Paediatric Anaesthesia
PHYSIOLOGY IN THE NEONATE
Before the age of 8 years, the calibre of the airways is relatively narrow. Airway resistance is therefore relatively high. Small decreases in the diameter of the airways as a result of oedema or respiratory secretions significantly increase the work of breathing. Elastic tissue in the lungs of small children is poorly developed. As a result of this, compliance is decreased. This has important consequences in that airway closure may occur during normal tidal ventilation, thereby bringing about an increase in alveolar–arterial oxygen tension difference (PA − aO2). This explains why PaO2 is lower in the infant than in the child. The decreased compliance results in ventilatory units with short time constants. Consequently, the infant is able to achieve adequate alveolar ventilation whilst maintaining a high respiratory rate. However, because of the increased resistance and decreased compliance, the work of breathing may represent up to 15% of total oxygen consumption (Table 36.1). The high respiratory rate is necessary because the metabolic rate of the infant is nearly twice that of the adult. The high alveolar minute ventilation explains why induction and emergence from inhalational anaesthesia are relatively rapid in small children. The high metabolic rate also explains why desaturation occurs very rapidly in children.
TABLE 36.1
Lung Mechanics of the Neonate Compared with the Adult
| Neonate | Adult | |
| Compliance (mL cmH2O−1) | 5 | 100 |
| Resistance (cmH2O L−1 s−1) | 30 | 2 |
| Time constant (s) | 0.5 | 1.3 |
| Respiratory rate (breath min−1) | 32 | 15 |
The ratio of physiological dead space to tidal volume (VD/VT) is similar to that of the adult at about 0.3. However, because the volumes are smaller, modest increases in VD produced by equipment such as humidification filters may have a disproportionately greater effect (Table 36.2).
TABLE 36.2
Respiratory Variables in the Neonate
| Tidal volume (V) | 7 mL kg−1 |
| Dead space (VD) | (VT) × 0.3 mL |
| Respiratory rate | 32 breath min−1 |
Cardiovascular System
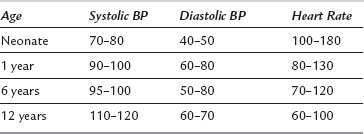
The process of growth demands a high metabolic rate. It is, therefore, not surprising that infants and children have a higher cardiac index compared with the adult, so that oxygen and nutrients may be delivered to actively growing tissues. The ventricles of neonates and infants are poorly compliant, so even though the ventricles of infants demonstrate the Frank-Starling mechanism, the main determinant of cardiac output is heart rate. Infants tolerate heart rates of 200 beat min−1 with ease (Table 36.3). Bradycardia may occur readily in response to hypoxaemia and vagal stimulation and it results in a decrease in cardiac output. Immediate cessation of the stimulus, and treatment with oxygen and atropine, are absolutely crucial. A heart rate of 60 beat min−1 in an infant is considered a cardiac arrest and requires cardiac massage. Arrhythmias are rare in the absence of cardiac disease. The usual cardiac arrest scenarios are electromechanical dissociation and asystole, not ventricular fibrillation.
Haemoglobin
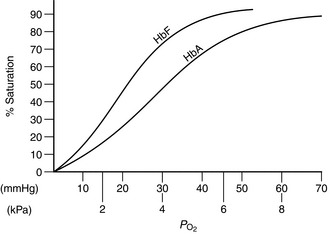
At birth, 75–80% of the neonate’s haemoglobin is fetal haemoglobin (HbF). By the age of 6 months, adult haemoglobin (HbA) haematopoiesis is fully established. HbF has a higher affinity for oxygen than HbA. This is demonstrated by the leftward shift of the oxygen haemoglobin dissociation curve (Fig. 36.1). Low tissue PO2 and metabolic acidosis in the tissues result in the avidity of HbF for oxygen being reduced, thereby aiding delivery of oxygen. Alkalosis produced by hyperventilation results in less oxygen being available and it is therefore sensible to maintain normocapnia.
