Pain and pain management
Introduction
Pain is a primitive, multi-dimensional experience with physiological, psychological, social and emotional components. The International Association for the Study of Pain (IASP) Subcommittee on Taxonomy (Merskey & Bogduk 1994) describes pain as ‘an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage’. Pain is also a valuable and necessary part of the body’s defence mechanism and usually indicates that something is wrong, for example, physical damage or disease (Scholz & Woolf 2002). Pain management meanwhile is a fundamental feature of healthcare with the aim to achieve comfort through some form of analgesia – taken from the Greek word for painlessness.
Feeling pain – transmission anatomy and physiology
Pain is felt when sensory nerve endings are stimulated and neurones relay information to the brain. Specialized pain receptors, known as nociceptors, are found in free nerve endings close to mast cells and small blood vessels that all work together to respond to pain (McHugh & McHugh 2000). Nociceptors are found in large numbers in the skin, arterial walls, periosteum and joint surfaces and in smaller numbers in all of the deep tissues of the body (Marieb & Hoehn 2007).
There appear to be two distinct types of nociceptors: high-threshold mechanoreceptors that respond to strong mechanical stimuli, and polymodal nociceptors that respond to mechanical, thermal and chemical stimuli (McHugh & McHugh 2000). Mechanical stimuli, e.g., compressing or stretching tissues and thermal stimuli, e.g., excess heat or cold, appear to stimulate the nociceptors through chemical mediator release (Allan 2005). Chemical stimuli occur as a result of the substances that are released from damaged tissues, e.g., prostaglandins, serotonin, bradykinin and histamine (McHugh & McHugh 2000). Examples of tissue damage when such chemicals are released are infection, ischaemia, inflammation, ulceration and nerve damage (Godfrey 2005).
• A-delta fibres respond to stimulation of the high-threshold mechanoreceptors. The fibres are large diameter and thinly myelinated. They transmit pain impulses rapidly (about 5–30 m/s) and are known as ‘first’ or ‘fast’ fibres. Pain sensation is usually sharp pricking, well-localized or stinging (Hudspith et al. 2006).
• C fibres connect to the polymodal receptors. They are smaller, unmyelinated fibres that conduct at 0.5–2 m/s and are known as ‘second’ or ‘slow’ pain fibres. Pain sensation may be burning, dull and poorly localized or aching.
Other fibres and receptors involved are:
• A-beta fibres are thicker and heavily myelinated, conducting information such as touch, pressure and temperature very rapidly (30–100 m/s) but not pain.
• opioid receptors are found throughout the brain and spinal cord and respond to naturally occurring endogenous opioids and to synthetic exogenous opioids.
A-delta fibres, C fibres and T (transmitter) neurones in the spinal cord
Information carried by the A-delta fibres and C fibres is relayed to the substantia gelatinosa in the dorsal horn of the spine where the neurones terminate and synapse with T (transmitter) neurones (Fig. 25.1). For the transfer of this information between the A-delta and C fibres to the T neurones excitatory neurochemical transmitters need to be released, since there is a synaptic cleft between the two (Strøma et al. 2012). These transmitters include adenosine triphosphate, glutamate, calcitonin gene-related peptide, bradykinin, nitrous oxide and substance P. The T neurones cross the spinal cord and ascend on the opposite side of the spino-thalmic tract carrying pain information to the medulla where they re-cross to the original side and synapse with secondary sensory neurones that transmit the sensation onto the thalamus in the brain. It is at this point that the sensation is experienced in a general manner without detail. From the thalmus a third group of neurones relay the information to the cerebral cortex and the somatosensory cortex to allow pain localization and stimulus interpretation. This perception of pain in the brain is vital as it allows us to act to relieve or alleviate the situation. Fibres from the thalamus also connect with the hypothalamus and reticular system, accounting for the changes in the autonomic nervous system outlined below and the motor response, and to the limbic system where emotional and behavioural responses are generated (Godfrey 2005).
