Pain
Definition of pain
Pain is the presenting symptom in almost every orthopaedic patient. A complaint of pain is always indicative of some variety or degree of dysfunction1 and results from a combination of physical and psychological causes, although sometimes one or the other predominates. All pain must be regarded as real. Pain entirely devoid of somatic cause is labelled ‘psychogenic pain’: although no peripheral tissue damage exists, the pain is just as distressing as somatic pain2 (see Section 16).
The taxonomy committee of the International Association for the Study of Pain defined pain as: ‘an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage’.3 Pain is thus not a ‘primary sensation’ in the sense that smell, taste, touch, vision and hearing are, but is an ‘emotional state’, like sorrow, love or hate. The consequence is that it is extremely difficult to explain one’s pain to another person. This is reflected in the numerous words that patients use to describe intensity and quality of pain: twinge, ache, distress, discomfort, soreness, cramp, suffering, misery, agony, torment, anguish.4 The fact that pain is always a subjective experience provides the first difficulty in its use in diagnosis. The language used is not always easy to understand, and the examiner usually needs a high level of competence and understanding to translate patients’ subjective descriptions into more objective and useful statements.
Perception and modulation of pain
The intensity of pain does not depend only on the intensity of irritation of the peripheral nociceptive system (receptors and their afferents). Centripetal transmission of peripheral nociceptive stimulation is subject to varying degrees of facilitatory and inhibitory modulation at different synapses during its course to the cerebral cortex. An important modulation site, of major concern to the orthopaedic physician, is the gateway synapse in the basal spinal nucleus, but there are also modulation systems in the spinal grey matter, in the thalamus and in the cerebral cortex itself.5
Peripheral nociceptive system
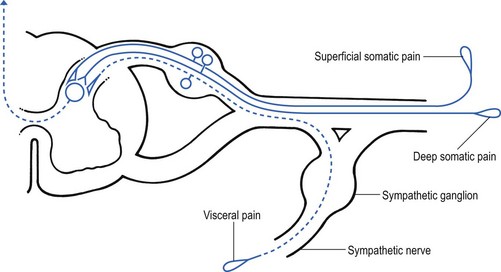
Nociceptive receptors are defined as nerve endings that are sensitive to noxious or potentially noxious (mechanical and chemical) stimuli. The perceptual aspect of the nociceptive system consists of unmyelinated free nerve endings, distributed three dimensionally throughout skin, subcutaneous and adipose tissue, fasciae, aponeuroses, ligaments, tendons, muscles, periosteum and bone.6,7 Clinically, three distinct areas of pain perception may be considered: the skin (superficial somatic pain); the locomotor system (deep somatic pain); and the viscera (visceral pain). Of these, only the skin is adapted to localize pain exactly in the region of injury. Deep somatic and visceral pain are often felt in unusual locations (see Referred pain, p. 6).
In normal circumstances, this nociceptive receptor system remains largely inactive. The unmyelinated free nerve endings are depolarized only by the application of mechanical forces sufficient to deform or damage the tissue that contains them or after exposure to sufficient concentrations of irritating chemical substances (lactic acid, serotonin, prostaglandins and histamine), released from local inflammatory cells and from the peripheral terminals of the primary afferent fibres themselves.8–10
Another important influence on nociceptor sensitivity is the pH of the tissue. High local concentrations of protons are known to occur in inflammation and the consequent reduction in pH contributes to the sensitization of nociceptors.11,12
Afferent nociceptive system
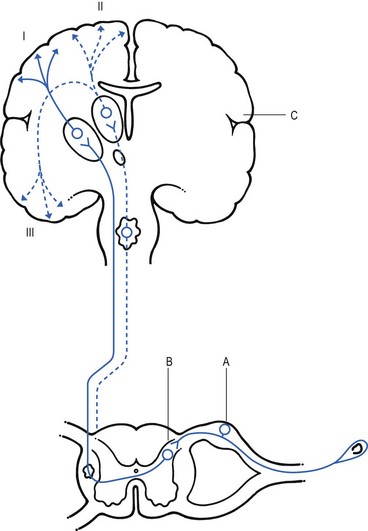
Nerve impulses generated at the nociceptive receptor system are delivered into the spinal cord by small myelinated and unmyelinated nerve fibres (5 µm or less in diameter), that mainly belong to the Ad and C groups of afferent nerve fibres (Fig. 1.1). Their cell bodies are located in the dorsal root ganglia of the spinal nerves. The very small diameter of the C nerves explains their slow conduction velocity (1 m/s), and their extreme sensitivity to blockade by local anaesthetic drugs. The myelinated Ad fibres are slightly larger and have a faster conduction velocity (10 m/s).13
The nociceptive afferents enter the spinal cord, where they divide into short ascending and descending branches, before they terminate at synapses on various groups of relay neurones in the dorsal horn of the spinal grey matter. Most of the connections are to the neurones in the basal spinal nucleus (at the base of the dorsal horn).14,15
The efferents of these cross the cord obliquely to turn upwards on the contralateral side and form the anterolateral spinal tract, which connects the basal spinal nucleus with the thalamic nuclei and has therefore traditionally been called the ‘spinothalamic tract’. Most of the fibres in this tract, however, do not directly ascend to the thalamus without interruption, but instead synapse with neurones in the brainstem reticular system, while others re-enter the spinal grey matter to synapse with internuncial neurones.16 However, the majority of the ascending nociceptive inputs terminate (sometimes after crossing several synapses) in the thalamic nuclear relay sites.17 It should be emphasized that not only do the neurones in the thalamic centres respond to peripheral noxious stimulation but they can also be activated by mechanoreceptor peripheral stimulation (see Pain modulation systems below).
The axons of the thalamic nuclei then ascend to the neurones of the cerebral cortex. Three thalamocortical projections can be defined: those responsible for perception; those related to the emotional experience; and those responsible for memory.18
The first project to the superior paracentral region of the cerebral cortex and seem to contribute to the so-called ‘perceptual component’ of pain – the patient’s ability to perceive whereabouts (in which segment of his body) the pain is localized.19
The activation of the second thalamocortical projection system, projections that pass from the medial and anterior thalamic nuclei to the frontal lobes, evokes the emotional disturbances related with pain.20 Thus, a stimulus ‘hurts’ only when the nociceptive afferent projections arrive at the frontal cortex.