Renal Function and Fluid Balance
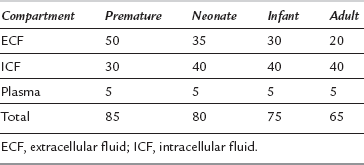
Body fluids constitute a greater proportion of body weight in the infant, particularly the premature infant, compared with the adult (Table 36.4). In an adult, most of the total body water is in the intracellular compartment. In a newborn infant, most of the total body water is in the extracellular compartment. With increasing age, the ratio reverses. Plasma volume remains constant throughout life at about 5% of body weight.
Fluid Therapy
An intravenous infusion delivering maintenance fluids should be in place for all neonates requiring surgery. Maintenance fluid requirements increase over the first few days of life (Tables 36.5, 36.6). The normal infant requires of the order of 3–5 mmol kg−1 of sodium and an equivalent amount of potassium per day to maintain normal serum electrolyte concentrations. The ability of the infant’s kidneys to eliminate excess sodium is limited. Exceeding this amount in the absence of loss results in hypernatraemia and its sequelae. Infants undergoing any procedure more than the briefest should also have their calorific needs addressed. This may be achieved by including glucose-containing fluids in the regimen; failure to do so results in hypoglycaemia and ketosis. This may occur rapidly because of the limited glycogen stores and high metabolic rate of the infant.
TABLE 36.5
Fluid Requirements in the First Week of Life
| Day | Rate (mL kg−1 Day−1) |
| 1 | 0 |
| 2, 3 | 50 |
| 4, 5 | 75 |
| 6 | 100 |
| 7 | 120 |
TABLE 36.6
Maintenance Fluid Requirements
| Weight (kg) | Rate (mL kg−1 Day−1) |
| Up to 10 kg | 100 |
| 10–20 kg | 1000 + 50 × [weight (kg) – 10] mL |
| 20–30 kg | 1500 + 25 × [weight (kg) – 20] mL |
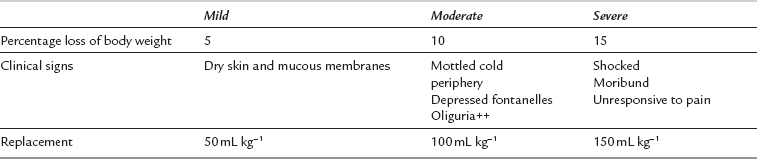
It is imperative that the anaesthetist recognizes and resuscitates the dehydrated infant appropriately before surgery. Clinical examination of skin turgor, capillary refill, tension of fontanelles, arterial pressure and venous filling may aid estimation of hydration, but electrolyte and haemoglobin concentrations and haematocrit, urine volumes and plasma and urine osmolalities should be monitored if problems of fluid balance exist (Table 36.7).
PHARMACOLOGY IN THE NEONATE
Specific Drugs in Paediatric Anaesthesia
Nitrous Oxide: Nitrous oxide is used as a carrier gas for most inhalational anaesthetic agents. It is also used for its MAC-sparing effect. The effect is most marked with halothane, with which a 60% reduction can be achieved. However, with the newer agents such as sevoflurane, only a 25% reduction may be produced. Thus, there would appear to be little to be gained by adding nitrous oxide to a sevoflurane anaesthetic. A major problem with nitrous oxide is its greater solubility compared with nitrogen. It diffuses into closed nitrogen-containing spaces at a greater rate than nitrogen leaves, thereby causing expansion. This effect is particularly important in lung lesions such as pneumothorax and congenital lobar emphysema. Expansion of the bowel in exomphalos or gastroschisis may make surgical reduction into the peritoneal cavity difficult.