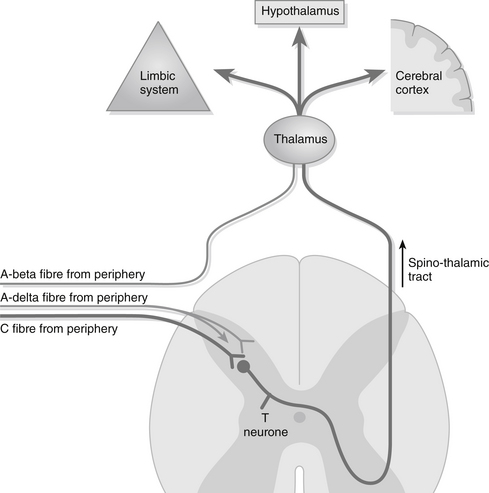
Figure 25.1 The pain pathway.
Opioids and opioid receptors
While substance P and glutamate have been implicated in the transmission of pain, other neurochemicals appear to possess analgesic properties. These include endorphins, enkephalins and dynorphins, which are produced by the body and have an analgesic action similar to that of morphine. Further research is required, but the existence and action of these chemicals help to explain phenomena such as the placebo response, where an individual perceives pain relief even though no analgesic agent has been given. It appears that, in such cases, the mere expectation of pain relief is sufficient to release psychogenically the endogenous opiates, which would then cause a genuine analgesia even without the administration of an analgesic drug (Allan 2005).
Pain theories
Specificity theory
The traditional specificity theory was developed by Descartes in 1644 (Godfrey 2005). Descartes thought there was a direct link from the point of pain to the brain, suggesting that pain is a specific sensation and that pain intensity is proportional to the extent of the tissue damage (Watt-Watson & Ivers Donovan 1992). According to this theory, pain associated with a minor cut gives minimal discomfort, whereas pain associated with major trauma hurts far more. It is now known that pain is not simply a function of the amount of bodily damage, but is influenced by attention, anxiety, suggestion, experience and other psychological variables (Melzack & Wall 1982). However, current research indicates that conduction of pain impulses is more complex than was originally proposed. The recognition of the pain pathways inherent in the specificity theory provides the basis for surgery of intractable pain where the pain pathway is interrupted so impulses cannot reach consciousness (Hallet 1992).
Gate control theory
The gate control theory of pain proposed by Melzack & Wall (1965) revolutionized the understanding of pain. They proposed the idea of a ‘gate’ in the substantia gelatinosa in the dorsal (or posterior) horn of the spinal cord through which pain information must pass on its way to the brain. The substantia gelatinosa is an area of special neurones located close to each posterior column of grey matter and extending the length of the spinal cord. A number of factors can block or close the ‘gate’ to pain messages, but equally other factors will open the gate and allow pain to be experienced by the individual. When nerve impulses from the nociceptors are brought to the dorsal horn by A-delta and C fibres and relayed to the T neurones, the gate is opened.
The T neurones can be inhibited by neurochemical transmitters released by tiny interneurones in the substantia gelatinosa. The A-beta fibres synapse with these interneurones and inhibit transmission of information to the T neurone (Fig. 25.2).
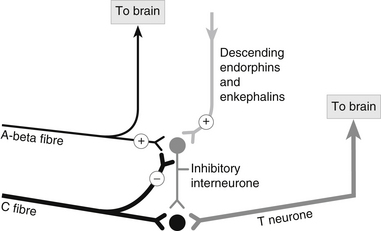
Figure 25.2 Inhibitory interneurone and pain transmission.
Inhibition of the T neurone reduces the flow of pain information to the brain, effectively ‘closing’ the pain gate. Increasing activity in the A-beta fibres (touch, pressure, temperature) lessens the pain felt, while an increase in activity in the small-diameter A-delta and C fibres means that more pain is perceived (Barasi 1991). This is the basis for many of the non-pharmacological pain-relieving measures, including ‘rubbing it better’, the application of heat or cold, electrical stimulation and counter-current irritation, all of which stimulate the A-beta fibres and so reduce the pain messages being relayed to the brain.