A third thalamocortical projection system links some of the medial thalamic nuclei to the cortex of the ipsilateral temporal lobe. Here the recent and long-term memory storage systems of the brain are located.21,22
A fourth projection system exists which relates some thalamic nuclei to subjacent hypothalamic nuclei in the ventral diencephalon. It is very probable that this thalamohypothalamic system provides the means whereby nociceptive afferent activity entering the brain evokes the complex of visceral reflex (cardiovascular and gastrointestinal) effects and hormonal changes that are so often associated with the experience of pain.23
Pain modulation systems
There are both peripheral and central pain modulation systems.
Peripheral modulation of pain
One of the most important sites at which a synaptic modulation operates from both the peripheral and central sources is at the synapses in the basal spinal nucleus. In 1965, Melzack and Wall,24 basing their theory mainly on the work of Noordenbos,25 published an article entitled: ‘Pain mechanism: a new theory’. They called their concept of peripheral pain modulation the gate control theory (Fig. 1.2), which is based on three premises:
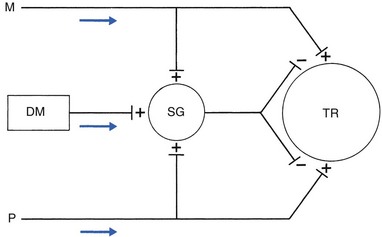
Fig 1.2 Gate control theory (after Melzack and Wall24): P, small nociceptive fibres (pain); M, large mechanoreceptive fibres; TR, transmission cells (relay neurones in the basal spinal nucleus); DM, descending modulation; SG, substantia gelatinosa; +, excitatory effect; –, inhibitory effect.
• Afferent nerves contain two types of fibres: small fibres (P), as described above, and large-diameter afferents (M), which are derived from the various mechanoreceptors in the articular capsule, ligaments and muscle spindles. These fibres produce information about static joint position, pressure changes in the joints, joint movement and stresses that develop in the joint at the extremes of movement. The fibres of mechanoreceptor transmission have a lower stimulation threshold and a faster conduction velocity than the smaller and mostly unmyelinated fibres of the nociceptive system.
• In the substantia gelatinosa (SG) of the dorsal horn, both afferent systems converge and interrelate, with the overall effect that the large-diameter afferents have an inhibitory effect on the relay neurones located in the basal spinal nucleus. This inhibition is presynaptic and is reduced only when there is a massive input from the small nociceptive afferent fibres. The latter thus facilitates central transmission of pain. The interaction between both systems is gate control: impulses travelling along the larger fibres close the gate, and those in the small fibres open the gate so that impulses to thalamus and cortex can pass through.
• The activity of the gate is not only modulated by impulses from nociceptive and mechanoreceptor systems but also receives a descending and regulating feedback from the reticular system, the thalamus and the cerebral cortex.
Central modulation of pain
Awareness of pain is also modulated at the central projection systems.
At the reticular formation
A modulation system at the reticular formation in the brainstem exerts a continuous inhibitory effect on the projection neurones in the spinal nucleus ganglion via the reticulospinal tract, which is discharging continuously at varying frequencies throughout life.26 The inhibitory effect on nociceptive afferent transmission is augmented when the attention of the patient is distracted from the site of pain. This is what occurs when another painful site elsewhere in the body is stimulated (counter-irritation), when the patient concentrates on work or other activities or when hypnosis is induced.27 The inhibitory effect of this reticular system also increases when the blood concentration of catecholamines is very high, as can be the case in states of great emotional tension.28 Also some drugs (chlorpromazine, diazepam and morphine) may selectively increase the activity of the reticular neurones that operate this inhibitory system.29
Inhibitory reticular activity is depressed and pain is enhanced when attention is concentrated on the painful site, or following the administration of barbiturates, caffeine or theophylline.30
Referred pain
Introduction
In 1905, Sir Henry Head described referred pain in the abdominal wall caused by a visceral disease.31 Using the dermatological appearances in herpes zoster, he constructed schemes of segmental innervation of the skin.32 He also described dermatomic zones that became painful in the event of provocation of a related visceral structure. His theory of pain reference was built on the concept of the segmental organization of the human body and its nociceptive system. Further experiments in this sphere were conducted by Sir Thomas Lewis in 1936.33 In 1938 and 1939, Kellgren published the results of a systematic examination of the phenomena of referred pain, demonstrating segmental radiation and failure to cross the midline.34,35
His experiments were confirmed by others.36–38 Later, the concept of segmental reference of pain was refined39,40 and exact borders of the different dermatomes mapped out.41–44
Possible mechanisms
The fact that referred pain is an error in perception was first pointed out by John Hunter in 1835 (cited by Cyriax).45 It was obvious that if pain is felt elsewhere than at its true site, the nociceptive mechanism is reacting inappropriately. However, since there seems to be logical consistency in the way the errors are made (pain from specific lesions is always referred to the same areas), there must also be a logical explanation for ‘failures’. (If a machine always makes the same mistake, a structural or functional disorder must exist.) The basis for the inadequacy must therefore be sought in a miscalculation in the pain mechanism. Theoretically, the defect can lie anywhere along the afferent pathway, from the peripheral receptors to the synapses in the spinal cord and the reticular area and projection zones in the sensory cortex.
• Error at the level of the spinal cord. Most authors have opted for this hypothesis. Mackenzie described an ‘irritable focus’ in the grey matter of the spinal cord as being responsible for the phenomenon.46 Also Livingston47 placed the basis of the error at synapses in the dorsal horns. Wedell et al48,49 and Pomeranz50 accepted a double origin for the sensitive neurone – afferent fibres of a somatic structure, and those coming from a related visceral structure synapse with the same spinal ganglion. Taylor et al51 and Wells et al52 also made a plea for the spinal explanation of referred pain. Their view is that separate peripheral sensory nerves (deep somatic, skin and visceral) converge on to the same cell in the dorsal horn of the spinal cord (Fig. 1.3).
• Failure at the sensory cortex. A number of authors have proposed that the misinterpretation is at the projection area of the sensory cortex rather than at the spinal level.33,53,54 The concept was clinically elaborated by Cyriax who based his theory on a number of premises:
 Referred pain is a pain experience felt elsewhere than at its true site of origin.
Referred pain is a pain experience felt elsewhere than at its true site of origin.
 Skin is an organ adapted to localize the pain accurately.
Skin is an organ adapted to localize the pain accurately.
 Pain is experienced in the sensory cortex, which is organized dermatome by dermatome.