Halothane: This agent has been the gold standard for induction of anaesthesia in children, because until recently its odour was one of the least pungent. It shares with all anaesthetic agents the ability to depress the myocardium. However, it also slows heart rate, causing a decrease in cardiac output. It is therefore prudent to give an anticholinergic before its administration. Induction with halothane is smooth, and because most vaporizers allow 5 × MAC to be administered, it may be given in almost 100% oxygen. This is a useful feature when anaesthetizing a child with an airway problem. Another property which makes halothane useful in this situation is its prolonged action compared with the newer volatile agents, as it is undesirable that anaesthesia ‘lightens’ during instrumentation of the airway. In adult anaesthetic practice, repeat administrations of halothane within a period of less than 3 months may be associated with hepatic dysfunction and occasionally with fulminant hepatic failure. The exact mechanism of this toxic effect is not clear, but some have speculated that a reductive metabolite of halothane is responsible. Reductive hepatic metabolism of drugs is developed poorly in children and this may explain why this problem is extremely rare in children. However, if a child needs a second anaesthetic within 3 months of a first halothane anaesthetic, a risk–benefit assessment has to be undertaken. Economic considerations dictate that this is the most widely used inhalational agent for induction and maintenance of anaesthesia in children worldwide. As one might predict from its physical characteristics, emergence from halothane anaesthesia tends to take longer compared with the newer agents. It is acceptable to induce anaesthesia with halothane and then to use a less lipid-soluble agent for maintenance.
Isoflurane: Cardiac output tends to be well maintained when less than 1 MAC of this agent is used for maintenance of anaesthesia. However, because of its pungency, it is not a suitable agent for induction. In spite of the fact that it has a low blood/gas partition coefficient, induction tends to be slow because of breath-holding. It may be used for maintenance of anaesthesia after sevoflurane or intravenous induction.
Sevoflurane: The blood/gas partition coefficient of 0.68 results in rapid induction of anaesthesia with this agent and also a quick recovery. It is the least pungent of the currently available agents. It is possible to turn the vaporizer to its maximum output of 8% without experiencing significant problems of coughing, breath-holding or laryngeal spasm. There is little to be gained by including nitrous oxide during induction, as the MAC-sparing effect on sevoflurane is not as great as with other agents. It is not unusual to observe slowing of the heart rate during induction, but it is not usually necessary to give an anticholinergic. Cardiac arrhythmias do not commonly occur during induction or maintenance with sevoflurane. Economic considerations dictate that the agent is used mainly for induction, followed by a cheaper agent such as isoflurane for maintenance. Sevoflurane is an excellent choice for induction in children with upper airway obstruction. The agent is partly degraded by soda lime to compound A, which is nephrotoxic in rats because they possess the enzyme β-lyase. This hazard would appear to be theoretical in humans.
Desflurane: The blood/gas partition coefficient of 0.42 suggests that induction should be rapid, but this is not so because desflurane is very irritant to the upper airway. It may be used for maintenance of anaesthesia with the benefit that emergence is very rapid. This may be particularly desirable in the ex-premature infant. Cost and the fact that the agent has to be delivered using a special vaporizer limit the use of desflurane in paediatric practice.
Intravenous Agents
Barbiturates: Thiopental is the most widely used in this class of agents. A dose of 5–6 mg kg−1 of a 2.5% solution is required in the healthy child. The main advantage is that injection into a small vein is pain-free. The agent can be given rectally using a 10% solution at a dose of 30 mg kg−1, but induction and recovery tend to be slow with this technique.
Propofol: Propofol may be used for both induction and maintenance of anaesthesia as part of a total intravenous technique. Although not common in paediatric anaesthetic practice, the technique of total intravenous anaesthesia using propofol is very useful in the child prone to malignant hyperthermia or the child with porphyria. Induction and maintenance doses tend to be larger in the younger healthy patient. The reasons for this are because of a larger central volume of distribution and because the clearance of the agent is higher compared with the adult. Pain on injection is a problem and this may be lessened by the addition of 0.2 mg kg−1 of lidocaine. Propofol stabilized in a medium-chain triglyceride emulsion appears to cause less pain on injection.