The gating mechanism is affected by information flowing from the brain through the descending inhibitory pathways. These pathways originate in a number of areas of the brain (i.e., reticular formation, periaqueductal grey matter, raphe nuclei) and synapse with the inhibitory neurones in the substantia gelatinosa, releasing the neurotransmitters serotonin and noradrenaline norepinephrine (Barasi 1991). These inhibitory neurotransmitters excite the interneurones in the dorsal horn of the spine secreting the body’s natural endogenous opioids (endorphins, enkephalins and dynorphins) that inhibit the T neurones suppressing pain transmission. They also block the release of substance P from the A-delta and C fibres and block the receptors for substance P on the T neurones (Sherwood 2010). Allowing the brain to release endogenous opiates is a key factor in pain relief and several methods can be employed in emergency settings to try to achieve this:
• use of sensory input, such as distraction, guided imagery and hypnotism
If the inhibitory interneurones are stimulated, either by the A-beta fibres or by input from descending brain pathways, fewer pain impulses will be relayed to the T fibres and so less pain information will be carried to the brain. Melzack & Wall (1965) felt that this theory explains why the relationship between pain and injury is so variable and why the location of pain can differ from the site of injury. It also explains how pain can persist in the absence of injury or after healing and why the nature and location of pain can change over time. Hallet (1992) suggested that the gate control theory expands the role of the spinal cord; it is not just a relay station, but a centre for filtering and integrating incoming sensory information.
Effects of pain
The physiological responses that occur when the nociceptors are stimulated are similar to those of the acute stress (‘flight-or-fight’) response as first described by Cannon (1915) also known as the freeze, fight, or flight response (Bracha et al. 2004). The sympathetic nervous system is activated causing general vasoconstriction, while dilating the arteries supplying vital organs such as the muscles (McArdle et al. 2006). This results in tachycardia, tachypnoea, hypertension, sweating and pallor. Tidal volume and alveolar ventilation may be reduced, as is gastric motility. Skeletal muscle spasm may occur and hormonal changes may cause electrolyte imbalances and hyperglycaemia (Sutcliffe 1993).
Non-physiological effects
There is evidence that everyone has the same pain threshold, i.e., they perceive pain at the same stimulus intensity. Sternback & Tursky (1965) found that there was no difference among four different ethnic groups in the level of electric shock that was first reported as producing a detectable sensation. Heat, for example, is perceived as painful by everyone at the 44–46°C range when it begins to damage tissues (Marieb & Hoehn 2007). The sensory conducting apparatus, in other words, appears to be essentially similar in all people so that a given level of input always elicits a sensation. However, an individual’s tolerance of and response to pain will be affected by a number of factors other than those described above. The different thresholds associated with pain are identified in Box 25.1.
Although there is evidence that everyone, regardless of cultural background, has a uniform threshold, cultural background does have a powerful effect on the pain tolerance levels. Sternback & Tursky (1965) reported that the levels at which subjects refused to tolerate electric shocks, even when they were encouraged by experimenters, depended in part on the ethnic origin of the subject. For the emergency nurse, this may explain the differing reactions to pain of individuals from different cultural backgrounds.
Anxiety and the experience of pain have also been linked (Hayward 1975, Cave 1994). Hayward (1975) demonstrated that if patients were given information regarding their post-operative pain, they experienced less pain and required less analgesia. Walsh (1993) found in a study of patients with relatively minor problems that of the 90% with pain, many were anxious. He suggested that this might be due to a variety of reasons:
• the sudden and unexpected disruption of the illness or injury
• fear of the possible long-term effects of the illness or injury
Pain is not simply a physiological response; rather it is a psycho-physiological phenomenon, explaining why the experience of pain is unique for each person (Sofaer 1992). As McCaffery (1983) noted, ‘pain is always a subjective experience and pain is what the patient says it is and exists when the patient says it does’.
Assessing pain
Uncontrolled acute pain is known to cause psychological distress, and it may also lead to adverse physiological changes in some organs and systems (Green & McGhie 2010). As a consequence, it needs to be assessed and addressed early and effectively.
Individuals not only feel and react differently to pain, but describe it differently as well (Sofaer 1998). It is difficult to measure pain as it is a subjective phenomenon especially in relation to the cultural values and norms regarding the expression of pain as well as a result of the psychological response. This is further complicated where patients are unable to describe their pain because of the location or severity of their injury, their age or cognitive ability or because they are unconscious or intubated. In part, this may explain why pain levels are often poorly documented in emergency settings (Chisolm et al. 2008, Easton et al. 2012). The aim of pain assessment is to take the patient’s subjective experience and transform it into objective data which health professionals can understand and use to plan relevant pain-relieving measures and monitor their effect.
In some situations it will be immediately obvious that the patient is profoundly distressed and requires urgent intervention, e.g., the patient suddenly presenting to the ED with severe crushing chest pain. Intervention in these circumstances should be immediate and only a brief assessment of the situation is required. In other circumstances, however, a more thorough assessment is required and should be ongoing in the light of the patient’s current and in some cases their already known clinical condition. Hallet (1992) identified the range of information that should be included when making pain assessments (Box 25.2).