Pain is experienced in the sensory cortex, which is organized dermatome by dermatome.
 The skin is represented accurately in the sensory cortex.
The skin is represented accurately in the sensory cortex.
 A memory storage system is located in the sensory cortex. This is fed by constant input from the skin. Input from deeper somatic structures is very rare in a normal and healthy individual.
A memory storage system is located in the sensory cortex. This is fed by constant input from the skin. Input from deeper somatic structures is very rare in a normal and healthy individual.
The ability to localize pain in the region of injury is limited to skin and does not apply when the source of the pain is in deep tissue. In due course, a certain pain memory is built up in the temporal lobes, and achieves a high degree of anatomical precision. The efficacy of the long-term memory storage system is not simply a function of the intensity of the painful experience but also relates to the length of time a painful experience lasts or to the frequency with which it is repeated.55,56 Since the frequency of painful stimuli coming from the skin is much higher than the frequency of stimuli coming from deeper structures, it will be obvious that pain memory will centre around painful experiences from the skin. When the same cortical cells receive a painful message arriving from a deep-seated structure, the memory will interpret it on the basis of past experience; in that the sensory cortex is arranged segmentally, the pain will be ascribed to the correct segment but the system will fail to localize it accurately at the site of the lesion. The brain therefore ‘places’ it in the tissue it has a reference for – the skin. Pain is thus felt under the surface area connected with the particular cells that belong to the same segment as the tissue from which the nociceptive afferents originate. The pain is felt deep to the skin of the relevant dermatome and not accurately in the skin.
Clinical consequences
Before this discussion of referred pain is continued, it should be stressed that root pain reference does not necessarily mean that a nerve is involved. The false idea that wide radiation of pain is evidence of involvement of nerves is still strongly held by some and is usually the most important obstacle to a logical understanding of referred pain. To approach the problem of referred pain with an open mind, the reader must constantly remember that referred pain is an error of perception. Although the nerve supply to these peripheral structures is distributed on a segmental basis, it does not indicate that referred pain ‘runs down’ a somatic nerve. For instance, pain at the anterior aspect of the leg does not necessarily mean that a nerve structure (L3, femoral nerve or peripheral branches of the femoral nerve) is involved. Although inflammation of the dural sleeve of the L3 nerve root does of course lead to pain extending in the L3 dermatome, the same pain can be provoked by a lesion in any other tissue belonging to the L3 segment (e.g. hip joint or psoas bursa). There will not be any difference in the nature and extent of the pain. The only distinction between pain as the result of a compressed and inflamed nerve root and pain originating from trauma to other structures is the appearance of paraesthesia (see Pressure on nerves, Ch. 2).
Rules of referred pain (Box 1.1)
To understand the segmental organization of the nociceptive mechanism, it is necessary to reconsider embryogenesis (Fig. 1.4).
Embryogenesis
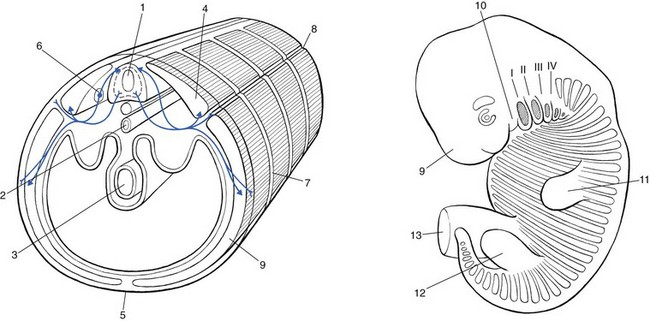
Primitive body
When a fetus is between 4 and 6 weeks old, 42 pairs of somites develop: four occipital, eight cervical, 12 thoracic, four to six lumbar, five sacral and eight to 10 coccygeal.57 The first two and the last seven or eight pairs disappear early in development. The ventral aspect of each somite differentiates into the sclerotome which, together with the chorda around the neural tube, forms the origin of the axial skeleton. The other part of the somite becomes the myotome, covered by the dermatome. Each pair of somites develops its own segmental innervation which later leads to the development of the spinal ganglion and spinal nerve.
Limb formation
After the first month of intra-uterine life, two pairs of buds originate at the lateral sides of the fetus. The proximal papules appear first at the base of the neck, followed by the formation of two distal buds in the caudal area. These extensions gradually project from the cylindrical fetal segments from which they originate. As the limbs grow further and further laterally, some dermatomes become totally disconnected from the trunk (Fig. 1.5).
Dermatomes
The cutaneous area supplied by one spinal nerve is a dermatome. The first clinicians to draw dermatomic maps were Head and Campbell.58 Their diagrams are the basis for the classical drawings in standard neurological textbooks. However, they did not take into consideration the significant degree of variability and overlap in dermatomic borders.57,58 Later investigators such as Keegan and Garrett, and Fukui et al59,60 have demonstrated that dermatomes of adjacent spinal nerves overlap markedly.
We use the charts drawn by Foerster41 in 1933 and corrected by Cyriax in 1982,45 in order to give the broadest possible information about the area to which pain from a particular segment may refer. The extreme importance of accurate localization of pain in orthopaedic medicine requires the physician to use a map of dermatomes that is as close as possible to clinical reality. The figures showing the dermatomes are based on Foerster,41 Cole et al,61 Cyriax,45 Conesa,62 Wakasugi,63 and Mitta.64
Cervical and thoracic dermatomes
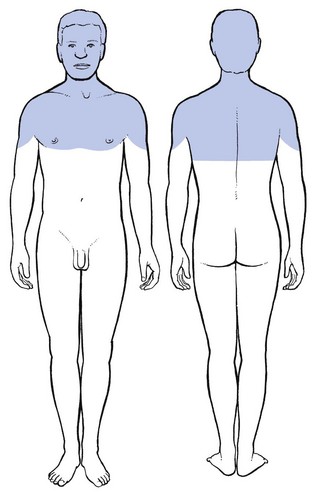
C1 to C4 occupy the scalp (C1), the back of the neck and the temporal area, the upper half of the ear and the upper half of the face (C2), the neck, lower mandibular area and chin (C3), and the lower half of the neck, shoulder area, front of the upper chest and the area above the spina scapulae (C4) (Fig. 1.6).