Ketamine: The current formulation of this drug is a racemic mixture of the S(+) and R(–) enantiomers. It is possible to separate the two enantiomers, although at present this does not appear to be a commercially viable proposition. The reason for doing this is that the S(+) enantiomer is twice as potent, recovery is quicker and the incidence of emergence reactions is lower. One of the major advantages of ketamine is the intense analgesia it provides. The analgesia has both spinal and supraspinal components. Epidural administration of the drug in combination with local anaesthetic significantly prolongs the duration of analgesia compared with local anaesthetic alone. The lack of cardiovascular depression in vivo is a feature which allows the drug to be used for inducing anaesthesia in children with congenital heart disease. Occasionally, pulmonary vascular resistance may increase, and as a consequence pulmonary pressures also increase. Even though upper airway reflexes are relatively well preserved, aspiration of gastric contents may still occur. The drug has bronchodilator properties and may be used for sedating the child with status asthmaticus to allow artificial ventilation in the ICU. It is prudent to administer an anti-cholinergic when the drug is used for maintenance of anaesthesia, because increased salivation and bronchial secretions are potential problems. Emergence from ketamine anaesthesia is slower than with other agents. It may be accompanied by emergence phenomena such as hallucinations and unpleasant dreams. The incidence of these can be reduced by concurrent administration of a benzodiazepine.
Opioids: These may be used in large doses as the sole agent to provide stable haemodynamic conditions for children with cardiac disease. The major disadvantage of using this technique is that drug effects persist into the postoperative period, causing respiratory depression. As a result, postoperative mechanical ventilation is mandatory. Morphine is the drug used most commonly for the management of severe pain in children. Hepatic glucuronidation is the process by which it is eliminated. Morphine is converted to morphine-3- and morphine-6-glucuronide. These metabolites are active. Morphine-3-glucuronide antagonizes the analgesic effects of morphine-6-glucuronide. The very young suffer from respiratory depression at lower morphine infusion rates. This is probably because of sensitivity of the brainstem and also because the ratio of morphine-3- to morphine-6-glucuronide is lower. Remifentanil is the newest of the synthetic opioids. This drug is unique in that its metabolism to a virtually inactive metabolite is by non-specific esterases in blood and tissue. The half-life of the drug is independent of the duration of infusion. There are few data on the use of this drug in infants and children, and at present it is unlicensed for use in children under the age of 2 years. However, it is likely to be valuable in infants because of its lack of accumulation and short half-life. Opioid-induced respiratory depression may be reversed by naloxone. The reversal is short-lived and it is probably wise to mechanically ventilate the lungs of children in this state.
Neuromuscular Blocking Drugs and their Antagonists
Succinylcholine: This depolarizing neuromuscular blocking drug has the most rapid onset of action of any currently readily available agent. It is therefore the drug of choice for the patient with a full stomach and also for the treatment of laryngeal spasm. In the infant, it has the ability to cause bradycardia after only a single dose. It is wise to administer an anticholinergic before administration. A hyperkalaemic response is not seen after administration to children with myelomeningocoele or cerebral palsy. It is one of the most potent triggers for malignant hyperthermia. The incidence of this increases if succinylcholine is preceded by induction of anaesthesia with halothane. Fatal cardiac arrest has occurred in a small number of patients. It is presumed that these patients had unsuspected muscular dystrophies and that the drug caused massive muscle breakdown. As it is not possible to predict which patients might exhibit this response, it is wise to limit the use of the drug to patients with a full stomach and for the relief of laryngospasm.
Non-Depolarizing Agents: Onset and duration of action and the response in patients with renal and hepatic disease are probably the most important considerations when choosing one of these agents. Rocuronium has the most rapid onset of all the currently available agents. The onset of all these drugs may be increased by giving larger doses, but this is counterbalanced by a correspondingly longer duration of action. Recently, a new amino-steroid, rapacuronium, was evaluated in paediatric practice. Data suggested that its onset of action was similar to that of succinylcholine and that its duration of action was comparable to that of mivacurium. However, there were several reports of bronchospasm and hypoxaemia, especially in small children, and the drug was withdrawn. Mivacurium has the shortest duration of action of the currently available drugs. Mivacurium is best suited for the short surgical procedure, which usually matches its duration of action. Very occasionally, a patient may be cholinesterase-deficient, in which case its duration is long. Atracurium, rocuronium and vecuronium have an intermediate duration of action, making them the most commonly used, as the duration of most paediatric operations falls into this category. Cisatracurium and pancuronium are best reserved for longer procedures. Pancuronium is excreted renally and should be used with caution in the patient with renal failure. In spite of the fact that vecuronium and rocuronium are excreted by the liver, their duration of action is minimally affected by hepatic disease. Atracurium or cisatracurium are the most obvious choices for patients with renal or hepatic disease because elimination is altered minimally by organ failure.