Pain assessment tools
A number of pain assessment tools are available (Green & McGhie 2010) that are potentially useful in the ED. Scales can be used to measure pain, treatment effectiveness and satisfaction. Three scores are the Verbal Rating Scale (VRS) scoring from ‘no pain’ to ‘severe pain’; the Visual Analogue Scale (VAS) consisting of a line that ranges from ‘no pain’ to ‘worst pain imaginable’ where the patient points to a position on the line; and the Numeric Rating Scale (NRS), using a scoring 0-10 or 0-5. Williamson & Hoggart (2005) argue that the VAS and NRS are not interchangeable; however, they continue to be used in combination. The VRS has the benefit of simplicity but is less sensitive than either the VAS or the NRS (Green & McGhie 2010). The advantages of the VAS combined with a NRS are as follows:
• it is sensitive to small changes
• it can be used to measure pain intensity
• it can be used to measure pain relief
• it is easy for the patient to use
• the numerical interpretation that facilitates documentation.
The disadvantages of VAS are that:
• pain is scored on a single dimension only
• some patient groups, such as the visually impaired, cognitively impaired, children or the elderly, may find it difficult to use.
For children under three years old the observational FLACC scale (Merkel et al. 1997) is available. This includes five categories: face, legs, activity, cry and consolability with each category scoring 0–2 with a maximum of 10 points awarded overall.
For children over the age of three years and those unable to grasp the concept of linear scales, pain rating scales using pictures of faces have become popular, e.g., Wong-Baker FACES™ (Hockenberry & Wilson 2009). The faces, which are in increasing degrees of distress, are shown to patients who are then asked to point to the one that depicts the amount of pain they are feeling. Each face has a numeric link to it making it easily recordable. However, concern about these pain scales has been expressed by Mather & Mackie (1983), who noted that children sometimes played down their pain because they did not want to be given an injection. They found that pain reporting has the potential to be inaccurate where the consequences were known or expected to be unpleasant, e.g., administration of an injection.
Pharmacological pain management
Most acute pain is managed solely with drugs. Analgesic drugs work in several ways: by altering the pain sensation, depressing pain perception or modifying the patient’s response to pain. As a general principle, drugs are used most effectively if their selection is based on the cause and intensity of pain. They can be delivered in a variety of routes (Box 25.3).
Analgesics act in the brain, spinal cord, nerve endings and at the site of tissue damage to reduce the amount of pain being felt. They are selective as they are able to diminish pain without affecting other sensations (O’Hara & Campling 1996). Moreover, analgesics do not ‘mask pain’, particularly that of abdominal pain hindering assessment (Helfand & Freeman 2009). Analgesics can be divided into two groups: opiates and non-opiates.
Opiates
Morphine
Morphine is a derivative of the opium poppy and is extremely effective. In the ED it is usually administered intravenously or orally. It alleviates pain and anxiety; however, the nurse should note the following (Greenstein & Gould 2004):
• the pupils of the eye are constricted due to an effect on the nucleus of the third facial nerve
• morphine stimulates the vagus nerve, which may present difficulties when morphine is used for the pain of coronary thrombosis as it may further reduce the pulse rate and blood pressure
• morphine causes spasms of the sphincters, including the sphincter of Oddi, and therefore should not be used in pancreatitis.
In trauma cases, oral or intramuscular morphine should not be given as the shocked patient will have poor perfusion leading to limited initial drug absorption and bolus absorption following resuscitation (Driscoll et al. 1994).
Diamorphine
The actions of diamorphine are similar to those of morphine, but it is 2.5 times more potent as an analgesic agent (Thompson & Webster 1992) with a similar rise in side effects. It is the drug of choice in the management of severe central chest pain in many countries. Intravenous (i.v.) administration should be accompanied with anti-emetics, and the depressant effects on the respiratory centre need to be monitored, particularly in patients with chronic chest disease. Diamorphine is usually administered in a dose of 2.5–5 mg i.v., repeated as necessary. Diamorphine, a class A controlled drug, is also known as heroin and the antidote naloxone 0.4 mg i.v. should always be available.