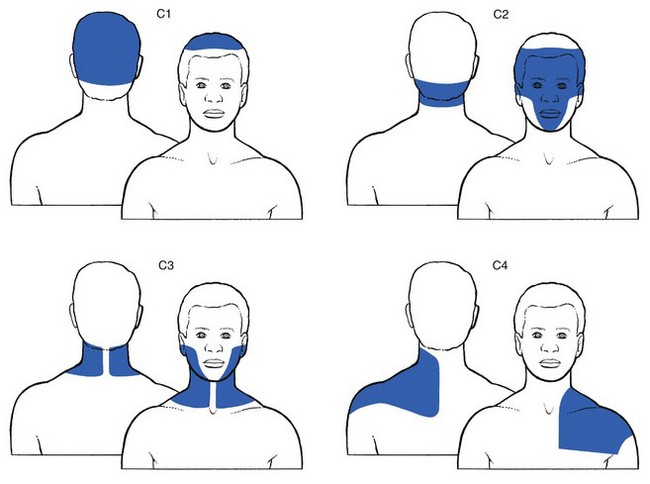
Fig 1.6 C1 to C4 dermatomes.
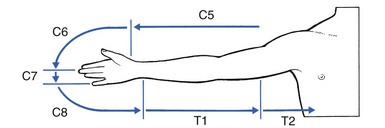
C5 to T2 are projected from the trunk to form the covering of the upper limb (Figs 1.7 and 1.8).
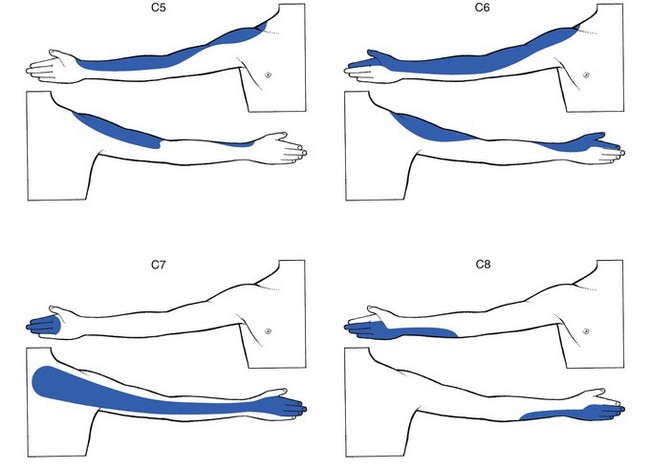
Fig 1.7 C5 to C8 dermatomes.
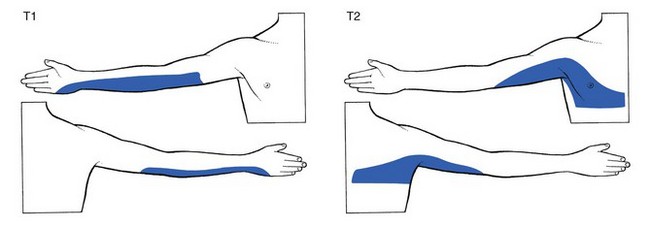
Fig 1.8 T1 and T2 dermatomes.
C5 covers the deltoid area and the outer aspect of the arm up to the base of the thumb.
C7 comprises the back of the arm and hand, together with index, long and ring fingers.
T1 includes the inner aspect of the forearm as far as the hypothenar eminence.
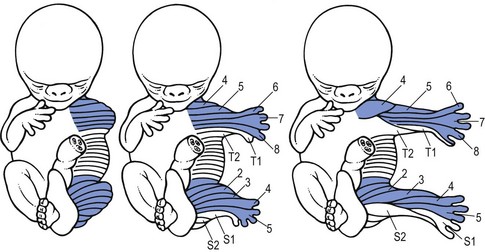
If the upper limb is held outstretched horizontally with the thumb pointing upwards, the original position of the embryological bud is recreated, and one can reconstruct the way the dermatomes project from the trunk. This is a good way to memorize the position of the separate dermatomes in the upper limb (see Fig. 1.9).
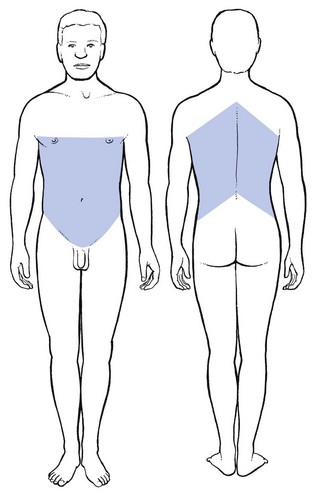
From T4 to T12, the dermatomes encircle the trunk, more or less following the original segmental construction of the embryo (Fig. 1.10).
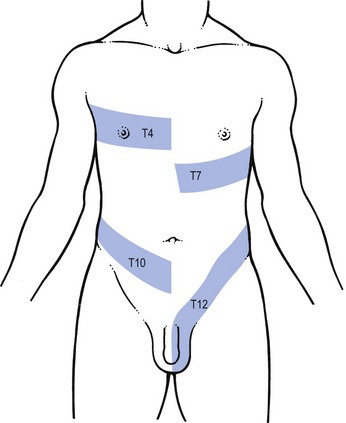
Fig 1.10 T4, T7, T10 and T12 dermatomes.
T4 constitutes the axilla and a patch on the front of the chest.
T4 encircles the trunk at the level of the nipple.
T7 reaches the lower costal margin and covers the xiphoid process.
Lumbar and sacral dermatomes
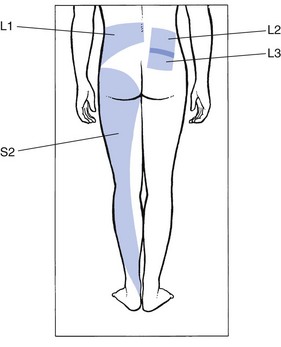
L1 is also more or less circular (Fig. 1.11). It comprises the lumbar region from the second to the fourth lumbar vertebrae, and runs along the upper aspect of the buttock and the iliac crest to the lower abdomen and the groin.
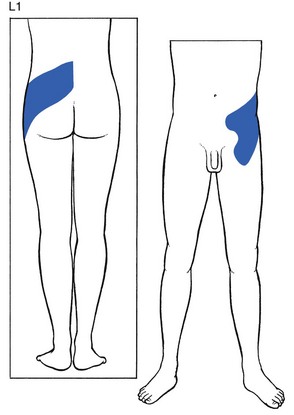
Fig 1.11 L1 dermatome.