ANAESTHETIC MANAGEMENT
It is extremely important that the child is weighed before arrival in theatre, because body weight is the simplest and most reliable guide to drug dosage. Veins suitable for insertion of a cannula should be identified and, if possible, local anaesthetic cream applied and covered with an occlusive dressing. If it has not been possible to weigh the child, the weight may be estimated from the child’s age (Table 36.8).
TABLE 36.8
Estimates of Children’s Weight
| Age | Approximate Body Weight (kg) |
| Neonate | 3 |
| 4 months | (Age in months × 0.5) + 4 = 6 |
| 1–8 years | (age in years × 2) + 8 |
| 9–13 years | (age in years × 3) + 7 |
Premedication
 EMLA (eutectic mixture of local anaesthetics) has been available for nearly two decades. Venepuncuture is usually painless if it has been applied and an occlusive dressing placed over the site at least 1 h before the planned procedure. It is wise to apply it over at least two locations marked by the anaesthetist in case the first attempt fails. It should not be used in the very small child or on mucous membranes because of the danger of systemic absorption of prilocaine that results in methaemoglobinaemia. It should not be left on the skin for more than 5 h. A major disadvantage of EMLA is that it causes some venoconstriction and this may obscure the vein.
EMLA (eutectic mixture of local anaesthetics) has been available for nearly two decades. Venepuncuture is usually painless if it has been applied and an occlusive dressing placed over the site at least 1 h before the planned procedure. It is wise to apply it over at least two locations marked by the anaesthetist in case the first attempt fails. It should not be used in the very small child or on mucous membranes because of the danger of systemic absorption of prilocaine that results in methaemoglobinaemia. It should not be left on the skin for more than 5 h. A major disadvantage of EMLA is that it causes some venoconstriction and this may obscure the vein.
 Tetracaine gel is the other agent available for this purpose. It has the advantage of a quicker onset of action and also provides analgesia for a considerable period of time after the occlusive dressing has been removed (4 h). This is an advantage in the day-care unit, because it may be applied as part of the admission procedure for all the children, left on for about 45 min and then removed, as small children often object to the presence of the occlusive dressing.
Tetracaine gel is the other agent available for this purpose. It has the advantage of a quicker onset of action and also provides analgesia for a considerable period of time after the occlusive dressing has been removed (4 h). This is an advantage in the day-care unit, because it may be applied as part of the admission procedure for all the children, left on for about 45 min and then removed, as small children often object to the presence of the occlusive dressing.
Induction
SPECIFIC OPERATIONS IN THE NEONATE
Anon. Postgraduate educational issue: the paediatric patient. Br. J. Anaesth. 1999;83:16–129.
Arthurs, G., Nicholls, B. Ultrasound in anaesthetic practice. Cambridge: Cambridge University Press; 2009.
Baum, V., O’Flaherty, J. Anesthesia for genetic, metabolic and dysmorphic syndromes of childhood. Baltimore: Lippincott Williams & Wilkins; 2006.
Dalens, B. Regional anesthesia for infants, children and adolescents, second ed. Baltimore: Williams & Wilkins; 2001.
McKenzie I., Gaukroger P.B., Ragg P., Brown T.C.K., eds. Manual of acute pain management in children. Edinburgh: Churchill Livingstone, 1997.
Motoyama, E.K., Davis, P.J. Smith’s anesthesia for infants and children. St Louis: Mosby; 2006.
Peutrell, J.M., Mather, S.J. Regional anaesthesia for babies and children. Oxford: Oxford University Press; 1997.
Rowney, D.A., Doyle, E. Epidural and subarachnoid blockade in children. Anaesthesia. 1998;53:980–1001.