details and results. Appl Neurophysiol. 1981;44:194-206.
313. Ray C.D., Burton C.V., Lifson A. Neurostimulation as used in a large clinical practice. Appl Neurophysiol. 1982;45:160-166.
314. Kupers R.C., Van den Oever R., Van Houdenhove B., et al. Spinal cord stimulation in Belgium: a nation-wide survey on the incidence, indications and therapeutic efficacy by the health insurer. Pain. 1994;56:211-216.
315. North R.B., Kidd D., Shipley J., Taylor R.S. Spinal cord stimulation versus reoperation for failed back surgery syndrome: a cost effectiveness and cost utility analysis based on a randomized, controlled trial. Neurosurgery. 2007;61:361-368.
316. Van Buyten J.P., Van Zundert J., Vueghs P., Vanduffel L. Efficacy of spinal cord stimulation: 10 years of experience in a pain centre in Belgium. Eur J Pain. 2001;5:299-307.
317. Burchiel K.J., Anderson V.C., Brown F.D., et al. Prospective, multicenter study of spinal cord stimulation for relief of chronic back and extremity pain. Spine (Phila Pa 1976). 1996;21:2786-2794.
318. Turner J.A., Loeser J.D., Bell K.G. Spinal cord stimulation for chronic low back pain: a systematic literature synthesis. Neurosurgery. 1995;37:1088-1095.
319. Lee A.W., Pilitsis J.G. Spinal cord stimulation: indications and outcomes. Neurosurg Focus. 2006;21:E3.
320. Deer T.R. Current and future trends in spinal cord stimulation for chronic pain. Curr Pain Headache Rep. 2001;5:503-509.
321. Taylor R.S., Taylor R.J., Van Buyten J.P., et al. The cost effectiveness of spinal cord stimulation in the treatment of pain: a systematic review of the literature. J Pain Symptom Manage. 2004;27:370-378.
322. Bel S., Bauer B.L. Dorsal column stimulation (DCS): cost to benefit analysis. Acta Neurochir Suppl (Wien). 1991;52:121-123.
323. Bell G.K., Kidd D., North R.B. Cost-effectiveness analysis of spinal cord stimulation in treatment of failed back surgery syndrome. J Pain Symptom Manage. 1997;13:286-295.
324. Devulder J., Crombez E., Brusselmans G. Efficacy of spinal cord stimulation for neuropathic pain: assessment by abstinence; Monhemius R, Simpson B, Eur J Pain 2003,7;513–519. Eur J Pain. 2005;9(1):95. author reply 97–98
325. Loge D., Devulder J.E., De Coster O., et al. The epidural fibrous sheath: a guide for the replacement of a spinal cord stimulation electrode. Reg Anesth Pain Med. 2002;27:353-356.
326. Devulder J., De Laat M., Van Bastelaere M., Rolly G. Spinal cord stimulation: a valuable treatment for chronic failed back surgery patients. J Pain Symptom Manage. 1997;13:296-301.
327. Kumar K., Hunter G., Demeria D.D. Treatment of chronic pain by using intrathecal drug therapy compared with conventional pain therapies: a cost-effectiveness analysis. J Neurosurg. 2002;97:803-810.
328. Manca A., Kumar K., Taylor R.S., et al. Quality of life, resource consumption and costs of spinal cord stimulation versus conventional medical management in neuropathic pain patients with failed back surgery syndrome (PROCESS trial). Eur J Pain. 2008;12:1047-1058.
329. Manchikanti L., Staats P.S., Singh V., et al. Evidence-based practice guidelines for interventional techniques in the management of chronic spinal pain. Pain Physician. 2003;6:3-81.
330. Oakley J.C. Spinal cord stimulation: patient selection, technique, and outcomes. Neurosurg Clin North Am. 2003;14:365-380.
331. Barolat G. Spinal cord stimulation for chronic pain management. Arch Med Res. 2000;31:258-262.
332. North R., Shipley J., Prager J., et al. Practice parameters for the use of spinal cord stimulation in the treatment of chronic neuropathic pain. Pain Med. 2007;8(Suppl 4):S200-S275.
333. Turner J.A., Loeser J.D., Deyo R.A., Sanders S.B. Spinal cord stimulation for patients with failed back surgery syndrome or complex regional pain syndrome: a systematic review of effectiveness and complications. Pain. 2004;108:137-147.