L2 and L3 are two discontinuous areas, one in the lower lumbar region and upper buttock, and one in the leg (Fig. 1.12). The areas in the buttock largely overlap. Also, in the leg, there is considerable overlap between L2 and L3: L2 involves the whole front of the thigh, from the groin to the patella. L3 also takes in the anterior aspect of the thigh, but spreads further down, as far as the anterior and medial aspects of the ankle.
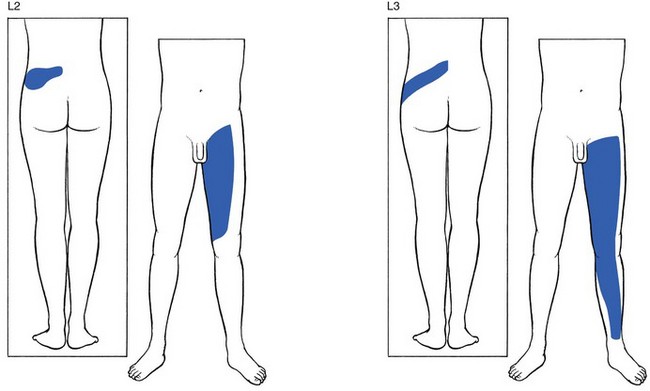
Fig 1.12 L2 and L3 dermatomes.
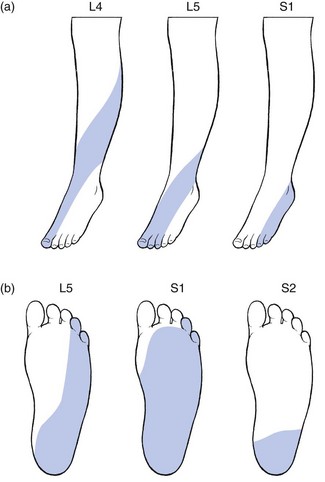
L4, L5 and S1 are completely disconnected from the trunk, overlying the surface of the leg and foot (Figs 1.13 and 1.14).64 In consequence, the third lumbar dermatome lies adjacent to the upper border of the second sacral dermatome at the lower buttock.
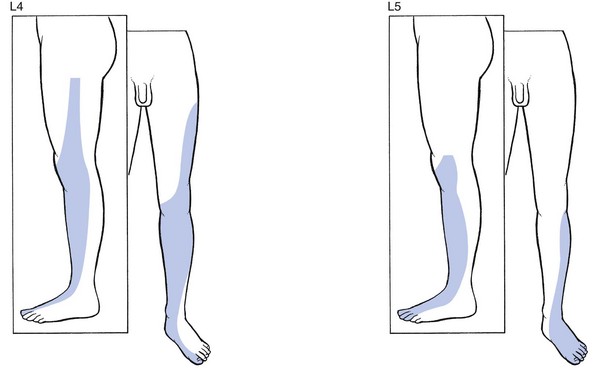
Fig 1.13 L4 and L5 dermatomes.
S1 includes the calf, the heel, the lateral malleolus and foot, the two outer toes and the whole sole of the foot (Fig. 1.15). Sicard and Leca65 demonstrated that the fifth lumbar and first sacral dermatomes also comprise a small vertical band at the posterior aspect of the thigh, which could account for the thigh pain commonly described by patients suffering from L5 or S1 sciatica.
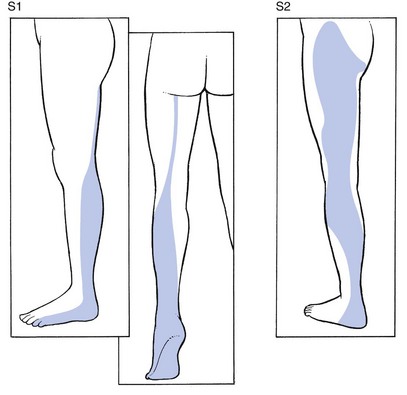
Fig 1.15 S1 and S2 dermatomes.
S2 is large and comprises the plantar aspect of the heel, the calf, the back of the whole thigh and the lower buttock. In the buttock, it borders with the lumbar part of L3 (Fig. 1.15).
S3 is a narrow zone at the inner side of the thigh, where it borders with L2 anteriorly and S2 posteriorly (Fig. 1.16). The tip ends just proximal to the knee. The upper extent reaches the inguinal ligament where it adjoins the 12th thoracic and first and second lumbar dermatomes. It follows that the groin is a confluence of dermatomes, and pain, apart from that of local origin, may be referred from a 12th thoracic, a first or second lumbar, or a third sacral origin. The groin is also a common site for extrasegmental dural pain reference.
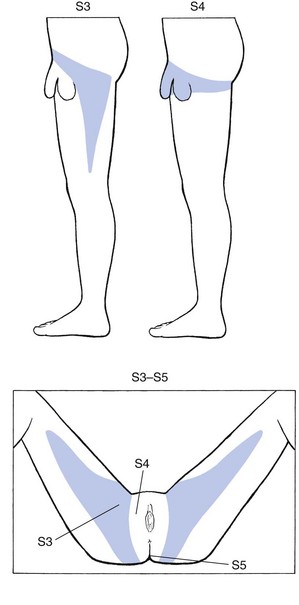
Fig 1.16 S3 to S5 dermatomes.
S4 comprises the saddle area, anus, perineum, and scrotum and penis or labia and vagina.
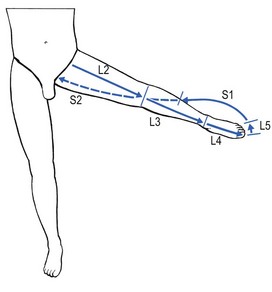
As in the upper limb, the original position of the distal embryological bud can be reconstructed by abducting the thigh to 90°, and by lateral rotation until the big toe points upwards. This position demonstrates the way the dermatomes were projected from the trunk and also constitutes a good way of memorizing the position of the various dermatomes in the lower limb (Fig. 1.17). Proximally to distally and then turning proximally again, the following are encountered: L2 at the anterior thigh, L3 at thigh and leg, L4 at the lateral aspect of the leg, anterior aspect of ankle and inner border of foot up to the big toe, L5 at the dorsum of the foot and the three inner toes, S1 at the lateral aspect of the foot, outer malleolus and calf, S2 at the posterior aspect of the leg; turning back to the trunk in the gluteal area, the boundary zone in the perineum, between leg and trunk is comprised of S3 and S4.