Fentanyl
The actions of fentanyl are similar to those of morphine although it has a faster peak, shorter duration of action, and a non-histamine release that can reduce adverse effects experienced by the patient, particularly hypotension (Braude & Richards 2004). Fentanyl lends itself well to situations where establishing i.v. access may be difficult, e.g., in young children, but where significant pain needs to be addressed. This is because it may be administered intranasally for absorption by the mucosa (Borland et al. 2007), or nebulized and inhaled for absorption across alveoli (Furyk et al. 2009).
Pethidine
Pethidine is a synthetically derived analgesic that has similar actions to diamorphine and is chemically related to atropine. It is less powerful than morphine but has a lower risk of respiratory depression and does not cause constriction of the pupils; it is therefore used in head injuries where observation of the pupil size may be important (Goldstein & Gould 2004). Unlike morphine, it does not cause spasms of the sphincters and is the analgesic of choice in pancreatitis. It is worthy of note that pethidine can react with monoamine oxidase inhibitor (MAOI) drugs and cause severe hypertension (Carr & Mann 2000).
Non-opiates
Entonox
This gaseous mixture of 50 % oxygen and 50 % nitrous oxide can be effective as a short-term analgesic agent. Its primary use is to provide pain relief to conscious patients who are able to use the demand valve system of delivery for analgesia during unpleasant procedures, e.g., during splinting (Adam & Osborne 1997). It is contraindicated when there is a pneumothorax or a fracture to the base of the skull (Driscoll et al. 1994) or in a prolonged manner for potential bowel obstruction (McKinnon 1980).
Paracetamol/Acetophinomen
This is most suitable for mild to moderate pain. Its mechanism of action remains in debate. It is thought to act by inhibiting the cycloxygenase (Cox-I, Cox-II, Cox-III) enzymes, therefore increasing the patient’s pain threshold. It has no impact on the inflammatory response nor on platelet function or gastric mucosa. It can be used in combination with other analgesia but caution needs to be used as many patients self-administer this pre-hospital which can lead to the unintentional self-poisoning (Bronstein et al. 2010).
NSAIDs
These are the most widely used analgesics. Commonly used NSAIDs include aspirin, indometacin and ibuprofen. They are effective particularly in relieving pain associated with inflammation, such as musculoskeletal disorders, trauma to peripheral tissues and headache. Some studies have shown that NSAIDs provided pain relief equal to or better than 10 mg intramuscular morphine (McQuay & Moore 1998). NSAIDs also act on the hypothalamus to reset the body’s thermostat during febrile episodes, reducing the temperature.
NSAIDs inhibit the enzyme cyclo-oxygenase and thus affect the body’s production of prostaglandins (Carr & Mann 2000), which cause pain and inflammation. However, prostaglandins are also responsible for maintaining the mucous lining of the gastric mucosa. As a consequence, the use of NSAIDs can lead to gastric damage, which may range from nausea or ‘heartburn’ to gastrointestinal bleeding. This can be ameliorated by recommending the patient to take the NSAIDs with meals and/or milk. NSAIDs can also trigger asthmatic reactions, and patients should be asked if they suffer from asthma before NSAIDs are prescribed or dispensed. In the past five years, selective cyclo-oxygenase II specific inhibitors (COX-II) have been developed which produce analgesia with fewer side effects.
Non-pharmacological pain management
In addition to pharmacological interventions, there is a wide range of pain-relieving strategies that can be employed by the nurse to relieve patient pain and suffering. These include information, positioning, thermal, mechanical and/or electrical stimuli and distraction and the placebo effect. McCaffery (1990) has suggested that a combination of pharmacological and non-pharmacological methods probably yields the most effective relief for the patient.
Information
Attention to factors identified by the patient and, for example, to his level of anxiety regarding pain is essential. Hayward’s classic study showed that patients who were kept informed about the level of discomfort and pain they could expect reported lower levels of pain (Hayward 1975). It is both unwise and unethical for the nurse to state that a patient will not feel pain before a painful procedure. This is especially true for children, who may subsequently lose trust in their health carers. Honesty with reassurance can do much to alleviate the mental suffering associated with current or impending pain from nursing and medical procedures.
Positioning
Immobilization and elevation are simple but effective measures can do much to alleviate pain and suffering and are particularly useful for patients who have sustained musculoskeletal injuries. The nurse can use slings, splints, pillows or blankets to place the injured limb in a comfortable position and should advise the patient to move it as little as possible. Swelling and pain, particularly when associated with soft tissue injury, can also be reduced by elevation of an injured limb (Walsh 1996).