Discrepancies between dermatomes and myotomes
We have mentioned already that, as the outcome of embryological development, dermatomes do not always precisely cover the underlying myotomes. Cyriax described eight areas in the human body where the skin and the structure it covers have completely different embryological derivations (Cyriax45). These are the head, scapular and pectoral region, hand, intrathoracic and intra-abdominal region, buttock and scrotum.
Intra-abdominal region
Most of the intra-abdominal content also has a mid- and lower thoracic origin. The embryological derivation of the stomach and duodenum (T6–T10), liver (T7–T9 right), gall bladder (T6–T10 right),66 pancreas (T7–T8) and small intestine (T9–T10), fit very well with their actual localization in the abdominal cavity, and therefore pain derived from these organs approximates with their surface representation. Structures of lower thoracic, lumbar or even sacral origin, however, show a more complicated pattern of referred pain. The kidney and ureter, for instance, have a T11–L1 derivation and, although they are localized high up in the abdomen, referred pain can reach the inguinal fossa and the groin (T12–L1). The colonic flexure is from L2 to L3, which allows the pain not only to radiate to the lower back but also to the front of the thigh. The sigmoid colon and rectum have S3–S5 origin. Hence, in diseases of the sigmoid and the rectum, pain can be felt in the iliac fossa, perineum, penis, vulva and inner aspect of the thigh.
The buttock
The skin of the lower lumbar area and the outer buttock is derived from L1. At the upper buttock, there is considerable overlap with the lumbar patches of the L2 and L3 segments (Fig. 1.18). The skin of the lower buttock is derived from S2.
Referred pain in visceral diseases
It is important to emphasize that referred pain is not a phenomenon of orthopaedic medicine only. As described earlier, many visceral diseases also cause referred pain. For the convenience of practitioners often faced with thoracic or abdominal pain, a list of the segmental derivations of the viscera, based on the work of Cyriax45 (see his p. 30), William and Warwick,67 Lindsay et al68 and Guyton (cited by Van Cranenburgh69 is given in Box 1.2).
Dura mater an ‘exception’ to segmental reference
A possible explanation for the misleading pain reference of the dura may lie in its multisegmental origin, which is reflected in the great overlap between the fibres of the consecutive sinuvertebral nerves innervating its anterior aspect.70–73 More recent researchers describe division of the nerves into ascending and descending branches which ramify variously, to give off longitudinally and transversely orientated branches.74,75 In a recent study that used the very sensitive acetylcholinesterase method, more ramifications between the nerve branches were demonstrated (Fig. 1.19).76 Ascending branches up to four segments cranial to the level of entry into the dural nerve plexus and also descending branches extending up to four segments caudally were observed. In addition, many vertical and horizontal interconnections between the various ascending and descending branches were seen. The conclusion is that dural nerves may spread over eight segments and that a great overlap exists between adjacent and contralateral dural nerves.
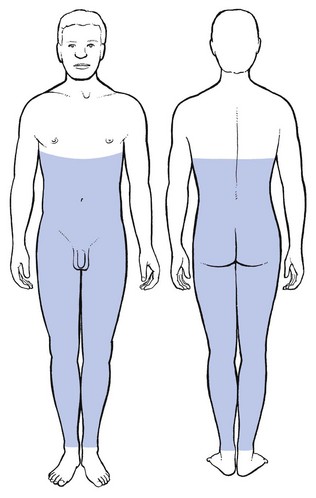
These findings may form an anatomical explanation for the clinical observations of Cyriax on the limits of extrasegmental reference of dural pain.77 Upwards, pain originating in the lower cervical part of the dura may spread to the occiput, skull and forehead (Fig. 1.20). Downwards it can descend to T7 which corresponds with the lower angles of the scapulae. Anteriorly the pain can occupy the whole pectoral area. Extrasegmental pain does not extend beyond the upper half of the arms.
A middle thoracic disc lesion can cause dural extrasegmental pain that may radiate to the base of the neck, and downwards to the whole abdomen and to the upper lumbar region (Fig. 1.21).
Dural extrasegmental reference from a low lumbar level may reach the lower thorax posteriorly, the lower abdomen deep to the umbilicus, the groins, the buttocks, and the sacrum and coccyx (Fig. 1.22). Unlike the cervical dura – which does not radiate far into the arms – extrasegmental reference from a lumbar origin may also involve the legs, both the anterior and the posterior aspects, and descend to the ankles.
Referred tenderness
• In cervical dural compression, the upper border of the trapezius, the scapular muscles or the base of the neck.
• In lower lumbar dural involvement, the sacroiliac region and the upper part of the buttocks.
If the tender area is palpated without the prior conduct of a proper functional examination of the neck or lumbar spine, the tenderness is regarded as the primary lesion, the more so because the patient insists that it is the apparent origin of the pain. It is no wonder then that ‘fibrositis’, ‘myofibrositis’ or ‘myofascial pain’ syndromes have long been regarded as primary lesions. The ‘fibrositis’ concept has been used to explain the cause of lumbago.78 Lewis33 was the first to recognize that trigger points and myalgic spots were not primary lesions and that the painful area, although tender to the touch, did not contain a focus.
Cyriax considered that the localized tender area is a secondary effect of pressure on the dura mater.79,80 He derived his theory from the simple clinical observation that the tender spot shifted from place to place over a few seconds after a successful manipulation and that, when a full and painless range of motion had been restored, the tenderness disappeared.
However, referred tenderness has also been demonstrated in muscular and fibrous tissue lesions,81 in visceral lesions such as ischaemic heart attacks82 and in pathological viscera. The phenomena are not completely understood as yet but it is reasonable to assume that trigger points are caused by summation mechanisms which can be understood in terms of gate-control mechanisms.79
Summation – the excitatory effect of converging inputs – is an important pain mechanism. Pain may be triggered by two sources of nerve impulses: a major one from the lesion; and a minor one from normal skin which add together. If one source is removed, it becomes more difficult for the other to trigger the feeling of pain.83
Factors determining reference of pain
The degree of reference – that is the distance between the localization of the perception and the site of the lesion – depends on four different factors (Box 1.3). Some can be explained on the basis of the known pathways, others remain unexplained and result purely from clinical observations.
The strength of the stimulus
The mechanism of this phenomenon is probably based on the fact that the more the peripheral sensory nerve fibres are stimulated, the more there is cerebral cortical activity.84
The depth of the affected structure
As early as 1939, Kellgren35, and Lewis and Kellgren85 stated that the localizing ability of a structure depended largely on its depth from the surface. This was confirmed later by other studies.38,44
References
1. Bonica, JJ, Albe Fessard, D. Advances in Pain Research and Therapy. New York: Raven Press; 1976.
2. Elton, D, Stuart, GV, Burrows, GD, Self-esteem and chronic pain. J Psychosom Res 1978; 22:25. ![]()
3. Merskey, H, Pain terms: a list with definitions and notes on usage. Recommended by the IASP Subcommittee on Taxonomy. Pain 1979; 6:249. ![]()
4. Melzack, R, Torgerson, WS, On the language of pain. Anaesthesiology 1971; 35:50. ![]()
5. Wyke, BD. Neurological mechanisms in the experience of pain. Acupunct Electrother Res J. 1979; 4:27.
6. Ralston, HJ, Miller, MR, Kasahara, M, Nerve endings in human fasciae, tendons, ligaments, periosteum and joint synovial membrane. Anat Rec 1960; 136:137. ![]()
7. Besson, JM, Guilbaud, G, Abdelmoumene, M, Chaouch, A, Physiologie de la nociception. J Physiol (Paris) 1982; 78:7–107. ![]()
8. Iggo, A. The case for ‘pain’ receptors. In: Janzen R, Keidel WD, Herz A, Steichele C, eds. Pain: Basic Principles, Pharmacology, Therapy. Stuttgart: Thieme; 1972:60.
9. Van Hees, J, Gybels, JM, Pain related to single afferent C fibres from human skin. Brain Res 1972; 48:397. ![]()
10. Sarkin, LS, Wallace, MS, Acute pain mechanisms. Surg Clin North Am. 1999;79(2):213–229. ![]()
11. Dray, A, Inflammatory mediators of pain. Br J Anaesth 1995; 75:125–131. ![]()
12. Reeh, PW, Steen, KH, Tissue acidosis in nociception and pain. Progress in Brain Research. Kunasawa, T, Kruger, C, Ulisumara, K, eds. Progress in Brain Research; vol. 13. Elsevier Science, Amsterdam, 1996:143–151.
13. Wall, PD, McMahon, SB, Microneuronography and its relation to perceived sensation. Pain 1985; 21:209. ![]()
14. Nathan, PW, The gate-control theory of pain: a critical review. Brain 1976; 99:123. ![]()
15. Price, DD, Dubner, R, Neurons that subserve the the sensory-discriminative aspects of pain. Pain 1977; 3:307. ![]()
16. Wyke, BD. The neurology of low back pain. In: Jayson MIV, ed. The Lumbar Spine and Back Pain. 2nd ed. Bath: Pitman Medical; 1980:265–339.
17. Yaksh, TL. Spinal Afferent Processing. New York: Plenum; 1986.
18. Melzack, R, Casey, KL. Sensory, motivational and central control determinants of pain: a new conceptual model. In: Kenshalo D, ed. The Skin Senses. Springfield: Thomas; 1968:923–993.
19. Hand, PJ, Morrison, AR, Thalamocortical projections from the ventrobasal complex to somatic sensory area I and II. Exp Neurol 1970; 27:291. ![]()
20. Desijaru, T, Purpura, DP, Organisation of specific–nonspecific thalamic internuclear synaptic pathways. Brain Res 1970; 21:169. ![]()
21. Penfield, W. The role of the temporal cortex in recall of past experience and interpretation of the present. In: Wolstenholme GEW, O’Connor CM, eds. The Neurological Basis of Behaviour. London: Churchill; 1958:149.
22. Newcombe, F. Memory. In: Critchley M, O’Leary JL, Jennett B, eds. Scientific Foundations of Neurology. London: Heinemann; 1972:205.
23. Black, P. Physiological Correlates of Emotion. New York: Academic Press; 1970.
24. Melzack, R, Wall, PD, Pain mechanism: a new theory. Science 1965; 150:971–979. ![]()
25. Noordenbos, W. Pain. Problems Pertaining to the Transmission of Nerve Impulses Which Give Rise to Pain. Amsterdam: Elsevier; 1959.
26. Mayer, DJ, Price, D, Central nervous system of analgesia. Pain 1976; 2:379. ![]()
27. Tan, S-Y, Cognitive and behavioural methods for pain control: a selective review. Pain 1982; 12:201–228. ![]()
28. Langen, D. Psychosomatic aspects in the treatment of pain. In: Janzen R, Keidel WD, Herz A, Steichele C, eds. Pain: Basic Principles, Pharmacology, Therapy. Stuttgart: Thieme; 1972:164.
29. Chapman, CR, Feather, BW, Effects of diazepam on human pain tolerance and pain sensitivity. Psychosom Med 1973; 35:330. ![]()
30. Fields, HL, Henricher, MH, Anatomy and physiology of a nociceptive modulatory system. Phil Trans Roy Soc B 1985; 308:361–379. ![]()
31. Head, H. The afferent nervous system from a new aspect. Brain. 1905; 28:99.
32. Head, H, Campbell, AW. The pathology of herpes zoster and its bearing on sensory location. Brain. 1900; 23:353–523.
33. Lewis, T. Pain. New York: MacMillan; 1942.
34. Kellgren, JH. Observations of referred pain arising from muscle. Clin Sci. 1938; 3:175.
35. Kellgren, JH. On the distribution of pain from deep somatic structures. Clin Sci. 1939; 4:35.
36. Inman, VT, Saunders, JB de CM. Referred pain from skeletal structures. J Nerv Ment Dis. 1944; 99:660.
37. Travell, J, Berry, C, Bigelow, N. Effects of referred somatic pain on structures in the reference zone. Fed Proc. 1944; 3:49.
38. McCall, IW, Park, WM, O’Brien, JP, Induced pain referred from posterior elements in normal subjects. Spine 1979; 4:441. ![]()
39. Hansen, K, Schliack, H. Segmental Innervation. Stuttgart: Thieme; 1962.
40. Kunert, W. Wirbelsäule and Innere Medizin. Stuttgart: F. Enke; 1975.
41. Foerster, O. Dermatomes in man. Brain. 1933; 56:1.
42. Lewis, T, Kellgren, JH. Observations relating to referred pain. Visceromotor reflexes and other associated phenomena. Clin Sci. 1939; 4:47.