Thermal and mechanical stimulation and electroanalgesia
For patients who have sustained musculoskeletal injuries, superficial burns or other injuries, the use of cold compresses can reduce swelling and alleviate pain. They act by reducing the release of pain-causing chemicals, such as lactic acid, potassium ions, serotonin and histamine (Lee & Warren 1978). The nurse should ensure that the cold compress does not cause further injury, such as frostbite, and therefore ice should be placed in a plastic bag and covered with a paper or cloth towel. The use of gel packs which can be kept cool in the fridge or warmed in hot water can reduce this risk.
Distraction
McCaffery (1990) defined distraction as simply focusing attention on stimuli other than the pain sensation. One of the most frequently used distraction techniques in the ED involves breathing exercises. Patients are directed to focus their breathing by concentrating on inhalations and exhalations. Appropriate use of humour is also a successful distraction strategy that has been shown to improve the release of the body’s natural endorphins (Watt-Watson & Ivers Donovan, 1992). More recent distractions include use of audio-visual media, such as artwork, music, movies and electronic games.
References
Adam, S.K., Osborne, S. Critical Care Nursing: Science and Practice. Oxford: Oxford Medical; 1997.
Allan, D. Sensory receptors and sense organs. In Montague S.E., Watson R., Herbert R.A., eds.: Physiology for Nursing Practice, third ed, London: Elsevier, 2005.
Barasi, S. The physiology of pain. Surgical Nurse. 1991;4(5):14–20.
Borland, M., Jacobs, I., King, B., et al. A randomized controlled trial comparing intranasal fentanyl to intravenous morphine for managing acute pain in children in the emergency department. Annals of Emergency Medicine. 2007;49(3):335–340.
Bracha, H.S., Ralston, T.C., Matsukawa, J.M. Does ‘fight or flight’ need updating? Psychomatics. 2004;45(5):448–449.
Braude, D., Richards, M. Appeal for fentanyl prehospital use. Prehospital Emergency Care. 2004;8:441–442.
Bronstein, A. C., Spyker, D. A., Cantilena, L. R., et al. 2009. Annual Report of the American Association of Poison Control Centres (NPDS) 27th Annual Report Clinical Toxicology 48, 979–1178.
Cannon, W.B. Bodily Changes in Pain, Hunger, Fear and Rage: An Account of Recent Researches into the Function of Emotional Excitement. New York: Appleton-Century-Crofts; 1915.
Carr, E.C.J., Mann, E.M. Pain: Creative Approaches to Effective Management. Basingstoke: Macmillan Press; 2000.
Cave, I. Pain in A&E: the patient’s view. Emergency Nurse. 1994;2(2):19–20.
Chisholm, C.D., Weaver, C.S., Whenmouth, L.F., et al. A comparison of observed versus documented physician assessment and treatment of pain: the physician record does not reflect the reality. Annals of Emergency Medicine. 2008;52:383–389.
Driscoll, P., Gwinnutt, C., Brook, S. Extremity trauma. In: Driscoll P.A., Gwinnutt C.L., LeDuc Jimmerson C., Goodall A., eds. Trauma Resuscitation: The Team Approach. Basingstoke: Macmillan, 1994.
Easton, R.M., Bendinelli, C., Sisak, K., et al. Recalled pain scores are not reliable after acute trauma. Injury. 2012;43(7):1029–1032.
Furyk, J.S., Grabowski, W.J., Black, L.H. Nebulized fentanyl versus intravenous morphine in children with suspected limb fractures in the emergency department: A randomized controlled trial. Emergency Medicine Australasia. 2009;21(3):203–209.
Godfrey, H. Understanding the Human Body: Biological Perspectives for Healthcare. Edinburgh: Churchill Livingstone; 2005.
Green, L., McGhie, J. Assessment of acute and chronic pain. Anaesthesia and Intensive Care Medicine. 2010;12(1):911.
Greenstein, B., Gould, D. Trounce’s Clinical Pharmacology for Nurses, seventeenth ed. Edinburgh: Elsevier; 2004.
Hallet, N. Pain: prevention and cure. In: Royle J.A., Walsh M., eds. Watson’s Medical-Surgical Nursing and Related Physiology. London: Baillière Tindall, 1992.