43. Keegan, JJ, Garett, FD, The segmental distribution of the cutaneous nerves in the limbs of man. Anat Rec 1948; 102:409. ![]()
44. Hockaday, JM, Whitty, CWM, Patterns of referred pain in the normal subject. Brain. 1967;90(3):481–496. ![]()
45. Cyriax, JH. Textbook of Orthopaedic Medicine, vol. 1, 8th ed. London: Baillière Tindall; 1982.
46. MacKenzie, J. Krankheitszeichen und ihre Auslegung 3. Würzburg: Kabitsch; 1917.
47. Livingston, WK. Pain Mechanisms. New York: Macmillan; 1944.
48. Wedell, G, Sinclair, DG, Feindel, WH, Anatomical basis for alterations in quality of pain sensibility. J Neurophys 1948; 11:99. ![]()
49. Wedell, G, Referred pain in relation to the mechanism of common sensibility. Proc Roy Soc Med 1957; 50:581. ![]()
50. Pomeranz, B, Wall, PD, Weber, WV, Cord cells responding to fine myelinated afferents from viscera, muscle and skin. J Physiol 1968; 199:511–532. ![]()
51. Taylor, DCM, Pierau, FR-K, Mizutain, M. Possible bases for referred pain. In: Holden AV, Winslow W, eds. The Neurobiology of Pain. Manchester: Manchester University Press; 1984:143.
52. Wells, PE, Frampton, V, Bowsher, D. Pain Management by Physiotherapy, 2nd ed. Oxford: Butterworth-Heinemann; 1994.
53. Cyriax, JH. Massage, Manipulation and Local Anaesthesia. London: Hamilton; 1941.
54. Ruch, TC. Visceral sensation and referred pain. In: Fulton JF, ed. Howell’s Textbook of Physiology. Philadelphia: WB Saunders, 1946.
55. Merskey, H, Spear, FG. Pain: Psychological and Psychiatric Aspects. London: Baillière Tindall and Cassell; 1967.
56. Neurological aspects of the diagnosis and treatment of facial pain. In: Cohen B, Kramer I, eds. Scientific Foundations of Dentistry. London: Heinemann; 1974:278.
57. Patten, BM. Human Embryology. New York: McGraw-Hill; 1968.
58. Head, H, Campbell, AW. The pathology of herpes zoster and its bearing on sensory location. Brain. 1900; 23:353–523.
59. Keegan, JJ, Garrett, ED, The segmental distribution of the cutaneous nerves in the limbs of man. Anat Rec 1949; 101:409. ![]()
60. Fukui, S, Ohseto, K, et al, Distribution of referred pain from the zygapophyseal joints and dorsal rami. Clin J Pain. 1997;13(4):303–307. ![]()
61. Cole, JP, Lesswing, AL, Cole, JR, Analysis of lumbosacral dermatomes in man. Clin Orthop 1968; 61:241. ![]()
62. Conesa, SH, Argote, ML. A Visual Aid to the Examination of Nerve Roots. London: Baillière Tindall; 1976.
63. Wakasugi, B. Dermatomes of the body and the extremeties. Surg Treatment. 1982; 132:270.
64. Mitta, H, Study on dermatomes by means of selective lumbar spinal nerve block. Spine 1993; 18:1782–1786. ![]()
65. Sicard, A, Leca, A, Place de rachiotomie dans le traitement chirurgical des sciatiques. Press Med 1954; 62:1737. ![]()
66. Doran, FSA, The sites to which pain is referred from the common bile duct in man and its implication for the theory of referred pain. Br J Surg 1967; 54:599–606. ![]()
67. Williams, PL, Warwick, R. Gray’s Anatomy. Edinburgh: Churchill Livingstone; 1980.
68. Lindsay, KW, Bone, I, Callander, R. Neurology and Neurosurgery Illustrated, 2nd ed. Edinburgh: Churchill Livingstone; 1991.
69. Van Cranenburgh, B. Segmentale verschijnselen. Utrecht: Bohn, Scheltema and Holkema; 1985.
70. Pedersen, HE, Conrad, FJ, Blunck, MD, Gartner, E, The anatomy of lumbosacral posterior rami and meningeal branches of spinal nerves (sinu-vertebral nerves). J Bone Joint Surg. 1956;38A(2):377–391. ![]()
71. Stillwell, DL, The nerve supply of the vertebral column and its associated structures in the monkey. Anat Rec. 1956;125(2):139–162. ![]()
72. Kimmel, DL, Innervation of spinal dura mater, and dura mater of the posterior cranial fossa. Neurology 1961; 11:800–809. ![]()
73. Edghar, MA, Nundy, S. Innervation of the spinal dura mater. J Neurol Neurosurg Psychiatr. 1966; 29:530–534.
74. Jackson, HC, Winkelmann, RK, Bickel, WH, Nerve endings in the human lumbar spinal column and related structures. J Bone Joint Surg 1966; 48A:1272–1281. ![]()
75. Edgar, MA, Ghadially, JA, Innervation of the lumbar spine. Clin Orthop Rel Res 1976; 115:35–41. ![]()
76. Groen, GJ, Baljet, B, Drukker, J, The innervation of the spinal dura mater: anatomy and clinical implications. Acta Neurochir 1988; 92:39–46. ![]()
77. Cyriax, JH, Dural pain. Lancet 1978; 1:919–921. ![]()
78. Gowers, W, Lumbago. BMJ 1904; i:117. ![]()
79. Travell, JG, Simons, DG, Myofascial Pain and Dysfunction. Williams and Wilkins, Baltimore, 1983.. ![]()
80. Cyriax, JH, Fibrositis. BMJ 1948; ii:251. ![]()
81. Simons, DG, Muscle pain syndromes, part II. Am J Phys Med 1976; 55:15–42. ![]()
82. Kennard, MA, Haugen, FP, The relation of subcutaneous focal sensitivity to referred pain of cardiac origin. Anaesthesiology 1955; 16:297–311. ![]()
83. Melzack, R, Wall, P, The Challenge of Pain. Penguin, London, 1991.. ![]()
84. Woolsey, CN, Marshall, WH, Bard, P. Observations on cortical somatic sensory mechanism of cat and monkey. J Neurophysiol. 1941; 4:1.
85. Lewis, T, Kellgren, JH. Observations relating referred pain, visceromotor reflexes and other associated phenomena. Clin Sci. 1939; 4:47.