Hayward, J. Information: A Prescription Against Pain. London: RCN; 1975.
Helfand, M., Freeman, M. Assessment and management of acute pain in adult medical inpatients: A systematic review. Pain Medicine. 2009;10(7):1183–1199.
Hockenberry, M.J., Wilson, D. Wong’s Essentials Of Pediatric Nursing, eighth ed. St. Louis: Mosby; 2009.
Hudspith, M.J., Siddall, P.J., Munglani, R. Physiology of pain. In Hemming H.C., Hopkins P.M., eds.: Foundations of Anesthesia, second ed, London: Mosby, 2006.
Lee, J.M., Warren, M.P. Clinical applications of cold for the musculoskeletal system. Cold Therapy in Rehabilitation. London: Bell & Hyman; 1978.
Marieb, E.N., Hoehn, K. Human Anatomy and Physiology, seventh ed. San Francisco: Pearson Benjamin Cummings; 2007.
Mather, L., Mackie, J. The incidence of post-operative pain in children. Pain. 1983;15:271–282.
McArdle, W.D., Katch, F.I., Katch, V.L. Essentials of Exercise Physiology Volume 1, third ed. Baltimore, MD: Lippincott Williams & Wilkins; 2006.
McCaffrey, M. Nursing the Patient in Pain. London: Chapman & Hall; 1983.
McCaffrey, M. Nursing approaches to non-pharmacological pain control. International Journal of Nursing Studies. 1990;27(1):1–5.
McHugh, J.M., McHugh, W.B. Pain: neuroanatomy, chemical mediators and clinical applications. AACN Clinical Issues. 2000;11(2):168–178.
McKinnon, K.D.L. Dr McKinnon replies. Canadian Family Physician. 1980;26:639–643.
McQuay, H., Moore, A. An Evidence-Based Resource for Pain Relief. Oxford: Oxford University Press; 1998.
Melzack, R., Wall, P.D. Pain mechanisms: a new theory. Science. 1965;150:971–979.
Melzack, R., Wall, P.D. The Challenge of Pain. New York: Basic Books; 1982.
Merkel, S.I., Voepel-Lewis, T., Shayevitz, J.R., et al. The FLACC: A behavioral scale for scoring postoperative pain in young children. Pediatric Nurse. 1997;23(3):293–297.
Merskey, H., Bogduk, N. Classification of chronic pain. In: Merskey H., Bogduk N., eds. Part III: Pain Terms, A Current List with Definitions and Notes on Usage. IASP Task Force On Taxonomy. Seattle: IASP Press, 1994.
O΄Hara, P., Campling, J. Pain Management for Health Professionals. London: Chapman and Hall; 1996.
Scholz, J., Woolf, C.J. Can we conquer pain? Nature Neuroscience. 2002;5(Suppl):1062–1067.
Sherwood, L. Human Physiology: From Cells to Systems. Peripheral nervous system, afferent division, special senses, seventh ed. Sydney: Cengage Learning; 2010.
Sofaer, B. Pain – A Handbook for Nurses, second ed. London: Chapman & Hall; 1992.
Sofaer, B. Pain – Principles, Practice and Patients. Cheltenham: Stanley Thorne; 1998.
Sternback, R.A., Tursky, B. Ethnic differences among housewives in psychophysical and skin potential responses to electrical shock. Psychophysiology. 1965;1:241–246.
Strøma, V., Røeb, C., Matrea, D., et al. Deep tissue hyperalgesia after computer work. Scandinavian Journal of Pain. 2012;3(1):53–60.
Sutcliffe, A.J. Pain relief for acutely ill and injured patients. Care of the Critically Ill. 1993;9(6):266–269.
Thompson, D.R., Webster, R. Caring for the Coronary Patient. Oxford: Butterworth Heinemann; 1992.
Walsh, M. Pain and anxiety in A&E attenders. Nursing Standard. 1993;17(7):40–42.
Walsh, M. Accident and Emergency Nursing: A New Approach, third ed. Oxford: Butterworth Heinemann; 1996.
Watt-Watson, J.H., Ivers Donovan, M. Pain Management: Nursing Perspectives. St Louis: Mosby Year Book; 1992.
Williamson, A., Hoggart, B. Pain: A review of three commonly used pain rating scales. Journal of Clinical Nursing. 2005;14:798–804.







